Exacerbation of Elastase-Induced Emphysema via Increased Oxidative Stress in Metallothionein-Knockout Mice
Abstract
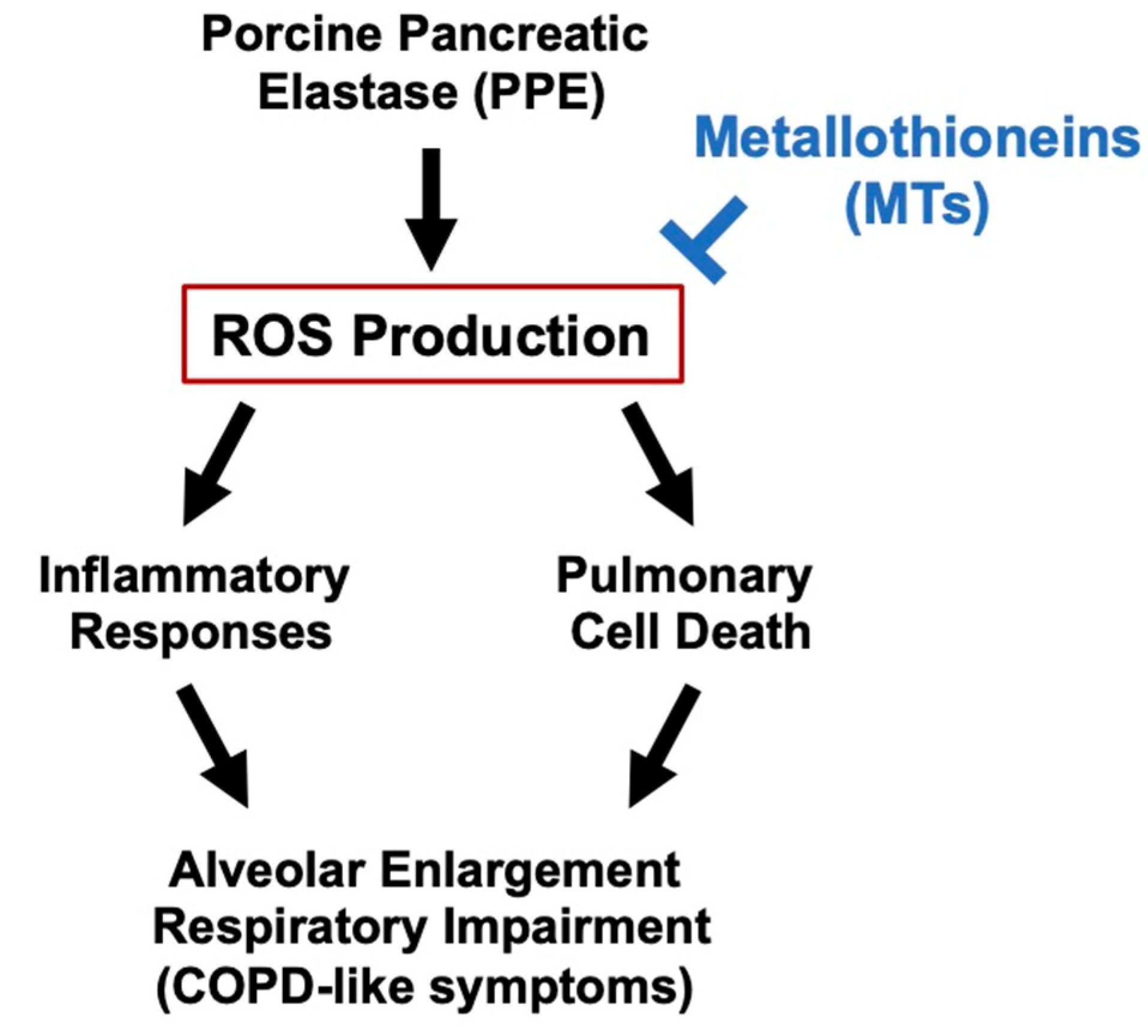
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Animals
2.2. Preparation of BALF
2.3. Measurement of ROS by In Vivo Imaging Analysis
2.4. Histological and Immunohistochemical Analyses
2.5. Real-Time Reverse Transcription (RT) Polymerase Chain Reaction (PCR) Analysis
2.6. Treatment of Mice with PPE
2.7. Analyses of Lung Mechanics and Respiratory Functions
2.8. Statistical Analysis
3. Results
3.1. Expression of Metallothionein in Lung Tissues
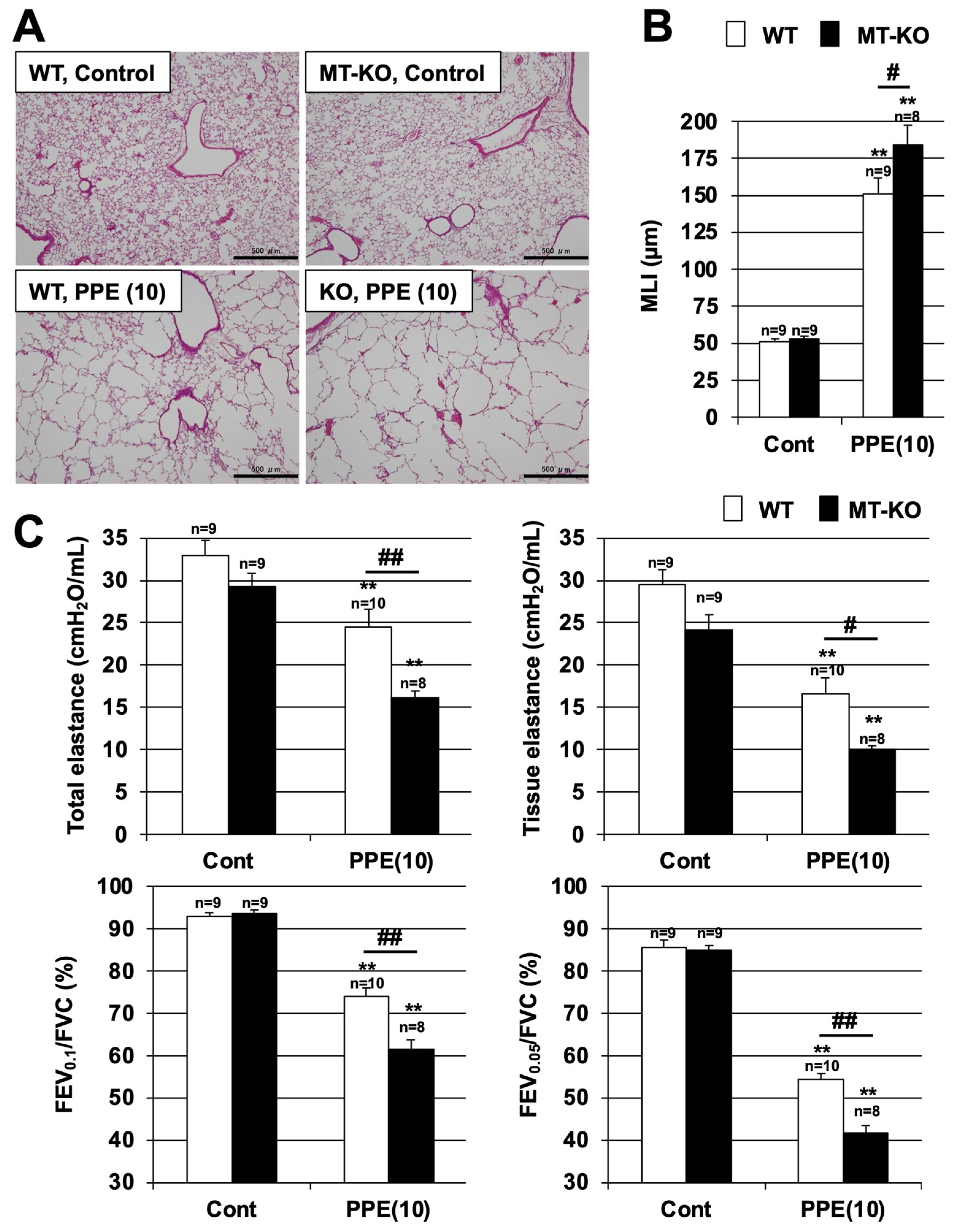
3.2. Effect of Metallothionein on PPE-Induced Lung Injury
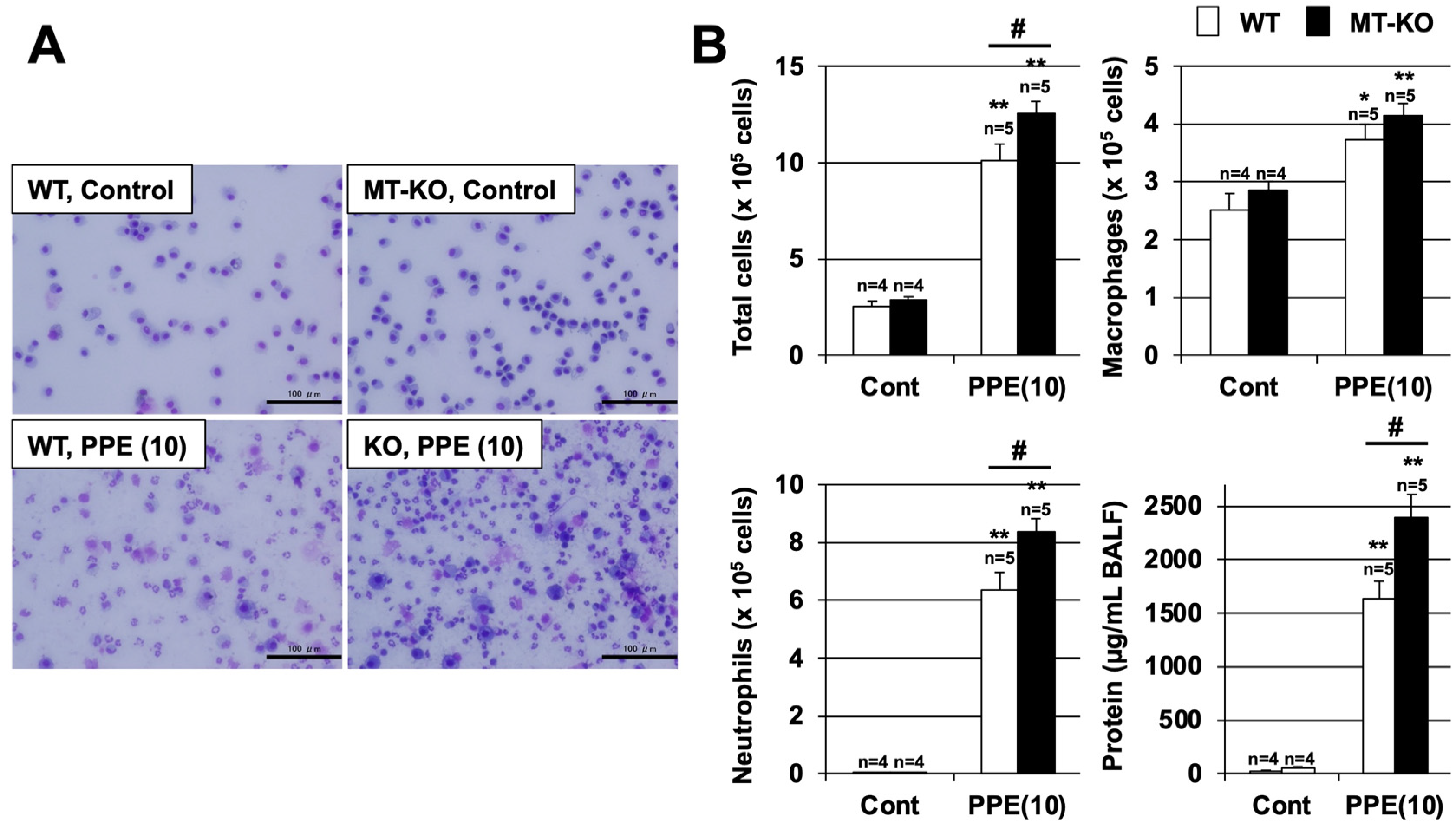
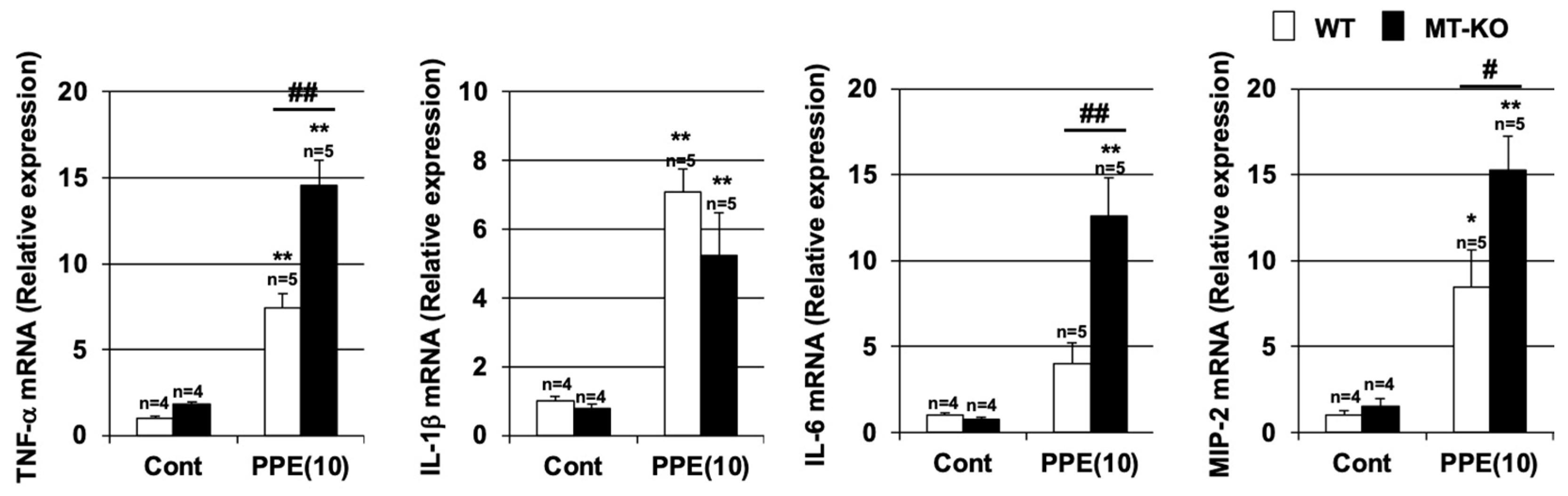
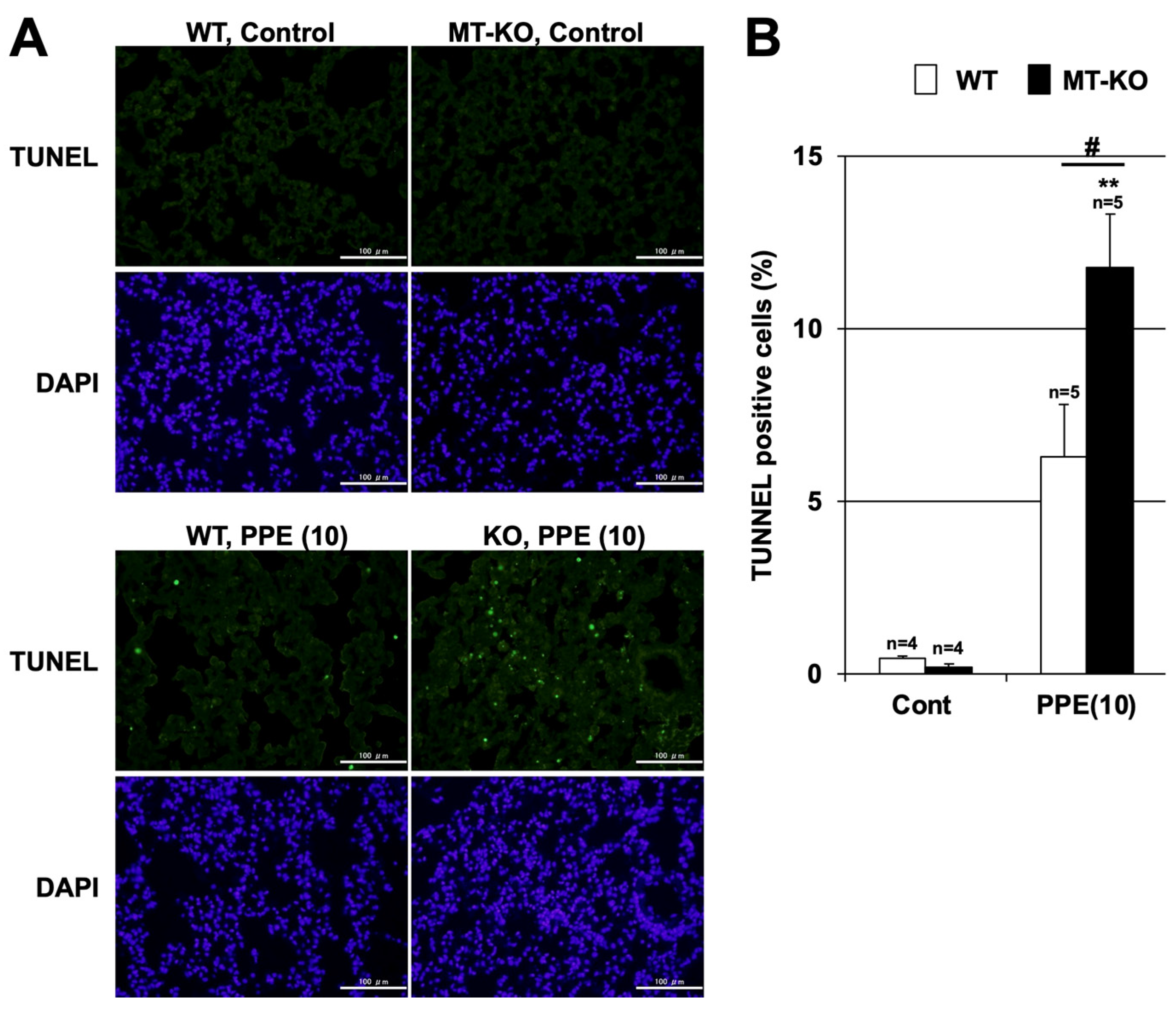
3.3. Effect of Metallothionein on the PPE-Induced Pulmonary Inflammatory Response and Pulmonary Cell Death
3.4. Effect of Metallothionein on PPE-Induced ROS Production
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barnes, P.J.; Stockley, R.A. COPD: Current therapeutic interventions and future approaches. Eur. Respir. J. 2005, 25, 1084–1106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vogelmeier, C.F.; Criner, G.J.; Martinez, F.J.; Anzueto, A.; Barnes, P.J.; Bourbeau, J.; Celli, B.R.; Chen, R.; Decramer, M.; Fabbri, L.M.; et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report. GOLD Executive Summary. Am. J. Respir. Crit. Care Med. 2017, 195, 557–582. [Google Scholar] [CrossRef] [PubMed]
- Alsaeedi, A.; Sin, D.D.; McAlister, F.A. The effects of inhaled corticosteroids in chronic obstructive pulmonary disease: A systematic review of randomized placebo-controlled trials. Am. J. Med. 2002, 113, 59–65. [Google Scholar] [CrossRef]
- Mak, J.C. Pathogenesis of COPD. Part II. Oxidative-antioxidative imbalance. Int. J. Tuberc. Lung Dis. 2008, 12, 368–374. [Google Scholar] [PubMed]
- Antus, B.; Harnasi, G.; Drozdovszky, O.; Barta, I. Monitoring oxidative stress during chronic obstructive pulmonary disease exacerbations using malondialdehyde. Respirology 2014, 19, 74–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drost, E.M.; Skwarski, K.M.; Sauleda, J.; Soler, N.; Roca, J.; Agusti, A.; MacNee, W. Oxidative stress and airway inflammation in severe exacerbations of COPD. Thorax 2005, 60, 293–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drummond, G.R.; Selemidis, S.; Griendling, K.K.; Sobey, C.G. Combating oxidative stress in vascular disease: NADPH oxidases as therapeutic targets. Nat. Rev. Drug. Discov. 2011, 10, 453–471. [Google Scholar] [CrossRef] [Green Version]
- Boukhenouna, S.; Wilson, M.A.; Bahmed, K.; Kosmider, B. Reactive Oxygen Species in Chronic Obstructive Pulmonary Disease. Oxid. Med. Cell. Longev. 2018, 2018, 5730395. [Google Scholar] [CrossRef]
- Tanaka, K.-I.; Tanaka, Y.; Miyazaki, Y.; Namba, T.; Sato, K.; Aoshiba, K.; Azuma, A.; Mizushima, T. Therapeutic Effect of Lecithinized Superoxide Dismutase on Pulmonary Emphysema. J. Pharmacol. Exp. Ther. 2011, 338, 810–818. [Google Scholar] [CrossRef]
- Tanaka, K.; Ishihara, T.; Sugizaki, T.; Kobayashi, D.; Yamashita, Y.; Tahara, K.; Yamakawa, N.; Iijima, K.; Mogushi, K.; Tanaka, H.; et al. Mepenzolate bromide displays beneficial effects in a mouse model of chronic obstructive pulmonary disease. Nat. Commun. 2013, 4, 2686. [Google Scholar] [CrossRef] [Green Version]
- Davis, S.R.; Cousins, R.J. Metallothionein expression in animals: A physiological perspective on function. J. Nutr. 2000, 130, 1085–1088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schanz, M.; Schaaf, L.; Dippon, J.; Biegger, D.; Fritz, P.; Alscher, M.D.; Kimmel, M. Renal effects of metallothionein induction by zinc in vitro and in vivo. BMC Nephrol. 2017, 18, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruttkay-Nedecky, B.; Nejdl, L.; Gumulec, J.; Zitka, O.; Masarik, M.; Eckschlager, T.; Stiborova, M.; Adam, V.; Kizek, R. The role of metallothionein in oxidative stress. Int. J. Mol. Sci. 2013, 14, 6044–6066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lian, Y.; Zhao, J.; Wang, Y.M.; Zhao, J.; Peng, S.Q. Metallothionein protects against isoniazid-induced liver injury through the inhibition of CYP2E1-dependent oxidative and nitrosative impairment in mice. Food Chem. Toxicol. 2017, 102, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zhou, S.; Kong, L.; Chen, J.; Feng, W.; Cai, J.; Miao, L.; Tan, Y. Metallothionein deletion exacerbates intermittent hypoxia-induced renal injury in mice. Toxicol. Lett. 2015, 232, 340–348. [Google Scholar] [CrossRef]
- Lv, Y.; Zhang, B.; Xing, G.; Wang, F.; Hu, Z. Protective effect of naringenin against acetaminophen-induced acute liver injury in metallothionein (MT)-null mice. Food Funct. 2013, 4, 297–302. [Google Scholar] [CrossRef]
- Ren, M.; Wang, Y.M.; Zhao, J.; Zhao, J.; Zhao, Z.M.; Zhang, T.F.; He, J.; Ren, S.P.; Peng, S.Q. Metallothioneins attenuate paraquat-induced acute lung injury in mice through the mechanisms of anti-oxidation and anti-apoptosis. Food Chem. Toxicol. 2014, 73, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Wesselkamper, S.C.; McDowell, S.A.; Medvedovic, M.; Dalton, T.P.; Deshmukh, H.S.; Sartor, M.A.; Case, L.M.; Henning, L.N.; Borchers, M.T.; Tomlinson, C.R.; et al. The role of metallothionein in the pathogenesis of acute lung injury. Am. J. Respir. Cell Mol. Biol. 2006, 34, 73–82. [Google Scholar] [CrossRef] [Green Version]
- Fuji, Y.; Matsura, T.; Kai, M.; Kawasaki, H.; Yamada, K. Protection by polaprezinc, an anti-ulcer drug, against indomethacin-induced apoptosis in rat gastric mucosal cells. Jpn. J. Pharmacol. 2000, 84, 63–70. [Google Scholar] [CrossRef] [Green Version]
- Kimura, K.; Nakano, Y.; Sugizaki, T.; Shimoda, M.; Kobayashi, N.; Kawahara, M.; Tanaka, K.I. Protective effect of polaprezinc on cadmium-induced injury of lung epithelium. Metallomics 2019, 11, 1310–1320. [Google Scholar] [CrossRef]
- Masters, B.A.; Kelly, E.J.; Quaife, C.J.; Brinster, R.L.; Palmiter, R.D. Targeted disruption of metallothionein I and II genes increases sensitivity to cadmium. Proc. Natl. Acad. Sci. USA 1994, 91, 584–588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, K.I.; Tamura, F.; Sugizaki, T.; Kawahara, M.; Kuba, K.; Imai, Y.; Mizushima, T. Evaluation of Lecithinized Superoxide Dismutase for the Prevention of Acute Respiratory Distress Syndrome in Animal Models. Am. J. Respir. Cell Mol. Biol. 2017, 56, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.I.; Kubota, M.; Shimoda, M.; Hayase, T.; Miyaguchi, M.; Kobayashi, N.; Ikeda, M.; Ishima, Y.; Kawahara, M. Thioredoxin-albumin fusion protein prevents urban aerosol-induced lung injury via suppressing oxidative stress-related neutrophil extracellular trap formation. Environ. Pollut. 2021, 268, 115787. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, Y.; Tanaka, K.I.; Yamakawa, N.; Asano, T.; Kanda, Y.; Takafuji, A.; Kawahara, M.; Takenaga, M.; Fukunishi, Y.; Mizushima, T. Chemical modification-mediated optimisation of bronchodilatory activity of mepenzolate, a muscarinic receptor antagonist with anti-inflammatory activity. Bioorg. Med. Chem. 2019, 27, 3339–3346. [Google Scholar] [CrossRef] [PubMed]
- Sugizaki, T.; Tanaka, K.I.; Asano, T.; Kobayashi, D.; Hino, Y.; Takafuji, A.; Shimoda, M.; Mogushi, K.; Kawahara, M.; Mizushima, T. Idebenone has preventative and therapeutic effects on pulmonary fibrosis via preferential suppression of fibroblast activity. Cell. Death. Discov. 2019, 5, 146. [Google Scholar] [CrossRef]
- Sarma, J.V.; Ward, P.A. Oxidants and redox signaling in acute lung injury. Compr. Physiol. 2011, 1, 1365–1381. [Google Scholar] [CrossRef]
- Tasaka, S.; Amaya, F.; Hashimoto, S.; Ishizaka, A. Roles of oxidants and redox signaling in the pathogenesis of acute respiratory distress syndrome. Antioxid. Redox. Sign. 2008, 10, 739–753. [Google Scholar] [CrossRef]
- Checa, J.; Aran, J.M. Airway Redox Homeostasis and Inflammation Gone Awry: From Molecular Pathogenesis to Emerging Therapeutics in Respiratory Pathology. Int. J. Mol. Sci. 2020, 21, 9317. [Google Scholar] [CrossRef]
- Kadota, Y.; Aki, Y.; Toriuchi, Y.; Mizuno, Y.; Kawakami, T.; Sato, M.; Suzuki, S. Deficiency of metallothionein-1 and -2 genes shortens the lifespan of the 129/Sv mouse strain. Exp. Gerontol. 2015, 66, 21–24. [Google Scholar] [CrossRef]
- Swindell, W.R. Metallothionein and the biology of aging. Ageing Res. Rev. 2011, 10, 132–145. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanaka, K.I.; Shiota, S.; Sakakibara, O.; Shimoda, M.; Takafuji, A.; Takabatake, M.; Kadota, Y.; Kawakami, T.; Suzuki, S.; Kawahara, M. Exacerbation of Elastase-Induced Emphysema via Increased Oxidative Stress in Metallothionein-Knockout Mice. Biomolecules 2022, 12, 583. https://doi.org/10.3390/biom12040583
Tanaka KI, Shiota S, Sakakibara O, Shimoda M, Takafuji A, Takabatake M, Kadota Y, Kawakami T, Suzuki S, Kawahara M. Exacerbation of Elastase-Induced Emphysema via Increased Oxidative Stress in Metallothionein-Knockout Mice. Biomolecules. 2022; 12(4):583. https://doi.org/10.3390/biom12040583
Chicago/Turabian StyleTanaka, Ken Ichiro, Sachie Shiota, Okina Sakakibara, Mikako Shimoda, Ayaka Takafuji, Misaki Takabatake, Yoshito Kadota, Takashige Kawakami, Shinya Suzuki, and Masahiro Kawahara. 2022. "Exacerbation of Elastase-Induced Emphysema via Increased Oxidative Stress in Metallothionein-Knockout Mice" Biomolecules 12, no. 4: 583. https://doi.org/10.3390/biom12040583
APA StyleTanaka, K. I., Shiota, S., Sakakibara, O., Shimoda, M., Takafuji, A., Takabatake, M., Kadota, Y., Kawakami, T., Suzuki, S., & Kawahara, M. (2022). Exacerbation of Elastase-Induced Emphysema via Increased Oxidative Stress in Metallothionein-Knockout Mice. Biomolecules, 12(4), 583. https://doi.org/10.3390/biom12040583





