Tau Protein Modulates Perineuronal Extracellular Matrix Expression in the TauP301L-acan Mouse Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Immunohistochemistry
2.3. Tissue Extraction for Biochemical Analysis
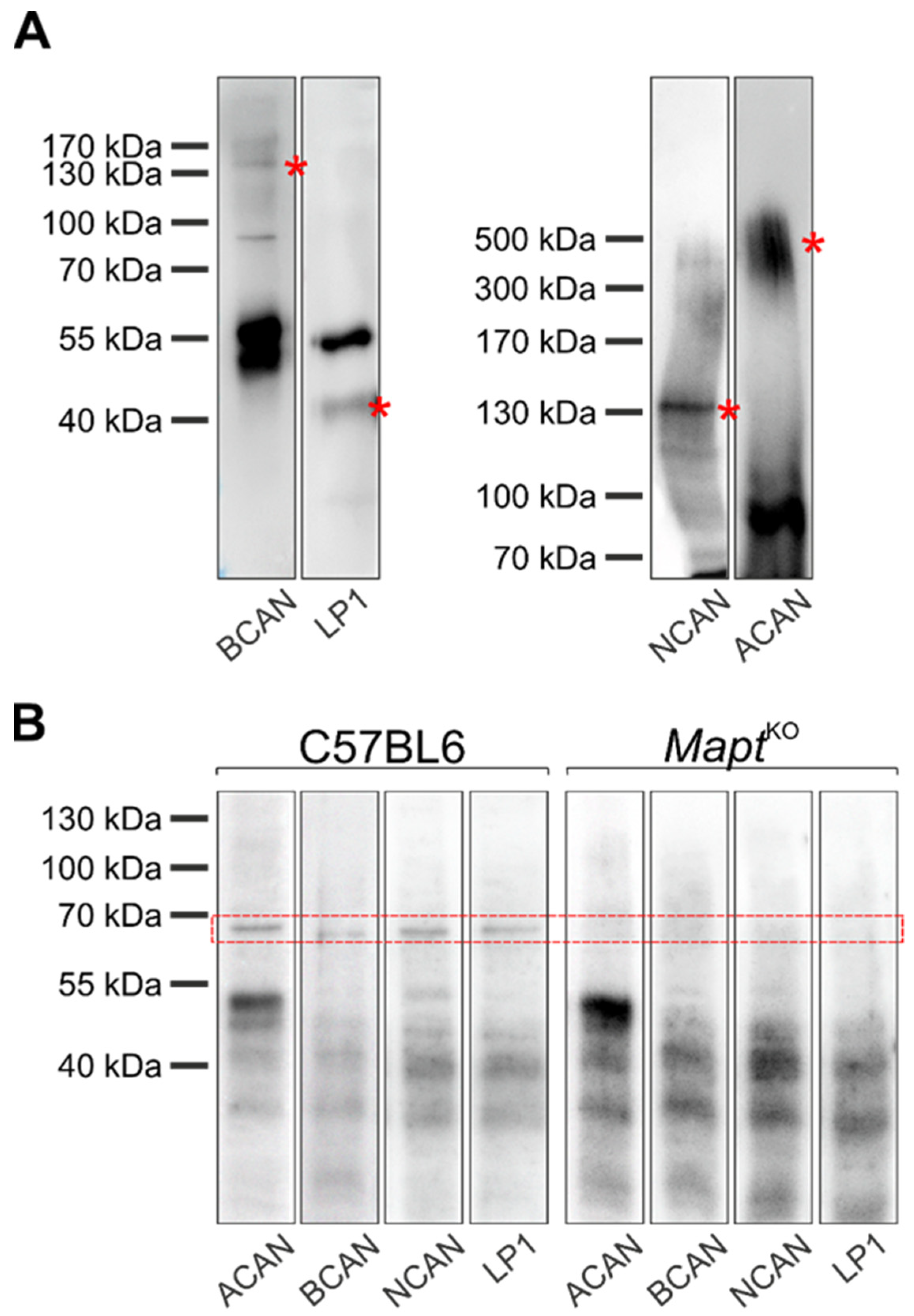
2.4. Western Blot
2.5. Enzyme-Linked Immunosorbent Assay (ELISA)
2.6. Co-Immunoprecipitation
2.7. Statistics
3. Results
3.1. Characterization of Perineuronal Nets in Brains of TauP301L-acan Mice
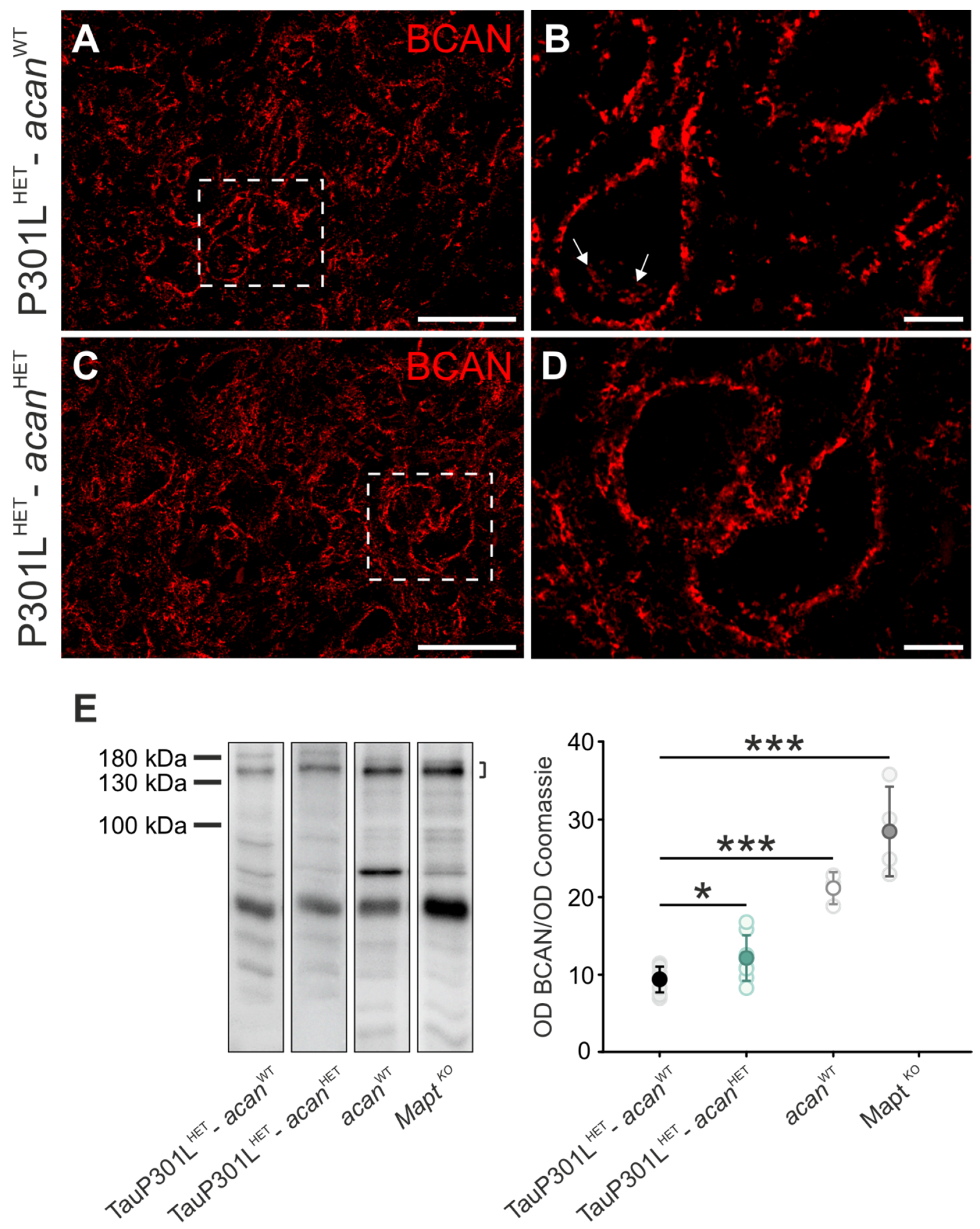
3.1.1. Brevican
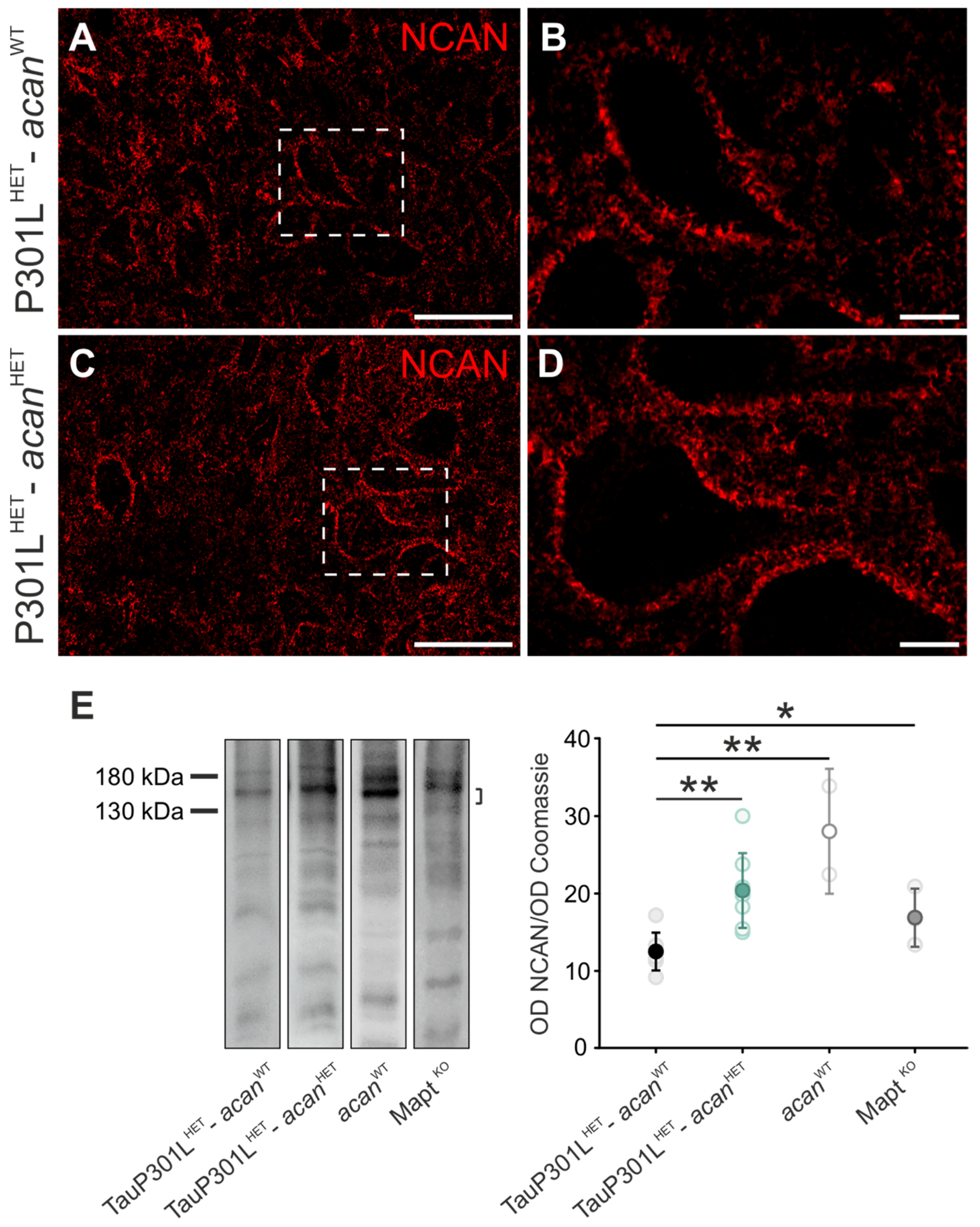
3.1.2. Neurocan
3.1.3. HAPLN1
3.1.4. Hyaluronic Acid
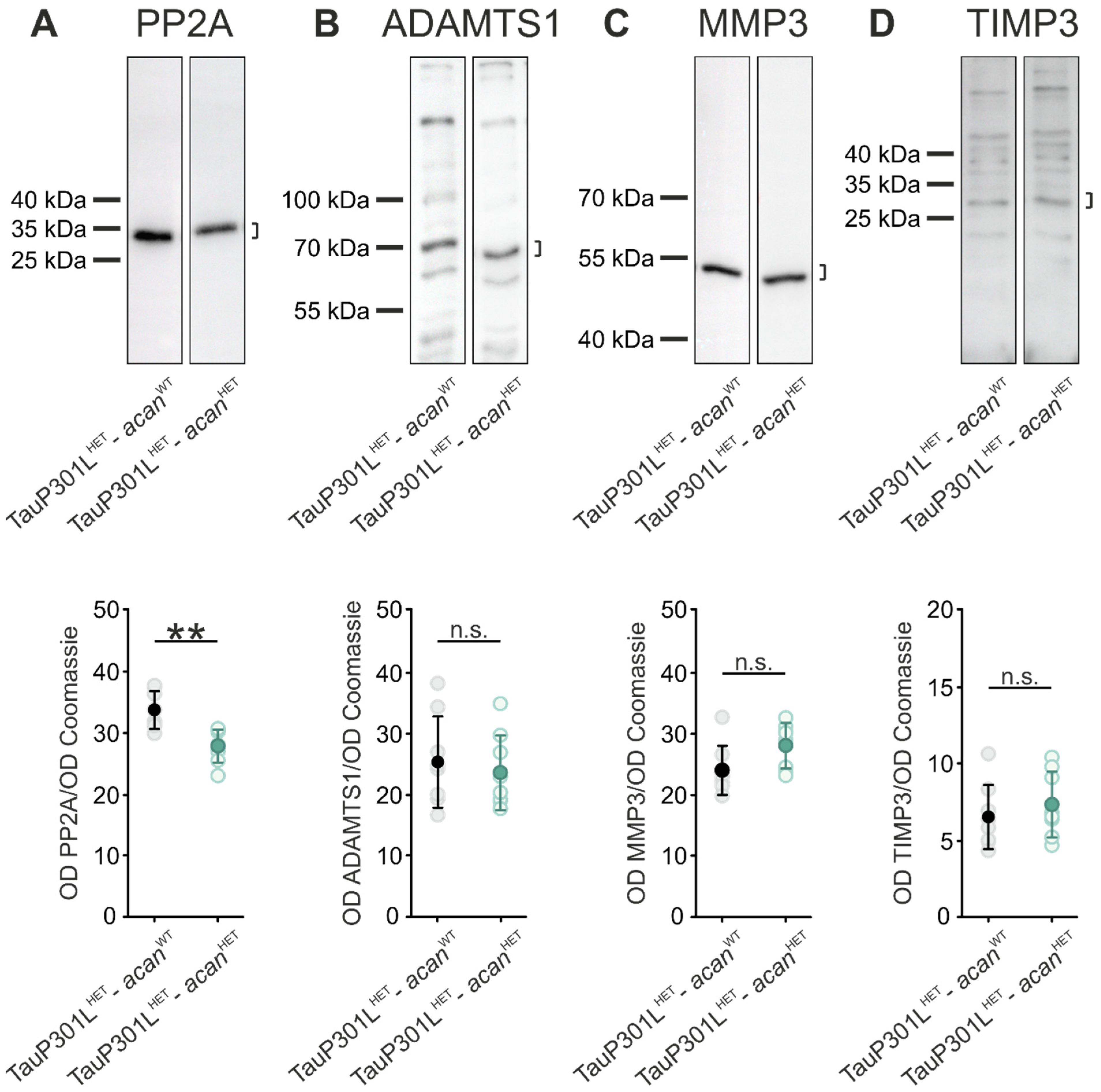
3.2. Characterization of Protein Levels of PP2A and PN Modulating Enzymes in Brains of TauP301L-acan Mice
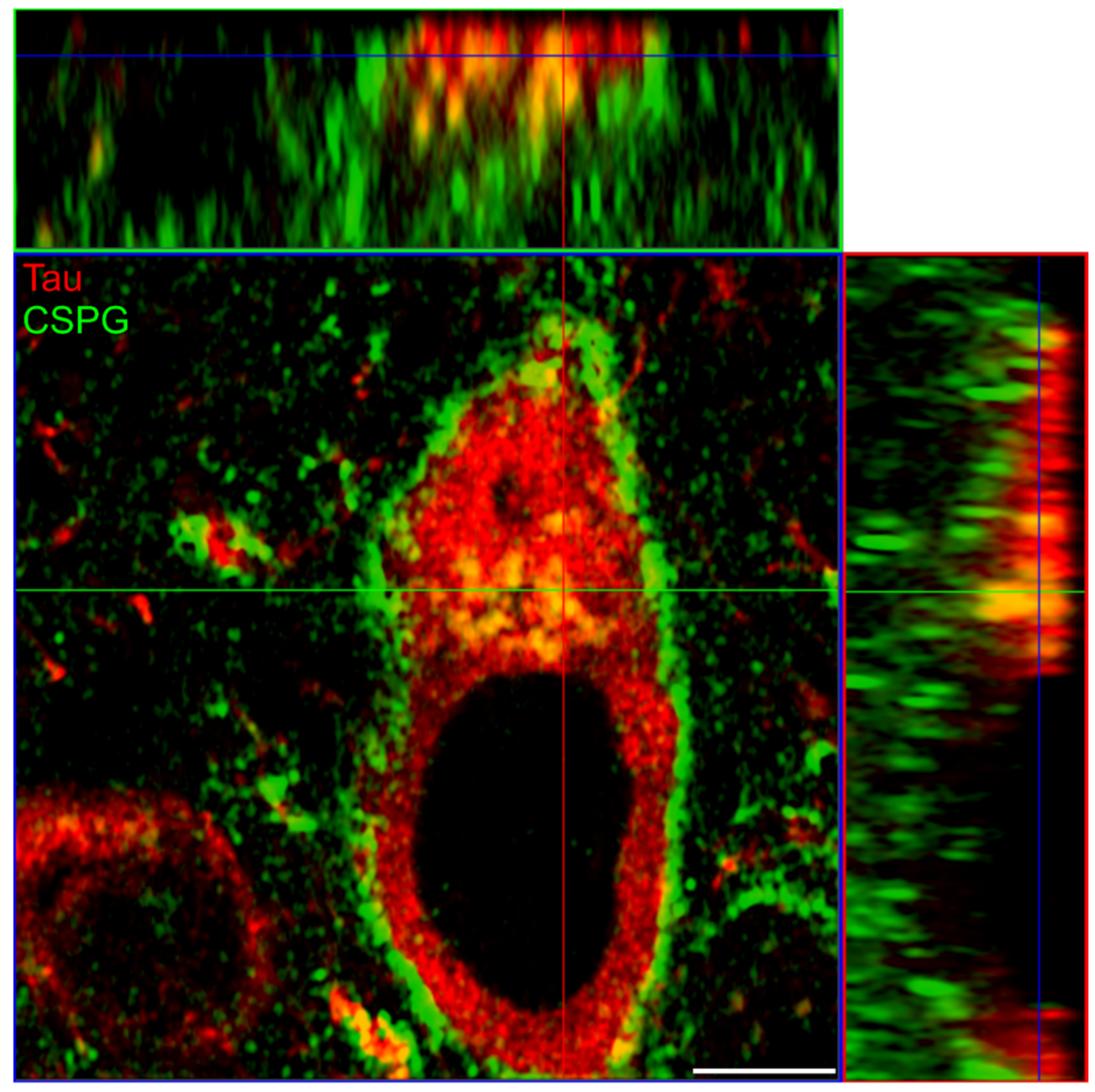
3.3. Co-Immunoprecipitation of Major Perineuronal Net Components and Tau Protein
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gu, Y.; Oyama, F.; Ihara, Y. Tau is widely expressed in rat tissues. J. Neurochem. 1996, 67, 1235–1244. [Google Scholar] [CrossRef]
- Binder, L.I.; Frankfurter, A.; Rebhun, L.I. The distribution of tau in the mammalian central nervous system. J. Cell Biol. 1985, 101, 1371–1378. [Google Scholar] [CrossRef] [Green Version]
- Hirokawa, N.; Funakoshi, T.; Sato-Harada, R.; Kanai, Y. Selective stabilization of tau in axons and microtubule-associated protein 2C in cell bodies and dendrites contributes to polarized localization of cytoskeletal proteins in mature neurons. J. Cell Biol. 1996, 132, 667–679. [Google Scholar] [CrossRef]
- Ittner, L.M.; Ke, Y.D.; Delerue, F.; Bi, M.; Gladbach, A.; van Eersel, J.; Wölfing, H.; Chieng, B.C.; Christie, M.J.; Napier, I.A.; et al. Dendritic function of tau mediates amyloid-beta toxicity in Alzheimer’s disease mouse models. Cell 2010, 142, 387–397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merino-Serrais, P.; Benavides-Piccione, R.; Blazquez-Llorca, L.; Kastanauskaite, A.; Rábano, A.; Avila, J.; DeFelipe, J. The influence of phospho-τ on dendritic spines of cortical pyramidal neurons in patients with Alzheimer’s disease. Brain 2013, 136, 1913–1928. [Google Scholar] [CrossRef]
- Zempel, H.; Luedtke, J.; Kumar, Y.; Biernat, J.; Dawson, H.; Mandelkow, E.; Mandelkow, E.-M. Amyloid-β oligomers induce synaptic damage via Tau-dependent microtubule severing by TTLL6 and spastin. EMBO J. 2013, 32, 2920–2937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mietelska-Porowska, A.; Wasik, U.; Goras, M.; Filipek, A.; Niewiadomska, G. Tau protein modifications and interactions: Their role in function and dysfunction. Int. J. Mol. Sci. 2014, 15, 4671–4713. [Google Scholar] [CrossRef] [Green Version]
- Buée, L.; Bussière, T.; Buée-Scherrer, V.; Delacourte, A.; Hof, P.R. Tau protein isoforms, phosphorylation and role in neurodegenerative disorders11These authors contributed equally to this work. Brain Res. Rev. 2000, 33, 95–130. [Google Scholar] [CrossRef]
- Eidenmüller, J.; Fath, T.; Hellwig, A.; Reed, J.; Sontag, E.; Brandt, R. Structural and functional implications of tau hyperphosphorylation: Information from phosphorylation-mimicking mutated tau proteins. Biochemistry 2000, 39, 13166–13175. [Google Scholar] [CrossRef]
- Bhaskar, K.; Yen, S.-H.; Lee, G. Disease-related modifications in tau affect the interaction between Fyn and Tau. J. Biol. Chem. 2005, 280, 35119–35125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magnani, E.; Fan, J.; Gasparini, L.; Golding, M.; Williams, M.; Schiavo, G.; Goedert, M.; Amos, L.A.; Spillantini, M.G. Interaction of tau protein with the dynactin complex. EMBO J. 2007, 26, 4546–4554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, E.M.; Dubey, M.; Camp, P.J.; Vernon, B.C.; Biernat, J.; Mandelkow, E.; Majewski, J.; Chi, E.Y. Interaction of tau protein with model lipid membranes induces tau structural compaction and membrane disruption. Biochemistry 2012, 51, 2539–2550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stefanoska, K.; Volkerling, A.; Bertz, J.; Poljak, A.; Ke, Y.D.; Ittner, L.M.; Ittner, A. An N-terminal motif unique to primate tau enables differential protein-protein interactions. J. Biol. Chem. 2018, 293, 3710–3719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boekhoorn, K.; Terwel, D.; Biemans, B.; Borghgraef, P.; Wiegert, O.; Ramakers, G.J.A.; de Vos, K.; Krugers, H.; Tomiyama, T.; Mori, H.; et al. Improved long-term potentiation and memory in young tau-P301L transgenic mice before onset of hyperphosphorylation and tauopathy. J. Neurosci. 2006, 26, 3514–3523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kremer, A.; Maurin, H.; Demedts, D.; Devijver, H.; Borghgraef, P.; van Leuven, F. Early improved and late defective cognition is reflected by dendritic spines in Tau.P301L mice. J. Neurosci. 2011, 31, 18036–18047. [Google Scholar] [CrossRef]
- Lee, V.M.; Goedert, M.; Trojanowski, J.Q. Neurodegenerative tauopathies. Annu. Rev. Neurosci. 2001, 24, 1121–1159. [Google Scholar] [CrossRef]
- Spillantini, M.G.; Goedert, M. Tau protein pathology in neurodegenerative diseases. Trends Neurosci. 1998, 21, 428–433. [Google Scholar] [CrossRef]
- Iqbal, K.; Liu, F.; Gong, C.-X.; Grundke-Iqbal, I. Tau in Alzheimer disease and related tauopathies. Curr. Alzheimer Res. 2010, 7, 656–664. [Google Scholar] [CrossRef] [Green Version]
- Mudher, A.; Colin, M.; Dujardin, S.; Medina, M.; Dewachter, I.; Alavi Naini, S.M.; Mandelkow, E.-M.; Mandelkow, E.; Buée, L.; Goedert, M.; et al. What is the evidence that tau pathology spreads through prion-like propagation? Acta Neuropathol. Commun. 2017, 5, 99. [Google Scholar] [CrossRef]
- Irwin, D.J. Tauopathies as clinicopathological entities. Parkinsonism Relat. Disord. 2016, 22 (Suppl. 1), S29–S33. [Google Scholar] [CrossRef] [Green Version]
- Ayers, J.I.; Giasson, B.I.; Borchelt, D.R. Prion-like Spreading in Tauopathies. Biol. Psychiatry 2018, 83, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Brückner, G.; Hausen, D.; Härtig, W.; Drlicek, M.; Arendt, T.; Brauer, K. Cortical areas abundant in extracellular matrix chondroitin sulphate proteoglycans are less affected by cytoskeletal changes in Alzheimer’s disease. Neuroscience 1999, 92, 791–805. [Google Scholar] [CrossRef]
- Bekku, Y.; Rauch, U.; Ninomiya, Y.; Oohashi, T. Brevican distinctively assembles extracellular components at the large diameter nodes of Ranvier in the CNS. J. Neurochem. 2009, 108, 1266–1276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bekku, Y.; Su, W.-D.; Hirakawa, S.; Fässler, R.; Ohtsuka, A.; Kang, J.S.; Sanders, J.; Murakami, T.; Ninomiya, Y.; Oohashi, T. Molecular cloning of Bral2, a novel brain-specific link protein, and immunohistochemical colocalization with brevican in perineuronal nets☆. Mol. Cell. Neurosci. 2003, 24, 148–159. [Google Scholar] [CrossRef]
- Giamanco, K.A.; Morawski, M.; Matthews, R.T. Perineuronal net formation and structure in aggrecan knockout mice. Neuroscience 2010, 170, 1314–1327. [Google Scholar] [CrossRef]
- Hagihara, K.; Miura, R.; Kosaki, R.; Berglund, E.; Ranscht, B.; Yamaguchi, Y. Immunohistochemical evidence for the brevican-tenascin-R interaction: Colocalization in perineuronal nets suggests a physiological role for the interaction in the adult rat brain. J. Comp. Neurol. 1999, 410, 256–264. [Google Scholar] [CrossRef]
- Matthews, R.T.; Kelly, G.M.; Zerillo, C.A.; Gray, G.; Tiemeyer, M.; Hockfield, S. Aggrecan Glycoforms Contribute to the Molecular Heterogeneity of Perineuronal Nets. J. Neurosci. 2002, 22, 7536–7547. [Google Scholar] [CrossRef]
- Deepa, S.S.; Carulli, D.; Galtrey, C.; Rhodes, K.; Fukuda, J.; Mikami, T.; Sugahara, K.; Fawcett, J.W. Composition of perineuronal net extracellular matrix in rat brain: A different disaccharide composition for the net-associated proteoglycans. J. Biol. Chem. 2006, 281, 17789–17800. [Google Scholar] [CrossRef] [Green Version]
- Köppe, G.; Brückner, G.; Härtig, W.; Delpech, B.; Bigl, V. Characterization of proteoglycan-containing perineuronal nets by enzymatic treatments of rat brain sections. Histochem. J. 1997, 29, 11–20. [Google Scholar] [CrossRef]
- Carulli, D.; Rhodes, K.E.; Fawcett, J.W. Upregulation of aggrecan, link protein 1, and hyaluronan synthases during formation of perineuronal nets in the rat cerebellum. J. Comp. Neurol. 2007, 501, 83–94. [Google Scholar] [CrossRef]
- Kwok, J.C.F.; Dick, G.; Wang, D.; Fawcett, J.W. Extracellular matrix and perineuronal nets in CNS repair. Dev. Neurobiol. 2011, 71, 1073–1089. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Fawcett, J. The perineuronal net and the control of CNS plasticity. Cell Tissue Res. 2012, 349, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Sonntag, M.; Blosa, M.; Schmidt, S.; Rübsamen, R.; Morawski, M. Perineuronal nets in the auditory system. Hear. Res. 2015, 329, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.; Arendt, T.; Morawski, M.; Sonntag, M. Neurocan Contributes to Perineuronal Net Development. Neuroscience 2020, 442, 69–86. [Google Scholar] [CrossRef]
- Brückner, G.; Morawski, M.; Arendt, T. Aggrecan-based extracellular matrix is an integral part of the human basal ganglia circuit. Neuroscience 2008, 151, 489–504. [Google Scholar] [CrossRef]
- Morawski, M.; Brückner, G.; Jäger, C.; Seeger, G.; Arendt, T. Neurons associated with aggrecan-based perineuronal nets are protected against tau pathology in subcortical regions in Alzheimer’s disease. Neuroscience 2010, 169, 1347–1363. [Google Scholar] [CrossRef]
- Morawski, M.; Brückner, G.; Jäger, C.; Seeger, G.; Matthews, R.T.; Arendt, T. Involvement of perineuronal and perisynaptic extracellular matrix in Alzheimer’s disease neuropathology. Brain Pathol. 2012, 22, 547–561. [Google Scholar] [CrossRef] [Green Version]
- Suttkus, A.; Holzer, M.; Morawski, M.; Arendt, T. The neuronal extracellular matrix restricts distribution and internalization of aggregated Tau-protein. Neuroscience 2016, 313, 225–235. [Google Scholar] [CrossRef]
- Suttkus, A.; Rohn, S.; Weigel, S.; Glöckner, P.; Arendt, T.; Morawski, M. Aggrecan, link protein and tenascin-R are essential components of the perineuronal net to protect neurons against iron-induced oxidative stress. Cell Death Dis. 2014, 5, e1119. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, S.; Stapf, C.; Schmutzler, S.; Lachmann, I.; Arendt, T.; Holzer, M.; Sonntag, M.; Morawski, M. Aggrecan modulates the expression and phosphorylation of tau in a novel bigenic TauP301L-Acan mouse model. Eur. J. Neurosci. 2021, 53, 3889–3904. [Google Scholar] [CrossRef]
- Lewis, J.; McGowan, E.; Rockwood, J.; Melrose, H.; Nacharaju, P.; van Slegtenhorst, M.; Gwinn-Hardy, K.; Paul Murphy, M.; Baker, M.; Yu, X.; et al. Neurofibrillary tangles, amyotrophy and progressive motor disturbance in mice expressing mutant (P301L) tau protein. Nat. Genet. 2000, 25, 402–405. [Google Scholar] [CrossRef] [PubMed]
- Tucker, K.L.; Meyer, M.; Barde, Y.A. Neurotrophins are required for nerve growth during development. Nat. Neurosci. 2001, 4, 29–37. [Google Scholar] [CrossRef]
- Watanabe, H.; Kimata, K.; Line, S.; Strong, D.; Gao, L.Y.; Kozak, C.A.; Yamada, Y. Mouse cartilage matrix deficiency (cmd) caused by a 7 bp deletion in the aggrecan gene. Nat. Genet. 1994, 7, 154–157. [Google Scholar] [CrossRef]
- Rittenhouse, E.; Dunn, L.C.; Cookingham, J.; Calo, C.; Spiegelman, M.; Dooher, G.B.; Bennett, D. Cartilage matrix deficiency (cmd): A new autosomal recessive lethal mutation in the mouse. J. Embryol. Exp. Morphol. 1978, 43, 71–84. [Google Scholar] [CrossRef]
- Morawski, M.; Brückner, G.; Arendt, T.; Matthews, R.T. Aggrecan: Beyond cartilage and into the brain. Int. J. Biochem. Cell Biol. 2012, 44, 690–693. [Google Scholar] [CrossRef]
- Végh, M.J.; Heldring, C.M.; Kamphuis, W.; Hijazi, S.; Timmerman, A.J.; Li, K.W.; van Nierop, P.; Mansvelder, H.D.; Hol, E.M.; Smit, A.B.; et al. Reducing hippocampal extracellular matrix reverses early memory deficits in a mouse model of Alzheimer’s disease. Acta Neuropathol. Commun. 2014, 2, 76. [Google Scholar] [CrossRef] [Green Version]
- Howell, M.D.; Bailey, L.A.; Cozart, M.A.; Gannon, B.M.; Gottschall, P.E. Hippocampal administration of chondroitinase ABC increases plaque-adjacent synaptic marker and diminishes amyloid burden in aged APPswe/PS1dE9 mice. Acta Neuropathol. Commun. 2015, 3, 54. [Google Scholar] [CrossRef] [PubMed]
- Holmes, B.B.; DeVos, S.L.; Kfoury, N.; Li, M.; Jacks, R.; Yanamandra, K.; Ouidja, M.O.; Brodsky, F.M.; Marasa, J.; Bagchi, D.P.; et al. Heparan sulfate proteoglycans mediate internalization and propagation of specific proteopathic seeds. Proc. Natl. Acad. Sci. USA 2013, 110, E3138–E3147. [Google Scholar] [CrossRef] [Green Version]
- Stopschinski, B.E.; Thomas, T.L.; Nadji, S.; Darvish, E.; Fan, L.; Holmes, B.B.; Modi, A.R.; Finnell, J.G.; Kashmer, O.M.; Estill-Terpack, S.; et al. A synthetic heparinoid blocks Tau aggregate cell uptake and amplification. J. Biol. Chem. 2020, 295, 2974–2983. [Google Scholar] [CrossRef]
- De Calignon, A.; Polydoro, M.; Suárez-Calvet, M.; William, C.; Adamowicz, D.H.; Kopeikina, K.J.; Pitstick, R.; Sahara, N.; Ashe, K.H.; Carlson, G.A.; et al. Propagation of tau pathology in a model of early Alzheimer’s disease. Neuron 2012, 73, 685–697. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Drouet, V.; Wu, J.W.; Witter, M.P.; Small, S.A.; Clelland, C.; Duff, K. Trans-synaptic spread of tau pathology in vivo. PLoS ONE 2012, 7, e31302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe, H.; Nakata, K.; Kimata, K.; Nakanishi, I.; Yamada, Y. Dwarfism and age-associated spinal degeneration of heterozygote cmd mice defective in aggrecan. Proc. Natl. Acad. Sci. USA 1997, 94, 6943–6947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inagaki, J.; Takahashi, K.; Ogawa, H.; Asano, K.; Faruk Hatipoglu, O.; Cilek, M.Z.; Obika, M.; Ohtsuki, T.; Hofmann, M.; Kusachi, S.; et al. ADAMTS1 inhibits lymphangiogenesis by attenuating phosphorylation of the lymphatic endothelial cell-specific VEGF receptor. Exp. Cell Res. 2014, 323, 263–275. [Google Scholar] [CrossRef]
- Blosa, M.; Sonntag, M.; Brückner, G.; Jäger, C.; Seeger, G.; Matthews, R.T.; Rübsamen, R.; Arendt, T.; Morawski, M. Unique features of extracellular matrix in the mouse medial nucleus of trapezoid body--implications for physiological functions. Neuroscience 2013, 228, 215–234. [Google Scholar] [CrossRef]
- Jakobs, C.; Perner, S.; Hornung, V. AIM2 Drives Joint Inflammation in a Self-DNA Triggered Model of Chronic Polyarthritis. PLoS ONE 2015, 10, e0131702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tammi, R.; MacCallum, D.; Hascall, V.C.; Pienimäki, J.P.; Hyttinen, M.; Tammi, M. Hyaluronan bound to CD44 on keratinocytes is displaced by hyaluronan decasaccharides and not hexasaccharides. J. Biol. Chem. 1998, 273, 28878–28888. [Google Scholar] [CrossRef] [Green Version]
- Croft, C.L.; Wade, M.A.; Kurbatskaya, K.; Mastrandreas, P.; Hughes, M.M.; Phillips, E.C.; Pooler, A.M.; Perkinton, M.S.; Hanger, D.P.; Noble, W. Membrane association and release of wild-type and pathological tau from organotypic brain slice cultures. Cell Death Dis. 2017, 8, e2671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borghgraef, P.; Menuet, C.; Theunis, C.; Louis, J.V.; Devijver, H.; Maurin, H.; Smet-Nocca, C.; Lippens, G.; Hilaire, G.; Gijsen, H.; et al. Increasing brain protein O-GlcNAc-ylation mitigates breathing defects and mortality of Tau.P301L mice. PLoS ONE 2013, 8, e84442. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Jing, Q.; Wu, L.; Chen, J.; Huang, M.; Qin, Y.; Wang, T. The prognostic and diagnostic value of tissue inhibitor of metalloproteinases gene family and potential function in gastric cancer. J. Cancer 2021, 12, 4086–4098. [Google Scholar] [CrossRef] [PubMed]
- Welinder, C.; Ekblad, L. Coomassie staining as loading control in Western blot analysis. J. Proteome Res. 2011, 10, 1416–1419. [Google Scholar] [CrossRef] [PubMed]
- Blosa, M.; Sonntag, M.; Jäger, C.; Weigel, S.; Seeger, J.; Frischknecht, R.; Seidenbecher, C.I.; Matthews, R.T.; Arendt, T.; Rübsamen, R.; et al. The extracellular matrix molecule brevican is an integral component of the machinery mediating fast synaptic transmission at the calyx of Held. J. Physiol. 2015, 593, 4341–4360. [Google Scholar] [CrossRef]
- De Luca, C.; Papa, M. Looking Inside the Matrix: Perineuronal Nets in Plasticity, Maladaptive Plasticity and Neurological Disorders. Neurochem. Res. 2016, 41, 1507–1515. [Google Scholar] [CrossRef]
- Sucha, P.; Chmelova, M.; Kamenicka, M.; Bochin, M.; Oohashi, T.; Vargova, L. The Effect of Hapln4 Link Protein Deficiency on Extracellular Space Diffusion Parameters and Perineuronal Nets in the Auditory System During Aging. Neurochem. Res. 2020, 45, 68–82. [Google Scholar] [CrossRef]
- Overk, C.R.; Kelley, C.M.; Mufson, E.J. Brainstem Alzheimer’s-like pathology in the triple transgenic mouse model of Alzheimer’s disease. Neurobiol. Dis. 2009, 35, 415–425. [Google Scholar] [CrossRef] [Green Version]
- Costa, C.; Tortosa, R.; Domènech, A.; Vidal, E.; Pumarola, M.; Bassols, A. Mapping of aggrecan, hyaluronic acid, heparan sulphate proteoglycans and aquaporin 4 in the central nervous system of the mouse. J. Chem. Neuroanat. 2007, 33, 111–123. [Google Scholar] [CrossRef]
- Watson, C. Motor Nuclei of the Cranial Nerves. In The Mouse Nervous System; Elsevier: Amsterdam, The Netherlands, 2012; pp. 490–498. ISBN 9780123694973. [Google Scholar]
- Li, X.; Yu, B.; Sun, Q.; Zhang, Y.; Ren, M.; Zhang, X.; Li, A.; Yuan, J.; Madisen, L.; Luo, Q.; et al. Generation of a whole-brain atlas for the cholinergic system and mesoscopic projectome analysis of basal forebrain cholinergic neurons. Proc. Natl. Acad. Sci. USA 2018, 115, 415–420. [Google Scholar] [CrossRef] [Green Version]
- Muir, J.L. Acetylcholine, Aging, and Alzheimer’s Disease. Pharmacol. Biochem. Behav. 1997, 56, 687–696. [Google Scholar] [CrossRef]
- Whitehouse, P.J.; Price, D.L.; Clark, A.W.; Coyle, J.T.; DeLong, M.R. Alzheimer disease: Evidence for selective loss of cholinergic neurons in the nucleus basalis. Ann. Neurol. 1981, 10, 122–126. [Google Scholar] [CrossRef]
- Park, H.-J.; Lee, K.-W.; Oh, S.; Yan, R.; Zhang, J.; Beach, T.G.; Adler, C.H.; Voronkov, M.; Braithwaite, S.P.; Stock, J.B.; et al. Protein Phosphatase 2A and Its Methylation Modulating Enzymes LCMT-1 and PME-1 Are Dysregulated in Tauopathies of Progressive Supranuclear Palsy and Alzheimer Disease. J. Neuropathol. Exp. Neurol. 2018, 77, 139–148. [Google Scholar] [CrossRef]
- Ward, M.P.; Spiers, J.P. Protein phosphatase 2A regulation of markers of extracellular matrix remodelling in hepatocellular carcinoma cells: Functional consequences for tumour invasion. Br. J. Pharmacol. 2017, 174, 1116–1130. [Google Scholar] [CrossRef] [Green Version]
- Stomrud, E.; Björkqvist, M.; Janciauskiene, S.; Minthon, L.; Hansson, O. Alterations of matrix metalloproteinases in the healthy elderly with increased risk of prodromal Alzheimer’s disease. Alzheimer’s Res. Ther. 2010, 2, 20. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.-X.; Tan, M.-S.; Yu, J.-T.; Tan, L. Matrix metalloproteinases and their multiple roles in Alzheimer’s disease. BioMed Res. Int. 2014, 2014, 908636. [Google Scholar] [CrossRef]
- Miguel, R.F.; Pollak, A.; Lubec, G. Metalloproteinase ADAMTS-1 but not ADAMTS-5 is manifold overexpressed in neurodegenerative disorders as Down syndrome, Alzheimer’s and Pick’s disease. Brain Res. Mol. Brain Res. 2005, 133, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Testa, D.; Prochiantz, A.; Di Nardo, A.A. Perineuronal nets in brain physiology and disease. Semin. Cell Dev. Biol. 2019, 89, 125–135. [Google Scholar] [CrossRef]
- Brelstaff, J.H.; Mason, M.; Katsinelos, T.; McEwan, W.A.; Ghetti, B.; Tolkovsky, A.M.; Spillantini, M.G. Microglia become hypofunctional and release metalloproteases and tau seeds when phagocytosing live neurons with P301S tau aggregates. Sci. Adv. 2021, 7, eabg4980. [Google Scholar] [CrossRef]
- Forostyak, S.; Homola, A.; Turnovcova, K.; Svitil, P.; Jendelova, P.; Sykova, E. Intrathecal delivery of mesenchymal stromal cells protects the structure of altered perineuronal nets in SOD1 rats and amends the course of ALS. Stem Cells 2014, 32, 3163–3172. [Google Scholar] [CrossRef] [Green Version]
- Gray, E.; Thomas, T.L.; Betmouni, S.; Scolding, N.; Love, S. Elevated matrix metalloproteinase-9 and degradation of perineuronal nets in cerebrocortical multiple sclerosis plaques. J. Neuropathol. Exp. Neurol. 2008, 67, 888–899. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Katagiri, Y.; McCann, T.E.; Unsworth, E.; Goldsmith, P.; Yu, Z.-X.; Tan, F.; Santiago, L.; Mills, E.M.; Wang, Y.; et al. Chondroitin-4-sulfation negatively regulates axonal guidance and growth. J. Cell Sci. 2008, 121, 3083–3091. [Google Scholar] [CrossRef] [Green Version]
- Carulli, D.; Pizzorusso, T.; Kwok, J.C.F.; Putignano, E.; Poli, A.; Forostyak, S.; Andrews, M.R.; Deepa, S.S.; Glant, T.T.; Fawcett, J.W. Animals lacking link protein have attenuated perineuronal nets and persistent plasticity. Brain 2010, 133, 2331–2347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sorg, B.A.; Berretta, S.; Blacktop, J.M.; Fawcett, J.W.; Kitagawa, H.; Kwok, J.C.F.; Miquel, M. Casting a Wide Net: Role of Perineuronal Nets in Neural Plasticity. J. Neurosci. 2016, 36, 11459–11468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duncan, J.A.; Foster, R.; Kwok, J.C.F. The potential of memory enhancement through modulation of perineuronal nets. Br. J. Pharmacol. 2019, 176, 3611–3621. [Google Scholar] [CrossRef]
- Zimmermann, D.R.; Dours-Zimmermann, M.T. Extracellular matrix of the central nervous system: From neglect to challenge. Histochem. Cell Biol. 2008, 130, 635–653. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Li, Z.-X.; Jin, T.; Wang, Z.-Y.; Zhao, P. Tau Pathology Promotes the Reorganization of the Extracellular Matrix and Inhibits the Formation of Perineuronal Nets by Regulating the Expression and the Distribution of Hyaluronic Acid Synthases. J. Alzheimers. Dis. 2017, 57, 395–409. [Google Scholar] [CrossRef] [Green Version]
- Lendvai, D.; Morawski, M.; Négyessy, L.; Gáti, G.; Jäger, C.; Baksa, G.; Glasz, T.; Attems, J.; Tanila, H.; Arendt, T.; et al. Neurochemical mapping of the human hippocampus reveals perisynaptic matrix around functional synapses in Alzheimer’s disease. Acta Neuropathol. 2013, 125, 215–229. [Google Scholar] [CrossRef]
- Hondius, D.C.; van Nierop, P.; Li, K.W.; Hoozemans, J.J.M.; van der Schors, R.C.; van Haastert, E.S.; van der Vies, S.M.; Rozemuller, A.J.M.; Smit, A.B. Profiling the human hippocampal proteome at all pathologic stages of Alzheimer’s disease. Alzheimer’s Dement. 2016, 12, 654–668. [Google Scholar] [CrossRef]
- Nakamura, H.; Fujii, Y.; Inoki, I.; Sugimoto, K.; Tanzawa, K.; Matsuki, H.; Miura, R.; Yamaguchi, Y.; Okada, Y. Brevican is degraded by matrix metalloproteinases and aggrecanase-1 (ADAMTS4) at different sites. J. Biol. Chem. 2000, 275, 38885–38890. [Google Scholar] [CrossRef] [Green Version]
- Pizzi, M.A.; Crowe, M.J. Matrix metalloproteinases and proteoglycans in axonal regeneration. Exp. Neurol. 2007, 204, 496–511. [Google Scholar] [CrossRef]
- Murphy, G. Tissue inhibitors of metalloproteinases. Genome Biol. 2011, 12, 233. [Google Scholar] [CrossRef]
- Fontanil, T.; Mohamedi, Y.; Moncada-Pazos, A.; Cobo, T.; Vega, J.A.; Cobo, J.L.; García-Suárez, O.; Cobo, J.; Obaya, Á.J.; Cal, S. Neurocan is a New Substrate for the ADAMTS12 Metalloprotease: Potential Implications in Neuropathies. Cell. Physiol. Biochem. 2019, 52, 1003–1016. [Google Scholar] [CrossRef]
- Rivera, S.; García-González, L.; Khrestchatisky, M.; Baranger, K. Metalloproteinases and their tissue inhibitors in Alzheimer’s disease and other neurodegenerative disorders. Cell. Mol. Life Sci. 2019, 76, 3167–3191. [Google Scholar] [CrossRef]
- Raeeszadeh-Sarmazdeh, M.; Do, L.D.; Hritz, B.G. Metalloproteinases and Their Inhibitors: Potential for the Development of New Therapeutics. Cells 2020, 9, 1313. [Google Scholar] [CrossRef] [PubMed]
- Hernandes-Alejandro, M.; Montaño, S.; Harrington, C.R.; Wischik, C.M.; Salas-Casas, A.; Cortes-Reynosa, P.; Pérez Salazar, E.; Cazares-Apatiga, J.; Apatiga-Perez, R.; Ontiveros Torres, M.Á.; et al. Analysis of the Relationship Between Metalloprotease-9 and Tau Protein in Alzheimer’s Disease. J. Alzheimer’s Dis. 2020, 76, 553–569. [Google Scholar] [CrossRef]
- Kaplan, A.; Spiller, K.J.; Towne, C.; Kanning, K.C.; Choe, G.T.; Geber, A.; Akay, T.; Aebischer, P.; Henderson, C.E. Neuronal matrix metalloproteinase-9 is a determinant of selective neurodegeneration. Neuron 2014, 81, 333–348. [Google Scholar] [CrossRef] [Green Version]
- Lo, E.H.; Wang, X.; Cuzner, M.L. Extracellular proteolysis in brain injury and inflammation: Role for plasminogen activators and matrix metalloproteinases. J. Neurosci. Res. 2002, 69, 1–9. [Google Scholar] [CrossRef]
- Asahina, M.; Yoshiyama, Y.; Hattori, T. Expression of matrix metalloproteinase-9 and urinary-type plasminogen activator in Alzheimer’s disease brain. Clin. Neuropathol. 2001, 20, 60–63. [Google Scholar]
- Brkic, M.; Balusu, S.; Libert, C.; Vandenbroucke, R.E. Friends or Foes: Matrix Metalloproteinases and Their Multifaceted Roles in Neurodegenerative Diseases. Mediat. Inflamm. 2015, 2015, 620581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, Y.; Liu, W.; Jiang, H.; Jiang, Q.; Feng, J. Selective vulnerability of dopaminergic neurons to microtubule depolymerization. J. Biol. Chem. 2005, 280, 34105–34112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mizoguchi, H.; Takuma, K.; Fukuzaki, E.; Ibi, D.; Someya, E.; Akazawa, K.; Alkam, T.; Tsunekawa, H.; Mouri, A.; Noda, Y.; et al. Matrix metalloprotease-9 inhibition improves amyloid beta-mediated cognitive impairment and neurotoxicity in mice. J. Pharmacol. Exp. Ther. 2009, 331, 14–22. [Google Scholar] [CrossRef] [Green Version]
- Agrawal, S.M.; Lau, L.; Yong, V.W. MMPs in the central nervous system: Where the good guys go bad. Semin. Cell Dev. Biol. 2008, 19, 42–51. [Google Scholar] [CrossRef]
- Fragkouli, A.; Tsilibary, E.C.; Tzinia, A.K. Neuroprotective role of MMP-9 overexpression in the brain of Alzheimer’s 5xFAD mice. Neurobiol. Dis. 2014, 70, 179–189. [Google Scholar] [CrossRef]
- Ethell, I.M.; Ethell, D.W. Matrix metalloproteinases in brain development and remodeling: Synaptic functions and targets. J. Neurosci. Res. 2007, 85, 2813–2823. [Google Scholar] [CrossRef] [PubMed]
- Lasek, A.W.; Chen, H.; Chen, W.-Y. Releasing Addiction Memories Trapped in Perineuronal Nets. Trends Genet. 2018, 34, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Wen, T.H.; Afroz, S.; Reinhard, S.M.; Palacios, A.R.; Tapia, K.; Binder, D.K.; Razak, K.A.; Ethell, I.M. Genetic Reduction of Matrix Metalloproteinase-9 Promotes Formation of Perineuronal Nets Around Parvalbumin-Expressing Interneurons and Normalizes Auditory Cortex Responses in Developing Fmr1 Knock-Out Mice. Cereb. Cortex 2018, 28, 3951–3964. [Google Scholar] [CrossRef] [Green Version]
- Satoh, K.; Suzuki, N.; Yokota, H. ADAMTS-4 (a disintegrin and metalloproteinase with thrombospondin motifs) is transcriptionally induced in beta-amyloid treated rat astrocytes. Neurosci. Lett. 2000, 289, 177–180. [Google Scholar] [CrossRef]
- Pehlivan, S.; Fedakar, R.; Eren, B.; Akyol, S.; Eren, F.; Inanir, N.T.; Gurses, M.S.; Ural, M.N.; Tagil, S.M.; Demircan, K. ADAMTS4, 5, 9, and 15 Expressions in the Autopsied Brain of Patients with Alzheimer’s Disease: A Preliminary Immunohistochemistry Study. Klin. Psikofarmakol. Bülteni-Bull. Clin. Psychopharmacol. 2016, 26, 7–14. [Google Scholar] [CrossRef] [Green Version]
- Horstmann, S.; Budig, L.; Gardner, H.; Koziol, J.; Deuschle, M.; Schilling, C.; Wagner, S. Matrix metalloproteinases in peripheral blood and cerebrospinal fluid in patients with Alzheimer’s disease. Int. Psychogeriatr. 2010, 22, 966–972. [Google Scholar] [CrossRef]
- Mroczko, B.; Groblewska, M.; Zboch, M.; Kulczyńska, A.; Koper, O.M.; Szmitkowski, M.; Kornhuber, J.; Lewczuk, P. Concentrations of matrix metalloproteinases and their tissue inhibitors in the cerebrospinal fluid of patients with Alzheimer’s disease. J. Alzheimers Dis. 2014, 40, 351–357. [Google Scholar] [CrossRef]
- Berg, G.; Miksztowicz, V.; Schreier, L. Metalloproteinases in metabolic syndrome. Clin. Chim. Acta 2011, 412, 1731–1739. [Google Scholar] [CrossRef]
- Minta, K.; Cullen, N.C.; Nimer, F.A.; Thelin, E.P.; Piehl, F.; Clarin, M.; Tullberg, M.; Jeppsson, A.; Portelius, E.; Zetterberg, H.; et al. Dynamics of extracellular matrix proteins in cerebrospinal fluid and serum and their relation to clinical outcome in human traumatic brain injury. Clin. Chem. Lab. Med. 2019, 57, 1565–1573. [Google Scholar] [CrossRef]
- Jonesco, D.S.; Karsdal, M.A.; Henriksen, K. The CNS-specific proteoglycan, brevican, and its ADAMTS4-cleaved fragment show differential serological levels in Alzheimer’s disease, other types of dementia and non-demented controls: A cross-sectional study. PLoS ONE 2020, 15, e0234632. [Google Scholar] [CrossRef]
- Suttkus, A.; Morawski, M.; Arendt, T. Protective Properties of Neural Extracellular Matrix. Mol. Neurobiol. 2016, 53, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Lee, S.; Jung, C.; Ahmed, A.; Lee, G.; Hall, G.F. Interneuronal transfer of human tau between Lamprey central neurons in situ. J. Alzheimer’s Dis. 2010, 19, 647–664. [Google Scholar] [CrossRef]
- Yamada, K.; Cirrito, J.R.; Stewart, F.R.; Jiang, H.; Finn, M.B.; Holmes, B.B.; Binder, L.I.; Mandelkow, E.-M.; Diamond, M.I.; Lee, V.M.-Y.; et al. In vivo microdialysis reveals age-dependent decrease of brain interstitial fluid tau levels in P301S human tau transgenic mice. J. Neurosci. 2011, 31, 13110–13117. [Google Scholar] [CrossRef] [PubMed]
- Pernègre, C.; Duquette, A.; Leclerc, N. Tau Secretion: Good and Bad for Neurons. Front. Neurosci. 2019, 13, 649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simón, D.; García-García, E.; Gómez-Ramos, A.; Falcón-Pérez, J.M.; Díaz-Hernández, M.; Hernández, F.; Avila, J. Tau overexpression results in its secretion via membrane vesicles. Neurodegener. Dis. 2012, 10, 73–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medina, M.; Avila, J. The role of extracellular Tau in the spreading of neurofibrillary pathology. Front. Cell. Neurosci. 2014, 8, 113. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Ramos, A.; Díaz-Hernández, M.; Cuadros, R.; Hernández, F.; Avila, J. Extracellular tau is toxic to neuronal cells. FEBS Lett. 2006, 580, 4842–4850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flach, K.; Hilbrich, I.; Schiffmann, A.; Gärtner, U.; Krüger, M.; Leonhardt, M.; Waschipky, H.; Wick, L.; Arendt, T.; Holzer, M. Tau oligomers impair artificial membrane integrity and cellular viability. J. Biol. Chem. 2012, 287, 43223–43233. [Google Scholar] [CrossRef] [Green Version]
- Yamada, K. Extracellular Tau and Its Potential Role in the Propagation of Tau Pathology. Front. Neurosci. 2017, 11, 667. [Google Scholar] [CrossRef]
- Takeda, S. Progression of Alzheimer’s disease, tau propagation, and its modifiable risk factors. Neurosci. Res. 2019, 141, 36–42. [Google Scholar] [CrossRef]


| Detected Components | Dilution | Source | References/RRID |
|---|---|---|---|
| ADAMTS1 (AF5867) | WB 1:100 | R&D Systems | [53]; RRID: AB_2044595 |
| Aggrecan, core protein (AB1031) | IHC 1:1000 | Merck Millipore | [54]; RRID: AB_90460 |
| WB 1:1000 | |||
| IP 1:50 | |||
| Brevican, core protein (clone 2) | IHC 1:1250 | BD Biosciences | [54]; RRID: AB_398212 |
| WB 1:1250 | |||
| IP 1:20 | |||
| MMP3, C-terminal part (clone EP1186Y) | WB 1:4000 | Abcam | [55]; RRID:AB_881243 |
| Neurocan, N-terminal part (AF5800) | IHC 1:400 | R&D Systems | [54]; RRID: AB_2149717 |
| WB 1:2500 | |||
| IP 1:8 | |||
| HAPLN1 (AF2608) | IHC 1:400 | R&D Systems | [54]; RRID: AB_2116135 |
| WB 1:750 | |||
| IP 1:8 | |||
| Hyaluronic acid binding protein | IHC 1:100 | Merck Millipore | [56]; RRID: AB_2861303 |
| pan tau, C-terminal tau epitope | WB 1:4000 | Dako | [57]; RRID: AB_10013724 |
| PP2A alpha + beta antibody (clone E155) | WB 1:15000 | Abcam | [58]; RRID:AB_777385 |
| human tau total (clone 7E5) | IHC 1:750 | Roboscreen | [40], 847-0102006301 |
| TIMP3, C-terminal TIMP3 epitope | WB 1:800 | Proteintech | [59]; RRID: AB_2204973 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmidt, S.; Holzer, M.; Arendt, T.; Sonntag, M.; Morawski, M. Tau Protein Modulates Perineuronal Extracellular Matrix Expression in the TauP301L-acan Mouse Model. Biomolecules 2022, 12, 505. https://doi.org/10.3390/biom12040505
Schmidt S, Holzer M, Arendt T, Sonntag M, Morawski M. Tau Protein Modulates Perineuronal Extracellular Matrix Expression in the TauP301L-acan Mouse Model. Biomolecules. 2022; 12(4):505. https://doi.org/10.3390/biom12040505
Chicago/Turabian StyleSchmidt, Sophie, Max Holzer, Thomas Arendt, Mandy Sonntag, and Markus Morawski. 2022. "Tau Protein Modulates Perineuronal Extracellular Matrix Expression in the TauP301L-acan Mouse Model" Biomolecules 12, no. 4: 505. https://doi.org/10.3390/biom12040505






