Investigation of the Repairing Effect and Mechanism of Oral Degraded Sericin on Liver Injury in Type II Diabetic Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Sericin Peptides
2.3. Rat Feed
2.4. Liver Weight and Organ Body Coefficients
2.5. Extraction of Total Protein from Liver Samples
2.6. Western Blotting
2.7. Determination of Related Inflammatory Factors
2.8. Data Processing
3. Results
3.1. Liver Organ Quality and Organ Coefficient
3.2. Carbohydrate Metabolism Pathway
3.3. Impact on PI3K/AKT Pathway
3.4. Impact on AMPK/ACC Pathway
3.5. Impact on Liver Damage
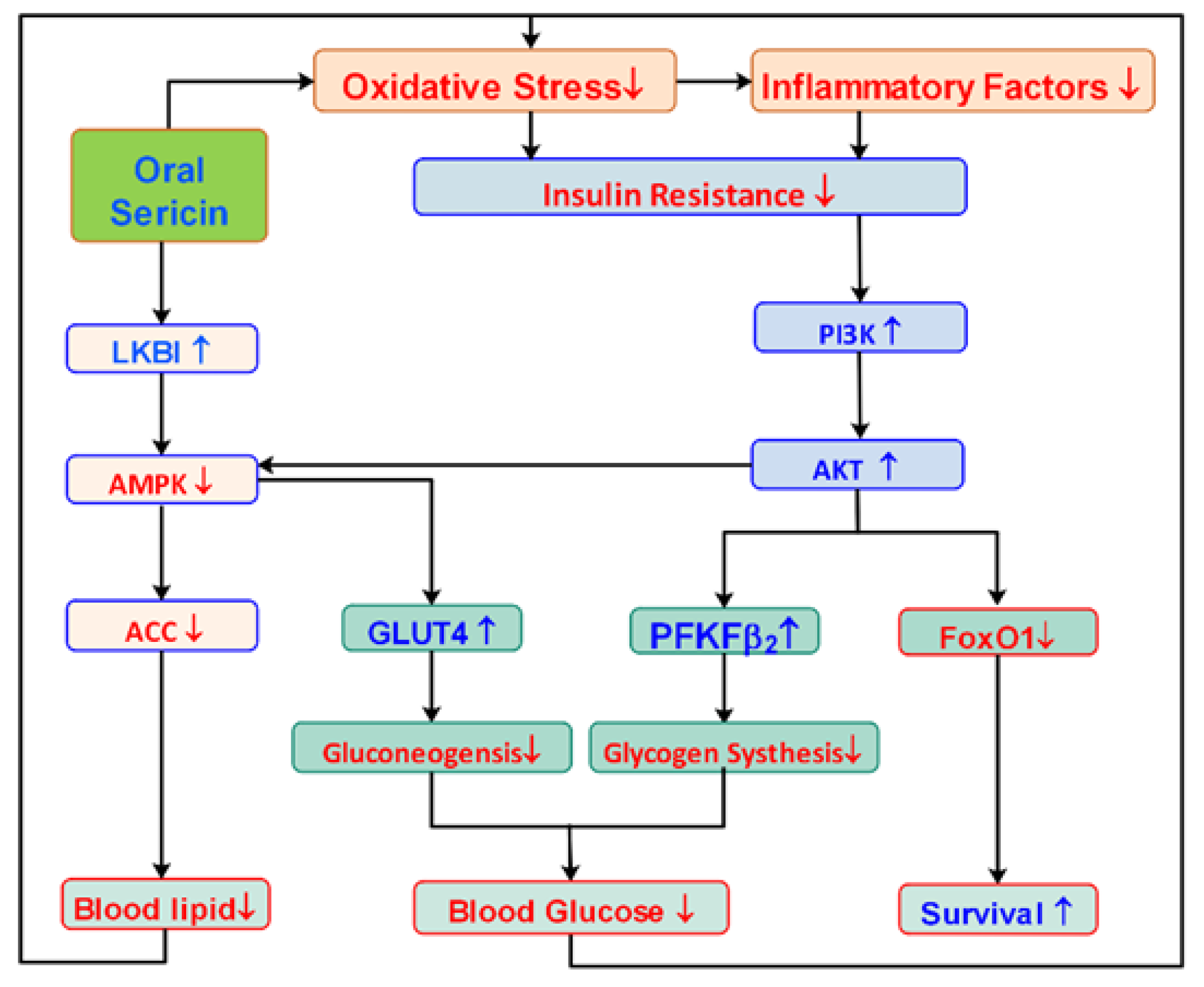
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hideki, U.; Akira, K.; Yasuhiro, S.; Esteban, C.G.; Shuichi, M.; Kohei, M.; Noriko, M.; Nagako, K.; Takashi, T.; Yasuko, H.; et al. Oxidative stress is associated with adiposity and insulin resistance in men. J. Clin. Endocrinol. Metab. 2003, 88, 4673–4676. [Google Scholar]
- Horita, S.; Nakamura, M.; Suzuki, M.; Satoh, N.; Suzuki, A.; Seki, G. Selective insulin resistance in the kidney. BioMed. Res. Int. 2016, 3, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gu, S. Differential regulation of amino acid transporter SNAT3 by insulin in hepatocytes. J. Biol. Chem. 2005, 280, 26055–26062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mariano, F.; Biancone, L. Metformin, chronic nephropathy and lactic acidosis: A multi-faceted issue for the nephrologist. J. Nephrol. 2021, 34, 1127–1135. [Google Scholar] [CrossRef] [PubMed]
- Kageyama, S.; Nakamichi, N.; Sekino, H.; Nakano, S. Comparison of the effects of acarbose and voglibose in healthy subjects. Clin. Ther. 1997, 19, 720–729. [Google Scholar] [CrossRef]
- Billington, E.O.; Grey, A.; Bolland, M.J. The effect of thiazolidinediones on bone mineral density and bone turnover: Systematic review and meta-analysis. Diabetologia 2015, 58, 2238–2246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, X.L.; Wang, W.Q.; Shi, C.Y.; Tong, Q.; Fang, J.G. Research progress in pharmacological effects of dihydromyricelin. Chin. Tradit. Herb. Drugs 2015, 46, 603–609. [Google Scholar]
- Cao, T.-T.; Zhang, Y.-Q. Processing and characterization of silk sericin from Bombyx mori and its application in biomaterials and biomedicines. Mater. Sci. Eng. C 2016, 61, 940–952. [Google Scholar] [CrossRef] [PubMed]
- Kato, N.; Sato, S.; Yamanaka, A.; Yamada, H.; Fuwa, N.; Nomura, M. Silk protein, sericin, inhibits lipid peroxidation and tyrosinase activity. Biosci. Biotechnol. Biochem. 1998, 62, 145–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ampawong, S.; Isarangkul, D.; Aramwit, P. Sericin ameliorated dysmorphic mitochondria in high-cholesterol diet/streptozotocin rat by antioxidative property. Exp. Biol. Med. 2017, 242, 411–421. [Google Scholar] [CrossRef] [Green Version]
- Sasaki, M.; Kato, N.; Watanabe, H.; Yamada, H. Silk protein, sericin, suppresses colon carcinogenesis induced by 1, 2-dimethylhydrazine in mice. Oncol. Rep. 2000, 7, 1049–1052. [Google Scholar] [CrossRef] [PubMed]
- Zhaorigetu, S.; Yanaka, N.; Sasaki, M.; Watanabe, H.; Kato, N. Silk protein, sericin, suppresses DMBA-TPA-induced mouse skin tumorigenesis by reducing oxidative stress, inflammatory responses and endogenous tumor promoter TNF-α. Oncol. Rep. 2003, 10, 537–543. [Google Scholar] [PubMed]
- Kumar, J.P.; Mandal, B.B. Silk sericin induced pro-oxidative stress leads to apoptosis in human cancer cells. Food Chem. Toxicol. 2019, 123, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Dash, R.; Mandal, M.; Ghosh, S.K.; Kundu, S.C. Silk sericin protein of tropical tasar silkworm inhibits UVB-induced apoptosis in human skin keratinocytes. Mol. Cell Biochem. 2008, 311, 111–119. [Google Scholar] [CrossRef] [PubMed]
- OKazaki, Y.S.; Akehi, S.K.; Yonghui, X.U. Consumption of sericin reduces serum lipids, ameliorates glucose tolerance, and elevates serum adiponectin in rats fed a high-fat diet. Biosci. Biotechnol. Biochem. 2010, 74, 1534–1538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aramwit, P.; Kanokpanont, S.; De-Eknamkul, W.; Srichana, T. Monitoring of inflammatory mediators induced by silk sericin. J. Biosci. Bioeng. 2009, 107, 556–561. [Google Scholar] [CrossRef] [PubMed]
- Nanteetip, L.; Kanittaporn, T.; Acharaporn, D.; Waree, T.; Sutatip, P.; Manote, S. Sericin Reduces Serum Cholesterol in Rats and Cholesterol Uptake into Caco-2 Cells. J. Agric. Food Chem. 2010, 58, 12519–12522. [Google Scholar]
- Napavichayanun, S.; Ampawong, S.; Aramwit, P. Hypocholesterolemic and antioxidative properties of Sericin on adrenal secretory granule biosynthesis in hyperlipidemic rats. Pharmacoeconomics 2017, 2, 4172. [Google Scholar]
- Yellamma, K. Silk Protein, Sericin as a Cognitive Enhancer in Alzheimer’s Disease. J. Alzheimers Dis. Park. 2014, 4, 1000163. [Google Scholar]
- Peera, K.; Yellamma, K. Sericin as a cholinergic modulator in Alzheimer’s disease induced rat. Int. J. Pharm. Pharm. Sci. 2015, 7, 108–112. [Google Scholar]
- Dong, X.; Zhao, S.X.; Yin, X.L.; Wang, H.Y.; Wei, Z.G.; Zhang, Y.Q. Silk sericin has significantly hypoglycaemic effect in type 2 diabetic mice via anti-oxidation and anti-inflammation. Int. J. Biol. Macromol. 2020, 150, 1061–1071. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-D.; Zhong, Z.-H.; Weng, Y.-J.; Wei, Z.-Z.; Zhang, Y.-Q. Degraded Sericin Significantly Regulates Blood Glucose Levels and Improves Impaired Liver Function in T2D Rats by Reducing Oxidative Stress. Biomolecules 2021, 11, 1255. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, L.; Li, Z.K.; Wu, F.; Hang, B.Y.; Cai, B.Y.; Weng, Z.F.; Zhao, L.; Ding, C.F.; Du, Y.G. Effect and mechanism of mulberry leaf polysaccharide on nephropathy in type 1 diabetic rats. Chin. J. Med. 2018, 98, 1792–1796. [Google Scholar]
- Zhao, Z.-L.; Li, W.-W.; Wang, F.; Zhang, Y.-Q. Using of hydrated lime water as a novel degumming agent of silk and sericin recycling from wastewater. J. Clean. Prod. 2018, 172, 2090–2096. [Google Scholar] [CrossRef]
- Zhang, F.; Ye, C.; Li, G.; Ding, W.; Zhou, W.; Zhu, H.; Chen, G.; Luo, T.; Guang, M.; Liu, Y.; et al. The Rat Model of Type 2 Diabetic Mellitus and Its Glycometabolism Characters. Exp. Anim. 2003, 52, 401–407. [Google Scholar] [CrossRef] [Green Version]
- Furman, B.L. Streptozotocin-Induced Diabetic Models in Mice and Rats. Curr. Protoc. Pharmacol. 2015, 70, 5–47. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Yang, Z.; Tang, Q.; Chen, Z. Effects of sericin on the testicular growth hormone/insulin-like growth factor-1 axis in a rat model of type 2 diabetes. Int. J. Clin. Exp. Med. 2015, 8, 10411–10419. [Google Scholar]
- Park, S.; Zhang, T.; Qiu, J.Y.; Wu, X.; Lee, J.-Y.; Lee, B.-Y. Acid Hydrolyzed Silk Peptide Consumption Improves Anti-Diabetic Symptoms by Potentiating Insulin Secretion and Preventing Gut Microbiome Dysbiosis in Non-Obese Type 2 Diabetic Animals. Nutrients 2020, 12, 311. [Google Scholar] [CrossRef] [Green Version]
- Bertola, A.; Bonnafous, S.; Cormont, M.; Anty, R.; Tanti, J.F.; Tran, A. Hepatocyte growth factor induces glucose uptake in 3T3-L1 adipocytes through A Gab1/phosphatidylinositol 3-kinase/Glut4 pathway. J. Biol. Chem. 2007, 282, 10325–10332. [Google Scholar] [CrossRef] [Green Version]
- Roebuck, K.A. Oxidant stress regulation of IL-8 and ICAM-1 gene expression: Differential activation and binding of the transcription factors AP-1 and NF-kappaB. Int. J. Mol. Med. 1999, 4, 223–230. [Google Scholar] [CrossRef]
- Arya, A.; Cheah, S.C.; Looi, C.Y.; Taha, H.; Mustafa, M.R.; Mohd, M.A. The methanolic fraction of Centratherum anthelminticum seed downregulates pro-inflammatory cytokines, oxidative stress, and hyperglycemia in STZ-nicotinamide–induced type 2 diabetic rats. Food Chem. Toxicol. 2012, 50, 4209–4220. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Li, X.; Lu, S.; Chen, G.; Xing, T. Prospects of Silk Sericin Membranes Fabricated with Tyrosinase. J. Fiber Bioeng. Inform. 2015, 8, 57–67. [Google Scholar] [CrossRef]
- Chen, Z.; Yang, S.; He, Y.; Song, C.; Liu, Y. Effect of sericin on diabetic hippocampal growth hormone/insulin-like growth factor 1 axis. Neural Regen. Res. 2013, 8, 1756–1764. [Google Scholar] [CrossRef] [PubMed]
- Preiss, D.; Sattar, N. Non-alcoholic fatty liver disease: An overview of prevalence, diagnosis, pathogenesis and treatment considerations. Clin. Sci. 2008, 115, 141–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamboni, L.; Li, Y.; Liu, J. Silk Sericin-Functionalized Bacterial Cellulose as a Potential Wound-Healing Biomaterial. Biomacromolecules 2016, 17, 3076–3084. [Google Scholar] [CrossRef] [PubMed]
- Seo, C.-W.; Um, I.C.; Rico, C.W.; Kang, M.Y. Antihyperlipidemic and Body Fat-Lowering Effects of Silk Proteins with Different Fibroin/Sericin Compositions in Mice Fed with High Fat Diet. J. Agric. Food Chem. 2011, 59, 4192–4197. [Google Scholar] [CrossRef] [PubMed]
- Furuya, S.; Tabata, T.; Mitoma, J.; Yamada, K.; Yamasaki, M.; Makino, A.; Yamamoto, T.; Watanabe, M.; Kano, M.; Hirabayashi, Y. L-Serine and glycine serve as major astroglia-derived trophic factors for cerebellar Purkinje neurons. Proc. Natl. Acad. Sci. USA 2000, 97, 11528–11533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marra, F.; Gastaldelli, A.; Baroni, G.S.; Tell, G.; Tiribelli, C. Molecular basis and mechanisms of progression of non-alcoholic steatohepatitis. Trends Mol. Med. 2008, 14, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.-Y.; Shi, C.-X.; Gao, R.; Sun, H.-J.; Xiong, X.-Q.; Ding, L.; Chen, Q.; Li, Y.-H.; Wang, J.-J.; Kang, Y.-M.; et al. Irisin inhibits hepatic gluconeogenesis and increases glycogen synthesis via the PI3K/Akt pathway in type 2 diabetic mice and hepatocytes. Clin. Sci. 2015, 129, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Ahrén, B.; Masmiquel, L.; Kumar, H.; Sargin, M.; Karsbøl, J.D.; Jacobsen, S.H.; Chow, F. Efficacy and safety of once-weekly semaglutide versus once-daily sitagliptin as an add-on to metformin, thiazolidinediones, or both, in patients with type 2 diabetes (sustain 2): A 56-week, double-blind, phase 3a, randomised trial. Lancet Diabetes Endocrinol. 2017, 5, 341–354. [Google Scholar] [CrossRef]
- Russell-Jones, D.; Cuddihy, R.M.; Hanefeld, M.; Kumar, A.; Gonzalez, J.G.; Chan, M.; Wolka, A.M.; Boardman, M.K. Efficacy and safety of exenatide once weekly versus metformin, pioglitazone, and sitagliptin used as monotherapy in drug-naive patients with type 2 diabetes (duration-4): A 26-week double-blind study. Diabetes Care 2011, 35, 252–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winder, W.W. AMP-Activated Protein Kinase: Possible Target for Treatment of Type 2 Diabetes. Diabetes Technol. Ther. 2000, 2, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ma, Y.; Xia, Y.; Shen, W.; Mao, J.; Xue, R. Silk sericin—Insulin bioconjugates: Synthesis, characterization and biological activity. J. Control Release 2006, 115, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-Q. Applications of natural silk protein sericin in biomaterials. Biotechnol. Adv. 2002, 20, 91–100. [Google Scholar] [CrossRef]
- Cao, T.-T.; Zhang, Y.-Q. Viability and proliferation of L929, tumour and hybridoma cells in the culture media containing sericin protein as a supplement or serum substitute. Appl. Microbiol. Biotechnol. 2015, 99, 7219–7228. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.-G.; Zhang, Y.-Q. A new estimation of the total flavonoids in silkworm cocoon sericin layer through aglycone determination by hydrolysis-assisted extraction and HPLC-DAD analysis. Food Nutr. Res. 2016, 60, 30932. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.-G.; Wang, H.-Y.; Wei, Z.-G.; Zhang, Y.-Q. Therapeutic effects of ethanolic extract from the green cocoon shell of silkworm Bombyx mori on type 2 diabetic mice and its hypoglycaemic mechanism. Toxicol. Res. 2019, 8, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.-L.; Zhang, Y.-Q. Greener degumming production of layered sericin peptides from a silkworm cocoon and their physicochemical characteristics and bioactivities in vitro. J. Clean. Prod. 2020, 261, 121080. [Google Scholar] [CrossRef]
- Song, C.; Liu, D.; Yang, S.; Cheng, L.; Xing, E.; Chen, Z. Sericin enhances the insulin-PI3K/AKT signaling pathway in the liver of a type 2 diabetes rat model. Exp. Ther. Med. 2018, 16, 3345–3352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; He, Y.; Song, C.; Dong, Z.; Su, Z.; Xue, J. Sericin can reduce hippocampal neuronal apoptosis by activating the Akt signal transduction pathway in a rat model of diabetes mellitus. Neural Regen. Res. 2012, 7, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; He, Y.; Fu, W.; Xue, J. Effects of sericin on heme oxygenase-1 expression in the hippocampus and cerebral cortex of type 2 diabetes mellitus rats. Neural Regen. Res. 2011, 6, 423–427. [Google Scholar] [CrossRef]
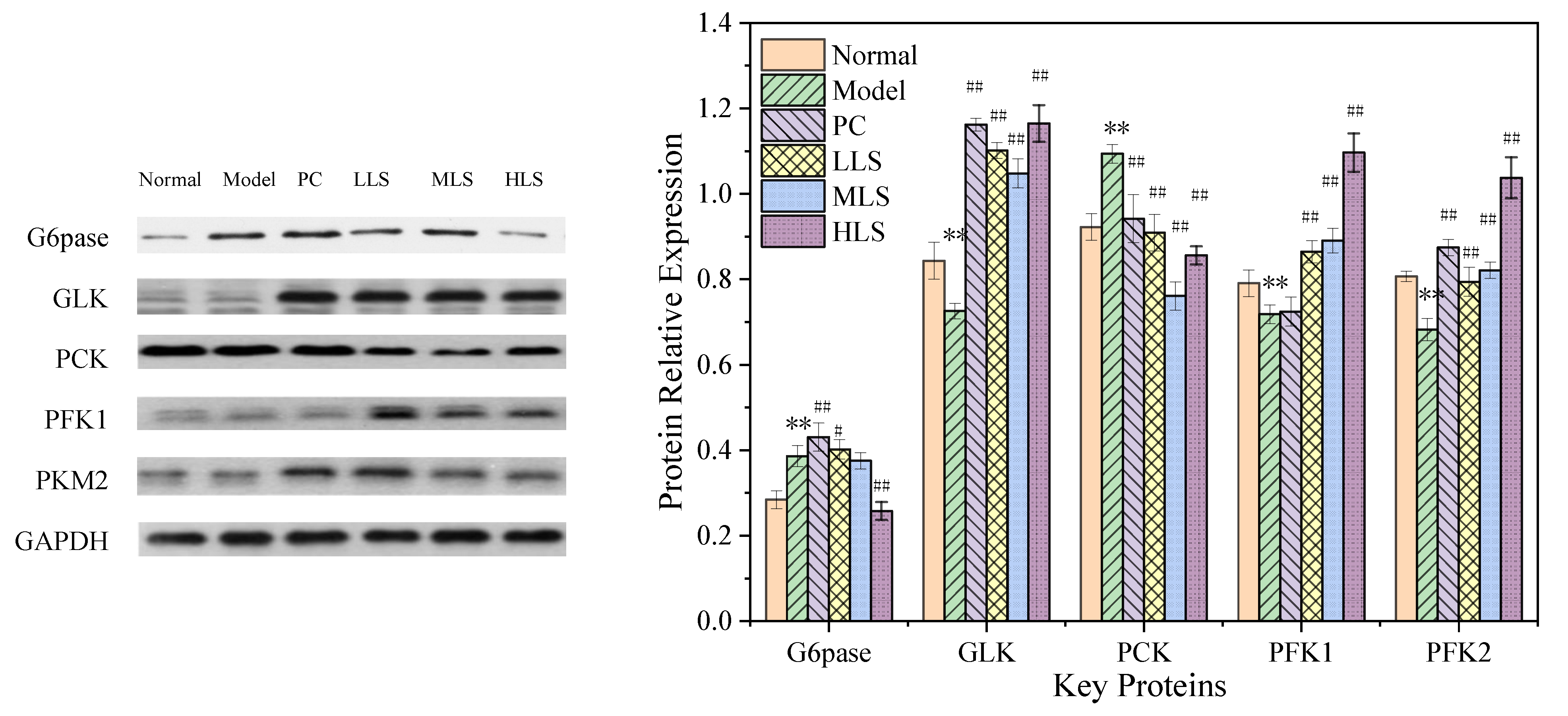

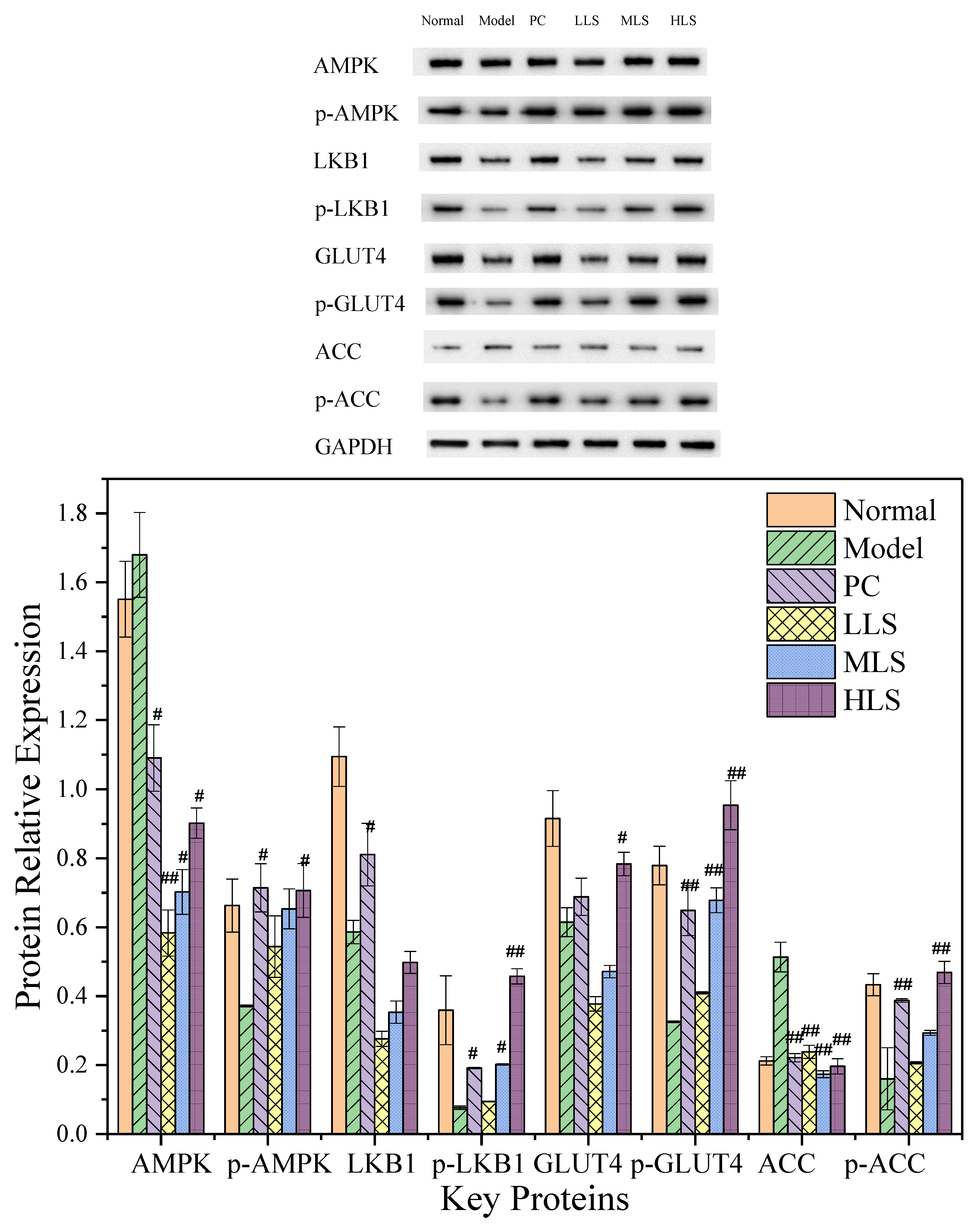
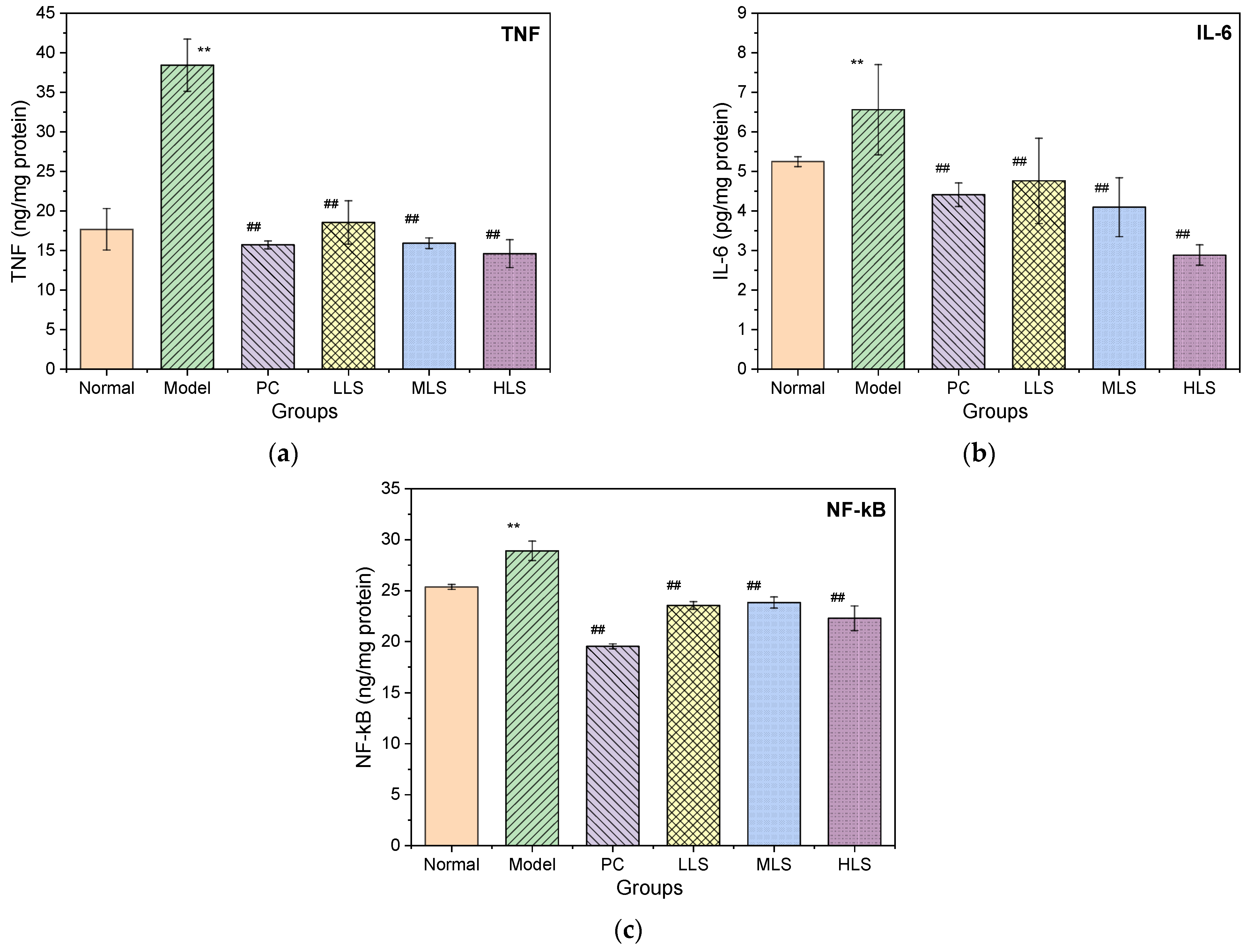
| Group | Liver Mass (g) | Coefficient (%) |
|---|---|---|
| Normal | 16.38 ± 1.70 | 3.25 ± 0.34 |
| Model | 10.86 ± 1.43 ** | 4.35 ± 0.54 ** |
| PC | 15.73 ± 2.92 ## | 4.23 ± 0.79 |
| LLS | 10.43 ± 1.20 | 4.11 ± 0.49 |
| MLS | 10.49 ± 1.33 | 4.23 ± 0.54 |
| HLS | 11.90 ± 1.34 # | 4.03 ± 0.73 # |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Z.-Z.; Weng, Y.-J.; Zhang, Y.-Q. Investigation of the Repairing Effect and Mechanism of Oral Degraded Sericin on Liver Injury in Type II Diabetic Rats. Biomolecules 2022, 12, 444. https://doi.org/10.3390/biom12030444
Wei Z-Z, Weng Y-J, Zhang Y-Q. Investigation of the Repairing Effect and Mechanism of Oral Degraded Sericin on Liver Injury in Type II Diabetic Rats. Biomolecules. 2022; 12(3):444. https://doi.org/10.3390/biom12030444
Chicago/Turabian StyleWei, Zhen-Zhen, Yu-Jie Weng, and Yu-Qing Zhang. 2022. "Investigation of the Repairing Effect and Mechanism of Oral Degraded Sericin on Liver Injury in Type II Diabetic Rats" Biomolecules 12, no. 3: 444. https://doi.org/10.3390/biom12030444






