Glucose Oxidase, an Enzyme “Ferrari”: Its Structure, Function, Production and Properties in the Light of Various Industrial and Biotechnological Applications
Abstract
1. Introduction
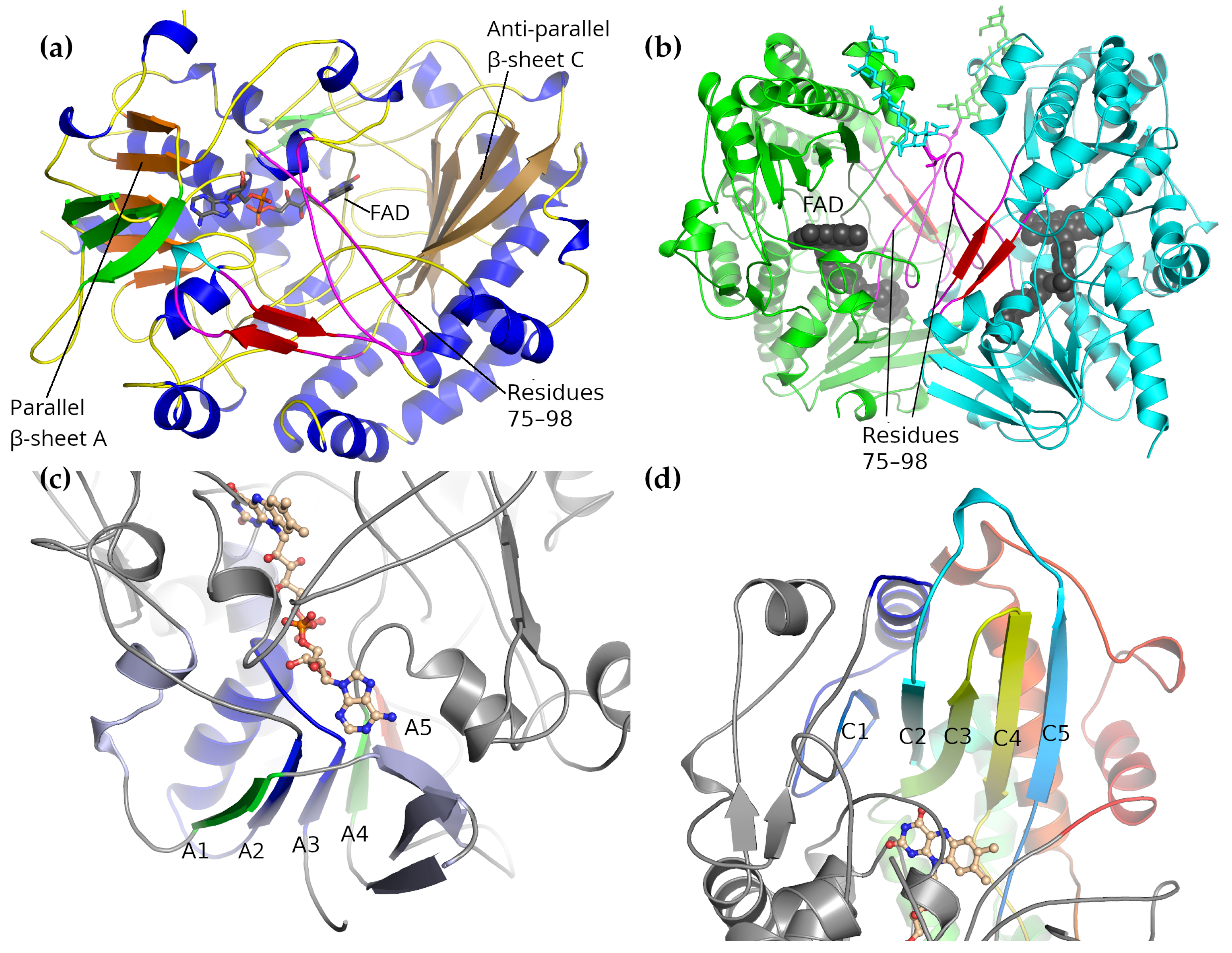
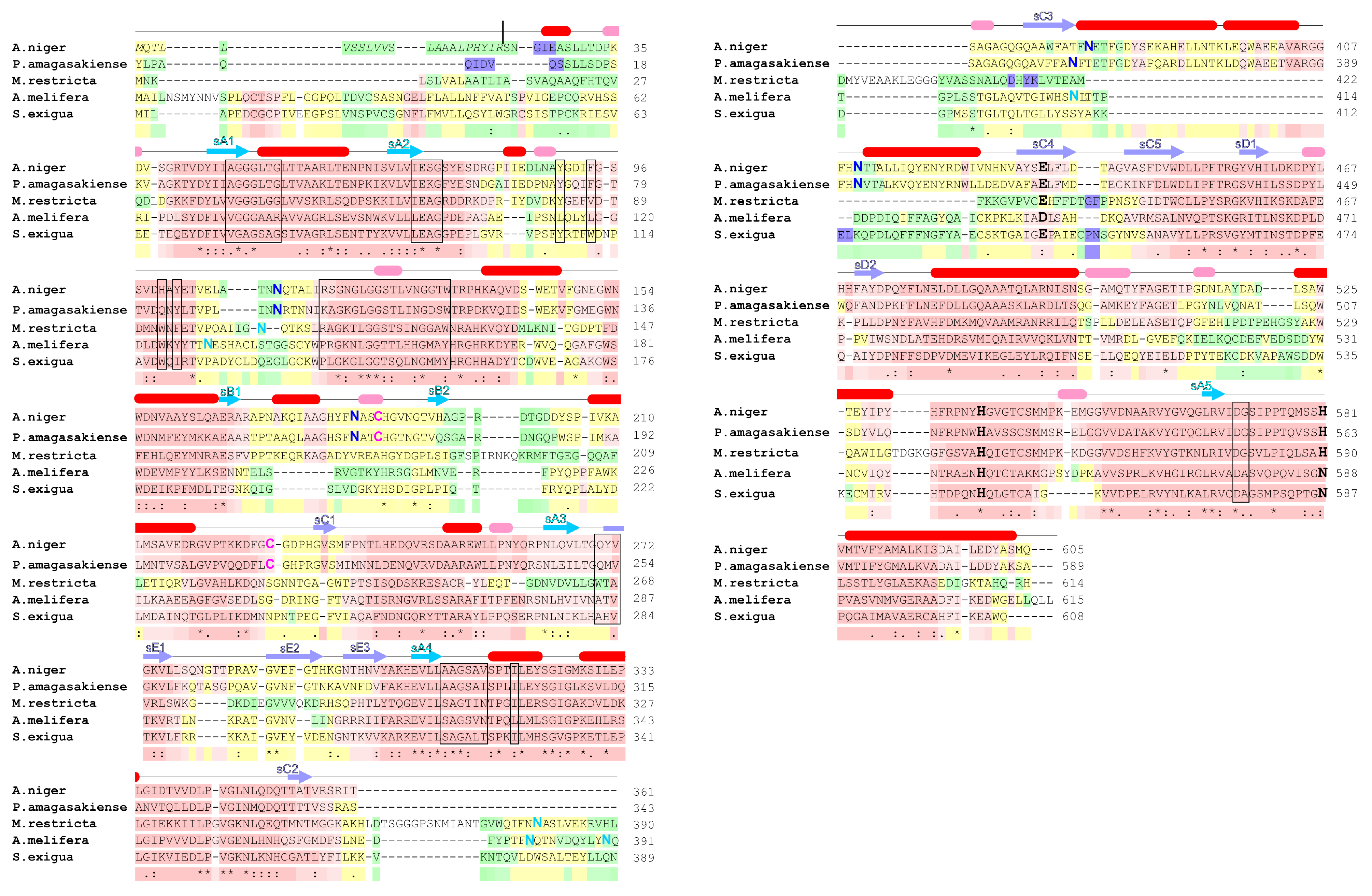
2. Glucose Oxidase Structure
2.1. Overall Structure
2.2. Dimer Interface
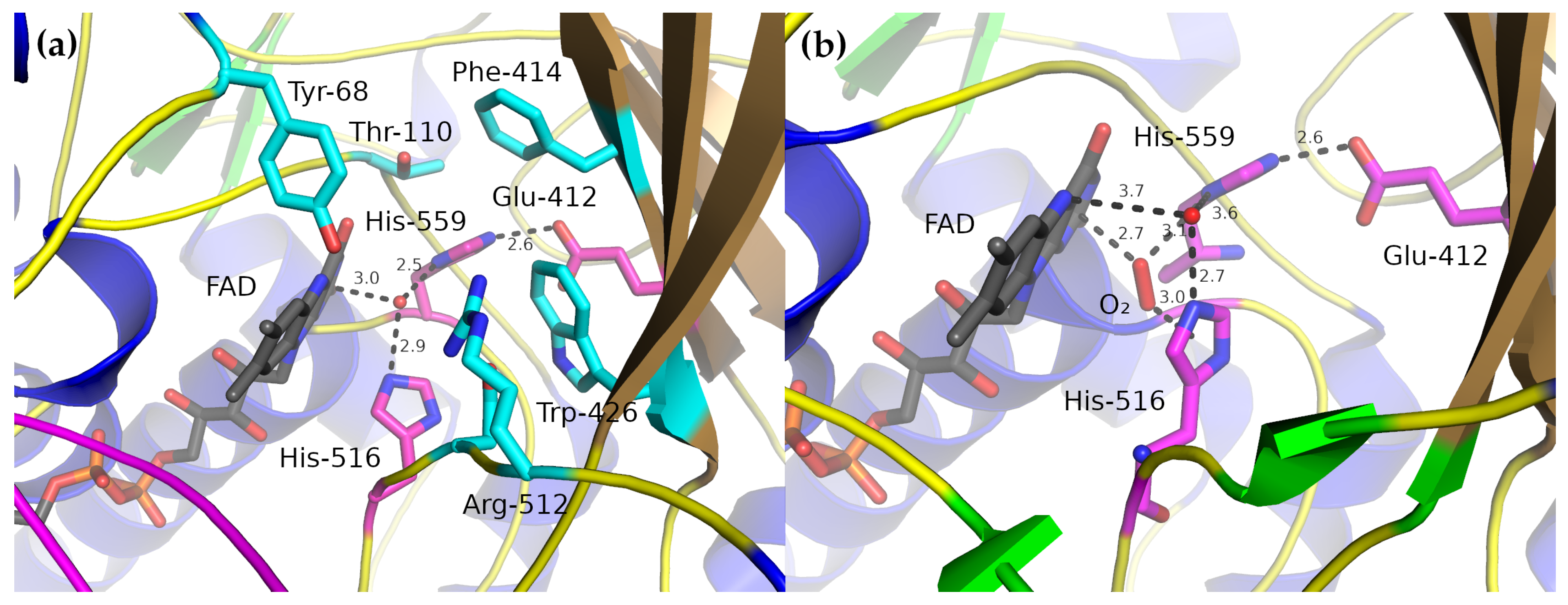
2.3. Active Site
3. Catalytic Mechanism
Glycosylation
4. Natural Sources of Glucose Oxidase
5. Industrial and Medical Applications of GOx
5.1. Industrial Production of GOx
5.2. Use of Glucose Oxidase in the Food Industry
5.3. Glucose Oxidase Biosensors in Medicine Applications
5.3.1. Cancer
5.3.2. Diabetes Treatment
5.4. GOx in Wound Healing: From Bench to Bedside
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| GOx | Glucose Oxidase |
| FAD | Flavin Adenine Dinucleotide |
| NAD(P) | Nicotinamide Adenine Dinucleotide (Phosphate) |
| PQQ | PyrroloQuinoline Quinone |
| GMC | Glucose-Methanol-Choline |
| RMSD | Root-Mean-Squared Deviation |
| NMR | Nuclear Magnetic Resonance |
| NCBI | National Center for Biotechnology Information |
| SSF | Solid State Fermentation |
| PDA | PolyDopAmine |
| HSP70/90 | Heat-Shock Protein 70/90 |
| TPZ | Tirapazamine |
| CDT | ChemoDynamic Therapy |
| GASA | 4-[(hydroxy-3-methoxyphenyl)-azo]-benzenesulfonic acid |
| ROS | Reactive Oxygen Species |
References
- Stolarczyk, K.; Rogalski, J.; Bilewicz, R. NAD(P)-dependent glucose dehydrogenase: Applications for biosensors, bioelectrodes, and biofuel cells. Bioelectrochemistry 2020, 135, 107574. [Google Scholar] [CrossRef]
- Anthony, C. The quinoprotein dehydrogenases for methanol and glucose. Arch. Biochem. Biophys. 2004, 428, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Bankar, S.B.; Bule, M.V.; Singhal, R.S.; Ananthanarayan, L. Glucose oxidase—An overview. Biotechnol. Adv. 2009, 27, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Abrera, A.T.; Sützl, L.; Haltrich, D. Pyranose oxidase: A versatile sugar oxidoreductase for bioelectrochemical applications. Bioelectrochemistry 2020, 132, 107409. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.; Turner, A.P.F. Glucose oxidase: An ideal enzyme. Biosens. Bioelectron. 1992, 7, 165–185. [Google Scholar] [CrossRef]
- Mattevi, A. To be or not to be an oxidase: Challenging the oxygen reactivity of flavoenzymes. Trends Biochem. Sci. 2006, 31, 276–283. [Google Scholar] [CrossRef]
- Cavener, D.R. GMC oxidoreductases: A newly defined family of homologous proteins with diverse catalytic activities. J. Mol. Biol. 1992, 223, 811–814. [Google Scholar] [CrossRef]
- Sützl, L.; Foley, G.; Gillam, E.M.J.; Bodén, M.; Haltrich, D. The GMC superfamily of oxidoreductases revisited: Analysis and evolution of fungal GMC oxidoreductases. Biotechnol. Biofuels 2019, 12, 118. [Google Scholar] [CrossRef]
- Hecht, H.J.; Kalisz, H.M.; Hendle, J.; Schmod, R.D.; Schomburg, D. Crystal Structure of Glucose Oxidase from Aspergillus niger Refined at 2.3 Å Resolution. J. Mol. Biol. 1993, 229, 153–172. [Google Scholar] [CrossRef]
- Wohlfahrt, G.; Witt, S.; Hendle, J.; Schomburg, D.; Kalisz, H.M.; Hecht, H.J. 1.8 and 1.9 Å resolution structures of the Penicillium amagasakiense and Aspergillus niger glucose oxidases as a basis for modelling substrate complexes. Acta Crystallogr. D Struct. Biol. 1999, 55, 969–977. [Google Scholar] [CrossRef]
- Kommoju, P.R.; Chen, Z.; Bruckner, R.C.; Mathews, F.S.; Jorns, M.S. Probing Oxygen Activation Sites in Two Flavoprotein Oxidases Using Chloride as an Oxygen Surrogate. Biochemistry 2011, 50, 5521–5534. [Google Scholar] [CrossRef] [PubMed]
- Petrović, D.; Frank, D.; Kamerlin, S.C.L.; Hoffmann, K.; Strodel, B. Shuffling Active Site Substate Populations Affects Catalytic Activity: The Case of Glucose Oxidase. ACS Catal. 2017, 7, 6188–6197. [Google Scholar] [CrossRef] [PubMed]
- Cerutti, G.; Gugole, E.; Montemiglio, L.C.; Turbé-Doan, A.; Chena, D.; Navarro, D.; Lomascolo, A.; Piumi, F.; Exertier, C.; Freda, I.; et al. Crystal structure and functional characterization of an oligosaccharide dehydrogenase from Pycnoporus cinnabarinus provides insights into fungal breakdown of lignocellulose. Biotechnol. Biofuels. 2021, 14, 161. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Sakai, G.; Mori, K.; Kojima, K.; Kamitori, S.; Sode, K. Structural analysis of fungus-derived FAD glucose dehydrogenase. Sci. Rep. 2015, 5, 13498. [Google Scholar] [CrossRef]
- Sorigué, D.; Hadjidemetriou, K.; Blangy, S.; Gotthard, G.; Bonvalet, A.; Coquelle, N.; Samire, P.; Aleksandrov, A.; Antonucci, L.; Benachir, A.; et al. Mechanism and dynamics of fatty acid photodecarboxylase. Science 2021, 372, eabd5687. [Google Scholar] [CrossRef] [PubMed]
- Carro, J.; Martínez-Júlvez, M.; Medina, M.; Martínez, A.T.; Ferreira, P. Protein dynamics promote hydride tunnelling in substrate oxidation by aryl-alcohol oxidase. Phys. Chem. Chem. Phys. 2017, 19, 28666–28675. [Google Scholar] [CrossRef] [PubMed]
- Švecová, L.; Østergaard, L.H.; Skálová, T.; Schnorr, K.M.; Kovaľ, T.; Kolenko, P.; Stránský, J.; Sedlák, D.; Dušková, J.; Trundová, M.; et al. Crystallographic fragment screening-based study of a novel FAD-dependent oxidoreductase from Chaetomium thermophilum. Acta Crystallogr. D Struct. Biol. 2021, 77, 755–775. [Google Scholar] [CrossRef]
- Tan, T.C.; Spadiut, O.; Wongnate, T.; Sucharitakul, J.; Krondorfer, I.; Sygmund, C.; Haltrich, D.; Chaiyen, P.; Peterbauer, C.K.; Divne, C. The 1.6 Å Crystal Structure of Pyranose Dehydrogenase from Agaricus meleagris Rationalizes Substrate Specificity and Reveals a Flavin Intermediate. PLoS ONE 2013, 8, e53567. [Google Scholar] [CrossRef]
- Koch, C.; Neumann, P.; Valerius, O.; Feussner, I.; Ficner, R. Crystal Structure of Alcohol Oxidase from Pichia pastoris. PLoS ONE 2016, 11, e0149846. [Google Scholar] [CrossRef]
- Nguyen, Q.T.; Romero, E.; Dijkman, W.P.; de Vasconcellos, S.P.; Binda, C.; Mattevi, A.; Fraaije, M.W. Structure-Based Engineering of Phanerochaete chrysosporium Alcohol Oxidase for Enhanced Oxidative Power toward Glycerol. Biochemistry 2018, 57, 6209–6218. [Google Scholar] [CrossRef]
- Finnegan, S.; Yuan, H.; Wang, Y.F.; Orville, A.M.; Weber, I.T.; Gadda, G. Structural and kinetic studies on the Ser101Ala variant of choline oxidase: Catalysis by compromise. Arch. Biochem. Biophys. 2010, 501, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Mugo, A.N.; Kobayashi, J.; Yamasaki, T.; Mikami, B.; Ohnishi, K.; Yoshikane, Y.; Yagi, T. Crystal structure of pyridoxine 4-oxidase from Mesorhizobium loti. Biochim. Biophys. Acta 2013, 1834, 953–963. [Google Scholar] [CrossRef] [PubMed]
- Pickl, M.; Swoboda, A.; Romero, E.; Winkler, C.K.; Binda, C.; Mattevi, A.; Faber, K.; Fraaije, M.W. Kinetic Resolution of sec-Thiols by Enantioselective Oxidation with Rationally Engineered 5-(Hydroxymethyl)furfural Oxidase. Angew. Chem. Int. Ed. Engl. 2018, 57, 2864–2868. [Google Scholar] [CrossRef] [PubMed]
- Doubayashi, D.; Ootake, T.; Maeda, Y.; Oki, M.; Tokunaga, Y.; Sakurai, A.; Nagaosa, Y.; Mikami, B.; Uchida, H. Formate Oxidase, an Enzyme of the Glucose-Methanol-Choline Oxidoreductase Family, Has a His-Arg Pair and 8-Formyl-FAD at the Catalytic Site. Biosci. Biotechnol. Biochem. 2011, 75, 1662–1667. [Google Scholar] [CrossRef][Green Version]
- Kadowaki, M.A.S.; Higasi, P.M.R.; de Godoy, M.O.; de Araújo, E.A.; Godoy, A.S.; Prade, R.A.; Polikarpov, I. Enzymatic versatility and thermostability of a new aryl-alcohol oxidase from Thermothelomyces thermophilus M77. Biochim. Biophys. Acta Gen. Subj. 2020, 1864, 129681. [Google Scholar] [CrossRef]
- Coulombe, R.; Yue, K.Q.; Ghisla, S.; Vrielink, A. Oxygen Access to the Active Site of Cholesterol Oxidase through a Narrow Channel Is Gated by an Arg-Glu Pair. J. Biol. Chem. 2001, 276, 30435–30441. [Google Scholar] [CrossRef]
- Tsuge, H.; Natsuaki, O.; Ohashi, K. Purification, properties, and molecular features of glucose oxidase from Aspergillus niger. J. Biochem. 1975, 78, 835–843. [Google Scholar] [CrossRef]
- Swoboda, B.E.P. The relationship between molecular conformation and the binding of flavin-adenine dinucleotide in glucose oxidase. Biochim. Biophys. Acta 1969, 175, 365–379. [Google Scholar] [CrossRef]
- Cioci, F.; Lavecchia, R. Effect of polyols and sugars on heat-induced flavin dissociation in glucose oxidase. Biochem. Mol. Biol. Int. 1994, 34, 705–712. [Google Scholar]
- Gouda, M.D.; Singh, S.A.; Rao, A.G.A.; Thakur, M.S.; Karanth, N.G. Thermal Inactivation of Glucose Oxidase. Mechanism and stabilization using additives. J. Biol. Chem. 2003, 278, 24324–24333. [Google Scholar] [CrossRef]
- Zoldák, G.; Zubrik, A.; Musatov, A.; Stupák, M.; Sedlák, E. Irreversible Thermal Denaturation of Glucose Oxidase from Aspergillus niger Is the Transition to the Denatured State with Residual Structure. J. Biol. Chem. 2004, 279, 47601–47609. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.N.; Manley, P.; Wilkinson, A. The dissociation of glucose oxidase by sodium n-dodecyl sulphate. Biochem. J. 1982, 203, 285–291. [Google Scholar] [CrossRef]
- Ye, W.N.; Combes, D. The relationship between the glucose oxidase subunit structure and its thermostability. Biochim. Biophys. Acta 1989, 999, 86–93. [Google Scholar] [CrossRef]
- Leskovac, V.; Trivić, S.; Wohlfahrt, G.; Kandrač, J.; Peričin, D. Glucose oxidase from Aspergillus niger: The mechanism of action with molecular oxygen, quinones, and one-electron acceptors. Int. J. Biochem. Cell Biol. 2005, 37, 731–750. [Google Scholar] [CrossRef]
- Gibson, Q.H.; Swoboda, B.E.P.; Massey, V. Kinetics and Mechanism of Action of Glucose Oxidase. J. Biol. Chem. 1964, 239, 3927–3934. [Google Scholar] [CrossRef]
- Swoboda, B.E.P.; Massey, V. Purification and Properties of the Glucose Oxidase from Aspergillus Niger. J. Biol. Chem. 1965, 240, 2209–2215. [Google Scholar] [CrossRef]
- Pazur, J.H.; Kleppe, K. The Oxidation of Glucose and Related Compounds by Glucose Oxidase from Aspergillus niger. Biochemistry 1964, 3, 578–583. [Google Scholar] [CrossRef]
- Kunst, A.; Draeger, B.; Ziegenhorn, J. Colorimetric methods with glucose oxidase and peroxidase. In Methods of Enzymatic Analysis, 3rd ed.; Bergmeyered, H.U., Ed.; Chemie: Weinheim, Germany, 1984. [Google Scholar]
- Voet, J.G.; Coe, J.; Epstein, J.; Matossian, V.; Shipley, T. Electrostatic Control of Enzyme Reactions: Effect of Ionic Strength on the pKa of an Essential Acidic Group on Glucose Oxidase. Biochemistry 1981, 20, 7182–7185. [Google Scholar] [CrossRef]
- Su, Q.; Klinman, J.P. Nature of Oxygen Activation in Glucose Oxidase from Aspergillus niger: The Importance of Electrostatic Stabilization in Superoxide Formation. Biochemistry 1999, 38, 8572–8581. [Google Scholar] [CrossRef]
- Roth, J.P.; Klinman, J.P. Catalysis of electron transfer during activation of O2 by the flavoprotein glucose oxidase. Proc. Natl. Acad. Sci. USA 2003, 100, 62–67. [Google Scholar] [CrossRef]
- Wohlfahrt, G.; Trivić, S.; Zeremski, J.; Peričin, D.; Leskovac, V. The chemical mechanism of action of glucose oxidase from Aspergillus niger. Mol. Cell. Biochem. 2004, 260, 69–83. [Google Scholar] [CrossRef]
- Bright, H.J.; Appleby, M. The pH Dependence of the Individual Steps in the Glucose Oxidase Reaction. J. Biol. Chem. 1969, 244, 3625–3634. [Google Scholar] [CrossRef]
- Bright, H.J.; Gibson, Q.H. The Oxidation of 1-Deuterated Glucose by Glucose Oxidase. J. Biol. Chem. 1967, 242, 994–1003. [Google Scholar] [CrossRef]
- Sanner, C.; Macheroux, P.; Rüterjans, H.; Müller, F.; Bacher, A. 15N- and 13C-NMR investigations of glucose oxidase from Aspergillus niger. Eur. J. Biochem. 1991, 196, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Weibel, M.K.; Bright, H.J. The Glucose Oxidase Mechanism. Interpretation of the pH dependence. J. Biol. Chem. 1971, 246, 2734–2744. [Google Scholar] [CrossRef]
- Sawyer, D.T. Oxygen Chemistry; Oxford University Press: Oxford, UK, 1991. [Google Scholar]
- Prabhakar, R.; Siegbahn, P.E.M.; Minaev, B.F.; Ågren, H. Activation of Triplet Dioxygen by Glucose Oxidase: Spin-Orbit Coupling in the Superoxide Ion. J. Phys. Chem. B 2002, 106, 3742–3750. [Google Scholar] [CrossRef]
- Palfey, B.A.; Ballou, D.P.; Massey, V. Oxygen Activation by Flavins and Pterins. In Active Oxygen in Biochemistry; Valentine, J.S., Foote, C.S., Greenberg, A., Liebman, J.F., Eds.; Springer: Dordrecht, The Netherlands, 1995; pp. 37–83. [Google Scholar] [CrossRef]
- Kalisz, H.M.; Hecht, H.J.; Schomburg, D.; Schmid, R.D. Effects of carbohydrate depletion on the structure, stability and activity of glucose oxidase from Aspergillus niger. Biochim. Biophys. Acta 1991, 1080, 138–142. [Google Scholar] [CrossRef]
- Kalisz, H.M.; Hecht, H.J.; Schomburg, D.; Schmid, R.D. Crystallization and preliminary X-ray diffraction studies of a deglycosylated glucose oxidase from Aspergillus niger. J. Mol. Biol. 1990, 213, 207–209. [Google Scholar] [CrossRef]
- Kohen, A.; Jonsson, T.; Klinman, J.P. Effects of Protein Glycosylation on Catalysis: Changes in Hydrogen Tunneling and Enthalpy of Activation in the Glucose Oxidase Reaction. Biochemistry 1997, 36, 2603–2611. [Google Scholar] [CrossRef]
- Edge, A.S.B. Deglycosylation of glycoproteins with trifluoromethanesulphonic acid: Elucidation of molecular structure and function. Biochem. J. 2003, 376, 339–350. [Google Scholar] [CrossRef]
- Frederick, K.R.; Tung, J.; Emerick, R.S.; Masiarz, F.R.; Chamberlain, S.H.; Vasavada, A.; Rosenberg, S. Glucose Oxidase from Aspergillus niger. Cloning, gene sequence, secretion from Saccharomyces cerevisiae and kinetic analysis of a yeast-derived enzyme. J. Biol. Chem. 1990, 265, 3793–3802. [Google Scholar] [CrossRef]
- Dubey, M.K.; Zehra, A.; Aamir, M.; Meena, M.; Ahirwal, L.; Singh, S.; Shukla, S.; Upadhyay, R.S.; Bueno-Mari, R.; Bajpai, V.K. Improvement Strategies, Cost Effective Production, and Potential Applications of Fungal Glucose Oxidase (GOD): Current Updates. Front. Microbiol. 2017, 8, 1032. [Google Scholar] [CrossRef] [PubMed]
- Bean, R.C.; Hassid, W.Z. Carbohydrate oxidase from a red alga, Iridophycus flaccidum. J. Biol. Chem. 1956, 218, 425–436. [Google Scholar] [CrossRef]
- Bean, R.C.; Porter, G.G.; Steinberg, B.M. Carbohydrate Metabolism of Citrus Fruits. II. Oxidation of sugars by an aerodehydrogenase from young orange fruits. J. Biol. Chem. 1961, 236, 1235–1240. [Google Scholar] [CrossRef]
- Heyningen, R.V. Metabolism of Xylose by the Lens. Calf lens in vitro. Biochem. J. 1956, 69, 481–491. [Google Scholar] [CrossRef]
- Dowling, J.H.; Levine, H.B. Hexose oxidation by an enzyme system of Malleomyces pseudomallei. J. Bacteriol. 1956, 72, 555–560. [Google Scholar] [CrossRef]
- King, T.E.; Cheldelin, V.H. Glucose oxidation and cytochromes in solubilized particulate fractions of Acetobacter suboxydans. J. Biol. Chem. 1957, 224, 579–590. [Google Scholar] [CrossRef]
- Bentley, R.; Slechta, L. Oxidation of mono- and disaccharides to aldonic acids by Pseudomonas species. J. Bacteriol. 1960, 79, 346–355. [Google Scholar] [CrossRef]
- Hauge, J.G. Glucose dehydrogenation in bacteria: A comparative study. J. Bacteriol. 1961, 82, 609–614. [Google Scholar] [CrossRef]
- Ohashi, K.; Natori, S.; Kubo, T. Expression of amylase and glucose oxidase in the hypopharyngeal gland with an age-dependent role change of the worker honeybee (Apis mellifera L.). Eur. J. Biochem. 1999, 265, 127–133. [Google Scholar] [CrossRef]
- Tang, Q.; Hu, Y.; Kang, L.; Wang, C.Z. Characterization of glucose-induced glucose oxidase gene and protein expression in Helicoverpa armigera larvae. Arch. Insect Biochem. Physiol. 2012, 79, 104–119. [Google Scholar] [CrossRef]
- Afshar, K.; Dufresne, P.J.; Pan, L.; Merkx-Jacques, M.; Bede, J.C. Diet-specific salivary gene expression and glucose oxidase activity in Spodoptera exigua (Lepidoptera: Noctuidae) larvae. J. Insect Physiol. 2010, 56, 1798–1806. [Google Scholar] [CrossRef] [PubMed]
- Caridis, K.A.; Christakopoulos, P.; Macris, B.J. Simultaneous production of glucose oxidase and catalase by Alternaria alternata. Appl. Microbiol. Biotechnol. 1991, 34, 794–797. [Google Scholar] [CrossRef]
- Yuivar, Y.; Barahona, S.; Alcaíno, J.; Cifuentes, V.; Baeza, M. Biochemical and Thermodynamical Characterization of Glucose Oxidase, Invertase, and Alkaline Phosphatase Secreted by Antarctic Yeasts. Front. Mol. Biosci. 2017, 4, 86. [Google Scholar] [CrossRef] [PubMed]
- Raghukumar, C.; Mohandass, C.; Kamat, S.; Shailaja, M.S. Simultaneous detoxification and decolorization of molasses spent wash by the immobilized white-rot fungus Flavodon flavus isolated from the marine habitat. Enzym. Microb. Technol. 2004, 35, 197–202. [Google Scholar] [CrossRef]
- Macías-Sánchez, K.; García-Soto, J.; López-Ramírez, A.; Martínez-Cadena, G. Rho1 and other GTP-binding proteins are associated with vesicles carrying glucose oxidase activity from Fusarium oxysporum f. sp. lycopersici. Antonie Leeuwenhoek 2011, 99, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Jiménez, R.; García-Soto, J.; Martínez-Cadena, G. Small GTP-binding proteins are associated with chitosomes and vesicles carrying glucose oxidase from Mucor circinelloides. Microbiology (Reading) 2008, 154, 842–851. [Google Scholar] [CrossRef][Green Version]
- Eremin, A.N.; Makarenko, M.V.; Zhukovskaia, L.A.; Mikhailova, R.V. Isolation and characterization of extracellular glucose oxidase from Penicillium adametzii LF F-2044.1. Prikl. Biokhim. Mikrobiol. 2006, 42, 345–352. [Google Scholar] [CrossRef]
- Kiess, M.; Hecht, H.J.; Kalisz, H.M. Glucose oxidase from Penicillium amagasakiense. Primary structure and comparisonwith other glucose-methanol-choline (GMC) oxidoreductases. Eur. J. Biochem. 1998, 252, 90–99. [Google Scholar] [CrossRef]
- Johnstone-Robertson, M.; Clarke, K.G.; Harrison, S.T.L. Characterization of the distribution of glucose oxidase in Penicillium sp. CBS 120262 and Aspergillus niger NRRL-3 cultures and its effect on integrated product recovery. Biotechnol. Bioeng. 2008, 99, 910–918. [Google Scholar] [CrossRef]
- Gao, Z.; Li, Z.; Zhang, Y.; Huang, H.; Li, M.; Zhou, L.; Tang, Y.; Yao, B.; Zhang, W. High-level expression of the Penicillium notatum glucose oxidase gene in Pichia pastoris using codon optimization. Biotechnol. Lett. 2012, 34, 507–514. [Google Scholar] [CrossRef]
- Kim, H.W.; Kimura, S.; Ohno, N.; Okadome, M.; Takahashi, H.; Amachi, S.; Shinoyama, H.; Fujii, T. Purification of Glucose Oxidase and Catalase Produced by the Apple Blue Mold, Penicillium expansum O-385-10, and Their Characteristics Including the Browning of Apple Fruit. Jpn. J. Food Microbiol. 2005, 22, 10–16. [Google Scholar] [CrossRef]
- Abalikhina, T.A.; Morozkin, A.D.; Bogdanov, V.P.; Kaverznera, E. Composition and structure of glucose oxidase from Penicillium vitale. Biokhimiya 1971, 36, 191–198. [Google Scholar]
- Chi, B.B.; Lu, Y.N.; Yin, P.C.; Liu, H.Y.; Chen, H.Y.; Shan, Y. Sequencing and Comparative Genomic Analysis of a Highly Metal-Tolerant Penicillium janthinellum P1 Provide Insights Into Its Metal Tolerance. Front. Microbiol. 2021, 12, 663217. [Google Scholar] [CrossRef]
- Zhao, J.; Janse, B.J.H. Comparison of H2O2-producing enzymes in selected white rot fungi. FEMS Microbiol. Lett. 1996, 139, 215–221. [Google Scholar] [CrossRef]
- Shin, K.S.; Youn, H.D.; Han, Y.H.; Kang, S.O.; Hah, Y.C. Purification and characterization of d-glucose oxidase from white-rot fungus Pleurotus ostreatus. Eur. J. Biochem. 1993, 215, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Levasseur, A.; Lomascolo, A.; Chabrol, O.; Ruiz-Dueñas, F.J.; Boukhris-Uzan, E.; Piumi, F.; Kües, U.; Ram, A.F.J.; Murat, C.; Haon, M.; et al. The genome of the white-rot fungus Pycnoporus cinnabarinus: A basidiomycete model with a versatile arsenal for lignocellulosic biomass breakdown. BMC Genom. 2014, 15, 486. [Google Scholar] [CrossRef]
- Guimarães, L.H.S.; Peixoto-Nogueira, S.C.; Michelin, M.; Rizzatti, A.C.S.; Sandrim, V.C.; Zanoelo, F.F.; Aquino, A.C.M.M.; Junior, A.B.; de Lourdes T. M. Polizeli, M. Screening of filamentous fungi for production of enzymes of biotechnological interest. Braz. J. Microbiol. 2006, 37, 474–480. [Google Scholar] [CrossRef]
- Ohm, R.A.; de Jong, J.F.; Lugones, L.G.; Aerts, A.; Kothe, E.; Stajich, J.E.; de Vries, R.P.; Record, E.; Levasseur, A.; Baker, S.E.; et al. Genome sequence of the model mushroom Schizophyllum commune. Nat. Biotechnol. 2010, 28, 957–963. [Google Scholar] [CrossRef]
- Kim, K.K.; Fravel, D.R.; Papavizas, G.C. Production, purification, and properties of glucose oxidase from the biocontrol fungus Talaromyces flavus. Can. J. Microbiol. 1990, 36, 199–205. [Google Scholar] [CrossRef]
- Semashko, T.V.; Mikhailova, R.V.; Eremin, A.N. Extracellular Glucose Oxidase of Penicillium funiculosum 46.1. Appl. Biochem. Microbiol. 2003, 39, 368–374. [Google Scholar] [CrossRef]
- Rando, D.; Kohring, G.W.; Giffhorn, F. Production, purification and characterization of glucose oxidase from a newly isolated strain of Penicillium pinophilum. Appl. Microbiol. Biotechnol. 1997, 48, 34–40. [Google Scholar] [CrossRef]
- Nakamatsu, T.; Akamatsu, T.; Miyajima, R.; Simo, I. Microbial Production of Glucose Oxidase. Agric. Biol. Chem. 1975, 39, 1803–1811. [Google Scholar] [CrossRef]
- Nierman, W.C.; Fedorova-Abrams, N.D.; Andrianopoulos, A. Genome Sequence of the AIDS-Associated Pathogen Penicillium marneffei (ATCC18224) and Its Near Taxonomic Relative Talaromyces stipitatus (ATCC10500). Genome Announc. 2015, 3, e01559-14. [Google Scholar] [CrossRef] [PubMed]
- Pulci, V.; D’Ovidio, R.; Petruccioli, M.; Federici, F. The glucose oxidase of Penicillium variabile P16: Gene cloning, sequencing and expression. Lett. Appl. Microbiol. 2004, 38, 233–238. [Google Scholar] [CrossRef]
- Wibberg, D.; Jelonek, L.; Rupp, O.; Hennig, M.; Eikmeyer, F.; Goesmann, A.; Hartmann, A.; Borriss, R.; Grosch, R.; Pühler, A.; et al. Establishment and interpretation of the genome sequence of the phytopathogenic fungus Rhizoctonia solani AG1-IB isolate 7/3/14. J. Biotechnol. 2013, 167, 142–155. [Google Scholar] [CrossRef] [PubMed]
- Gazis, R.; Kuo, A.; Riley, R.; LaButti, K.; Lipzen, A.; Lin, J.; Amirebrahimi, M.; Hesse, C.N.; Spatafora, J.W.; Henrissat, B.; et al. The genome of Xylona heveae provides a window into fungal endophytism. Fungal Biol. 2016, 120, 26–42. [Google Scholar] [CrossRef]
- Guindon, S.; Dufayard, J.F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New Algorithms and Methods to Estimate Maximum-Likelihood Phylogenies: Assessing the Performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef]
- Sievers, F.; Higgins, D.G. Clustal Omega for making accurate alignments of many protein sequences. Protein Sci. 2018, 27, 135–145. [Google Scholar] [CrossRef]
- Di Tommaso, P.; Moretti, S.; Xenarios, I.; Orobitg, M.; Montanyola, A.; Chang, J.M.; Taly, J.F.; Notredame, C. T-Coffee: A web server for the multiple sequence alignment of protein and RNA sequences using structural information and homology extension. Nucleic Acids Res. 2011, 39, W13–W17. [Google Scholar] [CrossRef]
- Allameh, S.; Rabbani, M. A Distance-Based Microfluidic Paper-Based Biosensor for Glucose Measurements in Tear Range. Appl. Biochem. Biotechnol. 2022. [Google Scholar] [CrossRef]
- Asrami, P.N.; Azar, P.A.; Tehrani, M.S.; Mozaffari, S.A. Glucose Oxidase/Nano-ZnO/Thin Film Deposit FTO as an Innovative Clinical Transducer: A Sensitive Glucose Biosensor. Front. Chem. 2020, 8, 503. [Google Scholar] [CrossRef]
- Devasenathipathy, R.; Mani, V.; Chen, S.M.; Huang, S.T.; Huang, T.T.; Lind, C.M.; Hwae, K.Y.; Chen, T.Y.; Chen, B.J. Glucose biosensor based on glucose oxidase immobilized at gold nanoparticles decorated graphene-carbon nanotubes. Enzym. Microb. Technol. 2015, 78, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Jędrzak, A.; Rębiś, T.; Kuznowicz, M.; Jesionowski, T. Bio-inspired magnetite/lignin/polydopamine-glucose oxidase biosensing nanoplatform. From synthesis, via sensing assays to comparison with others glucose testing techniques. Int. J. Biol. Macromol. 2019, 127, 677–682. [Google Scholar] [CrossRef] [PubMed]
- Kalaivani, G.J.; Suja, S.K. Nanomolar level sensing of glucose in food samples using glucose oxidase confined MWCNT-Inulin-TiO2 bio-nanocomposite. Food Chem. 2019, 298, 124981. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Xu, H.; Zhang, Y.; Yi, S.; Cui, R.; Xing, S.; Wei, C.; Lin, J.; Huang, P. Glucose Oxidase-Instructed Traceable Self-Oxygenation/Hyperthermia Dually Enhanced Cancer Starvation Therapy. Theranostics 2020, 10, 1544–1554. [Google Scholar] [CrossRef] [PubMed]
- Chai, Z.; Dong, H.; Sun, X.; Fan, Y.; Wang, Y.; Huang, F. Development of glucose oxidase-immobilized alginate nanoparticles for enhanced glucose-triggered insulin delivery in diabetic mice. Int. J. Biol. Macromol. 2020, 159, 640–647. [Google Scholar] [CrossRef]
- Shao, L.; Li, Y.; Huang, F.; Wang, X.; Lu, J.; Jia, F.; Pan, Z.; Cui, X.; Ge, G.; Deng, X.; et al. Complementary autophagy inhibition and glucose metabolism with rattle-structured polydopamine@mesoporous silica nanoparticles for augmented low-temperature photothermal therapy and in vivo photoacoustic imaging. Theranostics 2020, 10, 7273–7286. [Google Scholar] [CrossRef]
- German, N.; Ramanaviciene, A.; Ramanavicius, A. Formation of Polyaniline and Polypyrrole Nanocomposites with Embedded Glucose Oxidase and Gold Nanoparticles. Polymers 2019, 11, 377. [Google Scholar] [CrossRef]
- Wei, X.; Chen, J.; Ali, M.C.; Munyemana, J.C.; Qiu, H. Cadmium cobaltite nanosheets synthesized in basic deep eutectic solvents with oxidase-like, peroxidase-like, and catalase-like activities and application in the colorimetric assay of glucose. Mikrochim. Acta 2020, 187, 314. [Google Scholar] [CrossRef]
- Garcia-Hernandez, C.; Garcia-Cabezon, C.; Martin-Pedrosa, F.; Rodriguez-Mendez, M.L. Analysis of musts and wines by means of a bio-electronic tongue based on tyrosinase and glucose oxidase using polypyrrole/gold nanoparticles as the electron mediator. Food Chem. 2019, 289, 751–756. [Google Scholar] [CrossRef] [PubMed]
- Steffolani, M.E.; Ribotta, P.D.; Pérez, G.T.; León, A.E. Combinations of glucose oxidase, α-amylase and xylanase affect dough properties and bread quality. Int. J. Food Sci. Technol. 2012, 47, 525–534. [Google Scholar] [CrossRef]
- Mohammadnejad, P.; Asl, S.S.; Aminzadeh, S.; Haghbeen, K. A new sensitive spectrophotometric method for determination of saliva and blood glucose. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 229, 117897. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, Y.; Ye, Y.; DiSanto, R.; Sun, W.; Ranson, D.; Ligler, F.S.; Buse, J.B.; Gu, Z. Microneedle-array patches loaded with hypoxia-sensitive vesicles provide fast glucose-responsive insulin delivery. Proc. Natl. Acad. Sci. USA 2015, 112, 8260–8265. [Google Scholar] [CrossRef]
- Yu, J.; Qian, C.; Zhang, Y.; Cui, Z.; Zhu, Y.; Shen, Q.; Ligler, F.S.; Buse, J.B.; Gu, Z. Hypoxia and H2O2 Dual-Sensitive Vesicles for Enhanced Glucose-Responsive Insulin Delivery. Nano Lett. 2017, 17, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Jia, H.R.; Zhang, X.; Zhang, X.; Sun, Q.; Wang, S.Z.; Zhao, J.; Wu, F.G. A Glucose/Oxygen-Exhausting Nanoreactor for Starvation- and Hypoxia-Activated Sustainable and Cascade Chemo-Chemodynamic Therapy. Small 2020, 16, e2000897. [Google Scholar] [CrossRef] [PubMed]
- Kornecki, J.F.; Carballares, D.; Tardioli, P.W.; Rodrigues, R.C.; Berenguer-Murcia, Á.; Alcántarae, A.R.; Fernandez-Lafuente, R. Enzyme production of d-gluconic acid and glucose oxidase: Successful tales of cascade reactions. Catal. Sci. Technol. 2020, 10, 5740. [Google Scholar] [CrossRef]
- Khatami, S.H.; Vakili, O.; Ahmadi, N.; Fard, E.S.; Mousavi, P.; Khalvati, B.; Maleksabet, A.; Savardashtaki, A.; Taheri-Anganeh, M.; Movahedpour, A. Glucose oxidase: Applications, sources, and recombinant production. Biotechnol. Appl. Biochem. 2021. Online ahead of print. [Google Scholar] [CrossRef]
- FDA. Enzyme Preparations Used in Food (Partial List). 2018. Available online: https://www.fda.gov/food/generally-recognized-safe-gras/enzyme-preparations-used-food-partial-list (accessed on 27 February 2022).
- Kalisz, H.M.; Hendle, J.; Schmid, R.D. Structural and biochemical properties of glycosylated and deglycosylated glucose oxidase from Penicillium amagasakiense. Appl. Microbiol. Biotechnol. 1997, 47, 502–507. [Google Scholar] [CrossRef]
- Kusai, K.; Sekuzu, I.; Hagihara, B.; Okunuki, K.; Yamauchi, S.; Nakai, M. Crystallization of glucose oxidase from Penicillium amagasakiense. Biochim. Biophys. Acta 1960, 40, 555–557. [Google Scholar] [CrossRef]
- Holland, J.T.; Harper, J.C.; Dolan, P.L.; Manginell, M.M.; Arango, D.C.; Rawlings, J.A.; Apblett, C.A.; Brozik, S.M. Rational redesign of glucose oxidase for improved catalytic function and stability. PLoS ONE 2012, 7, e37924. [Google Scholar] [CrossRef] [PubMed]
- Song, H.T.; Xiao, W.J.; Yang, Y.M.; Zhao, Y.; Gao, Y.; Liu, S.H.; Liu, Z.L.; Xia, W.C.; Li, R.; Li, N.N.; et al. Improving the anti-oxidation of glucose oxidase with computer-aided structure optimization. J. Adv. Biotechnol. 2016, 53, 736–740. [Google Scholar] [CrossRef]
- Marín-Navarro, J.; Roupain, N.; Talens-Perales, D.; Polaina, J. Identification and Structural Analysis of Amino Acid Substitutions that Increase the Stability and Activity of Aspergillus niger Glucose Oxidase. PLoS ONE 2015, 10, e0144289. [Google Scholar] [CrossRef] [PubMed]
- Mirón, J.; González, M.P.; Pastrana, L.; Murado, M.A. Diauxic production of glucose oxidase by Aspergillus niger in submerged culture: A dynamic model. Enzym. Microb. Technol. 2002, 31, 615–620. [Google Scholar] [CrossRef]
- Mirón, J.; Vázquez, J.A.; González, P.; Murado, M.A. Enhancement glucose oxidase production by solid-state fermentation of Aspergillus niger on polyurethane foams using mussel processing wastewaters. Enzym. Microb. Technol. 2010, 46, 21–27. [Google Scholar] [CrossRef]
- Kriaa, M.; Kammoun, R. Producing Aspergillus tubingensis CTM507 glucose oxidase by solid state fermentation versus submerged fermentation: Process optimization and enzyme stability by an intermediary metabolite in relation with diauxic growth. J. Chem. Technol. Biotechnol. 2016, 91, 1540–1550. [Google Scholar] [CrossRef]
- Hatzinikolaou, D.G.; Macris, B.J. Factors regulating production of glucose oxidase by Aspergillus niger. Enzym. Microb. Technol. 1995, 17, 530–534. [Google Scholar] [CrossRef]
- Hatzinikolaou, D.G.; Hansen, O.C.; Macris, B.J.; Tingey, A.; Kekos, D.; Goodenough, P.; Stougaard, P. A new glucose oxidase from Aspergillus niger: Characterization and regulation studies of enzyme and gene. Appl. Microbiol. Biotechnol. 1996, 46, 371–381. [Google Scholar] [CrossRef]
- Liu, J.Z.; Huang, Y.Y.; Liu, J.; Weng, L.P.; Ji, L.N. Effects of metal ions on simultaneous production of glucose oxidase and catalase by Aspergillus niger. Lett. Appl. Microbiol. 2001, 32, 16–19. [Google Scholar] [CrossRef]
- Toren, E.C., Jr.; Burger, F.J. Trace determination of metal ion inhibitors of the glucose-glucose oxidase system. Mikrochim. Acta 1968, 56, 538–545. [Google Scholar] [CrossRef]
- Nakamura, S.; Ogura, Y. Mode of Inhibition of Glucose Oxidase by Metal Ions. J. Biochem. 1968, 64, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Khurshid, S.; Kashmiri, M.A.; Quershi, Z.; Ahmad, W. Optimization of glucose oxidase production by Aspergillus niger. Afr. J. Biotechnol. 2011, 10, 1674–1678. [Google Scholar] [CrossRef]
- Ramzan, M.; Mehmood, T. Enhanced production of glucose oxidase from UV-mutant of Aspergillus niger. Afr. J. Biotechnol. 2009, 8, 288–290. [Google Scholar] [CrossRef]
- Park, E.H.; Shin, Y.M.; Lim, Y.Y.; Kwon, T.H.; Kim, D.H.; Yang, M.S. Expression of glucose oxidase by using recombinant yeast. J. Biotechnol. 2000, 81, 35–44. [Google Scholar] [CrossRef]
- Kapat, A.; Jung, J.K.; Park, Y.H. Enhancement of glucose oxidase production in batch cultivation of recombinant Saccharomyces cerevisiae: Optimization of oxygen transfer condition. J. Appl. Microbiol. 2001, 90, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Malherbe, D.F.; du Toit, M.; Otero, R.R.C.; van Rensburg, P.; Pretorius, I.S. Expression of the Aspergillus niger glucose oxidase gene in Saccharomyces cerevisiae and its potential applications in wine production. Appl. Microbiol. Biotechnol. 2003, 61, 502–511. [Google Scholar] [CrossRef]
- Demain, A.L.; Vaishnav, P. Production of recombinant proteins by microbes and higher organisms. Biotechnol. Adv. 2009, 27, 297–306. [Google Scholar] [CrossRef]
- Courjean, O.; Mano, N. Recombinant glucose oxidase from Penicillium amagasakiense for efficient bioelectrochemical applications in physiological conditions. J. Biotechnol. 2011, 151, 122–129. [Google Scholar] [CrossRef]
- Romanos, M.A.; Scorer, C.A.; Clare, J.J. Foreign Gene Expression in Yeast: A Review. Yeast 1992, 8, 423–488. [Google Scholar] [CrossRef]
- Guo, Y.; Lu, F.; Zhao, H.; Tang, Y.; Lu, Z. Cloning and heterologous expression of glucose oxidase gene from Aspergillus niger Z-25 in Pichia pastoris. Appl. Biochem. Biotechnol. 2010, 162, 498–509. [Google Scholar] [CrossRef]
- Qiu, Z.; Guo, Y.; Bao, X.; Hao, J.; Sun, G.; Peng, B.; Bi, W. Expression of Aspergillus niger glucose oxidase in yeast Pichia pastoris SMD1168. Biotechnol. Biotechnol. Equip. 2016, 30, 998–1005. [Google Scholar] [CrossRef]
- Rasiah, I.A.; Sutton, K.H.; Low, F.L.; Lin, H.M.; Gerrard, J.A. Crosslinking of wheat dough proteins by glucose oxidase and the resulting effects on bread and croissants. Food Chem. 2005, 89, 325–332. [Google Scholar] [CrossRef]
- Wong, C.M.; Wong, K.H.; Chen, X.D. Glucose oxidase: Natural occurrence, function, properties and industrial applications. Appl. Microbiol. Biotechnol. 2008, 78, 927–938. [Google Scholar] [CrossRef] [PubMed]
- Steffolani, M.E.; Ribotta, P.D.; Pérez, G.T.; León, A.E. Effect of glucose oxidase, transglutaminase, and pentosanase on wheat proteins: Relationship with dough properties and bread-making quality. J. Cereal Sci. 2010, 51, 366–373. [Google Scholar] [CrossRef]
- Vemulapalli, V.; Miller, K.A.; Hoseney, R.C. Glucose Oxidase in Breadmaking Systems. Cereal Chem. 1998, 75, 439–442. [Google Scholar] [CrossRef]
- El-Rashidy, L.A.; Bahlol, H.E.M.; El-Desoky, A.A. Improving Quality of Pan Bread by Using Glucose Oxidase and Lipase Enzymes. Middle East J. Appl. Sci. 2015, 5, 1035–1043. [Google Scholar]
- Dagdelen, A.F.; Gocmen, D. Effects of glucose oxidase, hemicellulase and ascorbic acid on dough and bread quality. J. Food Qual. 2007, 30, 1009–1022. [Google Scholar] [CrossRef]
- Decamps, K.; Joye, I.J.; Rakotozafy, L.; Nicolas, J.; Courtin, C.M.; Delcour, J.A. The bread dough stability improving effect of pyranose oxidase from Trametes multicolour and glucose oxidase from Aspergillus niger: Unraveling the molecular mechanism. J. Agric. Food Chem. 2013, 61, 7848–7854. [Google Scholar] [CrossRef]
- Kriaa, M.; Ouhibi, R.; Graba, H.; Besbes, S.; Jardak, M.; Kammoun, R. Synergistic effect of Aspergillus tubingensis CTM 507 glucose oxidase in presence of ascorbic acid and alpha amylase on dough properties, baking quality and shelf life of bread. J. Food Sci. Technol. 2016, 53, 1259–1268. [Google Scholar] [CrossRef]
- Tozatti, P.; Hopkins, E.J.; Briggs, C.; Hucl, P.; Nickerson, M.T. Effect of chemical oxidizers and enzymatic treatments on the baking quality of doughs formulated with five Canadian spring wheat cultivars. Food Sci. Technol. Int. 2020, 26, 614–628. [Google Scholar] [CrossRef]
- Röcker, J.; Schmitt, M.; Pasch, L.; Ebert, K.; Grossmann, M. The use of glucose oxidase and catalase for the enzymatic reduction of the potential ethanol content in wine. Food Chem. 2016, 210, 660–670. [Google Scholar] [CrossRef] [PubMed]
- Valencia, P.; Espinoza, K.; Ramirez, C.; Franco, W.; Urtubia, A. Technical Feasibility of Glucose Oxidase as a Prefermentation Treatment for Lowering the Alcoholic Degree of Red Wine. Am. J. Enol. Vitic. 2017, 68, 386–389. [Google Scholar] [CrossRef]
- Bănică, F.G. Chemical Sensors and Biosensors: Fundamentals and Applications; John Wiley & Sons: Chichester, UK, 2012. [Google Scholar]
- Lopes, F.M.; de Aleluia Batista, K.; Batista, G.L.A.; Fernandes, K.F. Biosensor for determination of glucose in real samples of beverages. Food Sci. Technol. 2012, 32, 65–69. [Google Scholar] [CrossRef]
- Mason, M.; Longo, E.; Scampicchio, M. Monitoring of glucose in beer brewing by a carbon nanotubes based nylon nanofibrous biosensor. J. Nanomater. 2016, 2016, 5217023. [Google Scholar] [CrossRef]
- Zeeb, B.; Fischer, L.; Weiss, J. Stabilization of food dispersions by enzymes. Food Funct. 2014, 5, 198–213. [Google Scholar] [CrossRef]
- Golikova, E.P.; Lakina, N.V.; Grebennikova, O.V.; Matveeva, V.G.; Sulman, E.M. A study of biocatalysts based on glucose oxidase. Faraday Discuss. 2017, 202, 303–314. [Google Scholar] [CrossRef]
- Sisak, C.; Csanádi, Z.; Rónay, E.; Szajáni, B. Elimination of glucose in egg white using immobilized glucose oxidase. Enzym. Microb. Technol. 2006, 39, 1002–1007. [Google Scholar] [CrossRef]
- El-Hariri, M.; Al-Yazeed, H.A.; Samir, A.; Elhelw, R.; Soliman, R. Genetic and phenotypic diversity of naturally isolated wild strains of Aspergillus niger with hyper glucose oxidase production. J. Biosci. Biotechnol. 2015, 4, 245–253. [Google Scholar]
- Kirk, O.; Borchert, T.V.; Fuglsang, C.C. Industrial enzyme applications. Curr. Opin. Biotechnol. 2002, 13, 345–351. [Google Scholar] [CrossRef]
- Karimi, A.; Mahdizadeh, F.; Salari, D.; Vahabzadeh, F.; Khataee, A. Enzymatic scavenging of oxygen dissolved in water: Application of response surface methodology in optimization of conditions. Chem. Ind. Chem. Eng. Q. 2012, 18, 431–439. [Google Scholar] [CrossRef]
- Crueger, A.; Crueger, W. Glucose transforming enzymes. In Microbial Enzymes and Biotechnology; Fogarty, W.M., Kelly, C.T., Eds.; Elsevier: New York, NY, USA, 1990; pp. 177–226. [Google Scholar]
- Bhat, S.V.; Swathi, B.R.; Rosy, M.; Govindappa, M. Isolation and characterization of glucose oxidase (GOD) from Aspergillus flavus and Penicillium sp. Int. J. Curr. Microbiol. Appl. Sci. 2013, 2, 153–161. [Google Scholar]
- Isaksen, A.; Adler-Nissen, J. Antioxidative Effect of Glucose Oxidase and Catalase in Mayonnaises of Different Oxidative Susceptibility. I. Product Trials. Lebensm. Wiss. Technol. 1997, 30, 841–846. [Google Scholar] [CrossRef]
- Cichello, S.A. Oxygen absorbers in food preservation: A review. J. Food Sci. Technol. 2015, 52, 1889–1895. [Google Scholar] [CrossRef] [PubMed]
- Vartiainen, J.; Rättö, M.; Paulussen, S. Antimicrobial Activity of Glucose Oxidase-immobilized Plasma-activated Polypropylene Films. Packag. Technol. Sci. 2005, 18, 243–251. [Google Scholar] [CrossRef]
- Hanušová, K.; Vápenka, L.; Dobiáš, J.; Mišková, L. Development of antimicrobial packaging materials with immobilized glucose oxidase and lysozyme. Cent. Eur. J. Chem. 2013, 11, 1066–1078. [Google Scholar] [CrossRef]
- Yuan, H.; Bai, H.; Liu, L.; Lv, F.; Wang, S. A glucose-powered antimicrobial system using organic-inorganic assembled network materials. Chem. Commun. (Camb.) 2015, 51, 722–724. [Google Scholar] [CrossRef]
- Xu, M.; Wanga, R.; Li, Y. An electrochemical biosensor for rapid detection of E. coli O157:H7 with highly efficient bifunctional glucose oxidase-polydopamine nanocomposites and Prussian blue modified screen-printed interdigitated electrode. Analyst 2016, 141, 5441. [Google Scholar] [CrossRef]
- Wang, M.; Wang, D.; Chen, Q.; Li, C.; Li, Z.; Lin, J. Recent Advances in Glucose-Oxidase-Based Nanocomposites for Tumor Therapy. Small 2019, 15, e1903895. [Google Scholar] [CrossRef]
- Chu, K.F.; Dupuy, D.E. Thermal ablation of tumours: Biological mechanisms and advances in therapy. Nat. Rev. Cancer 2014, 14, 199–208. [Google Scholar] [CrossRef]
- Cheng, L.; Wang, C.; Feng, L.; Yang, K.; Liu, Z. Functional nanomaterials for phototherapies of cancer. Chem. Rev. 2014, 114, 10869–10939. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, H.; Liu, H.; Yuan, Q.; Ren, F.; Han, Y.; Sun, Q.; Li, Z.; Gao, M. Boosting H2O2-Guided Chemodynamic Therapy of Cancer by Enhancing Reaction Kinetics through Versatile Biomimetic Fenton Nanocatalysts and the Second Near-Infrared Light Irradiation. Adv. Funct. Mater. 2020, 30, 1906128. [Google Scholar] [CrossRef]
- Clark, L.C., Jr.; Lyons, C. Electrode systems for continuous monitoring in cardiovascular surgery. Ann. N. Y. Acad. Sci. 1962, 102, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Pullano, S.A.; Greco, M.; Bianco, M.G.; Foti, D.; Brunetti, A.; Fiorillo, A.S. Glucose biosensors in clinical practice: Principles, limits and perspectives of currently used devices. Theranostics 2022, 12, 493–511. [Google Scholar] [CrossRef] [PubMed]
- Klonoff, D.C.; Ahn, D.; Drincic, A. Continuous glucose monitoring: A review of the technology and clinical use. Diabetes Res. Clin. Pract. 2017, 133, 178–192. [Google Scholar] [CrossRef]
- Fokkert, M.; van Dijk, P.R.; Edens, M.A.; Hernández, A.D.; Slingerland, R.; Gans, R.; Álvarez, E.D.; Bilo, H. Performance of the Eversense versus the Free Style Libre Flash glucose monitor during exercise and normal daily activities in subjects with type 1 diabetes mellitus. BMJ Open Diabetes Res. Care 2020, 8, e001193. [Google Scholar] [CrossRef]
- Gutierrez, E.A.; Mundhada, H.; Meier, T.; Duefel, H.; Bocola, M.; Schwaneberg, U. Reengineered glucose oxidase for amperometric glucose determination in diabetes analytics. Biosens. Bioelectron. 2020, 50, 84–90. [Google Scholar] [CrossRef]
- Yang, F.; Li, M.; Liu, Y.; Wang, T.; Feng, Z.; Cui, H.; Gu, N. Glucose and magnetic-responsive approach toward in situ nitric oxide bubbles controlled generation for hyperglycemia theranostics. J. Control. Release 2016, 228, 87–95. [Google Scholar] [CrossRef]
- Spinas, G.A.; Laffranchi, R.; Francoys, I.; David, I.; Richter, C.; Reinecke, M. The early phase of glucose-stimulated insulin secretion requires nitric oxide. Diabetologia 1998, 41, 292–299. [Google Scholar] [CrossRef][Green Version]
- Ma, R.; Shi, L. Phenylboronic acid-based glucose-responsive polymeric nanoparticles: Synthesis and applications in drug delivery. Polym. Chem. 2014, 5, 1503–1518. [Google Scholar] [CrossRef]
- Molan, P.C. The evidence supporting the use of honey as a wound dressing. Int. J. Low. Extrem. Wounds 2006, 5, 40–54. [Google Scholar] [CrossRef]
- Bucekova, M.; Valachova, I.; Kohutova, L.; Prochazka, E.; Klaudiny, J.; Majtan, J. Honeybee glucose oxidase–its expression in honeybee workers and comparative analyses of its content and H2O2-mediated antibacterial activity in natural honeys. Naturwissenschaften 2014, 101, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Bucekova, M.; Jardekova, L.; Juricova, V.; Bugarova, V.; Marco, G.D.; Gismondi, A.; Leonardi, D.; Farkasovska, J.; Godocikova, J.; Laho, M.; et al. Antibacterial Activity of Different Blossom Honeys: New Findings. Molecules 2019, 24, 1573. [Google Scholar] [CrossRef] [PubMed]
- Bucekova, M.; Buriova, M.; Pekarik, L.; Majtan, V.; Majtan, J. Phytochemicals-mediated production of hydrogen peroxide is crucial for high antibacterial activity of honeydew honey. Sci. Rep. 2018, 8, 9061. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Du, X.; Jiang, L.; Luo, H.; Wang, F.; Wang, J.; Qiu, L.; Liu, L.; Liu, X.; Wang, X.; et al. Glucose Oxidase-Loaded Antimicrobial Peptide Hydrogels: Potential Dressings for Diabetic Wound. J. Nanosci. Nanotechnol. 2020, 20, 2087–2094. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Y.; Yang, X.; Cao, Z.; Nie, H.; Bian, Y.; Yang, G. Glucose-triggered in situ forming keratin hydrogel for the treatment of diabetic wounds. Acta Biomater. 2021, 125, 208–218. [Google Scholar] [CrossRef]
- Vasquez, J.M.; Idrees, A.; Carmagnola, I.; Sigen, A.; McMahon, S.; Marlinghaus, L.; Ciardelli, G.; Greiser, U.; Tai, H.; Wang, W.; et al. In situ Forming Hyperbranched PEG—Thiolated Hyaluronic Acid Hydrogels with Honey-Mimetic Antibacterial Properties. Front. Bioeng. Biotechnol. 2021, 9, 742135. [Google Scholar] [CrossRef]
- Davis, P.; Wood, L.; Wood, Z.; Eaton, A.; Wilkins, J. Clinical experience with a glucose oxidase-containing dressing on recalcitrant wounds. J. Wound Care 2009, 18, 116–121. [Google Scholar] [CrossRef]
- Rashaan, Z.M.; Krijnen, P.; Kwa, K.A.; van Baar, M.E.; Breederveld, R.S.; van den Akker-van Marle, M.E. Long-term quality of life and cost-effectiveness of treatment of partial thickness burns: A randomized controlled trial comparing enzyme alginogel vs silver sulfadiazine (FLAM study). Wound Repair Regen. 2020, 28, 375–384. [Google Scholar] [CrossRef]
- Huang, T.; Yuan, B.; Jiang, W.; Ding, Y.; Jiang, L.; Rena, H.; Tang, J. Glucose oxidase and Fe3O4/TiO2/Ag3PO4 co-embedded biomimetic mineralization hydrogels as controllable ROS generators for accelerating diabetic wound healing. J. Mater. Chem. B 2021, 9, 6190–6200. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, Z.; Hao, J.; Ding, F.; Li, Z.; Ren, X. Hollow nanosphere-doped bacterial cellulose and polypropylene wound dressings: Biomimetic nanocatalyst mediated antibacterial therapy. Chem. Eng. J. 2022, 432, 134309. [Google Scholar] [CrossRef]
- Du, X.; Jia, B.; Wang, W.; Zhang, C.; Liu, X.; Qu, Y.; Zhao, M.; Li, W.; Yang, Y.; Li, Y.Q. pH-switchable nanozyme cascade catalysis: A strategy for spatial-temporal modulation of pathological wound microenvironment to rescue stalled healing in diabetic ulcer. J. Nanobiotechnol. 2022, 20, 12. [Google Scholar] [CrossRef] [PubMed]
- Simões, D.; Miguel, S.P.; Ribeiro, M.P.; Coutinho, P.; Mendonça, A.G.; Correia, I.J. Recent advances on antimicrobial wound dressing: A review. Eur. J. Pharm. Biopharm. 2018, 127, 130–141. [Google Scholar] [CrossRef] [PubMed]



| PDB ID | Resolution (Å) | Source | Reference | Remarks |
|---|---|---|---|---|
| 1gal | 2.3 | A. niger | [9] | The earliest and lowest resolution structure. |
| 1cf3 | 1.9 | A. niger | [10] | |
| 1gpe | 1.8 | P. amagasakiense | [10] | The only structure with a complete dimer in the asymmetric unit. |
| 3qvp | 1.2 | A. niger | [11] | The highest resolution structure available. |
| 3qvr | 1.3 | A. niger | [11] | |
| 5nit | 1.9 | A. niger | [12] | A2 Mutant engineered for higher stability and turnover: T30V, I94V, A162T, R537K, M566V. |
| 5niw | 1.8 | A. niger | [12] | A9 mutant: T30V, R37K, I94V, V106I, A162T, M566V. |
| PDB ID | RMSD | Description | Identity (%) | Reference |
|---|---|---|---|---|
| 6xut | 1.2704 | Trametes cinnabarina Oligosaccharide dehydrogenase | 35.89 | [13] |
| 4ynt | 1.2841 | Aspergillus flavus FAD glucose dehydrogenase | 35.69 | [14] |
| 6zh7 | 1.4654 | Chlorella variabilis Fatty acid Photodecarboxylase | 27.99 | [15] |
| 5oc1 | 1.5672 | Pleurotus Eryngii aryl-alcohol oxidase | 29.67 | [16] |
| 6ze2 | 1.5827 | Chaetomium thermophilum FAD-dependent oxidoreductase | 31.24 | [17] |
| 4h7u | 1.6328 | Agaricus meleagris pyranose dehydrogenase | 28.60 | [18] |
| 5hsa | 1.6562 | Pichia pastoris Alcohol Oxidase | 23.56 | [19] |
| 6h3g | 1.6725 | Phanerodontia chrysosporium Alcohol oxidase | 24.81 | [20] |
| 3nne | 1.7055 | Arthrobacter globiformis choline oxidase | 26.31 | [21] |
| 4ha6 | 1.7182 | Mesorhizobium loti pyridoxine 4-oxidase | 26.85 | [22] |
| 6f97 | 1.7508 | Methylovorus sp. 5-(hydroxymethyl)furfural oxidase | 28.52 | [23] |
| 3q9t | 1.7866 | Aspergillus oryzae formate oxidase | 25.55 | [24] |
| 6o9n | 2.1470 | Myceliophthora thermophila aryl-alcohol oxidase | 30.75 | [25] |
| Substrate | GOx Activity (%) 1 | Reference |
|---|---|---|
| -d-glucose | 100 | [35,36,37,38] |
| 2-deoxy-d-glucose | 25–30 | [36,37,38] |
| 4-O-methyl-d-glucose | 15 | [37] |
| 6-deoxy-d-glucose | 10 | [37] |
| 4-deoxy-d-glucose | 2 | [37] |
| 2-deoxy-6-fluoro-d-glucose | 1.85 | [38] |
| 3,6-methyl-d-glucose | 1.85 | [38] |
| 4,6-dimethyl-d-glucose | 1.22 | [38] |
| 3-deoxy-d-glucose | 1 | [37] |
| 6-O-methyl-d-glucose | 1 | [37] |
| -d-glucose | 0.64 | [37,38] |
| mannose | 0.2; 1 | [36,37,38] |
| altrose | 0.16 | [37,38] |
| galactose | 0.08 | [36,37,38] |
| xylose | 0.03 | [36,37,38] |
| idose | 0.02 | [37,38] |
| Organism Name | Accession or Reference 1 | Identity 2 |
|---|---|---|
| Fungi | ||
| Alternaria alternata | [66] | – |
| Aspergillus carbonarius | U V9SH09 | 85.79 |
| Aspergillus niger | U P13006 [3,55] | 100.00 |
| Aureobasidium pullulans | U A0A221SAG9 | 34.09 |
| Aureobasidium sp. | U A0A1V0E5A9 | 31.69 |
| Ceratocystis fimbriata | U A0A0F8CXS8 | 28.28 |
| Cladosporium neopsychrotolerans | U A0A5Q2UVJ5, U A0A5Q2USS5 | 31.80, 32.56 |
| Cystobasidium laryngis | [67] | – |
| Dioszegia sp. | [67] | – |
| Flavodon flavus | [68] | – |
| Fusarium oxysporum | [69] | – |
| Goffeauzyma gastrica | [67] | – |
| Goffeauzyma gilvescens | [67] | – |
| Leucosporidium fragarium | [67] | – |
| Leucosporidium creatinivorum | [67] | – |
| Malassezia restricta | U A0A3G2S2X3, U A0A3G2SBT7 | 29.44, 32.64 |
| Mucor circinelloides | [70] | – |
| Penicillium adametzii | U A2I7K9 [71] | 64.13 |
| Penicillium amagasakiense | U P81156 [72] | 65.74 |
| Penicillium canescens | [73] | – |
| Penicillium chrysogenum | U K9L4P7 [74] | 62.91 |
| Penicillium expansum | [75] | – |
| Penicillium janthinellum | [76,77] | – |
| Penicillium viticola | U A0A0Y0IDS5 | 63.00 |
| Penicillium sp. | U A0A7L7T1A0 | 65.51 |
| Penicillium sp. RFL-2021a | N KAF7733001 | 34.33 |
| Phanerochaete chrysosporium | [78] | – |
| Pleurotus ostreatus | [79] | – |
| Pycnoporus cinnabarinus | [80] | – |
| Rasamsonia emersonii | U A0A0F4YPS7 | 34.78 |
| Rhizopus stolonifer | [81] | – |
| Schizophyllum commune | U D8QJE7 [82] | 34.31 |
| Serendiptia indica N CAG7851011 | 33.56 | |
| Sporidiobolus salmonicolor | [67] | – |
| Talaromyces flavus | U Q92452 [83] | 63.97 |
| Talaromyces funiculosus | [84] | – |
| Talaromyces pinophilus | [85] | – |
| Talaromyces purpureogenus | [86] | – |
| Talaromyces stipitatus | U B8MDS4 [87] | 63.85 |
| Talaromyces variabilis | U Q70FC9 [88] | 66.61 |
| Thanatephorus cucumeris | U M5BNG8 [89] | 30.98 |
| Wickerhamomyces anomalus | [67] | – |
| Xylona heveae | [90] | – |
| Yarrowia sp. B02 | N KAG5360348 | 26.87 |
| Insects | ||
| Apis melifera | U Q9U8X6 [63] | 26.66 |
| Helicoverpa armigera | U B2MW81 [64] | 29.40 |
| Heliothis viriplaca | U A0A142I707 | 29.27 |
| Mythimna separata | U A0A218N0E8 | 28.07 |
| Spodoptera exigua | U D9ZFI1 [65] | 28.74 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bauer, J.A.; Zámocká, M.; Majtán, J.; Bauerová-Hlinková, V. Glucose Oxidase, an Enzyme “Ferrari”: Its Structure, Function, Production and Properties in the Light of Various Industrial and Biotechnological Applications. Biomolecules 2022, 12, 472. https://doi.org/10.3390/biom12030472
Bauer JA, Zámocká M, Majtán J, Bauerová-Hlinková V. Glucose Oxidase, an Enzyme “Ferrari”: Its Structure, Function, Production and Properties in the Light of Various Industrial and Biotechnological Applications. Biomolecules. 2022; 12(3):472. https://doi.org/10.3390/biom12030472
Chicago/Turabian StyleBauer, Jacob A., Monika Zámocká, Juraj Majtán, and Vladena Bauerová-Hlinková. 2022. "Glucose Oxidase, an Enzyme “Ferrari”: Its Structure, Function, Production and Properties in the Light of Various Industrial and Biotechnological Applications" Biomolecules 12, no. 3: 472. https://doi.org/10.3390/biom12030472
APA StyleBauer, J. A., Zámocká, M., Majtán, J., & Bauerová-Hlinková, V. (2022). Glucose Oxidase, an Enzyme “Ferrari”: Its Structure, Function, Production and Properties in the Light of Various Industrial and Biotechnological Applications. Biomolecules, 12(3), 472. https://doi.org/10.3390/biom12030472







