Key Molecules of Fatty Acid Metabolism in Gastric Cancer
Abstract
:1. Introduction
2. Functions of Fatty Acids
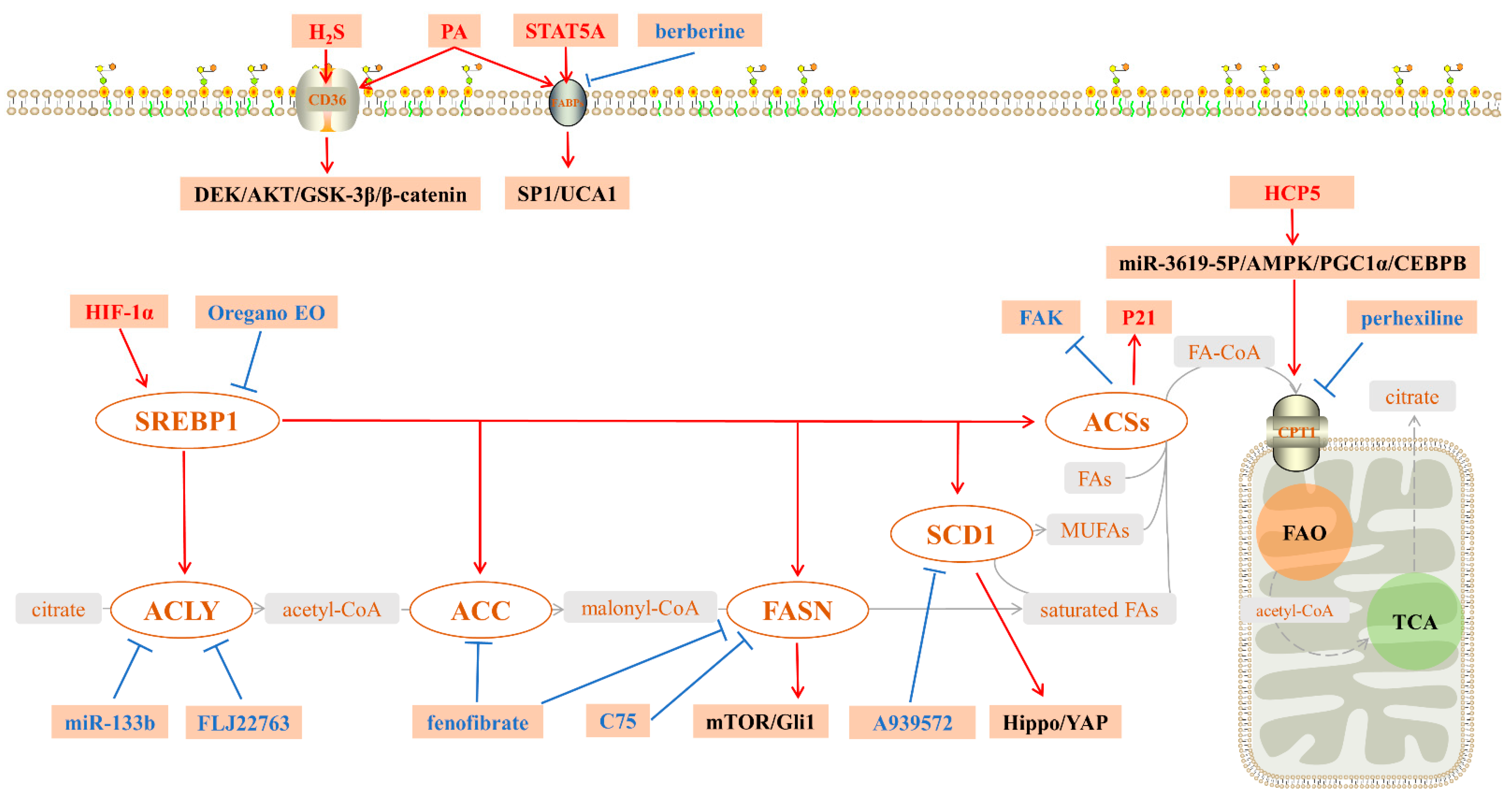
3. Key Molecules in Fatty Acid Metabolism
3.1. Key Molecules in the Endogenous Synthesis of FAs
3.1.1. Sterol Regulatory Element-Binding Protein 1 (SREBP1)
3.1.2. ACLY
3.1.3. Acetyl-CoA Synthases (ACSs)
3.1.4. ACC
3.1.5. FASN
3.1.6. SCD1
3.2. Key Molecules for Exogenous Uptake of FAs
3.2.1. CD36
3.2.2. FABPs
3.3. A Key Molecule in Fatty Acid Catabolism
Carnitine palmitoyltransferase 1 (CPT1)
4. Concluding Remarks
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Smyth, E.C.; Nilsson, M.; Grabsch, H.I.; van Grieken, N.C.T.; Lordick, F. Gastric cancer. Lancet 2020, 396, 635–648. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Reinfeld, B.I.; Madden, M.Z.; Wolf, M.M.; Chytil, A.; Bader, J.E.; Patterson, A.R.; Sugiura, A.; Cohen, A.S.; Ali, A.; Do, B.T.; et al. Cell-programmed nutrient partitioning in the tumour microenvironment. Nature 2021, 593, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [Green Version]
- Counihan, J.L.; Grossman, E.A.; Nomura, D.K. Cancer Metabolism: Current Understanding and Therapies. Chem. Rev. 2018, 118, 6893–6923. [Google Scholar] [CrossRef]
- Koundouros, N.; Poulogiannis, G. Reprogramming of fatty acid metabolism in cancer. Br. J. Cancer 2020, 122, 4–22. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.-H.; Ren, X.-H.; Liang, X.-H.; Tang, Y.-L. Roles of fatty acid metabolism in tumourigenesis: Beyond providing nutrition (Review). Mol. Med. Rep. 2018, 18, 5307–5316. [Google Scholar] [CrossRef] [Green Version]
- Serhan, C.N.; Yacoubian, S.; Yang, R. Anti-inflammatory and proresolving lipid mediators. Annu. Rev. Pathol. 2008, 3, 279–312. [Google Scholar] [CrossRef] [Green Version]
- Fagone, P.; Jackowski, S. Membrane phospholipid synthesis and endoplasmic reticulum function. J. Lipid Res. 2009, 50, S311–S316. [Google Scholar] [CrossRef] [Green Version]
- Beloribi-Djefaflia, S.; Vasseur, S.; Guillaumond, F. Lipid metabolic reprogramming in cancer cells. Oncogenesis 2016, 5, e189. [Google Scholar] [CrossRef] [PubMed]
- Duwaerts, C.C.; Maher, J.J. Macronutrients and the adipose-liver axis in obesity and fatty liver. Cell. Mol. Gastroenterol. Hepatol. 2019, 7, 749–761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fabian, C.J.; Kimler, B.F.; Hursting, S.D. Omega-3 fatty acids for breast cancer prevention and survivorship. Breast Cancer Res. 2015, 17, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corn, K.C.; Windham, M.A.; Rafat, M. Lipids in the tumor microenvironment: From cancer progression to treatment. Prog. Lipid Res. 2020, 80, 101055. [Google Scholar] [CrossRef]
- Rysman, E.; Brusselmans, K.; Scheys, K.; Timmermans, L.; Derua, R.; Munck, S.; van Veldhoven, P.P.; Waltregny, D.; Daniëls, V.W.; Machiels, J.; et al. De novo lipogenesis protects cancer cells from free radicals and chemotherapeutics by promoting membrane lipid saturation. Cancer Res. 2010, 70, 8117–8126. [Google Scholar] [CrossRef] [Green Version]
- Snaebjornsson, M.T.; Janaki-Raman, S.; Schulze, A. Greasing the wheels of the cancer machine: The role of lipid metabolism in cancer. Cell Metab. 2020, 31, 62–76. [Google Scholar] [CrossRef]
- Horton, J.D.; Goldstein, J.L.; Brown, M.S. SREBPs: Activators of the complete program of cholesterol and fatty acid synthesis in the liver. J. Clin. Investig. 2002, 109, 1125–1131. [Google Scholar] [CrossRef]
- Santos, C.R.; Schulze, A. Lipid metabolism in cancer. FEBS J. 2012, 279, 2610–2623. [Google Scholar] [CrossRef]
- Li, S.; Oh, Y.-T.; Yue, P.; Khuri, F.R.; Sun, S.-Y. Inhibition of mTOR complex 2 induces GSK3/FBXW7-dependent degradation of sterol regulatory element-binding protein 1 (SREBP1) and suppresses lipogenesis in cancer cells. Oncogene 2016, 35, 642–650. [Google Scholar] [CrossRef] [Green Version]
- Sato, R.; Okamoto, A.; Inoue, J.; Miyamoto, W.; Sakai, Y.; Emoto, N.; Shimano, H.; Maeda, M. Transcriptional regulation of the ATP citrate-lyase gene by sterol regulatory element-binding proteins. J. Biol. Chem. 2000, 275, 12497–12502. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Luo, J.; Ma, G.; Zhang, X.; Yao, D.; Li, M.; Loor, J.J. Acyl-CoA synthetase short-chain family member 2 (ACSS2) is regulated by SREBP-1 and plays a role in fatty acid synthesis in caprine mammary epithelial cells. J. Cell. Physiol. 2018, 233, 1005–1016. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, I.; Shimano, H.; Korn, B.S.; Bashmakov, Y.; Horton, J.D. Nuclear sterol regulatory element-binding proteins activate genes responsible for the entire program of unsaturated fatty acid biosynthesis in transgenic mouse liver. J. Biol. Chem. 1998, 273, 35299–35306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ezzeddini, R.; Taghikhani, M.; Somi, M.H.; Samadi, N.; Rasaee, M.J. Clinical importance of FASN in relation to HIF-1α and SREBP-1c in gastric adenocarcinoma. Life Sci. 2019, 224, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Enjoji, M.; Kohjima, M.; Ohtsu, K.; Matsunaga, K.; Murata, Y.; Nakamuta, M.; Imamura, K.; Tanabe, H.; Iwashita, A.; Nagahama, T.; et al. Intracellular mechanisms underlying lipid accumulation (white opaque substance) in gastric epithelial neoplasms: A pilot study of expression profiles of lipid-metabolism-associated genes. J. Gastroenterol. Hepatol. 2016, 31, 776–781. [Google Scholar] [CrossRef]
- Sun, Q.; Yu, X.; Peng, C.; Liu, N.; Chen, W.; Xu, H.; Wei, H.; Fang, K.; Dong, Z.; Fu, C.; et al. Activation of SREBP-1c alters lipogenesis and promotes tumor growth and metastasis in gastric cancer. Biomed. Pharmacother. 2020, 128, 110274. [Google Scholar] [CrossRef]
- Zhao, L.; Peng, Y.; He, S.; Li, R.; Wang, Z.; Huang, J.; Lei, X.; Li, G.; Ma, Q. Apatinib induced ferroptosis by lipid peroxidation in gastric cancer. Gastric Cancer 2021, 24, 642–654. [Google Scholar] [CrossRef]
- Balusamy, S.R.; Perumalsamy, H.; Huq, M.A.; Balasubramanian, B. Anti-proliferative activity of Origanum vulgare inhibited lipogenesis and induced mitochondrial mediated apoptosis in human stomach cancer cell lines. Biomed. Pharmacother. 2018, 108, 1835–1844. [Google Scholar] [CrossRef]
- Migita, T.; Narita, T.; Nomura, K.; Miyagi, E.; Inazuka, F.; Matsuura, M.; Ushijima, M.; Mashima, T.; Seimiya, H.; Satoh, Y.; et al. ATP citrate lyase: Activation and therapeutic implications in non-small cell lung cancer. Cancer Res. 2008, 68, 8547–8554. [Google Scholar] [CrossRef] [Green Version]
- Burke, A.C.; Huff, M.W. ATP-citrate lyase: Genetics, molecular biology and therapeutic target for dyslipidemia. Curr. Opin. Lipidol. 2017, 28, 193–200. [Google Scholar] [CrossRef]
- Feng, X.; Zhang, L.; Xu, S.; Shen, A.-Z. ATP-citrate lyase (ACLY) in lipid metabolism and atherosclerosis: An updated review. Prog. Lipid Res. 2020, 77, 101006. [Google Scholar] [CrossRef]
- El-Rifai, W.; Frierson, H.F.; Moskaluk, C.A.; Harper, J.C.; Petroni, G.R.; Bissonette, E.A.; Jones, D.R.; Knuutila, S.; Powell, S.M. Genetic differences between adenocarcinomas arising in Barrett’s esophagus and gastric mucosa. Gastroenterology 2001, 121, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Varis, A.; Wolf, M.; Monni, O.; Vakkari, M.-L.; Kokkola, A.; Moskaluk, C.; Frierson, H.; Powell, S.M.; Knuutila, S.; Kallioniemi, A.; et al. Targets of gene amplification and overexpression at 17q in gastric cancer. Cancer Res. 2002, 62, 2625–2629. [Google Scholar] [PubMed]
- Qian, X.; Hu, J.; Zhao, J.; Chen, H. ATP citrate lyase expression is associated with advanced stage and prognosis in gastric adenocarcinoma. Int. J. Clin. Exp. Med. 2015, 8, 7855–7860. [Google Scholar] [PubMed]
- Chen, P.; Li, L.; Wang, H.; Zhao, J.; Cheng, Y.; Xie, J.; Cao, M.; Huang, L.; Yang, F.; Chen, H.; et al. Omeprazole, an inhibitor of proton pump, suppresses De novo lipogenesis in gastric epithelial cells. Biomed. Pharmacother. 2020, 130, 110472. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Wang, X.; Zheng, L.; Zhao, H.; Li, W.; Wang, B.; Xue, L.; Tian, Y.; Xie, Y. Construction and Analysis of the Tumor-Specific mRNA-miRNA-lncRNA Network in Gastric Cancer. Front. Pharmacol. 2020, 11, 1112. [Google Scholar] [CrossRef] [PubMed]
- Icard, P.; Wu, Z.; Fournel, L.; Coquerel, A.; Lincet, H.; Alifano, M. ATP citrate lyase: A central metabolic enzyme in cancer. Cancer Lett. 2020, 471, 125–134. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, X.; Wang, T.; Xian, S.; Lu, Y. 3-Bromopyruvate and sodium citrate induce apoptosis in human gastric cancer cell line MGC-803 by inhibiting glycolysis and promoting mitochondria-regulated apoptosis pathway. Biochem. Biophys. Res. Commun. 2016, 475, 37–43. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.; Wang, Q.; Lu, J.; Ma, G.; Ge, Y.; Chu, H.; Du, M.; Wang, M.; Zhang, Z. Long non-coding RNA FLJ22763 is involved in the progression and prognosis of gastric cancer. Gene 2019, 693, 84–91. [Google Scholar] [CrossRef]
- Cheng, Y.; Jia, B.; Wang, Y.; Wan, S. MiR-133b acts as a tumor suppressor and negatively regulates ATP citrate lyase via PPARγ in gastric cancer. Oncol. Rep. 2017, 38, 3220–3226. [Google Scholar] [CrossRef] [Green Version]
- Watkins, P.A.; Maiguel, D.; Jia, Z.; Pevsner, J. Evidence for 26 distinct acyl-coenzyme A synthetase genes in the human genome. J. Lipid Res. 2007, 48, 2736–2750. [Google Scholar] [CrossRef] [Green Version]
- Ohkuni, A.; Ohno, Y.; Kihara, A. Identification of acyl-CoA synthetases involved in the mammalian sphingosine 1-phosphate metabolic pathway. Biochem. Biophys. Res. Commun. 2013, 442, 195–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, W.C.; Cheng, W.C.; Cheng, B.H.; Chen, L.; Ju, L.J.; Ou, Y.-J.; Jeng, L.B.; Yang, M.D.; Hung, Y.C.; Ma, W.L. Mitochondrial Acetyl-CoA Synthetase 3 is Biosignature of Gastric Cancer Progression. Cancer Med. 2018, 7, 1240–1252. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Yao, X.; Tang, S.; Zhang, J.; Yau, T.O.; Li, X.; Tang, C.-M.; Kang, W.; Lung, R.W.M.; Li, J.W.; et al. Integrative identification of Epstein-Barr virus-associated mutations and epigenetic alterations in gastric cancer. Gastroenterology 2014, 147, 1350–1362.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hur, H.; Kim, Y.-B.; Ham, I.-H.; Lee, D. Loss of ACSS2 expression predicts poor prognosis in patients with gastric cancer. J. Surg. Oncol. 2015, 112, 585–591. [Google Scholar] [CrossRef]
- Zaidi, N.; Royaux, I.; Swinnen, J.V.; Smans, K. ATP citrate lyase knockdown induces growth arrest and apoptosis through different cell- and environment-dependent mechanisms. Mol. Cancer Ther. 2012, 11, 1925–1935. [Google Scholar] [CrossRef] [Green Version]
- Ye, X.; Zhang, Y.; Wang, X.; Li, Y.; Gao, Y. Tumor-suppressive functions of long-chain acyl-CoA synthetase 4 in gastric cancer. IUBMB Life 2016, 68, 320–327. [Google Scholar] [CrossRef] [Green Version]
- Seo, E.H.; Kim, H.J.; Kim, J.H.; Lim, B.; Park, J.L.; Kim, S.Y.; Lee, S.I.; Jeong, H.Y.; Song, K.S.; Kim, Y.S. ONECUT2 upregulation is associated with CpG hypomethylation at promoter-proximal DNA in gastric cancer and triggers ACSL5. Int. J. Cancer 2020, 146, 3354–3368. [Google Scholar] [CrossRef] [Green Version]
- Wakil, S.J.; Abu-Elheiga, L.A. Fatty acid metabolism: Target for metabolic syndrome. J. Lipid Res. 2009, 50, S138–S143. [Google Scholar] [CrossRef] [Green Version]
- Padanad, M.S.; Konstantinidou, G.; Venkateswaran, N.; Melegari, M.; Rindhe, S.; Mitsche, M.; Yang, C.; Batten, K.; Huffman, K.E.; Liu, J.; et al. Fatty acid oxidation mediated by Acyl-CoA synthetase long chain 3 is required for mutant KRAS lung tumorigenesis. Cell Rep. 2016, 16, 1614–1628. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Wang, X. Identification of molecular features correlating with tumor immunity in gastric cancer by multi-omics data analysis. Ann. Transl. Med. 2020, 8, 1050. [Google Scholar] [CrossRef]
- Fang, W.; Cui, H.; Yu, D.; Chen, Y.; Wang, J.; Yu, G. Increased expression of phospho-acetyl-CoA carboxylase protein is an independent prognostic factor for human gastric cancer without lymph node metastasis. Med. Oncol. (Northwood Lond. Engl.) 2014, 31, 15. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G.; Pan, D.A. Regulation of fatty acid synthesis and oxidation by the AMP-activated protein kinase. Biochem. Soc. Trans. 2002, 30, 1064–1070. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Zheng, Y.; Zhang, M.; Wang, J.; Yu, G.; Fang, W. Reciprocal expression of p-AMPKa and p-S6 is strongly associated with the prognosis of gastric cancer. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2016, 37, 4803–4811. [Google Scholar] [CrossRef] [PubMed]
- Asturias, F.J.; Chadick, J.Z.; Cheung, I.K.; Stark, H.; Witkowski, A.; Joshi, A.K.; Smith, S. Structure and molecular organization of mammalian fatty acid synthase. Nat. Struct. Mol. Biol. 2005, 12, 225–232. [Google Scholar] [CrossRef]
- Zhou, Z.; Ibekwe, E.; Chornenkyy, Y. Metabolic Alterations in Cancer Cells and the Emerging Role of Oncometabolites as Drivers of Neoplastic Change. Antioxidants 2018, 7, 16. [Google Scholar] [CrossRef] [Green Version]
- Menendez, J.A.; Lupu, R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat. Rev. Cancer 2007, 7, 763–777. [Google Scholar] [CrossRef]
- Ito, T.; Sato, K.; Maekawa, H.; Sakurada, M.; Orita, H.; Shimada, K.; Daida, H.; Wada, R.; Abe, M.; Hino, O.; et al. Elevated levels of serum fatty acid synthase in patients with gastric carcinoma. Oncol. Lett. 2014, 7, 616–620. [Google Scholar] [CrossRef] [Green Version]
- Xiang, H.G.; Hao, J.; Zhang, W.J.; Lu, W.J.; Dong, P.; Liu, Y.B.; Chen, L. Expression of fatty acid synthase negatively correlates with PTEN and predicts peritoneal dissemination of human gastric cancer. Asian Pac. J. Cancer Prev. APJCP 2015, 16, 6851–6855. [Google Scholar] [CrossRef] [Green Version]
- Duan, J.; Sun, L.; Huang, H.; Wu, Z.; Wang, L.; Liao, W. Overexpression of fatty acid synthase predicts a poor prognosis for human gastric cancer. Mol. Med. Rep. 2016, 13, 3027–3035. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Yao, Y.; Pan, G.; Zhan, S.; Shi, W.; Lu, T.; Yuan, J.; Tian, K.; Jiang, L.; Song, S.; et al. Small interfering RNA-mediated knockdown of fatty acid synthase attenuates the proliferation and metastasis of human gastric cancer cells via the mTOR/Gli1 signaling pathway. Oncol. Lett. 2018, 16, 594–602. [Google Scholar] [CrossRef]
- Li, H.E.; Wang, X.; Tang, Z.; Liu, F.; Chen, W.; Fang, Y.; Wang, C.; Shen, K.; Qin, J.; Shen, Z.; et al. A concordant expression pattern of fatty acid synthase and membranous human epidermal growth factor receptor 2 exists in gastric cancer and is associated with a poor prognosis in gastric adenocarcinoma patients. Oncol. Lett. 2015, 10, 2107–2117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.; Su, W.; Liu, H.; Chen, T.; Höti, N.; Pei, H.; Zhu, H. Fatty acid synthase is a prognostic marker and associated with immune infiltrating in gastric cancers precision medicine. Biomark. Med. 2020, 14, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Chen, L.; Zhou, M.; Zhang, J.; Sun, L.; Huang, N.; Bin, J.; Liao, Y.; Liao, W. MACC1 decreases the chemosensitivity of gastric cancer cells to oxaliplatin by regulating FASN expression. Oncol. Rep. 2017, 37, 2583–2592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, B.; Deng, H.; Cui, H.; Zhao, R.; Li, H.; Wei, B.; Chen, L. Knockdown of PGM1 enhances anticancer effects of orlistat in gastric cancer under glucose deprivation. Cancer Cell Int. 2021, 21, 481. [Google Scholar] [CrossRef] [PubMed]
- Attie, A.D.; Krauss, R.M.; Gray-Keller, M.P.; Brownlie, A.; Miyazaki, M.; Kastelein, J.J.; Lusis, A.J.; Stalenhoef, A.F.H.; Stoehr, J.P.; Hayden, M.R.; et al. Relationship between stearoyl-CoA desaturase activity and plasma triglycerides in human and mouse hypertriglyceridemia. J. Lipid Res. 2002, 43, 1899–1907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tutino, V.; Gigante, I.; Scavo, M.P.; Refolo, M.G.; De Nunzio, V.; Milella, R.A.; Caruso, M.G.; Notarnicola, M. Stearoyl-CoA Desaturase-1 enzyme inhibition by grape skin extracts affects membrane fluidity in human colon cancer cell lines. Nutrients 2020, 12, 693. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Zhou, T.; Li, C.; Dai, Z.; Che, D.; Yao, Y.; Li, L.; Ma, J.; Yang, X.; Gao, G. High metastaticgastric and breast cancer cells consume oleic acid in an AMPK dependent manner. PLoS ONE 2014, 9, e97330. [Google Scholar] [CrossRef]
- Xiang, F.; Wu, K.; Liu, Y.; Shi, L.; Wang, D.; Li, G.; Tao, K.; Wang, G. Omental adipocytes enhance the invasiveness of gastric cancer cells by oleic acid-induced activation of the PI3K-Akt signaling pathway. Int. J. Biochem. Cell Biol. 2017, 84, 14–21. [Google Scholar] [CrossRef]
- Ntambi, J. Regulation of stearoyl-CoA desaturases and role in metabolism. Prog. Lipid Res. 2004, 43, 91–104. [Google Scholar] [CrossRef]
- Gao, Y.; Li, J.; Xi, H.; Cui, J.; Zhang, K.; Zhang, J.; Zhang, Y.; Xu, W.; Liang, W.; Zhuang, Z.; et al. Stearoyl-CoA-desaturase-1 regulates gastric cancer stem-like properties and promotes tumour metastasis via Hippo/YAP pathway. Br. J. Cancer 2020, 122, 1837–1847. [Google Scholar] [CrossRef]
- Wang, C.; Shi, M.; Ji, J.; Cai, Q.; Zhao, Q.; Jiang, J.; Liu, J.; Zhang, H.; Zhu, Z.; Zhang, J. Stearoyl-CoA desaturase 1 (SCD1) facilitates the growth and anti-ferroptosis of gastric cancer cells and predicts poor prognosis of gastric cancer. Aging 2020, 12, 15374–15391. [Google Scholar] [CrossRef] [PubMed]
- Roongta, U.V.; Pabalan, J.G.; Wang, X.; Ryseck, R.-P.; Fargnoli, J.; Henley, B.J.; Yang, W.-P.; Zhu, J.; Madireddi, M.T.; Lawrence, R.M.; et al. Cancer cell dependence on unsaturated fatty acids implicates stearoyl-CoA desaturase as a target for cancer therapy. Mol. Cancer Res. MCR 2011, 9, 1551–1561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Li, Y. CD36 tango in cancer: Signaling pathways and functions. Theranostics 2019, 9, 4893–4908. [Google Scholar] [CrossRef] [PubMed]
- Kuemmerle, N.B.; Rysman, E.; Lombardo, P.S.; Flanagan, A.J.; Lipe, B.C.; Wells, W.A.; Pettus, J.R.; Froehlich, H.M.; Memoli, V.A.; Morganelli, P.M.; et al. Lipoprotein lipase links dietary fat to solid tumor cell proliferation. Mol. Cancer Ther. 2011, 10, 427–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pepino, M.Y.; Kuda, O.; Samovski, D.; Abumrad, N.A. Structure-function of CD36 and importance of fatty acid signal transduction in fat metabolism. Annu. Rev. Nutr. 2014, 34, 281–303. [Google Scholar] [CrossRef] [Green Version]
- Ibrahimi, A.; Bonen, A.; Blinn, W.D.; Hajri, T.; Li, X.; Zhong, K.; Cameron, R.; Abumrad, N.A. Muscle-specific overexpression of FAT/CD36 enhances fatty acid oxidation by contracting muscle, reduces plasma triglycerides and fatty acids, and increases plasma glucose and insulin. J. Biol. Chem. 1999, 274, 26761–26766. [Google Scholar] [CrossRef] [Green Version]
- Dai, J.; Li, Z.-X.; Zhang, Y.; Ma, J.-L.; Zhou, T.; You, W.-C.; Li, W.-Q.; Pan, K.-F. Whole Genome Messenger RNA Profiling Identifies a Novel Signature to Predict Gastric Cancer Survival. Clin. Transl. Gastroenterol. 2019, 10, e00004. [Google Scholar] [CrossRef]
- Wang, J.; Wen, T.; Li, Z.; Che, X.; Gong, L.; Jiao, Z.; Qu, X.; Liu, Y. CD36 upregulates DEK transcription and promotes cell migration and invasion via GSK-3β/β-catenin-mediated epithelial-to-mesenchymal transition in gastric cancer. Aging 2020, 13, 1883–1897. [Google Scholar] [CrossRef]
- Chen, C.N.; Lin, J.J.; Chen, J.J.W.; Lee, P.H.; Yang, C.Y.; Kuo, M.L.; Chang, K.J.; Hsieh, F.J. Gene expression profile predicts patient survival of gastric cancer after surgical resection. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2005, 23, 7286–7295. [Google Scholar] [CrossRef]
- Jiang, M.; Wu, N.; Xu, B.; Chu, Y.; Li, X.; Su, S.; Chen, D.; Li, W.; Shi, Y.; Gao, X.; et al. Fatty acid-induced CD36 expression via O-GlcNAcylation drives gastric cancer metastasis. Theranostics 2019, 9, 5359–5373. [Google Scholar] [CrossRef]
- Pan, J.; Fan, Z.; Wang, Z.; Dai, Q.; Xiang, Z.; Yuan, F.; Yan, M.; Zhu, Z.; Liu, B.; Li, C. CD36 mediates palmitate acid-induced metastasis of gastric cancer via AKT/GSK-3β/β-catenin pathway. J. Exp. Clin. Cancer Res. 2019, 38, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, Y.; Lin, K.; Zhao, Y.; Wu, Q.; Chen, D.; Wang, J.; Liang, Y.; Li, J.; Hu, J.; Wang, H.; et al. Adipocytes fuel gastric cancer omental metastasis via PITPNC1-mediated fatty acid metabolic reprogramming. Theranostics 2018, 8, 5452–5468. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Tao, B.; Fan, Q.; Wang, S.; Chen, L.; Zhang, J.; Hao, Y.; Dong, S.; Wang, Z.; Wang, W.; et al. Fatty-acid receptor CD36 functions as a hydrogen sulfide-targeted receptor with its Cys333-Cys272 disulfide bond serving as a specific molecular switch to accelerate gastric cancer metastasis. EBioMedicine 2019, 45, 108–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, D.; Li, Y.; Xing, L.; Tan, Y.; Sun, J.; Zeng, B.; Xiang, T.; Tan, J.; Ren, G.; Wang, Y. Utilization of adipocyte-derived lipids and enhanced intracellular trafficking of fatty acids contribute to breast cancer progression. Cell Commun. Signal. CCS 2018, 16, 32. [Google Scholar] [CrossRef] [Green Version]
- Veerkamp, J.H.; Peeters, R.A.; Maatman, R. Structural and functional features of different types of cytoplasmic fatty acid-binding proteins. Biochim. Biophys. Acta (BBA) 1991, 1081, 1–24. [Google Scholar] [CrossRef]
- Zimmerman, A.W.; Veerkamp, J.H. New insights into the structure and function of fatty acid-binding proteins. Cell. Mol. Life Sci. CMLS 2002, 59, 1096–1116. [Google Scholar] [CrossRef]
- Furuhashi, M.; Hotamisligil, G.S. Fatty acid-binding proteins: Role in metabolic diseases and potential as drug targets. Nat. Reviews. Drug Discov. 2008, 7, 489–503. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Hao, J.; Zeng, J.; Sauter, E.R. SnapShot: FABP Functions. Cell 2020, 182, 1066.e1. [Google Scholar] [CrossRef]
- Satoh, Y.; Mori, K.; Kitano, K.; Kitayama, J.; Yokota, H.; Sasaki, H.; Uozaki, H.; Fukayama, M.; Seto, Y.; Nagawa, H.; et al. Analysis for the combination expression of CK20, FABP1 and MUC2 is sensitive for the prediction of peritoneal recurrence in gastric cancer. Jpn. J. Clin. Oncol. 2012, 42, 148–152. [Google Scholar] [CrossRef] [Green Version]
- Hashimoto, T.; Kusakabe, T.; Watanabe, K.; Sugino, T.; Fukuda, T.; Nashimoto, A.; Honma, K.-I.; Sato, Y.; Kimura, H.; Fujii, H.; et al. Liver-type fatty acid-binding protein is highly expressed in intestinal metaplasia and in a subset of carcinomas of the stomach without association with the fatty acid synthase status in the carcinoma. Pathobiol. J. Immunopathol. Mol. Cell. Biol. 2004, 71, 115–122. [Google Scholar] [CrossRef]
- Jiang, Z.; Shen, H.; Tang, B.; Yu, Q.; Ji, X.; Wang, L. Quantitative proteomic analysis reveals that proteins required for fatty acid metabolism may serve as diagnostic markers for gastric cancer. Clin. Chim. Acta Int. J. Clin. Chem. 2017, 464, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Kusakabe, T.; Sugino, T.; Fukuda, T.; Watanabe, K.; Sato, Y.; Nashimoto, A.; Honma, K.; Kimura, H.; Fujii, H.; et al. Expression of heart-type fatty acid-binding protein in human gastric carcinoma and its association with tumor aggressiveness, metastasis and poor prognosis. Pathobiol. J. Immunopathol. Mol. Cell. Biol. 2004, 71, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yuan, Z.; Shen, R.; Jiang, Y.; Xu, W.; Gu, M.; Gu, X. Identification of biomarkers predicting the chemotherapeutic outcomes of capecitabine and oxaliplatin in patients with gastric cancer. Oncol. Lett. 2020, 20, 290. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Cho, S.M.; Kim, S.; Cheong, J.-H.; Kwon, H.J. Functional inhibition of fatty acid binding protein 4 ameliorates impaired ciliogenesis in GCs. Biochem. Biophys. Res. Commun. 2021, 539, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liu, Z.; Chen, X.; Lu, Y.; Wang, B.; Li, F.; Lu, S.; Zhou, X. Downregulation of FABP5 Suppresses the Proliferation and Induces the Apoptosis of Gastric Cancer Cells Through the Hippo Signaling Pathway. DNA Cell Biol. 2021, 40, 1076–1086. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, Z.W.; Su, W.H.; Chen, J.; Wang, Y.L. Prognostic Value and Immune Infiltrates of ABCA8 and FABP4 in Stomach Adenocarcinoma. BioMed Res. Int. 2020, 2020, 4145164. [Google Scholar] [CrossRef]
- Lin, R.; Zhang, H.; Yuan, Y.; He, Q.; Zhou, J.; Li, S.; Sun, Y.; Li, D.Y.; Qiu, H.-B.; Wang, W.; et al. Fatty Acid Oxidation Controls CD8(+) Tissue-Resident Memory T-cell Survival in Gastric Adenocarcinoma. Cancer Immunol. Res. 2020, 8, 479–492. [Google Scholar] [CrossRef] [Green Version]
- Dong, S.-R.; Ju, X.-L.; Yang, W.-Z. STAT5A reprograms fatty acid metabolism and promotes tumorigenesis of gastric cancer cells. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 8360–8370. [Google Scholar]
- Zhao, G.; Wu, M.; Wang, X.; Du, Z.; Zhang, G. Effect of FABP5 gene silencing on the proliferation, apoptosis and invasion of human gastric SGC-7901 cancer cells. Oncol. Lett. 2017, 14, 4772–4778. [Google Scholar] [CrossRef] [Green Version]
- Pan, J.; Dai, Q.; Zhang, T.; Li, C. Palmitate acid promotes gastric cancer metastasis via FABP5/SP1/UCA1 pathway. Cancer Cell Int. 2019, 19, 69. [Google Scholar] [CrossRef]
- Li, L.; Peng, Z.; Hu, Q.; Xu, L.; Zou, X.; Yu, Y.; Huang, D.; Yi, P. Berberine Suppressed Tumor Growth through Regulating Fatty Acid Metabolism and Triggering Cell Apoptosis via Targeting FABPs. Evid.-Based Complement. Altern. Med. 2020, 2020, 6195050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Wang, Y.; Guan, L.; Chen, Y.; Chen, P.; Sun, J.; Gonzalez, F.J.; Huang, M.; Bi, H. Lipidomics reveals carnitine palmitoyltransferase 1C protects cancer cells from lipotoxicity and senescence. J. Pharm. Anal. 2021, 11, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Casals, N.; Zammit, V.; Herrero, L.; Fadó, R.; Rodríguez-Rodríguez, R.; Serra, D. Carnitine palmitoyltransferase 1C: From cognition to cancer. Prog. Lipid Res. 2016, 61, 134–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Currie, E.; Schulze, A.; Zechner, R.; Walther, T.C.; Farese, R.V.J.R. Cellular fatty acid metabolism and cancer. Cell Metab. 2013, 18, 153–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, H.; Liu, B.; Chen, Z.; Li, G.; Zhang, Z. MSC-induced lncRNA HCP5 drove fatty acid oxidation through miR-3619-5p/AMPK/PGC1α/CEBPB axis to promote stemness and chemo-resistance of gastric cancer. Cell Death Dis. 2020, 11, 233. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Li, C.; Song, Y.; Yan, Z. Inhibition of carnitine palmitoyl transferase 1A-induced fatty acid oxidation suppresses cell progression in gastric cancer. Arch. Biochem. Biophys. 2020, 696, 108664. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, C.; Li, X.; Shen, J.; Xu, Y.; Shi, H.; Mu, X.; Pan, J.; Zhao, T.; Li, M.; et al. CPT1A-mediated succinylation of S100A10 increases human gastric cancer invasion. J. Cell. Mol. Med. 2019, 23, 293–305. [Google Scholar] [CrossRef]
- Li, X.; Zhang, C.; Zhao, T.; Su, Z.; Li, M.; Hu, J.; Wen, J.; Shen, J.; Wang, C.; Pan, J.; et al. Lysine-222 succinylation reduces lysosomal degradation of lactate dehydrogenase a and is increased in gastric cancer. J. Exp. Clin. Cancer Res. 2020, 39, 172. [Google Scholar] [CrossRef]
- Chen, L.; Peng, J.; Wang, Y.; Jiang, H.; Wang, W.; Dai, J.; Tang, M.; Wei, Y.; Kuang, H.; Xu, G.; et al. Fenofibrate-induced mitochondrial dysfunction and metabolic reprogramming reversal: The anti-tumor effects in gastric carcinoma cells mediated by the PPAR pathway. Am. J. Transl. Res. 2020, 12, 428–446. [Google Scholar]
- Wang, Y.; Lu, J.-H.; Wang, F.; Wang, Y.-N.; He, M.-M.; Wu, Q.-N.; Lu, Y.-X.; Yu, H.-E.; Chen, Z.-H.; Zhao, Q.; et al. Inhibition of fatty acid catabolism augments the efficacy of oxaliplatin-based chemotherapy in gastrointestinal cancers. Cancer Lett. 2020, 473, 74–89. [Google Scholar] [CrossRef]
- Chen, T.; Wu, G.; Hu, H.; Wu, C. Enhanced fatty acid oxidation mediated by CPT1C promotes gastric cancer progression. J. Gastrointest. Oncol. 2020, 11, 695–707. [Google Scholar] [CrossRef] [PubMed]
- Batchuluun, B.; Pinkosky, S.L.; Steinberg, G.R. Lipogenesis inhibitors: Therapeutic opportunities and challenges. Nat. Rev. Drug Discov. 2022, 21, 283–305. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Kang, B.; Li, C.; Chen, T.; Zhang, Z. GEPIA2: An enhanced web server for large-scale expression profiling and interactive analysis. Nucleic Acids Res. 2019, 47, W556–W560. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Molecules | Expression | Findings | Influence |
|---|---|---|---|
| SREBP1 | Upregulate [24,25] | Activation of SREBP-1c in GC resulted in upregulation of SCD and FASN and downregulation of ELOVL6. Knockdown of SREBP1c significantly inhibited the proliferation, invasiveness, and migration of GC cells [25] | Tumor promotion [25] |
| ACLY | Upregulate [33] | Inhibition of ACLY by high-dose sodium citrate reduced the growth of GC in mice [37] | Poor prognosis [33] Tumor promotion [37] |
| ACSS3 | - | ACSS3 knockdown could suppress colony formation under a regular culture, inhibit wound-healing ability under starvation conditions, and increase the basal level of cell death, even more dramatically under starvation conditions [42] | Poor prognosis [42] Tumor promotion [42] |
| ACSL4 | Downregulate [46] | ACSL4 knockdown enhanced cell growth, colony formation, and cell migration in vitro and promoted subcutaneous xenografts’ growth in vivo [46] | Tumor suppressing [46] |
| ACSL5 | - | SiRNA-mediated repression of ACSL5 inhibits the oncogenicity of MKN01 cells [47] | Tumor promotion [47] |
| pACC | Downregulate [53] | Metformin-induced pACC upregulation resulted in significant inhibition of GC cell proliferation and colony formation [51] | Poor prognosis [51,53] Tumor promotion [51] |
| FASN | Upregulate [24,58,59,60,62] | The FASN inhibitor C75 or siFASN blocked endogenous fatty acid metabolism in GC and attenuated MACC1 upregulation-induced cell proliferation and chemo-resistance to oxaliplatin to varying degrees [63] | Poor prognosis [59,61] Tumor promotion [60,63] Oxaliplatin resistance [63] |
| Inhibition of FASN also inhibited GC proliferation and metastasis by targeting the mTOR/Gli1 signaling pathway [60] | |||
| SCD1 | Upregulate [70,71] | SCD1 regulates cell stemness through the Hippo/YAP pathway, which influences gastric carcinogenesis, chemo-resistance, and metastasis [70] | Oxaliplatin resistance [70] Tumor promotion [70,71,72] |
| SCD1 promotes the proliferative capacity, migratory capacity, and stemness of GC cells, and SCD1 also has an anti-iron death effect and accelerates the growth of transplanted tumors in mice [71] | |||
| The mean tumor volume in the A939572-treated group was reduced by nearly 50% relative to vehicle-treated animals [72] | |||
| CD36 | Upregulate [77] | CD36 mediates c-Myc-induced DEK transcription in GC cells, then upregulation of DEK enhances GSK-3β/β-catenin signaling [78] | Poor prognosis [78] Tumor promotion [78,80,81] |
| Palmitic acid promoted GC metastasis through phosphorylation of AKT and CD36 promoted GC metastasis as a key mediator of AKT/GSK-3β/β-catenin signaling [81] | |||
| FABP4 | Downregulate [96] Upregulate [97] | Regulation of FABP4 by a small-molecule FABP4 inhibitor or siFABP4 restores primary cilia to inhibit the proliferation and migration of GCs, thus exhibiting potential anticancer effects [94] | Tumor promotion [94] Poor prognosis [94,96] |
| FABP5 | Upregulate [97] | Silencing of the FABP5 gene attenuated the invasiveness of GC cells, prevented cell proliferation, and stalled the cell cycle in the G0/G1 phase, leading to a significant increase in apoptosis [95,99] | Tumor promotion [95,97,98,99,101] |
| PA enters GC cells, promotes the nuclear transport of FABP5, which then increases the GC nuclear protein levels of SP1 and PA-induced GC metastasis via FABP5/SP1/UCA1 signaling, contributing efficient prevention and therapeutic strategies for GC [100] | |||
| CPT1 | Upregulate [106,107] | CPT1A overexpression activates fatty acid oxidation in GC cells by increasing the NADP/NADPH ratio and thus increases the proliferation, invasion, and epithelial–mesenchymal transition (EMT) of GC cells [106] | Poor prognosis [106,111] Enhanced tumorigenesis [106,108,110] |
| CPT1A succinylates LDHA on K222, which thereby reduces the binding and inhibits the degradation of LDHA and promotes GC invasion and proliferation [108] | |||
| The CPT1 inhibitor perhexiline and oxaliplatin synergistically inhibit tumor xenograft progression, suggesting that CPT1-mediated fatty acid translocation and further fatty acid oxidation may be associated with oxaliplatin resistance [110] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Zhang, L.; Qiu, Z.; Deng, W.; Wang, W. Key Molecules of Fatty Acid Metabolism in Gastric Cancer. Biomolecules 2022, 12, 706. https://doi.org/10.3390/biom12050706
Li C, Zhang L, Qiu Z, Deng W, Wang W. Key Molecules of Fatty Acid Metabolism in Gastric Cancer. Biomolecules. 2022; 12(5):706. https://doi.org/10.3390/biom12050706
Chicago/Turabian StyleLi, Chunlei, Lilong Zhang, Zhendong Qiu, Wenhong Deng, and Weixing Wang. 2022. "Key Molecules of Fatty Acid Metabolism in Gastric Cancer" Biomolecules 12, no. 5: 706. https://doi.org/10.3390/biom12050706






