High Lipid Content of Prey Fish and n−3 PUFA Peroxidation Impair the Thiamine Status of Feeding-Migrating Atlantic Salmon (Salmo salar) and Is Reflected in Hepatic Biochemical Indices
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fish and Sample Preparation
2.1.1. Prey Fish
2.1.2. Salmon
2.2. Chemical Analyses
2.2.1. Analysis of Thiamine Components
2.2.2. Analysis of Carotenoids
2.2.3. Fatty Acid Analyses
2.2.4. Enzyme Activity and Malondialdehyde Determinations
2.2.5. Quality Control
2.3. Calculations and Statistical Analyses
3. Results
3.1. Nutritional State
3.1.1. Nutritional Characteristics of Prey Fish
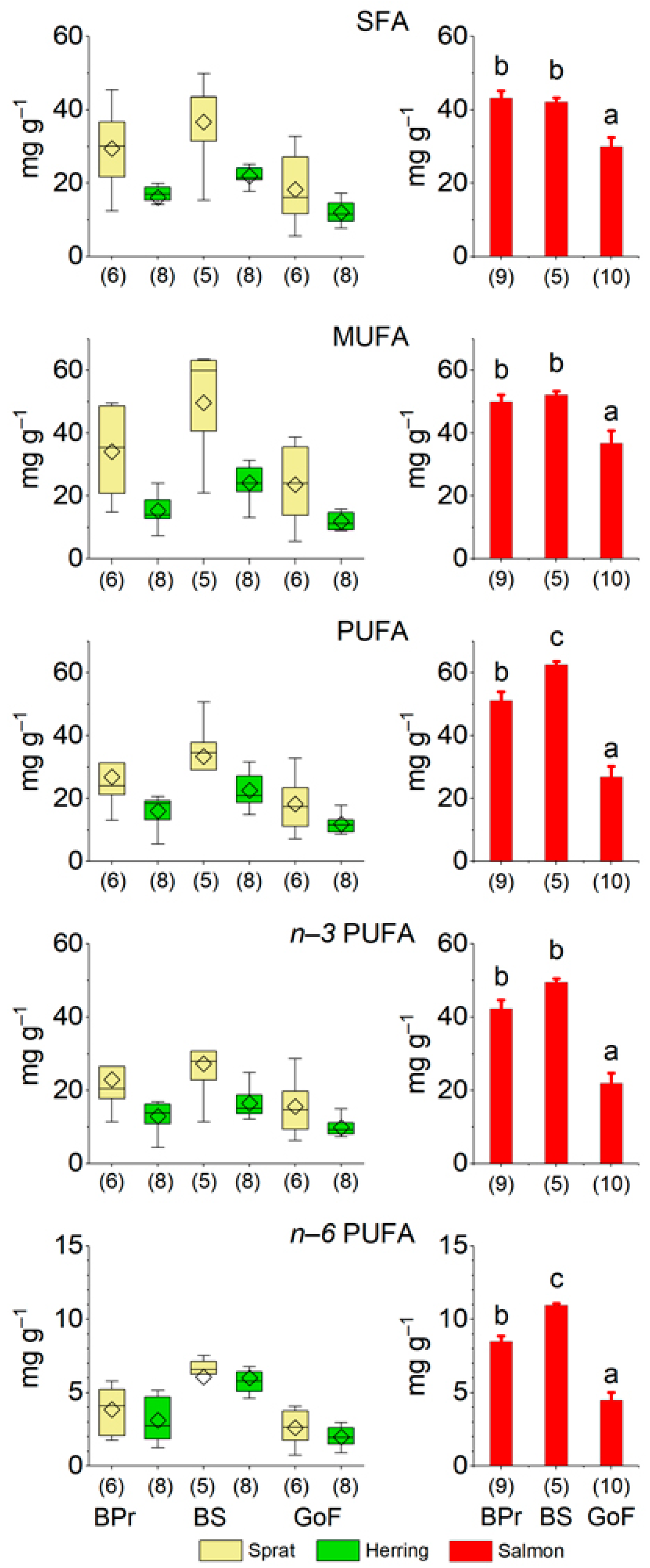
3.1.2. Reflection of Prey Fish Nutrients in Salmon
| Baltic Proper (BPr) | Bothnian Sea (BS) | Gulf of Finland (GoF) | p | F2,21 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Saturated FAs (SFA) | |||||||||||
| 14:0 | 5.82 | ± | 0.28 b | 5.31 | ± | 0.24 b | 2.88 | ± | 0.31 a | <0.0001 | 30.6 |
| 16:0 | 29.67 | ± | 1.32 b | 29.49 | ± | 0.84 b | 20.10 | ± | 1.78 a | 0.0002 | 13.1 |
| 17:0 | 0.81 | ± | 0.04 b | 0.87 | ± | 0.02 b | 0.54 | ± | 0.03 a | <0.0001 | 25.6 |
| 18:0 | 5.56 | ± | 0.26 | 5.66 | ± | 0.15 | 5.49 | ± | 0.42 | 0.950 | 0.05 |
| SFA | 43.20 | ± | 1.93 b | 42.11 | ± | 1.20 b | 29.92 | ± | 2.56 a | 0.0004 | 11.5 |
| Monounsaturated FAs (MUFA) | |||||||||||
| 16:1n−7 | 6.91 | ± | 0.27 b | 7.38 | ± | 0.18 b | 5.08 | ± | 0.64 a | 0.010 | 5.86 |
| 17:1n−8 | 1.00 | ± | 0.04 b | 0.69 | ± | 0.04 a | 0.62 | ± | 0.05 a | <0.0001 | 22.1 |
| 18:1n−9 | 34.85 | ± | 1.59 b | 32.97 | ± | 1.11 b | 25.01 | ± | 2.94 a | 0.013 | 5.34 |
| 18:1n−7 | 4.84 | ± | 0.22 a | 7.74 | ± | 0.34 b | 4.15 | ± | 0.36 a | <0.0001 | 26.8 |
| 20:1n−9 | 1.74 | ± | 0.26 | 2.24 | ± | 0.10 | 1.39 | ± | 0.25 | 0.116 | 2.39 |
| MUFA | 50.03 | ± | 2.22 b | 52.14 | ± | 1.16 b | 36.71 | ± | 4.11 a | 0.007 | 6.45 |
| Polyunsaturated FAs (PUFA) | |||||||||||
| 18:2n−6 | 5.33 | ± | 0.26 b | 7.58 | ± | 0.13 c | 2.29 | ± | 0.34 a | <0.0001 | 68.6 |
| 18:3n−3 | 4.25 | ± | 0.21 b | 4.29 | ± | 0.14 b | 1.66 | ± | 0.24 a | <0.0001 | 47.5 |
| 20:2n−6 | 1.19 | ± | 0.07 b | 2.14 | ± | 0.04 c | 0.61 | ± | 0.08 a | <0.0001 | 86.0 |
| 20:4n−6 (ARA) | 0.96 | ± | 0.04 | 1.24 | ± | 0.03 | 0.93 | ± | 0.12 | 0.113 | 2.42 |
| 20:5n−3 (EPA) | 9.36 | ± | 0.54 b | 9.91 | ± | 0.30 b | 4.20 | ± | 0.57 a | <0.0001 | 33.8 |
| 22:5n−3 (DPA) | 4.58 | ± | 0.23 b | 5.51 | ± | 0.20 b | 2.99 | ± | 0.36 a | <0.0001 | 15.7 |
| 22:6n−3 (DHA) | 23.82 | ± | 1.49 b | 28.53 | ± | 0.71 b | 12.91 | ± | 1.68 a | <0.0001 | 24.6 |
| PUFA | 51.20 | ± | 2.73 b | 62.53 | ± | 1.13 c | 26.86 | ± | 3.44 a | <0.0001 | 33.0 |
| n−3 PUFA | 42.25 | ± | 2.37 b | 49.53 | ± | 0.96 b | 21.94 | ± | 2.85 a | <0.0001 | 29.4 |
| n−6 PUFA | 8.47 | ± | 0.40 b | 10.96 | ± | 0.12c | 4.47 | ± | 0.57 a | <0.0001 | 41.1 |
| Weight, kg | 3.6 (2.6–4.6) | 3.7 (3.1–4.5) | 2.7 (1.1–6.2) | ||||||||
| Length, cm | 71.1 (64.3–77.8) | 70.9 (67.4–74.3) | 60.1 (49.3–85.8) | ||||||||
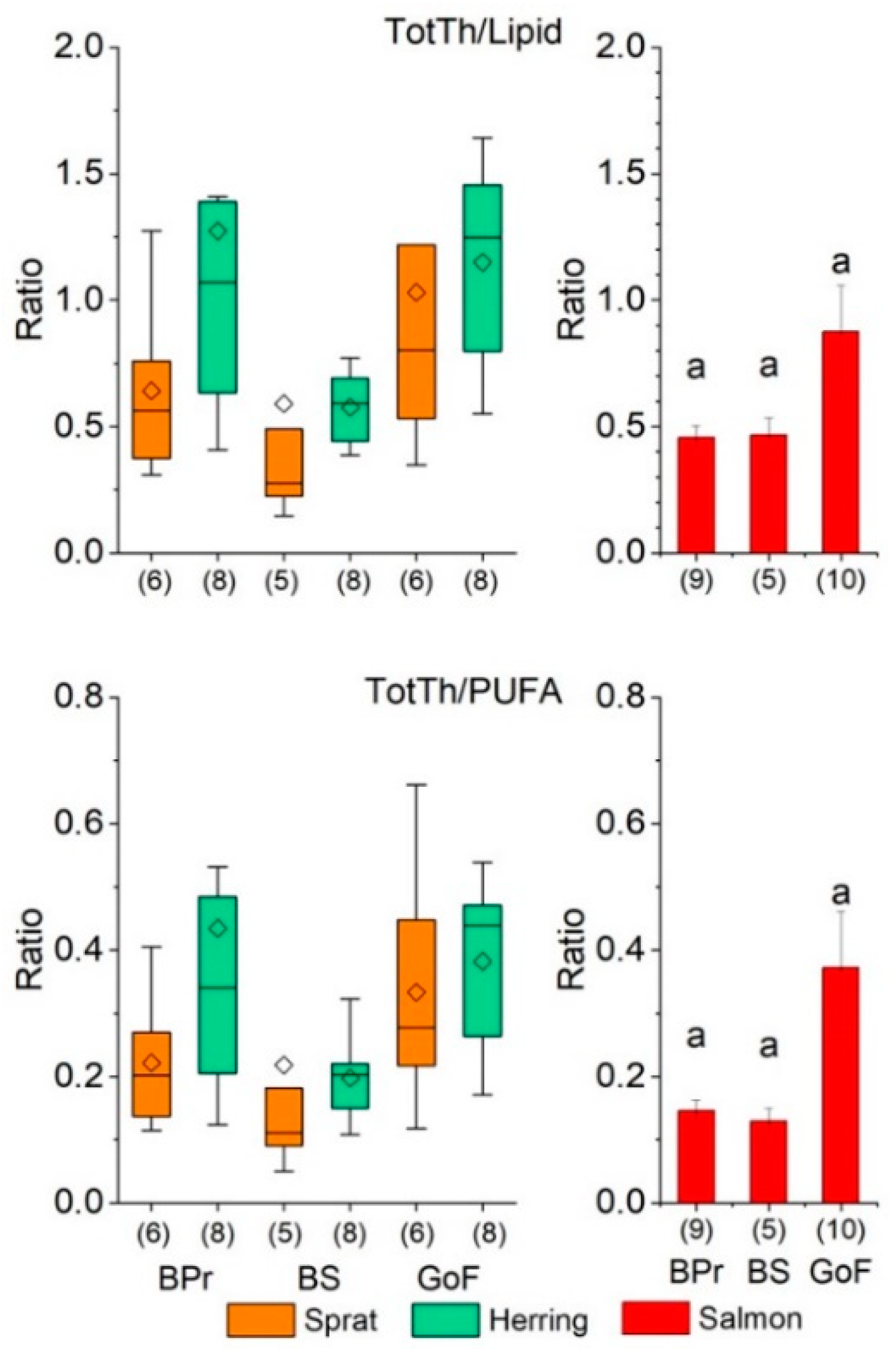
3.1.3. Thiamine per Lipid and PUFA in Prey Fish and Salmon
3.2. Thiamine Components
3.2.1. Thiamine Components in Prey Fish
3.2.2. Thiamine Components in Salmon
3.3. Salmon Liver Biochemistry
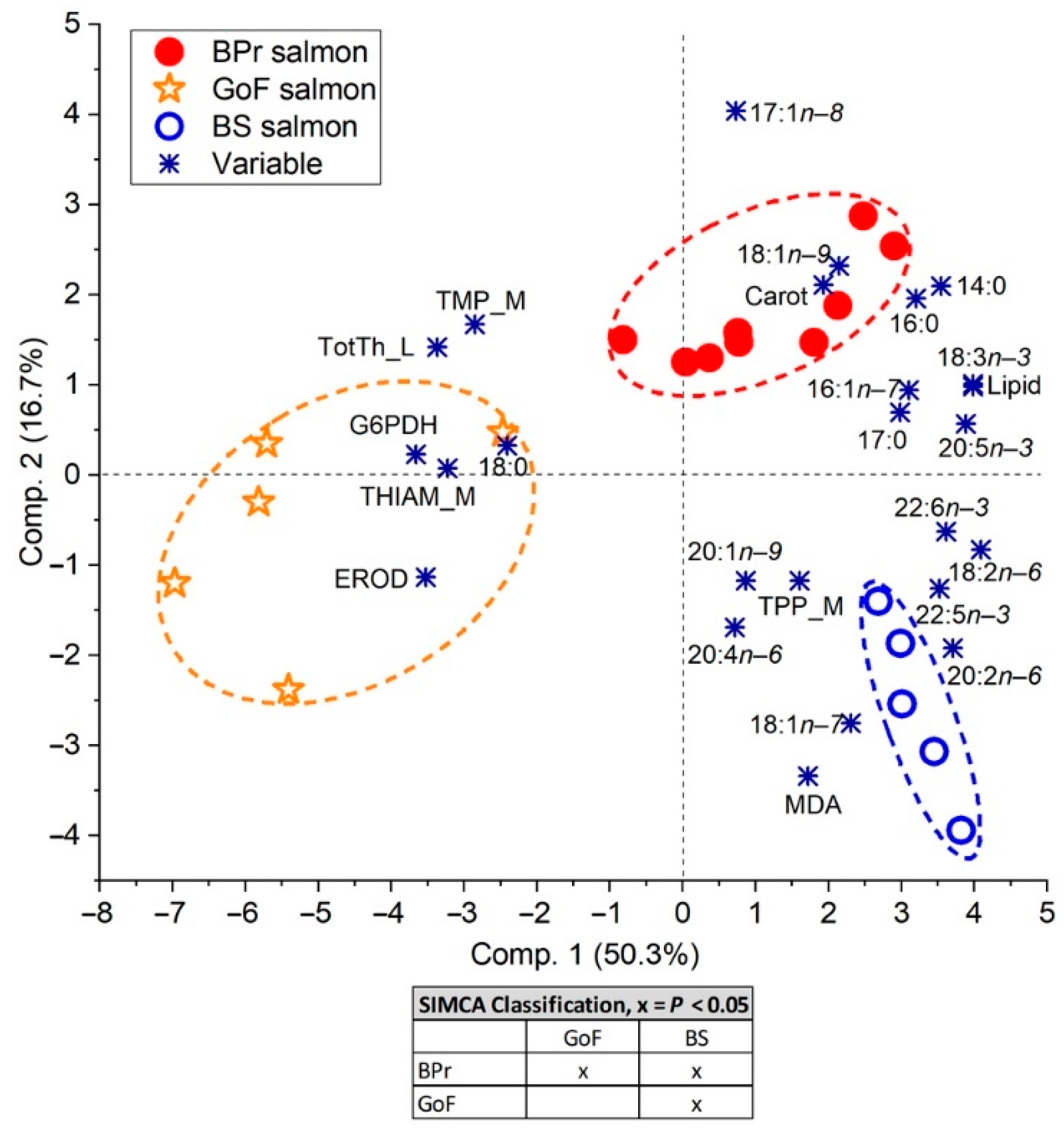
3.4. Relationships between the Indices
3.4.1. Relationship of Salmon Lipid and Fatty Acid Content with Prey Fish Lipid Content
3.4.2. Relationship of Thiamine and Its Components with Lipid Content and Fatty Acid Content within Species
3.4.3. Relationship of Liver Biochemical Indices with Lipid and Fatty Acid Content in Salmon
3.4.4. Carotenoid Relationships in Salmon
3.4.5. Organochlorine Relationships with the Hepatic Parameters in Salmon
3.5. Spatial Differences in Salmon Biochemistry
4. Discussion
4.1. High G6PDH Activity Was Associated with Low Lipid Content
4.2. Lipid Peroxidation Product MDA Was Associated with n−3 PUFAs
4.3. Thiamine Status Was Negatively Associated with Lipids and n−3 PUFAs
4.4. Thiamine Component Composition in Relation to Lipids and PUFAs
4.5. Lipid Peroxidation Impairs Thiamine Status
4.6. The Least Carotenoids from the Leanest Prey Fish
4.7. EROD Was Associated with PCBs and PCDDs, but MDA and Thiamine Were Not
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karlsson, L.; Karlström, Ö. The Baltic salmon (Salmo salar L.): Its history, present situation and future. Dana 1994, 10, 61–85. [Google Scholar]
- Vuorinen, P.J.; Roots, O.; Keinänen, M. Review of organohalogen toxicants in fish from the Gulf of Finland. J. Mar. Syst. 2017, 171, 141–150. [Google Scholar] [CrossRef]
- Keinänen, M.; Käkelä, R.; Ritvanen, T.; Pönni, J.; Harjunpää, H.; Myllylä, T.; Vuorinen, P.J. Fatty acid signatures connect thiamine deficiency with the diet of the Atlantic salmon (Salmo salar) feeding in the Baltic Sea. Mar. Biol. 2018, 165, 161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vuorinen, P.J.; Rokka, M.; Nikonen, S.; Juntunen, E.-P.; Ritvanen, T.; Heinimaa, P.; Keinänen, M. Model for estimating thiamine deficiency-related mortality of Atlantic salmon (Salmo salar) offspring and variation in the Baltic salmon M74 syndrome. Mar Freshw. Behav. Physiol. 2021, 54, 97–131. [Google Scholar] [CrossRef]
- Karlström, Ö. Development of the M74 syndrome in wild populations of Baltic salmon (Salmo salar) in Swedish rivers. Ambio 1999, 28, 82–86. Available online: https://www.jstor.org/stable/4314852 (accessed on 23 March 2022).
- Romakkaniemi, A.; Pera, I.; Karlsson, L.; Jutila, E.; Carlsson, U.; Pakarinen, T. Development of wild Atlantic salmon stocks in the rivers of the northern Baltic Sea in response to management measures. ICES J. Mar. Sci. 2003, 60, 329–442. [Google Scholar] [CrossRef]
- ICES. Baltic salmon and trout assessment working group (WGBAST). ICES Sci. Rep. 2020, 2, 261. [Google Scholar] [CrossRef]
- Keinänen, M.; Iivari, J.; Juntunen, E.-P.; Kannel, R.; Heinimaa, P.; Nikonen, S.; Pakarinen, T.; Romakkaniemi, A.; Vuorinen, P.J. Thiamine Deficiency M74 of Salmon Can Be Prevented; Riista-ja kalatalous–Selvityksiä; Riista-ja Kalatalouden Tutkimuslaitos: Helsinki, Finland, 2014; (In Finnish with English Abstract). [Google Scholar]
- Keinänen, M.; Uddström, A.; Mikkonen, J.; Casini, M.; Pönni, J.; Myllylä, T.; Aro, E.; Vuorinen, P.J. The thiamine deficiency syndrome M74, a reproductive disorder of Atlantic salmon (Salmo salar) feeding in the Baltic Sea, is related to the fat and thiamine content of prey fish. ICES J. Mar. Sci. 2012, 69, 516–528. [Google Scholar] [CrossRef] [Green Version]
- Keinänen, M.; Käkelä, R.; Ritvanen, T.; Myllylä, T.; Pönni, J.; Vuorinen, P.J. Fatty acid composition of sprat (Sprattus sprattus) and herring (Clupea harengus) in the Baltic Sea as potential prey for salmon (Salmo salar). Helgol. Mar. Res. 2017, 71, 4. [Google Scholar] [CrossRef] [Green Version]
- Futia, M.H.; Connerton, M.J.; Weidel, B.C.; Rinchard, J. Diet predictions of Lake Ontario salmonines based on fatty acids and correlations between their fat content and thiamine concentrations. J. Great Lakes Res. 2019, 45, 934–948. [Google Scholar] [CrossRef]
- Futia, M.H.; Rinchard, J. Evaluation of adult and offspring thiamine deficiency in salmonine species from Lake Ontario. J. Great Lakes Res. 2019, 45, 811–820. [Google Scholar] [CrossRef]
- Amcoff, P.; Börjeson, H.; Landergren, P.; Vallin, L.; Norrgren, L. Thiamine (vitamin B1) concentrations in salmon (Salmo salar), brown trout (Salmo trutta) and cod (Gadus morhua) from the Baltic sea. Ambio 1999, 28, 48–54. Available online: http://www.jstor.org/stable/4314848 (accessed on 23 March 2022).
- Futia, M.H.; Hallenbeck, S.; Noyes, A.D.; Honeyfield, D.C.; Eckerlin, G.E.; Rinchard, J. Thiamine deficiency and the effectiveness of thiamine treatments through broodstock injections and egg immersion on Lake Ontario steelhead trout. J. Great Lakes Res. 2017, 43, 352–358. [Google Scholar] [CrossRef]
- Harbicht, A.B.; Castro-Santos, T.; Gorsky, D.; Hand, D.M.; Fraser, D.J.; Ardren, W.R. Environmental, anthropogenic, and dietary influences on fine-scale movement patterns of Atlantic salmon through challenging waters. Can. J. Fish Aquat. Sci. 2018, 75, 2198–2210. [Google Scholar] [CrossRef]
- Brown, S.B.; Honeyfield, D.C.; Hnath, J.G.; Wolgamood, M.; Marcquenski, S.V.; Fitzsimons, J.D.; Tillitt, D.E. Thiamine status in adult salmonines in the Great Lakes. J. Aquat. Anim. Health 2005, 17, 59–64. [Google Scholar] [CrossRef]
- Mikkonen, J.; Keinänen, M.; Casini, M.; Pönni, J.; Vuorinen, P.J. Relationships between fish stock changes in the Baltic Sea and the M74 syndrome, a reproductive disorder of Atlantic salmon (Salmo salar). ICES J. Mar. Sci. 2011, 68, 2134–2144. [Google Scholar] [CrossRef] [Green Version]
- Tocher, D.R. Metabolism and functions of lipids and fatty acids in teleost fish. Rev. Fish Sci. 2003, 11, 107–184. [Google Scholar] [CrossRef]
- Lonsdale, D. A review of the biochemistry, metabolism and clinical benefits of thiamin(e) and its derivatives. Evid-Based Compl. Alt. 2006, 3, 49–59. [Google Scholar] [CrossRef]
- Lonsdale, D.; Marrs, C. Thiamine Deficiency Disease, Dysautonomia, and High Calorie Malnutrition; Academic Press: London, UK; San Diego, CA, USA; Cambridge, MA, USA; Kidlington, UK, 2019. [Google Scholar]
- Combs, G.F., Jr.; McClung, J.P. The Vitamins, Fundamental Aspects in Nutrition and Health, 5th ed.; Academic Press: London, UK; San Diego, CA, USA; Cambridge, MA, USA; Kidlington, UK, 2017; pp. 297–314. [Google Scholar]
- Gibson, G.E.; Zhang, H. Interactions of oxidative stress with thiamine homeostasis promote neurodegeneration. Neurochem. Int. 2002, 40, 493–504. [Google Scholar] [CrossRef]
- Frank, R.A.W.; Leeper, F.J.; Luisi, B.F. Structure, mechanism and catalytic duality of thiamine-dependent enzymes. Cell. Mol. Life Sci. 2007, 64, 892–905. [Google Scholar] [CrossRef]
- Depeint, F.; Bruce, W.R.; Shangari, N.; Mehta, R.; O’Brien, P.J. Mitochondrial function and toxicity: Role of the B vitamin family on mitochondrial energy metabolism. Chem. Biol. Interact. 2006, 163, 94–112. [Google Scholar] [CrossRef] [PubMed]
- Casteels, M.; Sniekers, M.; Fraccascia, P.; Mannaerts, G.P.; VanVeldhoven, P.P. The role of 2-hydroxyacyl-CoA lyase, a thiamin pyrophosphate-dependent enzyme, in the peroxisomal metabolism of 3-methyl-branched fatty acids and 2-hydroxy straight-chain fatty acids. Biochem. Soc. Trans. 2007, 35, 876–880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woodward, B. Dietary vitamin requirements of cultured young fish, with emphasis on quantitative estimates for salmonids. Aquaculture 1994, 124, 133–168. [Google Scholar] [CrossRef]
- Kriketos, A.D.; Peters, J.C.; Hill, J.O. Cellular and whole-animal energetics. In Biochemical and Physiological Aspects of Human Nutrition; Stipanuk, M.H., Ed.; Saunders: Philadelphia, PA, USA; Elsevier: Philadelphia, PA, USA, 2000; pp. 411–424. [Google Scholar]
- Tacon, A.G.J. Lipid nutritional pathology in farmed fish. Arch. Anim. Nutr. 1996, 49, 33–39. [Google Scholar] [CrossRef]
- Spector, A.A. Lipid metabolism: Essential fatty acids. In Biochemical and Physiological Aspects of Human Nutrition; Stipanuk, M.H., Ed.; Saunders: Philadelphia, PA, USA; Elsevier: Philadelphia, PA, USA, 2000; pp. 365–383. [Google Scholar]
- Kjær, M.; Todorcevic, M.; Torstensen, B.; Vegusdal, A.; Ruyter, B. Dietary n−3 HUFA affects mitochondrial fatty acid β-oxidation capacity and susceptibility to oxidative stress in Atlantic salmon. Lipids 2008, 43, 813–827. [Google Scholar] [CrossRef]
- Sargent, J.; Tocher, D.R.; Bell, J.G. The lipids. In Fish Nutrition, 3rd ed.; Halver, J.E., Hardy, R.W., Eds.; Academic Press: San Diego, CA, USA, 2002; pp. 181–257. [Google Scholar]
- Corraze, G.; Kaushik, S. Lipids from marine and freshwater fish, Les lipides des poissons marins et d’eau douce. OCL 1999, 6, 111–115. [Google Scholar]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Van der Oost, R.; Beyer, J.; Vermeulen, N.P.E. Fish bioaccumulation and biomarkers in environmental risk assessment: A review. Environ. Toxicol. Phar. 2003, 13, 57–149. [Google Scholar] [CrossRef]
- Moore, K.; Roberts, L.J. Measurement of lipid peroxidation. Free Radic. Res. 1998, 28, 659–671. [Google Scholar] [CrossRef]
- Del Rio, D.; Stewart, A.J.; Pellegrini, N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr. Metab. Cardiovasc. Dis. 2005, 15, 316–328. [Google Scholar] [CrossRef]
- Lukienko, P.I.; Mel’nichenko, N.G.; Zverinskii, I.V.; Zabrodskaya, S.V. Antioxidant properties of thiamine. Bull. Exp. Biol. Med. 2000, 130, 874–876. [Google Scholar] [CrossRef] [PubMed]
- ICES. Baltic fisheries assessment working group (WGBFAS). ICES Sci. Rep. 2021, 3, 732. [Google Scholar] [CrossRef]
- Hansson, S.; Karlsson, L.; Ikonen, E.; Christensen, O.; Mitans, A.; Uzars, D.; Petersson, E.; Ragnarsson, B. Stomach analyses of Baltic salmon from 1959–1962 and 1994–1997: Possible relations between diet and yolk-sac-fry mortality (M74). J. Fish Biol. 2001, 58, 1730–1745. [Google Scholar] [CrossRef]
- Jacobson, P.; Gådmark, A.; Huss, M. Population and size-specific distribution of Atlantic salmon Salmo salar in the Baltic Sea over five decades. J. Fish Biol. 2020, 96, 408–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aro, E. A review of fish migration patterns in the Baltic. Rapp. P-v Réun Cons. Int. Explor. Mer. 1989, 190, 72–96. [Google Scholar]
- Salminen, M.; Kuikka, S.; Erkamo, E. Divergence in feeding migration of Baltic salmon (Salmo salar L.); the significance of smolt size. Nordic J. Freshw. Res. 1994, 69, 32–42. [Google Scholar]
- Jacobson, P.; Gårdmark, A.; Östergren, J.; Casini, M.; Huss, M. Size-dependent prey availability affects diet and performance of predatory fish at sea: A case study of Atlantic salmon. Ecosphere 2018, 9, e02081. [Google Scholar] [CrossRef] [Green Version]
- Salminen, M.; Erkamo, E.; Salmi, J. Diet of post-smolt and one-sea-winter Atlantic salmon in the Bothnian Sea, northern Baltic. J. Fish Biol. 2001, 58, 16–35. [Google Scholar] [CrossRef]
- Kallio-Nyberg, I.; Saloniemi, I.; Jutila, E.; Jokikokko, E. Effect of hatchery rearing and environmental factors on the survival, growth and migration of Atlantic salmon in the Baltic Sea. Fish Res. 2011, 109, 285–294. [Google Scholar] [CrossRef]
- Keinänen, M.; Uddström, A.; Mikkonen, J.; Rytilahti, J.; Juntunen, E.-P.; Nikonen, S.; Vuorinen, P.J. The M74 Syndrome of Baltic Salmon: The Monitoring Results from Finnish Rivers Up Until 2007; Riista-ja Kalatalous–Selvityksiä; Riista-ja Kalatalouden Tutkimuslaitos: Helsinki, Finland, 2008; (In Finnish with English abstract). [Google Scholar]
- Keinänen, M.; Tolonen, T.; Ikonen, E.; Parmanne, R.; Tigerstedt, C.; Rytilahti, J.; Soivio, A.; Vuorinen, P.J. Reproduction Disorder of Baltic Salmon—M74; Riista-ja Kalatalouden Tutkimuslaitos, Kalatutkimuksia–Fiskundersökningar; Riista-ja Kalatalouden Tutkimuslaitos: Helsinki, Finland, 2000; (In Finnish with English abstract). [Google Scholar]
- Pönni, J. Silakka: Kalakantojen Tila Vuonna 2017 Sekä Ennuste Vuosille 2018 ja 2019, Silakka, Kilohaili, Turska, Lohi, Siika, Kuha ja Ahven; Raitaniemi, J., Ed.; Luonnonvara-ja Biotalouden Tutkimus: Helsinki, Finland, 2018; pp. 6–16. (In Finnish) [Google Scholar]
- Vuorinen, P.J.; Keinänen, M.; Kiviranta, H.; Koistinen, J.; Kiljunen, M.; Myllylä, T.; Pönni, J.; Peltonen, H.; Verta, M.; Karjalainen, J. Biomagnification of organohalogens in Atlantic salmon (Salmo salar) from its main prey species in three areas of the Baltic Sea. Sci. Total Environ. 2012, 421–422, 129–143. [Google Scholar] [CrossRef]
- Røjbek, M.C.; Tomkiewicz, J.; Jacobsen, C.; Støttrup, J.G. Forage fish quality: Seasonal lipid dynamics of herring (Clupea harengus L.) and sprat (Sprattus sprattus L.) in the Baltic Sea. ICES J. Mar. Sci. 2014, 71, 56–71. [Google Scholar] [CrossRef] [Green Version]
- Vuorinen, P.J.; Parmanne, R.; Vartiainen, T.; Keinänen, M.; Kiviranta, H.; Kotovuori, O.; Halling, F. PCDD, PCDF, PCB and thiamine in Baltic herring (Clupea harengus L.) and sprat [Sprattus sprattus (L.)] as a background to the M74 syndrome of Baltic salmon (Salmo salar L.). ICES J. Mar. Sci. 2002, 59, 480–496. [Google Scholar] [CrossRef]
- Vuorinen, P.J.; Kiviranta, H.; Koistinen, J.; Pöyhönen, O.; Ikonen, E.; Keinänen, M. Organohalogen concentrations and feeding status in Atlantic salmon (Salmo salar L.) of the Baltic Sea during the spawning run. Sci. Total Environ. 2014, 468–469, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Dias, J.; Alvarez, M.J.; Diez, A.; Arzel, J.; Corraze, G.; Bautista, J.M.; Kaushik, S.J. Regulation of hepatic lipogenesis by dietary protein/energy in juvenile European seabass (Dicentrarchus labrax). Aquaculture 1998, 161, 169–186. [Google Scholar] [CrossRef]
- Varghese, S.; Oommen, O.V. Long-term feeding of dietary oils alters lipid metabolism, lipid peroxidation, and antioxidant enzyme activities in a teleost (Anabas testudineus Bloch). Lipids 2000, 35, 757–762. [Google Scholar] [CrossRef] [PubMed]
- Gélineau, A.; Corraze, G.; Boujard, T.; Larroquet, L.; Kaushik, S. Relation between dietary lipid level and voluntary feed intake, growth, nutrient gain, lipid deposition and hepatic lipogenesis in rainbow trout. Reprod. Nutr. Dev. 2001, 41, 487–503. [Google Scholar] [CrossRef] [Green Version]
- Chauhan, A.; Srivastva, N.; Bubber, P. Thiamine deficiency induced dietary disparity promotes oxidative stress and neurodegeneration. Indian J. Clin. Biochem. 2018, 33, 422–428. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine, 4th ed.; Oxford University Press: Oxford, UK, 2007. [Google Scholar]
- Miki, W. Biological functions and activities of animal carotenoids. Pure Appl. Chem. 1991, 63, 141–146. [Google Scholar] [CrossRef]
- Nie, X.P.; Zie, J.; Haeubner, N.; Tallmark, B.; Snoeijs, P. Why Baltic herring and sprat are weak conduits for astaxanthin from zooplankton to piscivorous fish. Limnol. Oceanogr. 2011, 56, 1155–1167. [Google Scholar] [CrossRef]
- Mikolajczyk, S.; Warenik-Bany, M.; Pajurek, M. PCDD/Fs and PCBs in Baltic fish—Recent data, risk for consumers. Mar. Pollut. Bull. 2021, 171, 112763. [Google Scholar] [CrossRef]
- Svendsen, T.C.; Vorkamp, K.; Ronsholdt, B.; Frier, J.O. Retrospective determination of primary feeding areas of Atlantic salmon (Salmo salar) using fingerprinting of chlorinated organic contaminants. ICES J. Mar. Sci. 2008, 65, 921–929. [Google Scholar] [CrossRef] [Green Version]
- Vuori, K.; Kiljunen, M.; Kanerva, M.; Koljonen, M.L.; Nikinmaa, M. Stock-specific variation of trophic position, diet and environmental stress markers in Atlantic salmon Salmo salar during feeding migrations in the Baltic Sea. J. Fish Biol. 2012, 81, 1815–1833. [Google Scholar] [CrossRef] [PubMed]
- Broeg, K.; Zander, S.; Diamant, A.; Korting, W.; Kruner, G.; Paperna, I.; Westernhagen, H.V. The use of fish metabolic, pathological and parasitological indices in pollution monitoring-1. North Sea. Helgol. Mar. Res. 1999, 53, 171–194. [Google Scholar] [CrossRef] [Green Version]
- Whyte, J.J.; Jung, R.E.; Schmitt, C.J.; Tillitt, D.E. Ethoxyresorufin-O-deethylase (EROD) activity in fish as a biomarker of chemical exposure. Crit. Rev. Toxicol. 2000, 30, 347–570. [Google Scholar] [CrossRef]
- Livingstone, D.R. Contaminant-stimulated reactive oxygen species production and oxidative damage in aquatic organisms. Mar. Pollut. Bull. 2001, 42, 656–666. [Google Scholar] [CrossRef]
- Grotto, D.; Maria, L.S.; Valentini, J.; Paniz, C.; Schmitt, G.; Garcia, S.C.; Pomblum, V.J.; Rocha, J.B.T.; Farina, M. Importance of the lipid peroxidation biomarkers and methodological aspects FOR malondialdehyde quantification. Quím Nova. 2009, 32, 169–174. [Google Scholar] [CrossRef] [Green Version]
- Lind, Y.; Huovila, T.; Käkelä, R. A retrospective study of fatty acid composition in Baltic herring (Clupea harengus membras) caught at three locations in the Baltic Sea (1973–2009). ICES J. Mar. Sci. 2018, 75, 330–339. [Google Scholar] [CrossRef]
- Richter, H.; McDermott, J.G. The staining of fish otoliths for age determination. J. Fish Biol. 1990, 36, 773–779. [Google Scholar] [CrossRef]
- Hiilivirta, P.; Ikonen, E.; Lappalainen, J. Comparison of two methods for distinguishing wild from hatchery-reared salmon (Salmo salar Linnaeus, 1758) in the Baltic Sea. ICES J. Mar. Sci. 1998, 55, 981–986. [Google Scholar] [CrossRef]
- Pettersson, A.; Lignell, Å. Astaxanthin deficiency in eggs and fry of Baltic salmon (Salmo salar) with the M74 syndrome. Ambio 1999, 28, 43–47. Available online: http://www.jstor.org/stable/4314847 (accessed on 23 March 2022).
- Vuorinen, P.J.; Rokka, M.; Ritvanen, T.; Käkelä, R.; Nikonen, S.; Pakarinen, T.; Keinänen, M. Changes in thiamine concentrations, fatty acid composition, and some other lipid-related biochemical indices in Baltic Sea Atlantic salmon (Salmo salar) during the spawning run and pre-spawning fasting. Helgol. Mar. Res. 2020, 74, 10. [Google Scholar] [CrossRef]
- Kvalheim, O.M.; Karstang, T.V. A general-purpose program for multivariate data-analysis. Chemometr. Intell. Lab. 1987, 2, 235–237. [Google Scholar] [CrossRef]
- Wold, S.; Sjöström, M. SIMCA: A method for analyzing chemical data in terms of similarity and analogy. In Chemometrics: Theory and Application; Kowalski, B., Ed.; American Chemical Society: Washington, DC, USA, 1977; pp. 243–282. [Google Scholar]
- SAS Inc. SAS/STAT 9.2 User’s Guide; SAS Institute Inc.: Cary, NC, USA, 2008. [Google Scholar]
- Salati, L.M.; Amir-Ahmady, B. Dietary regulation of expression of glucose-6-phosphate dehydrogenase. Annu. Rev. Nutr. 2001, 21, 121–140. [Google Scholar] [CrossRef] [PubMed]
- Casini, M.; Cardinale, M.; Arrhenius, F. Feeding preferences of herring (Clupea harengus) and sprat (Sprattus sprattus) in the southern Baltic Sea. ICES J. Mar. Sci. 2004, 61, 1267–1277. [Google Scholar] [CrossRef]
- Arrhenius, F.; Hansson, S. Food consumption of larval, young and adult herring and sprat in the Baltic Sea. Mar. Ecol. Progr. Ser. 1993, 96, 125–137. [Google Scholar] [CrossRef]
- Möllmann, C.; Kornilovs, G.; Fetter, M.; Köster, F.W. Feeding ecology of central Baltic Sea herring and sprat. J. Fish Biol. 2004, 65, 1563–1581. [Google Scholar] [CrossRef]
- Möllmann, C.; Kornilovs, G.; Fetter, M.; Köster, F.W. Climate, zooplankton, and pelagic fish growth in the central Baltic Sea. ICES J. Mar. Sci. 2005, 62, 1270–1280. [Google Scholar] [CrossRef]
- Happel, A.; Jonas, J.L.; McKenna, P.R.; Rinchard, J.; He, J.X.; Czesny, S.J. Spatial variability of lake trout diets in Lakes Huron and Michigan revealed by stomach content and fatty acid profiles. Can. J. Fish Aquat. Sci. 2018, 75, 95–105. [Google Scholar] [CrossRef]
- Kanerva, M.; Vehmas, A.; Nikinmaa, M.; Vuori, K.A. Spatial variation in transcript and protein abundance of Atlantic salmon during feeding migration in the Baltic Sea. Environ. Sci. Technol. 2014, 48, 13969–13977. [Google Scholar] [CrossRef]
- Bilska-Wilkosz, A.; Iciek, M.; Górny, M. Chemistry and Biochemistry Aspects of the 4-Hydroxy-2,3-trans-nonenal. Biomolecules 2022, 12, 145. [Google Scholar] [CrossRef]
- Guichardant, M.; Chantegrel, B.; Deshayes, C.; Doutheau, A.; Moliere, P.; Lagarde, M. Specific markers of lipid peroxidation issued from n−3 and n−6 fatty acids. Biochem. Soc. Trans. 2004, 32, 139–140. [Google Scholar] [CrossRef] [PubMed]
- Catalá, A. Five decades with polyunsaturated fatty acids: Chemical synthesis, enzymatic formation, lipid peroxidation and its biological effects. J. Lipids 2013, 2013, 710290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koski, P.; Soivio, A.; Hartikainen, K.; Hirvi, T.; Myllylä, T. M74 syndrome and thiamine in salmon broodfish and offspring. Boreal. Environ. Res. 2001, 6, 79–92. [Google Scholar]
- Salminen, M. Marine Survival of Atlantic Salmon in the Baltic Sea; NPAFC Technical Report No. 4; North Pacific Anadromous Fish Commission: Vancouver, BC, Canada, 2002; pp. 27–29. [Google Scholar]
- Welch, D.W.; Futia, M.H.; Rinchard, J.; Teffer, A.K.; Miller, K.M.; Hinch, S.G.; Honeyfield, D.C. Thiamine levels in muscle and eggs of adult Pacific salmon from the Fraser River, British Columbia. J. Aquat. Anim. Health 2018, 30, 191–200. [Google Scholar] [CrossRef]
- Manzetti, S.; Zhang, J.; van der Spoel, D. Thiamin function, metabolism, uptake, and transport. Biochemistry 2014, 53, 821–835. [Google Scholar] [CrossRef]
- Graham, C.; Pakhomov, E.A.; Hunt, B.P.V. A salmon diet database for the North Pacific Ocean. Sci. Data 2020, 7, 332. [Google Scholar] [CrossRef]
- McCormick, D.B. Niacin, riboflavin and thiamin. In Biochemical and Physiological Aspects of Human Nutrition; Stipanuk, M.H., Ed.; Saunders: Philadelphia, PA, USA; Elsevier: Philadelphia, PA, USA, 2000; pp. 458–482. [Google Scholar]
- Alvarez, M.J.; Lopez-Bote, C.J.; Diez, A.; Corraze, G.; Arzel, J.; Dias, J.; Kaushik, S.J.; Bautista, J.M. Dietary fish oil and digestible protein modify susceptibility to lipid peroxidation in the muscle of rainbow trout (Oncorhynchus mykiss) and sea bass (Dicentrarchus labrax). Br. J. Nutr. 1998, 80, 281–289. [Google Scholar] [CrossRef] [Green Version]
- Østbye, T.K.; Kjær, M.A.; Rørå, A.M.B.; Torstensen, B.; Ruyter, B. High n−3 HUFA levels in the diet of Atlantic salmon affect muscle and mitochondrial membrane lipids and their susceptibility to oxidative stress. Aquacult. Nutr. 2011, 17, 177–190. [Google Scholar] [CrossRef]
- Snoeijs, P.; Häubner, N. Astaxanthin dynamics in Baltic Sea mesozooplankton communities. J. Sea Res. 2014, 85, 131–143. [Google Scholar] [CrossRef]
- Brett, J.R. Environmental factors and growth. In Fish Physiology, Bioenergetics and Growth; Hoar, W.S., Randall, D.J., Brett, J.R., Eds.; Academic Press: New York, NY, USA; San Francisco, CA, USA; London, UK, 1979; Volume VIII, pp. 599–675. [Google Scholar]
- Salo, S.; Verta, M.; Malve, O.; Korhonen, M.; Lehtoranta, J.; Kiviranta, H.; Isosaari, P.; Ruokojärvi, P.; Koistinen, J.; Vartiainen, T. Contamination of River Kymijoki sediments with polychlorinated dibenzo-p-dioxins, dibenzofurans and mercury and their transport to the Gulf of Finland in the Baltic Sea. Chemosphere 2008, 73, 1675–1683. [Google Scholar] [CrossRef]


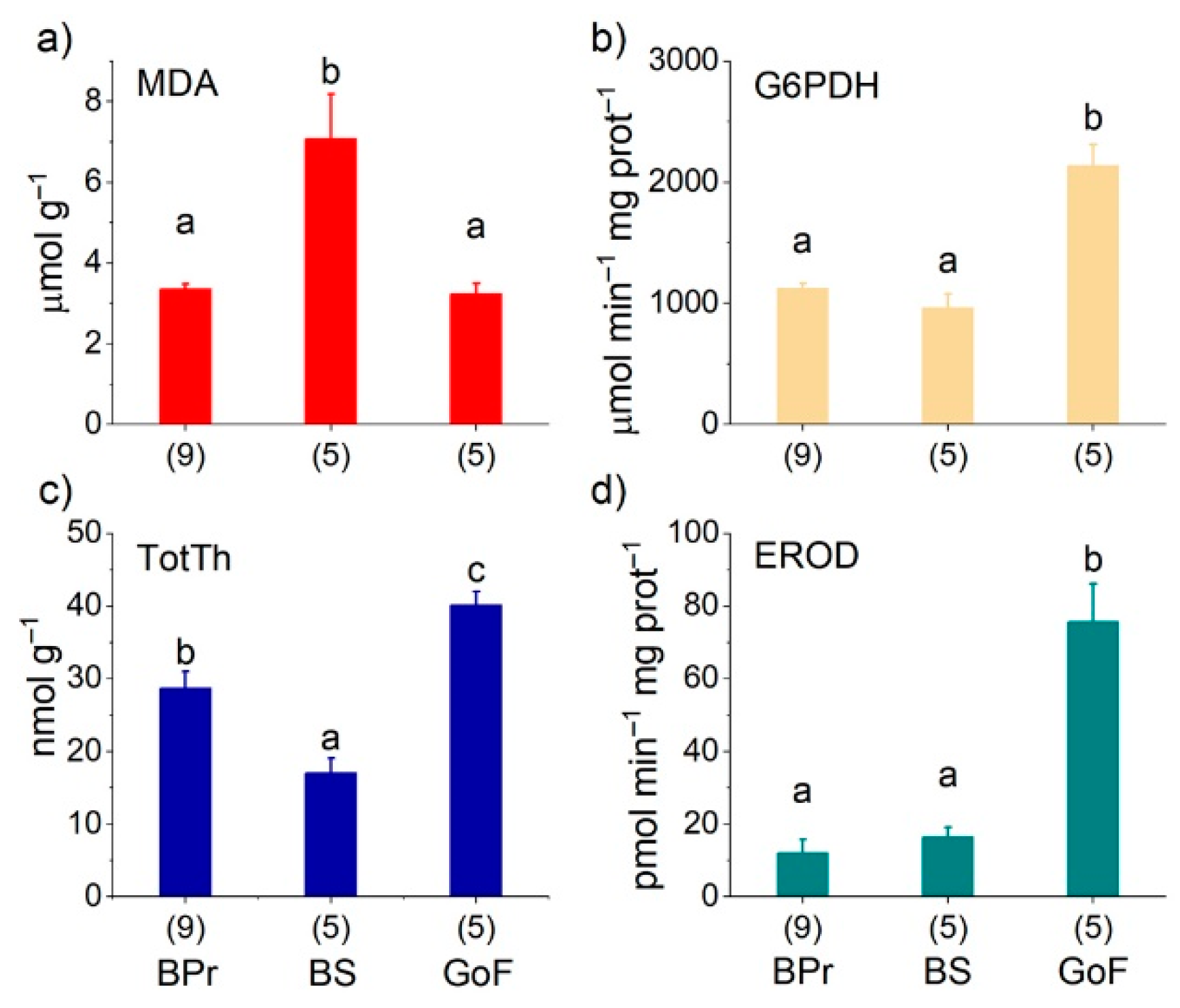



| TPP nmol g−1 | TMP nmol g−1 | THIAM nmol g−1 | TotTh nmol g−1 | N | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Muscle | |||||||||||||
| BPr | 4.530 | ± | 0.366 a | 2.352 | ± | 0.286 b | 0.322 | ± | 0.149 a | 7.204 | ± | 0.549 | 9 |
| BS | 6.581 | ± | 1.032 b | 1.196 | ± | 0.226 a | 0.075 | ± | 0.005 a | 7.851 | ± | 1.255 | 5 |
| GoF | 3.302 | ± | 0.439 a | 2.626 | ± | 0.241 b | 1.028 | ± | 0.177 b | 6.956 | ± | 0.528 | 10 |
| Liver | |||||||||||||
| BPr | 21.926 | ± | 1.676 b | 6.694 | ± | 0.698 b | 0.129 | ± | 0.053 a | 28.748 | ± | 2.264 b | 9 |
| BS | 12.236 | ± | 1.519 a | 4.220 | ± | 0.475 a | 0.557 | ± | 0.062 b | 17.014 | ± | 2.024 a | 5 |
| GoF | 31.914 | ± | 1.942 c | 8.069 | ± | 0.541 b | 0.137 | ± | 0.091 a | 40.120 | ± | 1.892 c | 5 |
| TPP | TMP | THIAM | TotTh | TPP% | THIAM% | ||
|---|---|---|---|---|---|---|---|
| Salmon | Body lipid | 0.458 | −0.583 | −0.662 | −0.203 | 0.680 | −0.664 |
| 0.024 | 0.003 | 0.0004 | 0.341 | 0.0003 | 0.0004 | ||
| SFA | 0.378 | −0.595 | −0.656 | −0.277 | 0.624 | −0.624 | |
| 0.069 | 0.002 | 0.001 | 0.190 | 0.001 | 0.001 | ||
| MUFA | 0.445 | −0.551 | −0.595 | −0.171 | 0.628 | −0.584 | |
| 0.029 | 0.005 | 0.002 | 0.425 | 0.001 | 0.003 | ||
| PUFA | 0.503 | −0.553 | −0.644 | −0.140 | 0.706 | −0.680 | |
| 0.012 | 0.005 | 0.001 | 0.515 | 0.0001 | 0.0003 | ||
| n−3 PUFA | 0.490 | −0.541 | −0.635 | −0.140 | 0.690 | −0.671 | |
| 0.015 | 0.006 | 0.001 | 0.514 | 0.0002 | 0.0003 | ||
| n−6 PUFA | 0.574 | −0.611 | −0.673 | −0.125 | 0.784 | −0.715 | |
| 0.003 | 0.002 | 0.0003 | 0.562 | <0.0001 | <0.0001 | ||
| Sprat | Body lipid | 0.453 | 0.447 | −0.488 | −0.193 | 0.754 | −0.755 |
| 0.068 | 0.072 | 0.047 | 0.458 | 0.001 | 0.001 | ||
| Herring | Body lipid | 0.584 | 0.599 | −0.339 | −0.172 | 0.635 | −0.733 |
| 0.003 | 0.002 | 0.106 | 0.420 | 0.001 | <0.0001 |
| G6PDH | MDA | TotTh | |
|---|---|---|---|
| Lipid | −0.625 | 0.319 | −0.537 |
| 0.004 | 0.183 | 0.018 | |
| SFA | −0.440 | 0.106 | −0.299 |
| 0.059 | 0.667 | 0.213 | |
| MUFA | −0.480 | 0.216 | −0.448 |
| 0.038 | 0.374 | 0.054 | |
| n−3 PUFA | −0.719 | 0.526 | −0.681 |
| 0.001 | 0.021 | 0.001 | |
| n−6 PUFA | −0.774 | 0.594 | −0.758 |
| 0.0001 | 0.007 | 0.0002 | |
| PUFA | −0.732 | 0.534 | −0.695 |
| 0.0004 | 0.019 | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keinänen, M.; Nikonen, S.; Käkelä, R.; Ritvanen, T.; Rokka, M.; Myllylä, T.; Pönni, J.; Vuorinen, P.J. High Lipid Content of Prey Fish and n−3 PUFA Peroxidation Impair the Thiamine Status of Feeding-Migrating Atlantic Salmon (Salmo salar) and Is Reflected in Hepatic Biochemical Indices. Biomolecules 2022, 12, 526. https://doi.org/10.3390/biom12040526
Keinänen M, Nikonen S, Käkelä R, Ritvanen T, Rokka M, Myllylä T, Pönni J, Vuorinen PJ. High Lipid Content of Prey Fish and n−3 PUFA Peroxidation Impair the Thiamine Status of Feeding-Migrating Atlantic Salmon (Salmo salar) and Is Reflected in Hepatic Biochemical Indices. Biomolecules. 2022; 12(4):526. https://doi.org/10.3390/biom12040526
Chicago/Turabian StyleKeinänen, Marja, Soili Nikonen, Reijo Käkelä, Tiina Ritvanen, Mervi Rokka, Timo Myllylä, Jukka Pönni, and Pekka J. Vuorinen. 2022. "High Lipid Content of Prey Fish and n−3 PUFA Peroxidation Impair the Thiamine Status of Feeding-Migrating Atlantic Salmon (Salmo salar) and Is Reflected in Hepatic Biochemical Indices" Biomolecules 12, no. 4: 526. https://doi.org/10.3390/biom12040526
APA StyleKeinänen, M., Nikonen, S., Käkelä, R., Ritvanen, T., Rokka, M., Myllylä, T., Pönni, J., & Vuorinen, P. J. (2022). High Lipid Content of Prey Fish and n−3 PUFA Peroxidation Impair the Thiamine Status of Feeding-Migrating Atlantic Salmon (Salmo salar) and Is Reflected in Hepatic Biochemical Indices. Biomolecules, 12(4), 526. https://doi.org/10.3390/biom12040526






