Harnessing Unconventional T Cells and Innate Lymphoid Cells to Prevent and Treat Hematological Malignancies: Prospects for New Immunotherapy
Abstract
:1. Introduction
1.1. General Aspects on Unconventional T Cells
- rapid responses to innate immune cells due to antigen-independent activation thanks to cytokines and ligand recognition;
- non-classical MHC to innate immune cells;
- the exhibition of limited T cell antigen receptor (TCR) repertoires, recognizing unconventional peptide antigens as a distinctive feature.
1.2. Activation and Functions of Unconventional T Cells
1.3. Search Strategy
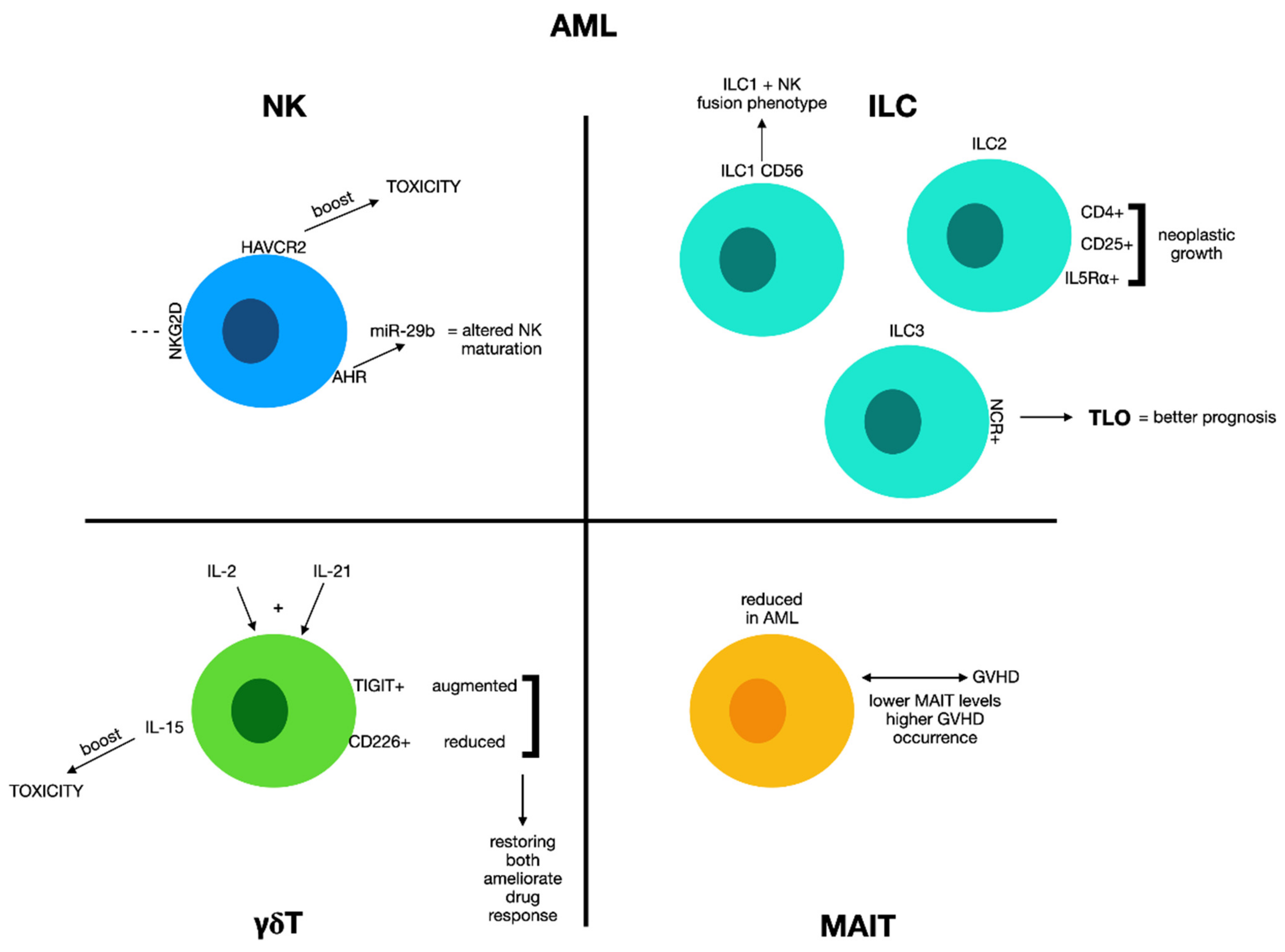
2. Acute Leukemia
2.1. Natural Killer Cells and Acute Myeloid Leukemia
2.2. Innate Lymphoid Cells and Acute Myeloid Leukemia
2.3. γδ T Cells and Acute Myeloid Leukemia
2.4. MAIT Cells and Acute Myeloid Leukemia
2.5. Acute Lymphoblastic Leukemia
2.5.1. Natural Killer Cells and Acute Lymphoblastic Leukemia
2.5.2. γδ Cells and Acute Lymphoblastic Leukemia
2.6. Chronic Myeloid Leukemia
2.6.1. Natural Killer Cells and Chronic Myeloid Leukemia
2.6.2. γδ Cells and Chronic Myeloid Leukemia
2.7. Natural Killer Cells and ILCs in Chronic Myeloproliferative Diseases Ph−
2.8. Multiple Myeloma
2.8.1. Natural Killer Cells and Multiple Myeloma
2.8.2. Innate Lymphoid Cells 1 and Multiple Myeloma
2.8.3. Innate Lymphoid Cells 2 and Multiple Myeloma
2.8.4. γδ T Cells and Multiple Myeloma
2.8.5. MAIT Cells and Multiple Myeloma
2.9. Chronic Lymphocytic Leukemia
2.9.1. Natural Killer Cells and Innate Lymphoid Cells in Chronic Lymphocytic Leukemia
2.9.2. γδ T Cells in Chronic Lymphocytic Leukemia
2.10. Unconventional T Cells in Lymphomas
3. Therapeutical Perspectives and Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Davey, M.S.; Willcox, C.R.; Hunter, S.; Kasatskaya, S.; Remmerswaal, E.; Salim, M.; Mohammed, F.; Bemelman, F.J.; Chudakov, D.M.; Oo, Y.H.; et al. The human Vδ2+ T-cell compartment comprises distinct innate-like Vγ9+ and adaptive Vγ9− subsets. Nat. Commun. 2018, 9, 1760. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, D.I.; Koay, H.-F.; McCluskey, J.; Gherardin, N. The biology and functional importance of MAIT cells. Nat. Immunol. 2019, 20, 1110–1128. [Google Scholar] [CrossRef] [PubMed]
- Morita, H.; Moro, K.; Koyasu, S. Innate lymphoid cells in allergic and nonallergic inflammation. J. Allergy Clin. Immunol. 2016, 138, 1253–1264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bostick, J.; Zhou, L. Innate lymphoid cells in intestinal immunity and inflammation. Cell. Mol. Life Sci. 2016, 73, 237–252. [Google Scholar] [CrossRef] [PubMed]
- Teunissen, M.B.; Munneke, J.M.; Bernink, J.H.; Spuls, P.I.; Res, P.C.; Velde, A.T.; Cheuk, S.H.; Brouwer, M.W.; Menting, S.P.; Eidsmo, L.; et al. Composition of Innate Lymphoid Cell Subsets in the Human Skin: Enrichment of NCR + ILC3 in Lesional Skin and Blood of Psoriasis Patients. J. Investig. Dermatol. 2014, 134, 2351–2360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clottu, A.S.; Humbel, M.; Fluder, N.; Karampetsou, M.P.; Comte, D. Innate Lymphoid Cells in Autoimmune Diseases. Front. Immunol. 2022, 12, 789788. [Google Scholar] [CrossRef]
- Poccia, F.; Agrati, C.; Martini, F.; Mejia, G.; Wallace, M.; Malkovsky, M. Vγ9Vδ2 T cell-mediated non-cytolytic antiviral mechanisms and their potential for cell-based therapy. Immunol. Lett. 2005, 100, 14–20. [Google Scholar] [CrossRef]
- Presti, E.L.; De Gaetano, A.; Pioggia, G.; Gangemi, S. Comprehensive Analysis of the ILCs and Unconventional T Cells in Virus Infection: Profiling and Dynamics Associated with COVID-19 Disease for a Future Monitoring System and Therapeutic Opportunities. Cells 2022, 11, 542. [Google Scholar] [CrossRef]
- Carissimo, G.; Xu, W.; Kwok, I.; Abdad, M.Y.; Chan, Y.-H.; Fong, S.-W.; Puan, K.J.; Lee, C.Y.-P.; Yeo, N.K.-W.; Amrun, S.N.; et al. Whole blood immunophenotyping uncovers immature neutrophil-to-VD2 T-cell ratio as an early marker for severe COVID-19. Nat. Commun. 2020, 11, 5243. [Google Scholar] [CrossRef]
- Van Wilgenburg, B.; Scherwitzl, I.; Hutchinson, E.C.; Leng, T.; Kurioka, A.; Kulicke, C.; De Lara, C.; Cole, S.; Vasanawathana, S.; Limpitikul, W.; et al. MAIT cells are activated during human viral infections. Nat. Commun. 2016, 7, 11653. [Google Scholar] [CrossRef] [Green Version]
- Orumaa, K.; Dunne, M.R. The role of unconventional T cells in COVID-19. Ir. J. Med Sci. 2021, 191, 519–528. [Google Scholar] [CrossRef]
- Eslauenwhite, D.; Ejohnston, B. Regulation of NKT Cell Localization in Homeostasis and Infection. Front. Immunol. 2015, 6, 255. [Google Scholar] [CrossRef] [Green Version]
- Knight, A.; Mackinnon, S.; Lowdell, M.W. Human Vdelta1 gamma-delta T cells exert potent specific cytotoxicity against primary multiple myeloma cells. Cytotherapy 2012, 14, 1110–1118. [Google Scholar] [CrossRef]
- Liu, C.; Emami, S.N.; Pettersson, J.; Ranford-Cartwright, L.; Faye, I.; Parmryd, I. Vγ9Vδ2 T cells proliferate in response to phosphoantigens released from erythrocytes infected with asexual and gametocyte stage Plasmodium falciparum. Cell. Immunol. 2018, 334, 11–19. [Google Scholar] [CrossRef]
- Rigau, M.; Ostrouska, S.; Fulford, T.S.; Johnson, D.N.; Woods, K.; Ruan, Z.; McWilliam, H.E.; Hudson, C.; Tutuka, C.; Wheatley, A.K.; et al. Butyrophilin 2A1 is essential for phosphoantigen reactivity by γδ T cells. Science 2020, 367, eaay5516. [Google Scholar] [CrossRef]
- Gully, B.S.; Rossjohn, J.; Davey, M.S. Our evolving understanding of the role of the γδ T cell receptor in γδ T cell mediated immunity. Biochem. Soc. Trans. 2021, 49, 1985–1995. [Google Scholar] [CrossRef]
- Zeng, X.; Wei, Y.-L.; Huang, J.; Newell, E.W.; Yu, H.; Kidd, B.A.; Kuhns, M.S.; Waters, R.W.; Davis, M.M.; Weaver, C.; et al. γδ T Cells Recognize a Microbial Encoded B Cell Antigen to Initiate a Rapid Antigen-Specific Interleukin-17 Response. Immunity 2012, 37, 524–534. [Google Scholar] [CrossRef] [Green Version]
- Harly, C.; Joyce, S.P.; Domblides, C.; Bachelet, T.; Pitard, V.; Mannat, C.; Pappalardo, A.; Couzi, L.; Netzer, S.; Massara, L.; et al. Human γδ T cell sensing of AMPK-dependent metabolic tumor reprogramming through TCR recognition of EphA2. Sci. Immunol. 2021, 6, eaba9010. [Google Scholar] [CrossRef]
- Marlin, R.; Pappalardo, A.; Kaminski, H.; Willcox, C.R.; Pitard, V.; Netzer, S.; Khairallah, C.; Lomenech, A.-M.; Harly, C.; Bonneville, M.; et al. Sensing of cell stress by human γδ TCR-dependent recognition of annexin A2. Proc. Natl. Acad. Sci. USA 2017, 114, 3163–3168. [Google Scholar] [CrossRef] [Green Version]
- Willcox, C.R.; Pitard, V.; Netzer, S.; Couzi, L.; Salim, M.; Silberzahn, T.; Moreau, J.-F.; Hayday, A.; Willcox, B.E.; Déchanet-Merville, J. Cytomegalovirus and tumor stress surveillance by binding of a human γδ T cell antigen receptor to endothelial protein C receptor. Nat. Immunol. 2012, 13, 872–879. [Google Scholar] [CrossRef]
- Treiner, E.; Duban, L.; Bahram, S.; Radosavljevic, M.; Wanner, V.; Tilloy, F.; Affaticati, P.; Gilfillan, S.; Lantz, O. Selection of evolutionarily conserved mucosal-associated invariant T cells by MR1. Nature 2003, 422, 164–169. [Google Scholar] [CrossRef]
- Jahng, A.; Maricic, I.; Aguilera, C.; Cardell, S.; Halder, R.C.; Kumar, V. Prevention of Autoimmunity by Targeting a Distinct, Noninvariant CD1d-reactive T Cell Population Reactive to Sulfatide. J. Exp. Med. 2004, 199, 947–957. [Google Scholar] [CrossRef] [Green Version]
- Forkel, M.; Van Tol, S.; Höög, C.; Michaëlsson, J.; Almer, S.; Mjösberg, J. Distinct Alterations in the Composition of Mucosal Innate Lymphoid Cells in Newly Diagnosed and Established Crohn’s Disease and Ulcerative Colitis. J. Crohn’s Colitis 2019, 13, 67–78. [Google Scholar] [CrossRef]
- Presti, E.L.; Di Mitri, R.; Mocciaro, F.; di Stefano, A.B.; Scibetta, N.; Unti, E.; Cicero, G.; Pecoraro, G.; Conte, E.; Dieli, F.; et al. Characterization of γδ T Cells in Intestinal Mucosa from Patients with Early-Onset or Long-Standing Inflammatory Bowel Disease and Their Correlation with Clinical Status. J. Crohn’s Colitis 2019, 13, 873–883. [Google Scholar] [CrossRef]
- Fergusson, J.; Hühn, M.; Swadling, L.; Walker, L.; Kurioka, A.; Llibre, A.; Bertoletti, A.; Holländer, G.; Newell, E.; Davis, M.; et al. CD161(int)CD8+ T cells: A novel population of highly functional, memory CD8+ T cells enriched within the gut. Mucosal Immunol. 2016, 9, 401–413. [Google Scholar] [CrossRef] [Green Version]
- Lo Presti, E.; Dieli, F.; Fournie, J.J.; Meraviglia, S. Deciphering human γδ T cell response in cancer: Lessons from tumor-infiltrating γδ T cells. Immunol. Rev. 2020, 298, 153–164. [Google Scholar] [CrossRef]
- Crome, S.; Nguyen, L.T.; Lopez-Verges, S.; Yang, S.Y.C.; Martin, B.; Yam, J.Y.; Johnson, D.J.; Nie, J.; Pniak, M.; Yen, P.H.; et al. A distinct innate lymphoid cell population regulates tumor-associated T cells. Nat. Med. 2017, 23, 368–375. [Google Scholar] [CrossRef]
- Allison, M.; Mathews, J.; Gilliland, T.; Mathew, S.O. Natural Killer Cell-Mediated Immunotherapy for Leukemia. Cancers 2022, 14, 843. [Google Scholar] [CrossRef]
- Boeck, C.L.; Amberger, D.C.; Doraneh-Gard, F.; Sutanto, W.; Guenther, T.; Schmohl, J.; Schuster, F.; Salih, H.; Babor, F.; Borkhardt, A.; et al. Significance of Frequencies, Compositions, and/or Antileukemic Activity of (DC-stimulated) Invariant NKT, NK and CIK Cells on the Outcome of Patients With AML, ALL and CLL. J. Immunother. 2017, 40, 224–248. [Google Scholar] [CrossRef]
- Chretien, A.-S.; Fauriat, C.; Orlanducci, F.; Galseran, C.; Rey, J.; Borg, G.B.; Gautherot, E.; Granjeaud, S.; Hamel-Broza, J.-F.; Demerle, C.; et al. Natural Killer Defective Maturation Is Associated with Adverse Clinical Outcome in Patients with Acute Myeloid Leukemia. Front. Immunol. 2017, 8, 573. [Google Scholar] [CrossRef] [PubMed]
- Morvan, M.; Lanier, L.L. NK cells and cancer: You can teach innate cells new tricks. Nat. Cancer 2016, 16, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Khaznadar, Z.; Boissel, N.; Agaugué, S.; Henry, G.; Cheok, M.; Vignon, M.; Geromin, D.; Cayuela, J.-M.; Castaigne, S.; Pautas, C.; et al. Defective NK Cells in Acute Myeloid Leukemia Patients at Diagnosis Are Associated with Blast Transcriptional Signatures of Immune Evasion. J. Immunol. 2015, 195, 2580–2590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stringaris, K.; Sekine, T.; Khoder, A.; Alsuliman, A.; Razzaghi, B.; Sargeant, R.; Pavlu, J.; Brisley, G.; de Lavallade, H.; Sarvaria, A.; et al. Leukemia-induced phenotypic and functional defects in natural killer cells predict failure to achieve remission in acute myeloid leukemia. Haematologica 2014, 99, 836–847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hahn, C.; Chong, C.E.; Carmichael, C.; Wilkins, E.J.; Brautigan, P.J.; Li, X.-C.; Babic, M.; Lin, M.; Carmagnac, A.; Lee, Y.K.; et al. Heritable GATA2 mutations associated with familial myelodysplastic syndrome and acute myeloid leukemia. Nat. Genet. 2011, 43, 1012–1017. [Google Scholar] [CrossRef]
- Ostergaard, P.; Simpson, M.; Connell, F.C.; Steward, C.; Brice, G.; Woollard, W.J.; Dafou, D.; Kilo, T.; Smithson, S.; Lunt, P.; et al. Mutations in GATA2 cause primary lymphedema associated with a predisposition to acute myeloid leukemia (Emberger syndrome). Nat. Genet. 2011, 43, 929–931. [Google Scholar] [CrossRef] [Green Version]
- Mace, E.M.; Hsu, A.P.; Monaco-Shawver, L.; Makedonas, G.; Rosen, J.B.; Dropulic, L.; Cohen, J.I.; Frenkel, E.P.; Bagwell, J.C.; Sullivan, J.L.; et al. Mutations in GATA2 cause human NK cell deficiency with specific loss of the CD56 bright subset. Blood 2013, 121, 2669–2677. [Google Scholar] [CrossRef] [Green Version]
- Pizzolo, G.; Trentin, L.; Vinante, F.; Agostini, C.; Zambello, R.; Masciarelli, M.; Feruglio, C.; Dazzi, F.; Todeschini, G.; Chilosi, M.; et al. Natural killer cell function and lymphoid subpopulations in acute non-lymphoblastic leukaemia in com-plete remission. Br. J. Cancer 1988, 58, 368–372. [Google Scholar] [CrossRef] [Green Version]
- Hilpert, J.; Grosse-Hovest, L.; Grünebach, F.; Buechele, C.; Nuebling, T.; Raum, T.; Steinle, A.; Salih, H.R. Comprehensive Analysis of NKG2D Ligand Expression and Release in Leukemia: Implications for NKG2D-Mediated NK Cell Responses. J. Immunol. 2012, 189, 1360–1371. [Google Scholar] [CrossRef] [Green Version]
- Clayton, A.; Mitchell, J.P.; Court, J.; Linnane, S.; Mason, M.D.; Tabi, Z. Human Tumor-Derived Exosomes Down-Modulate NKG2D Expression. J. Immunol. 2008, 180, 7249–7258. [Google Scholar] [CrossRef] [Green Version]
- Szczepanski, M.J.; Szajnik, M.; Welsh, A.; Whiteside, T.L.; Boyiadzis, M. Blast-derived microvesicles in sera from patients with acute myeloid leukemia suppress natural killer cell function via membrane-associated transforming growth factor-β1. Haematologica 2011, 96, 1302–1309. [Google Scholar] [CrossRef]
- Rakova, J.; Truxova, I.; Holicek, P.; Salek, C.; Hensler, M.; Kasikova, L.; Pasulka, J.; Holubova, M.; Kovar, M.; Lysak, D.; et al. TIM-3 levels correlate with enhanced NK cell cytotoxicity and improved clinical outcome in AML patients. OncoImmunology 2021, 10, 1889822. [Google Scholar] [CrossRef]
- Scoville, S.D.; Nalin, A.P.; Chen, L.; Chen, L.; Zhang, M.; McConnell, K.; Casas, S.B.; Ernst, G.; Traboulsi, A.A.-R.; Hashi, N.; et al. Human AML activates the aryl hydrocarbon receptor pathway to impair NK cell development and function. Blood 2018, 132, 1792–1804. [Google Scholar] [CrossRef]
- Rosenberg, S.A.; Lotze, M.T.; Muul, L.M.; Chang, A.E.; Avis, F.P.; Leitman, S.; Linehan, W.M.; Robertson, C.N.; Lee, R.E.; Rubin, J.T.; et al. A Progress Report on the Treatment of 157 Patients with Advanced Cancer Using Lymphokine-Activated Killer Cells and Interleukin-2 or High-Dose Interleukin-2 Alone. New Engl. J. Med. 1987, 316, 889–897. [Google Scholar] [CrossRef]
- Innao, V.; Allegra, A.G.; Musolino, C.; Allegra, A. New Frontiers about the Role of Human Microbiota in Immunotherapy: The Immune Checkpoint Inhibitors and CAR T-Cell Therapy Era. Int. J. Mol. Sci. 2020, 21, 8902. [Google Scholar] [CrossRef]
- Allegra, A.; Innao, V.; Gerace, D.; Vaddinelli, D.; Musolino, C. Adoptive immunotherapy for hematological malignancies: Current status and new insights in chimeric antigen receptor T cells. Blood Cells Mol. Dis. 2016, 62, 49–63. [Google Scholar] [CrossRef]
- Björklund, A.T.; Carlsten, M.; Sohlberg, E.; Liu, L.L.; Clancy, T.; Karimi, M.; Cooley, S.; Miller, J.S.; Klimkowska, M.; Schaffer, M.; et al. Complete Remission with Reduction of High-Risk Clones following Haploidentical NK-Cell Therapy against MDS and AML. Clin. Cancer Res. 2018, 24, 1834–1844. [Google Scholar] [CrossRef] [Green Version]
- Dolstra, H.; Roeven, M.W.; Spanholtz, J.; Hangalapura, B.N.; Tordoir, M.; Maas, F.; Leenders, M.; Bohme, F.; Kok, N.; Trilsbeek, C.; et al. Successful Transfer of Umbilical Cord Blood CD34+ Hematopoietic Stem and Progenitor-derived NK Cells in Older Acute Myeloid Leukemia Patients. Clin. Cancer Res. 2017, 23, 4107–4118. [Google Scholar] [CrossRef] [Green Version]
- Ciurea, S.O.; Schafer, J.R.; Bassett, R.; Denman, C.J.; Cao, K.; Willis, D.; Rondon, G.; Chen, J.; Soebbing, D.; Kaur, I.; et al. Phase 1 clinical trial using mbIL21 ex vivo–expanded donor-derived NK cells after haploidentical transplantation. Blood 2017, 130, 1857–1868. [Google Scholar] [CrossRef]
- Sivori, S.; Pende, D.; Quatrini, L.; Pietra, G.; Della Chiesa, M.; Vacca, P.; Tumino, N.; Moretta, F.; Mingari, M.C.; Locatelli, F.; et al. NK cells and ILCs in tumor immunotherapy. Mol. Asp. Med. 2021, 80, 100870. [Google Scholar] [CrossRef]
- Ruggeri, L.; Vago, L.; Eikema, D.-J.; de Wreede, L.C.; Ciceri, F.; Diaz, M.A.; Locatelli, F.; Jindra, P.; Milone, G.; Diez-Martin, J.L.; et al. Natural killer cell alloreactivity in HLA-haploidentical hematopoietic transplantation: A study on behalf of the CTIWP of the EBMT. Bone Marrow Transplant. 2021, 56, 1900–1907. [Google Scholar] [CrossRef]
- Krieger, E.; Qayyum, R.; Keating, A.; Toor, A. Increased donor inhibitory KIR with known HLA interactions provide protection from relapse following HLA matched unrelated donor HCT for AML. Bone Marrow Transplant. 2021, 56, 2714–2722. [Google Scholar] [CrossRef]
- Montaldo, E.; Vacca, P.; Moretta, L.; Mingari, M.C. Understanding human NK cell differentiation: Clues for improving the haploidentical hematopoietic stem cell transplantation. Immunol. Lett. 2013, 155, 2–5. [Google Scholar] [CrossRef]
- Bertaina, A.; Merli, P.; Rutella, S.; Pagliara, D.; Bernardo, M.E.; Masetti, R.; Pende, D.; Falco, M.; Handgretinger, R.; Moretta, F.; et al. HLA-haploidentical stem cell transplantation after removal of αβ+ T and B cells in children with nonmalignant disorders. Blood 2014, 124, 822–826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Locatelli, F.; Merli, P.; Pagliara, D.; Pira, G.L.; Falco, M.; Pende, D.; Rondelli, R.; Lucarelli, B.; Brescia, L.P.; Masetti, R.; et al. Outcome of children with acute leukemia given HLA-haploidentical HSCT after αβ T-cell and B-cell depletion. Blood 2017, 130, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Pistoia, V.; Tumino, N.; Vacca, P.; Veneziani, I.; Moretta, A.; Locatelli, F.; Moretta, L. Human γδ T-Cells: From Surface Receptors to the Therapy of High-Risk Leukemias. Front. Immunol. 2018, 9, 984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Locatelli, F.; Pende, D.; Falco, M.; Della Chiesa, M.; Moretta, A.; Moretta, L. NK Cells Mediate a Crucial Graft-versus-Leukemia Effect in Haploidentical-HSCT to Cure High-Risk Acute Leukemia. Trends Immunol. 2018, 39, 577–590. [Google Scholar] [CrossRef]
- Tang, X.; Yang, L.; Li, Z.; Nalin, A.P.; Dai, H.; Xu, T.; Yin, J.; You, F.; Zhu, M.; Shen, W.; et al. First-in-man clinical trial of CAR NK-92 cells: Safety test of CD33-CAR NK-92 cells in patients with relapsed and refractory acute myeloid leukemia. Am. J. Cancer Res. 2018, 8, 1083–1089. [Google Scholar]
- Munneke, J.M.; Björklund, A.T.; Mjösberg, J.; Garming-Legert, K.; Bernink, J.H.; Blom, B.; Huisman, C.; Van Oers, M.H.J.; Spits, H.; Malmberg, K.-J.; et al. Activated innate lymphoid cells are associated with a reduced susceptibility to graft-versus-host disease. Blood 2014, 124, 812–821. [Google Scholar] [CrossRef]
- Trabanelli, S.; Curti, A.; Lecciso, M.; Salomé, B.; Riether, C.; Ochsenbein, A.; Romero, P.; Jandus, C. CD127+ innate lymphoid cells are dysregulated in treatment naïve acute myeloid leukemia patients at diagnosis. Haematologica 2015, 100, e257–e260. [Google Scholar] [CrossRef] [Green Version]
- Salomé, B.; Gomez-Cadena, A.; Loyon, R.; Suffiotti, M.; Salvestrini, V.; Wyss, T.; Vanoni, G.; Ruan, D.F.; Rossi, M.; Tozzo, A.; et al. CD56 as a marker of an ILC1-like population with NK cell properties that is functionally impaired in AML. Blood Adv. 2019, 3, 3674–3687. [Google Scholar] [CrossRef]
- Wu, L.; Lin, Q.; Ma, Z.; Chowdhury, F.A.; Mazumder, H.H.; Du, W. Mesenchymal PGD2 activates an ILC2-Treg axis to promote proliferation of normal and malignant HSPCs. Leukemia 2020, 34, 3028–3041. [Google Scholar] [CrossRef]
- Trabanelli, S.; Chevalier, M.F.; Martinez-Usatorre, A.; Gomez-Cadena, A.; Salomé, B.; Lecciso, M.; Salvestrini, V.; Verdeil, G.; Racle, J.; Papayannidis, C.; et al. Tumour-derived PGD2 and NKp30-B7H6 engagement drives an immunosuppressive ILC2-MDSC axis. Nat. Commun. 2017, 8, 59. [Google Scholar] [CrossRef] [Green Version]
- Musolino, C.; Allegra, A.; Pioggia, G.; Gangemi, S. Immature myeloid-derived suppressor cells: A bridge between inflammation and cancer. Oncol. Rep. 2016, 37, 671–683. [Google Scholar] [CrossRef] [Green Version]
- Bruce, D.W.; Stefanski, H.E.; Vincent, B.G.; Dant, T.A.; Reisdorf, S.; Bommiasamy, H.; Serody, D.A.; Wilson, J.E.; McKinnon, K.P.; Shlomchik, W.D.; et al. Type 2 innate lymphoid cells treat and prevent acute gastrointestinal graft-versus-host disease. J. Clin. Investig. 2017, 127, 1813–1825. [Google Scholar] [CrossRef] [Green Version]
- Bruce, D.W.; Kolupaev, O.V.; Laurie, S.J.; Bommiasamy, H.; Stefanski, H.; Blazar, B.R.; Coghill, J.M.; Serody, J.S. Third-party type 2 innate lymphoid cells prevent and treat GI tract GvHD. Blood Adv. 2021, 5, 4578–4589. [Google Scholar] [CrossRef]
- Vivier, E.; Artis, D.; Colonna, M.; Diefenbach, A.; Di Santo, J.P.; Eberl, G.; Koyasu, S.; Locksley, R.M.; McKenzie, A.N.J.; Mebius, R.E.; et al. Innate Lymphoid Cells: 10 Years On. Cell 2018, 174, 1054–1066. [Google Scholar] [CrossRef] [Green Version]
- Mohty, M.; Malard, F.; Savani, B.N. High-dose total body irradiation and myeloablative conditioning before allogeneic hematopoietic cell transplantation: Time to rethink? Biol. Blood Marrow. Transpl. 2015, 21, 620–624. [Google Scholar] [CrossRef] [Green Version]
- Shlomchik, W.D. Graft-versus-host disease. Nat. Rev. Immunol. 2007, 7, 340–352. [Google Scholar] [CrossRef]
- Rangel-Moreno, J.; Garcia-Hernandez, M.D.L.L.; Ramos-Payan, R.; Biear, J.; Hernady, E.; Sangster, M.; Randall, T.D.; Johnston, C.J.; Finkelstein, J.N.; Williams, J.P. Long-Lasting Impact of Neonatal Exposure to Total Body Gamma Radiation on Secondary Lymphoid Organ Structure and Function. Radiat. Res. 2015, 184, 352–366. [Google Scholar] [CrossRef] [Green Version]
- Dudakov, J.A.; Hanash, A.M.; Jenq, R.R.; Young, L.F.; Ghosh, A.; Singer, N.V.; West, M.L.; Smith, O.M.; Holland, A.M.; Tsai, J.J.; et al. Interleukin-22 Drives Endogenous Thymic Regeneration in Mice. Science 2012, 336, 91–95. [Google Scholar] [CrossRef] [Green Version]
- Moretta, F.; Petronelli, F.; Lucarelli, B.; Pitisci, A.; Bertaina, A.; Locatelli, F.; Mingari, M.C.; Moretta, L.; Montaldo, E. The generation of human innate lymphoid cells is influenced by the source of hematopoietic stem cells and by the use of G-CSF. Eur. J. Immunol. 2016, 46, 1271–1278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vacca, P.; Montaldo, E.; Croxatto, D.; Moretta, F.; Bertaina, A.; Vitale, C.; Locatelli, F.; Mingari, M.C.; Moretta, L. NK Cells and Other Innate Lymphoid Cells in Hematopoietic Stem Cell Transplantation. Front. Immunol. 2016, 7, 188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Xia, P.; Chen, Y.; Qu, Y.; Xiong, Z.; Ye, B.; Du, Y.; Tian, Y.; Yin, Z.; Xu, Z.; et al. Regulatory Innate Lymphoid Cells Control Innate Intestinal Inflammation. Cell 2017, 171, 201–216.e18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakaguchi, S.; Yamaguchi, T.; Nomura, T.; Ono, M. Regulatory T cells and immune tolerance. Cell 2008, 133, 775–787. [Google Scholar] [CrossRef] [Green Version]
- Gury-BenAri, M.; Thaiss, C.A.; Serafini, N.; Winter, D.R.; Giladi, A.; Lara-Astiaso, D.; Levy, M.; Salame, T.M.; Weiner, A.; David, E.; et al. The spectrum and regulatory landscape of intestinal innate lymphoid cells are shaped by the microbiome. Cell 2016, 166, 1231–1246.e13. [Google Scholar] [CrossRef]
- Yu, J.; Li, Y.; Pan, Y.; Liu, Y.; Xing, H.; Xie, X.; Wan, D.; Jiang, Z. Deficient Regulatory Innate Lymphoid Cells and Differential Expression of miRNAs in Acute Myeloid Leukemia Quantified by Next Generation Sequence. Cancer Manag. Res. 2019, 11, 10969–10982. [Google Scholar] [CrossRef] [Green Version]
- Jin, Z.; Lan, T.; Zhao, Y.; Du, J.; Chen, J.; Lai, J.; Xu, L.; Chen, S.; Zhong, X.; Wu, X.; et al. Higher TIGIT+CD226- γδ T cells in Patients with Acute Myeloid Leukemia. Immunol. Investig. 2020, 51, 40–50. [Google Scholar] [CrossRef]
- Gentles, A.J.; Newman, A.M.; Liu, C.L.; Bratman, S.V.; Feng, W.; Kim, D.; Nair, V.S.; Xu, Y.; Khuong, A.; Hoang, C.D.; et al. The prognostic landscape of genes and infil-trating immune cells across human cancers. Nat. Med. 2015, 21, 938–945. [Google Scholar] [CrossRef]
- Simoni, Y.; Becht, E.; Fehlings, M.; Loh, C.Y.; Koo, S.-L.; Teng, K.W.W.; Yeong, J.P.S.; Nahar, R.; Zhang, T.; Kared, H.; et al. Bystander CD8+ T cells are abundant and phenotypically distinct in human tumour infiltrates. Nature 2018, 557, 575–579. [Google Scholar] [CrossRef]
- Tosolini, M.; Pont, F.; Poupot, M.; Vergez, F.; Nicolau-Travers, M.L.; Vermijlen, D.; Sarry, J.E.; Dieli, F.; Fournié, J.J. Assess-ment of Tumor-Infiltrating Tcrvg9vd2 gd Lymphocyte Abundance by Deconvolution of Human Cancers Microarrays. Coimmunology 2017, 6, e1284723. [Google Scholar] [CrossRef]
- Kabelitz, D.; Serrano, R.; Kouakanou, L.; Peters, C.; Kalyan, S. Cancer immunotherapy with γδ T cells: Many paths ahead of us. Cell. Mol. Immunol. 2020, 17, 925–939. [Google Scholar] [CrossRef]
- Deniger, D.C.; Moyes, J.S.; Cooper, L.J.N. Clinical Applications of Gamma Delta T Cells with Multivalent Immunity. Front. Immunol. 2014, 5, 636. [Google Scholar] [CrossRef] [Green Version]
- Buccheri, S.; Guggino, G.; Caccamo, N.; Li Donni, P.; Dieli, F. Efficacy and safety of gammadeltaT cell-based tumor im-munotherapy: A meta-analysis. J. Biol. Regul. Homeost. Agents 2014, 28, 81–90. [Google Scholar]
- Godder, K.T.; Henslee-Downey, P.J.; Mehta, J.; Park, B.S.; Chiang, K.Y.; Abhyankar, S.; Lamb, L.S. Long term disease-free survival in acute leukemia patients recovering with increased gammadelta T cells after partially mismatched related donor bone marrow transplantation. Bone Marrow. Transpl. 2007, 39, 751–757. [Google Scholar] [CrossRef] [Green Version]
- Blazar, B.R.; Taylor, P.A.; Panoskaltsis-Mortari, A.; Barrett, T.A.; Bluestone, J.A.; Vallera, D.A. Lethal murine graft-versus-host disease induced by donor gamma/delta expressing T cells with specificity for host nonclassical major his-tocompatibility complex class Ib antigens. Blood 1996, 87, 827–837. [Google Scholar] [CrossRef] [Green Version]
- Maeda, Y.; Reddy, P.; Lowler, K.P.; Liu, C.; Bishop, D.K.; Ferrara, J.L.M. Critical role of host γδ T cells in experimental acute graft-versus-host disease. Blood 2005, 106, 749–755. [Google Scholar] [CrossRef]
- Van Acker, H.H.; Anguille, S.; Willemen, Y.; Bergh, J.M.V.D.; Berneman, Z.N.; Lion, E.; Smits, E.L.; Van Tendeloo, V.F. Interleukin-15 enhances the proliferation, stimulatory phenotype, and antitumor effector functions of human gamma delta T cells. J. Hematol. Oncol. 2016, 9, 101. [Google Scholar] [CrossRef] [Green Version]
- Van Acker, H.H.; Anguille, S.; Van Tendeloo, V.; Lion, E. Empowering gamma delta T cells with antitumor immunity by dendritic cell-based immunotherapy. OncoImmunology 2015, 4, e1021538. [Google Scholar] [CrossRef] [Green Version]
- Van Acker, H.H.; Anguille, S.; De Reu, H.; Berneman, Z.; Smits, E.; Van Tendeloo, V.F. Interleukin-15-Cultured Dendritic Cells Enhance Anti-Tumor Gamma Delta T Cell Functions through IL-15 Secretion. Front. Immunol. 2018, 9, 658. [Google Scholar] [CrossRef] [Green Version]
- Deniger, D.C.; Maiti, S.N.; Mi, T.; Switzer, K.C.; Ramachandran, V.; Hurton, L.V.; Ang, S.; Olivares, S.; Rabinovich, B.A.; Huls, M.H.; et al. Activating and Propagating Polyclonal Gamma Delta T Cells with Broad Specificity for Malignancies. Clin. Cancer Res. 2014, 20, 5708–5719. [Google Scholar] [CrossRef] [Green Version]
- Rozenbaum, M.; Meir, A.; Aharony, Y.; Itzhaki, O.; Schachter, J.; Bank, I.; Jacoby, E.; Besser, M.J. Gamma-Delta CAR-T Cells Show CAR-Directed and Independent Activity Against Leukemia. Front Immunol. 2020, 11, 1347. [Google Scholar] [CrossRef] [PubMed]
- Comont, T.; Nicolau-Travers, M.-L.; Bertoli, S.; Recher, C.; Vergez, F.; Treiner, E. MAIT cells numbers and frequencies in patients with acute myeloid leukemia at diagnosis: Association with cytogenetic profile and gene mutations. Cancer Immunol. Immunother. 2021, 71, 875–887. [Google Scholar] [CrossRef] [PubMed]
- Solders, M.; Erkers, T.; Gorchs, L.; Poiret, T.; Remberger, M.; Magalhaes, I.; Kaipe, H. Mucosal-Associated Invariant T Cells Display a Poor Reconstitution and Altered Phenotype after Allogeneic Hematopoietic Stem Cell Transplantation. Front. Immunol. 2017, 8, 1861. [Google Scholar] [CrossRef] [Green Version]
- Ben Youssef, G.; Tourret, M.; Salou, M.; Ghazarian, L.; Houdouin, V.; Mondot, S.; Mburu, Y.; Lambert, M.; Azarnoush, S.; Diana, J.-S.; et al. Ontogeny of human mucosal-associated invariant T cells and related T cell subsets. J. Exp. Med. 2018, 215, 459–479. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, K.; Umeda, K.; Hiejima, E.; Iwai, A.; Mikami, M.; Nodomi, S.; Saida, S.; Kato, I.; Hiramatsu, H.; Yasumi, T.; et al. Influence of post-transplant mucosal-associated invariant T cell recovery on the development of acute graft-versus-host disease in allogeneic bone marrow transplantation. Int. J. Hematol. 2018, 108, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Konuma, T.; Kohara, C.; Watanabe, E.; Takahashi, S.; Ozawa, G.; Suzuki, K.; Mizukami, M.; Nagai, E.; Jimbo, K.; Kaito, Y.; et al. Reconstitution of Circulating Mucosal-Associated Invariant T Cells after Allogeneic Hematopoietic Cell Transplantation: Its Association with the Riboflavin Synthetic Pathway of Gut Microbiota in Cord Blood Transplant Recipients. J. Immunol. 2020, 204, 1462–1473. [Google Scholar] [CrossRef] [PubMed]
- Stikvoort, A.; Chen, Y.; Rådestad, E.F.S.; Törlén, J.K.; Lakshmikanth, T.; Björklund, A.; Mikes, J.; Achour, A.; Gertow, J.; Sundberg, B.; et al. Combining Flow and Mass Cytometry in the Search for Biomarkers in Chronic Graft-versus-Host Disease. Front. Immunol. 2017, 8, 717. [Google Scholar] [CrossRef]
- Xin, Q.; Chen, Z.; Wei, W.; Wu, Y. Animal models of acute lymphoblastic leukemia: Recapitulating the human disease to evaluate drug efficacy and discover therapeutic targets. Biochem. Pharmacol. 2022, 198, 114970. [Google Scholar] [CrossRef]
- Mizia-Malarz, A.; Sobol-Milejska, G. NK Cells as Possible Prognostic Factor in Childhood Acute Lymphoblastic Leukemia. Dis. Markers 2019, 2019, 3596983. [Google Scholar] [CrossRef]
- Sullivan, E.M.; Jeha, S.; Kang, G.; Cheng, C.; Rooney, B.; Holladay, M.; Bari, R.; Schell, S.; Tuggle, M.; Pui, C.-H.; et al. NK Cell Genotype and Phenotype at Diagnosis of Acute Lymphoblastic Leukemia Correlate with Postinduction Residual Disease. Clin. Cancer Res. 2014, 20, 5986–5994. [Google Scholar] [CrossRef] [Green Version]
- Lafont, V.; Liautard, J.; Liautard, J.P.; Favero, J. Production of TNF-a by Human Vg9Vd2 T Cells Via Engagement of FcgRIIIA, the Low Affinity Type 3 Receptor for the Fc Portion of IgG, Expressed upon TCR Activation by Nonpeptidic An-tigen. J. Immunol. 2001, 166, 7190–7199. [Google Scholar] [CrossRef] [Green Version]
- Seidel, U.J.E.; Vogt, F.; Grosse-Hovest, L.; Jung, G.; Handgretinger, R.; Lang, P. T Cell-Mediated Antibody-Dependent Cellular Cytotoxicity with CD19 Antibodies Assessed by an Impedance-Based Label-Free Real-Time Cytotoxicity Assay. Front. Immunol. 2014, 5, 618. [Google Scholar] [CrossRef] [Green Version]
- Schiller, C.B.; Braciak, T.A.; Fenn, N.C.; Seidel, U.J.E.; Roskopf, C.C.; Wildenhain, S.; Honegger, A.; Schubert, I.A.; Schele, A.; Lämmermann, K.; et al. CD19-specific triplebody SPM-1 engages NK and γδ T cells for rapid and efficient lysis of malignant B-lymphoid cells. Oncotarget 2016, 7, 83392–83408. [Google Scholar] [CrossRef] [Green Version]
- Cortes, J.; Pavlovsky, C.; Saußele, S. Chronic myeloid leukaemia. Lancet 2021, 398, 1914–1926. [Google Scholar] [CrossRef]
- Pierson, B.; Miller, J.S. CD56+bright and CD56+dim natural killer cells in patients with chronic myelogenous leukemia progressively decrease in number, respond less to stimuli that recruit clonogenic natural killer cells, and exhibit decreased proliferation on a per cell basis. Blood 1996, 88, 2279–2287. [Google Scholar] [CrossRef] [Green Version]
- Cervantes, F.; Pierson, B.A.; McGlave, P.B.; Verfaillie, C.; Miller, J.S. Autologous activated natural killer cells suppress primitive chronic myelogenous leukemia progenitors in long-term culture. Blood 1996, 87, 2476–2485. [Google Scholar] [CrossRef] [Green Version]
- Silla, L.M.R.; Pincus, S.M.; Locker, J.D.; Glover, J.; Elder, E.M.; Donnenberg, A.D.; Nardi, N.B.; Bryant, J.; Ball, E.D.; Whiteside, T.L. Generation of activated natural killer (A-NK) cells in patients with chronic myelogenous leukaemia and their role in the in vitro disappearance of BCR/abl-positive targets. Br. J. Haematol. 1996, 93, 375–385. [Google Scholar] [CrossRef]
- Ilander, M.; Olsson-Strömberg, U.; Schlums, H.; Guilhot, J.; Brück, O.; Lähteenmäki, H.; Kasanen, T.; Koskenvesa, P.; Söder-lund, S.; Höglund, M.; et al. Increased proportion of mature NK cells is associated with successful imatinib discontinuation in chronic myeloid leukemia. Leukemia 2017, 31, 1108–1116. [Google Scholar] [CrossRef]
- Dumas, P.; Bérard, E.; Bréal, C.; Dulucq, S.; Réa, D.; Nicolini, F.; Forcade, E.; Dufossée, M.; Pasquet, J.; Turcq, B.; et al. Killer immunoglobulin-like receptor genotypes and chronic myeloid leukemia outcomes after imatinib cessation for treatment-free remission. Cancer Med. 2019, 8, 4976–4985. [Google Scholar] [CrossRef] [Green Version]
- Marin, D.; Gabriel, I.H.; Ahmad, S.; Foroni, L.; De Lavallade, H.; Clark, R.; O’Brien, S.; Sergeant, R.; Hedgley, C.; Milojkovic, D.; et al. KIR2DS1 genotype predicts for complete cytogenetic response and survival in newly diagnosed chronic myeloid leukemia patients treated with imatinib. Leukemia 2012, 26, 296–302. [Google Scholar] [CrossRef]
- Yeung, D.T.; Tang, C.; Vidovic, L.; White, D.L.; Branford, S.; Hughes, T.P.; Yong, A.S. KIR2DL5B genotype predicts out-comes in CML patients treated with response-directed sequential imatinib/nilotinib strategy. Blood 2015, 126, 2720–2723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barros, M.D.S.; de Araújo, N.D.; Magalhães-Gama, F.; Ribeiro, T.L.P.; Hanna, F.S.A.; Tarragô, A.M.; Malheiro, A.; Costa, A.G. γδ T Cells for Leukemia Immunotherapy: New and Expanding Trends. Front. Immunol. 2021, 12, 729085. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, N.; Narita, M.; Yokoyama, A.; Sekiguchi, A.; Saito, A.; Tochiki, N.; Furukawa, T.; Toba, K.; Aizawa, Y.; Takahashi, M. Type I IFN-mediated enhancement of anti-leukemic cytotoxicity of γδ T cells expanded from peripheral blood cells by stimulation with zoledronate. Cytotherapy 2006, 8, 118–129. [Google Scholar] [CrossRef] [PubMed]
- D’Asaro, M.; La Mendola, C.; Di Liberto, D.; Orlando, V.; Todaro, M.; Spina, M.; Guggino, G.; Meraviglia, S.; Caccamo, N.; Messina, A.; et al. Vg9vd2 T Lymphocytes Efficiently Recognize and Kill Zoledronate-Sensitized, Imatinib-Sensitive, and Imatinib-Resistant Chronic Myelogenous Leukemia Cells. J. Immunol. 2010, 184, 3260–3268. [Google Scholar] [CrossRef] [Green Version]
- Siegers, G.M.; Felizardo, T.C.; Mathieson, A.M.; Kosaka, Y.; Wang, X.-H.; Medin, J.A.; Keating, A. Anti-Leukemia Activity of In Vitro-Expanded Human Gamma Delta T Cells in a Xenogeneic Ph+ Leukemia Model. PLoS ONE 2011, 6, e16700. [Google Scholar] [CrossRef]
- Baumeister, J.; Chatain, N.; Sofias, A.M.; Lammers, T.; Koschmieder, S. Progression of Myeloproliferative Neoplasms (MPN): Diagnostic and Therapeutic Perspectives. Cells 2021, 10, 3551. [Google Scholar] [CrossRef]
- Froom, P.; Aghai, E.; Kinarty, A.; Lahat, N. Decreased natural killer (NK) activity in patients with myeloproliferative dis-orders. Cancer 1989, 64, 1038–1040. [Google Scholar] [CrossRef]
- Briard, D.; Brouty-Boyé, D.; Giron-Michel, J.; Azzarone, B.; Jasmin, C.; Le Bousse-Kerdilès, C. Impaired NK cell differentiation of blood-derived CD34+ progenitors from patients with myeloid metaplasia with myelofibrosis. Clin. Immunol. 2003, 106, 201–212. [Google Scholar] [CrossRef]
- Romano, M.; Sollazzo, D.; Trabanelli, S.; Barone, M.; Polverelli, N.; Perricone, M.; Forte, D.; Luatti, S.; Cavo, M.; Vianelli, N.; et al. Mutations in JAK2 and Calreticulin genes are associated with specific alterations of the immune system in myelofibrosis. OncoImmunology 2017, 6, e1345402. [Google Scholar] [CrossRef] [Green Version]
- Allegra, A.; Sant’Antonio, E.; Penna, G.; Alonci, A.; D’Angelo, A.; Russo, S.; Cannavò, A.; Gerace, D.; Musolino, C. Novel therapeutic strategies in multiple myeloma: Role of the heat shock protein inhibitors. Eur. J. Haematol. 2011, 86, 93–110. [Google Scholar] [CrossRef]
- Allegra, A.; Penna, G.; Alonci, A.; Russo, S.; Greve, B.; Innao, V.; Minardi, V.; Musolino, C. Monoclonal antibodies: Potential new therapeutic treatment against multiple myeloma. Eur. J. Haematol. 2013, 90, 441–468. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Sanz, R.; González, M.; Orfão, A.; Moro, M.J.; Hernández, J.M.; Borrego, D.; Carnero, M.; Casanova, F.; Bárez, A.; Jiménez, R.; et al. Analysis of natural killer-associated antigens in peripheral blood and bone marrow of multiple myeloma patients and prognostic implications. Br. J. Haematol. 1996, 93, 81–88. [Google Scholar] [CrossRef]
- Viel, S.; Charrier, E.; Marçais, A.; Rouzaire, P.; Bienvenu, J.; Karlin, L.; Salles, G.; Walzer, T. Monitoring NK cell activity in patients with hematological malignancies. OncoImmunology 2013, 2, e26011. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, Y.; Lin, Y.; Zhang, A.; Zou, R.; Xu, H.; Wang, S. Prognostic significance of CD56 expression in patients with multiple myeloma: A meta-analysis. Hematology 2022, 27, 122–131. [Google Scholar] [CrossRef]
- Wu, J.; Gao, F.-X.; Wang, C.; Qin, M.; Han, F.; Xu, T.; Hu, Z.; Long, Y.; He, X.-M.; Deng, X.; et al. IL-6 and IL-8 secreted by tu-mour cells impair the function of NK cells via the STAT3 pathway in oesophageal squamous cell carcinoma. J. Exp. Clin. Cancer Res. 2019, 38, 321. [Google Scholar] [CrossRef]
- Holt, D.; Ma, X.; Kundu, N.; Fulton, A. Prostaglandin E2 (PGE2) suppresses natural killer cell function primarily through the PGE2 receptor EP4. Cancer Immunol. Immunother. 2011, 60, 1577–1586. [Google Scholar] [CrossRef] [Green Version]
- Lin, L.; Benson, D.M.; DeAngelis, S.; Bakan, C.E.; Li, P.-K.; Li, C.; Lin, J. A small molecule, LLL12 inhibits constitutive STAT3 and IL-6-induced STAT3 signaling and exhibits potent growth suppressive activity in human multiple myeloma cells. Int. J. Cancer 2011, 130, 1459–1469. [Google Scholar] [CrossRef] [Green Version]
- Tsuruma, T.; Yagihashi, A.; Hirata, K.; Torigoe, T.; Araya, J.; Watanabe, N.; Sato, N. Interleukin-10 Reduces Natural Killer (NK) Sensitivity of Tumor Cells by Downregulating NK Target Structure Expression. Cell. Immunol. 1999, 198, 103–110. [Google Scholar] [CrossRef]
- Martinet, L.; Jean, C.; Dietrich, G.; Fournié, J.-J.; Poupot, R. PGE2 inhibits natural killer and γδ T cell cytotoxicity triggered by NKR and TCR through a cAMP-mediated PKA type I-dependent signaling. Biochem. Pharmacol. 2010, 80, 838–845. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, H.; Nielsen, H.J.; Tvede, N.; Klarlund, K.; Mansa, B.; Moesgaard, F.; Drivsholm, A.; Nielsen, H.; Nielsen, H.J. Immune dysfunction in multiple myeloma. Reduced natural killer cell activity and increased levels of soluble interleukin-2 receptors. APMIS 1991, 99, 340–346. [Google Scholar] [CrossRef]
- Zavidij, O.; Haradhvala, N.J.; Mouhieddine, T.H.; Sklavenitis-Pistofidis, R.; Cai, S.; Reidy, M.; Rahmat, M.; Flaifel, A.; Ferland, B.; Su, N.K.; et al. Single-cell RNA sequencing reveals compromised immune microenvironment in precursor stages of multiple myeloma. Nat. Cancer 2020, 1, 493–506. [Google Scholar] [CrossRef]
- Fionda, C.; Stabile, H.; Molfetta, R.; Soriani, A.; Bernardini, G.; Zingoni, A.; Gismondi, A.; Paolini, R.; Cippitelli, M.; Santoni, A. Translating the anti-myeloma activity of Natural Killer cells into clinical application. Cancer Treat. Rev. 2018, 70, 255–264. [Google Scholar] [CrossRef]
- Moloudizargari, M.; Abdollahi, M.; Asghari, M.H.; Zimta, A.A.; Neagoe, I.B.; Nabavi, S.M. The emerging role of exosomes in multiple myeloma. Blood Rev. 2019, 38, 100595. [Google Scholar] [CrossRef]
- Benson, D.M., Jr.; Bakan, C.E.; Mishra, A.; Hofmeister, C.C.; Efebera, Y.; Becknell, B.; Baiocchi, R.A.; Zhang, J.; Yu, J.; Smith, M.K.; et al. The PD-1/PD-L1 axis modulates the natural killer cell versus multiple myeloma effect: A therapeutic target for CT-011, a novel monoclonal anti–PD-1 antibody. Blood 2010, 116, 2286–2294. [Google Scholar] [CrossRef] [PubMed]
- Famularo, G.; D’Ambrosio, A.; Quintieri, F.; Di, S.G.; Parzanese, I.; Pizzuto, F.; Giacomelli, R.; Pugliese, O.; Tonietti, G. Nat-ural killer cell frequency and function in patients with monoclonal gammopathies. J. Clin. Lab. Immunol. 1992, 37, 99–109. [Google Scholar] [PubMed]
- Schütt, P.; Brandhorst, D.; Stellberg, W.; Poser, M.; Ebeling, P.; Müller, S.; Buttkereit, U.; Opalka, B.; Lindemann, M.; Grosse-Wilde, H.; et al. Immune parameters in multiple myeloma patients: Influence of treatment and correlation with opportunistic infections. Leuk. Lymphoma 2006, 47, 1570–1582. [Google Scholar] [CrossRef] [PubMed]
- De Rossi, G.; De Sanctis, G.; Bottari, V.; Tribalto, M.; Lopez, M.; Petrucci, M.T.; Fontana, L. Surface markers and cytotoxic activities of lymphocytes in monoclonal gammopathy of undetermined significance and untreated multiple myeloma. Cancer Immunol. Immunother. 1987, 25, 133–136. [Google Scholar] [CrossRef]
- Omede, P.; Boccadoro, M.; Gallone, G.; Frieri, R.; Battaglio, S.; Redoglia, V.; Pileri, A. Multiple myeloma: Increased circulating lymphocytes carrying plasma cell-associated antigens as an indicator of poor survival. Blood 1990, 76, 1375–1379. [Google Scholar] [CrossRef] [Green Version]
- Tienhaara, A.; Pelliniemi, T.T. Peripheral blood lymphocyte subsets in multiple myeloma and monoclonal gammopathy of undetermined significance. Clin. Lab. Haematol. 1994, 16, 213–223. [Google Scholar] [CrossRef]
- Fauriat, C.; Mallet, F.; Olive, D.; Costello, R. Impaired activating receptor expression pattern in natural killer cells from patients with multiple myeloma. Leukemia 2006, 20, 732–733. [Google Scholar] [CrossRef]
- Jurisic, V.; Srdic, T.; Konjevic, G.; Markovic, O.; Colovic, M. Clinical stage-depending decrease of NK cell activity in multiple myeloma patients. Med Oncol. 2007, 24, 312–317. [Google Scholar] [CrossRef]
- Barberi, C.; De Pasquale, C.; Allegra, A.; Migliore, G.S.; Oliveri, D.; LoIacono, F.; Innao, V.; Musolino, C.; Pende, D.; Cantoni, C.; et al. Myeloma cells induce the accumulation of activated CD94low NK cells by cell-to-cell contacts involving CD56 molecules. Blood Adv. 2020, 4, 2297–2307. [Google Scholar] [CrossRef]
- Alfarra, H.; Weir, J.; Grieve, S.; Reiman, T. Targeting NK Cell Inhibitory Receptors for Precision Multiple Myeloma Immunotherapy. Front. Immunol. 2020, 11, 575609. [Google Scholar] [CrossRef]
- Romagné, F.; André, P.; Spee, P.; Zahn, S.; Anfossi, N.; Gauthier, L.; Capanni, M.; Ruggeri, L.; Benson, D.M., Jr.; Blaser, B.W.; et al. Preclinical characterization of 1-7F9, a novel human anti–KIR receptor therapeutic antibody that augments natural killer–mediated killing of tumor cells. Blood 2009, 114, 2667–2677. [Google Scholar] [CrossRef]
- Benson, D.M.; Bakan, C.E.; Zhang, S.; Collins, S.M.; Liang, J.; Srivastava, S.; Hofmeister, C.; Efebera, Y.; Andre, P.; Romagne, F.; et al. IPH2101, a novel anti-inhibitory KIR antibody, and lenalidomide combine to enhance the natural killer cell versus multiple myeloma effect. Blood 2011, 118, 6387–6391. [Google Scholar] [CrossRef] [Green Version]
- Benson, D.M., Jr.; Hofmeister, C.C.; Padmanabhan, S.; Suvannasankha, A.; Jagannath, S.; Abonour, R.; Bakan, C.; Andre, P.; Efebera, Y.; Tiollier, J.; et al. A phase 1 trial of the anti-KIR antibody IPH2101 in patients with relapsed/refractory multiple myeloma. Blood 2012, 120, 4324–4333. [Google Scholar] [CrossRef]
- Benson, D.M., Jr.; Cohen, A.; Jagannath, S.; Munshi, N.C.; Spitzer, G.; Hofmeister, C.C.; Efebera, Y.A.; Andre, P.; Zerbib, R.; Caligiuri, M.A. A Phase I Trial of the Anti-KIR Antibody IPH2101 and Lenalidomide in Patients with Relapsed/Refractory Multiple Myeloma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2015, 21, 4055–4061. [Google Scholar] [CrossRef] [Green Version]
- Björklund, K.; Forkel, M.; Picelli, S.; Konya, V.; Theorell, J.; Friberg, D.; Sandberg, K.B.S.P.R.; Mjösberg, J. The heterogeneity of human CD127+ innate lymphoid cells revealed by single-cell RNA sequencing. Nat. Immunol. 2016, 17, 451–460. [Google Scholar] [CrossRef]
- Kini Bailur, J.; Mehta, S.; Zhang, L.; Neparidze, N.; Parker, T.L.; Bar, N.; Anderson, T.; Xu, M.; Dhodapkar, K.; Dhodapkar, M.V. Early Enrichment and Subsequent Dysfunction of Marrow ILC Subsets in Monoclonal Gammopathy: Target for IMiD Therapy. Blood 2017, 130 (Suppl. 1), 4370. [Google Scholar]
- Szudy-Szczyrek, A.; Ahern, S.; Kozioł, M.; Majowicz, D.; Szczyrek, M.; Krawczyk, J.; Hus, M. Therapeutic Potential of Innate Lymphoid Cells for Multiple Myeloma Therapy. Cancers 2021, 13, 4806. [Google Scholar] [CrossRef]
- Sarkar, S.; Chauhan, S.K.S.; Daly, J.; Natoni, A.; Fairfield, H.; Henderson, R.; Nolan, E.; Swan, D.; Hu, J.; Reagan, M.R.; et al. The CD38low natural killer cell line KHYG1 transiently expressing CD16F158V in combination with daratumumab targets multiple myeloma cells with minimal effector NK cell fratricide. Cancer Immunol. Immunother. 2020, 69, 421–434. [Google Scholar] [CrossRef] [PubMed]
- Bigley, A.B.; Spade, S.; Agha, N.H.; Biswas, S.; Tang, S.; Malik, M.H.; Dai, L.; Masoumi, S.; Patiño-Escobar, B.; Hale, M.; et al. FcεRIγ-negative NK cells persist in vivo and enhance efficacy of therapeutic monoclonal antibodies in multiple myeloma. Blood Adv. 2021, 5, 3021–3031. [Google Scholar] [CrossRef] [PubMed]
- Guillerey, C.; Stannard, K.; Chen, J.; Krumeich, S.; Miles, K.; Nakamura, K.; Smith, J.; Yu, Y.; Ng, S.; Harjunpää, H.; et al. Systemic administration of IL-33 induces a population of circulating KLRG1 hi type 2 innate lymphoid cells and inhibits type 1 innate immunity against multiple myeloma. Immunol. Cell Biol. 2021, 99, 65–83. [Google Scholar] [CrossRef] [PubMed]
- Mariani, S.; Muraro, M.; Pantaleoni, F.; Fiore, F.; Nuschak, B.; Peola, S.; Foglietta, M.; Palumbo, A.; Coscia, M.; Castella, B.; et al. Effector γδ T cells and tumor cells as immune targets of zoledronic acid in multiple myeloma. Leukemia 2005, 19, 664–670. [Google Scholar] [CrossRef]
- Kunzmann, V.; Bauer, E.; Feurle, J.; Weissinger, F.; Tony, H.P.; Wilhelm, M. Stimulation of gammadelta T cells by aminobi-sphosphonates and induction of antiplasma cell activity in multiple myeloma. Blood 2000, 96, 384–392. [Google Scholar] [CrossRef]
- Girlanda, S.; Fortis, C.; Belloni, D.; Ferrero, E.; Ticozzi, P.; Sciorati, C.; Tresoldi, M.; Vicari, A.; Spies, T.; Groh, V.; et al. MICA Expressed by Multiple Myeloma and Monoclonal Gammopathy of Undetermined Significance Plasma Cells Costimulates Pamidronate-Activated γδ Lymphocytes. Cancer Res. 2005, 65, 7502–7508. [Google Scholar] [CrossRef] [Green Version]
- Burjanadzé, M.; Condomines, M.; Rème, T.; Quittet, P.; Latry, P.; Lugagne, C.; Romagné, F.; Morel, Y.; Rossi, J.F.; Klein, B.; et al. In vitro expansion of gamma delta T cells with anti-myeloma cell activity by Phosphostim and IL-2 in patients with multiple myeloma. Br. J. Haematol. 2007, 139, 206–216. [Google Scholar] [CrossRef] [Green Version]
- Garg, T.K.; Szmania, S.M.; Khan, J.A.; Hoering, A.; Malbrough, P.A.; Moreno-Bost, A.; Greenway, A.D.; Lingo, J.D.; Li, X.; Yaccoby, S.; et al. Highly activated and expanded natural killer cells for multiple myeloma immunotherapy. Haematologica 2012, 97, 1348–1356. [Google Scholar] [CrossRef]
- Szmania, S.; Lapteva, N.; Garg, T.; Greenway, A.; Lingo, J.; Nair, B.; Stone, K.; Woods, E.; Khan, J.; Stivers, J.; et al. Ex Vivo–expanded Natural Killer Cells Demonstrate Robust Proliferation In Vivo in High-risk Relapsed Multiple Myeloma Patients. J. Immunother. 2015, 38, 24–36. [Google Scholar] [CrossRef] [Green Version]
- Uchida, R.; Ashihara, E.; Sato, K.; Kimura, S.; Kuroda, J.; Takeuchi, M.; Kawata, E.; Taniguchi, K.; Okamoto, M.; Shimura, K.; et al. γδT cells kill myeloma cells by sensing mevalonate metabolites and ICAM-1 molecules on cell surface. Biochem. Biophys. Res. Commun. 2007, 354, 613–618. [Google Scholar] [CrossRef]
- Niu, C.; Jin, H.; Li, M.; Zhu, S.; Zhou, L.; Jin, F.; Zhou, Y.; Xu, D.; Xu, J.; Zhao, L.; et al. Low-dose bortezomib increases the expression of NKG2D and DNAM-1 ligands and enhances induced NK and γδ T cell-mediated lysis in multiple myeloma. Oncotarget 2017, 8, 5954–5964. [Google Scholar] [CrossRef] [Green Version]
- Collins, S.M.; Bakan, C.E.; Swartzel, G.D.; Hofmeister, C.; Efebera, Y.A.; Kwon, H.; Starling, G.; Ciarlariello, D.; Bhaskar, S.S.; Briercheck, E.L.; et al. Elotuzumab directly enhances NK cell cytotoxicity against myeloma via CS1 ligation: Evidence for augmented NK cell function complementing ADCC. Cancer Immunol. Immunother. 2013, 62, 1841–1849. [Google Scholar] [CrossRef] [Green Version]
- Balasa, B.; Yun, R.; Belmar, N.A.; Fox, M.; Chao, D.T.; Robbins, M.D.; Starling, G.; Rice, A.G. Elotuzumab enhances natural killer cell activation and myeloma cell killing through interleukin-2 and TNF-α pathways. Cancer Immunol. Immunother. 2015, 64, 61–73. [Google Scholar] [CrossRef] [Green Version]
- Lagrue, K.; Carisey, A.; Morgan, D.J.; Chopra, R.; Davis, D.M. Lenalidomide augments actin remodeling and lowers NK-cell activation thresholds. Blood 2015, 126, 50–60. [Google Scholar] [CrossRef] [Green Version]
- Yang, G.; Gao, M.; Zhang, Y.; Kong, Y.; Gao, L.; Tao, Y.; Han, Y.; Wu, H.; Meng, X.; Xu, H.; et al. Carfil-zomib enhances natural killer cell-mediated lysis of myeloma linked with decreasing expression of HLA class I. Oncotar-Get 2015, 6, 26982–26994. [Google Scholar] [CrossRef]
- Gherardin, N.; Loh, L.; Admojo, L.; Davenport, A.J.; Richardson, K.; Rogers, A.; Darcy, P.K.; Jenkins, M.R.; Prince, H.M.; Harrison, S.J.; et al. Enumeration, functional responses and cytotoxic capacity of MAIT cells in newly diagnosed and relapsed multiple myeloma. Sci. Rep. 2018, 8, 4159. [Google Scholar] [CrossRef] [Green Version]
- Walsh, M.; White, G.; Romeril, K.; Buyck, H.; Stephens, M.; Brooks, C.; Weinkove, R. Innate-like T cell profile in myeloma: Severe deficiency of Vγ9Vδ2 T cells in aminobisphosphonate-treated patients. Leuk. Lymphoma 2016, 57, 977–980. [Google Scholar] [CrossRef]
- Forconi, F.; Moss, P. Perturbation of the normal immune system in patients with CLL. Blood 2015, 126, 573–581. [Google Scholar] [CrossRef] [Green Version]
- Allegra, A.; Tonacci, A.; Musolino, C.; Pioggia, G.; Gangemi, S. Secondary Immunodeficiency in Hematological Malignancies: Focus on Multiple Myeloma and Chronic Lymphocytic Leukemia. Front. Immunol. 2021, 12, 738915. [Google Scholar] [CrossRef]
- Riches, J.C.; Gribben, J.G. Understanding the immunodeficiency in chronic lymphocytic leukemia: Potential clinical impli-cations. Hematol. Oncol. Clin. N. Am. 2013, 27, 207–235. [Google Scholar] [CrossRef]
- Wild, J.J.; Schmiedel, B.J.; Maurer, A.; Raab, S.S.; Prokop, L.J.; Stevanović, S.; Dorfel, D.; Schneider, P.; Salih, H.R. Neutralization of (NK-cell-derived) B-cell activating factor by Belimumab restores sensitivity of chronic lymphoid leukemia cells to direct and Rituximab-induced NK lysis. Leukemia 2015, 29, 1676–1683. [Google Scholar] [CrossRef] [Green Version]
- Huergo-Zapico, L.; Acebes-Huerta, A.; Gonzalez-Rodriguez, A.P.; Contesti, J.; Gonzalez-García, E.; Payer, A.R.; Vil-la-Alvarez, M.; Fernández-Guizán, A.; López-Soto, A.; Gonzalez, S. Expansion of NK cells and reduction of NKG2D expres-sion in chronic lymphocytic leukemia. Correlation with progressive disease. PLoS ONE 2014, 9, e108326. [Google Scholar] [CrossRef]
- Ziegler, H.W.; Kay, N.E.; Zarling, J.M. Deficiency of natural killer cell activity in patients with chronic lymphocytic leuke-mia. Int. J. Cancer 1981, 27, 321–327. [Google Scholar] [CrossRef]
- Eskelund, C.W.; Nederby, L.; Thysen, A.H.; Skovbo, A.; Roug, A.S.; Hokland, M.E. Interleukin-21 and rituximab enhance NK cell functionality in patients with B-cell chronic lymphocytic leukaemia. Leuk. Res. 2011, 35, 914–920. [Google Scholar] [CrossRef]
- Reiners, K.S.; Topolar, D.; Henke, A.; Simhadri, V.R.; Kessler, J.; Sauer, M.; Bessler, M.; Hansen, H.P.; Tawadros, S.; Herling, M.; et al. Soluble ligands for NK cell receptors promote evasion of chronic lymphocytic leukemia cells from NK cell anti-tumor activity. Blood 2013, 121, 3658–3665. [Google Scholar] [CrossRef]
- De Weerdt, I.; van Hoeven, V.; Munneke, J.M.; Endstra, S.; Hofland, T.; Hazenberg, M.D.; Kater, A.P. Innate lymphoid cells are expanded and functionally altered in chronic lymphocytic leukemia. Haematologica 2016, 101, e461–e464. [Google Scholar] [CrossRef] [Green Version]
- Simões, C.; Silva, I.; Carvalho, A.; Silva, S.; Santos, S.; Marques, G.; Ribeiro, A.; Roque, A.; Carda, J.; Sarmento-Ribeiro, A.B.; et al. Quantification and Phenotypic Characterization of Pe-ripheral Blood Vd1 + T Cells in Chronic Lymphocytic Leukemia and Monoclonal B Cell Lymphocytosis. Cytom. Part B—Clin. Cytom. 2019, 96, 164–168. [Google Scholar] [CrossRef]
- Poggi, A.; Venturino, C.; Catellani, S.; Clavio, M.; Miglino, M.; Gobbi, M.; Steinle, A.; Ghia, P.; Stella, S.; Caligaris-Cappio, F.; et al. Vδ1 T Lymphocytes from B-CLL Patients Recognize ULBP3 Expressed on Leukemic B Cells and Up-Regulated by Trans-Retinoic Acid. Cancer Res. 2004, 64, 9172–9179. [Google Scholar] [CrossRef] [Green Version]
- Siegers, G.M.; Dhamko, H.; Wang, X.H.; Mathieson, A.M.; Kosaka, Y.; Felizardo, T.C.; Medin, J.A.; Tohda, S.; Schueler, J.; Fisch, P.; et al. Human Vd1 gd T Cells Expanded from Peripheral Blood Exhibit Specific Cytotoxicity Against B-Cell Chronic Lymphocytic Leukemia-Derived Cells. Cytotherapy 2011, 13, 753–764. [Google Scholar] [CrossRef]
- Almeida, A.; Correia, D.; Fernandes-Platzgummer, A.; Da Silva, C.L.; da Silva, M.G.; Anjos, D.R.; Silva-Santos, B. Delta One T Cells for Immunotherapy of Chronic Lymphocytic Leukemia: Clinical-Grade Expansion/Differentiation and Preclinical Proof of Concept. Clin. Cancer Res. 2016, 22, 5795–5804. [Google Scholar] [CrossRef] [Green Version]
- Coscia, M.; Vitale, C.; Peola, S.; Foglietta, M.; Rigoni, M.; Griggio, V.; Castella, B.; Angelini, D.; Chiaretti, S.; Riganti, C.; et al. Dysfunctional Vg9vd2 T Cells are Negative Prognosticators and Markers of Dysregulated Mevalonate Pathway Activity in Chronic Lymphocytic Leukemia Cells. Blood 2012, 120, 3271–3279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Correia, D.; Fogli, M.; Hudspeth, K.; da Silva, M.G.; Mavilio, D.; Silva-Santos, B. Differentiation of human peripheral blood Vδ1+ T cells expressing the natural cytotoxicity receptor NKp30 for recognition of lymphoid leukemia cells. Blood 2011, 118, 992–1001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lança, T.; Correia, D.V.; Moita, C.F.; Raquel, H.; Neves-Costa, A.; Ferreira, C.; Ramalho, J.S.; Barata, J.T.; Moita, L.F.; Gomes, A.Q.; et al. The MHC class Ib protein ULBP1 is a nonredundant determinant of leukemia/lymphoma susceptibility to γδ T-cell cytotoxicity. Blood 2010, 115, 2407–2411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Weerdt, I.; Hofland, T.; Lameris, R.; Endstra, S.; Jongejan, A.; Moerland, P.D.; de Bruin, R.C.G.; Remmerswaal, E.B.M.; ten Berge, I.J.M.; Liu, N.; et al. Improving CLL Vγ9Vδ2-T–cell fitness for cellular therapy by ex vivo activation and ibrutinib. Blood 2019, 134, 1271–1272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joalland, N.; Lafrance, L.; Oullier, T.; Marionneau-Lambot, S.; Loussouarn, D.; Jarry, U.; Scotet, E. Combined chemotherapy and allogeneic human Vγ9Vδ2 T lymphocyte-immunotherapies efficiently control the development of human epithelial ovarian cancer cells in vivo. OncoImmunology 2019, 8, e1649971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Weerdt, I.; Terpstra, S.; Hofland, T.; Lameris, R.; De Bruin, R.C.; Levin, M.-D.; Remmerswaal, E.B.; Berge, I.J.T.; Tonino, S.H.; De Gruijl, T.D.; et al. Chronic Lymphocytic Leukemia (CLL) Cells Are Susceptible to γδ-T Cell Mediated Killing, Provided CLL-Derived γδ-T Cell Dysfunction Can be Reversed. Blood 2015, 126, 2914. [Google Scholar] [CrossRef]
- Vela-Ojeda, J.; Perez-Retiguin, F.D.C.; Olivas-Bejarano, A.C.; Esparza, M.A.G.-R.; Garcia-Chavez, J.; Majluf-Cruz, A.; Reyes-Maldonado, E.; Montiel-Cervantes, L.A. Clinical relevance of NKT cells and soluble MIC-A in Hodgkin lymphoma. Leuk. Lymphoma 2020, 62, 801–809. [Google Scholar] [CrossRef]
- Reboursiere, E.; Gac, A.-C.; Garnier, A.; Salaun, V.; Reman, O.; Pham, A.-D.; Cabrera, Q.; Khoy, K.; Vilque, J.-P.; Fruchart, C.; et al. Increased frequencies of circulating and tumor-resident V?1 + T cells in patients with diffuse large B-cell lymphoma. Leuk. Lymphoma 2018, 59, 187–195. [Google Scholar] [CrossRef]
- Qi, J.; Xu, L.; Huang, D.; He, H.; Yao, J.; Zhang, J.; Xu, Y.; Yang, L. Defining Diffuse Large B-Cell Lymphoma Immunotypes by CD8+ T Cells and Natural Killer Cells. J. Oncol. 2022, 2022, 3168172. [Google Scholar] [CrossRef]
- Gleason, M.K.; Ross, J.A.; Warlick, E.D.; Lund, T.C.; Verneris, M.R.; Wiernik, A.; Spellman, S.; Haagenson, M.D.; Lenvik, A.J.; Litzow, M.R.; et al. CD16xCD33 bispecific killer cell engager (BiKE) activates NK cells against primary MDS and MDSC CD33+ targets. Blood 2014, 123, 3016–3026. [Google Scholar] [CrossRef]
- Vallera, D.A.; Felices, M.; McElmurry, R.; McCullar, V.; Zhou, X.; Schmohl, J.U.; Zhang, B.; Lenvik, A.J.; Panoskaltsis-Mortari, A.; Verneris, M.R.; et al. IL15 Trispecific Killer Engagers (TriKE) Make Natural Killer Cells Specific to CD33+ Targets While Also Inducing Persistence, In Vivo Expansion, and Enhanced Function. Clin. Cancer Res. 2016, 22, 3440–3450. [Google Scholar] [CrossRef] [Green Version]
- Felices, M.; Kodal, B.; Hinderlie, P.; Kaminski, M.F.; Cooley, S.; Weisdorf, D.J.; Vallera, D.A.; Miller, J.S.; Bachanova, V. Novel CD19-targeted TriKE restores NK cell function and proliferative capacity in CLL. Blood Adv. 2019, 3, 897–907. [Google Scholar] [CrossRef]
- Arvindam, U.S.; van Hauten, P.M.M.; Schirm, D.; Schaap, N.; Hobo, W.; Blazar, B.R.; Vallera, D.A.; Dolstra, H.; Felices, M.; Miller, J.S. A trispecific killer engager molecule against CLEC12A effectively induces NK-cell mediated killing of AML cells. Leukemia 2021, 35, 1586–1596. [Google Scholar] [CrossRef]
- Kouakanou, L.; Xu, Y.; Peters, C.; He, J.; Wu, Y.; Yin, Z.; Kabelitz, D. Vitamin C promotes the proliferation and effector functions of human γδ T cells. Cell. Mol. Immunol. 2020, 17, 462–473. [Google Scholar] [CrossRef] [Green Version]
- Van Gorkom, G.N.; Lookermans, E.L.; Van Elssen, C.H.; Bos, G.M. The Effect of Vitamin C (Ascorbic Acid) in the Treatment of Patients with Cancer: A Systematic Review. Nutrients 2019, 11, E977. [Google Scholar] [CrossRef] [Green Version]
- Vacca, P.; Munari, E.; Tumino, N.; Moretta, F.; Pietra, G.; Vitale, M.; Del Zotto, G.; Mariotti, F.R.; Mingari, M.C.; Moretta, L. Human natural killer cells and other innate lymphoid cells in cancer: Friends or foes? Immunol. Lett. 2018, 201, 14–19. [Google Scholar] [CrossRef]
- Davis, Z.B.; Felices, M.; Verneris, M.R.; Miller, J.S. Natural Killer Cell Adoptive Transfer Therapy: Exploiting the First Line of Defense Against Cancer. Cancer J. 2015, 21, 486–491. [Google Scholar] [CrossRef] [Green Version]
- Choi, I.; Yoon, S.R.; Park, S.-Y.; Kim, H.; Jung, S.-J.; Kang, Y.-L.; Lee, J.-H.; Lee, J.-H.; Kim, D.-Y.; Lee, J.-L.; et al. Donor-Derived Natural Killer Cell Infusion after Human Leukocyte Antigen–Haploidentical Hematopoietic Cell Transplantation in Patients with Refractory Acute Leukemia. Biol. Blood Marrow Transplant. 2016, 22, 2065–2076. [Google Scholar] [CrossRef] [Green Version]
- Story, J.Y.; Zoine, J.T.; Burnham, R.E.; Hamilton, J.A.; Spencer, H.T.; Doering, C.B.; Raikar, S.S. Bortezomib enhances cytotoxicity of ex vivo-expanded gamma delta T cells against acute myeloid leukemia and T-cell acute lymphoblastic leukemia. Cytotherapy 2021, 23, 12–24. [Google Scholar] [CrossRef]
- Allegra, A.; Penna, G.; Innao, V.; Greve, B.; Maisano, V.; Russo, S.; Musolino, C. Vaccination of multiple myeloma: Current strategies and future prospects. Crit. Rev. Oncol. 2015, 96, 339–354. [Google Scholar] [CrossRef]
- Allegra, A.; Russo, S.; Gerace, D.; Calabrò, L.; Maisano, V.; Innao, V.; Musolino, C. Vaccination strategies in lymphoproliferative disorders: Failures and successes. Leuk. Res. 2015, 39, 1006–1019. [Google Scholar] [CrossRef]
- Goldstein, M.J.; Varghese, B.; Brody, J.D.; Rajapaksa, R.; Kohrt, H.; Czerwinski, D.K.; Levy, S.; Levy, R. A CpG-loaded tumor cell vaccine induces antitumor CD4 T cells that are effective in adoptive therapy for large and established tumors. Blood 2010, 117, 118–127. [Google Scholar] [CrossRef] [Green Version]
- Mattarollo, S.R.; West, A.C.; Steegh, K.; Duret, H.; Paget, C.; Martin, B.; Matthews, G.M.; Shortt, J.; Chesi, M.; Bergsagel, P.L.; et al. NKT cell adjuvant-based tumor vaccine for treatment of myc oncogene-driven mouse B-cell lymphoma. Blood 2012, 120, 3019–3029. [Google Scholar] [CrossRef] [Green Version]
- Yang, P.; Peng, Y.; Feng, Y.; Xu, Z.; Feng, P.; Cao, J.; Chen, Y.; Chen, X.; Cao, X.; Yang, Y.; et al. Immune Cell-Derived Extracellular Vesicles—New Strategies in Cancer Immunotherapy. Front. Immunol. 2021, 12, 771551. [Google Scholar] [CrossRef]
- Varesano, S.; Zocchi, M.R.; Poggi, A. Zoledronate triggers Vδ2 T cells to destroy and kill spheroids of colon carcinoma: Quantitative image analysis of three-dimensional cultures. Front. Immunol. 2018, 9, 998. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allegra, A.; Casciaro, M.; Lo Presti, E.; Musolino, C.; Gangemi, S. Harnessing Unconventional T Cells and Innate Lymphoid Cells to Prevent and Treat Hematological Malignancies: Prospects for New Immunotherapy. Biomolecules 2022, 12, 754. https://doi.org/10.3390/biom12060754
Allegra A, Casciaro M, Lo Presti E, Musolino C, Gangemi S. Harnessing Unconventional T Cells and Innate Lymphoid Cells to Prevent and Treat Hematological Malignancies: Prospects for New Immunotherapy. Biomolecules. 2022; 12(6):754. https://doi.org/10.3390/biom12060754
Chicago/Turabian StyleAllegra, Alessandro, Marco Casciaro, Elena Lo Presti, Caterina Musolino, and Sebastiano Gangemi. 2022. "Harnessing Unconventional T Cells and Innate Lymphoid Cells to Prevent and Treat Hematological Malignancies: Prospects for New Immunotherapy" Biomolecules 12, no. 6: 754. https://doi.org/10.3390/biom12060754
APA StyleAllegra, A., Casciaro, M., Lo Presti, E., Musolino, C., & Gangemi, S. (2022). Harnessing Unconventional T Cells and Innate Lymphoid Cells to Prevent and Treat Hematological Malignancies: Prospects for New Immunotherapy. Biomolecules, 12(6), 754. https://doi.org/10.3390/biom12060754





