Revisiting the miR-200 Family: A Clan of Five Siblings with Essential Roles in Development and Disease
Abstract
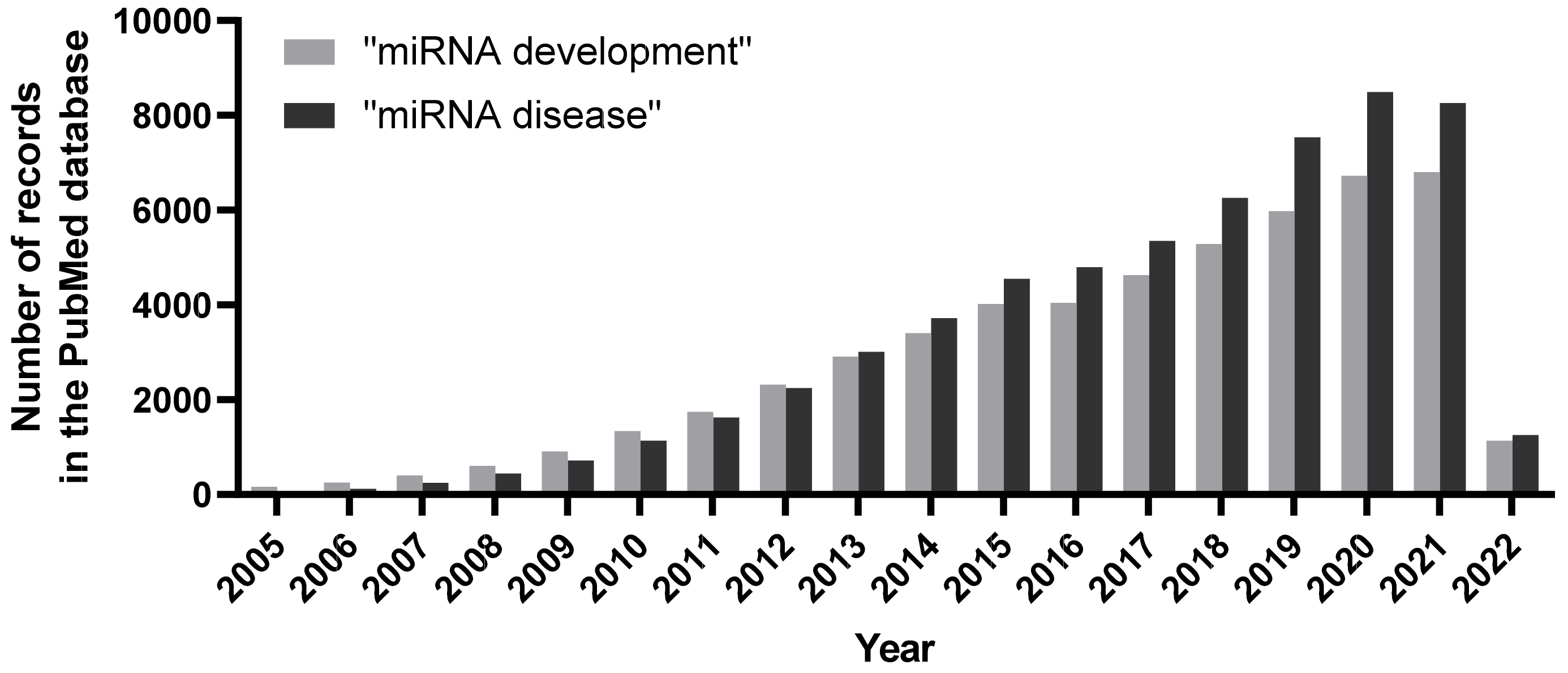
1. Introduction
2. miR-200 Family in Development
3. miR-200 Family in Pathophysiology
3.1. miR-200 Family in Neurodegenerative Diseases
3.1.1. miR-200 Family in Alzheimer’s Disease
3.1.2. miR-200 Family in Parkinson’s Disease
3.1.3. miR-200 Family in Amyotrophic Lateral Sclerosis
3.1.4. miR-200 Family in Multiple Sclerosis and Prion Disease
3.2. miR-200 Family in Fibrosis
3.3. miR-200 Family in Cancer
3.3.1. miR-200 Family in Cancer-Associated EMT and MET
3.3.2. miR-200 Family’s Tumor-Promoting Roles during Tumorigenesis and Metastasis
3.3.3. miR200 Family in Radiotherapy
3.3.4. miR-200 Family in Chemotherapy
4. miR-200 Family as Predictive Markers
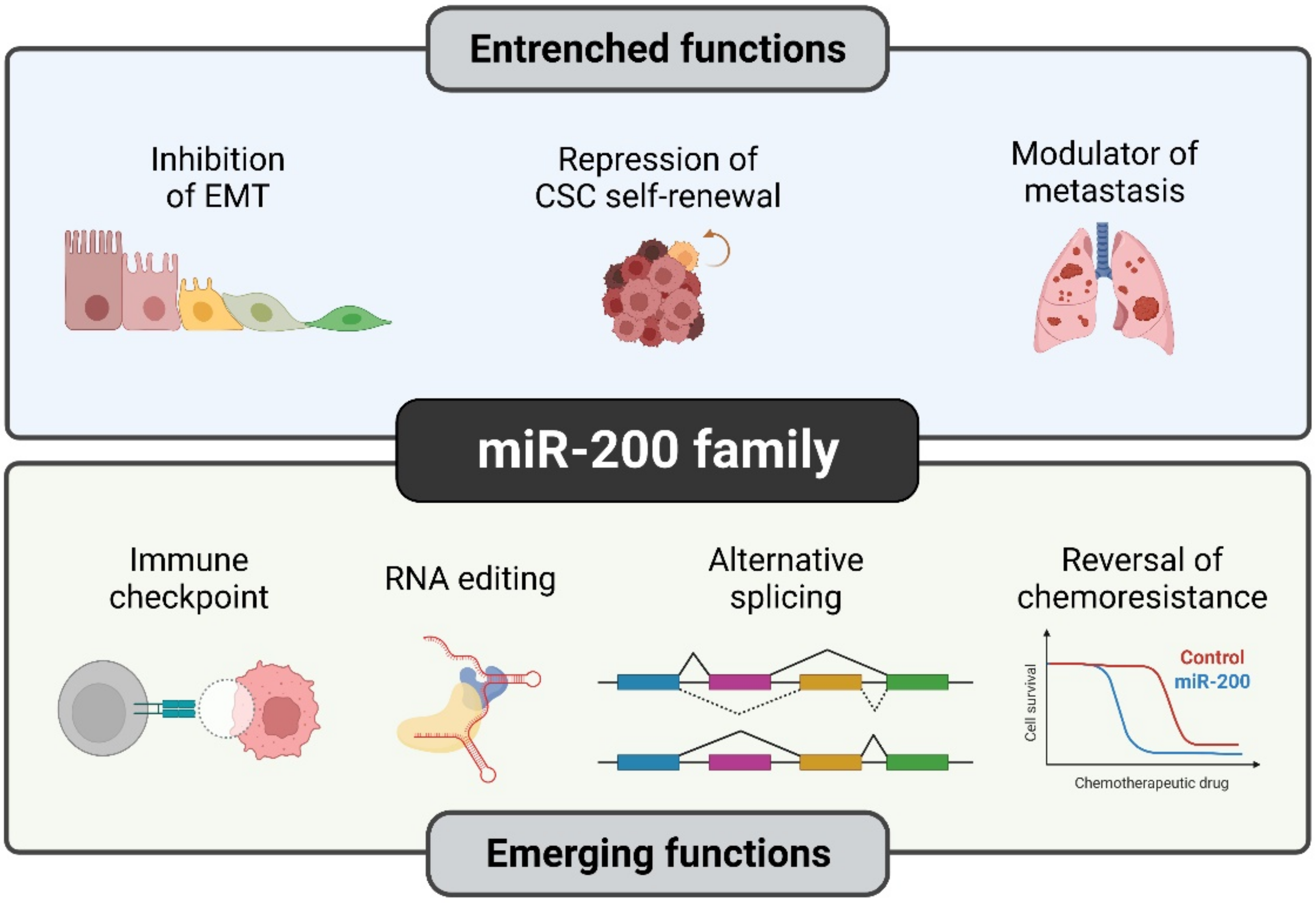
5. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Ohno, S. So Much “Junk” DNA in Our Genome. Brookhaven Symp. Biol. 1972, 23, 366–370. [Google Scholar] [PubMed]
- Lander, E.S. Initial Impact of the Sequencing of the Human Genome. Nature 2011, 470, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Lander, E.S.; Linton, L.M.; Birren, B.; Nusbaum, C.; Zody, M.C.; Baldwin, J.; Devon, K.; Dewar, K.; Doyle, M.; FitzHugh, W.; et al. Initial Sequencing and Analysis of the Human Genome. Nature 2001, 409, 860–921. [Google Scholar] [CrossRef] [PubMed]
- Pennisi, E. Genomics. ENCODE Project Writes Eulogy for Junk DNA. Science 2012, 337, 1159–1161. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Genomics, Biogenesis, Mechanism, and Function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Lewis, B.P.; Shih, I.; Jones-Rhoades, M.W.; Bartel, D.P.; Burge, C.B. Prediction of Mammalian MicroRNA Targets. Cell 2003, 115, 787–798. [Google Scholar] [CrossRef]
- Kozomara, A.; Birgaoanu, M.; Griffiths-Jones, S. MiRBase: From MicroRNA Sequences to Function. Nucleic Acids Res. 2019, 47, D155–D162. [Google Scholar] [CrossRef]
- Esquela-Kerscher, A.; Slack, F.J. Oncomirs—MicroRNAs with a Role in Cancer. Nat. Rev. Cancer 2006, 6, 259–269. [Google Scholar] [CrossRef]
- Dragomir, M.P.; Knutsen, E.; Calin, G.A. SnapShot: Unconventional MiRNA Functions. Cell 2018, 174, 1038–1038.e1. [Google Scholar] [CrossRef]
- Jie, M.; Feng, T.; Huang, W.; Zhang, M.; Feng, Y.; Jiang, H.; Wen, Z. Subcellular Localization of MiRNAs and Implications in Cellular Homeostasis. Genes 2021, 12, 856. [Google Scholar] [CrossRef]
- Dexheimer, P.J.; Cochella, L. MicroRNAs: From Mechanism to Organism. Front. Cell Dev. Biol. 2020, 8, 409. [Google Scholar] [CrossRef]
- Xu, M.; Chen, Y.; Xu, Z.; Zhang, L.; Jiang, H.; Pian, C. MiRLoc: Predicting MiRNA Subcellular Localization by Incorporating MiRNA-MRNA Interactions and MRNA Subcellular Localization. Brief. Bioinform. 2022, 23, bbac044. [Google Scholar] [CrossRef]
- Chang, T.-C.; Mendell, J.T. MicroRNAs in Vertebrate Physiology and Human Disease. Annu. Rev. Genom. Hum. Genet. 2007, 8, 215–239. [Google Scholar] [CrossRef]
- Cimmino, A.; Calin, G.A.; Fabbri, M.; Iorio, M.V.; Ferracin, M.; Shimizu, M.; Wojcik, S.E.; Aqeilan, R.I.; Zupo, S.; Dono, M.; et al. MiR-15 and MiR-16 Induce Apoptosis by Targeting BCL2. Proc. Natl. Acad. Sci. USA 2005, 102, 13944–13949. [Google Scholar] [CrossRef]
- Michael, M.Z.; O’ Connor, S.M.; van Holst Pellekaan, N.G.; Young, G.P.; James, R.J. Reduced Accumulation of Specific MicroRNAs in Colorectal Neoplasia. Mol. Cancer Res. 2003, 1, 882–891. [Google Scholar]
- Shi, L.; Middleton, J.; Jeon, Y.-J.; Magee, P.; Veneziano, D.; Laganà, A.; Leong, H.-S.; Sahoo, S.; Fassan, M.; Booton, R.; et al. KRAS Induces Lung Tumorigenesis through MicroRNAs Modulation. Cell Death Dis. 2018, 9, 219. [Google Scholar] [CrossRef]
- Zhao, Q.; Chen, S.; Zhu, Z.; Yu, L.; Ren, Y.; Jiang, M.; Weng, J.; Li, B. MiR-21 Promotes EGF-Induced Pancreatic Cancer Cell Proliferation by Targeting Spry2. Cell Death Dis. 2018, 9, 1157. [Google Scholar] [CrossRef]
- Huang, T.-H.; Wu, F.; Loeb, G.B.; Hsu, R.; Heidersbach, A.; Brincat, A.; Horiuchi, D.; Lebbink, R.J.; Mo, Y.-Y.; Goga, A.; et al. Up-Regulation of MiR-21 by HER2/Neu Signaling Promotes Cell Invasion. J. Biol. Chem. 2009, 284, 18515–18524. [Google Scholar] [CrossRef]
- Xia, H.; Zhang, W.; Zhang, B.; Zhao, Y.; Zhao, Y.; Li, S.; Liu, Y. MiR-21 Modulates the Effect of EZH2 on the Biological Behavior of Human Lung Cancer Stem Cells in Vitro. Oncotarget 2017, 8, 85442–85451. [Google Scholar] [CrossRef]
- Gao, Z.; Liu, H.; Shi, Y.; Yin, L.; Zhu, Y.; Liu, R. Identification of Cancer Stem Cell Molecular Markers and Effects of has-MiR-21-3p on Stemness in Esophageal Squamous Cell Carcinoma. Cancers 2019, 11, 518. [Google Scholar] [CrossRef]
- Li, A.; Yang, P.-M. Overexpression of MiR-21-5p in Colorectal Cancer Cells Promotes Self-Assembly of E-Cadherin-Dependent Multicellular Tumor Spheroids. Tissue Cell 2020, 65, 101365. [Google Scholar] [CrossRef]
- Mortoglou, M.; Miralles, F.; Arisan, E.D.; Dart, A.; Jurcevic, S.; Lange, S.; Uysal-Onganer, P. MicroRNA-21 Regulates Stemness in Pancreatic Ductal Adenocarcinoma Cells. Int. J. Mol. Sci. 2022, 23, 1275. [Google Scholar] [CrossRef]
- Sheng, S.; Su, W.; Mao, D.; Li, C.; Hu, X.; Deng, W.; Yao, Y.; Ji, Y. MicroRNA-21 Induces Cisplatin Resistance in Head and Neck Squamous Cell Carcinoma. PLoS ONE 2022, 17, e0267017. [Google Scholar] [CrossRef]
- Najjary, S.; Mohammadzadeh, R.; Mokhtarzadeh, A.; Mohammadi, A.; Kojabad, A.B.; Baradaran, B. Role of MiR-21 as an Authentic Oncogene in Mediating Drug Resistance in Breast Cancer. Gene 2020, 738, 144453. [Google Scholar] [CrossRef]
- Vandewalle, V.; Essaghir, A.; Bollaert, E.; Lenglez, S.; Graux, C.; Schoemans, H.; Saussoy, P.; Michaux, L.; Valk, P.J.M.; Demoulin, J.-B.; et al. MiR-15a-5p and MiR-21-5p Contribute to Chemoresistance in Cytogenetically Normal Acute Myeloid Leukaemia by Targeting PDCD4, ARL2 and BTG2. J. Cell Mol. Med. 2021, 25, 575–585. [Google Scholar] [CrossRef]
- Zhang, X.-Y.; Li, Y.-F.; Ma, H.; Gao, Y.-H. Regulation of MYB Mediated Cisplatin Resistance of Ovarian Cancer Cells Involves MiR-21-Wnt Signaling Axis. Sci. Rep. 2020, 10, 6893. [Google Scholar] [CrossRef]
- Sun, L.-H.; Tian, D.; Yang, Z.-C.; Li, J.-L. Exosomal MiR-21 Promotes Proliferation, Invasion and Therapy Resistance of Colon Adenocarcinoma Cells through Its Target PDCD4. Sci. Rep. 2020, 10, 8271. [Google Scholar] [CrossRef]
- Mitchell, P.S.; Parkin, R.K.; Kroh, E.M.; Fritz, B.R.; Wyman, S.K.; Pogosova-Agadjanyan, E.L.; Peterson, A.; Noteboom, J.; O’Briant, K.C.; Allen, A.; et al. Circulating MicroRNAs as Stable Blood-Based Markers for Cancer Detection. Proc. Natl. Acad. Sci. USA 2008, 105, 10513–10518. [Google Scholar] [CrossRef] [PubMed]
- Van Zandwijk, N.; Pavlakis, N.; Kao, S.C.; Linton, A.; Boyer, M.J.; Clarke, S.; Huynh, Y.; Chrzanowska, A.; Fulham, M.J.; Bailey, D.L.; et al. Safety and Activity of MicroRNA-Loaded Minicells in Patients with Recurrent Malignant Pleural Mesothelioma: A First-in-Man, Phase 1, Open-Label, Dose-Escalation Study. Lancet Oncol. 2017, 18, 1386–1396. [Google Scholar] [CrossRef]
- Anandappa, G.; Lampis, A.; Cunningham, D.; Khan, K.H.; Kouvelakis, K.; Vlachogiannis, G.; Hedayat, S.; Tunariu, N.; Rao, S.; Watkins, D.; et al. MiR-31-3p Expression and Benefit from Anti-EGFR Inhibitors in Metastatic Colorectal Cancer Patients Enrolled in the Prospective Phase II PROSPECT-C Trial. Clin. Cancer Res. 2019, 25, 3830–3838. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.-Y.; Wright, J.A.; Attema, J.L.; Gregory, P.A.; Bert, A.G.; Smith, E.; Thomas, D.; Lopez, A.F.; Drew, P.A.; Khew-Goodall, Y.; et al. Epigenetic Modulation of the MiR-200 Family Is Associated with Transition to a Breast Cancer Stem-Cell-like State. J. Cell Sci. 2013, 126, 2256–2266. [Google Scholar] [CrossRef]
- Humphries, B.; Wang, Z.; Oom, A.L.; Fisher, T.; Tan, D.; Cui, Y.; Jiang, Y.; Yang, C. MicroRNA-200b Targets Protein Kinase Cα and Suppresses Triple-Negative Breast Cancer Metastasis. Carcinogenesis 2014, 35, 2254–2263. [Google Scholar] [CrossRef]
- Green, K.J.; Jaiganesh, A.; Broussard, J.A. Desmosomes: Essential Contributors to an Integrated Intercellular Junction Network. F1000Research 2019, 8, F1000. [Google Scholar] [CrossRef]
- Landschaft, D. Gaps and Barriers: Gap Junctions as a Channel of Communication between the Soma and the Germline. Semin. Cell Dev. Biol. 2020, 97, 167–171. [Google Scholar] [CrossRef]
- Otani, T.; Furuse, M. Tight Junction Structure and Function Revisited. Trends Cell Biol. 2020, 30, 805–817. [Google Scholar] [CrossRef]
- Veeraval, L.; O’Leary, C.J.; Cooper, H.M. Adherens Junctions: Guardians of Cortical Development. Front. Cell Dev. Biol. 2020, 8, 6. [Google Scholar] [CrossRef]
- Akhtar, N.; Streuli, C.H. An Integrin-ILK-Microtubule Network Orients Cell Polarity and Lumen Formation in Glandular Epithelium. Nat. Cell Biol. 2013, 15, 17–27. [Google Scholar] [CrossRef]
- Rodriguez-Boulan, E.; Macara, I.G. Organization and Execution of the Epithelial Polarity Programme. Nat. Rev. Mol. Cell Biol. 2014, 15, 225–242. [Google Scholar] [CrossRef]
- Hay, E.D. The Mesenchymal Cell, Its Role in the Embryo, and the Remarkable Signaling Mechanisms That Create It. Dev. Dyn. 2005, 233, 706–720. [Google Scholar] [CrossRef]
- Yang, D.; Wu, X.; Zhou, Y.; Wang, W.; Wang, Z. The MicroRNA/TET3/REST Axis Is Required for Olfactory Globose Basal Cell Proliferation and Male Behavior. EMBO Rep. 2020, 21, e49431. [Google Scholar] [CrossRef]
- Kapsimali, M.; Kaushik, A.-L.; Gibon, G.; Dirian, L.; Ernest, S.; Rosa, F.M. Fgf Signaling Controls Pharyngeal Taste Bud Formation through MiR-200 and Delta-Notch Activity. Development 2011, 138, 3473–3484. [Google Scholar] [CrossRef]
- Banerjee, P.; Dutta, S.; Pal, R. Dysregulation of Wnt-Signaling and a Candidate Set of MiRNAs Underlie the Effect of Metformin on Neural Crest Cell Development. Stem Cells 2016, 34, 334–345. [Google Scholar] [CrossRef][Green Version]
- Shimono, Y.; Zabala, M.; Cho, R.W.; Lobo, N.; Dalerba, P.; Qian, D.; Diehn, M.; Liu, H.; Panula, S.P.; Chiao, E.; et al. Downregulation of MiRNA-200c Links Breast Cancer Stem Cells with Normal Stem Cells. Cell 2009, 138, 592–603. [Google Scholar] [CrossRef]
- Rebustini, I.T.; Hayashi, T.; Reynolds, A.D.; Dillard, M.L.; Carpenter, E.M.; Hoffman, M.P. MiR-200c Regulates FGFR-Dependent Epithelial Proliferation via Vldlr during Submandibular Gland Branching Morphogenesis. Development 2012, 139, 191–202. [Google Scholar] [CrossRef]
- Cao, H.; Jheon, A.; Li, X.; Sun, Z.; Wang, J.; Florez, S.; Zhang, Z.; McManus, M.T.; Klein, O.D.; Amendt, B.A. The Pitx2:MiR-200c/141:Noggin Pathway Regulates Bmp Signaling and Ameloblast Differentiation. Development 2013, 140, 3348–3359. [Google Scholar] [CrossRef]
- Beclin, C.; Follert, P.; Stappers, E.; Barral, S.; Coré, N.; de Chevigny, A.; Magnone, V.; Lebrigand, K.; Bissels, U.; Huylebroeck, D.; et al. MiR-200 Family Controls Late Steps of Postnatal Forebrain Neurogenesis via Zeb2 Inhibition. Sci. Rep. 2016, 6, 35729. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, N.; Park, S.-W.; Kim, H.; Park, H.-J.; Han, Y.-M. Lineage-Specific Expression of MiR-200 Family in Human Embryonic Stem Cells during In Vitro Differentiation. Int. J. Stem Cells 2017, 10, 28–37. [Google Scholar] [CrossRef]
- Jimenez, P.T.; Mainigi, M.A.; Word, R.A.; Kraus, W.L.; Mendelson, C.R. MiR-200 Regulates Endometrial Development During Early Pregnancy. Mol. Endocrinol. 2016, 30, 977–987. [Google Scholar] [CrossRef] [PubMed]
- Garaffo, G.; Conte, D.; Provero, P.; Tomaiuolo, D.; Luo, Z.; Pinciroli, P.; Peano, C.; D’Atri, I.; Gitton, Y.; Etzion, T.; et al. The Dlx5 and Foxg1 Transcription Factors, Linked via MiRNA-9 and -200, Are Required for the Development of the Olfactory and GnRH System. Mol. Cell Neurosci. 2015, 68, 103–119. [Google Scholar] [CrossRef] [PubMed]
- Hilmarsdóttir, B.; Briem, E.; Sigurdsson, V.; Franzdóttir, S.R.; Ringnér, M.; Arason, A.J.; Bergthorsson, J.T.; Magnusson, M.K.; Gudjonsson, T. MicroRNA-200c-141 and ∆Np63 Are Required for Breast Epithelial Differentiation and Branching Morphogenesis. Dev. Biol. 2015, 403, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Hoefert, J.E.; Bjerke, G.A.; Wang, D.; Yi, R. The MicroRNA-200 Family Coordinately Regulates Cell Adhesion and Proliferation in Hair Morphogenesis. J. Cell Biol. 2018, 217, 2185–2204. [Google Scholar] [CrossRef]
- Suh, H.N.; Han, H.J. Sonic Hedgehog Increases the Skin Wound-Healing Ability of Mouse Embryonic Stem Cells through the MicroRNA 200 Family. Br. J. Pharmacol. 2015, 172, 815–828. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, L.; Li, X.; Han, W.; Yang, K.; Wang, H.; Zhang, Y.; Su, R.; Liu, Z.; Wang, R.; et al. High-Throughput Sequencing of Hair Follicle Development-Related Micrornas in Cashmere Goat at Various Fetal Periods. Saudi J. Biol. Sci. 2018, 25, 1494–1508. [Google Scholar] [CrossRef]
- Sun, Z.; Yu, W.; Sanz Navarro, M.; Sweat, M.; Eliason, S.; Sharp, T.; Liu, H.; Seidel, K.; Zhang, L.; Moreno, M.; et al. Sox2 and Lef-1 Interact with Pitx2 to Regulate Incisor Development and Stem Cell Renewal. Development 2016, 143, 4115–4126. [Google Scholar] [CrossRef]
- Sanz-Navarro, M.; Seidel, K.; Sun, Z.; Bertonnier-Brouty, L.; Amendt, B.A.; Klein, O.D.; Michon, F. Plasticity within the Niche Ensures the Maintenance of a Sox2+ Stem Cell Population in the Mouse Incisor. Development 2018, 145, dev155929. [Google Scholar] [CrossRef]
- Michon, F.; Tummers, M.; Kyyrönen, M.; Frilander, M.J.; Thesleff, I. Tooth Morphogenesis and Ameloblast Differentiation Are Regulated by Micro-RNAs. Dev. Biol. 2010, 340, 355–368. [Google Scholar] [CrossRef]
- Jheon, A.H.; Li, C.-Y.; Wen, T.; Michon, F.; Klein, O.D. Expression of MicroRNAs in the Stem Cell Niche of the Adult Mouse Incisor. PLoS ONE 2011, 6, e24536. [Google Scholar] [CrossRef]
- Sweat, M.; Sweat, Y.; Yu, W.; Su, D.; Leonard, R.J.; Eliason, S.L.; Amendt, B.A. The MiR-200 Family Is Required for Ectodermal Organ Development through the Regulation of the Epithelial Stem Cell Niche. Stem Cells 2021, 39, 761–775. [Google Scholar] [CrossRef]
- Samavarchi-Tehrani, P.; Golipour, A.; David, L.; Sung, H.; Beyer, T.A.; Datti, A.; Woltjen, K.; Nagy, A.; Wrana, J.L. Functional Genomics Reveals a BMP-Driven Mesenchymal-to-Epithelial Transition in the Initiation of Somatic Cell Reprogramming. Cell Stem Cell 2010, 7, 64–77. [Google Scholar] [CrossRef]
- Trinh, K.; Storm, D.R. Detection of Odorants through the Main Olfactory Epithelium and Vomeronasal Organ of Mice. Nutr. Rev. 2004, 62, S189–S192. [Google Scholar] [CrossRef]
- Choi, P.S.; Zakhary, L.; Choi, W.-Y.; Caron, S.; Alvarez-Saavedra, E.; Miska, E.A.; McManus, M.; Harfe, B.; Giraldez, A.J.; Horvitz, H.R.; et al. Members of the MiRNA-200 Family Regulate Olfactory Neurogenesis. Neuron 2008, 57, 41–55. [Google Scholar] [CrossRef]
- Morante, J.; Vallejo, D.M.; Desplan, C.; Dominguez, M. Conserved MiR-8/MiR-200 Defines a Glial Niche That Controls Neuroepithelial Expansion and Neuroblast Transition. Dev. Cell 2013, 27, 174–187. [Google Scholar] [CrossRef]
- Hyun, S.; Lee, J.H.; Jin, H.; Nam, J.; Namkoong, B.; Lee, G.; Chung, J.; Kim, V.N. Conserved MicroRNA MiR-8/MiR-200 and Its Target USH/FOG2 Control Growth by Regulating PI3K. Cell 2009, 139, 1096–1108. [Google Scholar] [CrossRef]
- Jin, H.; Kim, V.N.; Hyun, S. Conserved MicroRNA MiR-8 Controls Body Size in Response to Steroid Signaling in Drosophila. Genes Dev. 2012, 26, 1427–1432. [Google Scholar] [CrossRef]
- Kennell, J.A.; Cadigan, K.M.; Shakhmantsir, I.; Waldron, E.J. The MicroRNA MiR-8 Is a Positive Regulator of Pigmentation and Eclosion in Drosophila. Dev. Dyn. 2012, 241, 161–168. [Google Scholar] [CrossRef]
- Lucas, K.J.; Roy, S.; Ha, J.; Gervaise, A.L.; Kokoza, V.A.; Raikhel, A.S. MicroRNA-8 Targets the Wingless Signaling Pathway in the Female Mosquito Fat Body to Regulate Reproductive Processes. Proc. Natl. Acad. Sci. USA 2015, 112, 1440–1445. [Google Scholar] [CrossRef]
- Pentón-Rol, G.; Cervantes-Llanos, M. Report on the Symposium “Molecular Mechanisms Involved in Neurodegeneration”. Behav. Sci. 2018, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Karres, J.S.; Hilgers, V.; Carrera, I.; Treisman, J.; Cohen, S.M. The Conserved MicroRNA MiR-8 Tunes Atrophin Levels to Prevent Neurodegeneration in Drosophila. Cell 2007, 131, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Dong, L.; Liu, N.; Luo, X.; He, Z. Mir-141-3p Regulates Apoptosis and Mitochondrial Membrane Potential via Targeting Sirtuin1 in a 1-Methyl-4-Phenylpyridinium in Vitro Model of Parkinson’s Disease. BioMed Res. Int. 2020, 2020, e7239895. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.-S.; Liu, W.; Lu, G.-X. MiR-200a-3p Promotes b-Amyloid-Induced Neuronal Apoptosis through down-Regulation of SIRT1 in Alzheimer’s Disease. J. Biosci. 2017, 42, 397–404. [Google Scholar] [CrossRef]
- Liu, C.-G.; Wang, J.-L.; Li, L.; Xue, L.-X.; Zhang, Y.-Q.; Wang, P.-C. MicroRNA-135a and -200b, Potential Biomarkers for Alzheimer׳s Disease, Regulate β Secretase and Amyloid Precursor Protein. Brain Res. 2014, 1583, 55–64. [Google Scholar] [CrossRef]
- Liu, R.; Wang, M.; Su, L.; Li, X.; Zhao, S.; Yu, M. The Expression Pattern of MicroRNAs and the Associated Pathways Involved in the Development of Porcine Placental Folds That Contribute to the Expansion of the Exchange Surface Area. Biol. Reprod. 2015, 93, 62. [Google Scholar] [CrossRef]
- Higaki, S.; Muramatsu, M.; Matsuda, A.; Matsumoto, K.; Satoh, J.-I.; Michikawa, M.; Niida, S. Defensive Effect of MicroRNA-200b/c against Amyloid-Beta Peptide-Induced Toxicity in Alzheimer’s Disease Models. PLoS ONE 2018, 13, e0196929. [Google Scholar] [CrossRef]
- Salimian, N.; Peymani, M.; Ghaedi, K.; Nasr Esfahani, M.H. Modulation in MiR-200a/SIRT1axis Is Associated with Apoptosis in MPP+-Induced SH-SY5Y Cells. Gene 2018, 674, 25–30. [Google Scholar] [CrossRef]
- Rostamian Delavar, M.; Baghi, M.; Safaeinejad, Z.; Kiani-Esfahani, A.; Ghaedi, K.; Nasr-Esfahani, M.H. Differential Expression of MiR-34a, MiR-141, and MiR-9 in MPP+-Treated Differentiated PC12 Cells as a Model of Parkinson’s Disease. Gene 2018, 662, 54–65. [Google Scholar] [CrossRef]
- Svetoni, F.; De Paola, E.; La Rosa, P.; Mercatelli, N.; Caporossi, D.; Sette, C.; Paronetto, M.P. Post-Transcriptional Regulation of FUS and EWS Protein Expression by MiR-141 during Neural Differentiation. Hum. Mol. Genet. 2017, 26, 2732–2746. [Google Scholar] [CrossRef]
- Zhang, T.; Wu, Y.-C.; Mullane, P.; Ji, Y.J.; Liu, H.; He, L.; Arora, A.; Hwang, H.-Y.; Alessi, A.F.; Niaki, A.G.; et al. FUS Regulates Activity of MicroRNA-Mediated Gene Silencing. Mol. Cell 2018, 69, 787–801.e8. [Google Scholar] [CrossRef]
- Wu, Q.; Ye, X.; Xiong, Y.; Zhu, H.; Miao, J.; Zhang, W.; Wan, J. The Protective Role of MicroRNA-200c in Alzheimer’s Disease Pathologies Is Induced by Beta Amyloid-Triggered Endoplasmic Reticulum Stress. Front. Mol. Neurosci. 2016, 9, 140. [Google Scholar] [CrossRef]
- Vinters, H.V. Emerging Concepts in Alzheimer’s Disease. Annu. Rev. Pathol. 2015, 10, 291–319. [Google Scholar] [CrossRef]
- Cornejo, V.H.; Hetz, C. The Unfolded Protein Response in Alzheimer’s Disease. Semin. Immunopathol. 2013, 35, 277–292. [Google Scholar] [CrossRef]
- Schipper, H.M.; Maes, O.C.; Chertkow, H.M.; Wang, E. MicroRNA Expression in Alzheimer Blood Mononuclear Cells. Gene Regul. Syst. Biol. 2007, 1, 263–274. [Google Scholar] [CrossRef]
- Shen, L.; Ji, H.-F. Associations between Homocysteine, Folic Acid, Vitamin B12 and Alzheimer’s Disease: Insights from Meta-Analyses. J. Alzheimer’s Dis 2015, 46, 777–790. [Google Scholar] [CrossRef]
- Goh, S.Y.; Chao, Y.X.; Dheen, S.T.; Tan, E.-K.; Tay, S.S.-W. Role of MicroRNAs in Parkinson’s Disease. Int. J. Mol. Sci. 2019, 20, 5649. [Google Scholar] [CrossRef]
- Jankovic, J. Parkinson’s Disease: Clinical Features and Diagnosis. J. Neurol. Neurosurg. Psychiatry 2008, 79, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Hallett, P.J.; Engelender, S.; Isacson, O. Lipid and Immune Abnormalities Causing Age-Dependent Neurodegeneration and Parkinson’s Disease. J. Neuroinflamm. 2019, 16, 153. [Google Scholar] [CrossRef]
- Li, J.; Uversky, V.N.; Fink, A.L. Conformational Behavior of Human Alpha-Synuclein Is Modulated by Familial Parkinson’s Disease Point Mutations A30P and A53T. Neurotoxicology 2002, 23, 553–567. [Google Scholar] [CrossRef]
- Puentes, L.N.; Lengyel-Zhand, Z.; Lee, J.Y.; Hsieh, C.-J.; Schneider, M.E.; Edwards, K.J.; Luk, K.C.; Lee, V.M.-Y.; Trojanowski, J.Q.; Mach, R.H. Poly (ADP-Ribose) Interacts With Phosphorylated α-Synuclein in Post Mortem PD Samples. Front. Aging Neurosci. 2021, 13, 704041. [Google Scholar] [CrossRef]
- Mo, M.; Xiao, Y.; Huang, S.; Cen, L.; Chen, X.; Zhang, L.; Luo, Q.; Li, S.; Yang, X.; Lin, X.; et al. MicroRNA Expressing Profiles in A53T Mutant Alpha-Synuclein Transgenic Mice and Parkinsonian. Oncotarget 2017, 8, 15–28. [Google Scholar] [CrossRef]
- Zhao, Y.J.; Wee, H.L.; Chan, Y.-H.; Seah, S.H.; Au, W.L.; Lau, P.N.; Pica, E.C.; Li, S.C.; Luo, N.; Tan, L.C.S. Progression of Parkinson’s Disease as Evaluated by Hoehn and Yahr Stage Transition Times. Mov. Disord. 2010, 25, 710–716. [Google Scholar] [CrossRef]
- Singh, P.; Hanson, P.S.; Morris, C.M. SIRT1 Ameliorates Oxidative Stress Induced Neural Cell Death and Is Down-Regulated in Parkinson’s Disease. BMC Neurosci. 2017, 18, 46. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.-L.; Mostoslavsky, R.; Saito, S.; Manis, J.P.; Gu, Y.; Patel, P.; Bronson, R.; Appella, E.; Alt, F.W.; Chua, K.F. Developmental Defects and P53 Hyperacetylation in Sir2 Homolog (SIRT1)-Deficient Mice. Proc. Natl. Acad. Sci. USA 2003, 100, 10794–10799. [Google Scholar] [CrossRef]
- Vaziri, H.; Dessain, S.K.; Eaton, E.N.; Imai, S.-I.; Frye, R.A.; Pandita, T.K.; Guarente, L.; Weinberg, R.A. HSIR2SIRT1 Functions as an NAD-Dependent P53 Deacetylase. Cell 2001, 107, 149–159. [Google Scholar] [CrossRef]
- Kim, E.-J.; Kho, J.-H.; Kang, M.-R.; Um, S.-J. Active Regulator of SIRT1 Cooperates with SIRT1 and Facilitates Suppression of P53 Activity. Mol. Cell 2007, 28, 277–290. [Google Scholar] [CrossRef]
- Talepoor Ardakani, M.; Rostamian Delavar, M.; Baghi, M.; Nasr-Esfahani, M.H.; Kiani-Esfahani, A.; Ghaedi, K. Upregulation of MiR-200a and MiR-204 in MPP+ -Treated Differentiated PC12 Cells as a Model of Parkinson’s Disease. Mol. Genet. Genom. Med. 2019, 7, e548. [Google Scholar] [CrossRef]
- Dini Modigliani, S.; Morlando, M.; Errichelli, L.; Sabatelli, M.; Bozzoni, I. An ALS-Associated Mutation in the FUS 3’-UTR Disrupts a MicroRNA-FUS Regulatory Circuitry. Nat. Commun. 2014, 5, 4335. [Google Scholar] [CrossRef]
- Figueroa-Romero, C.; Hur, J.; Lunn, J.S.; Paez-Colasante, X.; Bender, D.E.; Yung, R.; Sakowski, S.A.; Feldman, E.L. Expression of MicroRNAs in Human Post-Mortem Amyotrophic Lateral Sclerosis Spinal Cords Provides Insight into Disease Mechanisms. Mol. Cell Neurosci. 2016, 71, 34–45. [Google Scholar] [CrossRef]
- Taylor, J.P.; Brown, R.H.; Cleveland, D.W. Decoding ALS: From Genes to Mechanism. Nature 2016, 539, 197–206. [Google Scholar] [CrossRef]
- Zhou, F.; Zhang, C.; Guan, Y.; Chen, Y.; Lu, Q.; Jie, L.; Gao, H.; Du, H.; Zhang, H.; Liu, Y.; et al. Screening the Expression Characteristics of Several MiRNAs in G93A-SOD1 Transgenic Mouse: Altered Expression of MiRNA-124 Is Associated with Astrocyte Differentiation by Targeting Sox2 and Sox9. J. Neurochem. 2018, 145, 51–67. [Google Scholar] [CrossRef]
- Brownlee, W.J.; Hardy, T.A.; Fazekas, F.; Miller, D.H. Diagnosis of Multiple Sclerosis: Progress and Challenges. Lancet 2017, 389, 1336–1346. [Google Scholar] [CrossRef]
- Milo, R.; Kahana, E. Multiple Sclerosis: Geoepidemiology, Genetics and the Environment. Autoimmun. Rev. 2010, 9, A387–A394. [Google Scholar] [CrossRef]
- Waubant, E.; Lucas, R.; Mowry, E.; Graves, J.; Olsson, T.; Alfredsson, L.; Langer-Gould, A. Environmental and Genetic Risk Factors for MS: An Integrated Review. Ann. Clin. Transl. Neurol. 2019, 6, 1905–1922. [Google Scholar] [CrossRef] [PubMed]
- Naghavian, R.; Ghaedi, K.; Kiani-Esfahani, A.; Ganjalikhani-Hakemi, M.; Etemadifar, M.; Nasr-Esfahani, M.H. MiR-141 and MiR-200a, Revelation of New Possible Players in Modulation of Th17/Treg Differentiation and Pathogenesis of Multiple Sclerosis. PLoS ONE 2015, 10, e0124555. [Google Scholar] [CrossRef] [PubMed]
- Izadi, M.; Tahmasebi, S.; Pustokhina, I.; Yumashev, A.V.; Lakzaei, T.; Alvanegh, A.G.; Roshangar, L.; Dadashpour, M.; Yousefi, M.; Ahmadi, M. Changes in Th17 Cells Frequency and Function after Ozone Therapy Used to Treat Multiple Sclerosis Patients. Mult. Scler. Relat. Disord. 2020, 46, 102466. [Google Scholar] [CrossRef] [PubMed]
- Vallabh, S.M.; Minikel, E.V.; Schreiber, S.L.; Lander, E.S. Towards a Treatment for Genetic Prion Disease: Trials and Biomarkers. Lancet Neurol. 2020, 19, 361–368. [Google Scholar] [CrossRef]
- Thompson, A.G.B.; Lowe, J.; Fox, Z.; Lukic, A.; Porter, M.-C.; Ford, L.; Gorham, M.; Gopalakrishnan, G.S.; Rudge, P.; Walker, A.S.; et al. The Medical Research Council Prion Disease Rating Scale: A New Outcome Measure for Prion Disease Therapeutic Trials Developed and Validated Using Systematic Observational Studies. Brain 2013, 136, 1116–1127. [Google Scholar] [CrossRef]
- Boese, A.S.; Saba, R.; Campbell, K.; Majer, A.; Medina, S.; Burton, L.; Booth, T.F.; Chong, P.; Westmacott, G.; Dutta, S.M.; et al. MicroRNA Abundance Is Altered in Synaptoneurosomes during Prion Disease. Mol. Cell Neurosci. 2016, 71, 13–24. [Google Scholar] [CrossRef]
- Maisonneuve, P.; Marshall, B.C.; Knapp, E.A.; Lowenfels, A.B. Cancer Risk in Cystic Fibrosis: A 20-Year Nationwide Study From the United States. J. Natl. Cancer Inst. 2013, 105, 122–129. [Google Scholar] [CrossRef]
- Kalluri, R.; Weinberg, R.A. The Basics of Epithelial-Mesenchymal Transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef]
- Li, M.; Luan, F.; Zhao, Y.; Hao, H.; Zhou, Y.; Han, W.; Fu, X. Epithelial-Mesenchymal Transition: An Emerging Target in Tissue Fibrosis. Exp. Biol. Med. 2016, 241, 1–13. [Google Scholar] [CrossRef]
- Oba, S.; Kumano, S.; Suzuki, E.; Nishimatsu, H.; Takahashi, M.; Takamori, H.; Kasuya, M.; Ogawa, Y.; Sato, K.; Kimura, K.; et al. MiR-200b Precursor Can Ameliorate Renal Tubulointerstitial Fibrosis. PLoS ONE 2010, 5, e13614. [Google Scholar] [CrossRef]
- Hajarnis, S.; Yheskel, M.; Williams, D.; Brefort, T.; Glaudemans, B.; Debaix, H.; Baum, M.; Devuyst, O.; Patel, V. Suppression of MicroRNA Activity in Kidney Collecting Ducts Induces Partial Loss of Epithelial Phenotype and Renal Fibrosis. JASN 2018, 29, 518–531. [Google Scholar] [CrossRef]
- Kato, M.; Arce, L.; Wang, M.; Putta, S.; Lanting, L.; Natarajan, R. A MicroRNA Circuit Mediates Transforming Growth Factor-Β1 Autoregulation in Renal Glomerular Mesangial Cells. Kidney Int. 2011, 80, 358–368. [Google Scholar] [CrossRef]
- Xiong, M.; Jiang, L.; Zhou, Y.; Qiu, W.; Fang, L.; Tan, R.; Wen, P.; Yang, J. The MiR-200 Family Regulates TGF-Β1-Induced Renal Tubular Epithelial to Mesenchymal Transition through Smad Pathway by Targeting ZEB1 and ZEB2 Expression. Am. J. Physiol. Renal. Physiol. 2012, 302, F369–F379. [Google Scholar] [CrossRef]
- Huang, Y.; Tong, J.; He, F.; Yu, X.; Fan, L.; Hu, J.; Tan, J.; Chen, Z. MiR-141 Regulates TGF-Β1-Induced Epithelial-Mesenchymal Transition through Repression of HIPK2 Expression in Renal Tubular Epithelial Cells. Int. J. Mol. Med. 2015, 35, 311–318. [Google Scholar] [CrossRef]
- Yang, S.; Banerjee, S.; de Freitas, A.; Sanders, Y.Y.; Ding, Q.; Matalon, S.; Thannickal, V.J.; Abraham, E.; Liu, G. Participation of MiR-200 in Pulmonary Fibrosis. Am. J. Pathol. 2012, 180, 484–493. [Google Scholar] [CrossRef]
- Chilosi, M.; Caliò, A.; Rossi, A.; Gilioli, E.; Pedica, F.; Montagna, L.; Pedron, S.; Confalonieri, M.; Doglioni, C.; Ziesche, R.; et al. Epithelial to Mesenchymal Transition-Related Proteins ZEB1, β-Catenin, and β-Tubulin-III in Idiopathic Pulmonary Fibrosis. Mod. Pathol. 2017, 30, 26–38. [Google Scholar] [CrossRef]
- Moimas, S.; Salton, F.; Kosmider, B.; Ring, N.; Volpe, M.C.; Bahmed, K.; Braga, L.; Rehman, M.; Vodret, S.; Graziani, M.L.; et al. MiR-200 Family Members Reduce Senescence and Restore Idiopathic Pulmonary Fibrosis Type II Alveolar Epithelial Cell Transdifferentiation. ERJ Open Res. 2019, 5, 00138–02019. [Google Scholar] [CrossRef]
- Hassan, Z.K.; Al-Olayan, E.M. Curcumin Reorganizes MiRNA Expression in a Mouse Model of Liver Fibrosis. Asian Pac. J. Cancer Prev. 2012, 13, 5405–5408. [Google Scholar] [CrossRef]
- Wang, Y.; Zeng, Z.; Guan, L.; Ao, R. GRHL2 Induces Liver Fibrosis and Intestinal Mucosal Barrier Dysfunction in Non-Alcoholic Fatty Liver Disease via MicroRNA-200 and the MAPK Pathway. J. Cell Mol. Med. 2020, 24, 6107–6119. [Google Scholar] [CrossRef]
- Sundararajan, V.; Pang, Q.Y.; Choolani, M.; Huang, R.Y.-J. Spotlight on the Granules (Grainyhead-Like Proteins)—From an Evolutionary Conserved Controller of Epithelial Trait to Pioneering the Chromatin Landscape. Front. Mol. Biosci. 2020, 7, 213. [Google Scholar] [CrossRef]
- Feng, X.; Wang, Z.; Fillmore, R.; Xi, Y. MiR-200, a New Star MiRNA in Human Cancer. Cancer Lett. 2014, 344, 166–173. [Google Scholar] [CrossRef]
- Lamouille, S.; Subramanyam, D.; Blelloch, R.; Derynck, R. Regulation of Epithelial-Mesenchymal and Mesenchymal-Epithelial Transitions by Micrornas. Curr. Opin. Cell Biol. 2013, 25, 200–207. [Google Scholar] [CrossRef]
- Gollavilli, P.N.; Parma, B.; Siddiqui, A.; Yang, H.; Ramesh, V.; Napoli, F.; Schwab, A.; Natesan, R.; Mielenz, D.; Asangani, I.A.; et al. The Role of MiR-200b/c in Balancing EMT and Proliferation Revealed by an Activity Reporter. Oncogene 2021, 40, 2309. [Google Scholar] [CrossRef]
- Uhlmann, S.; Zhang, J.D.; Schwäger, A.; Mannsperger, H.; Riazalhosseini, Y.; Burmester, S.; Ward, A.; Korf, U.; Wiemann, S.; Sahin, Ö. MiR-200bc/429 Cluster Targets PLCγ1 and Differentially Regulates Proliferation and EGF-Driven Invasion than MiR-200a/141 in Breast Cancer. Oncogene 2010, 29, 4297–4306. [Google Scholar] [CrossRef]
- Cochrane, D.R.; Spoelstra, N.S.; Howe, E.N.; Nordeen, S.K.; Richer, J.K. MicroRNA-200c Mitigates Invasiveness and Restores Sensitivity to Microtubule-Targeting Chemotherapeutic Agents. Mol. Cancer Ther. 2009, 8, 1055–1066. [Google Scholar] [CrossRef]
- Ceppi, P.; Peter, M.E. MicroRNAs Regulate Both Epithelial-to-Mesenchymal Transition and Cancer Stem Cells. Oncogene 2014, 33, 269–278. [Google Scholar] [CrossRef]
- Noman, M.Z.; Janji, B.; Abdou, A.; Hasmim, M.; Terry, S.; Tan, T.Z.; Mami-Chouaib, F.; Thiery, J.P.; Chouaib, S. The Immune Checkpoint Ligand PD-L1 Is Upregulated in EMT-Activated Human Breast Cancer Cells by a Mechanism Involving ZEB-1 and MiR-200. OncoImmunology 2017, 6, e1263412. [Google Scholar] [CrossRef]
- Chen, L.; Gibbons, D.L.; Goswami, S.; Cortez, M.A.; Ahn, Y.-H.; Byers, L.A.; Zhang, X.; Yi, X.; Dwyer, D.; Lin, W.; et al. Metastasis Is Regulated via MicroRNA-200/ZEB1 Axis Control of Tumour Cell PD-L1 Expression and Intratumoral Immunosuppression. Nat. Commun. 2014, 5, 5241. [Google Scholar] [CrossRef]
- Raue, R.; Frank, A.-C.; Fuhrmann, D.C.; de la Cruz-Ojeda, P.; Rösser, S.; Bauer, R.; Cardamone, G.; Weigert, A.; Syed, S.N.; Schmid, T.; et al. MicroRNA-200c Attenuates the Tumor-Infiltrating Capacity of Macrophages. Biology 2022, 11, 349. [Google Scholar] [CrossRef]
- Hurteau, G.J.; Carlson, J.A.; Spivack, S.D.; Brock, G.J. Overexpression of the MicroRNA Hsa-MiR-200c Leads to Reduced Expression of Transcription Factor 8 and Increased Expression of E-Cadherin. Cancer Res. 2007, 67, 7972–7976. [Google Scholar] [CrossRef]
- Burk, U.; Schubert, J.; Wellner, U.; Schmalhofer, O.; Vincan, E.; Spaderna, S.; Brabletz, T. A Reciprocal Repression between ZEB1 and Members of the MiR-200 Family Promotes EMT and Invasion in Cancer Cells. EMBO Rep. 2008, 9, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Gregory, P.A.; Bert, A.G.; Paterson, E.L.; Barry, S.C.; Tsykin, A.; Farshid, G.; Vadas, M.A.; Khew-Goodall, Y.; Goodall, G.J. The MiR-200 Family and MiR-205 Regulate Epithelial to Mesenchymal Transition by Targeting ZEB1 and SIP1. Nat. Cell Biol. 2008, 10, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Korpal, M.; Lee, E.S.; Hu, G.; Kang, Y. The MiR-200 Family Inhibits Epithelial-Mesenchymal Transition and Cancer Cell Migration by Direct Targeting of E-Cadherin Transcriptional Repressors ZEB1 and ZEB2. J. Biol. Chem. 2008, 283, 14910–14914. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-M.; Gaur, A.B.; Lengyel, E.; Peter, M.E. The MiR-200 Family Determines the Epithelial Phenotype of Cancer Cells by Targeting the E-Cadherin Repressors ZEB1 and ZEB2. Genes Dev. 2008, 22, 894–907. [Google Scholar] [CrossRef]
- Bracken, C.P.; Gregory, P.A.; Kolesnikoff, N.; Bert, A.G.; Wang, J.; Shannon, M.F.; Goodall, G.J. A Double-Negative Feedback Loop between ZEB1-SIP1 and the MicroRNA-200 Family Regulates Epithelial-Mesenchymal Transition. Cancer Res. 2008, 68, 7846–7854. [Google Scholar] [CrossRef]
- Brabletz, S.; Brabletz, T. The ZEB/MiR-200 Feedback Loop—A Motor of Cellular Plasticity in Development and Cancer? EMBO Rep. 2010, 11, 670–677. [Google Scholar] [CrossRef]
- Brabletz, S.; Bajdak, K.; Meidhof, S.; Burk, U.; Niedermann, G.; Firat, E.; Wellner, U.; Dimmler, A.; Faller, G.; Schubert, J.; et al. The ZEB1/MiR-200 Feedback Loop Controls Notch Signalling in Cancer Cells: ZEB1 Activates Notch Signalling. EMBO J. 2011, 30, 770–782. [Google Scholar] [CrossRef]
- Yang, Y.; Ahn, Y.-H.; Gibbons, D.L.; Zang, Y.; Lin, W.; Thilaganathan, N.; Alvarez, C.A.; Moreira, D.C.; Creighton, C.J.; Gregory, P.A.; et al. The Notch Ligand Jagged2 Promotes Lung Adenocarcinoma Metastasis through a MiR-200-Dependent Pathway in Mice. J. Clin. Investig. 2011, 121, 1373–1385. [Google Scholar] [CrossRef]
- Xue, B.; Chuang, C.H.; Prosser, H.M.; Fuziwara, C.S.; Chan, C.; Sahasrabudhe, N.; Kühn, M.; Wu, Y.; Chen, J.; Biton, A.; et al. MiR-200 Deficiency Promotes Lung Cancer Metastasis by Activating Notch Signaling in Cancer-Associated Fibroblasts. Genes Dev. 2021, 35, 1109–1122. [Google Scholar] [CrossRef]
- Sundararajan, V.; Gengenbacher, N.; Stemmler, M.P.; Kleemann, J.A.; Brabletz, T.; Brabletz, S. The ZEB1/MiR-200c Feedback Loop Regulates Invasion via Actin Interacting Proteins MYLK and TKS5. Oncotarget 2015, 6, 27083–27096. [Google Scholar] [CrossRef]
- Li, X.; Roslan, S.; Johnstone, C.N.; Wright, J.A.; Bracken, C.P.; Anderson, M.; Bert, A.G.; Selth, L.A.; Anderson, R.L.; Goodall, G.J.; et al. MiR-200 Can Repress Breast Cancer Metastasis through ZEB1-Independent but Moesin-Dependent Pathways. Oncogene 2014, 33, 4077–4088. [Google Scholar] [CrossRef]
- Jurmeister, S.; Baumann, M.; Balwierz, A.; Keklikoglou, I.; Ward, A.; Uhlmann, S.; Zhang, J.D.; Wiemann, S.; Sahin, Ö. MicroRNA-200c Represses Migration and Invasion of Breast Cancer Cells by Targeting Actin-Regulatory Proteins FHOD1 and PPM1F. Mol. Cell Biol. 2012, 32, 633–651. [Google Scholar] [CrossRef]
- Huang, R.Y.-J.; Wong, M.K.; Tan, T.Z.; Kuay, K.T.; Ng, A.H.C.; Chung, V.Y.; Chu, Y.-S.; Matsumura, N.; Lai, H.-C.; Lee, Y.F.; et al. An EMT Spectrum Defines an Anoikis-Resistant and Spheroidogenic Intermediate Mesenchymal State That Is Sensitive to e-Cadherin Restoration by a Src-Kinase Inhibitor, Saracatinib (AZD0530). Cell Death Dis. 2013, 4, e915. [Google Scholar] [CrossRef]
- Hong, T.; Watanabe, K.; Ta, C.H.; Villarreal-Ponce, A.; Nie, Q.; Dai, X. An Ovol2-Zeb1 Mutual Inhibitory Circuit Governs Bidirectional and Multi-Step Transition between Epithelial and Mesenchymal States. PLoS Comput. Biol. 2015, 11, e1004569. [Google Scholar] [CrossRef] [PubMed]
- Pastushenko, I.; Brisebarre, A.; Sifrim, A.; Fioramonti, M.; Revenco, T.; Boumahdi, S.; Van Keymeulen, A.; Brown, D.; Moers, V.; Lemaire, S.; et al. Identification of the Tumour Transition States Occurring during EMT. Nature 2018, 556, 463–468. [Google Scholar] [CrossRef]
- Grosse-Wilde, A.; Fouquier d’Hérouël, A.; McIntosh, E.; Ertaylan, G.; Skupin, A.; Kuestner, R.E.; del Sol, A.; Walters, K.-A.; Huang, S. Stemness of the Hybrid Epithelial/Mesenchymal State in Breast Cancer and Its Association with Poor Survival. PLoS ONE 2015, 10, e0126522. [Google Scholar] [CrossRef]
- Yamashita, N.; Tokunaga, E.; Iimori, M.; Inoue, Y.; Tanaka, K.; Kitao, H.; Saeki, H.; Oki, E.; Maehara, Y. Epithelial Paradox: Clinical Significance of Coexpression of E-Cadherin and Vimentin With Regard to Invasion and Metastasis of Breast Cancer. Clin. Breast Cancer 2018, 18, e1003–e1009. [Google Scholar] [CrossRef]
- Ruscetti, M.; Quach, B.; Dadashian, E.L.; Mulholland, D.J.; Wu, H. Tracking and Functional Characterization of Epithelial-Mesenchymal Transition and Mesenchymal Tumor Cells during Prostate Cancer Metastasis. Cancer Res. 2015, 75, 2749–2759. [Google Scholar] [CrossRef]
- Fustaino, V.; Presutti, D.; Colombo, T.; Cardinali, B.; Papoff, G.; Brandi, R.; Bertolazzi, P.; Felici, G.; Ruberti, G. Characterization of Epithelial-Mesenchymal Transition Intermediate/Hybrid Phenotypes Associated to Resistance to EGFR Inhibitors in Non-Small Cell Lung Cancer Cell Lines. Oncotarget 2017, 8, 103340–103363. [Google Scholar] [CrossRef]
- Kröger, C.; Afeyan, A.; Mraz, J.; Eaton, E.N.; Reinhardt, F.; Khodor, Y.L.; Thiru, P.; Bierie, B.; Ye, X.; Burge, C.B.; et al. Acquisition of a Hybrid E/M State Is Essential for Tumorigenicity of Basal Breast Cancer Cells. Proc. Natl. Acad. Sci. USA 2019, 116, 7353–7362. [Google Scholar] [CrossRef]
- Jolly, M.K.; Somarelli, J.A.; Sheth, M.; Biddle, A.; Tripathi, S.C.; Armstrong, A.J.; Hanash, S.M.; Bapat, S.A.; Rangarajan, A.; Levine, H. Hybrid Epithelial/Mesenchymal Phenotypes Promote Metastasis and Therapy Resistance across Carcinomas. Pharmacol. Ther. 2019, 194, 161–184. [Google Scholar] [CrossRef] [PubMed]
- Siemens, H.; Jackstadt, R.; Hünten, S.; Kaller, M.; Menssen, A.; Götz, U.; Hermeking, H. MiR-34 and SNAIL Form a Double-Negative Feedback Loop to Regulate Epithelial-Mesenchymal Transitions. Cell Cycle 2011, 10, 4256–4271. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Jolly, M.K.; Levine, H.; Onuchic, J.N.; Ben-Jacob, E. MicroRNA-Based Regulation of Epithelial-Hybrid-Mesenchymal Fate Determination. Proc. Natl. Acad. Sci. USA 2013, 110, 18144–18149. [Google Scholar] [CrossRef]
- Jolly, M.K.; Tripathi, S.C.; Jia, D.; Mooney, S.M.; Celiktas, M.; Hanash, S.M.; Mani, S.A.; Pienta, K.J.; Ben-Jacob, E.; Levine, H. Stability of the Hybrid Epithelial/Mesenchymal Phenotype. Oncotarget 2016, 7, 27067–27084. [Google Scholar] [CrossRef]
- Burger, G.A.; Danen, E.H.J.; Beltman, J.B. Deciphering Epithelial–Mesenchymal Transition Regulatory Networks in Cancer through Computational Approaches. Front. Oncol. 2017, 7, 162. [Google Scholar] [CrossRef]
- Jia, W.; Tripathi, S.; Chakraborty, P.; Chedere, A.; Rangarajan, A.; Levine, H.; Jolly, M.K. Epigenetic Feedback and Stochastic Partitioning during Cell Division Can Drive Resistance to EMT. Oncotarget 2020, 11, 2611–2624. [Google Scholar] [CrossRef]
- Garinet, S.; Didelot, A.; Denize, T.; Perrier, A.; Beinse, G.; Leclere, J.-B.; Oudart, J.-B.; Gibault, L.; Badoual, C.; Le Pimpec-Barthes, F.; et al. Clinical Assessment of the MiR-34, MiR-200, ZEB1 and SNAIL EMT Regulation Hub Underlines the Differential Prognostic Value of EMT MiRs to Drive Mesenchymal Transition and Prognosis in Resected NSCLC. Br. J. Cancer 2021, 125, 1544–1551. [Google Scholar] [CrossRef]
- Deshmukh, A.P.; Vasaikar, S.V.; Tomczak, K.; Tripathi, S.; den Hollander, P.; Arslan, E.; Chakraborty, P.; Soundararajan, R.; Jolly, M.K.; Rai, K.; et al. Identification of EMT Signaling Cross-Talk and Gene Regulatory Networks by Single-Cell RNA Sequencing. Proc. Natl. Acad. Sci. USA 2021, 118, e2102050118. [Google Scholar] [CrossRef]
- Mateescu, B.; Batista, L.; Cardon, M.; Gruosso, T.; de Feraudy, Y.; Mariani, O.; Nicolas, A.; Meyniel, J.-P.; Cottu, P.; Sastre-Garau, X.; et al. MiR-141 and MiR-200a Act on Ovarian Tumorigenesis by Controlling Oxidative Stress Response. Nat. Med. 2011, 17, 1627–1635. [Google Scholar] [CrossRef]
- Hua, Y.; Choi, P.-W.; Trachtenberg, A.J.; Ng, A.C.; Kuo, W.P.; Ng, S.-K.; Dinulescu, D.M.; Matzuk, M.M.; Berkowitz, R.S.; Ng, S.-W. Epithelialization of Mouse Ovarian Tumor Cells Originating in the Fallopian Tube Stroma. Oncotarget 2016, 7, 66077–66086. [Google Scholar] [CrossRef]
- Ansenberger, K.; Zhuge, Y.; Lagman, J.A.J.; Richards, C.; Barua, A.; Bahr, J.M.; Hales, D.B. E-Cadherin Expression in Ovarian Cancer in the Laying Hen, Gallus Domesticus, Compared to Human Ovarian Cancer. Gynecol. Oncol. 2009, 113, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Hales, K.H.; Speckman, S.C.; Kurrey, N.K.; Hales, D.B. Uncovering Molecular Events Associated with the Chemosuppressive Effects of Flaxseed: A Microarray Analysis of the Laying Hen Model of Ovarian Cancer. BMC Genom. 2014, 15, 709. [Google Scholar] [CrossRef] [PubMed]
- Choi, P.-W.; So, W.W.; Yang, J.; Liu, S.; Tong, K.K.; Kwan, K.M.; Kwok, J.S.-L.; Tsui, S.K.W.; Ng, S.-K.; Hales, K.H.; et al. MicroRNA-200 Family Governs Ovarian Inclusion Cyst Formation and Mode of Ovarian Cancer Spread. Oncogene 2020, 39, 4045–4060. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Wang, J.; Yang, P.; Lu, Q.; Zhang, T.; Yang, Y. MicroRNA-200 Promotes Lung Cancer Cell Growth through FOG2-Independent AKT Activation. IUBMB Life 2015, 67, 720–725. [Google Scholar] [CrossRef]
- Damaghi, M.; West, J.; Robertson-Tessi, M.; Xu, L.; Ferrall-Fairbanks, M.C.; Stewart, P.A.; Persi, E.; Fridley, B.L.; Altrock, P.M.; Gatenby, R.A.; et al. The Harsh Microenvironment in Early Breast Cancer Selects for a Warburg Phenotype. Proc. Natl. Acad. Sci. USA 2021, 118, e2011342118. [Google Scholar] [CrossRef]
- Sinha, V.C.; Piwnica-Worms, H. Intratumoral Heterogeneity in Ductal Carcinoma In Situ: Chaos and Consequence. J. Mammary Gland Biol. Neoplasia 2018, 23, 191–205. [Google Scholar] [CrossRef]
- Haakensen, V.D.; Nygaard, V.; Greger, L.; Aure, M.R.; Fromm, B.; Bukholm, I.R.K.; Lüders, T.; Chin, S.-F.; Git, A.; Caldas, C.; et al. Subtype-Specific Micro-RNA Expression Signatures in Breast Cancer Progression. Int. J. Cancer 2016, 139, 1117–1128. [Google Scholar] [CrossRef]
- Dykxhoorn, D.M.; Wu, Y.; Xie, H.; Yu, F.; Lal, A.; Petrocca, F.; Martinvalet, D.; Song, E.; Lim, B.; Lieberman, J. MiR-200 Enhances Mouse Breast Cancer Cell Colonization to Form Distant Metastases. PLoS ONE 2009, 4, e7181. [Google Scholar] [CrossRef]
- Korpal, M.; Ell, B.J.; Buffa, F.M.; Ibrahim, T.; Blanco, M.A.; Celià-Terrassa, T.; Mercatali, L.; Khan, Z.; Goodarzi, H.; Hua, Y.; et al. Direct Targeting of Sec23a by MiR-200s Influences Cancer Cell Secretome and Promotes Metastatic Colonization. Nat. Med. 2011, 17, 1101–1108. [Google Scholar] [CrossRef]
- Ast, V.; Korda, T.; Oswald, M.; Kolte, A.; Eisel, D.; Osen, W.; Eichmüller, S.B.; Berndt, A.; König, R. MiR-192, MiR-200c and MiR-17 Are Fibroblast-Mediated Inhibitors of Colorectal Cancer Invasion. Oncotarget 2018, 9, 35559–35580. [Google Scholar] [CrossRef]
- Oghabi Bakhshaiesh, T.; Esmaeili, R. Effects of Noncoding RNAs in Radiotherapy Response in Breast Cancer: A Systematic Review. Cell Cycle 2022, 21, 883–893. [Google Scholar] [CrossRef]
- Kozak, J.; Jonak, K.; Maciejewski, R. The Function of MiR-200 Family in Oxidative Stress Response Evoked in Cancer Chemotherapy and Radiotherapy. Biomed. Pharm. 2020, 125, 110037. [Google Scholar] [CrossRef]
- Lin, J.; Liu, C.; Gao, F.; Mitchel, R.E.J.; Zhao, L.; Yang, Y.; Lei, J.; Cai, J. MiR-200c Enhances Radiosensitivity of Human Breast Cancer Cells. J. Cell Biochem. 2013, 114, 606–615. [Google Scholar] [CrossRef]
- Sun, Q.; Liu, T.; Yuan, Y.; Guo, Z.; Xie, G.; Du, S.; Lin, X.; Xu, Z.; Liu, M.; Wang, W.; et al. MiR-200c Inhibits Autophagy and Enhances Radiosensitivity in Breast Cancer Cells by Targeting UBQLN1. Int. J. Cancer 2015, 136, 1003–1012. [Google Scholar] [CrossRef]
- Wang, B.; Zheng, J.; Li, R.; Tian, Y.; Lin, J.; Liang, Y.; Sun, Q.; Xu, A.; Zheng, R.; Liu, M.; et al. Long Noncoding RNA LINC02582 Acts Downstream of MiR-200c to Promote Radioresistance through CHK1 in Breast Cancer Cells. Cell Death Dis. 2019, 10, 764. [Google Scholar] [CrossRef]
- Cortez, M.A.; Valdecanas, D.; Zhang, X.; Zhan, Y.; Bhardwaj, V.; Calin, G.A.; Komaki, R.; Giri, D.K.; Quini, C.C.; Wolfe, T.; et al. Therapeutic Delivery of MiR-200c Enhances Radiosensitivity in Lung Cancer. Mol. Ther. 2014, 22, 1494–1503. [Google Scholar] [CrossRef]
- Du, M.; Wang, J.; Chen, H.; Wang, S.; Chen, L.; Xu, Y.; Su, F.; Lu, X. MicroRNA-200a Suppresses Migration and Invasion and Enhances the Radiosensitivity of NSCLC Cells by Inhibiting the HGF/C-Met Signaling Pathway. Oncol. Rep. 2019, 41, 1497–1508. [Google Scholar] [CrossRef]
- Greither, T.; Vorwerk, F.; Kappler, M.; Bache, M.; Taubert, H.; Kuhnt, T.; Hey, J.; Eckert, A.W. Salivary MiR-93 and MiR-200a as Post-Radiotherapy Biomarkers in Head and Neck Squamous Cell Carcinoma. Oncol. Rep. 2017, 38, 1268–1275. [Google Scholar] [CrossRef]
- Tao, J.; Fan, M.; Zhou, D.; Hong, Y.; Zhang, J.; Liu, H.; Sharma, S.; Wang, G.; Dong, Q. MiR-200c Modulates the Pathogenesis of Radiation-Induced Oral Mucositis. Oxid. Med. Cell Longev. 2019, 2019, 2352079. [Google Scholar] [CrossRef]
- Lee, Y.-C.; Lin, C.-H.; Chang, W.-L.; Lin, W.-D.; Pan, J.-K.; Wang, W.-J.; Su, B.-C.; Chung, H.-H.; Tsai, C.-H.; Lin, F.-C.; et al. Concurrent Chemoradiotherapy-Driven Cell Plasticity by MiR-200 Family Implicates the Therapeutic Response of Esophageal Squamous Cell Carcinoma. Int. J. Mol. Sci. 2022, 23, 4367. [Google Scholar] [CrossRef]
- Nilsen, A.; Hillestad, T.; Skingen, V.E.; Aarnes, E.-K.; Fjeldbo, C.S.; Hompland, T.; Evensen, T.S.; Stokke, T.; Kristensen, G.B.; Grallert, B.; et al. MiR-200a/b/-429 Downregulation Is a Candidate Biomarker of Tumor Radioresistance and Independent of Hypoxia in Locally Advanced Cervical Cancer. Mol. Oncol. 2022, 16, 1402–1419. [Google Scholar] [CrossRef]
- Loret, N.; Denys, H.; Tummers, P.; Berx, G. The Role of Epithelial-to-Mesenchymal Plasticity in Ovarian Cancer Progression and Therapy Resistance. Cancers 2019, 11, 838. [Google Scholar] [CrossRef]
- Liu, S.; Tetzlaff, M.T.; Cui, R.; Xu, X. MiR-200c Inhibits Melanoma Progression and Drug Resistance through Down-Regulation of Bmi-1. Am. J. Pathol. 2012, 181, 1823–1835. [Google Scholar] [CrossRef]
- Mezencev, R.; Wartell, R.M. Cisplatin Binds to Pre-MiR-200b and Impairs Its Processing to Mature MicroRNA. Neoplasma 2018, 65, 222–227. [Google Scholar] [CrossRef]
- Crudele, F.; Bianchi, N.; Astolfi, A.; Grassilli, S.; Brugnoli, F.; Terrazzan, A.; Bertagnolo, V.; Negrini, M.; Frassoldati, A.; Volinia, S. The Molecular Networks of MicroRNAs and Their Targets in the Drug Resistance of Colon Carcinoma. Cancers 2021, 13, 4355. [Google Scholar] [CrossRef]
- Meidhof, S.; Brabletz, S.; Lehmann, W.; Preca, B.-T.; Mock, K.; Ruh, M.; Schüler, J.; Berthold, M.; Weber, A.; Burk, U.; et al. ZEB1-associated Drug Resistance in Cancer Cells Is Reversed by the Class I HDAC Inhibitor Mocetinostat. EMBO Mol. Med. 2015, 7, 831–847. [Google Scholar] [CrossRef]
- San, K.; Horita, M.; Ganapathy, A.; Chinnadurai, G.; Ezekiel, U.R. Deregulated Expression of MicroRNA-200b/c and SUZ12, a Polycomb Repressive Complex 2 Subunit, in Chemoresistant Colorectal Cancer Cells. Genes Cancer 2017, 8, 673–681. [Google Scholar] [CrossRef]
- Knezevic, J.; Pfefferle, A.D.; Petrovic, I.; Greene, S.B.; Perou, C.M.; Rosen, J.M. Expression of MiR-200c in Claudin-Low Breast Cancer Alters Stem Cell Functionality, Enhances Chemosensitivity and Reduces Metastatic Potential. Oncogene 2015, 34, 5997–6006. [Google Scholar] [CrossRef]
- Heydari, K.; Saidijam, M.; Reza Sharifi, M.; Dermani, F.K.; Soleimani Asl, S.; Shabab, N.; Najafi, R. The Effect of MiR-200c Inhibition on Chemosensitivity (5- FluoroUracil) in Colorectal Cancer. Pathol. Oncol. Res. 2018, 24, 145–151. [Google Scholar] [CrossRef]
- Brozovic, A.; Duran, G.E.; Wang, Y.C.; Francisco, E.B.; Sikic, B.I. The MiR-200 Family Differentially Regulates Sensitivity to Paclitaxel and Carboplatin in Human Ovarian Carcinoma OVCAR-3 and MES-OV Cells. Mol. Oncol. 2015, 9, 1678–1693. [Google Scholar] [CrossRef]
- Haenisch, S.; Werk, A.N.; Cascorbi, I. MicroRNAs and Their Relevance to ABC Transporters. Br. J. Clin. Pharmacol. 2014, 77, 587. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.J.; Yang, L.; Hong, Q.; Kuang, X.Y.; Di, G.H.; Shao, Z.M. MicroRNA-200a Confers Chemoresistance by Antagonizing TP53INP1 and YAP1 in Human Breast Cancer. BMC Cancer 2018, 18, 74. [Google Scholar] [CrossRef] [PubMed]
- Tomasini, R.; Seux, M.; Nowak, J.; Bontemps, C.; Carrier, A.; Dagorn, J.C.; Pébusque, M.J.; Iovanna, J.L.; Dusetti, N.J. TP53INP1 Is a Novel P73 Target Gene That Induces Cell Cycle Arrest and Cell Death by Modulating P73 Transcriptional Activity. Oncogene 2005, 24, 8093–8104. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kim, C.; Kang, H.; Tak, H.; Ahn, S.; Yoon, S.K.; Kuh, H.J.; Kim, W.; Lee, E.K. MicroRNA-200a-3p Increases 5-Fluorouracil Resistance by Regulating Dual Specificity Phosphatase 6 Expression. Exp. Mol. Med. 2017, 49, e327. [Google Scholar] [CrossRef] [PubMed]
- Choi, P.-W.; Ng, S.-W. The Functions of MicroRNA-200 Family in Ovarian Cancer: Beyond Epithelial-Mesenchymal Transition. Int. J. Mol. Sci. 2017, 18, 1207. [Google Scholar] [CrossRef]
- Yu, S.-J.; Hu, J.-Y.; Kuang, X.-Y.; Luo, J.-M.; Hou, Y.-F.; Di, G.-H.; Wu, J.; Shen, Z.-Z.; Song, H.-Y.; Shao, Z.-M. MicroRNA-200a Promotes Anoikis Resistance and Metastasis by Targeting YAP1 in Human Breast Cancer. Clin. Cancer Res. 2013, 19, 1389–1399. [Google Scholar] [CrossRef]
- Van Jaarsveld, M.T.M.; Helleman, J.; Boersma, A.W.M.; Van Kuijk, P.F.; Van Ijcken, W.F.; Despierre, E.; Vergote, I.; Mathijssen, R.H.J.; Berns, E.M.J.J.; Verweij, J.; et al. MiR-141 Regulates KEAP1 and Modulates Cisplatin Sensitivity in Ovarian Cancer Cells. Oncogene 2012, 32, 4284–4293. [Google Scholar] [CrossRef]
- Eades, G.; Yang, M.; Yao, Y.; Zhang, Y.; Zhou, Q. MiR-200a Regulates Nrf2 Activation by Targeting Keap1 MRNA in Breast Cancer Cells. J. Biol. Chem. 2011, 286, 40725–40733. [Google Scholar] [CrossRef]
- Cavallari, I.; Ciccarese, F.; Sharova, E.; Urso, L.; Raimondi, V.; Silic-Benussi, M.; D’Agostino, D.M.; Ciminale, V. The MiR-200 Family of MicroRNAs: Fine Tuners of Epithelial-Mesenchymal Transition and Circulating Cancer Biomarkers. Cancers 2021, 13, 5874. [Google Scholar] [CrossRef]
- Chen, J.; Tian, W.; Cai, H.; He, H.; Deng, Y. Down-Regulation of MicroRNA-200c Is Associated with Drug Resistance in Human Breast Cancer. Med. Oncol. 2012, 29, 2527–2534. [Google Scholar] [CrossRef]
- Men, D.; Liang, Y.; Chen, L. Decreased Expression of MicroRNA-200b Is an Independent Unfavorable Prognostic Factor for Glioma Patients. Cancer Epidemiol. 2014, 38, 152–156. [Google Scholar] [CrossRef]
- Thi Chung Duong, T.; Nguyen, T.H.N.; Thi Ngoc Nguyen, T.; Huynh, L.H.; Ngo, H.P.; Thi Nguyen, H. Diagnostic and Prognostic Value of MiR-200 Family in Breast Cancer: A Meta-Analysis and Systematic Review. Cancer Epidemiol. 2022, 77, 102097. [Google Scholar] [CrossRef]
- Mei, Y.; Zheng, J.; Xiang, P.; Liu, C.; Fan, Y. Prognostic Value of the MiR-200 Family in Bladder Cancer: A Systematic Review and Meta-Analysis. Medicine 2020, 99, e22891. [Google Scholar] [CrossRef]
- Madhavan, D.; Zucknick, M.; Wallwiener, M.; Cuk, K.; Modugno, C.; Scharpff, M.; Schott, S.; Heil, J.; Turchinovich, A.; Yang, R.; et al. Circulating MiRNAs as Surrogate Markers for Circulating Tumor Cells and Prognostic Markers in Metastatic Breast Cancer. Clin. Cancer Res. 2012, 18, 5972–5982. [Google Scholar] [CrossRef]
- Madhavan, D.; Peng, C.; Wallwiener, M.; Zucknick, M.; Nees, J.; Schott, S.; Rudolph, A.; Riethdorf, S.; Trumpp, A.; Pantel, K.; et al. Circulating MiRNAs with Prognostic Value in Metastatic Breast Cancer and for Early Detection of Metastasis. Carcinogenesis 2016, 37, 461–470. [Google Scholar] [CrossRef]
- Maierthaler, M.; Benner, A.; Hoffmeister, M.; Surowy, H.; Jansen, L.; Knebel, P.; Chang-Claude, J.; Brenner, H.; Burwinkel, B. Plasma MiR-122 and MiR-200 Family Are Prognostic Markers in Colorectal Cancer. Int. J. Cancer Res. 2017, 140, 176–187. [Google Scholar] [CrossRef]
- Santasusagna, S.; Moreno, I.; Navarro, A.; Martinez Rodenas, F.; Hernández, R.; Castellano, J.J.; Muñoz, C.; Monzo, M. Prognostic Impact of MiR-200 Family Members in Plasma and Exosomes from Tumor-Draining versus Peripheral Veins of Colon Cancer Patients. Oncology 2018, 95, 309–318. [Google Scholar] [CrossRef]
- Reese, M.; Flammang, I.; Yang, Z.; Dhayat, S.A. Potential of Exosomal MicroRNA-200b as Liquid Biopsy Marker in Pancreatic Ductal Adenocarcinoma. Cancers 2020, 12, 197. [Google Scholar] [CrossRef]
- Cheng, H.; Zhang, L.; Cogdell, D.E.; Zheng, H.; Schetter, A.J.; Nykter, M.; Harris, C.C.; Chen, K.; Hamilton, S.R.; Zhang, W. Circulating Plasma MiR-141 Is a Novel Biomarker for Metastatic Colon Cancer and Predicts Poor Prognosis. PLoS ONE 2011, 6, e17745. [Google Scholar] [CrossRef]
- Tejero, R.; Navarro, A.; Campayo, M.; Viñolas, N.; Marrades, R.M.; Cordeiro, A.; Ruíz-Martínez, M.; Santasusagna, S.; Molins, L.; Ramirez, J.; et al. MiR-141 and MiR-200c as Markers of Overall Survival in Early Stage Non-Small Cell Lung Cancer Adenocarcinoma. PLoS ONE 2014, 9, e101899. [Google Scholar] [CrossRef]
- Záveský, L.; Jandáková, E.; Weinberger, V.; Minář, L.; Hanzíková, V.; Dušková, D.; Drábková, L.Z.; Svobodová, I.; Hořínek, A. Ascites-Derived Extracellular MicroRNAs as Potential Biomarkers for Ovarian Cancer. Reprod. Sci. 2019, 26, 510–522. [Google Scholar] [CrossRef]
- Márton, É.; Lukács, J.; Penyige, A.; Janka, E.; Hegedüs, L.; Soltész, B.; Méhes, G.; Póka, R.; Nagy, B.; Szilágyi, M. Circulating Epithelial-Mesenchymal Transition-Associated MiRNAs Are Promising Biomarkers in Ovarian Cancer. J. Biotechnol. 2019, 297, 58–65. [Google Scholar] [CrossRef]
- Meng, X.; Müller, V.; Milde-Langosch, K.; Trillsch, F.; Pantel, K.; Schwarzenbach, H. Diagnostic and Prognostic Relevance of Circulating Exosomal MiR-373, MiR-200a, MiR-200b and MiR-200c in Patients with Epithelial Ovarian Cancer. Oncotarget 2016, 7, 16923–16935. [Google Scholar] [CrossRef]
- Choi, P.-W.; Bahrampour, A.; Ng, S.-K.; Liu, S.K.; Qiu, W.; Xie, F.; Kuo, W.P.; Kwong, J.; Hales, K.H.; Hales, D.B.; et al. Characterization of MiR-200 Family Members as Blood Biomarkers for Human and Laying Hen Ovarian Cancer. Sci. Rep. 2020, 10, 20071. [Google Scholar] [CrossRef]
- Savolainen, K.; Scaravilli, M.; Ilvesmäki, A.; Staff, S.; Tolonen, T.; Mäenpää, J.U.; Visakorpi, T.; Auranen, A. Expression of the MiR-200 Family in Tumor Tissue, Plasma and Urine of Epithelial Ovarian Cancer Patients in Comparison to Benign Counterparts. BMC Res. Notes 2020, 13, 311. [Google Scholar] [CrossRef] [PubMed]
- Zuberi, M.; Mir, R.; Das, J.; Ahmad, I.; Javid, J.; Yadav, P.; Masroor, M.; Ahmad, S.; Ray, P.C.; Saxena, A. Expression of Serum MiR-200a, MiR-200b, and MiR-200c as Candidate Biomarkers in Epithelial Ovarian Cancer and Their Association with Clinicopathological Features. Clin. Transl. Oncol. 2015, 17, 779–787. [Google Scholar] [CrossRef]
- Kan, C.W.S.; Hahn, M.A.; Gard, G.B.; Maidens, J.; Huh, J.Y.; Marsh, D.J.; Howell, V.M. Elevated Levels of Circulating MicroRNA-200 Family Members Correlate with Serous Epithelial Ovarian Cancer. BMC Cancer 2012, 12, 627. [Google Scholar] [CrossRef]
- Oliveira, D.N.P.; Carlsen, A.L.; Heegaard, N.H.H.; Prahm, K.P.; Christensen, I.J.; Høgdall, C.K.; Høgdall, E.V. Diagnostic Plasma MiRNA-Profiles for Ovarian Cancer in Patients with Pelvic Mass. PLoS ONE 2019, 14, e0225249. [Google Scholar] [CrossRef] [PubMed]
- Toiyama, Y.; Hur, K.; Tanaka, K.; Inoue, Y.; Kusunoki, M.; Boland, C.R.; Goel, A. Serum MiR-200c Is a Novel Prognostic and Metastasis-Predictive Biomarker in Patients with Colorectal Cancer. Ann. Surg. 2014, 259, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Stevic, I.; Müller, V.; Ni, Q.; Oliveira-Ferrer, L.; Pantel, K.; Schwarzenbach, H. Exosomal MicroRNAs as Tumor Markers in Epithelial Ovarian Cancer. Mol. Oncol. 2018, 12, 1935–1948. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Choi, M.C.; Jeong, J.-Y.; Hwang, S.; Jung, S.G.; Joo, W.D.; Park, H.; Song, S.H.; Lee, C.; Kim, T.H.; et al. Serum Exosomal MiRNA-145 and MiRNA-200c as Promising Biomarkers for Preoperative Diagnosis of Ovarian Carcinomas. J. Cancer 2019, 10, 1958–1967. [Google Scholar] [CrossRef]
- Shen, L.; Chen, G.; Xia, Q.; Shao, S.; Fang, H. Exosomal MiR-200 Family as Serum Biomarkers for Early Detection and Prognostic Prediction of Cholangiocarcinoma. Int. J. Clin. Exp. Pathol. 2019, 12, 3870–3876. [Google Scholar]
- Hulstaert, E.; Morlion, A.; Levanon, K.; Vandesompele, J.; Mestdagh, P. Candidate RNA Biomarkers in Biofluids for Early Diagnosis of Ovarian Cancer: A Systematic Review. Gynecol. Oncol. 2021, 160, 633–642. [Google Scholar] [CrossRef]
- Hydbring, P.; De Petris, L.; Zhang, Y.; Brandén, E.; Koyi, H.; Novak, M.; Kanter, L.; Hååg, P.; Hurley, J.; Tadigotla, V.; et al. Exosomal RNA-Profiling of Pleural Effusions Identifies Adenocarcinoma Patients through Elevated MiR-200 and LCN2 Expression. Lung Cancer 2018, 124, 45–52. [Google Scholar] [CrossRef]
- Gebert, L.F.R.; MacRae, I.J. Regulation of MicroRNA Function in Animals. Nat. Rev. Mol. Cell Biol. 2019, 20, 21–37. [Google Scholar] [CrossRef]
- Zhao, Y.; Qin, F.; Han, S.; Li, S.; Zhao, Y.; Wang, H.; Tian, J.; Cen, X. MicroRNAs in Drug Addiction: Current Status and Future Perspectives. Pharmacol. Ther. 2022, 108215. [Google Scholar] [CrossRef]
- Jouza, M.; Bohosova, J.; Stanikova, A.; Pecl, J.; Slaby, O.; Jabandziev, P. MicroRNA as an Early Biomarker of Neonatal Sepsis. Front. Pediatr. 2022, 10, 854324. [Google Scholar] [CrossRef]
- Gao, Y.-N.; Zhang, Y.-Q.; Wang, H.; Deng, Y.-L.; Li, N.-M. A New Player in Depression: MiRNAs as Modulators of Altered Synaptic Plasticity. Int. J. Mol. Sci. 2022, 23, 4555. [Google Scholar] [CrossRef]
- Żurawek, D.; Turecki, G. The MiRNome of Depression. Int. J. Mol. Sci. 2021, 22, 11312. [Google Scholar] [CrossRef]
- Hu, C.; Liang, X.; Fang, S.; Xu, L.; Gong, M.; Wang, Y.; Bi, Y.; Hong, S.; He, Y. ATRA Induces the Differentiation of Hepatic Progenitor Cells by Upregulating MicroRNA-200a. In Vitro Cell Dev. Biol. Anim. 2019, 55, 713–722. [Google Scholar] [CrossRef]
- Milevskiy, M.J.G.; Gujral, U.; Del Lama Marques, C.; Stone, A.; Northwood, K.; Burke, L.J.; Gee, J.M.W.; Nephew, K.; Clark, S.; Brown, M.A. MicroRNA-196a Is Regulated by ER and Is a Prognostic Biomarker in ER+ Breast Cancer. Br. J. Cancer 2019, 120, 621–632. [Google Scholar] [CrossRef]
- Nuñez-Olvera, S.I.; Puente-Rivera, J.; Ramos-Payán, R.; Pérez-Plasencia, C.; Salinas-Vera, Y.M.; Aguilar-Arnal, L.; López-Camarillo, C. Three-Dimensional Genome Organization in Breast and Gynecological Cancers: How Chromatin Folding Influences Tumorigenic Transcriptional Programs. Cells 2021, 11, 75. [Google Scholar] [CrossRef]
- Kang, S.Y.; Kim, D.G.; Kim, H.; Cho, Y.A.; Ha, S.Y.; Kwon, G.Y.; Jang, K.-T.; Kim, K.-M. Direct Comparison of the Next-Generation Sequencing and ITERT PCR Methods for the Diagnosis of TERT Hotspot Mutations in Advanced Solid Cancers. BMC Med. Genom. 2022, 15, 25. [Google Scholar] [CrossRef]
- Lim, G.X.Y.; Yeo, M.; Koh, Y.Y.; Winarni, T.I.; Rajan-Babu, I.-S.; Chong, S.S.; Faradz, S.M.H.; Guan, M. Validation of a Commercially Available Test That Enables the Quantification of the Numbers of CGG Trinucleotide Repeat Expansion in FMR1 Gene. PLoS ONE 2017, 12, e0173279. [Google Scholar] [CrossRef]

| miRNA | Target Gene | Function in Development | References |
|---|---|---|---|
| miR-200a/-200b | TET3 | Olfactory-mediated behaviors and globose basal cell proliferation and differentiation in the mouse main olfactory epithelium (MOE) | [40] |
| miR-200a/-200b/-429 | Sox2 | Taste bud formation | [41] |
| miR-200c | Sox-1 | Neural crest cell migration | [42] |
| BMI | Regulating self-renewal and differentiation of stem cells | [43] | |
| Vldlr | FGFR-mediated epithelial end bud proliferation during branching morphogenesis | [44] | |
| miR-141/-200c | noggin | Epithelial cell differentiation and tooth development | [45] |
| All members of the miR-200 family | ZEB1 | Differentiation of human embryonic stem cells into hepatocytes | [46] |
| ZEB2 | Promote late steps of postnatal forebrain neurogenesis | [47] | |
| ZEB1, ZEB2, PTCH/GLI | Endometrial development of embryo implantation | [48] | |
| Foxg1 | Olfactory receptor neuron differentiation, extension and connectivity of the olfactory axons, migration of the GnRH neurons | [49] |
| Disease | miRNA | Target Gene | Function in Neurodegenerative Diseases | References |
|---|---|---|---|---|
| Alzheimer’s disease | miR-141 | SIRT1 | Promote Aβ-induced neuronal apoptosis | [69] |
| miR-200a | [70] | |||
| miR-200b/-429 | APP | High expression of APP correlating with accelerated accumulation of the Aβ in brain and take part in the progression of AD | [71,72] | |
| miR-200b/c | S6K1 | Reduction in Aβ secretion and/or Aβ-induced spatial memory impairment by promoting activation of the insulin signaling pathway | [73] | |
| Parkinson’s disease | miR-200a | SIRT | Involved in DA neurons cell death via P53 and FOXO signaling pathways as a possible reason for PD pathogenesis | [74] |
| miR-141 | Induce neuronal apoptosis and oxidative stress | [75] | ||
| Amyotrophic lateral sclerosis | miR-141 | FUS, EWS, TAF15 | Involved in the differentiation of neuronal cells | [76] |
| miR-200c | FUS | Promote miR-200c-mediated gene silencing | [77] |
| miRNA | Target Gene | Mechanism Affected | Result | Cancer Types | References |
|---|---|---|---|---|---|
| miR-141, -200a | p38α | Response to oxidative stress | Paclitaxel sensitivity | Ovarian cancer | [159,195] |
| miR-200a | DUSP6 | ERK signaling | Promotes drug resistance to 5-FU, doxorubicin, and cisplatin | Hepatocellular carcinoma | [194] |
| TP53INP1 | Cell cycle arrest and apoptosis | Resistance to chemotherapy | Breast cancer | [192,193] | |
| YAP1 | Hippo signaling pathway; cell proliferation and suppression of apoptosis | Resistance to chemotherapy | Breast cancer | [192,196] | |
| miR-200b | MEOSIN | Organization of cytoskeleton (actin filaments) | Remodeling of cytoskeleton independent of ZEB1/miR-200 axis through a moesin-dependent pathway | Breast cancer | [141] |
| miR-200c | BMI1 | Regulation of cell cycle, stem cell self-renewal | Alteration of stem cell functionality | Breast cancer | [43] |
| FHOD1/PPM1F | Organization of cytoskeleton (actin filaments) | Remodeling of cytoskeleton independent of ZEB1/miR-200 axis. Regulation of stress fiber formation; repression of migration and invasion. | [142] | ||
| MYLK, TKS5 | Remodeling of cytoskeleton dependent of ZEB1/miR-200 axis. Invasive potential, formation of invadopodia. | [140] | |||
| TUBB3 | Organization of cytoskeleton (microtubuli) | Increased sensitivity to microtubule-binding chemotherapeutic agents (paclitaxel and others) | Ovarian cancer, endometrial cancer | [125] | |
| miR-200s | FOG2 | PI3K/AKT pathway | Survival and proliferation | Lung cancer | [195] |
| Jag1, Jag2, Maml2, Maml3 | Notch signaling pathway | Suppression of cell proliferation and metastasis | Pancreatic and lung adenocarcinoma and basal type of breast cancer | [137,138,139] | |
| KEAP1 | Keap1/Nrf2 signaling pathway | Oxidative stress response | Breast cancer and ovarian cancer | [197,198] | |
| SEC23A | Cancer cell secretome | Targeting secretion of metastasis-suppressive proteins; influencing tumor microenvironment; promoting metastatic colonization | Breast cancer | [168,169] | |
| ZEB1 and ZEB2 | EMT inducing transcription factors; repression of E-Cadherin | Repression of EMT | Non-small cell lung cancer and breast cancer | [130] | |
| Pancreatic cancer, colorectal cancer, and breast cancer | [131] | ||||
| Breast cancer | [132,133] | ||||
| NCI60 panel of cancer cell lines | [134] |
| Sample Used | Method of Detection | miRNA Detected | Pattern of Expression | Cancer | References |
|---|---|---|---|---|---|
| Ascitic fluid | RT-qPCR | All members of the miR-200 family | Upregulated | Ovarian cancer | [211] |
| Serum/plasma | RT-qPCR | All members of the miR-200 family | Upregulated | Ovarian cancer | [212] |
| miR-200a, -200b & -200c | [213,214,215,216,217] | ||||
| miR-200c | [218] | ||||
| Serum | RT-qPCR | miR-200c | Upregulated | Colorectal cancer | [219] |
| Serum exosomes | Microarray | miR-200b | Upregulated | Ovarian cancer | [220] |
| RT-qPCR | miR-200c | [221] | |||
| Plasma and exosomes from tumor-draining mesenteric vein | RT-qPCR | All members of the miR-200 family | Upregulated | Colon cancer | [207] |
| Serum | RT-qPCR | miR-141, -200a, -200b, and -200c | Upregulated | Cholangiocarcinoma | [222] |
| Serum exosomes | RT-qPCR | miR-200b, and -200c | Upregulated | Pancreatic ductal adenocarcinoma | [208] |
| Serum | TaqMan low density array | miR-200a, -200b, and -200c | Upregulated | Breast cancer | [204,205] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sundararajan, V.; Burk, U.C.; Bajdak-Rusinek, K. Revisiting the miR-200 Family: A Clan of Five Siblings with Essential Roles in Development and Disease. Biomolecules 2022, 12, 781. https://doi.org/10.3390/biom12060781
Sundararajan V, Burk UC, Bajdak-Rusinek K. Revisiting the miR-200 Family: A Clan of Five Siblings with Essential Roles in Development and Disease. Biomolecules. 2022; 12(6):781. https://doi.org/10.3390/biom12060781
Chicago/Turabian StyleSundararajan, Vignesh, Ulrike C. Burk, and Karolina Bajdak-Rusinek. 2022. "Revisiting the miR-200 Family: A Clan of Five Siblings with Essential Roles in Development and Disease" Biomolecules 12, no. 6: 781. https://doi.org/10.3390/biom12060781
APA StyleSundararajan, V., Burk, U. C., & Bajdak-Rusinek, K. (2022). Revisiting the miR-200 Family: A Clan of Five Siblings with Essential Roles in Development and Disease. Biomolecules, 12(6), 781. https://doi.org/10.3390/biom12060781







