Effect of Trimethine Cyanine Dye- and Folate-Conjugation on the In Vitro Biological Activity of Proapoptotic Peptides
Abstract
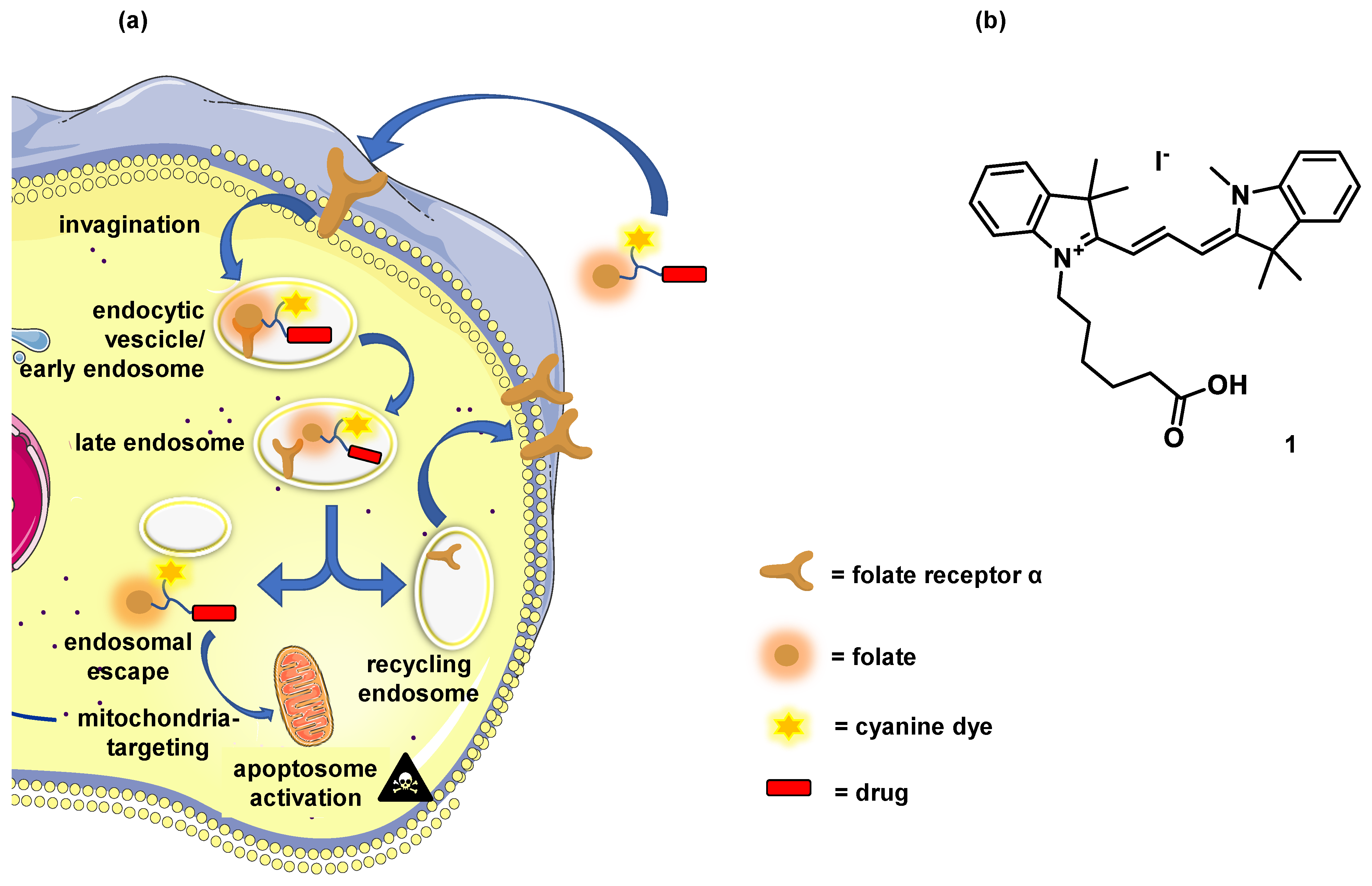
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Cell Viability Assay
2.3. Confocal Microscopy
2.4. Flow Cytometry
2.5. Statistical Analysis
3. Results
3.1. Synthesis of Peptides and Peptide Conjugates 2–13
3.2. Cytotoxicity of 1–13
3.3. Mitochondrial Localization and Cellular Uptake of 5–10
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interests
References
- Wang, H.; Naghavi, M.; Allen, C.; Barber, R.M.; Bhutta, Z.A.; Carter, A.; Casey, D.C.; Charlson, F.J.; Chen, A.Z.; Coates, M.M.; et al. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1459–1544. [Google Scholar] [CrossRef] [Green Version]
- Torre, L.A.; Siegel, R.L.; Ward, E.M.; Jemal, A. Global Cancer Incidence and Mortality Rates and Trends—An Update. Cancer Epidemiol. Biomark. Prev. 2016, 25, 16–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, A.S.; Ormiston-Smith, N.; Sasieni, P.D. Trends in the lifetime risk of developing cancer in Great Britain: Comparison of risk for those born from 1930 to 1960. Br. J. Cancer 2015, 112, 943–947. [Google Scholar] [CrossRef] [PubMed]
- Pucci, C.; Martinelli, C.; Ciofani, G. Innovative approaches for cancer treatment: Current perspectives and new challenges. Ecancermedicalscience 2019, 13, 961. [Google Scholar] [CrossRef]
- Dagogo-Jack, I.; Shaw, A.T. Tumour heterogeneity and resistance to cancer therapies. Nat. Rev. Clin. Oncol. 2018, 15, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Langer, R. Drugs on Target. Science 2001, 293, 58. [Google Scholar] [CrossRef]
- Roth, K.G.; Mambetsariev, I.; Kulkarni, P.; Salgia, R. The Mitochondrion as an Emerging Therapeutic Target in Cancer. Trends Mol. Med. 2020, 26, 119–134. [Google Scholar] [CrossRef]
- Usama, S.M.; Park, G.K.; Nomura, S.; Baek, Y.; Choi, H.S.; Burgess, K. Role of Albumin in Accumulation and Persistence of Tumor-Seeking Cyanine Dyes. Bioconj. Chem. 2020, 31, 248–259. [Google Scholar] [CrossRef]
- Yang, X.; Shi, C.; Tong, R.; Qian, W.; Zhau, H.E.; Wang, R.; Zhu, G.; Cheng, J.; Yang, V.W.; Cheng, T.; et al. Near IR heptamethine cyanine dye-mediated cancer imaging. Clin. Cancer Res. 2010, 16, 2833–2844. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Z.; Pflug, K.; Usama, S.M.; Kuai, D.; Yan, X.; Sitcheran, R.; Burgess, K. Cyanine–Gemcitabine Conjugates as Targeted Theranostic Agents for Glioblastoma Tumor Cells. J. Med. Chem. 2019, 62, 9236–9245. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, J.; Wang, L.; Hu, X.; Liu, X.; Liu, M.; Cao, Z.; Shangguan, D.; Tan, W. A Cyanine Dye to Probe Mitophagy: Simultaneous Detection of Mitochondria and Autolysosomes in Live Cells. J. Am. Chem. Soc. 2016, 138, 12368–12374. [Google Scholar] [CrossRef] [PubMed]
- Onoe, S.; Temma, T.; Shimizu, Y.; Ono, M.; Saji, H. Investigation of cyanine dyes for in vivo optical imaging of altered mitochondrial membrane potential in tumors. Cancer Med. 2014, 3, 775–786. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.-B.; Liu, J.-g.; Bai, X.-Y.; Huang, Y.-J.; Xu, N.; Ren, T. A Novel Fluorescent Dye Invades Mitochondria to Selectively Kill Cancer Stem Cells via Increased ROS Production. Bioinorg. Chem. Appl. 2021, 2021, 4763944. [Google Scholar] [CrossRef] [PubMed]
- Nödling, A.R.; Mills, E.M.; Li, X.; Cardella, D.; Sayers, E.J.; Wu, S.-H.; Jones, A.T.; Luk, L.Y.P.; Tsai, Y.-H. Cyanine dye mediated mitochondrial targeting enhances the anti-cancer activity of small-molecule cargoes. Chem. Commun. 2020, 56, 4672–4675. [Google Scholar] [CrossRef]
- Leamon, C.P.; Pastan, I.; Low, P.S. Cytotoxicity of folate-Pseudomonas exotoxin conjugates toward tumor cells. Contribution of translocation domain. J. Biol. Chem. 1993, 268, 24847–24854. [Google Scholar] [CrossRef]
- Cheung, A.; Bax, H.J.; Josephs, D.H.; Ilieva, K.M.; Pellizzari, G.; Opzoomer, J.; Bloomfield, J.; Fittall, M.; Grigoriadis, A.; Figini, M.; et al. Targeting folate receptor alpha for cancer treatment. Oncotarget 2016, 7, 52553–52574. [Google Scholar] [CrossRef] [Green Version]
- Fernández, M.; Javaid, F.; Chudasama, V. Advances in targeting the folate receptor in the treatment/imaging of cancers. Chem. Sci. 2018, 9, 790–810. [Google Scholar] [CrossRef] [Green Version]
- Sudimack, J.; Lee, R.J. Targeted drug delivery via the folate receptor. Adv. Drug Del. Rev. 2000, 41, 147–162. [Google Scholar] [CrossRef]
- Vlahov, I.R.; Leamon, C.P. Engineering Folate–Drug Conjugates to Target Cancer: From Chemistry to Clinic. Bioconj. Chem. 2012, 23, 1357–1369. [Google Scholar] [CrossRef]
- Leamon, C.P.; Vlahov, I.R.; Reddy, J.A.; Vetzel, M.; Santhapuram, H.K.R.; You, F.; Bloomfield, A.; Dorton, R.; Nelson, M.; Kleindl, P.; et al. Folate–Vinca Alkaloid Conjugates for Cancer Therapy: A Structure–Activity Relationship. Bioconj. Chem. 2014, 25, 560–568. [Google Scholar] [CrossRef]
- Seitz, J.D.; Vineberg, J.G.; Herlihy, E.; Park, B.; Melief, E.; Ojima, I. Design, synthesis and biological evaluation of a highly-potent and cancer cell selective folate–taxoid conjugate. Biorg. Med. Chem. 2015, 23, 2187–2194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vergel Galeano, C.F.; Rivera Monroy, Z.J.; Rosas Pérez, J.E.; García Castañeda, J.E. Efficient Synthesis of Peptides with 4-Methylpiperidine as Fmoc Removal Reagent by Solid Phase Synthesis. J. Mex. Chem. Soc. 2014, 58, 386–392. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.-H.; Xu, X.-D.; Luo, G.-F.; Jia, H.-Z.; Lei, Q.; Cheng, S.-X.; Zhuo, R.-X.; Zhang, X.-Z. Dual-Targeting Pro-apoptotic Peptide for Programmed Cancer Cell Death via Specific Mitochondria Damage. Sci. Rep. 2013, 3, 3468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leamon, C.P.; Deprince, R.B.; Hendren, R.W. Folate-mediated Drug Delivery: Effect of Alternative Conjugation Chemistry. J. Drug Target. 1999, 7, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, L.; Glänzel, W.; Korch, C.; Capes-Davis, A. Widespread Use of Misidentified Cell Line KB (HeLa): Incorrect Attribution and Its Impact Revealed through Mining the Scientific Literature. Cancer Res. 2017, 77, 2784–2788. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Hou, S.; Mao, S.; Wei, D.; Song, X.; Lu, Y. Uptake of folate-conjugated albumin nanoparticles to the SKOV3 cells. Int. J. Pharm. 2004, 287, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.; Xu, Y.; Sun, K.; Wang, H.; Zhang, F.; Zhou, Z.; Zhang, J.; Zhang, F.; Caliskan, B.; Qiu, Z.; et al. Identification of a peptide for folate receptor alpha by phage display and its tumor targeting activity in ovary cancer xenograft. Sci. Rep. 2018, 8, 8426. [Google Scholar] [CrossRef] [Green Version]
- Elwood, P.C. Molecular cloning and characterization of the human folate-binding protein cDNA from placenta and malignant tissue culture (KB) cells. J. Biol. Chem. 1989, 264, 14893–14901. [Google Scholar] [CrossRef]
- Horton, K.L.; Kelley, S.O. Engineered Apoptosis-Inducing Peptides with Enhanced Mitochondrial Localization and Potency. J. Med. Chem. 2009, 52, 3293–3299. [Google Scholar] [CrossRef]
- Law, B.; Quinti, L.; Choi, Y.; Weissleder, R.; Tung, C.H. A mitochondrial targeted fusion peptide exhibits remarkable cytotoxicity. Mol. Cancer Ther. 2006, 5, 1944–1949. [Google Scholar] [CrossRef] [Green Version]
- Ellerby, H.M.; Arap, W.; Ellerby, L.M.; Kain, R.; Andrusiak, R.; Rio, G.D.; Krajewski, S.; Lombardo, C.R.; Rao, R.; Ruoslahti, E.; et al. Anti-cancer activity of targeted pro-apoptotic peptides. Nat. Med. 1999, 5, 1032–1038. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.K.; Kim, S.; Park, R.W.; Park, J.Y.; Kim, I.S.; Lee, B. Bladder tumor-targeted delivery of pro-apoptotic peptide for cancer therapy. J. Control. Release 2016, 235, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Z.-Y.; Lai, W.-J.; Lin, Y.-X.; Li, D.; Nan, X.-H.; Wang, Y.; Wang, H.; Fang, Q.-J. Polymer–KLAK Peptide Conjugates Induce Cancer Cell Death through Synergistic Effects of Mitochondria Damage and Autophagy Blockage. Bioconj. Chem. 2017, 28, 1709–1721. [Google Scholar] [CrossRef] [PubMed]
- Smolarczyk, R.; Cichoń, T.; Graja, K.; Hucz, J.; Sochanik, A.; Szala, S. Antitumor effect of RGD-4C-GG-D(KLAKLAK)2 peptide in mouse B16(F10) melanoma model. Acta Biochim. Pol. 2006, 53, 801–805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mai, J.C.; Mi, Z.; Kim, S.H.; Ng, B.; Robbins, P.D. A proapoptotic peptide for the treatment of solid tumors. Cancer Res. 2001, 61, 7709–7712. [Google Scholar]
- Kim, H.Y.; Kim, S.; Youn, H.; Chung, J.-K.; Shin, D.H.; Lee, K. The cell penetrating ability of the proapoptotic peptide, KLAKLAKKLAKLAK fused to the N-terminal protein transduction domain of translationally controlled tumor protein, MIIYRDLISH. Biomaterials 2011, 32, 5262–5268. [Google Scholar] [CrossRef]
- Horton, K.L.; Stewart, K.M.; Fonseca, S.B.; Guo, Q.; Kelley, S.O. Mitochondria-penetrating peptides. ACS Chem. Biol. 2008, 15, 375–382. [Google Scholar] [CrossRef] [Green Version]
- Horton, K.L.; Pereira, M.P.; Stewart, K.M.; Fonseca, S.B.; Kelley, S.O. Tuning the activity of mitochondria-penetrating peptides for delivery or disruption. ChemBioChem 2012, 13, 476–485. [Google Scholar] [CrossRef]
- Marverti, G.; Marraccini, C.; Martello, A.; D’Arca, D.; Pacifico, S.; Guerrini, R.; Spyrakis, F.; Gozzi, G.; Lauriola, A.; Santucci, M.; et al. Folic Acid–Peptide Conjugates Combine Selective Cancer Cell Internalization with Thymidylate Synthase Dimer Interface Targeting. J. Med. Chem. 2021, 64, 3204–3221. [Google Scholar] [CrossRef]
- Kim, W.H.; Kim, C.G.; Kim, M.H.; Kim, D.-W.; Park, C.R.; Park, J.Y.; Lee, Y.-S.; Youn, H.; Kang, K.W.; Jeong, J.M.; et al. Preclinical evaluation of isostructural Tc-99m- and Re-188-folate-Gly-Gly-Cys-Glu for folate receptor-positive tumor targeting. Ann. Nucl. Med. 2016, 30, 369–379. [Google Scholar] [CrossRef]
- Leamon, C.P.; Reddy, J.A.; Vlahov, I.R.; Westrick, E.; Dawson, A.; Dorton, R.; Vetzel, M.; Santhapuram, H.K.; Wang, Y. Preclinical Antitumor Activity of a Novel Folate-Targeted Dual Drug Conjugate. Mol. Pharm. 2007, 4, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Dharmatti, R.; Miyatake, H.; Nandakumar, A.; Ueda, M.; Kobayashi, K.; Kiga, D.; Yamamura, M.; Ito, Y. Enhancement of Binding Affinity of Folate to Its Receptor by Peptide Conjugation. Int. J. Mol. Sci. 2019, 20, 2152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Low, P.S. Folate-mediated targeting of antineoplastic drugs, imaging agents, and nucleic acids to cancer cells. J. Control. Release 1998, 53, 39–48. [Google Scholar] [CrossRef]
- Leamon, C.P.; Low, P.S. Delivery of macromolecules into living cells: A method that exploits folate receptor endocytosis. Proc. Natl. Acad. Sci. USA 1991, 88, 5572–5576. [Google Scholar] [CrossRef] [Green Version]
- Kvach, M.V.; Ustinov, A.V.; Stepanova, I.A.; Malakhov, A.D.; Skorobogatyi, M.V.; Shmanai, V.V.; Korshun, V.A. A Convenient Synthesis of Cyanine Dyes: Reagents for the Labeling of Biomolecules. Eur. J. Org. Chem. 2008, 2008, 2107–2117. [Google Scholar] [CrossRef]



 | |||||||||
| EC50 in µM | |||||||||
| Compound | R1 | R2 | R3 | * | ** | KB | SK-OV-3 | MCF7 | HEK293 |
| 1 | - | - | - | - | - | 36.5 (3.6) | 8.6 (6.70) | 110 (13.2) | 221 (53.8) |
| 2 | H | isopropyl | - | (S) | (S) | >400 | >400 | >400 | >400 |
| 3 | H | isopropyl | - | (R) | (R) | 331 (152) | >400 | >400 | >400 |
| 4 | H | cyclohexyl | - | (R) | (S) | 6.5 (0.5) | 11.6 (2.37) | 50.9 (3.0) | 15.6 (2.6) |
| 5 | Cy3 | isopropyl | - | (S) | (S) | 6.7 (0.2) | 11.0 (2.56) | 7.37 (0.2) | 44.3 (7.0) |
| 6 | Cy3 | isopropyl | - | (R) | (R) | 3.5 (0.1) | 6.39 (0.79) | 5.7 (0.7) | 5.3 (0.2) |
| 7 | Cy3 | cyclohexyl | - | (R) | (S) | 5.5 (0.5) | 23.0 (1.64) | 11.6 (1.5) | 8.2 (0.5) |
| 8 | K(Fol) | isopropyl | Cy3 | (S) | (S) | 38.8 (6.6) | 8.8 (3.7) | 49.4 (4.3) | 35.6 (3.4) |
| 9 | K(Fol) | isopropyl | Cy3 | (R) | (R) | 22.4 (1.4) | 8.0 (2.2) | 13.2 (5.6) | 6.9 (1.8) |
| 10 | K(Fol) | cyclohexyl | Cy3 | (R) | (S) | 3.4 (0.7) | 6.0 (1.1) | 6.9 (0.9) | 6.8 (1.4) |
| 11 | K(Fol) | isopropyl | Ac | (S) | (S) | 242 (33.2) | >400 | >400 | >400 |
| 12 | K(Fol) | isopropyl | Ac | (R) | (R) | 151 (38.7) | 388.4 (21.3) | >400 | >400 |
| 13 | K(Fol) | cyclohexyl | Ac | (R) | (S) | 9.8 (0.6) | 41.5 (4.0) | 26.9 (7.2) | 20.4 (5.7) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cardella, D.; Deng, W.; Luk, L.Y.P.; Tsai, Y.-H. Effect of Trimethine Cyanine Dye- and Folate-Conjugation on the In Vitro Biological Activity of Proapoptotic Peptides. Biomolecules 2022, 12, 725. https://doi.org/10.3390/biom12050725
Cardella D, Deng W, Luk LYP, Tsai Y-H. Effect of Trimethine Cyanine Dye- and Folate-Conjugation on the In Vitro Biological Activity of Proapoptotic Peptides. Biomolecules. 2022; 12(5):725. https://doi.org/10.3390/biom12050725
Chicago/Turabian StyleCardella, Davide, Wenjing Deng, Louis Y. P. Luk, and Yu-Hsuan Tsai. 2022. "Effect of Trimethine Cyanine Dye- and Folate-Conjugation on the In Vitro Biological Activity of Proapoptotic Peptides" Biomolecules 12, no. 5: 725. https://doi.org/10.3390/biom12050725
APA StyleCardella, D., Deng, W., Luk, L. Y. P., & Tsai, Y.-H. (2022). Effect of Trimethine Cyanine Dye- and Folate-Conjugation on the In Vitro Biological Activity of Proapoptotic Peptides. Biomolecules, 12(5), 725. https://doi.org/10.3390/biom12050725






