Immunometabolic Markers in a Small Patient Cohort Undergoing Immunotherapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Clinical Blood Parameters, Blood Metabolites and Hormones
2.3. Flow Cytometry
3. Results
3.1. Identification of Target Analytes in Responders and Non-Responders
3.2. Correlation with PFS-Introduced PD-1+ Monocytes and the Free Androgen Index as Potentially Novel Markers in Immunotherapy
3.3. Combining Immune Subset Markers with Metabolic Markers Enhanced Correlation with PFS
3.4. Multiple Correlation Analysis Revealed a Strong Inverse Correlation between PD-1+ Monocytes and Hemoglobin
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zang, X. 2018 Nobel Prize in medicine awarded to cancer immunotherapy: Immune checkpoint blockade—A personal account. Genes Dis. 2018, 5, 302–303. [Google Scholar] [CrossRef] [PubMed]
- Lei, Q.; Wang, D.; Sun, K.; Wang, L.; Zhang, Y. Resistance Mechanisms of Anti-PD1/PDL1 Therapy in Solid Tumors. Front. Cell Dev. Biol. 2020, 8, 672. [Google Scholar] [CrossRef] [PubMed]
- Kok, V.C. Current Understanding of the Mechanisms Underlying Immune Evasion From PD-1/PD-L1 Immune Checkpoint Blockade in Head and Neck Cancer. Front. Oncol. 2020, 10, 268. [Google Scholar] [CrossRef]
- Kambayashi, Y.; Fujimura, T.; Hidaka, T.; Aiba, S. Biomarkers for Predicting Efficacies of Anti-PD1 Antibodies. Front. Med. 2019, 6, 174. [Google Scholar] [CrossRef]
- Riedl, J.M.; Barth, D.A.; Brueckl, W.M.; Zeitler, G.; Foris, V.; Mollnar, S.; Stotz, M.; Rossmann, C.H.; Terbuch, A.; Balic, M.; et al. C-Reactive Protein (CRP) Levels in Immune Checkpoint Inhibitor Response and Progression in Advanced Non-Small Cell Lung Cancer: A Bi-Center Study. Cancers 2020, 12, 2319. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, F.; Yuan, F.; Li, Y.; Ma, J.; Ou, Q.; Liu, Z.; Yang, B.; Wang, L.; Tao, H.; et al. Pretreatment hemoglobin level as a predictor to evaluate the efficacy of immune checkpoint inhibitors in patients with advanced non-small cell lung cancer. Ther. Adv. Med. Oncol. 2020, 12, 1758835920970049. [Google Scholar] [CrossRef]
- Krieg, C.; Nowicka, M.; Guglietta, S.; Schindler, S.; Hartmann, F.J.; Weber, L.M.; Dummer, R.; Robinson, M.D.; Levesque, M.P.; Becher, B. High-dimensional single-cell analysis predicts response to anti-PD-1 immunotherapy. Nat. Med. 2018, 24, 144–153. [Google Scholar] [CrossRef]
- Mengos, A.E.; Gastineau, D.A.; Gustafson, M.P. The CD14+HLA-DRlo/neg Monocyte: An Immunosuppressive Phenotype That Restrains Responses to Cancer Immunotherapy. Front. Immunol. 2019, 10, 1147. [Google Scholar] [CrossRef]
- Peranzoni, E.; Ingangi, V.; Masetto, E.; Pinton, L.; Marigo, I. Myeloid Cells as Clinical Biomarkers for Immune Checkpoint Blockade. Front. Immunol. 2020, 11, 1590. [Google Scholar] [CrossRef]
- Perrone, F.; Minari, R.; Bersanelli, M.; Bordi, P.; Tiseo, M.; Favari, E.; Sabato, R.; Buti, S. The Prognostic Role of High Blood Cholesterol in Advanced Cancer Patients Treated With Immune Checkpoint Inhibitors. J. Immunother. 2020, 43, 196–203. [Google Scholar] [CrossRef]
- Ichihara, E.; Harada, D.; Inoue, K.; Sato, K.; Hosokawa, S.; Kishino, D.; Watanabe, K.; Ochi, N.; Oda, N.; Hara, N.; et al. The impact of body mass index on the efficacy of anti-PD-1/PD-L1 antibodies in patients with non-small cell lung cancer. Lung Cancer 2020, 139, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Cortellini, A.; Bersanelli, M.; Buti, S.; Cannita, K.; Santini, D.; Perrone, F.; Giusti, R.; Tiseo, M.; Michiara, M.; Di Marino, P.; et al. A Multicenter Study of Body Mass Index in Cancer Patients Treated with Anti-PD-1/PD-L1 Immune Checkpoint Inhibitors: When Overweight Becomes Favorable. Available online: https://jitc.biomedcentral.com/articles/10.1186/s40425-019-0527-y (accessed on 26 March 2021).
- Renner, K.; Bruss, C.; Schnell, A.; Koehl, G.; Becker, H.M.; Fante, M.; Menevse, A.-N.; Kauer, N.; Blazquez, R.; Hacker, L.; et al. Restricting Glycolysis Preserves T Cell Effector Functions and Augments Checkpoint Therapy. Cell Rep. 2019, 29, 135–150.e9. [Google Scholar] [CrossRef] [PubMed]
- Irelli, A.; Sirufo, M.M.; D’Ugo, C.; Ginaldi, L.; de Martinis, M. Sex and Gender Influences on Cancer Immunotherapy Response. Biomedicines 2020, 8, 232. [Google Scholar] [CrossRef] [PubMed]
- Fornarini, G.; Rebuzzi, S.E.; Banna, G.L.; Calabrò, F.; Scandurra, G.; de Giorgi, U.; Masini, C.; Baldessari, C.; Naglieri, E.; Caserta, C.; et al. Immune-inflammatory biomarkers as prognostic factors for immunotherapy in pretreated advanced urinary tract cancer patients: An analysis of the Italian SAUL cohort. ESMO Open 2021, 6, 100118. [Google Scholar] [CrossRef]
- Imai, H.; Kishikawa, T.; Minemura, H.; Yamada, Y.; Ibe, T.; Yamaguchi, O.; Mouri, A.; Hamamoto, Y.; Kanazawa, K.; Kasai, T.; et al. Pretreatment Glasgow prognostic score predicts survival among patients with high PD-L1 expression administered first-line pembrolizumab monotherapy for non-small cell lung cancer. Cancer Med. 2021, 10, 6971–6984. [Google Scholar] [CrossRef]
- Banna, G.L.; Signorelli, D.; Metro, G.; Galetta, D.; de Toma, A.; Cantale, O.; Banini, M.; Friedlaender, A.; Pizzutillo, P.; Garassino, M.C.; et al. Neutrophil-to-lymphocyte ratio in combination with PD-L1 or lactate dehydrogenase as biomarkers for high PD-L1 non-small cell lung cancer treated with first-line pembrolizumab. Transl. Lung Cancer Res. 2020, 9, 1533–1542. [Google Scholar] [CrossRef]
- Banna, G.L.; Tiseo, M.; Cortinovis, D.L.; Facchinetti, F.; Aerts, J.G.J.V.; Baldessari, C.; Giusti, R.; Bria, E.; Grossi, F.; Berardi, R.; et al. Host immune-inflammatory markers to unravel the heterogeneous outcome and assessment of patients with PD-L1 ≥ 50% metastatic non-small cell lung cancer and poor performance status receiving first-line immunotherapy. Thorac. Cancer 2022, 13, 483–488. [Google Scholar] [CrossRef]
- Benzekry, S.; Grangeon, M.; Karlsen, M.; Alexa, M.; Bicalho-Frazeto, I.; Chaleat, S.; Tomasini, P.; Barbolosi, D.; Barlesi, F.; Greillier, L. Machine Learning for Prediction of Immunotherapy Efficacy in Non-Small Cell Lung Cancer from Simple Clinical and Biological Data. Cancers 2021, 13, 6210. [Google Scholar] [CrossRef]
- Hatae, R.; Chamoto, K.; Kim, Y.H.; Sonomura, K.; Taneishi, K.; Kawaguchi, S.; Yoshida, H.; Ozasa, H.; Sakamori, Y.; Akrami, M.; et al. Combination of host immune metabolic biomarkers for the PD-1 blockade cancer immunotherapy. JCI Insight 2020, 5, e133501. [Google Scholar] [CrossRef]
- Hurkmans, D.P.; Kuipers, M.E.; Smit, J.; van Marion, R.; Mathijssen, R.H.J.; Postmus, P.E.; Hiemstra, P.S.; Aerts, J.G.J.V.; von der Thüsen, J.H.; van der Burg, S.H. Tumor mutational load, CD8+ T cells, expression of PD-L1 and HLA class I to guide immunotherapy decisions in NSCLC patients. Cancer Immunol. Immunother. 2020, 69, 771–777. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. Available online: https://www.bibsonomy.org/bibtex/b38b0e6655978ad8c7d8455b175c2cbf (accessed on 3 April 2022). [CrossRef]
- Tanizaki, J.; Haratani, K.; Hayashi, H.; Chiba, Y.; Nakamura, Y.; Yonesaka, K.; Kudo, K.; Kaneda, H.; Hasegawa, Y.; Tanaka, K.; et al. Peripheral Blood Biomarkers Associated with Clinical Outcome in Non-Small Cell Lung Cancer Patients Treated with Nivolumab. J. Thorac. Oncol. 2018, 13, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Weide, B.; Martens, A.; Hassel, J.C.; Berking, C.; Postow, M.A.; Bisschop, K.; Simeone, E.; Mangana, J.; Schilling, B.; Di Giacomo, A.M.; et al. Baseline Biomarkers for Outcome of Melanoma Patients Treated with Pembrolizumab. Clin. Cancer Res. 2016, 22, 5487–5496. [Google Scholar] [CrossRef] [PubMed]
- Rosner, S.; Kwong, E.; Shoushtari, A.N.; Friedman, C.F.; Betof, A.S.; Brady, M.S.; Coit, D.G.; Callahan, M.K.; Wolchok, J.D.; Chapman, P.B.; et al. Peripheral blood clinical laboratory variables associated with outcomes following combination nivolumab and ipilimumab immunotherapy in melanoma. Cancer Med. 2018, 7, 690–697. [Google Scholar] [CrossRef] [PubMed]
- Galli, G.; Corsetto, P.; Ferrara, R.; Prelaj, A.; Proto, C.; Signorelli, D.; Zilembo, N.; de Toma, A.; Pagani, F.; Randon, G.; et al. Impact of cholesterolemia and body mass index on outcome of metastatic non small cell lung cancer treated with immunotherapy. J. Clin. Oncol. 2019, 37, e20691. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, W.; Li, X.; He, Y. Cholesterol Metabolism as a Potential Therapeutic Target and a Prognostic Biomarker for Cancer Immunotherapy. OncoTargets Ther. 2021, 14, 3803–3812. [Google Scholar] [CrossRef]
- Tong, J.; Mao, Y.; Yang, Z.; Xu, Q.; Zheng, Z.; Zhang, H.; Wang, J.; Zhang, S.; Rong, W.; Zheng, L. Baseline Serum Cholesterol Levels Predict the Response of Patients with Advanced Non-Small Cell Lung Cancer to Immune Checkpoint Inhibitor-Based Treatment. Cancer Manag. Res. 2021, 13, 4041–4053. [Google Scholar] [CrossRef]
- Brustugun, O.T.; Sprauten, M.; Helland, A. C-reactive protein (CRP) as a predictive marker for immunotherapy in lung cancer. JCO 2016, 34, e20623. [Google Scholar] [CrossRef]
- Oya, Y.; Yoshida, T.; Kuroda, H.; Mikubo, M.; Kondo, C.; Shimizu, J.; Horio, Y.; Sakao, Y.; Hida, T.; Yatabe, Y. Predictive clinical parameters for the response of nivolumab in pretreated advanced non-small-cell lung cancer. Oncotarget 2017, 8, 103117–103128. [Google Scholar] [CrossRef]
- Iivanainen, S.; Ahvonen, J.; Knuuttila, A.; Tiainen, S.; Koivunen, J.P. Elevated CRP levels indicate poor progression-free and overall survival on cancer patients treated with PD-1 inhibitors. ESMO Open 2019, 4, e000531. [Google Scholar] [CrossRef]
- Laino, A.S.; Woods, D.; Vassallo, M.; Qian, X.; Tang, H.; Wind-Rotolo, M.; Weber, J. Serum interleukin-6 and C-reactive protein are associated with survival in melanoma patients receiving immune checkpoint inhibition. J. Immunother. Cancer 2020, 8, e000842. [Google Scholar] [CrossRef] [PubMed]
- Lechner, M.G.; Megiel, C.; Russell, S.M.; Bingham, B.; Arger, N.; Woo, T.; Epstein, A.L. Functional characterization of human Cd33+ and Cd11b+ myeloid-derived suppressor cell subsets induced from peripheral blood mononuclear cells co-cultured with a diverse set of human tumor cell lines. J. Transl. Med. 2011, 9, 90. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.; Gibney, G.; Kudchadkar, R.; Yu, B.; Cheng, P.; Martinez, A.J.; Kroeger, J.; Richards, A.; McCormick, L.; Moberg, V.; et al. Phase I/II Study of Metastatic Melanoma Patients Treated with Nivolumab Who Had Progressed after Ipilimumab. Cancer Immunol. Res. 2016, 4, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Strauss, L.; Mahmoud, M.A.A.; Weaver, J.D.; Tijaro-Ovalle, N.M.; Christofides, A.; Wang, Q.; Pal, R.; Yuan, M.; Asara, J.; Patsoukis, N.; et al. Targeted deletion of PD-1 in myeloid cells induces antitumor immunity. Sci. Immunol. 2020, 5, eaay1863. [Google Scholar] [CrossRef]
- Columbus, G. PD-1 Expression on Monocytes Potential Immunotherapy Biomarker in RCC. OncLive [Online], 13 May 2016. Available online: https://www.onclive.com/view/pd-1-expression-on-monocytes-potential-immunotherapy-biomarker-in-rcc (accessed on 12 March 2022).
- Bai, R.; Lv, Z.; Xu, D.; Cui, J. Predictive biomarkers for cancer immunotherapy with immune checkpoint inhibitors. Biomark. Res. 2020, 8, 34. [Google Scholar] [CrossRef]
- Koh, J.; Lee, K.Y.; Kim, B.; Kim, M.S.; Cho, H.J.; Sun, J.-M.; Ahn, J.S.; Park, K.; Ahn, M.-J. Abstract A138: CD39 increase on cytotoxic T-cell induced by myeloid-derived suppressor cell correlated with poor prognosis in patients with non-small cell lung cancer. In Maintenance of Immune Balance: Effects of Targeted and Immune Therapies, Proceedings of the Abstracts: Fourth CRI-CIMT-EATI-AACR International Cancer Immunotherapy Conference: Translating Science into Survival, New York, NY, USA, 30 September–3 October 2018; American Association for Cancer Research: Philadelphia, PA, USA; p. A138.
- Li, X.-Y.; Moesta, A.K.; Xiao, C.; Nakamura, K.; Casey, M.; Zhang, H.; Madore, J.; Lepletier, A.; Aguilera, A.R.; Sundarrajan, A.; et al. Targeting CD39 in Cancer Reveals an Extracellular ATP- and Inflammasome-Driven Tumor Immunity. Cancer Discov. 2019, 9, 1754–1773. [Google Scholar] [CrossRef]
- Guo, H.; Xun, L.; Zhang, R.; Gou, X. Ratio of CD147high/CD147low in CD4+CD25+ T cells: A potential biomarker for early diagnosis and prediction of response to therapy for autoimmune diseases. Med. Hypotheses 2018, 115, 1–4. [Google Scholar] [CrossRef]
- Landras, A.; Reger de Moura, C.; Jouenne, F.; Lebbe, C.; Menashi, S.; Mourah, S. CD147 Is a Promising Target of Tumor Progression and a Prognostic Biomarker. Cancers 2019, 11, 1803. [Google Scholar] [CrossRef]
- Solstad, T.; Bains, S.J.; Landskron, J.; Aandahl, E.M.; Thiede, B.; Taskén, K.; Torgersen, K.M. CD147 (Basigin/Emmprin) identifies FoxP3+CD45RO+CTLA4+-activated human regulatory T cells. Blood 2011, 118, 5141–5151. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, J.; Wu, X.; Yao, H.; Yan, Z.; Guo, T.; Wang, W.; Wang, P.; Li, Y.; Yang, X.; et al. CD147 regulates antitumor CD8+ T-cell responses to facilitate tumor-immune escape. Cell. Mol. Immunol. 2020, 18, 1995–2009. [Google Scholar] [CrossRef]
- Gomes, A.L.; Carvalho, T.; Serpa, J.; Torre, C.; Dias, S. Hypercholesterolemia promotes bone marrow cell mobilization by perturbing the SDF-1:CXCR4 axis. Blood 2010, 115, 3886–3894. [Google Scholar] [CrossRef] [PubMed]
- Oda, E. Longitudinal associations between lymphocyte count and LDL cholesterol in a health screening population. J. Clin. Transl. Endocrinol. 2014, 1, 49–53. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Eacker, S.M.; Agrawal, N.; Qian, K.; Dichek, H.L.; Gong, E.-Y.; Lee, K.; Braun, R.E. Hormonal regulation of testicular steroid and cholesterol homeostasis. Mol. Endocrinol. 2008, 22, 623–635. [Google Scholar] [CrossRef] [PubMed]
- Buitenwerf, E.; Dullaart, R.P.F.; Muller Kobold, A.C.; Links, T.P.; Sluiter, W.J.; Connelly, M.A.; Kerstens, M.N. Cholesterol delivery to the adrenal glands estimated by adrenal venous sampling: An in vivo model to determine the contribution of circulating lipoproteins to steroidogenesis in humans. J. Clin. Lipidol. 2017, 11, 733–738. [Google Scholar] [CrossRef]
- Blom, D.J.; Djedjos, C.S.; Monsalvo, M.L.; Bridges, I.; Wasserman, S.M.; Scott, R.; Roth, E. Effects of Evolocumab on Vitamin E and Steroid Hormone Levels: Results From the 52-Week, Phase 3, Double-Blind, Randomized, Placebo-Controlled DESCARTES Study. Circ. Res. 2015, 117, 731–741. [Google Scholar] [CrossRef]
- Dev, R.; Del Fabbro, E.; Dalal, S. Endocrinopathies and cancer cachexia. Curr. Opin. Support. Palliat. Care 2019, 13, 286–291. [Google Scholar] [CrossRef]
- Rounis, K.; Makrakis, D.; Tsigkas, A.-P.; Georgiou, A.; Galanakis, N.; Papadaki, C.; Monastirioti, A.; Vamvakas, L.; Kalbakis, K.; Vardakis, N.; et al. Cancer cachexia syndrome and clinical outcome in patients with metastatic non-small cell lung cancer treated with PD-1/PD-L1 inhibitors: Results from a prospective, observational study. Transl. Lung Cancer Res. 2021, 10, 3538–3549. [Google Scholar] [CrossRef]
- Fernández-Pombo, A.; Rodríguez-Carnero, G.; Castro, A.I.; Cantón-Blanco, A.; Seoane, L.M.; Casanueva, F.F.; Crujeiras, A.B.; Martínez-Olmos, M.A. Relevance of nutritional assessment and treatment to counteract cardiac cachexia and sarcopenia in chronic heart failure. Clin. Nutr. (Edinb. Scotl.) 2021, 40, 5141–5155. [Google Scholar] [CrossRef]
- Cai, Z.; Xi, H.; Pan, Y.; Jiang, X.; Chen, L.; Cai, Y.; Zhu, K.; Chen, C.; Xu, X.; Chen, M. Effect of testosterone deficiency on cholesterol metabolism in pigs fed a high-fat and high-cholesterol diet. Lipids Health Dis. 2015, 14, 18. [Google Scholar] [CrossRef]
- Kenny, A.M.; Prestwood, K.M.; Gruman, C.A.; Fabregas, G.; Biskup, B.; Mansoor, G. Effects of transdermal testosterone on lipids and vascular reactivity in older men with low bioavailable testosterone levels. J. Gerontology. Ser. A Biol. Sci. Med. Sci. 2002, 57, M460–M465. [Google Scholar] [CrossRef]
- Salam, R.; Kshetrimayum, A.S.; Keisam, R. Testosterone and metabolic syndrome: The link. Indian J. Endocrinol. Metab. 2012, 16 (Suppl. S1), S12–S19. [Google Scholar] [CrossRef] [PubMed]
- Bersanelli, M.; Cortellini, A.; Buti, S. The interplay between cholesterol (and other metabolic conditions) and immune-checkpoint immunotherapy: Shifting the concept from the “inflamed tumor” to the “inflamed patient”. Hum. Vaccines Immunother. 2021, 17, 1930–1934. [Google Scholar] [CrossRef] [PubMed]
- Khojandi, N.; Kuehm, L.M.; Piening, A.; Donlin, M.J.; Hsueh, E.C.; Schwartz, T.L.; Farrell, K.; Richart, J.M.; Geerling, E.; Pinto, A.K.; et al. Oxidized Lipoproteins Promote Resistance to Cancer Immunotherapy Independent of Patient Obesity. Cancer Immunol. Res. 2021, 9, 214–226. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Polesso, F.; Wang, C.; Sehrawat, A.; Hawkins, R.M.; Murray, S.E.; Thomas, G.V.; Caruso, B.; Thompson, R.F.; Wood, M.A.; et al. Androgen receptor activity in T cells limits checkpoint blockade efficacy. Nature 2022, 603, 587–598. [Google Scholar] [CrossRef]
- Kwon, H.; Schafer, J.M.; Song, N.-J.; Kaneko, S.; Li, A.; Xiao, T.; Ma, A.; Allen, C.; Das, K.; Zhou, L.; et al. Androgen conspires with the CD8+ T cell exhaustion program and contributes to sex bias in cancer. Sci. Immunol. 2022. [Google Scholar] [CrossRef]
- Ma, X.; Bi, E.; Lu, Y.; Su, P.; Huang, C.; Liu, L.; Wang, Q.; Yang, M.; Kalady, M.F.; Qian, J.; et al. Cholesterol Induces CD8+ T Cell Exhaustion in the Tumor Microenvironment. Cell Metab. 2019, 30, 143–156. [Google Scholar] [CrossRef]
- Markman, J.L.; Porritt, R.A.; Wakita, D.; Lane, M.E.; Martinon, D.; Noval Rivas, M.; Luu, M.; Posadas, E.M.; Crother, T.R.; Arditi, M. Loss of testosterone impairs anti-tumor neutrophil function. Nat. Commun. 2020, 11, 1613. [Google Scholar] [CrossRef]
- Tulchiner, G.; Pichler, R.; Ulmer, H.; Staudacher, N.; Lindner, A.K.; Brunner, A.; Zelger, B.; Steinkohl, F.; Aigner, F.; Horninger, W.; et al. Sex-specific hormone changes during immunotherapy and its influence on survival in metastatic renal cell carcinoma. Cancer Immunol. Immunother. 2021, 70, 2805–2817. [Google Scholar] [CrossRef]
- Haupt, S.; Caramia, F.; Klein, S.L.; Rubin, J.B.; Haupt, Y. Sex disparities matter in cancer development and therapy. Nat. Rev. Cancer 2021, 21, 393–407. [Google Scholar] [CrossRef]
- Fang, F.; Qin, Y.; Hao, F.; Li, Q.; Zhang, W.; Zhao, C.; Chen, S.; Zhao, L.; Wang, L.; Cai, J. CD147 modulates androgen receptor activity through the Akt/Gsk-3β/β-catenin/AR pathway in prostate cancer cells. Oncol. Lett. 2016, 12, 1124–1128. [Google Scholar] [CrossRef]
- Li, X.; Yu, X.; Dai, D.; Song, X.; Xu, W. The altered glucose metabolism in tumor and a tumor acidic microenvironment associated with extracellular matrix metalloproteinase inducer and monocarboxylate transporters. Oncotarget 2016, 7, 23141–23155. [Google Scholar] [CrossRef] [PubMed]
- Grass, G.D.; Toole, B.P. How, with whom and when: An overview of CD147-mediated regulatory networks influencing matrix metalloproteinase activity. Biosci. Rep. 2015, 36, e00283. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Jin, R.; Zhu, X.; Yan, J.; Li, G. Function of CD147 in atherosclerosis and atherothrombosis. J. Cardiovasc. Transl. Res. 2015, 8, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Landskron, J.; Taskén, K. CD147 in regulatory T cells. Cell. Immunol. 2013, 282, 17–20. [Google Scholar] [CrossRef]
- Haschka, D.; Petzer, V.; Kocher, F.; Tschurtschenthaler, C.; Schaefer, B.; Seifert, M.; Sopper, S.; Sonnweber, T.; Feistritzer, C.; Arvedson, T.L.; et al. Classical and intermediate monocytes scavenge non-transferrin-bound iron and damaged erythrocytes. JCI Insight 2019, 4, e98867. [Google Scholar] [CrossRef]
- Tymoszuk, P.; Nairz, M.; Brigo, N.; Petzer, V.; Heeke, S.; Kircher, B.; Hermann-Kleiter, N.; Klepsch, V.; Theurl, I.; Weiss, G.; et al. Iron Supplementation Interferes With Immune Therapy of Murine Mammary Carcinoma by Inhibiting Anti-Tumor T Cell Function. Front. Oncol. 2020, 10, 584477. [Google Scholar] [CrossRef]
- Yan, Z.; Xu, G. A Novel Choice to Correct Inflammation-Induced Anemia in CKD: Oral Hypoxia-Inducible Factor Prolyl Hydroxylase Inhibitor Roxadustat. Front. Med. 2020, 7, 393. [Google Scholar] [CrossRef]
- Stansfield, B.K.; Ingram, D.A. Clinical significance of monocyte heterogeneity. Clin. Transl. Med. 2015, 4, 5. [Google Scholar] [CrossRef]
- Skrzeczyńska-Moncznik, J.; Bzowska, M.; Loseke, S.; Grage-Griebenow, E.; Zembala, M.; Pryjma, J. Peripheral blood CD14high CD16+ monocytes are main producers of IL-10. Scand. J. Immunol. 2008, 67, 152–159. [Google Scholar] [CrossRef]
- Prat, M.; Le Naour, A.; Coulson, K.; Lemée, F.; Leray, H.; Jacquemin, G.; Rahabi, M.C.; Lemaitre, L.; Authier, H.; Ferron, G.; et al. Circulating CD14high CD16low intermediate blood monocytes as a biomarker of ascites immune status and ovarian cancer progression. J. Immunother. Cancer 2020, 8, e000472. [Google Scholar] [CrossRef]
- Ando, K.; Hamada, K.; Shida, M.; Ohkuma, R.; Kubota, Y.; Horiike, A.; Matsui, H.; Ishiguro, T.; Hirasawa, Y.; Ariizumi, H.; et al. A high number of PD-L1+ CD14+ monocytes in peripheral blood is correlated with shorter survival in patients receiving immune checkpoint inhibitors. Cancer Immunol. Immunother. 2021, 70, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Gubbels Bupp, M.R.; Jorgensen, T.N. Androgen-Induced Immunosuppression. Front. Immunol. 2018, 9, 794. [Google Scholar] [CrossRef] [PubMed]




| Variables | Patients (32 = 100%) | Responders (18 = 56.25%) | Non-Responders (14 = 43.75%) |
|---|---|---|---|
| Female | 8 (25%) | 6 (18.75%) | 2 (6.25%) |
| Male | 24 (75%) | 12 (37.5%) | 12 (37.5%) |
| Age (years) | 64.78 (34–85) | 67.94 (46–85) | 60.71 (34–82) |
| Body mass index (kg/m2) | 24.19 (17.6–35.35) | 25.35 (18.8–35.35) | 22.86 (17.6–28.10) |
| Statin | 7 (21.88%) | 3 (9.38%) | 4 (12.5%) |
| Prednisolone ≤ 20 mg | 7 (21.88%) | 5 (15.63%) | 2 (6.25%) |
| NSAID | 18 (56.25%) | 8 (25%) | 10 (31.25%) |
| Primary tumor | |||
| NSCLC | 12 (37.5%) | 5 (15.63%) | 7 (21.88%) |
| Melanoma | 8 (25%) | 8 (25%) | 0 (0%) |
| HNSCC | 6 (18.75%) | 2 (6.25%) | 4 (12.5%) |
| Others | 6 (18.75%) | 3 (9.38%) | 3 (9.38%) |
| Previous treatments | |||
| <1 | 8 (25%) | 7 (21.88%) | 1 (3.13%) |
| ≥1 | 24 (75%) | 11 (34.38%) | 13 (40.63%) |
| Pembrolizumab | 10 (31.25%) | 9 (28.13%) | 1 (3.13%) |
| Nivolumab | 22 (68.75%) | 9 (28.13%) | 13 (40.63%) |
| Adverse events | 16 (50%) | 13 (40.63%) | 3 (9.38%) |
| Immune Subset | Standard Value or % Population | Responders | Non-Responders | Significance (p) |
|---|---|---|---|---|
| Absolute lymphocyte counts | (1.18–3.74/nL) | 1.49/nL (±0.66) | 0.74/nL (±0.26) | 0.0002 *** |
| Absolute basophil counts | (0.01–0.08/nL) | 0.03/nL (0.02/0.05) | 0.02/nL (0.01/0.02) | 0.0071 ** |
| LDL | (<100 mg/dL) | 131.5 mg/dL (108.75/165.5) | 95 mg/dL (75.75/116) | 0.0009 *** |
| HDL | (40–60 mg/dL) | 50 mg/dL (40/67.50) | 39.5 mg/dL (31/51.50) | 0.0253 * |
| HB | (11.2–15.7 g/dL) | 12.94 g/dL (±1.48) | 11.29 g/dL (±1.83) | 0.0109 * |
| CRP | (<5 mg/L) | 5.25 mg/L (2.9/13.58) | 11.25 mg/L (7/35.5) | 0.0162 * |
| CD33high CD11b+ monocytes | % of leukocytes | 1.89% (1.61/4.02) | 4.94% (2.74/7.0) | 0.0238 * |
| Myeloid Subsets | % from Population | Responders | Non-Responders | Significance (p) |
|---|---|---|---|---|
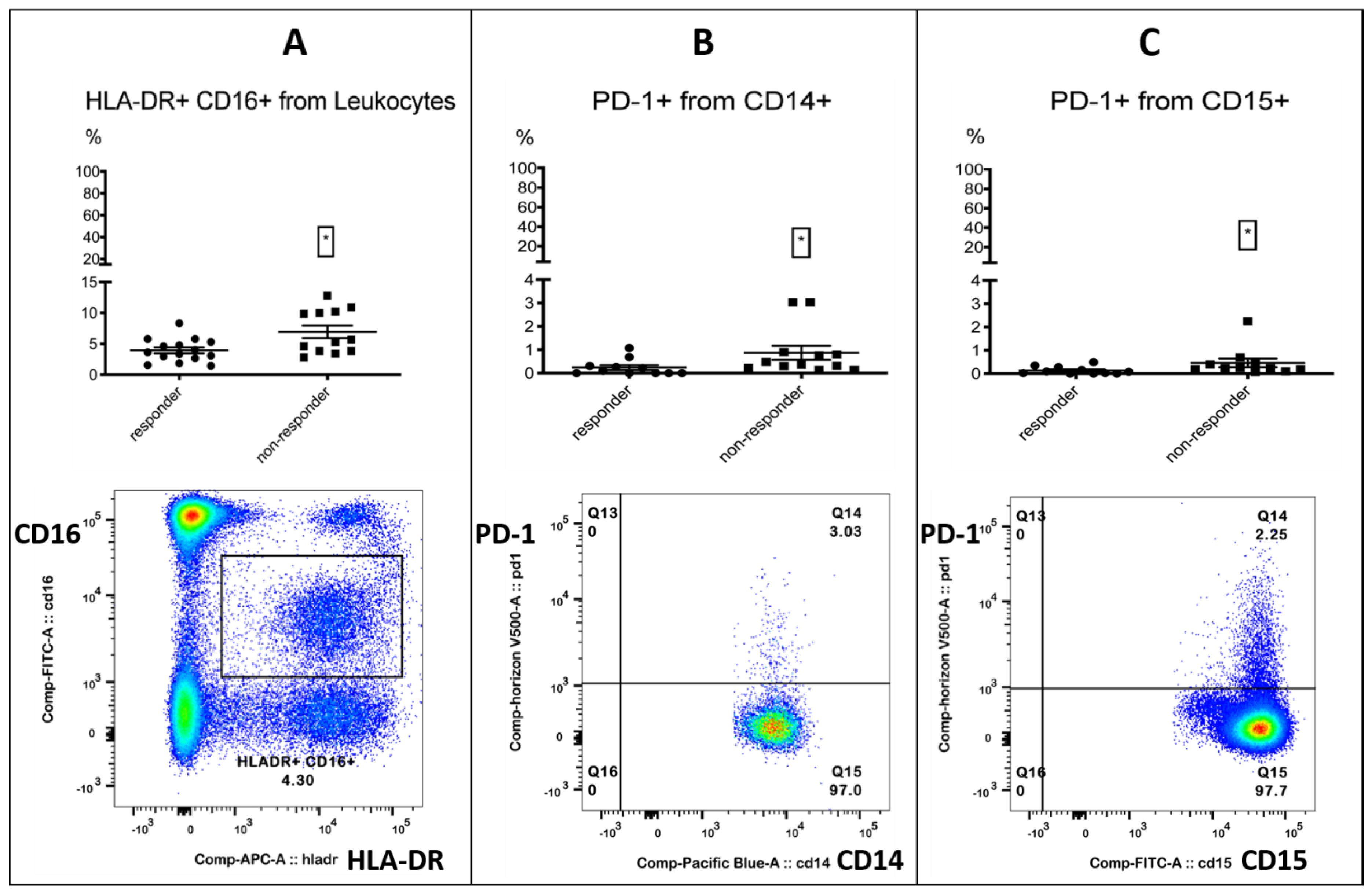
| PD-1+ granulocytes | % CD15+ granulocytes | 0.08% (0/0.28) | 0.24% (0.19/0.44) | 0.0266 * |
| PD-1+ monocytes | % CD14+ monocytes | 0.1% (0/0.31) | 0.43% (0.25/0.88) | 0.0119 * |
| HLA-DR+CD16+ | % leukocytes | 3.62% (2.68/5.31) | 5.49% (3.84/10.15) | 0.0291 * |
| Lymphocyte Subsets | % from Population | Responders | Non-Responders | Significance (p) |
|---|---|---|---|---|
| CD147+ CD19- | % from lymphocytes | 4.61% (3.32/7.9) | 10.1% (8.04/12.2) | 0.0376 * |
| CD39+ CD19- | % from lymphocytes | 1.3% (0.85/2.69) | 3.07% (1.62/6.39) | 0.0481 * |
| CD39+ CD19+ | % from lymphocytes | 3.88% (2.06/5.85) | 1.3% (0.71/2.36) | 0.0246 * |
| CD33- CD11b- | % from leukocytes | 14.7% (9.34/39.3) | 7.25% (5.1/10.55) | 0.0321 * |
| Hormonal Metabolites | Responders | Non-Responders | Significance (p) |
|---|---|---|---|
| Testosterone | 4.55 µg/L (3.84/5.43) | 3.58 µg/L (2.28/4.33) | 0.0317 * |
| Free androgen index | 36.08% (+/−7.4) | 25.02 % (+/−8.48) | 0.0031 ** |
| Target Analyte | Spearman rs | Significance (p) | Corrected p |
|---|---|---|---|
| Lymphocytes | 0.51 | 0.0039 ** | 0.0224 * |
| Basophils | 0.43 | 0.0175 * | 0.04 * |
| CD33 high+ CD11b+ | −0.28 | 0.1648 | 0.1758 |
| CD33- CD11b- | 0.31 | 0.1168 | 0.1335 |
| HLADR+ CD16+ | −0.43 | 0.0244 * | 0.0471 (*) |
| PD-1+ granulocytes | −0.40 | 0.0662 | 0.0883 |
| PD-1+ monocytes | −0.49 | 0.0166 * | 0.04 * |
| Hemoglobin | 0.44 | 0.0148 * | 0.04 * |
| CD147+ CD19- | −0.36 | 0.0652 | 0.0883 |
| CD39+ CD19- | −0.19 | 0.3369 | 0.3369 |
| CD39+ CD19+ | 0.33 | 0.0918 | 0.1130 |
| CRP | −0.46 | 0.0088 ** | 0.0352 * |
| LDL | 0.53 | 0.0021 ** | 0.0224 * |
| HDL | 0.39 | 0.0265 * | 0.0471 (*) |
| Testosterone | 0.45 | 0.0317 * | 0.0507 |
| Free androgen index | 0.57 | 0.0042 ** | 0.0224 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hofbauer, J.; Hauck, A.; Matos, C.; Babl, N.; Decking, S.-M.; Rechenmacher, M.; Schulz, C.; Regotta, S.; Mickler, M.; Haferkamp, S.; et al. Immunometabolic Markers in a Small Patient Cohort Undergoing Immunotherapy. Biomolecules 2022, 12, 716. https://doi.org/10.3390/biom12050716
Hofbauer J, Hauck A, Matos C, Babl N, Decking S-M, Rechenmacher M, Schulz C, Regotta S, Mickler M, Haferkamp S, et al. Immunometabolic Markers in a Small Patient Cohort Undergoing Immunotherapy. Biomolecules. 2022; 12(5):716. https://doi.org/10.3390/biom12050716
Chicago/Turabian StyleHofbauer, Joshua, Andreas Hauck, Carina Matos, Nathalie Babl, Sonja-Maria Decking, Michael Rechenmacher, Christian Schulz, Sabine Regotta, Marion Mickler, Sebastian Haferkamp, and et al. 2022. "Immunometabolic Markers in a Small Patient Cohort Undergoing Immunotherapy" Biomolecules 12, no. 5: 716. https://doi.org/10.3390/biom12050716
APA StyleHofbauer, J., Hauck, A., Matos, C., Babl, N., Decking, S.-M., Rechenmacher, M., Schulz, C., Regotta, S., Mickler, M., Haferkamp, S., Siska, P. J., Herr, W., Renner, K., Kreutz, M., & Schnell, A. (2022). Immunometabolic Markers in a Small Patient Cohort Undergoing Immunotherapy. Biomolecules, 12(5), 716. https://doi.org/10.3390/biom12050716






