Functional and Conformational Plasticity of an Animal Group 1 LEA Protein
Abstract
:1. Introduction
2. Material and Methods
2.1. Chemicals
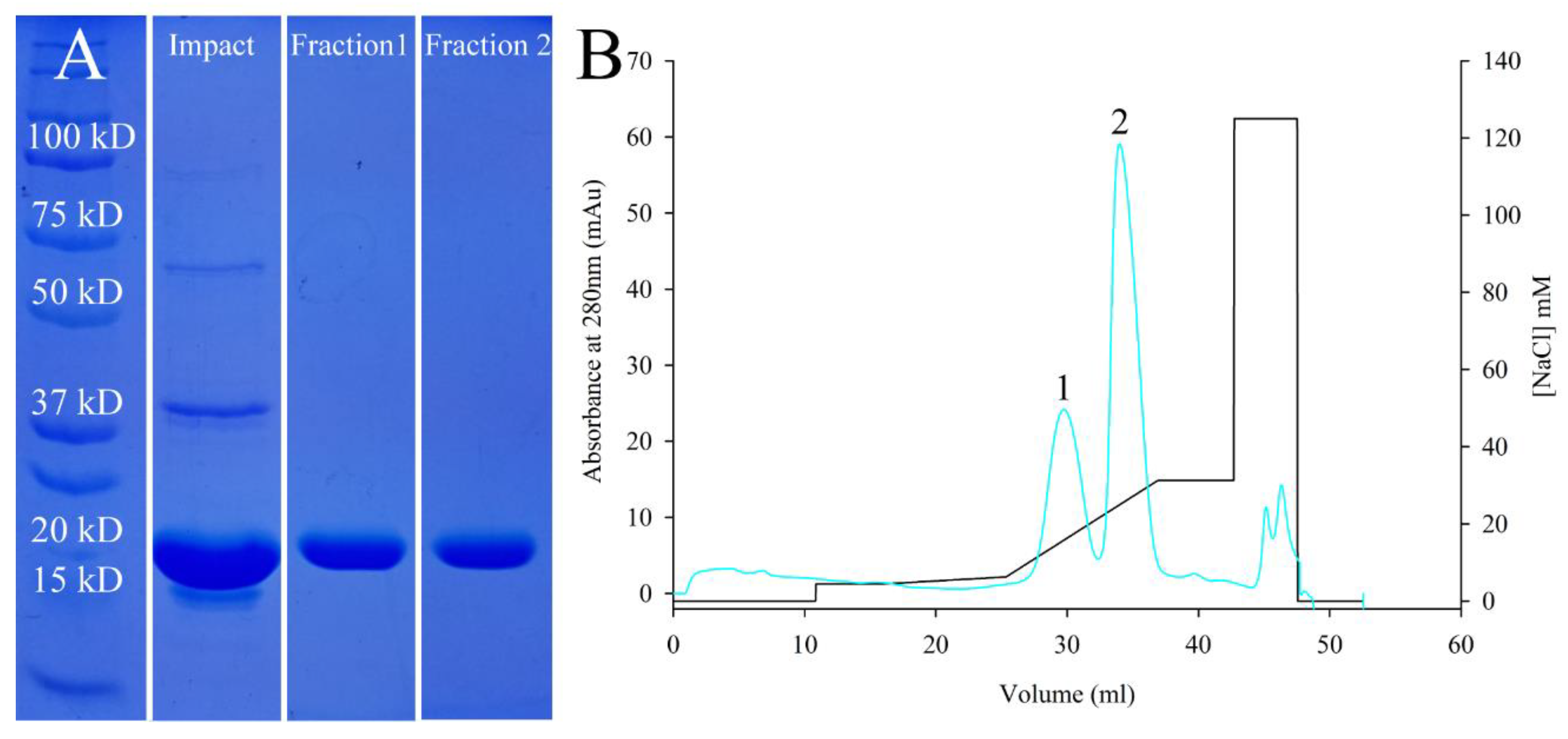
2.2. Protein Cloning, Expression, and Purification
2.3. Size-Exclusion Chromatography
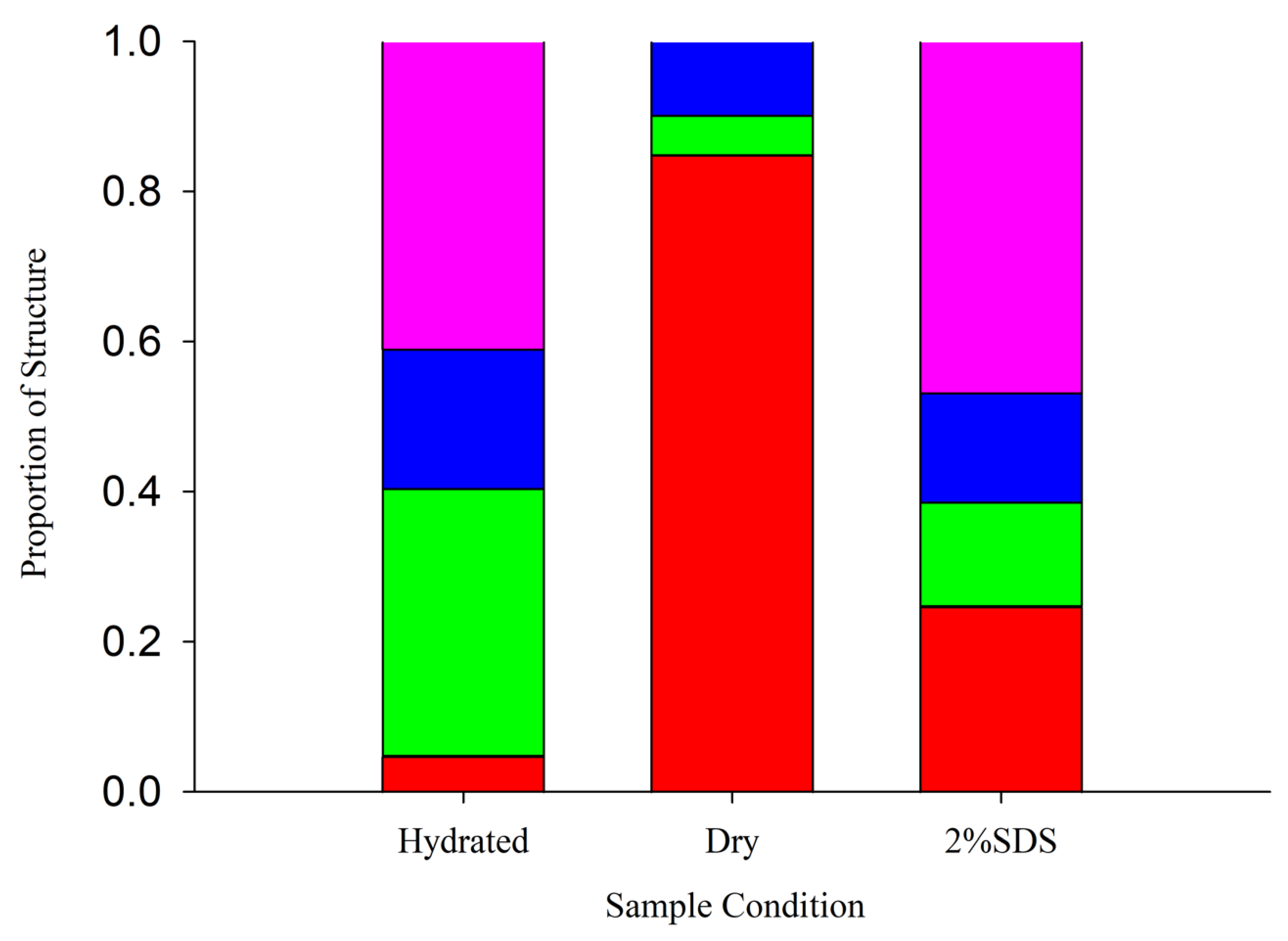
2.4. Circular Dichroism
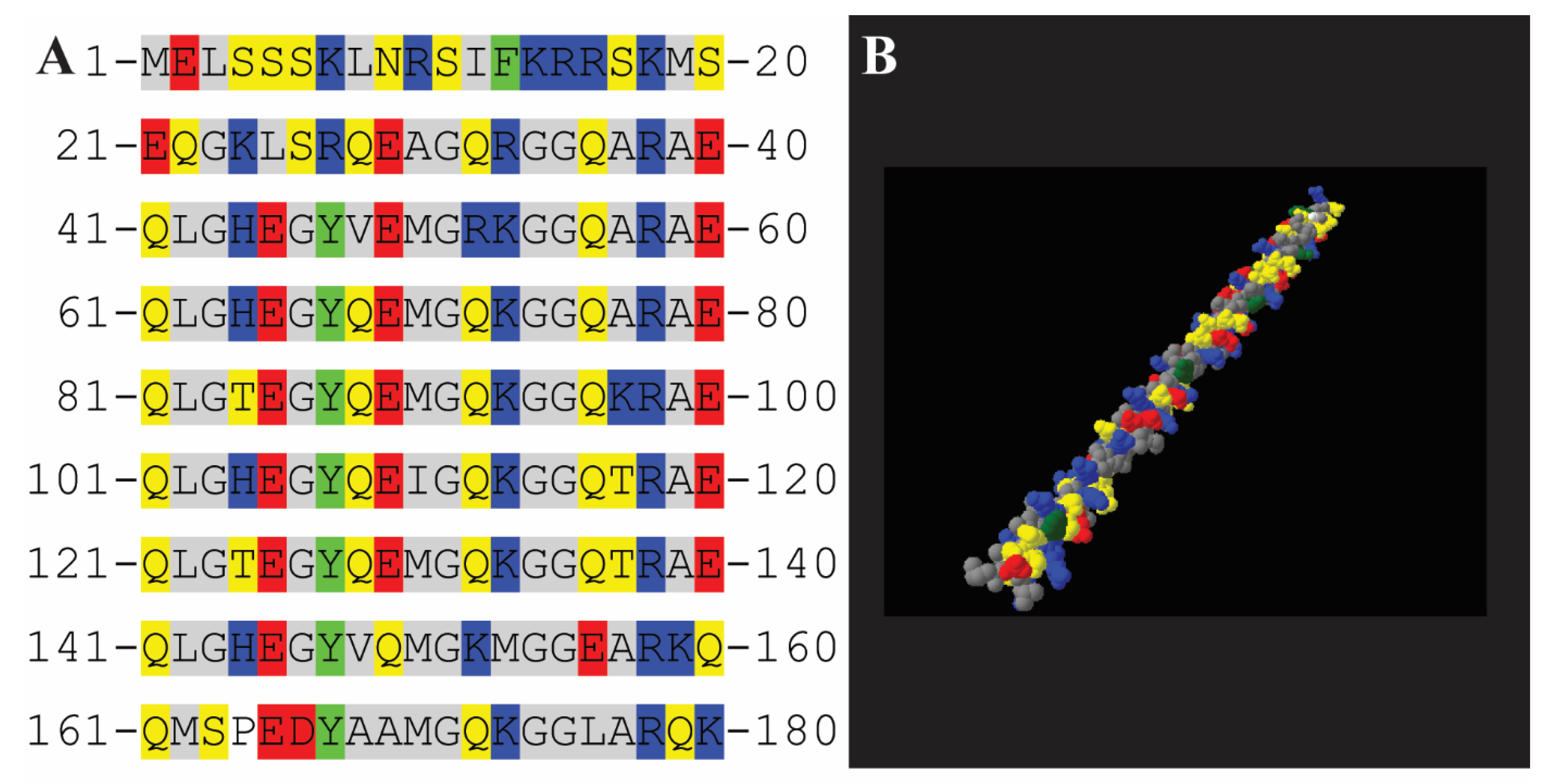
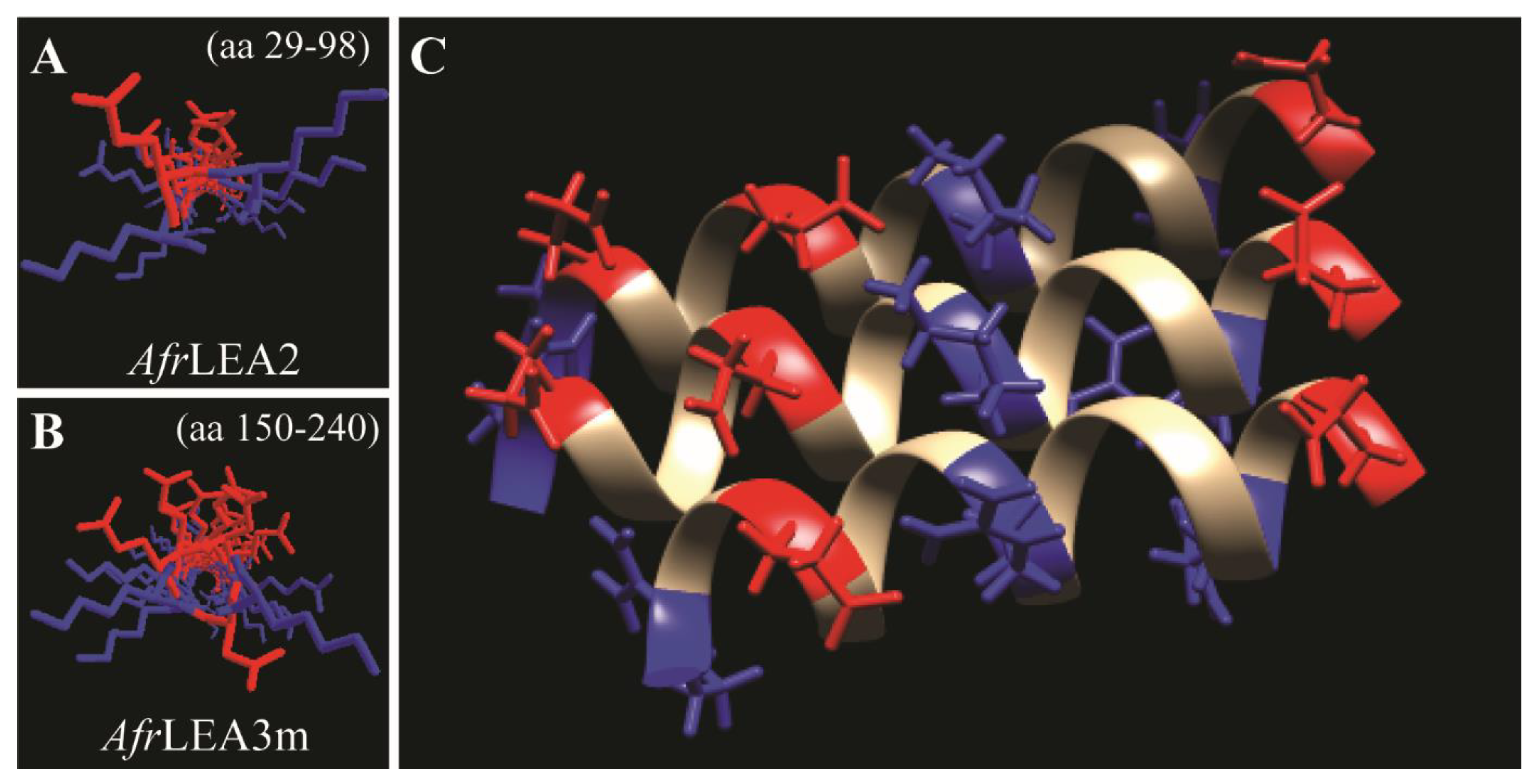
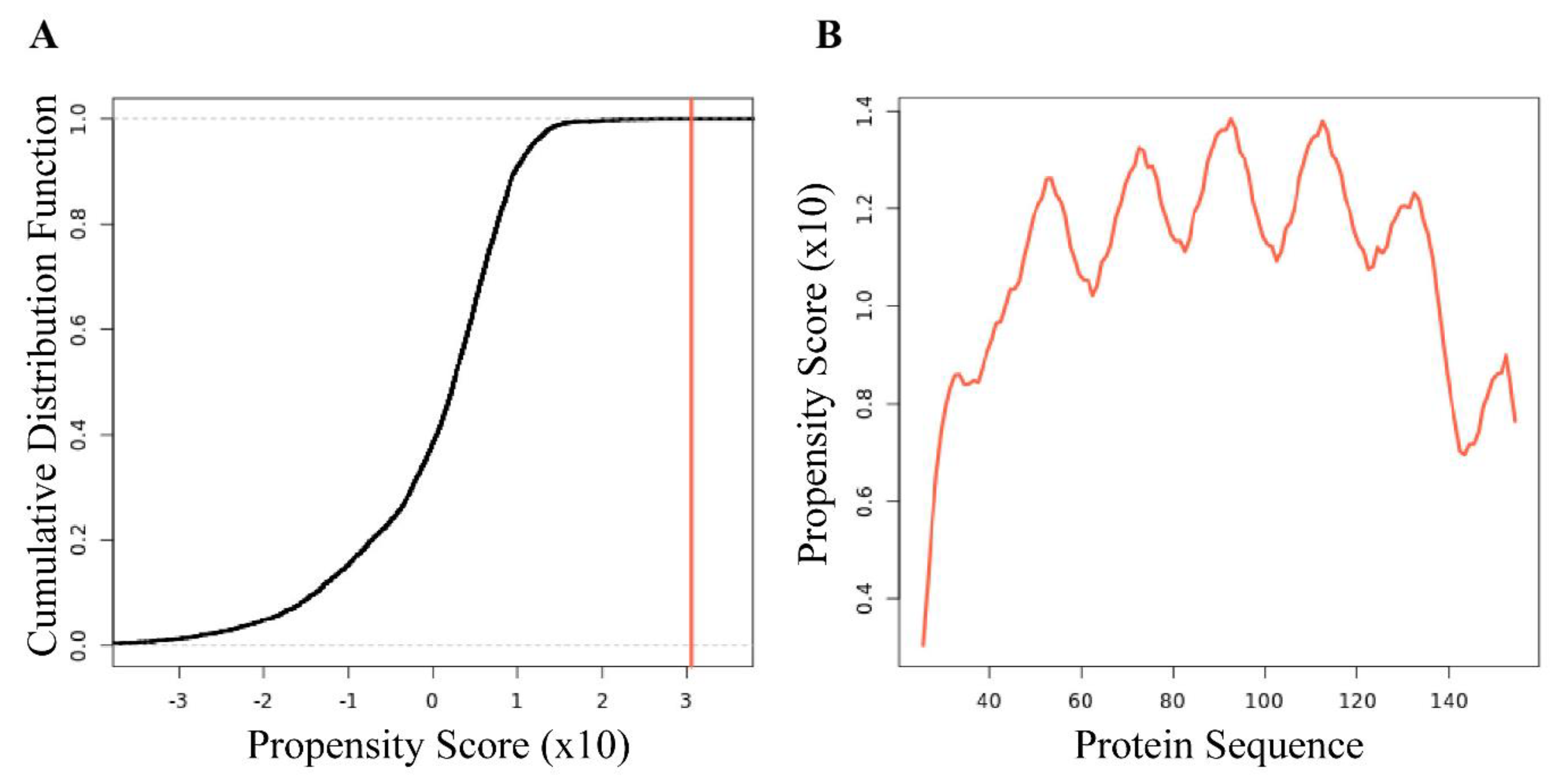
2.5. Bioinformatics Structural Predictions
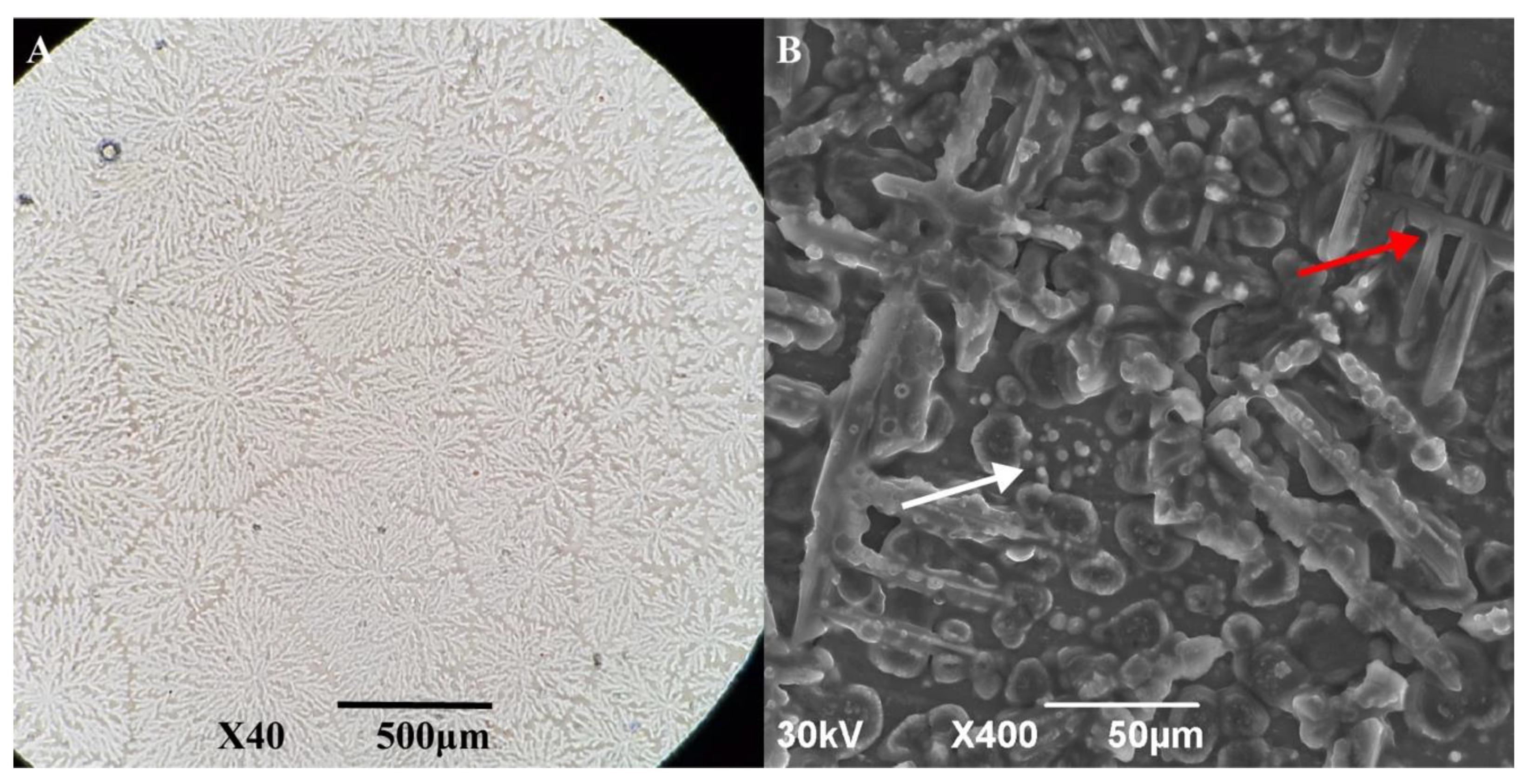
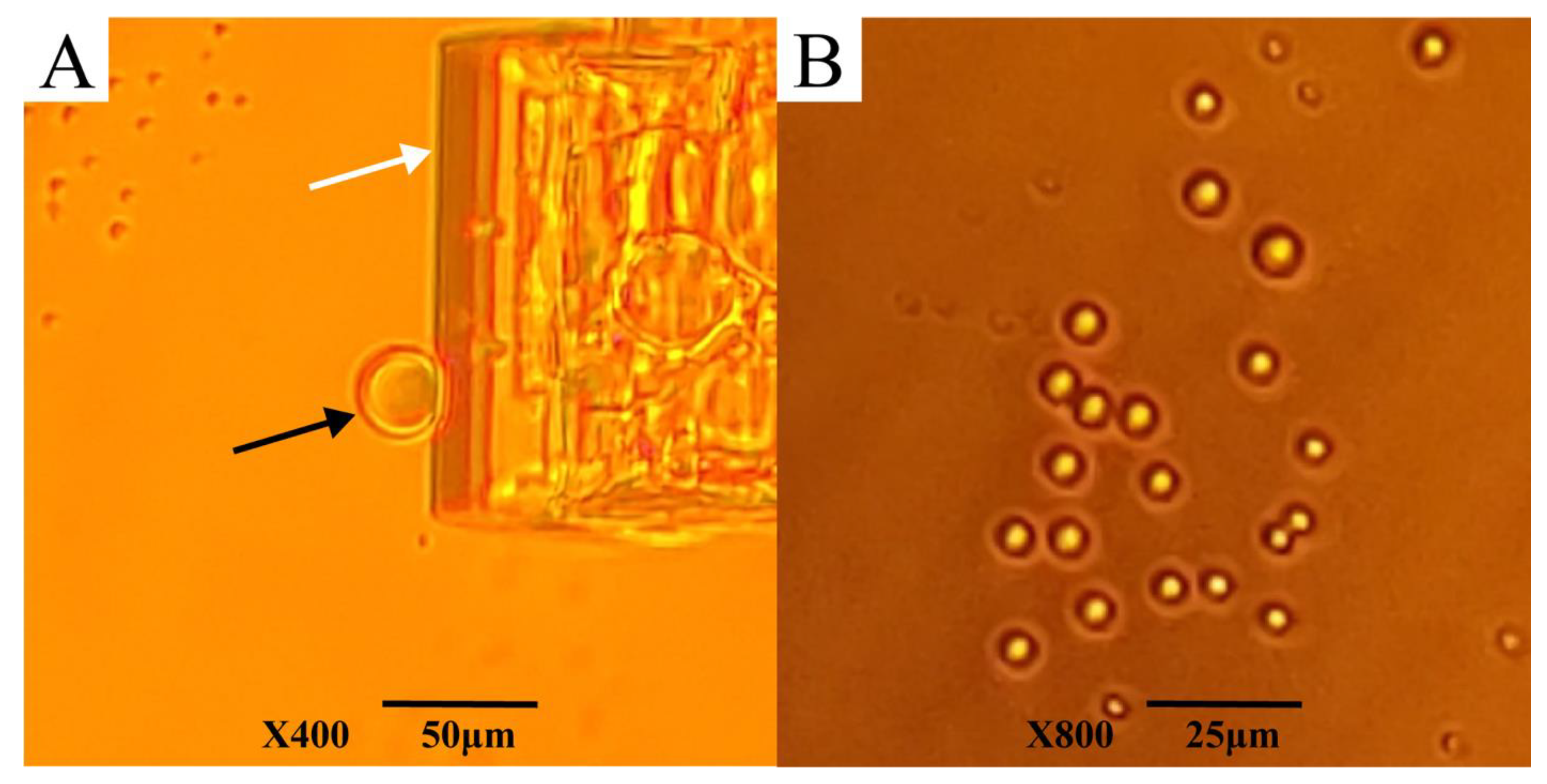
2.6. Sample Preparation for Light Microscopy and Electron Scanning Microscopy (SEM)
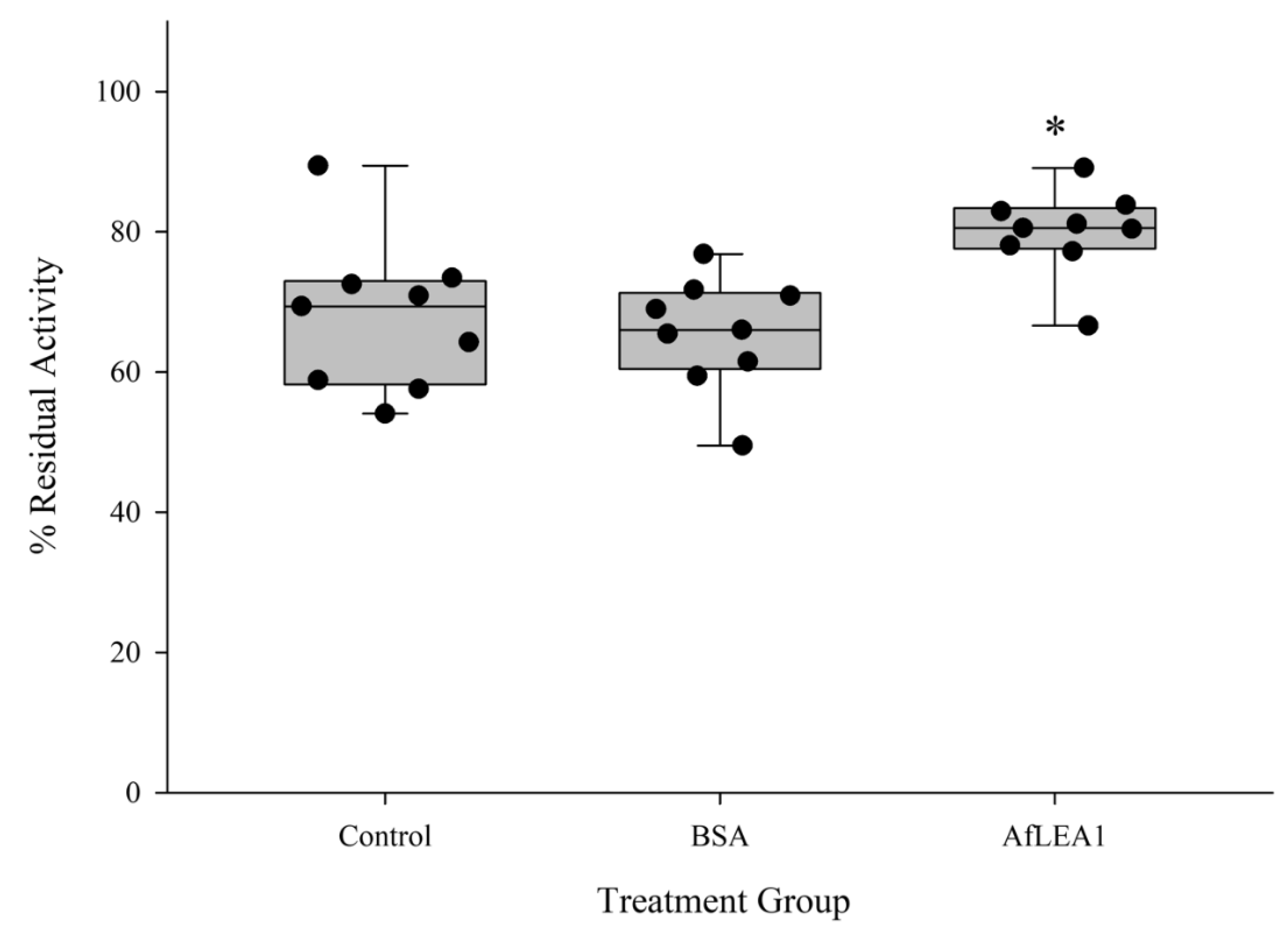
2.7. Lactate Dehydrogenase Activity Assays
2.8. Screening AfLEA1 for RNA-Induced Liquid-Liquid Phase Separation
2.9. SDS-PAGE
2.10. Cell Culture, Transgenic Cell Line, and Confocal Microscopy
3. Results and Discussion
3.1. Protein Cloning, Expression, and Purification
3.2. AfLEA1 Secondary Structural Analysis
3.3. Computational Interpretation of the Structure of AfLEA1 in the Desiccated State
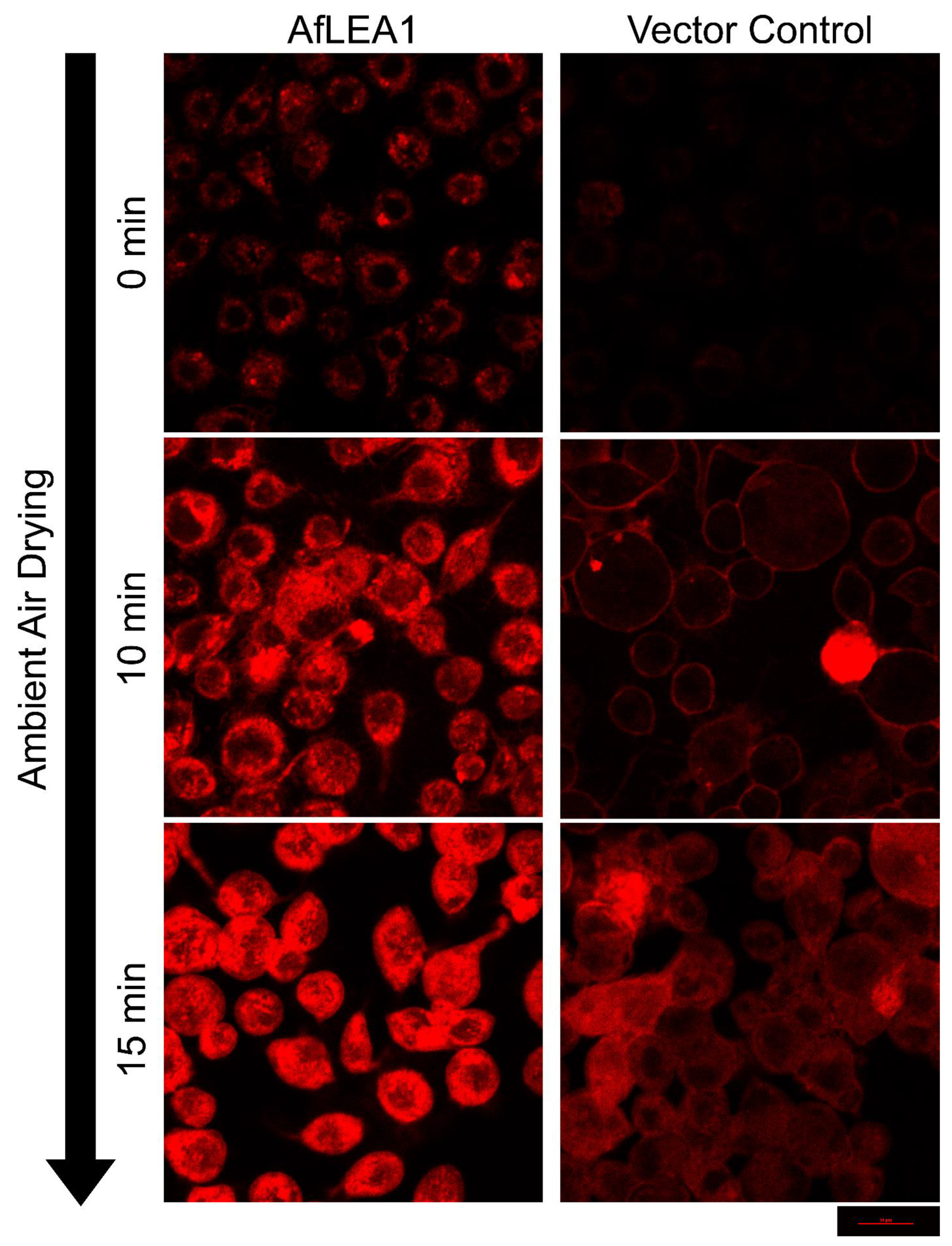
3.4. Behavior of AfLEA1 during Desiccation
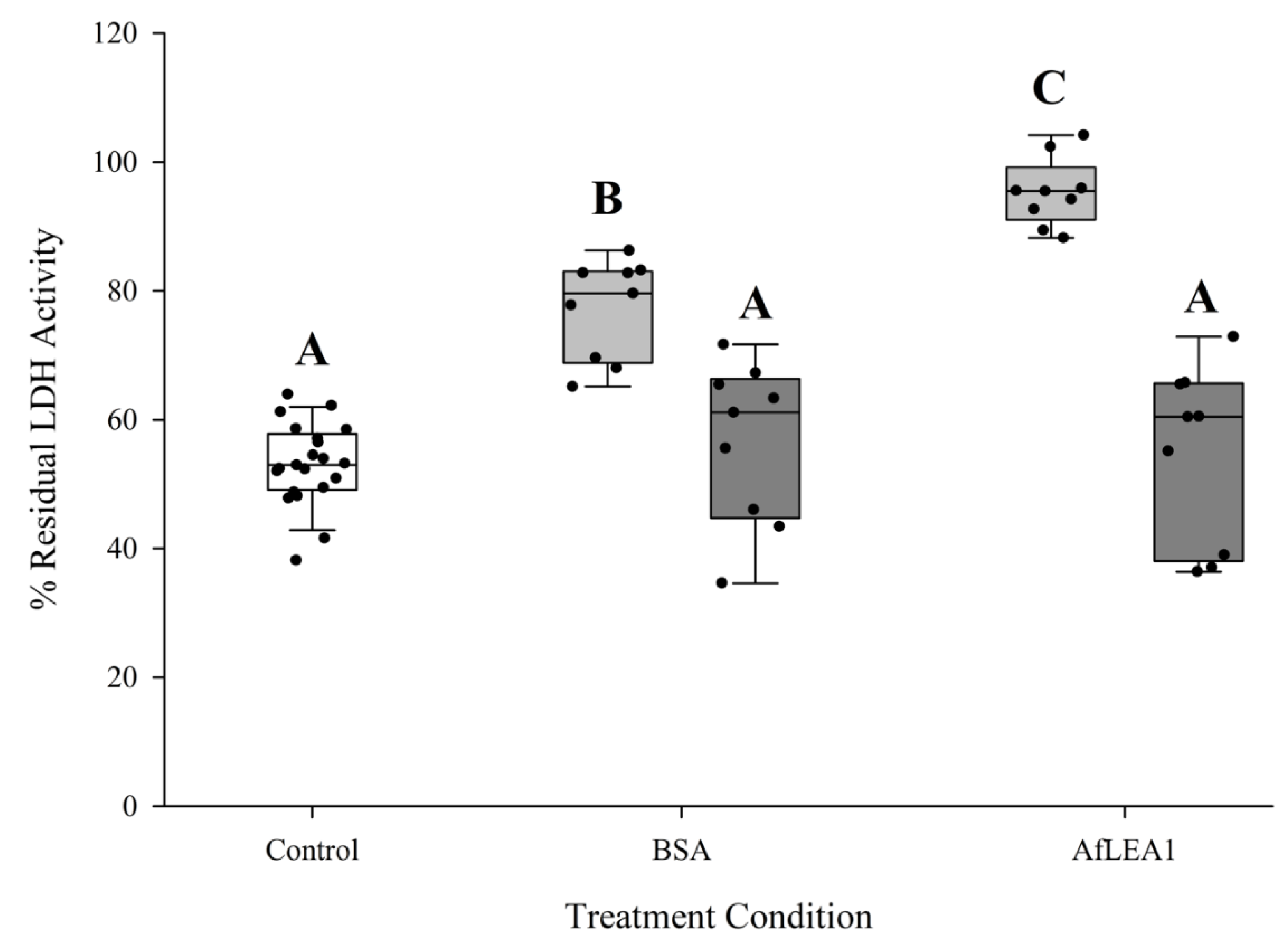
3.5. AfLEA1 Protection of Lactate Dehydrogenase (LDH) Activity during Desiccation and Rehydration
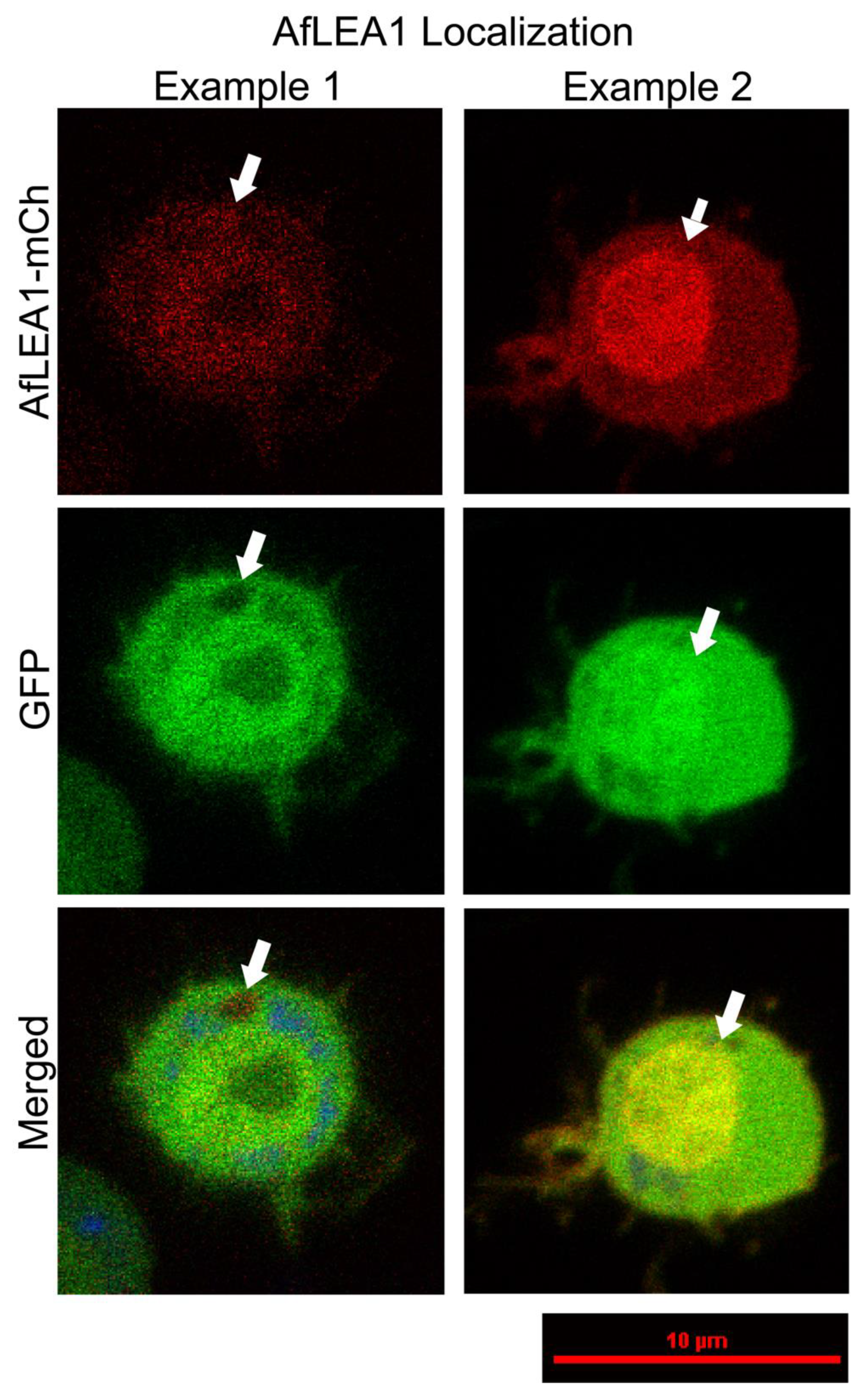
3.6. AfLEA1 Expressed in Kc167 Cells from D. melanogaster Cells Localizes to the Cytoplasm and Improves Cellular Structural Integrity during Desiccation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| IDP | Intrinsically Disordered Protein |
| LEA | Late Embryogenesis Abundant |
| LLPS | Liquid–Liquid Phase Separation |
| LDH | Lactate Dehydrogenase |
References
- Baker, J.; Van Dennsteele, C.; Dure, L., 3rd. Sequence and characterization of 6 Lea proteins and their genes from cotton. Plant Mol. Biol. 1988, 11, 277–291. [Google Scholar] [CrossRef] [PubMed]
- Hincha, D.K.; Thalhammer, A. LEA proteins: IDPs with versatile functions in cellular dehydration tolerance. Biochem. Soc. Trans. 2012, 40, 1000–1003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tunnacliffe, A.; Wise, M.J. The continuing conundrum of the LEA proteins. Naturwissenschaften 2007, 94, 791–812. [Google Scholar] [CrossRef] [PubMed]
- Graether, S.P.; Boddington, K.F. Disorder and function: A review of the dehydrin protein family. Front. Plant Sci. 2014, 5, 576. [Google Scholar] [CrossRef] [Green Version]
- Hand, S.C.; Menze, M.A.; Toner, M.; Boswell, L.; Moore, D. LEA proteins during water stress: Not just for plants anymore. Annu. Rev. Physiol. 2011, 73, 115–134. [Google Scholar] [CrossRef] [Green Version]
- Janis, B.; Belott, C.; Menze, M.A. Role of Intrinsic Disorder in Animal Desiccation Tolerance. Proteomics 2018, 18, e1800067. [Google Scholar] [CrossRef] [Green Version]
- Hunault, G.; Jaspard, E. LEAPdb: A database for the late embryogenesis abundant proteins. BMC Genom. 2010, 11, 221. [Google Scholar] [CrossRef] [Green Version]
- Hand, S.C.; Jones, D.; Menze, M.A.; Witt, T.L. Life without water: Expression of plant LEA genes by an anhydrobiotic arthropod. J. Exp. Zool. Part A Ecol. Genet. Physiol. 2007, 307, 62–66. [Google Scholar] [CrossRef] [Green Version]
- Wu, G.; Zhang, H.; Sun, J.; Liu, F.; Ge, X.; Chen, W.H.; Yu, J.; Wang, W. Diverse LEA (late embryogenesis abundant) and LEA-like genes and their responses to hypersaline stress in post-diapause embryonic development of Artemia franciscana. Comp Biochem. Physiol. B Biochem. Mol. Biol 2011, 160, 32–39. [Google Scholar] [CrossRef]
- Warner, A.H.; Miroshnychenko, O.; Kozarova, A.; Vacratsis, P.O.; MacRae, T.H.; Kim, J.; Clegg, J.S. Evidence for multiple group 1 late embryogenesis abundant proteins in encysted embryos of Artemia and their organelles. J. Biochem. 2010, 148, 581–592. [Google Scholar] [CrossRef]
- Toxopeus, J.; Warner, A.H.; MacRae, T.H. Group 1 LEA proteins contribute to the desiccation and freeze tolerance of Artemia franciscana embryos during diapause. Cell Stress Chaperones 2014, 19, 939–948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marunde, M.R.; Samarajeewa, D.A.; Anderson, J.; Li, S.; Hand, S.C.; Menze, M.A. Improved tolerance to salt and water stress in Drosophila melanogaster cells conferred by late embryogenesis abundant protein. J. Insect Physiol. 2013, 59, 377–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warner, A.H.; Guo, Z.H.; Moshi, S.; Hudson, J.W.; Kozarova, A. Study of model systems to test the potential function of Artemia group 1 late embryogenesis abundant (LEA) proteins. Cell Stress Chaperones 2016, 21, 139–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clegg, J.S. The origin of trehalose and its significance during the formation of encysted dormant embryos of Artemia salina. Comp. Biochem. Physiol. 1965, 14, 135–143. [Google Scholar] [CrossRef]
- Clegg, J.S. Free glycerol in dormant cysts of the brine shrimp Artemia salina, and its disappearance during development. Biol. Bull. 1962, 123, 295–301. [Google Scholar] [CrossRef]
- Candotto Carniel, F.; Fernandez-Marín, B.; Arc, E.; Craighero, T.; Laza, J.M.; Incerti, G.; Tretiach, M.; Kranner, I. How dry is dry? Molecular mobility in relation to thallus water content in a lichen. J. Exp. Bot. 2021, 72, 1576–1588. [Google Scholar] [CrossRef]
- Janis, B.; Uversky, V.N.; Menze, M.A. Potential functions of LEA proteins from the brine shrimp Artemia franciscana—Anhydrobiosis meets bioinformatics. J. Biomol. Struct. Dyn. 2017, 36, 3291–3309. [Google Scholar] [CrossRef] [Green Version]
- Anderson, J.M.; Hand, S.C. Transgenic expression of late embryogenesis abundant proteins improves tolerance to water stress in Drosophila melanogaster. J. Exp. Biol. 2021, 224, jeb238204. [Google Scholar] [CrossRef]
- Knox-Brown, P.; Rindfleisch, T.; Günther, A.; Balow, K.; Bremer, A.; Walther, D.; Miettinen, M.S.; Hincha, D.K.; Thalhammer, A. Similar Yet Different-Structural and Functional Diversity among Arabidopsis thaliana LEA_4 Proteins. Int. J. Mol. Sci. 2020, 21, 2794. [Google Scholar] [CrossRef] [Green Version]
- Navarro-Retamal, C.; Bremer, A.; Alzate-Morales, J.; Caballero, J.; Hincha, D.K.; González, W.; Thalhammer, A. Molecular dynamics simulations and CD spectroscopy reveal hydration-induced unfolding of the intrinsically disordered LEA proteins COR15A and COR15B from Arabidopsis thaliana. Phys. Chem. Chem. Phys. 2016, 18, 25806–25816. [Google Scholar] [CrossRef] [Green Version]
- Belott, C.; Janis, B.; Menze, M.A. Liquid-liquid phase separation promotes animal desiccation tolerance. Proc. Natl. Acad. Sci. USA 2020, 117, 27676–27684. [Google Scholar] [CrossRef] [PubMed]
- Chakrabortee, S.; Tripathi, R.; Watson, M.; Schierle, G.S.; Kurniawan, D.P.; Kaminski, C.F.; Wise, M.J.; Tunnacliffe, A. Intrinsically disordered proteins as molecular shields. Mol. Biosyst. 2012, 8, 210–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mouillon, J.M.; Gustafsson, P.; Harryson, P. Structural investigation of disordered stress proteins. Comparison of full-length dehydrins with isolated peptides of their conserved segments. Plant Physiol. 2006, 141, 638–650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Centner, C.S.; Moore, J.T.; Baxter, M.E.; Long, Z.T.; Miller, J.M.; Kovatsenko, E.S.; Xie, B.; Menze, M.A.; Berson, R.E.; Bates, P.J.; et al. Acoustofluidic-mediated molecular delivery to human T cells with a three-dimensional-printed flow chamber. J. Acoust. Soc. Am. 2021, 150, 4534–4547. [Google Scholar] [CrossRef]
- Yang, J.; Yan, R.; Roy, A.; Xu, D.; Poisson, J.; Zhang, Y. The I-TASSER Suite: Protein structure and function prediction. Nat. Methods 2015, 12, 7–8. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Zhang, Y. I-TASSER server: New development for protein structure and function predictions. Nucleic Acids Res. 2015, 43, W174–W181. [Google Scholar] [CrossRef] [Green Version]
- Roy, A.; Kucukural, A.; Zhang, Y. I-TASSER: A unified platform for automated protein structure and function prediction. Nat. Protoc. 2010, 5, 725–738. [Google Scholar] [CrossRef] [Green Version]
- Guex, N.; Peitsch, M.C. SWISS-MODEL and the Swiss-PdbViewer: An environment for comparative protein modeling. Electrophoresis 1997, 18, 2714–2723. [Google Scholar] [CrossRef]
- Bolognesi, B.; Lorenzo Gotor, N.; Dhar, R.; Cirillo, D.; Baldrighi, M.; Tartaglia, G.G.; Lehner, B. A Concentration-Dependent Liquid Phase Separation Can Cause Toxicity upon Increased Protein Expression. Cell Rep. 2016, 16, 222–231. [Google Scholar] [CrossRef] [Green Version]
- Uverskii, V.N. How many molten globules states exist? Biofizika 1998, 43, 416–421. [Google Scholar]
- Boswell, L.C.; Menze, M.A.; Hand, S.C. Group 3 late embryogenesis abundant proteins from embryos of Artemia franciscana: Structural properties and protective abilities during desiccation. Physiol. Biochem. Zool. 2014, 87, 640–651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greenfield, N.J. Using circular dichroism spectra to estimate protein secondary structure. Nat. Protoc. 2006, 1, 2876–2890. [Google Scholar] [CrossRef] [PubMed]
- Sharon, M.A.; Kozarova, A.; Clegg, J.S.; Vacratsis, P.O.; Warner, A.H. Characterization of a group 1 late embryogenesis abundant protein in encysted embryos of the brine shrimp Artemia franciscana. Biochem. Cell Biol. 2009, 87, 415–430. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, M.; Olvera-Carrillo, Y.; Garciarrubio, A.; Campos, F.; Covarrubias, A.A. The Enigmatic LEA Proteins and Other Hydrophilins. Plant Physiol. 2008, 148, 6–24. [Google Scholar] [CrossRef] [Green Version]
- Forood, B.; Feliciano, E.J.; Nambiar, K.P. Stabilization of alpha-helical structures in short peptides via end capping. Proc. Natl. Acad. Sci. USA 1993, 90, 838–842. [Google Scholar] [CrossRef] [Green Version]
- Presta, L.G.; Rose, G.D. Helix signals in proteins. Science 1988, 240, 1632–1641. [Google Scholar] [CrossRef]
- Aurora, R.; Rose, G.D. Helix capping. Protein Sci. A Publ. Protein Soc. 1998, 7, 21–38. [Google Scholar] [CrossRef]
- Moore, D.S.; Hansen, R.; Hand, S.C. Liposomes with diverse compositions are protected during desiccation by LEA proteins from Artemia franciscana and trehalose. Biochim. Biophys. Acta Biomembr. 2016, 1858, 104–115. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.; Wang, J.; Bai, Y.; Lang, J.W.; Liu, S.; Lin, Y.; Cheng, J. Ionic polypeptides with unusual helical stability. Nat. Commun. 2011, 2, 206. [Google Scholar] [CrossRef] [Green Version]
- Dalgicdir, C.; Globisch, C.; Peter, C.; Sayar, M. Tipping the Scale from Disorder to Alpha-helix: Folding of Amphiphilic Peptides in the Presence of Macroscopic and Molecular Interfaces. PLoS Comput. Biol. 2015, 11, e1004328. [Google Scholar] [CrossRef]
- Lotan, N.; Yaron, A.; Berger, A. The stabilization of the α-helix in aqueous solution by hydrophobic side-chain interaction. Biopolymers 1966, 4, 365–368. [Google Scholar] [CrossRef]
- Burley, S.K.; Petsko, G.A. Amino-aromatic interactions in proteins. FEBS Lett. 1986, 203, 139–143. [Google Scholar] [CrossRef] [Green Version]
- Brunette, T.J.; Parmeggiani, F.; Huang, P.-S.; Bhabha, G.; Ekiert, D.C.; Tsutakawa, S.E.; Hura, G.L.; Tainer, J.A.; Baker, D. Exploring the repeat protein universe through computational protein design. Nature 2015, 528, 580–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coates, J.C. Armadillo repeat proteins: Beyond the animal kingdom. Trends Cell Biol. 2003, 13, 463–471. [Google Scholar] [CrossRef]
- Tewari, R.; Bailes, E.; Bunting, K.A.; Coates, J.C. Armadillo-repeat protein functions: Questions for little creatures. Trends Cell Biol. 2010, 20, 470–481. [Google Scholar] [CrossRef]
- Arakawa, T.; Timasheff, S.N. Mechanism of protein salting in and salting out by divalent cation salts: Balance between hydration and salt binding. Biochemistry 1984, 23, 5912–5923. [Google Scholar] [CrossRef]
- Zaslavsky, B.Y.; Uversky, V.N. In Aqua Veritas: The Indispensable yet Mostly Ignored Role of Water in Phase Separation and Membrane-less Organelles. Biochemistry 2018, 57, 2437–2451. [Google Scholar] [CrossRef]
- Dignon, G.L.; Best, R.B.; Mittal, J. Biomolecular Phase Separation: From Molecular Driving Forces to Macroscopic Properties. Annu. Rev. Phys. Chem. 2020, 71, 53–75. [Google Scholar] [CrossRef] [Green Version]
- Ginsawaeng, O.; Heise, C.; Sangwan, R.; Karcher, D.; Hernández-Sánchez, I.E.; Sampathkumar, A.; Zuther, E. Subcellular Localization of Seed-Expressed LEA_4 Proteins Reveals Liquid-Liquid Phase Separation for LEA9 and for LEA48 Homo- and LEA42-LEA48 Heterodimers. Biomolecules 2021, 11, 1770. [Google Scholar] [CrossRef]
- Yagi-Utsumi, M.; Aoki, K.; Watanabe, H.; Song, C.; Nishimura, S.; Satoh, T.; Yanaka, S.; Ganser, C.; Tanaka, S.; Schnapka, V.; et al. Desiccation-induced fibrous condensation of CAHS protein from an anhydrobiotic tardigrade. Sci. Rep. 2021, 11, 21328. [Google Scholar] [CrossRef]
- Greenspan, P.; Mayer, E.P.; Fowler, S.D. Nile red: A selective fluorescent stain for intracellular lipid droplets. J. Cell Biol. 1985, 100, 965–973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haidekker, M.A.; Theodorakis, E.A. Environment-sensitive behavior of fluorescent molecular rotors. J. Biol. Eng. 2010, 4, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levitt, J.A.; Chung, P.H.; Suhling, K. Spectrally resolved fluorescence lifetime imaging of Nile red for measurements of intracellular polarity. J. Biomed. Opt. 2015, 20, 096002. [Google Scholar] [CrossRef] [Green Version]
- Swain, J.; Mishra, A.K. Nile red fluorescence for quantitative monitoring of micropolarity and microviscosity of pluronic F127 in aqueous media. Photochem. Photobiol. Sci. 2016, 15, 1400–1407. [Google Scholar] [CrossRef] [PubMed]
- Yablon, D.G.; Schilowitz, A.M. Solvatochromism of Nile red in nonpolar solvents. Appl. Spectrosc. 2004, 58, 843–847. [Google Scholar] [CrossRef] [PubMed]
- Buitink, J.; Leprince, O. Intracellular glasses and seed survival in the dry state. Comptes Rendus Biol. 2008, 331, 788–795. [Google Scholar] [CrossRef]
- Leprince, O.; Hendry, G.A.F.; McKersie, B.D. The mechanisms of desiccation tolerance in developing seeds. Seed Sci. Res. 2008, 3, 231–246. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janis, B.; Belott, C.; Brockman, T.; Menze, M.A. Functional and Conformational Plasticity of an Animal Group 1 LEA Protein. Biomolecules 2022, 12, 425. https://doi.org/10.3390/biom12030425
Janis B, Belott C, Brockman T, Menze MA. Functional and Conformational Plasticity of an Animal Group 1 LEA Protein. Biomolecules. 2022; 12(3):425. https://doi.org/10.3390/biom12030425
Chicago/Turabian StyleJanis, Brett, Clinton Belott, Tyler Brockman, and Michael A. Menze. 2022. "Functional and Conformational Plasticity of an Animal Group 1 LEA Protein" Biomolecules 12, no. 3: 425. https://doi.org/10.3390/biom12030425
APA StyleJanis, B., Belott, C., Brockman, T., & Menze, M. A. (2022). Functional and Conformational Plasticity of an Animal Group 1 LEA Protein. Biomolecules, 12(3), 425. https://doi.org/10.3390/biom12030425





