3D Printed Cell Culture Chamber for Testing the Effect of Pump-Based Chronic Drug Delivery on Inner Ear Tissue
Abstract
1. Introduction
2. Materials and Methods
2.1. NOC Design and Manufacture
2.2. NOC Preparation for Cell Cultivation
2.3. Setup for Pump-Based Drug Delivery
2.4. Validation
2.5. Preparation of Spiral Ganglion Cells
2.6. Cultivation of SGEs
2.7. Immunocytochemistry
2.8. SGE Analysis
2.9. Statistical Analysis
3. Results
3.1. NOC Manufacture
3.2. Cultivation of SGEs in the NOCs
3.3. SGE Analysis
3.4. Neurite Outgrowth Analysis
3.4.1. Number of Neurites
3.4.2. Guided Neurite Outgrowth
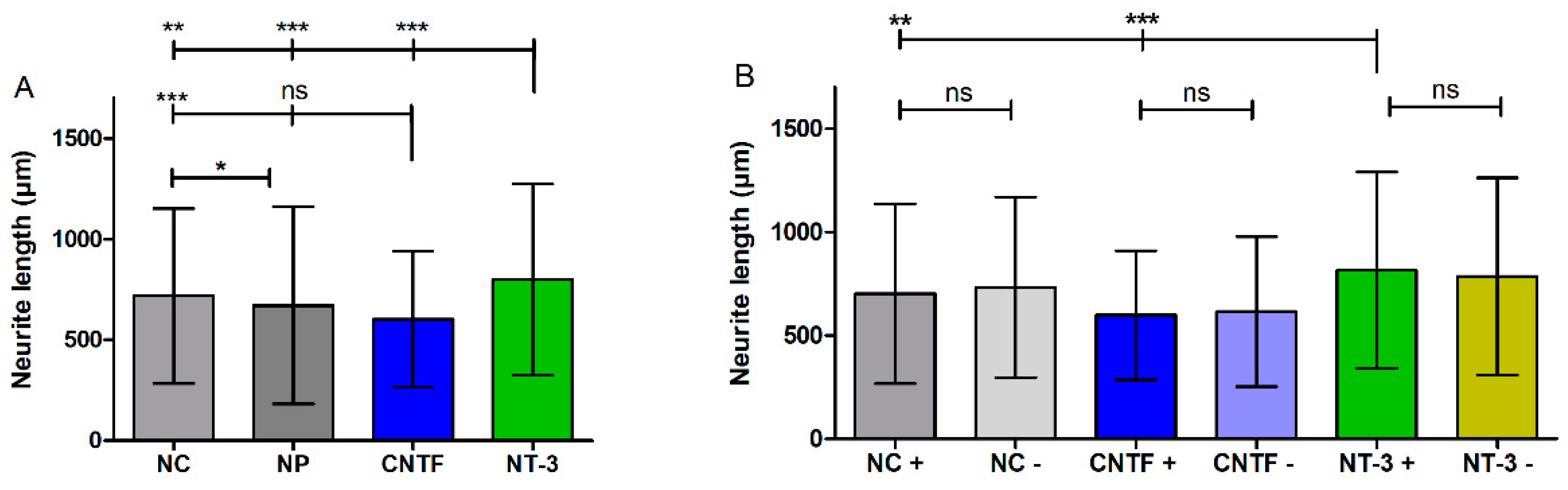
3.4.3. Neurite Length
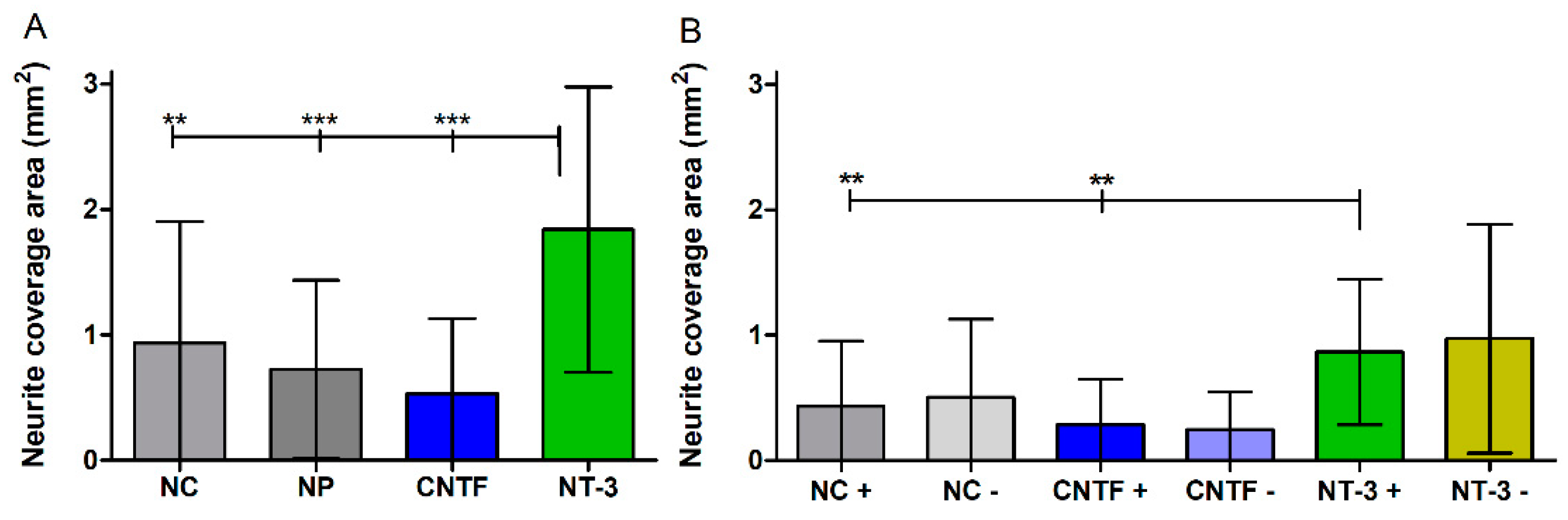
3.4.4. Neurite Coverage Area
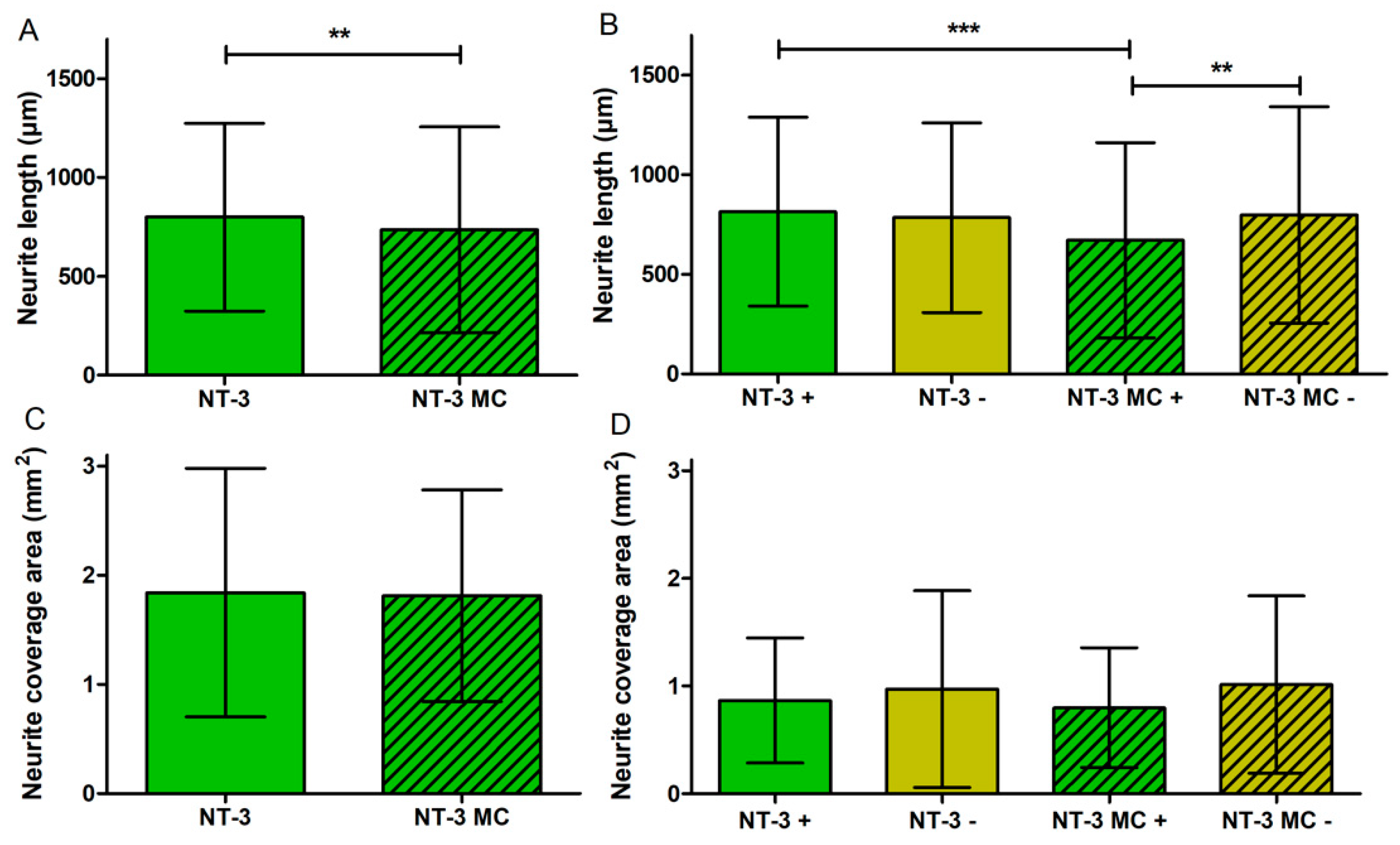
3.5. Influence of Medium Change on NOC Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lenarz, T. Cochlear Implant—State of the Art. Laryngorhinootologie 2017, 96, S123–S151. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.S.; Dorman, M.F. Cochlear implants: A remarkable past and a brilliant future. Hear. Res. 2008, 242, 3–21. [Google Scholar] [CrossRef] [PubMed]
- Plontke, S.K.; Götze, G.; Rahne, T.; Liebau, A. Intracochlear drug delivery in combination with cochlear implants: Current aspects. HNO 2017, 65, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Nyberg, S.; Abbott, N.J.; Shi, X.; Steyger, P.S.; Dabdoub, A. Delivery of therapeutics to the inner ear: The challenge of the blood-labyrinth barrier. Sci. Transl. Med. 2019, 11, eaao0935. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Li, S.K. Inner ear drug delivery: Recent advances, challenges, and perspective. Eur. J. Pharm. Sci. Off. J. Eur. Fed. 2019, 126, 82–92. [Google Scholar] [CrossRef]
- Prenzler, N.K.; Salcher, R.; Timm, M.; Gaertner, L.; Lenarz, T.; Warnecke, A. Intracochlear administration of steroids with a catheter during human cochlear implantation: A safety and feasibility study. Drug Deliv. Transl. Res. 2018, 8, 1191–1199. [Google Scholar] [CrossRef]
- Meyer, H.; Stöver, T.; Fouchet, F.; Bastiat, G.; Saulnier, P.; Bäumer, W.; Lenarz, T.; Scheper, V. Lipidic nanocapsule drug delivery: Neuronal protection for cochlear implant optimization. Int. J. Nanomed. 2012, 7, 2449–2464. [Google Scholar] [CrossRef]
- Brown, J.N.; Miller, J.M.; Altschuler, R.A.; Nuttall, A.L. Osmotic pump implant for chronic infusion of drugs into the inner ear. Hear. Res. 1993, 70, 167–172. [Google Scholar] [CrossRef]
- Borenstein, J.T. Intracochlear drug delivery systems. Expert Opin. Drug Deliv. 2011, 8, 1161–1174. [Google Scholar] [CrossRef]
- Homsy, A.; Laux, E.; Brossard, J.; Whitlow, H.J.; Roccio, M.; Hahnewald, S.; Senn, P.; MistrÍk, P.; Hessler, R.; Melchionna, T.; et al. Fine control of drug delivery for cochlear implant applications. Hear. Balanc. Commun. 2015, 13, 153–159. [Google Scholar] [CrossRef]
- Paasche, G.; Gibson, P.; Averbeck, T.; Becker, H.; Lenarz, T.; Stöver, T. Technical report: Modification of a cochlear implant electrode for drug delivery to the inner ear. Otol. Neurotol. 2003, 24, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Scheper, V.; Hessler, R.; Hütten, M.; Wilk, M.; Jolly, C.; Lenarz, T.; Paasche, G. Local inner ear application of dexamethasone in cochlear implant models is safe for auditory neurons and increases the neuroprotective effect of chronic electrical stimulation. PLoS ONE 2017, 12, e0183820. [Google Scholar] [CrossRef] [PubMed]
- Scheper, V.; Paasche, G.; Miller, J.M.; Warnecke, A.; Berkingali, N.; Lenarz, T.; Stöver, T. Effects of delayed treatment with combined GDNF and continuous electrical stimulation on spiral ganglion cell survival in deafened guinea pigs. J. Neurosci. Res. 2009, 87, 1389–1399. [Google Scholar] [CrossRef] [PubMed]
- Bas, E.; Anwar, M.R.; Goncalves, S.; Dinh, C.T.; Bracho, O.R.; Chiossone, J.A.; Van De Water, T.R. Laminin-coated electrodes improve cochlear implant function and post-insertion neuronal survival. Neuroscience 2019, 410, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Edin, F.; Jin, Z.; Alexsson, A.; Gudjonsson, O.; Liu, W.; Rask-Andersen, H.; Karlsson, M.; Li, H. Strategy towards independent electrical stimulation from cochlear implants: Guided auditory neuron growth on topographically modified nanocrystalline diamond. Acta Biomater. 2016, 31, 211–220. [Google Scholar] [CrossRef]
- Kikkawa, Y.S.; Nakagawa, T.; Ying, L.; Tabata, Y.; Tsubouchi, H.; Ido, A.; Ito, J. Growth factor-eluting cochlear implant electrode: Impact on residual auditory function, insertional trauma, and fibrosis. J. Transl. Med. 2014, 12, 280. [Google Scholar] [CrossRef]
- Scheper, V.; Hoffmann, A.; Gepp, M.M.; Schulz, A.; Hamm, A.; Pannier, C.; Hubka, P.; Lenarz, T.; Schwieger, J. Stem cell based drug delivery for protection of auditory neurons in a guinea pig model of cochlear implantation. Front. Cell. Neurosci. 2019, 13, 177. [Google Scholar] [CrossRef]
- Liu, Y.; Jolly, C.; Braun, S.; Janssen, T.; Scherer, E.; Steinhoff, J.; Ebenhoch, H.; Lohner, A.; Stark, T.; Kiefer, J. Effects of a dexamethasone-releasing implant on cochleae: A functional, morphological and pharmacokinetic study. Hear. Res. 2015, 327, 89–101. [Google Scholar] [CrossRef]
- Douchement, D.; Terranti, A.; Lamblin, J.; Salleron, J.; Siepmann, F.; Siepmann, J.; Vincent, C. Dexamethasone eluting electrodes for cochlear implantation: Effect on residual hearing. Cochlear Implants Int. 2015, 16, 195–200. [Google Scholar] [CrossRef]
- Wilk, M.; Hessler, R.; Mugridge, K.; Jolly, C.; Fehr, M.; Lenarz, T.; Scheper, V. Impedance Changes and Fibrous Tissue Growth after Cochlear Implantation are Correlated and Can Be Reduced Using a Dexamethasone Eluting Electrode. PLoS ONE 2016, 11, e0147552. [Google Scholar] [CrossRef]
- Schwieger, J.; Hamm, A.; Gepp, M.M.; Schulz, A.; Hoffmann, A.; Lenarz, T.; Scheper, V. Alginate-encapsulated brain-derived neurotrophic factor–overexpressing mesenchymal stem cells are a promising drug delivery system for protection of auditory neurons. J. Tissue Eng. 2020, 11, 2041731420911313. [Google Scholar] [CrossRef] [PubMed]
- Lousteau, R.J. Increased spiral ganglion cell survival in electrically stimulated, deafened guinea pig cochleae. Laryngoscope 1987, 97, 836–842. [Google Scholar] [CrossRef] [PubMed]
- Schwieger, J.; Warnecke, A.; Lenarz, T.; Esser, K.H.; Scheper, V.; Forsythe, J. Neuronal survival, morphology and outgrowth of spiral ganglion neurons using a defined growth factor combination. PLoS ONE 2015, 10, e0133680. [Google Scholar] [CrossRef][Green Version]
- Staecker, H.; Liu, W.; Hartnick, C.; Lefebvre, P.; Malgrange, B.; Moonen, G.; Van de Water, T.R. NT-3 combined with CNTF promotes survival of neurons in modiolus-spiral ganglion explants. Neuroreport 1995, 6, 1533–1537. [Google Scholar] [CrossRef] [PubMed]
- Hartnick, C.J.; Staecker, H.; Malgrange, B.; Lefebvre, P.P.; Liu, W.; Moonen, G.; Van De Water, T.R. Neurotrophic effects of BDNF and CNTF, alone and in combination, on postnatal day 5 rat acoustic ganglion neurons. J. Neurobiol. 1996, 30, 246–254. [Google Scholar] [CrossRef]
- Richardson, R.T.; Thompson, B.; Moulton, S.; Newbold, C.; Lum, M.G.; Cameron, A.; Wallace, G.; Kapsa, R.; Clark, G.; O’Leary, S. The effect of polypyrrole with incorporated neurotrophin-3 on the promotion of neurite outgrowth from auditory neurons. Biomaterials 2007, 28, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Wittig, J.H.; Ryan, A.F.; Asbeck, P.M. A reusable microfluidic plate with alternate-choice architecture for assessing growth preference in tissue culture. J. Neurosci. Methods 2005, 144, 79–89. [Google Scholar] [CrossRef]
- Xie, J.; Pak, K.; Evans, A.; Kamgar-Parsi, A.; Fausti, S.; Mullen, L.; Ryan, A.F. Neurotrophins differentially stimulate the growth of cochlear neurites on collagen surfaces and in gels. Neural Regen. Res. 2013, 8, 1541–1550. [Google Scholar] [CrossRef]
- Szobota, S.; Mathur, P.D.; Siegel, S.; Black, K.; Saragovi, H.U.; Foster, A.C. BDNF, NT-3 and Trk receptor agonist monoclonal antibodies promote neuron survival, neurite extension, and synapse restoration in rat cochlea ex vivo models relevant for hidden hearing loss. PLoS ONE 2019, 14, e0224022. [Google Scholar] [CrossRef]
- Lefebvre, P.P.; Malgrange, B.; Staecker, H.; Moghadass, M.; Van de Water, T.R.; Moonen, G. Neurotrophins affect survival and neuritogenesis by adult injured auditory neurons in vitro. Neuroreport 1994, 5, 865–868. [Google Scholar] [CrossRef]
- Gillespie, L.N.; Clark, G.M.; Bartlett, P.F.; Marzella, P.L. BDNF-induced survival of auditory neurons in vivo: Cessation of treatment leads to accelerated loss of survival effects. J. Neurosci. Res. 2003, 71, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, R.K.; Coco, A.; Epp, S.B. Neurotrophins and electrical stimulation for protection and repair of spiral ganglion neurons following sensorineural hearing loss. Hear. Res. 2008, 242, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Abdoul, T.; Schwieger, J.; Lenarz, T.; Nogueira, W.; Scheper, V. Investigation of auditory neuronal survival and outgrowth using a cochlear implant in an artificial cochlear model. Curr. Dir. Biomed. Eng. 2021, 7, 449–452. [Google Scholar] [CrossRef]
- Kawano, A.; Seldon, H.L.; Clark, G.M.; Ramsden, R.T.; Raine, C.H. Intracochlear factors contributing to psychophysical percepts following cochlear implantation. Acta Otolaryngol. 1998, 118, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Whitlon, D.S. Drug discovery for hearing loss: Phenotypic screening of chemical compounds on primary cultures of the spiral ganglion. Hear. Res. 2017, 349, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Campenot, R.B.; Lund, K.; Mok, S.A. Production of compartmented cultures of rat sympathetic neurons. Nat. Protoc. 2009, 4, 1869–1887. [Google Scholar] [CrossRef]
- Taylor, A.M.; Rhee, S.W.; Tu, C.H.; Cribbs, D.H.; Cotman, C.W.; Jeon, N.L. Microfluidic Multicompartment Device for Neuroscience Research. Langmuir 2003, 19, 1551–1556. [Google Scholar] [CrossRef]
- Taylor, A.M.; Blurton-Jones, M.; Rhee, S.W.; Cribbs, D.H.; Cotman, C.W.; Jeon, N.L. A microfluidic culture platform for CNS axonal injury, regeneration and transport. Nat. Methods 2005, 2, 599–605. [Google Scholar] [CrossRef]
- Lu, X.; Kim-Han, J.S.; O’Malley, K.L.; Sakiyama-Elbert, S.E. A microdevice platform for visualizing mitochondrial transport in aligned dopaminergic axons. J. Neurosci. Methods 2012, 209, 35–39. [Google Scholar] [CrossRef][Green Version]
- Shive, M.S.; Anderson, J.M. Biodegradation and biocompatibility of PLA and PLGA microspheres. Adv. Drug Deliv. Rev. 1997, 28, 5–24. [Google Scholar] [CrossRef]
- Ramot, Y.; Haim-Zada, M.; Domb, A.J.; Nyska, A. Biocompatibility and safety of PLA and its copolymers. Adv. Drug Deliv. Rev. 2016, 107, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Mondal, K.; Treadway, R.M.; Kumar, V.; Ma, S.; Holbery, J.D.; Dickey, M.D. Silicones for Stretchable and Durable Soft Devices: Beyond Sylgard-184. ACS Publ. 2018, 10, 11261–11268. [Google Scholar] [CrossRef] [PubMed]
- Zare, M.; Ghomi, E.R.; Venkatraman, P.D.; Ramakrishna, S. Silicone-based biomaterials for biomedical applications: Antimicrobial strategies and 3D printing technologies. J. Appl. Polym. Sci. 2021, 138, 50969. [Google Scholar] [CrossRef]
- Moučka, R.; Sedlačík, M.; Osička, J.; Pata, V. Mechanical properties of bulk Sylgard 184 and its extension with silicone oil. Sci. Rep. 2021, 11, 19090. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, Z.; Mateos-Timoneda, M.A.; Hyroššová, P.; Castaño, O.; Planell, J.A.; Perales, J.C.; Engel, E.; Alcántara, S. The effect of the composition of PLA films and lactate release on glial and neuronal maturation and the maintenance of the neuronal progenitor niche. Biomaterials 2013, 34, 2221–2233. [Google Scholar] [CrossRef] [PubMed]
- Quigley, A.F.; Bulluss, K.J.; Kyratzis, I.L.B.; Gilmore, K.; Mysore, T.; Schirmer, K.S.U.; Kennedy, E.L.; O’Shea, M.; Truong, Y.B.; Edwards, S.L.; et al. Engineering a multimodal nerve conduit for repair of injured peripheral nerve. J. Neural Eng. 2013, 10, 16008. [Google Scholar] [CrossRef]
- Kopach, O.; Zheng, K.; Sindeeva, O.A.; Gai, M.; Sukhorukov, G.B.; Rusakov, D.A. Polymer microchamber arrays for geometry-controlled drug release: A functional study in human cells of neuronal phenotype. Biomater. Sci. 2019, 7, 2358–2371. [Google Scholar] [CrossRef]
- Wang, Q.; Green, S.H. Functional role of neurotrophin-3 in synapse regeneration by spiral ganglion neurons on inner hair cells after excitotoxic trauma in vitro. J. Neurosci. 2011, 31, 7938–7949. [Google Scholar] [CrossRef]
- Liang, Q.; Shen, N.; Lai, B.; Xu, C.; Sun, Z.; Wang, Z.; Li, S. Electrical Stimulation Degenerated Cochlear Synapses Through Oxidative Stress in Neonatal Cochlear Explants. Front. Neurosci. 2019, 13, 1073. [Google Scholar] [CrossRef]
- Hahnewald, S.; Roccio, M.; Tscherter, A.; Streit, J.; Ambett, R.; Senn, P. Spiral ganglion neuron explant culture and electrophysiology on multi electrode arrays. J. Vis. Exp. 2016, 2016, e54538. [Google Scholar] [CrossRef]
- Theeuwes, F.; Yum, S.I. Principles of the design and operation of generic osmotic pumps for the delivery of semisolid or liquid drug formulations. Ann. Biomed. Eng. 1976, 4, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Whitlon, D.S.; Grover, M.; Tristano, J.; Williams, T.; Coulson, M.T. Culture conditions determine the prevalence of bipolar and monopolar neurons in cultures of dissociated spiral ganglion. Neuroscience 2007, 146, 833–840. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nayagam, B.A.; Muniak, M.A.; Ryugo, D.K. The spiral ganglion: Connecting the peripheral and central auditory systems. Hear. Res. 2011, 278, 2–20. [Google Scholar] [CrossRef] [PubMed]
- Fritzsch, B.; Fariñas, I.; Reichardt, L.F. Lack of neurotrophin 3 causes losses of both classes of spiral ganglion neurons in the cochlea in a region-specific fashion. J. Neurosci. 1997, 17, 6213–6225. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Kondo, K.; Ushio, M.; Kaga, K.; Ryan, A.F.; Yamasoba, T. Developmental changes in the responsiveness of rat spiral ganglion neurons to neurotrophic factors in dissociated culture: Differential responses for survival, neuritogenesis and neuronal morphology. Cell Tissue Res. 2013, 351, 15–27. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Frick, C.; Fink, S.; Schmidbauer, D.; Rousset, F.; Eickhoff, H.; Tropitzsch, A.; Kramer, B.; Senn, P.; Glueckert, R.; Rask-Andersen, H.; et al. Age-Dependency of Neurite Outgrowth in Postnatal Mouse Cochlear Spiral Ganglion Explants. Brain Sci. 2020, 10, 580. [Google Scholar] [CrossRef]
- Kocsis, J.D.; Rand, M.N.; Lankford, K.L.; Waxman, S.G. Intracellular calcium mobilization and neurite outgrowth in mammalian neurons. J. Neurobiol. 1994, 25, 252–264. [Google Scholar] [CrossRef]
- Chu, G.K.T.; Tator, C.H. Calcium influx is necessary for optimal regrowth of transected neurites of rat sympathetic ganglion neurons in vitro. Neuroscience 2001, 102, 945–957. [Google Scholar] [CrossRef]
- Altschuler, R.A.; Cho, Y.; Ylikoski, J.; Pirvola, U.; Magal, E.; Miller, J.M. Rescue and regrowth of sensory nerves following deafferentation by neurotrophic factors. Ann. N. Y. Acad. Sci. 1999, 884, 305–311. [Google Scholar] [CrossRef]
- Scheper, V.; Seidel-Effenberg, I.; Lenarz, T.; Stöver, T.; Paasche, G. Consecutive treatment with brain-derived neurotrophic factor and electrical stimulation has a protective effect on primary auditory neurons. Brain Sci. 2020, 10, 559. [Google Scholar] [CrossRef]
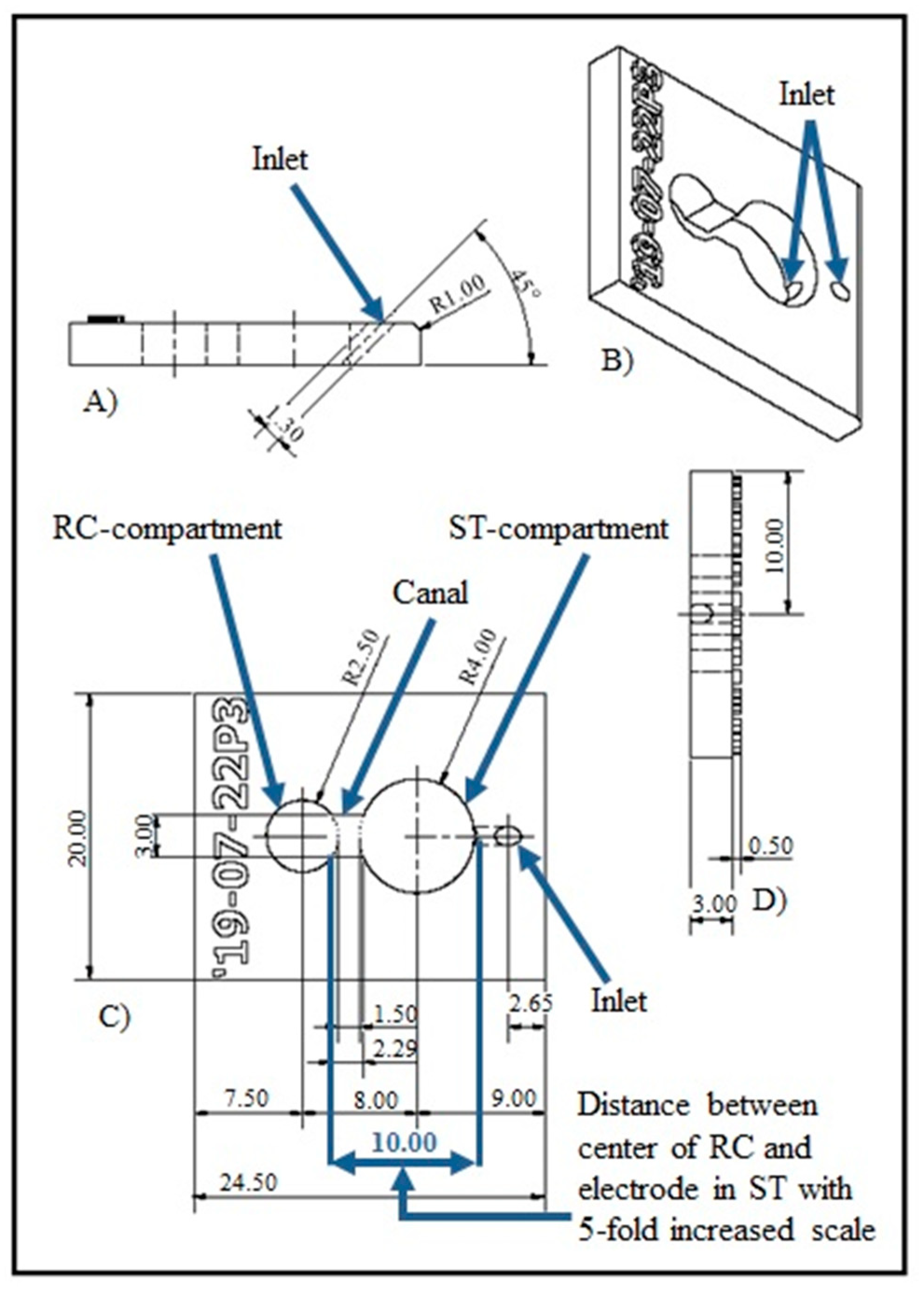
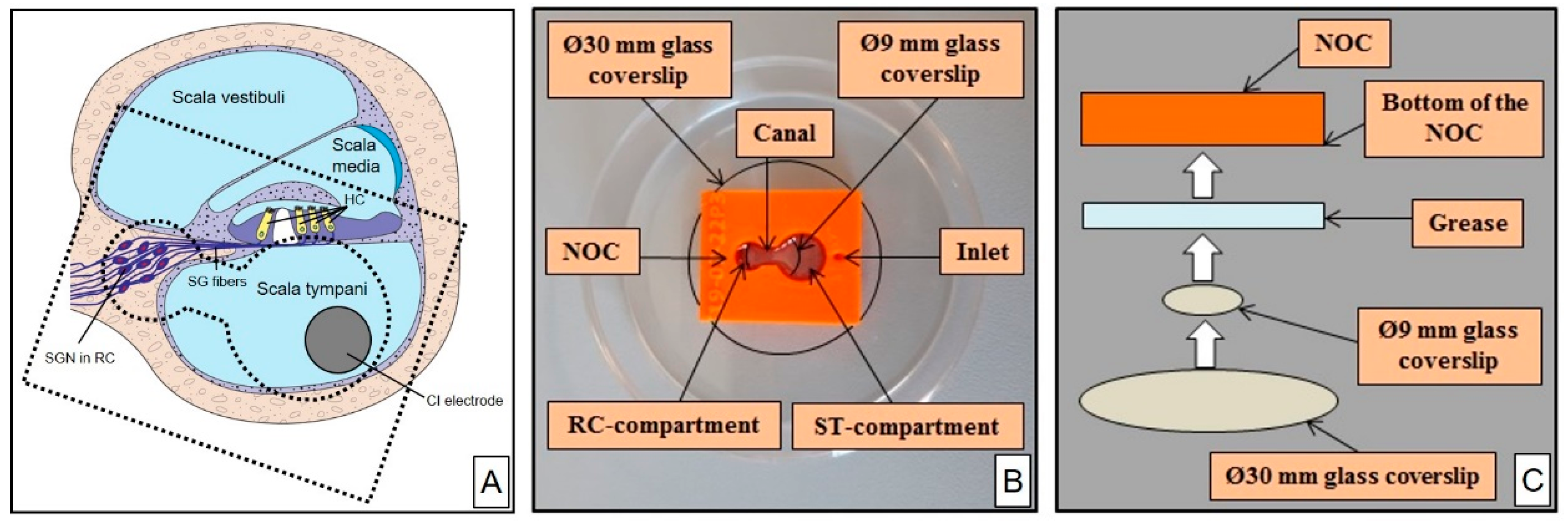
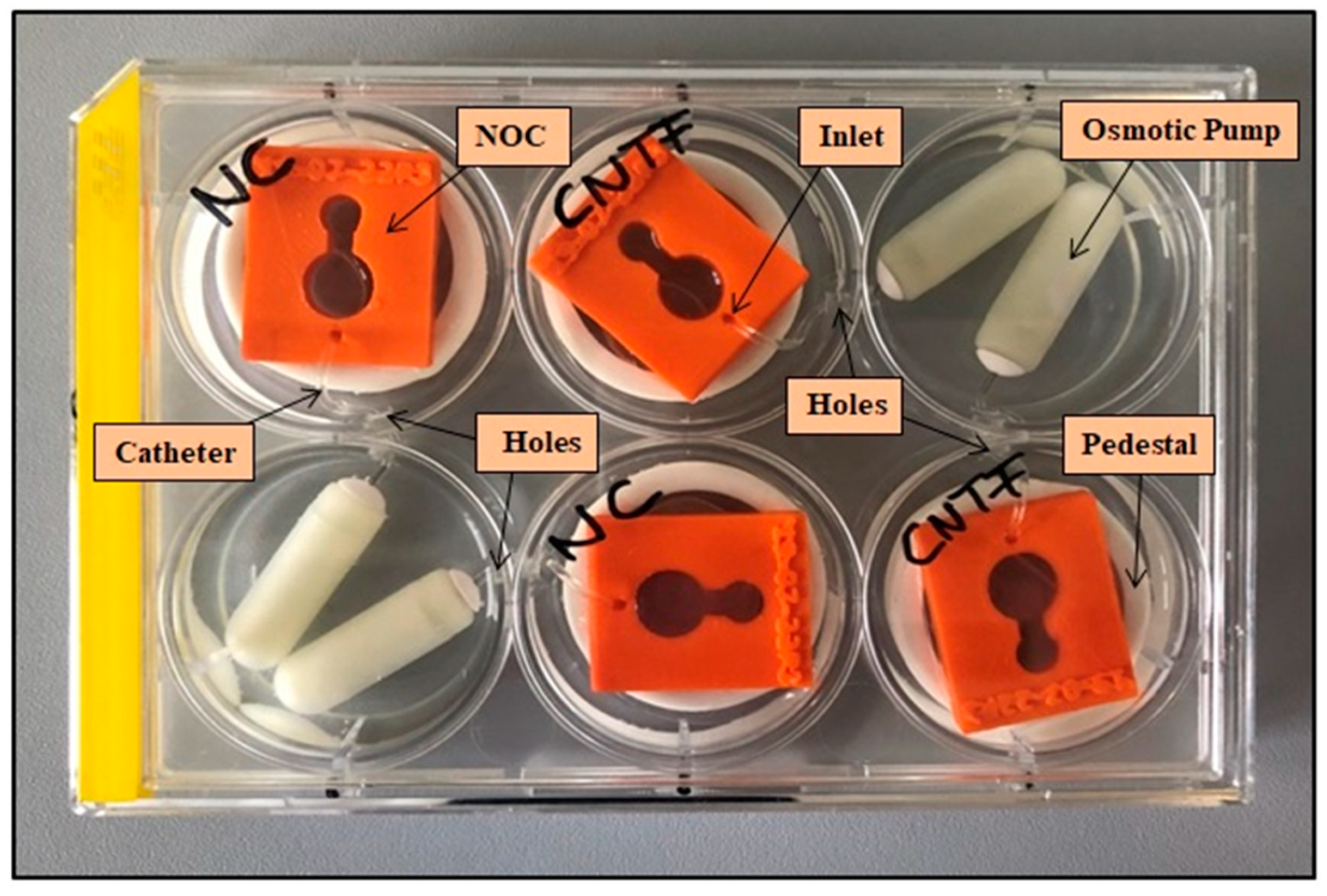
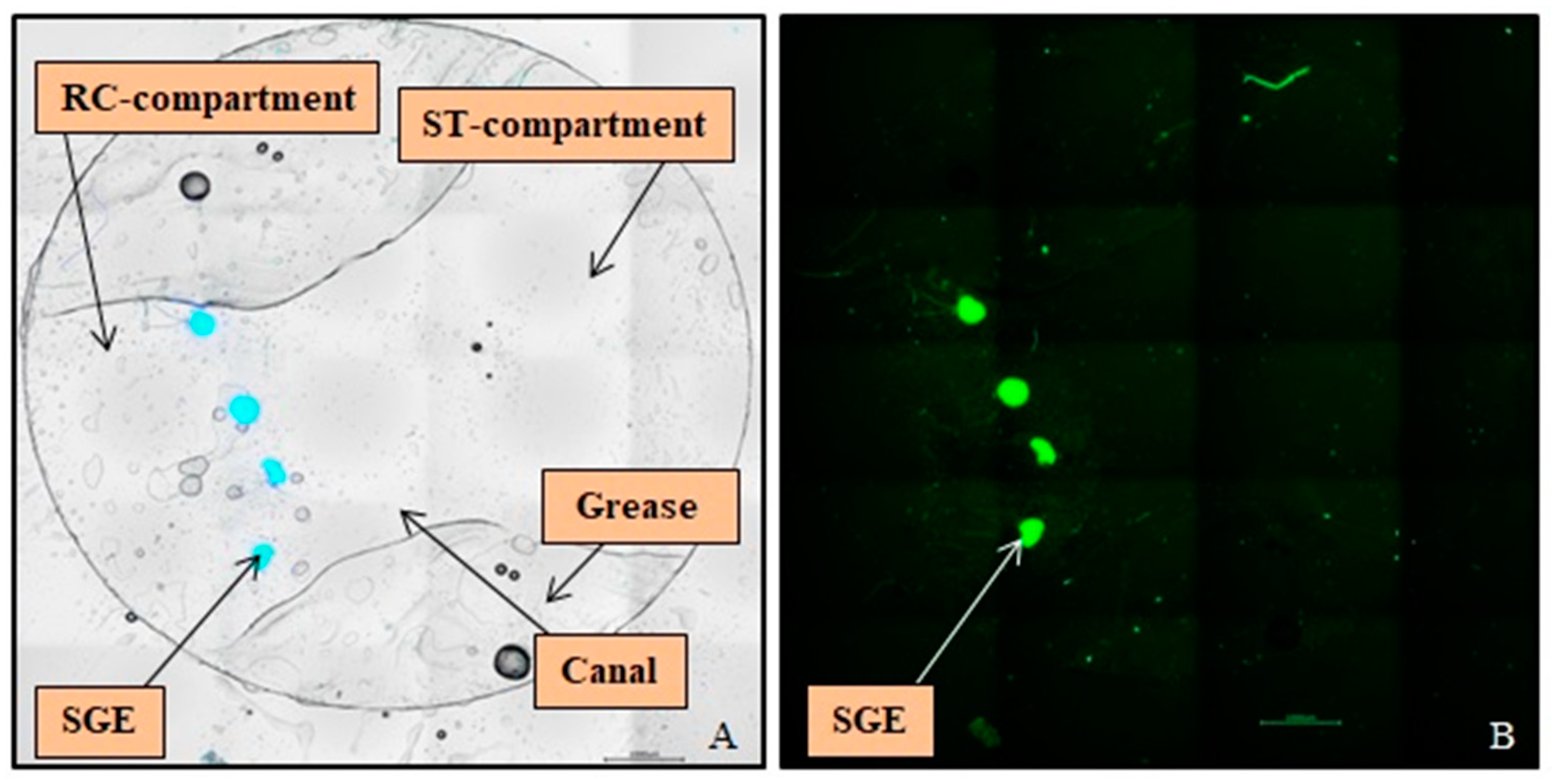




| Printing Technology | Fused Deposition Modeling |
| Printing time | 5 h 25 min printing process, 6 h including pre- and post-set up such as removing residuals of material and checking the consistency of the inlet, ~33 min/NOC |
| Nozzle temperature | 210 °C |
| Nozzle diameter | 0.4 mm |
| Layer thickness | 0.06 mm |
| Fill density | 100% |
| Overlap | 25% |
| Material amount | Orange-colored PLA, ~18 g (excluding PLA extrusion at the start of the process), ~1.64 g/NOC |
| Material costs | ~25.95 €/750 g filament role (excluding shipment), ~6 cent/NOC |
| Treatment Condition | |||||||
|---|---|---|---|---|---|---|---|
| NC | NP | CNTF | NT-3 | NC MC | NP MC | NT-3 MC | |
| N | 11 | 8 | 7 | 4 | 5 | 5 | 5 |
| NC | NP | CNTF | NT-3 | |
|---|---|---|---|---|
| Minimum | 35 | 20 | 53 | 25 |
| Maximum | 2602 | 3631 | 2377 | 2403 |
| Mean | 717 ± 434 | 670 ± 489 | 603 ± 336 | 799 ± 475 |
| NC + | NC − | CNTF + | CNTF − | NT-3 + | NT-3 − | |
|---|---|---|---|---|---|---|
| Minimum | 35 | 47 | 59 | 53 | 25 | 37 |
| Maximum | 2077 | 2602 | 1609 | 2377 | 2344 | 2403 |
| Mean | 701 ± 433 | 732 ± 436 | 597 ± 311 | 614 ± 363 | 814 ± 474 | 784 ± 476 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schwieger, J.; Frisch, A.S.; Rau, T.S.; Lenarz, T.; Hügl, S.; Scheper, V. 3D Printed Cell Culture Chamber for Testing the Effect of Pump-Based Chronic Drug Delivery on Inner Ear Tissue. Biomolecules 2022, 12, 589. https://doi.org/10.3390/biom12040589
Schwieger J, Frisch AS, Rau TS, Lenarz T, Hügl S, Scheper V. 3D Printed Cell Culture Chamber for Testing the Effect of Pump-Based Chronic Drug Delivery on Inner Ear Tissue. Biomolecules. 2022; 12(4):589. https://doi.org/10.3390/biom12040589
Chicago/Turabian StyleSchwieger, Jana, Anna Sophie Frisch, Thomas S. Rau, Thomas Lenarz, Silke Hügl, and Verena Scheper. 2022. "3D Printed Cell Culture Chamber for Testing the Effect of Pump-Based Chronic Drug Delivery on Inner Ear Tissue" Biomolecules 12, no. 4: 589. https://doi.org/10.3390/biom12040589
APA StyleSchwieger, J., Frisch, A. S., Rau, T. S., Lenarz, T., Hügl, S., & Scheper, V. (2022). 3D Printed Cell Culture Chamber for Testing the Effect of Pump-Based Chronic Drug Delivery on Inner Ear Tissue. Biomolecules, 12(4), 589. https://doi.org/10.3390/biom12040589







