Effects of the Leptin-Mediated MAPK/ERK Signaling Pathway on Collagen II Expression in Knee Cartilage of Newborn Male Mice from Obese Maternal Offspring
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Model
2.2. Histological Analysis
2.3. Immunohistochemistry
2.4. Elisa Assay
2.5. TdT-Mediated dUTP Nick End Labeling (TUNEL) Staining
2.6. Transmission Electron Microscopy
2.7. Cell Culture
2.8. Immunofluorescence
2.9. Flow Cytometry for Detecting Cell Apoptosis
2.10. RNA Extraction and Real-Time RT-PCR
2.11. Protein Extraction and Western Blot Analysis
2.12. Statistical Analysis
3. Results
3.1. Effect of Maternal Obesity on Baseline and Knee Cartilage in the Offspring of Neonatal 7-Day-Old Male Mice
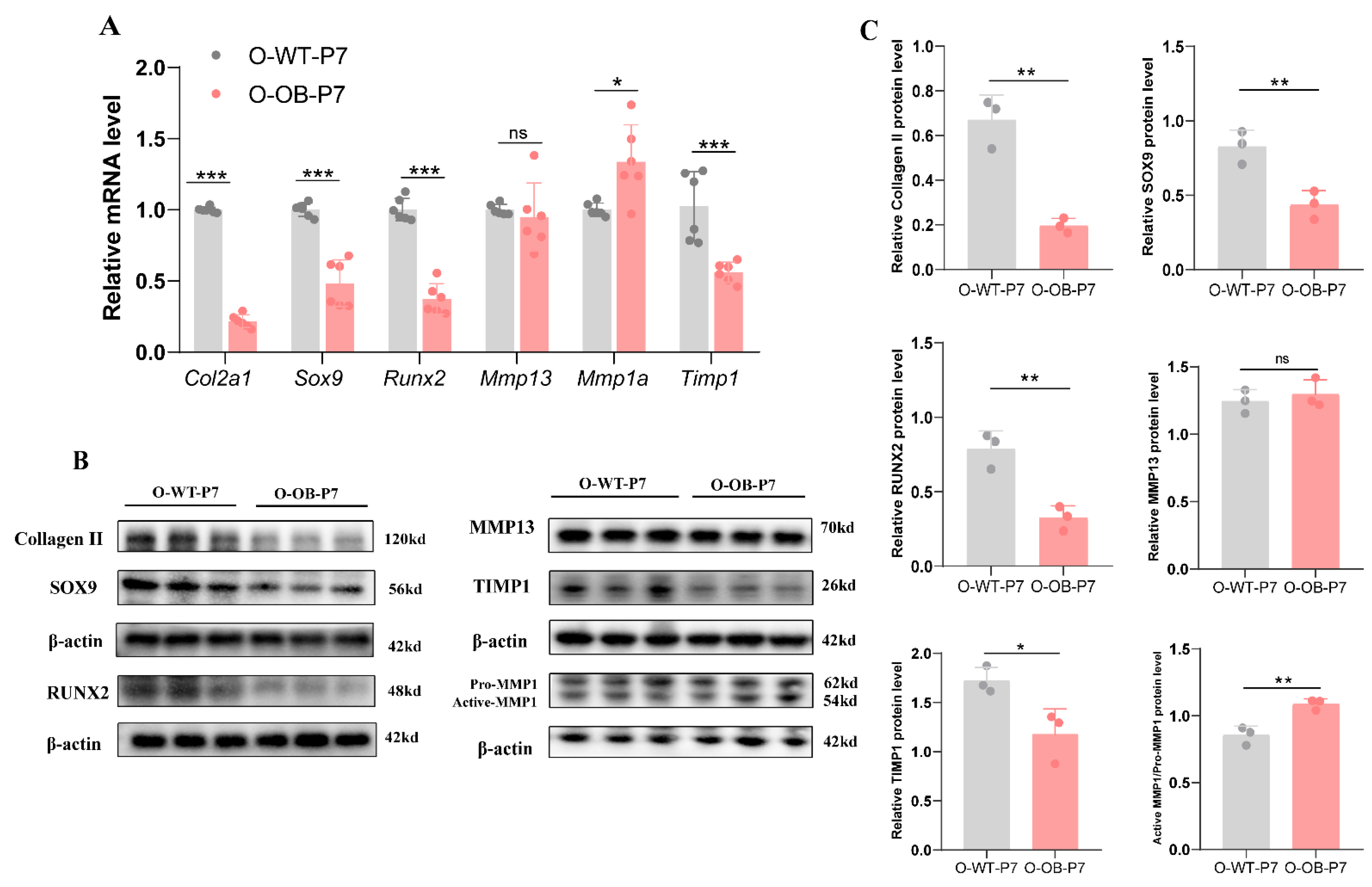
3.2. Alterations in Important Indicators Associated with Knee Cartilage in Neonatal 7-Day Male Mice from Maternal Obesity Group
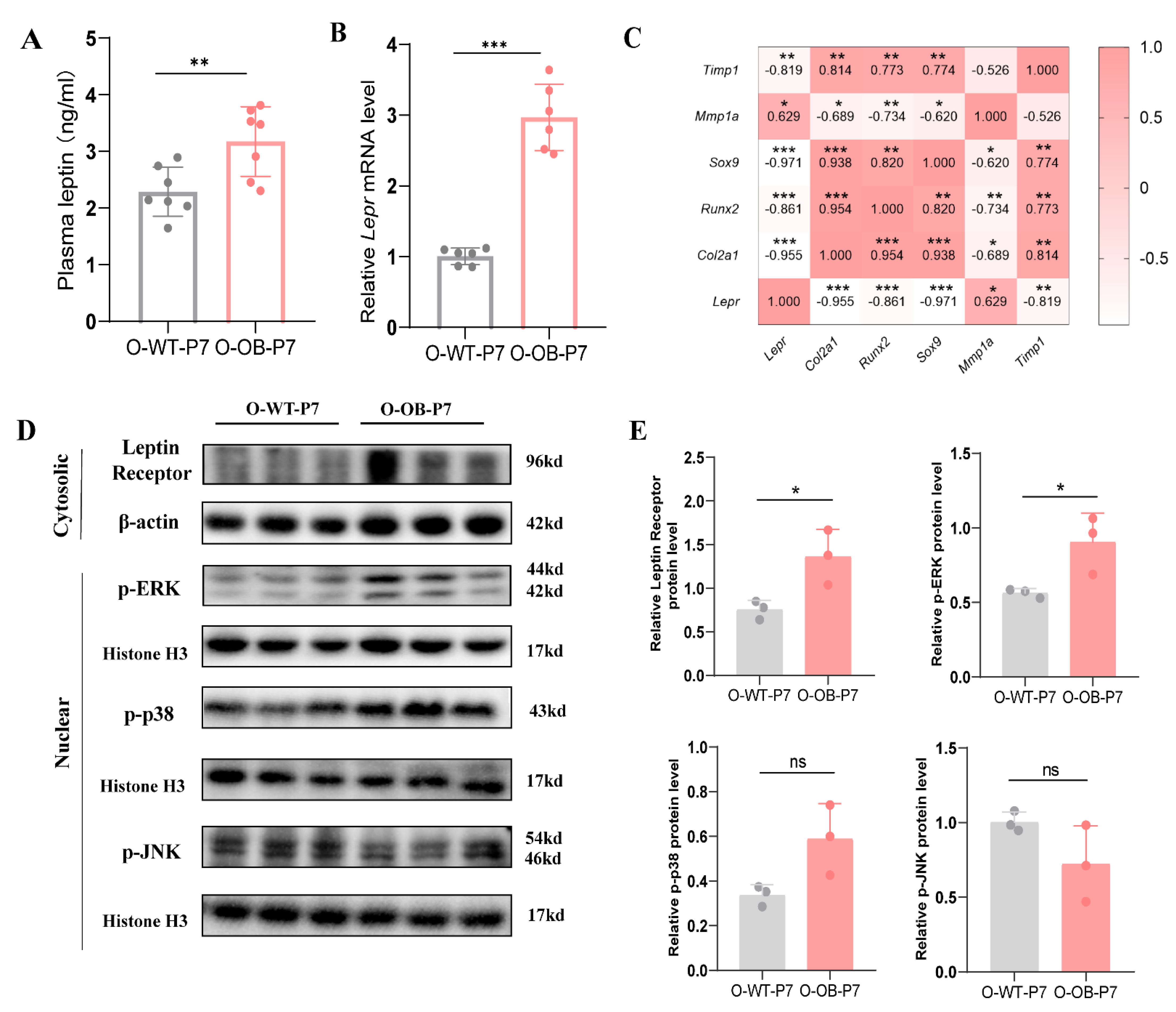
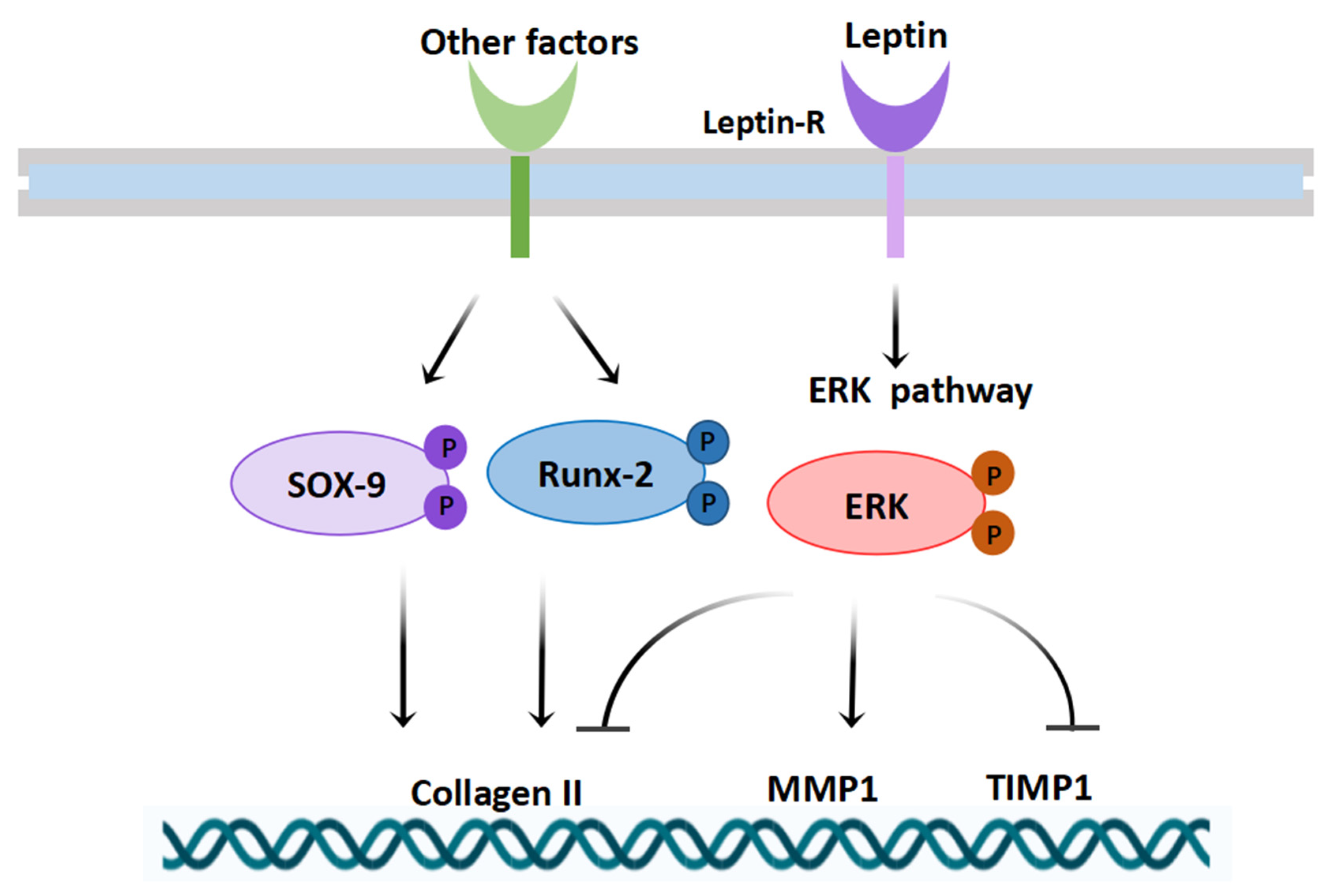
3.3. Leptin Signaling Pathway (MAPK/ERK) Was Activated in the Knee Cartilage of Male Neonatal Mice from the Maternal Obesity Group
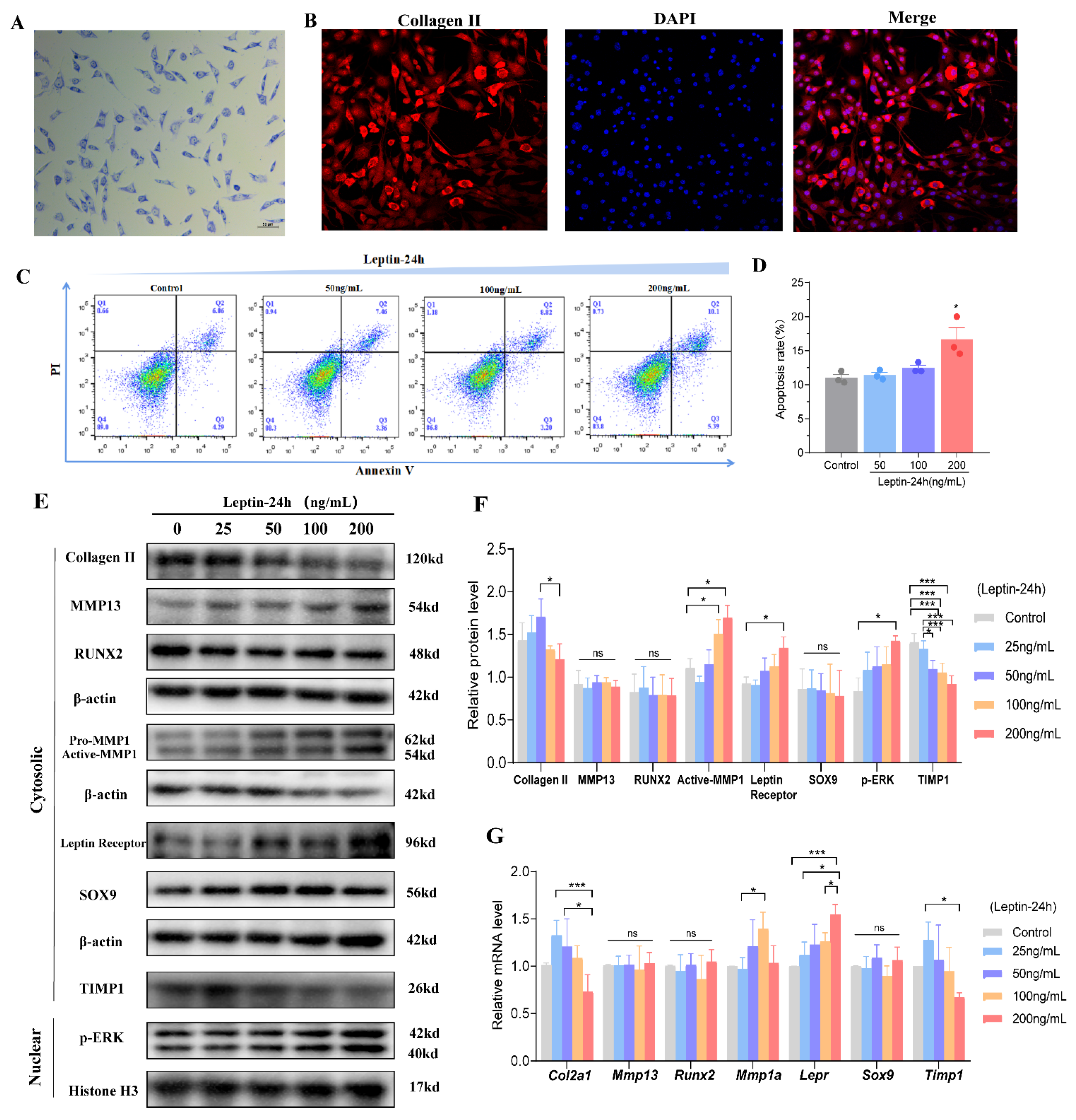
3.4. Expression of Key Cartilage-Related Indicators under the Effect of Different Doses of Leptin In Vitro Experiments
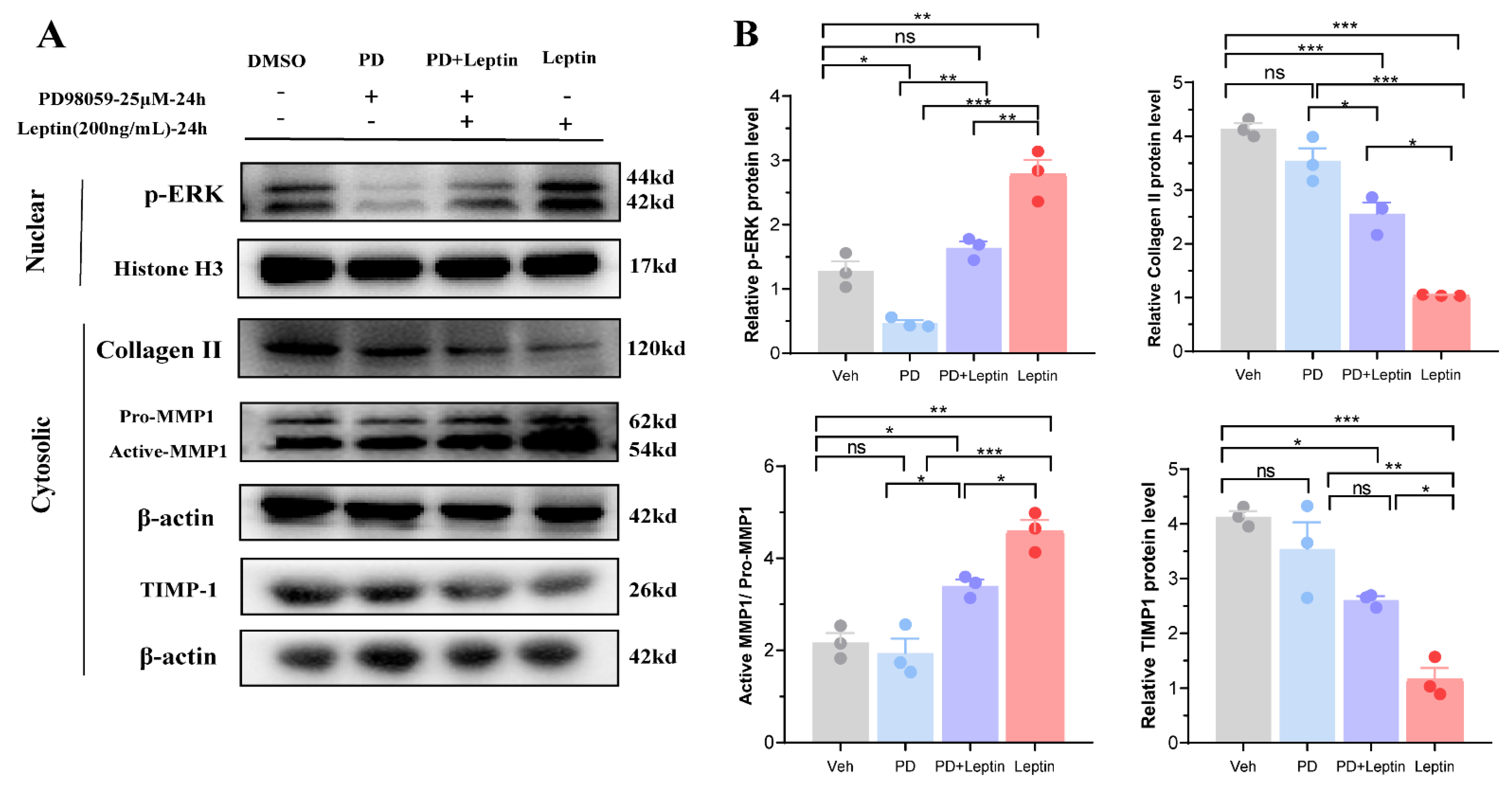
3.5. Inhibition of MAPK/ERK Signaling Pathway Altered the Expression of Collagen II, MMP1, and TIMP1 in Mouse Chondrocytes
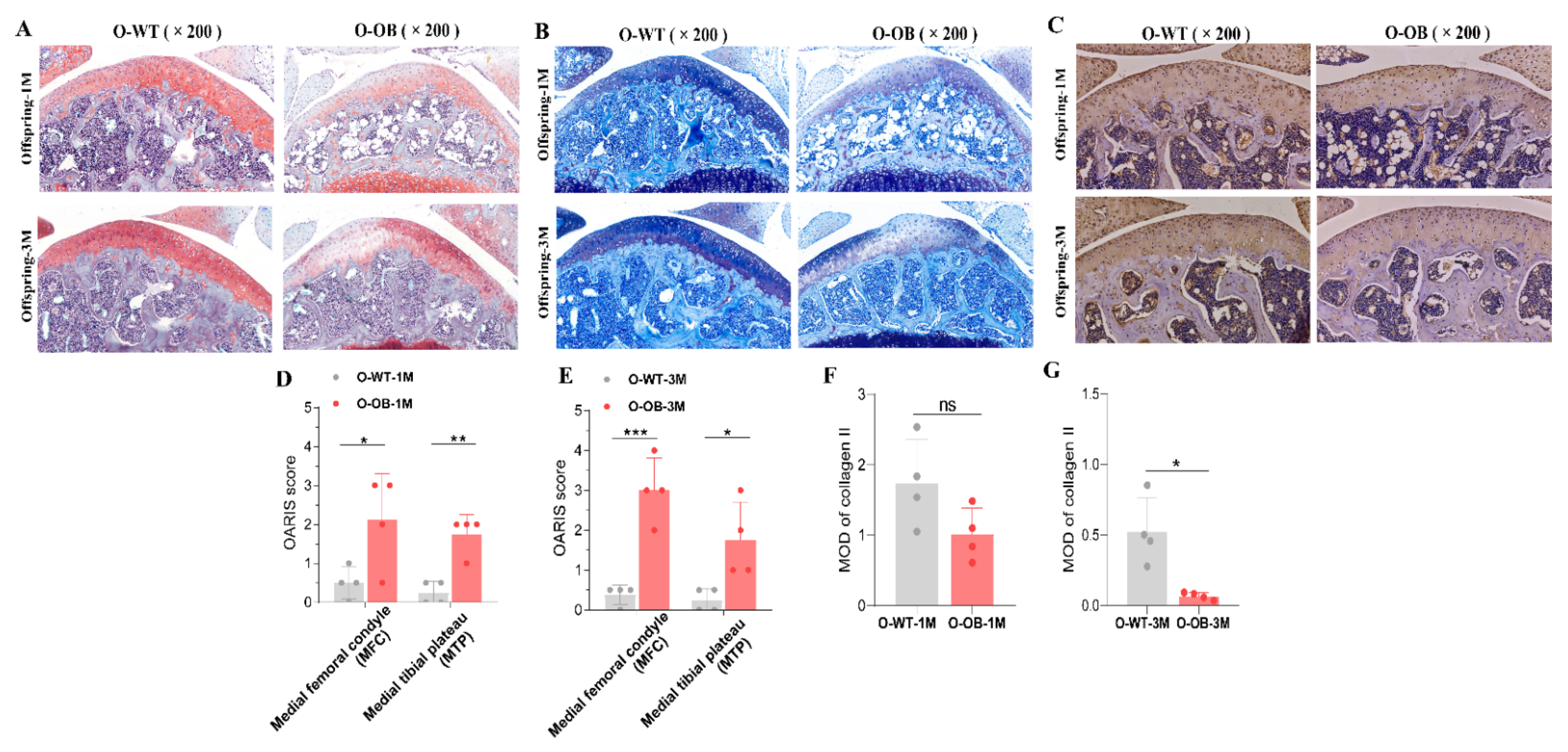
3.6. Reduced Expression of Proteoglycan and Collagen II in Knee Cartilage of Male Mice from the Maternal Obesity Group Persisted into Adulthood
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barker, D.J. Developmental origins of adult health and disease. J. Epidemiol. Community Health 2004, 58, 114–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buckels, E.J.; Bolam, S.M.; Tay, M.L.; Matthews, B.G. The impact of maternal high-fat diet on bone microarchitecture in offspring. Front. Nutr. 2021, 8, 730037. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J.; Osmond, C.; Law, C.M. The intrauterine and early postnatal origins of cardiovascular disease and chronic bronchitis. J. Epidemiol. Community Health 1989, 43, 237–240. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Yan, L.; Chen, S.; Pei, M. Functionality of decellularized matrix in cartilage regeneration: A comparison of tissue versus cell sources. Acta Biomater. 2018, 74, 56–73. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; GolDin, P.; Herdina, A.N.; Estefa, J.; Medvedeva, E.V.; Li, L.; Newton, P.T.; Kotova, S.; Shavkuta, B.; Saxena, A.; et al. Secondary ossification center induces and protects growth plate structure. eLife 2020, 9, e55212. [Google Scholar] [CrossRef]
- Cotterill, S.L.; Jackson, G.C.; Leighton, M.P.; Wagener, R.; Mäkitie, O.; Cole, W.G.; Briggs, M.D. Multiple epiphyseal dysplasia mutations in MATN3 cause misfolding of the A-domain and prevent secretion of mutant matrilin-3. Hum. Mutat. 2005, 26, 557–565. [Google Scholar] [CrossRef] [Green Version]
- Camarero-Espinosa, S.; Rothen-Rutishauser, B.; Foster, E.J.; Weder, C. Articular cartilage: From formation to tissue engineering. Biomater. Sci. 2016, 4, 734–767. [Google Scholar] [CrossRef]
- Chen, D.; Shen, J.; Zhao, W.; Wang, T.; Han, L.; Hamilton, J.L.; Im, H.J. Osteoarthritis: Toward a comprehensive understanding of pathological mechanism. Bone Res. 2017, 5, 16044. [Google Scholar] [CrossRef]
- Huang, J.; Zhao, L.; Fan, Y.; Liao, L.; Ma, P.X.; Xiao, G.; Chen, D. The microRNAs miR-204 and miR-211 maintain joint homeostasis and protect against osteoarthritis progression. Nat. Commun. 2019, 10, 2876. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Xiao, H.; Luo, H.; Tan, Y.; Ni, Q.; He, C.; Magdalou, J.; Chen, L.; Wang, H. GR/HDAC2/TGFβR1 pathway contributes to prenatal caffeine induced-osteoarthritis susceptibility in male adult offspring rats. Food Chem. Toxicol. 2020, 140, 111279. [Google Scholar] [CrossRef]
- Wu, Z.; Pan, Z.; Wen, Y.; Xiao, H.; Shangguan, Y.; Wang, H.; Chen, L. Egr1/p300/ACE signal mediates postnatal osteopenia in female rat offspring induced by prenatal ethanol exposure. Food Chem. Toxicol. 2020, 136, 111083. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Z.; Li, Q.X.; Zhang, D.M.; Chen, L.B.; Wang, H. Ryanodine receptor 1 mediated dexamethasone-induced chondrodysplasia in fetal rats. Biochim. Biophys. Acta. Mol. Cell Res. 2020, 1867, 118791. [Google Scholar] [CrossRef] [PubMed]
- Neogi, T. The epidemiology and impact of pain in osteoarthritis. Osteoarthr. Cartil. 2013, 21, 1145–1153. [Google Scholar] [CrossRef] [Green Version]
- Ortega, F.B.; Lee, D.C.; Katzmarzyk, P.T.; Ruiz, J.R.; Sui, X.; Church, T.S.; Blair, S.N. The intriguing metabolically healthy but obese phenotype: Cardiovascular prognosis and role of fitness. Eur. Heart J. 2013, 34, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Pikó, P.; Pál, L.; Szűcs, S.; Kósa, Z.; Sándor, J.; Ádány, R. Obesity-Related Changes in Human Plasma Lipidome Determined by the Lipidyzer Platform. Biomolecules 2021, 11, 326. [Google Scholar] [CrossRef]
- Chang, J.; Liao, Z.; Lu, M.; Meng, T.; Han, W.; Ding, C. Systemic and local adipose tissue in knee osteoarthritis. Osteoarthr. Cartil. 2018, 26, 864–871. [Google Scholar] [CrossRef] [Green Version]
- Thijssen, E.; van Caam, A.; van der Kraan, P.M. Obesity and osteoarthritis, more than just wear and tear: Pivotal roles for inflamed adipose tissue and dyslipidaemia in obesity-induced osteoarthritis. Rheumatology 2015, 54, 588–600. [Google Scholar] [CrossRef] [Green Version]
- Jéquier, E. Leptin signaling, adiposity, and energy balance. Ann. N. Y. Acad. Sci. 2002, 967, 379–388. [Google Scholar] [CrossRef]
- Park, H.K.; Ahima, R.S. Physiology of leptin: Energy homeostasis, neuroendocrine function and metabolism. Metab. Clin. Exp. 2015, 64, 24–34. [Google Scholar] [CrossRef] [Green Version]
- Yan, M.; Zhang, J.; Yang, H.; Sun, Y. The role of leptin in osteoarthritis. Medicine 2018, 97, e0257. [Google Scholar] [CrossRef]
- Ran, C.J.; Lazarenko, O.P.; Zhao, H.J.; Alund, A.W.; Kartik, S. Maternal obesity impairs skeletal development in adult offspring. J. Endocrinol. 2018, 239, 33–47. [Google Scholar]
- Zhang, H.J.; Liao, H.Y.; Bai, D.Y.; Wang, Z.Q.; Xie, X.W. MAPK/ERK signaling pathway: A potential target for the treatment of intervertebral disc degeneration. Biomed. Pharmacother. Biomed. Pharmacother. 2021, 143, 112170. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.C.; Ma, B.; Guo, S.; Yang, M.; Li, L.J.; Wang, S.J.; Tan, J. Leptin regulates disc cartilage endplate degeneration and ossification through activation of the MAPK-ERK signalling pathway in vivo and in vitro. J. Cell Mol. Med. 2018, 22, 2098–2109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Cao, L.; Tan, Y.; Zheng, Y.; Gui, Y. N-acetylcysteine protects neonatal mice from ventricular hypertrophy induced by maternal obesity in a sex-specific manner. Biomed. Pharmacother. Biomed. Pharmacother. 2021, 133, 110989. [Google Scholar] [CrossRef] [PubMed]
- Glasson, S.S.; Chambers, M.G.; Van Den Berg, W.B.; Little, C.B. The OARSI histopathology initiative—Recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthr. Cartil. 2010, 18 (Suppl. 3), S17–S23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Ji, Y.; Yang, W.; Zhang, C.; Angwa, L.; Jin, B.; Liu, J.; Lv, M.; Ma, W.; Yang, J.; et al. Inhibitors of apoptosis proteins (IAPs) are associated with T-2 toxin-induced decreased Collagen II in mouse chondrocytes in vitro. Toxicon 2020, 176, 34–43. [Google Scholar] [CrossRef]
- Foley, C.J.; Kuliopulos, A. Mouse matrix metalloprotease-1a (Mmp1a) gives new insight into MMP function. J. Cell. Physiol. 2014, 229, 1875–1880. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Yu, J.; Liu, H.; Liu, Y.; Sun, D. Endemic Kashin-Beck disease: A food-sourced osteoarthropathy. Semin. Arthritis Rheum. 2019, 50, 366–372. [Google Scholar] [CrossRef]
- Fernandes, T.L.; Gomoll, A.H.; Lattermann, C.; Hernandez, A.J.; Bueno, D.F.; Amano, M.T. Macrophage: A potential target on cartilage regeneration. Front. Immunol. 2020, 11, 111. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.; Sinkeviciute, D.; He, Y.; Karsdal, M.; Henrotin, Y.; Mobasheri, A.; Önnerfjord, P.; Bay-Jensen, A. The minor collagens in articular cartilage. Protein Cell 2017, 8, 560–572. [Google Scholar] [CrossRef] [Green Version]
- Dearden, L.; Bouret, S.G.; Ozanne, S.E. Sex and gender differences in developmental programming of metabolism. Mol. Metab. 2018, 15, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Sundrani, D.P.; Roy, S.S.; Jadhav, A.T.; Joshi, S.R. Sex-specific differences and developmental programming for diseases in later life. Reprod. Fertil. Dev. 2017, 29, 2085–2099. [Google Scholar] [CrossRef] [PubMed]
- Ji, Q.; Xu, X.; Kang, L.; Xu, Y.; Xiao, J.; Goodman, S.B.; Zhu, X.; Li, W.; Liu, J.; Gao, X.; et al. Hematopoietic PBX-interacting protein mediates cartilage degeneration during the pathogenesis of osteoarthritis. Nat. Commun. 2019, 10, 313. [Google Scholar] [CrossRef] [Green Version]
- Stevens-Lapsley, J.E.; Kohrt, W.M. Osteoarthritis in women: Effects of estrogen, obesity and physical activity. Women’s Health 2010, 6, 601–615. [Google Scholar] [CrossRef] [PubMed]
- Asarian, L.; Geary, N. Sex differences in the physiology of eating. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 305, R1215–R1267. [Google Scholar] [CrossRef] [Green Version]
- Bell, D.M.; Leung, K.K.; Wheatley, S.C.; Ng, L.J.; Zhou, S.; Ling, K.W.; Sham, M.H.; Koopman, P.; Tam, P.P.; Cheah, K.S. SOX9 directly regulates the type-II Collagen gene. Nat. Genet. 1997, 16, 174–178. [Google Scholar] [CrossRef]
- Haseeb, A.; Kc, R.; Angelozzi, M.; de Charleroy, C.; Rux, D.; Tower, R.J.; Yao, L.; Pellegrino da Silva, R.; Pacifici, M.; Qin, L.; et al. SOX9 keeps growth plates and articular cartilage healthy by inhibiting chondrocyte dedifferentiation/osteoblastic redifferentiation. Proc. Natl. Acad. Sci. USA 2021, 118, e2019152118. [Google Scholar] [CrossRef]
- Chen, D.; Kim, D.J.; Shen, J.; Zou, Z.; O’Keefe, R.J. Runx2 plays a central role in Osteoarthritis development. J. Orthop. Transl. 2020, 23, 132–139. [Google Scholar] [CrossRef]
- Murphy, G.; Lee, M.H. What are the roles of metalloproteinases in cartilage and bone damage. Ann. Rheum. Dis. 2005, 64 (Suppl. 4), iv44–iv47. [Google Scholar] [CrossRef]
- Kuroki, K.; Cook, J.L.; Kreeger, J.M.; Tomlinson, J.L. The effects of TIMP-1 and -2 on canine chondrocytes cultured in three-dimensional agarose culture system. Osteoarthr. Cartil. 2003, 11, 625–635. [Google Scholar] [CrossRef] [Green Version]
- Ohba, S.; Lanigan, T.M.; Roessler, B.J. Leptin receptor JAK2/STAT3 signaling modulates expression of Frizzled receptors in articular chondrocytes. Osteoarthr. Cartil. 2010, 18, 1620–1629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hui, W.; Litherland, G.J.; Elias, M.S.; Kitson, G.I.; Cawston, T.E.; Rowan, A.D.; Young, D.A. Leptin produced by joint white adipose tissue induces cartilage degradation via upregulation and activation of matrix metalloproteinases. Ann. Rheum. Dis. 2012, 71, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.P.; Chen, C.N.; Huang, K.C.; Chang, H.I.; Lee, K.C.; Lo, C.M.; Chang, S.F. Leptin induces MMP1/13 and ADAMTS 4 expressions through bone morphogenetic protein-2 autocrine effect in human chondrocytes. J. Cell. Biochem. 2018, 119, 3716–3724. [Google Scholar] [CrossRef] [PubMed]
- Boucherat, O.; Landry-Truchon, K.; Aoidi, R.; Houde, N.; Nadeau, V.; Charron, J.; Jeannotte, L. Lung development requires an active ERK/MAPK pathway in the lung mesenchyme. Dev. Dyn. 2017, 246, 72–82. [Google Scholar] [CrossRef] [Green Version]
- Dai, X.; Song, R.; Xiong, Y. The expression of ERK and JNK in patients with an endemic osteochondropathy, Kashin-Beck disease. Exp. Cell Res. 2017, 359, 337–341. [Google Scholar] [CrossRef] [PubMed]


| mRNA | Sequence (5′-3′) |
|---|---|
| Col2a1 | F:GCTGGTGAAGAAGGCAAACGAG R:CCATCTTGACCTGGGAATCCAC |
| Lepr | F: GGATGTGCGTTGGAGGACTATG R: GAAATACGCCAGTTCCCGACAG |
| Sox9 | F:CACACGTCAAGCGACCCATGAA R: TCTTCTCGCTCTCGTTCAGCAG |
| Runx2 | F:CCTGAACTCTGCACCAAGTCCT R:TCATCTGGCTCAGATAGGAGGG |
| Mmp13 | F: GATGACCTGTCTGAGGAAGACC R: GCATTTCTCGGAGCCTGTCAAC |
| Mmp1a | F:AGGAAGGCGATATTGTGCTCTCC R:TGGCTGGAAAGTGTGAGCAAGC |
| Timp1 | F: TCTTGGTTCCCTGGCGTACTCT R:GTGAGTGTCACTCTCCAGTTTGC |
| Gapdh | F:CATCACTGCCACCCAGAAGACTG R: ATGCCAGTGAGCTTCCCGTTCAG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Zhang, J.; Huo, Y.; Zheng, Y.; Gui, Y. Effects of the Leptin-Mediated MAPK/ERK Signaling Pathway on Collagen II Expression in Knee Cartilage of Newborn Male Mice from Obese Maternal Offspring. Biomolecules 2022, 12, 477. https://doi.org/10.3390/biom12030477
Wang W, Zhang J, Huo Y, Zheng Y, Gui Y. Effects of the Leptin-Mediated MAPK/ERK Signaling Pathway on Collagen II Expression in Knee Cartilage of Newborn Male Mice from Obese Maternal Offspring. Biomolecules. 2022; 12(3):477. https://doi.org/10.3390/biom12030477
Chicago/Turabian StyleWang, Wenji, Jialing Zhang, Yu Huo, Yuanzheng Zheng, and Yonghao Gui. 2022. "Effects of the Leptin-Mediated MAPK/ERK Signaling Pathway on Collagen II Expression in Knee Cartilage of Newborn Male Mice from Obese Maternal Offspring" Biomolecules 12, no. 3: 477. https://doi.org/10.3390/biom12030477






