Abstract
Mitochondrial function is dependent on molecular chaperones, primarily due to their necessity in the formation of respiratory complexes and clearance of misfolded proteins. Heat shock proteins (Hsps) are a subset of molecular chaperones that function in all subcellular compartments, both constitutively and in response to stress. The Hsp90 chaperone TNF-receptor-associated protein-1 (TRAP1) is primarily localized to the mitochondria and controls both cellular metabolic reprogramming and mitochondrial apoptosis. TRAP1 upregulation facilitates the growth and progression of many cancers by promoting glycolytic metabolism and antagonizing the mitochondrial permeability transition that precedes multiple cell death pathways. TRAP1 attenuation induces apoptosis in cellular models of cancer, identifying TRAP1 as a potential therapeutic target in cancer. Similar to cytosolic Hsp90 proteins, TRAP1 is also subject to post-translational modifications (PTM) that regulate its function and mediate its impact on downstream effectors, or ‘clients’. However, few effectors have been identified to date. Here, we will discuss the consequence of TRAP1 deregulation in cancer and the impact of post-translational modification on the known functions of TRAP1.
Keywords:
TRAP1; Hsp90; chaperone; post-translational modification; cancer; mitochondria; metabolism; Warburg effect 1. Introduction
Molecular chaperones of the heat shock protein-90 (Hsp90) family are involved in signal integration and the cellular stress response. These chaperones mediate cell signaling through the stabilization and activation of their substrate proteins, known as clients (https://www.picard.ch/downloads/Hsp90interactors.pdf, accessed 28 February 2022) [1]. The Hsp90 chaperone function is coupled to the ability to hydrolyze ATP, and chaperone activity can be precisely regulated by a heterogeneous group of proteins known as co-chaperones [2], as well as a diverse array of post-translational modifications (PTM) [3].
TNF-receptor-associated protein-1 (TRAP1) is the mitochondrial-dedicated Hsp90 family member and is localized to the mitochondrial matrix, inner mitochondrial membrane, and the intermembrane space [4,5,6]. TRAP1 was first identified through its interaction with the intracellular domain of the Type I TNF receptor [7], and early characterization of TRAP1 demonstrated ATP-binding ability and sensitivity to ATP-competitive Hsp90 inhibitors [8]. Despite this, TRAP1 was unable to form complexes with known cytosolic Hsp90 co-chaperones, nor could it promote the maturation of Hsp90 client proteins, suggesting a distinct mechanism of action for TRAP1 [8].
From this time, work has concentrated on the impact of TRAP1 on cellular processes, however identification of TRAP1 effectors and regulatory mechanisms of TRAP1 expression and activity are critical to understanding its biological function. TRAP1 has an established role as a master regulator of metabolic flux, and a large body of evidence has demonstrated that TRAP1 expression serves to suppress oxidative phosphorylation [9,10,11]. Further, TRAP1 also contributes to cell survival through complex formation with cyclophilin D (CypD), which regulates the opening of the permeability transition pore (PTP) [12]. These two known roles suggest a critical function for TRAP1 in maintaining cellular homeostasis [13]. Despite the critical importance of TRAP1 to these processes, the molecular mechanisms of TRAP1 function remain largely unresolved. Here, we will discuss recent advances in understanding the mechanisms of TRAP1 regulation, the impact of this regulation on TRAP1 function and downstream cellular processes, and the role of TRAP1 in cancer.
2. Structural Basis of TRAP1 Activity
Hsp90 family chaperones are characterized by their dimeric structure. Each of the two protomers are composed of an amino-terminal ATP-binding domain, followed by a middle domain, the primary interface for client interaction, and a C-terminal domain that allows constitutive dimerization of the protomers [14]. Hsp90 chaperone activity is coupled to its ability to hydrolyze ATP [15,16]. The ‘chaperone cycle’ begins with ATP binding to the ‘open’ conformation of Hsp90, followed by transient dimerization of the N-terminal domains of each protomer and ATP hydrolysis, and subsequent release of mature client proteins and regeneration of the ‘open’ Hsp90 dimer [17]. TRAP1 is broadly structurally similar to cytosolic Hsp90, with some notable exceptions, including a cleavable N-terminal mitochondrial localization signal and an N-terminal extension or ‘strap’ that stabilizes the ‘closed’ conformation of TRAP1 [18,19]. Asymmetrical post-translational modification and co-chaperone binding are important determinants of Hsp90 molecular chaperone function [18,20,21,22,23,24]. Interestingly, TRAP1 dimers are inherently asymmetric, and uniquely composed of one ‘straight’ and one ‘buckled’ protomer, with the buckled protomer demonstrating increased rates of ATP hydrolysis [25] (Figure 1). Recently, structural and cell-based studies have described a tetrameric form of TRAP1 induced in response to dysregulation of oxidative metabolism, although the impact of this TRAP1 state on its activity is as yet unknown [26]. Interestingly, whether TRAP1 ATPase activity is essential for the entire scope of its biological role also remains an open question [26].
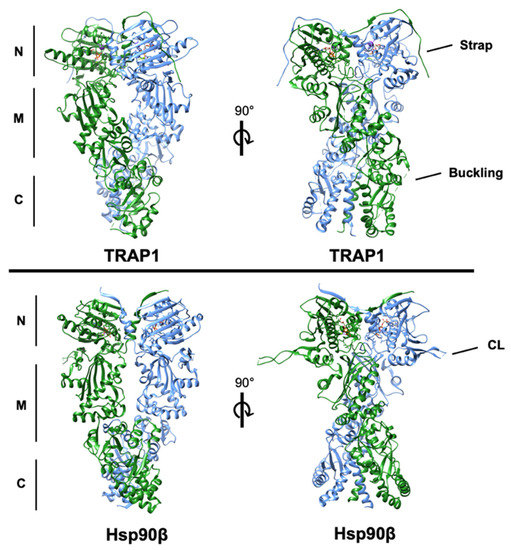
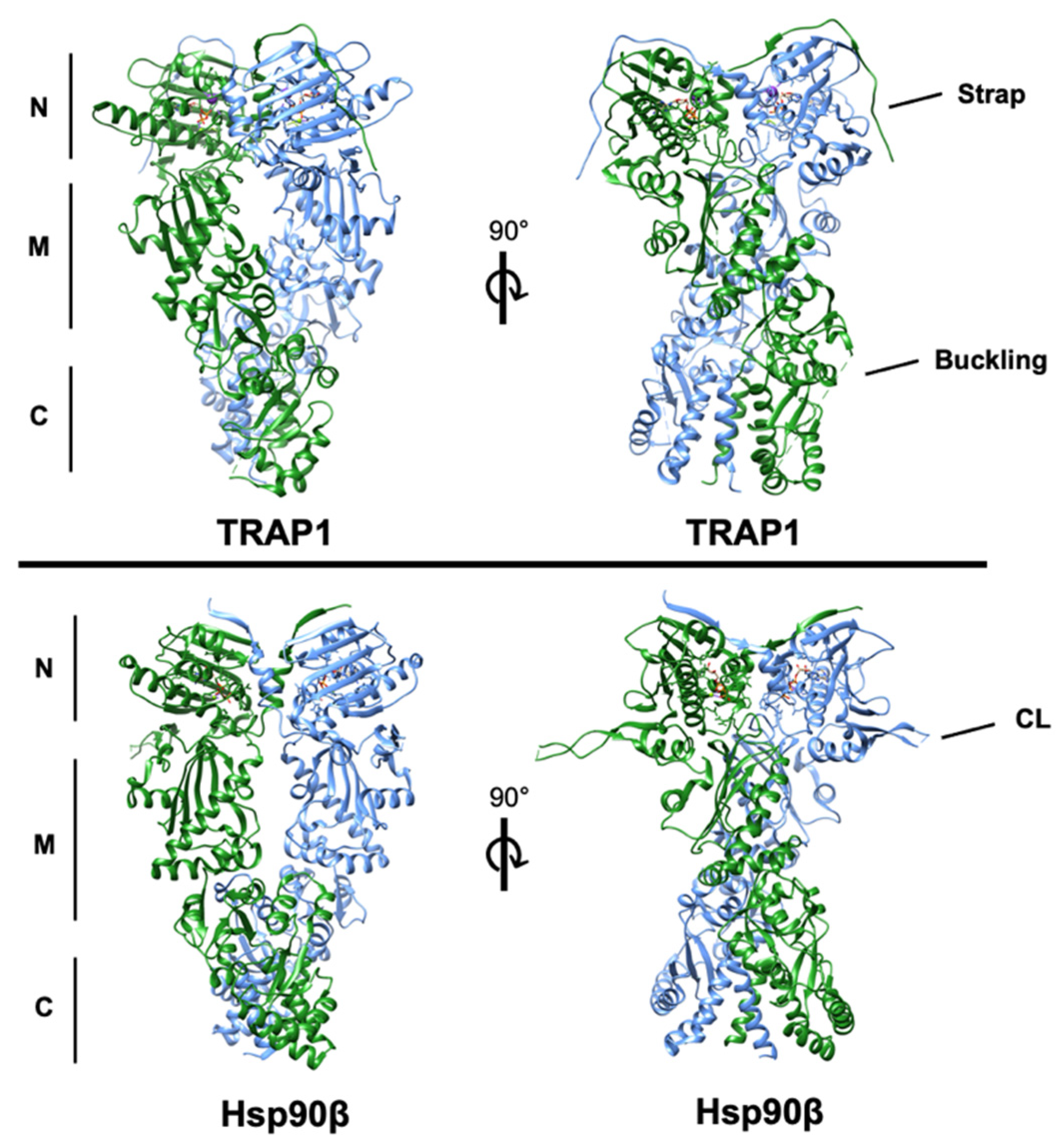
Figure 1.
Structures of human TRAP1 (PDB: 6xg6) and human Hsp90β (PDB: 5fwp) bound to nucleotide with the conserved N-, middle-, and C-domains denoted. One protomer of each is colored blue and the second is colored green. The regulatory N-terminal extension (strap) of each TRAP1 protomer can be observed overlapping the opposite protomer. The region of TRAP1 near the M-C boundary that ‘buckles’ during conformational rearrangement is incompletely resolved in the structure. Additionally, the resolved residues of the charged linker domain (CL) of cytosolic Hsp90, which is absent in TRAP1, are labeled in the lower right quadrant.
3. Impact of TRAP1 on Cancer Metabolism
Controversially, TRAP1 has alternately been characterized as an oncogene and tumor suppressor, and it has been suggested that TRAP1 is essential for malignant transformation of cells but dispensable at later stages of tumor development [6,27]. Despite this controversy, much of the literature supports the idea that TRAP1 regulates metabolic transformation during tumorigenesis, TRAP1 is overexpressed in many cancers, and TRAP1 attenuation is detrimental to tumor cell survival [28,29,30,31,32,33]. It may be more appropriate to suggest that, similar to cytosolic Hsp90, many cancers may be ‘addicted’ to TRAP1 [34,35,36]. In fact, multiple pathways in which TRAP1 activity can drive tumorigenesis have been described (Figure 2) and will be reviewed in the following section.
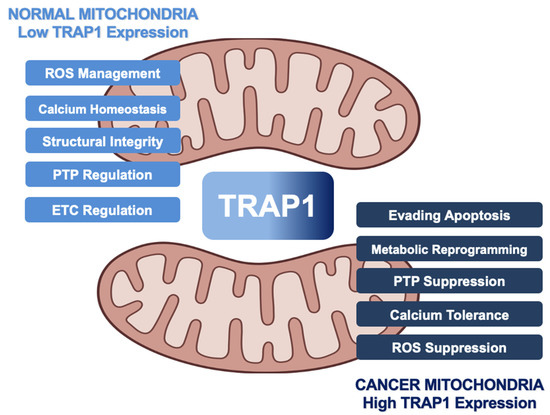
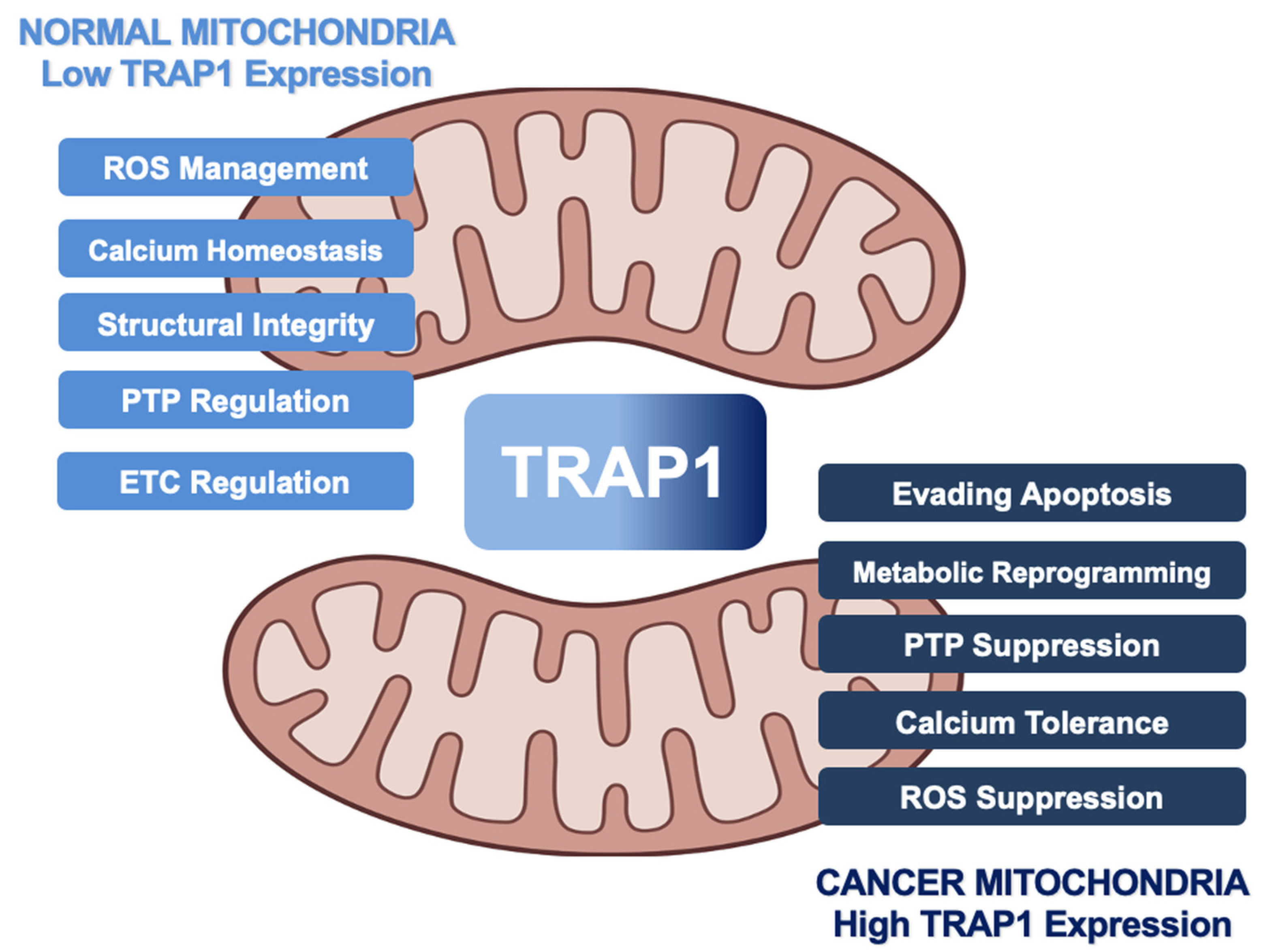
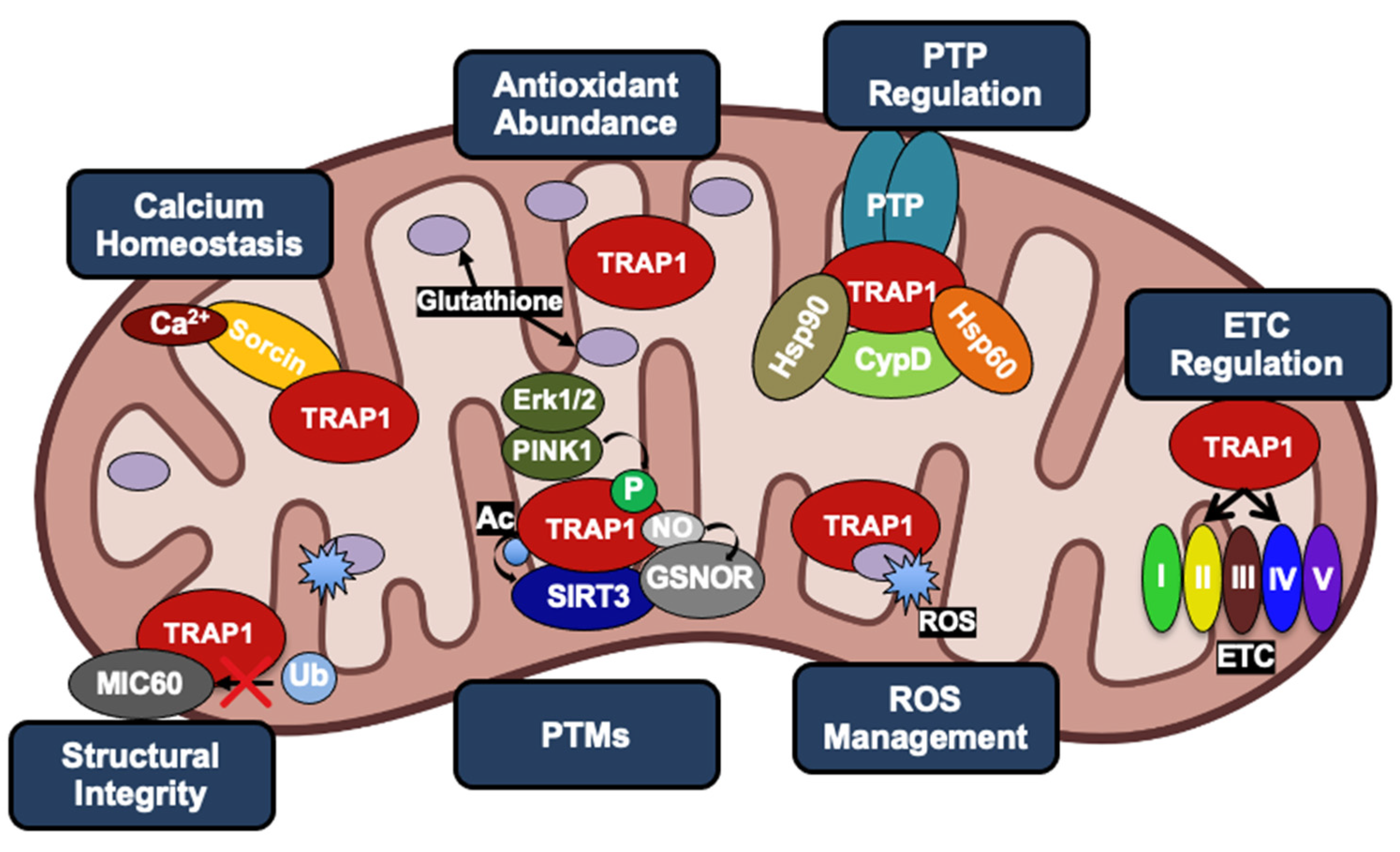
Figure 2.
Role of human TRAP1 in mitochondria of normal cells and cancer cells. Normal expression levels (light blue) lead to TRAP1 regulation of ROS and calcium levels, integrity of cristae, function of ETC, and oversight of the PTP. As TRAP1 expression increases (dark blue), mitochondria lose calcium sensitivity, downregulate ROS, and prevent PTP opening, leading to metabolic reprogramming and evasion of apoptosis in cancer.
3.1. Metabolic Regulation
The cellular energy currency adenosine triphosphate (ATP) is generated as a consequence of the complete oxidation of glucose to CO2 and H2O, and each molecule of glucose can maximally result in 36–38 ATP molecules [37]. Normal cells produce ATP primarily through cellular respiration, which describes a process in which glucose metabolism by glycolysis is coupled to the tricarboxylic acid cycle (TCA). Concurrent mitochondrial electron transport generates the electrochemical gradient that provides the force by which ATP is disseminated throughout the cell [38]. ATP generation is highly dysregulated in cancers, and many cancer subtypes supplement their ATP supply by upregulating cytosolic glycolysis, simultaneously generating additional ATP driven by the terminal fermentation of pyruvate to lactate [39]. This hyperactive glycolytic phenotype is known as the Warburg effect, and serves to support the accelerated growth of cancers through the increased synthesis of intermediates for anaplerotic metabolism and hypertrophy [40,41]. The phenotypic manifestations of metabolic dysregulation are variable and dependent on cell type and genotype, and many of the details and nuances of this differential regulation remain obscured.
Few specific biological roles and binding partners have been described for TRAP1, despite the broad understanding of its impact on metabolic flux. Two of the few described bona fide clients of TRAP1 however are subunits of electron transport chain (ETC) complexes, Complex II components succinate dehydrogenase subunit A/B (SDHA/B) [42,43,44,45], and Complex IV cytochrome c oxidase subunit 2 (COXII) [6,46,47]. Complex II/SDH is an iron–sulfur cluster-containing protein complex that functions to transfer electrons from succinate to coenzyme Q10-ubiquinone (Complex III) [48]. In agreement with the understanding of Hsp90 function, TRAP1 maintains SDH in a partially unfolded state [49], and TRAP1 inhibition releases active SDH, leading to an increase in its activity [27,44,50,51,52]. Further, SDH activity [44,53,54] and the oxygen consumption rate [6,55] are inversely correlated with TRAP1 expression, implicating TRAP1 in promoting the Warburg effect [56]. Notably, SDH also oxidizes succinate to fumarate and thus integrates the TCA cycle and the ETC, indicative of the broad influence of TRAP1 on mitochondrial metabolism [56,57,58].
Complex IV of the ETC converts molecular oxygen to water, and in doing so enacts the final step in generating the electrochemical gradient that supports ATP production by Complex V (ATP synthase) [59]. COXII is a downstream effector of TRAP1 function in the regulation of apoptosis, and TRAP1 regulates COXII expression [47] and activity [6]. As downregulation or inhibition of TRAP1 has been shown to destabilize COXII [46,50] and deletion of TRAP1 was associated with decreased COXIV subunit levels [60], it is possible that TRAP1 chaperoning of COXII/IV is mechanistically similar to SDHA/B. TRAP1 has also been shown to interact with the Complex V subunit ATPB, although little is known about this interaction [27].
Mitochondrial respiration drives the production of reactive oxygen species (ROS) and is responsible for most cellular ROS (Figure 3) [61]. In considering the role of TRAP1 in chaperoning SDH and COXII, TRAP1-mediated regulation of mitochondrial respiration suppresses ROS production [62], thereby contributing to the regulation of redox homeostasis, metabolic flux, and mitochondrial apoptosis.
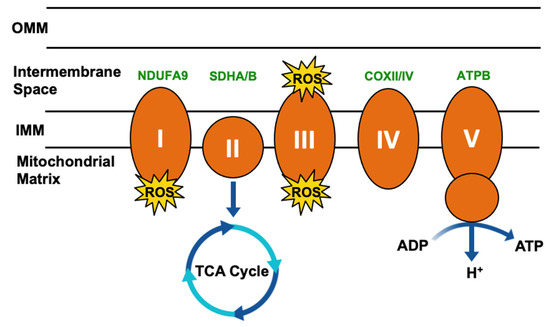
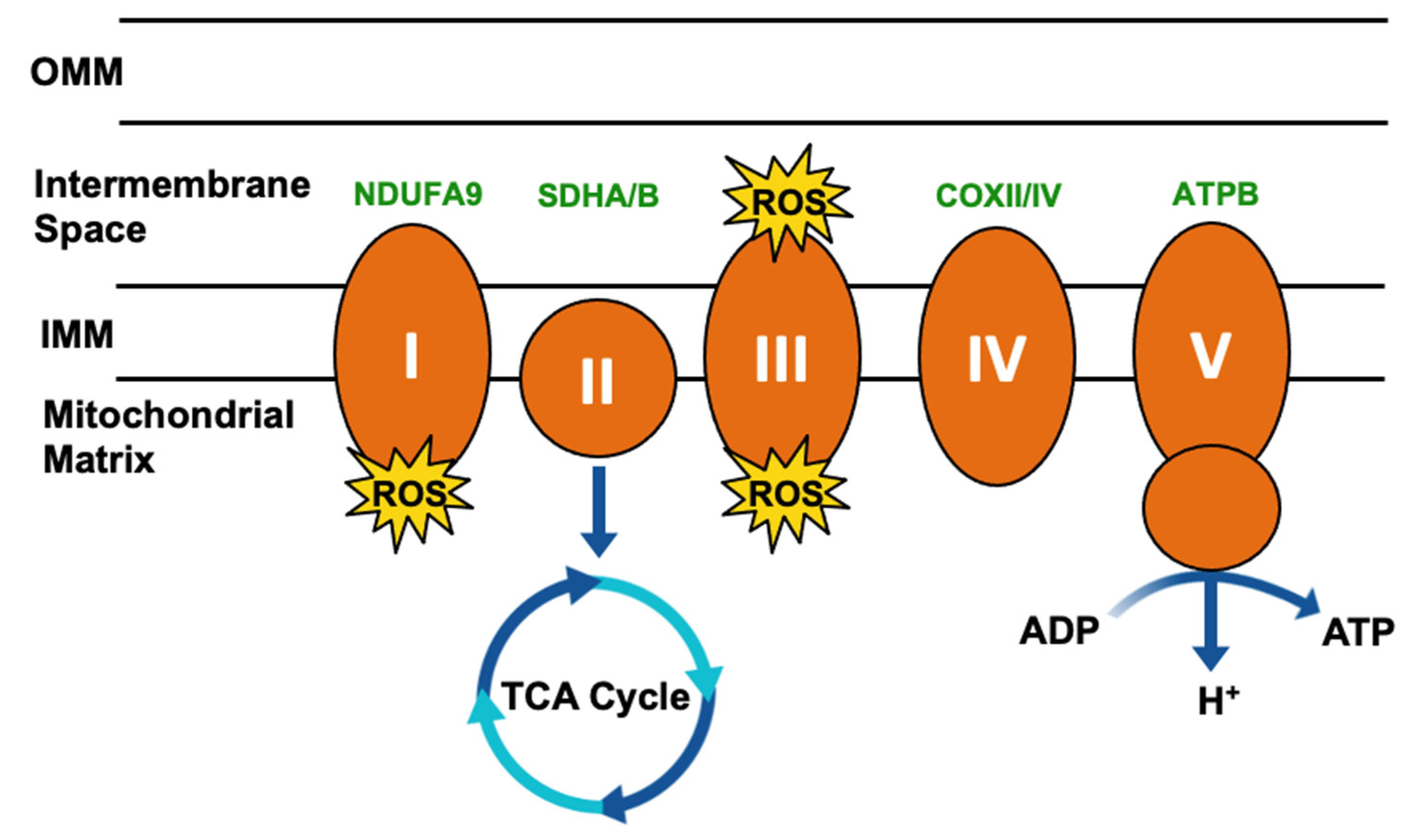
Figure 3.
Simplified mitochondrial respiration schematic. Electron transport chain (ETC) complexes (I–V) are represented by orange ovals, and reactive oxygen species (ROS) generated as a byproduct of Complex I and III activity is represented by yellow starbursts. Succinate dehydrogenase (SDH)/Complex II connects the ETC to the tricarboxylic acid (TCA) cycle. TRAP1 interactors involved in this process have been highlighted in green.
3.2. Contribution to Tumorigenesis
Cancer-associated increases in TRAP1 expression suggest a role for TRAP1 in oncogenesis [30,63,64]. Indeed, TRAP1 deletion delayed tumor formation in a mouse model of breast cancer, providing direct evidence of the role of TRAP1 in tumor initiation [65]. Further, TRAP1-mediated SDH inhibition leads to accumulation of the oncometabolite succinate [58]. Increased succinate inhibits the activity of prolyl hydroxylases, which are responsible for the hydroxylation of the transcription factor hypoxia inducible factor (HIF1α), a prerequisite for recognition by the VHL-dependent E3-ubiquitin ligase machinery [66]. Succinate-dependent HIF1α stabilization and activation promotes a well-established glycolytic transcriptional program [67], demonstrating yet another function of TRAP1 in the regulation of cancer-associated metabolic dysregulation.
TRAP1 expression was found to be elevated in aggressive pre-neoplastic lesions in a rat model of hepatocarcinogenesis [68]. The master antioxidant transcription factor NRF2 was also activated in this model, and given the established role of TRAP1 in regulating intracellular ROS, TRAP1 likely participates in NRF2-driven ROS mitigation during tumor development [68]. NRF2 inhibition led to decreased TRAP1 levels independent of TRAP1 transcription [68], suggesting that post-translational regulation is essential for sustained TRAP1 expression in pre-cancerous and cancerous cells. Interestingly, pentose phosphate pathway (PPP) flux was found to be increased in this model, and was determined to be a consequence of elevated citrate synthase activity in aggressive pre-neoplastic lesions [68]. Citrate accumulation inhibits downstream metabolic enzymes phosphofructokinase and SDH and activates the anaplerotic PPP [69]. This increase in citrate synthase activity was alleviated following TRAP1 knockdown or inhibition, suggesting that citrate synthase may also be a TRAP1 client [68].
Cell cycle dysregulation is a well-established driver of tumorigenesis [70]. TRAP1 impacts the cell cycle through regulation of protein quality control in cooperation with the proteasome regulator TBP7 [71,72]. Loss of the TRAP1/TBP7 machinery leads to increased ubiquitination and degradation of the G2-M checkpoint proteins CDK1 and MAD2 and dysregulation of mitotic entry [72]. However, whether TBP7 is a client or perhaps even the first co-chaperone of TRAP1 remains to be seen.
Taken together, these data describe multiple mechanisms through which TRAP1 dysregulation can impact cellular metabolic flux and, potentially, tumorigenesis.
3.3. Evasion of Apoptosis
Mitochondrial involvement in cell death is mediated by the release of cytochrome c [73,74]. Sustained opening of the permeability transition pore (PTP) within the inner mitochondrial membrane (IMM) initiates a series of events that lead to cytochrome c release and apoptosis or necrosis. Upon PTP opening, particles under 1500 Da, such as ions (Ca2+, K+, and H+), water, and other solutes, flood the IMM, causing swelling and unfolding of the cristae and eventual outer mitochondrial membrane (OMM) rupture. Subsequent efflux of cytochrome c through the compromised OMM into the cytosol induces the caspase cascade [75,76]. This sustained PTP opening is known as the mitochondrial permeability transition (PT) [77], and it can be triggered by several mechanisms, including elevated ROS, Ca2+, or inorganic phosphate levels, as well as decreased pH or ATP depletion [78]. Interplay between these elements also plays a role in its regulation, as elevated ROS has been shown to decrease the amount of Ca2+ required to trigger the PTP [76].
TRAP1 attenuation induces opening of the PTP and release of cytochrome c [47], and expression of TRAP1 likely discourages the initiation of apoptosis through two distinct, but potentially overlapping mechanisms: (1) regulation of triggers that signal into the PTP, and (2) direct disruption of the physical mechanism of PTP opening. TRAP1 knockdown has been shown to lead to increased ROS accumulation under oxidative stress [79] and TRAP1 overexpression insulates cells against iron chelation-mediated ROS production [80]. These effects are likely a consequence of both direct and indirect roles of TRAP1 in minimizing ROS generation. TRAP1 is a direct regulator of oxidative phosphorylation through its chaperoning of Complexes II and IV of the ETC [6,44,46] and has an indirect role in quenching existing ROS, as TRAP1 expression is associated with increased levels of the reduced form of the antioxidant glutathione (GSH) [81]. TRAP1-dependent regulation of ROS generation also results in decreased oxidation of the phospholipid cardiolipin. This phospholipid is responsible for the binding of cytochrome c to the inner folds of cristae, and its oxidation results in an increase of free cytochrome c in the inner membrane space that can potentially escape into the cytosol [78].
Furthermore, TRAP1 has been shown to chaperone the calcium-binding protein Sorcin [82]. TRAP1 is also thought to be responsible for Sorcin translocation into the mitochondria, given that Sorcin lacks its own mitochondrial localization sequence [8,82]. Overexpression of Sorcin in neonatal cardiac myocytes has been shown to increase mitochondrial Ca2+ levels, while simultaneously decreasing cytochrome c release, indicating an increase in mitochondrial Ca2+ tolerance [83]. Therefore, the chaperoning of Sorcin by TRAP1 is important for desensitizing the PTP to Ca2+ levels. Understanding this regulation is particularly important for TRAP1, as Ca2+ can replace Mg2+ as a co-factor and induce an increased rate of TRAP1 ATP hydrolysis [84]. TRAP1 has also been shown to decrease ubiquitination of the mitochondrial contact site and cristae organizing system subunit 60 (MIC60) under conditions of extracellular acidosis [85]. MIC60 is a critical component of the protein complex MICOS, which is regarded as the master organizer of the IMM through the formation of contact sites with the outer membrane and maintenance of cristae junctions [86,87]. Thus, TRAP1 regulation of MIC60 contributes to its anti-apoptotic function through the preservation of mitochondrial integrity.
Proposals for the structure of the PTP have gone through various iterations, however the prevailing model is that the PTP is formed by coordinated activities of the adenine nucleotide translocator (ANT) and the F-ATP synthase [88,89,90]. Furthermore, cyclophilin D (CypD) is key to PTP regulation [12,91]. Though its role in this process is controversial, CypD peptidyl-prolyl isomerase activity is required, as is its binding to the mitochondrial peripheral stalk subunit of the F-ATP synthase [63,90,92]. In addition to attenuating the triggers that lead to PTP opening, TRAP1 has been shown to antagonize the opening of the PTP itself. There is a general consensus that TRAP1 accomplishes this by forming a complex with CypD, interfering with the ability of CypD to interact with the PTP [12,63,93] potentially at the peripheral stalk of F-ATP synthase [90].
Further, the mitochondrial chaperones Hsp60 and Hsp90 have been implicated in this process, as their association with CypD also prevents PTP opening; however, the architecture of this complex has yet to be characterized [12,63,93,94,95,96].
4. Post-Translational Regulation of TRAP1
Post-translational modification is critically important to mitochondrial function [97] and has previously been shown to regulate TRAP1, though relatively little is known about individual PTM sites (Table 1, Figure 4) [5,6,98,99]. A comprehensive study of cytosolic Hsp90 has demonstrated the importance of post-translational regulation to Hsp90 chaperone activity (reviewed in [3,100]), and in the absence of certain co-chaperone regulatory proteins, specific PTM events have been shown to functionally recapitulate their activity [101]. This phenomenon may be critically important for TRAP1 biology, as TRAP1 is thought to act without the assistance of co-chaperones [8,10].

Table 1.
Reported PTMs of TRAP1. Paralog identifies conserved residues in Hsp90α. GSNOR—S-nitrosoglutathione reductase, ERK—extracellular signal-regulated kinase.
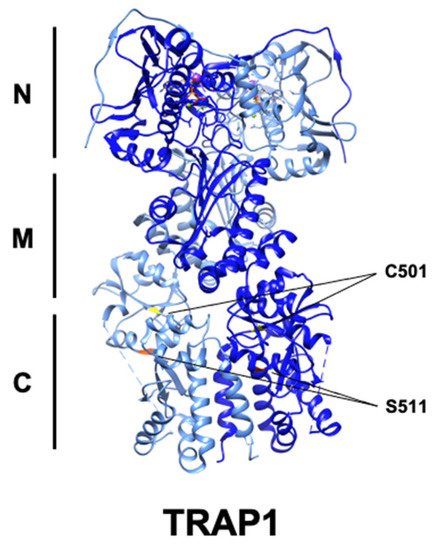
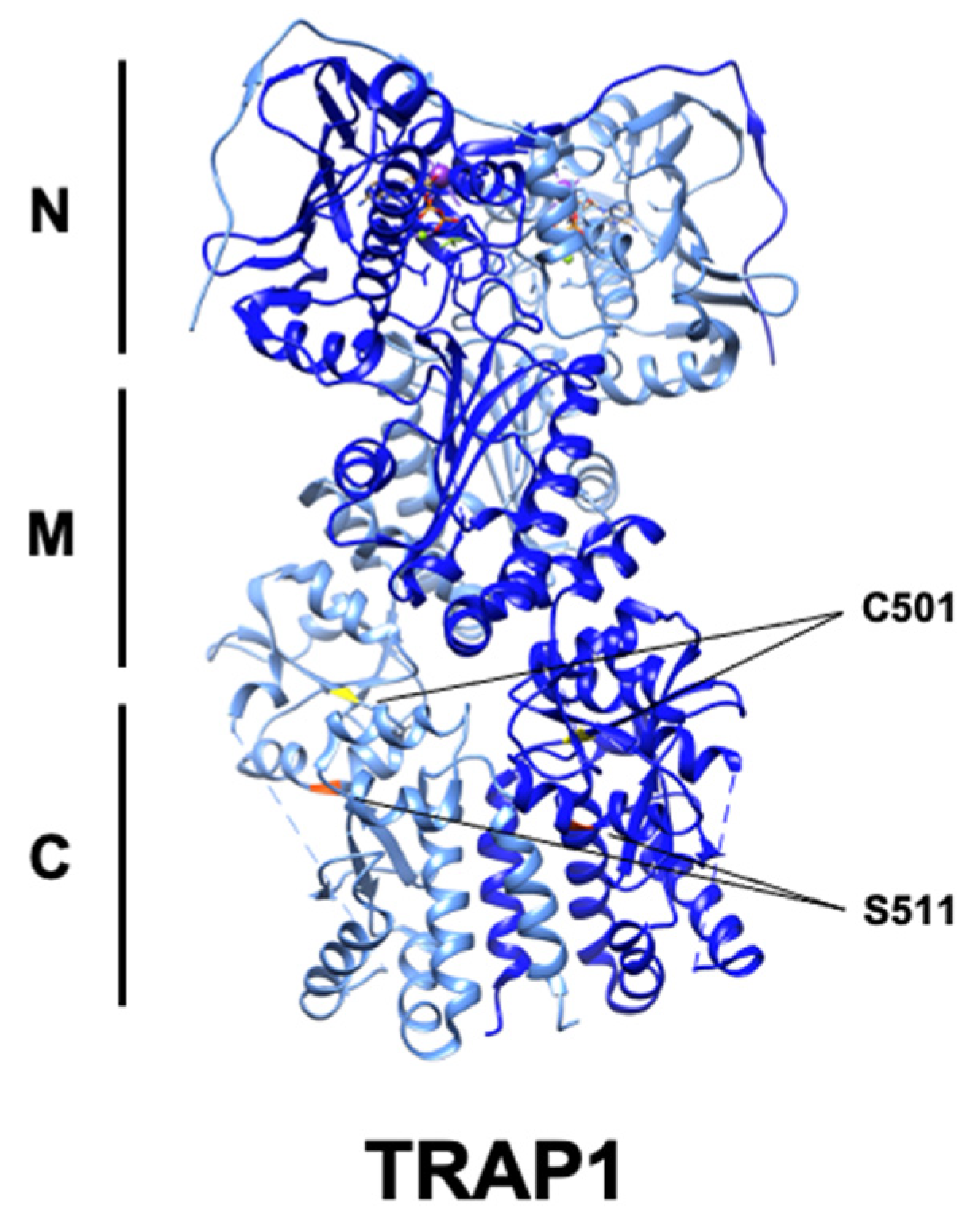
Figure 4.
Ribbon structure of human TRAP1 (PDB: 6xg6) with known PTM sites. C501 (yellow) and S511 (red) are highlighted, while S568 is absent.
4.1. Phosphorylation
PINK1 is a mitochondrially targeted serine/threonine kinase whose mutation and inactivation is linked to Parkinson’s disease [102]. PINK1 activity has previously been shown to be cytoprotective [103], and when exposed to H2O2, cells transfected with siRNA targeting PINK1 showed significant increases in cytochrome c release and apoptosis [5]. TRAP1 was shown to be phosphorylated by PINK1 and mediate PINK1 anti-apoptotic activity, as evidenced by the observation that TRAP1 knockdown sensitized cells to PINK1 attenuation [5,104,105]. Interestingly, TRAP1 inhibition leads to activation of PINK1, suggesting a reciprocal regulatory relationship [106].
TRAP1 has also been shown to interact with the mitochondrial serine protease HTRA2 in Parkinson’s disease [55]. Canonically, HTRA2 participates in mitochondrial and cellular quality control through inhibition of IAPs (inhibitor of apoptosis proteins) and induction of cell death, while loss of HTRA2 is associated with aberrant mitochondrial function and Parkinson’s disease (PD). Overexpression of HTRA2 led to decreased levels of TRAP1, suggesting that HTRA2 may play a role in regulating TRAP1 stability [55]. However, the effect of HTRA2 was independent of its protease activity and the interaction between HTRA2 and TRAP1 was abrogated through treatment with mitochondrial respiratory inhibitors [55]. TRAP1 overexpression is also capable of rescuing mitochondrial dysfunction-associated PINK1 and HTRA2 loss. Interestingly, HTRA2 is also a substrate of PINK1, demonstrating that further work is needed to understand the mechanistic regulation of TRAP1 by HTRA2 and the role of PINK1 in this system.
Neurofibromatosis is caused by mutation and inactivation of the Ras regulatory protein neurofibromin and is characterized by elevated Erk1/2 activity [10]. Active Erk1/2 is associated with TRAP1-SDH in the mitochondria of these cells, and Erk1/2-mediated phosphorylation of TRAP1-S511/S568 strengthens their association, suggestive of a chaperone–client relationship. Association of TRAP1 and SDH decreases SDH activity, leading to accumulation of the oncometabolite succinate [10]. TRAP1 attenuation or loss of phosphorylation at these residues prevents tumor growth, in a succinate-dependent manner [10]. Mitochondrial Erk1/2 was previously shown to antagonize PTP opening [107], perhaps indicating a role for TRAP1 phosphorylation in PTP regulation as well. Taken together, these data suggest that TRAP1 inhibition or combined TRAP1-Erk1/2 targeting may be a viable therapeutic strategy in neurofibromatosis and other cancers.
Interaction with mitochondrially localized c-Src remains the only described TRAP1–tyrosine kinase relationship [6]. Previous work has shown that mitochondrial c-Src is involved in the phosphorylation-mediated activation of ETC Complexes I, II, and IV [108,109]. TRAP1 binds to and maintains c-Src in an inactive state, providing a potential mechanism for TRAP1 suppression of oxidative metabolism and ROS mitigation [6]. Though TRAP1 tyrosine phosphorylation is induced by c-Src expression and abrogated by c-Src inhibition, direct phosphorylation of TRAP1 by c-Src remains to be demonstrated. Taken together, TRAP1 and c-Src play opposing roles in the regulation of mitochondrial metabolism, though the reciprocal impact of c-Src on TRAP1 remains unresolved.
4.2. Acetylation–Deacetylation
Acetylation modulates protein–protein interactions via neutralization of Lys residues and can be reversed by the activity of deacetylases. TRAP1 directly stabilizes one such deacetylase, sirtuin-3 (SIRT3), and augments SIRT3 activity in vitro and in glioma cells [27]. Interestingly, SIRT3 overexpression was also able to rescue the effects of TRAP1 inhibition by the TRAP1 inhibitor gamitrinib [27]. One potential explanation for this observation is that SIRT3-mediated deacetylation of TRAP1 modulates TRAP1 activity or its affinity for gamitrinib, though no direct evidence was reported [27]. SIRT3 knockdown was also shown to increase ROS levels, and SIRT3 overexpression reversed an increase in ROS caused by gamitrinib [27]. Interestingly, attenuation of SIRT3 specifically destabilized TRAP1 substrates NDUFA9 (CI) and SDHB (CII), but not SIRT3 substrates SOD2 and GDH, suggesting that SIRT3-mediated deacetylation of TRAP1 is important for TRAP1 chaperone activity [27]. Interestingly, these interactions were observed in glioblastoma (GBM) cancer stem cells (CSC), which showed a preference for mitochondrial respiration over glycolysis. This work provides a new paradigm for understanding the role of SIRT3 in cancer [110]. Given this context and the known role of both proteins in regulating mitochondrial metabolism, reciprocal regulation of SIRT3 and TRAP1 may provide a positive feedback mechanism that impacts the ability of TRAP1 to chaperone its dependent proteins.
4.3. Nitrosylation
The PTM S-nitrosylation (SNO) is the result of the covalent addition of -NO to the thiol group of cysteine residues [111]. SNO is enzymatically catalyzed by nitrosylases and reversed by the activity of denitrosylases, including S-nitrosoglutathione reductase (GSNOR) [112]. GSNOR is commonly deleted in hepatocellular carcinoma (HCC), and GSNOR-KO mice develop HCC, linking aberrant nitrosylation to cancer [113]. TRAP1-C501-SNO was identified by mass spectrometry [54,114] and this modification was found to decrease TRAP1 ATPase activity, modulate conformational rearrangement, and promote its proteasomal degradation [54,98]. TRAP1 degradation also led to increased SDH activity, in agreement with previous work [44], and sensitized cells to SDH inhibitors, identifying TRAP1-SNO as a predictor of tumor cell response to this class of drugs [54]. It follows that mutation of this residue to TRAP1-C501S provided protection from apoptosis in the presence of nitric oxide donors, demonstrating that disruption of TRAP1-SNO is essential for its anti-apoptotic role [98]. Curiously, however, TRAP1 is overexpressed in many cancers, allowing for the possibility that TRAP1-SNO is context-specific and perhaps also under temporal regulation.
Taken together, PTMs exert influence on TRAP1 through regulating the kinetics of ATP hydrolysis and associated conformational rearrangements, interaction with client proteins, and promoting TRAP1 degradation.
5. Current State of TRAP1 Inhibitor Development
Inhibition of cell metabolism is a re-emerging anti-cancer strategy [115]. TRAP1 control of cellular metabolic flux and mitochondrial apoptosis outlined herein identifies TRAP1 inhibition as a potential anti-cancer therapeutic target. Efforts towards the development of ATP-competitive inhibitors for cytosolic Hsp90 have provided lead compounds for optimization to address the dual challenges of mitochondrial localization and TRAP1 specificity. Conjugation to a chemical scaffold such as the mitochondrial-targeting moiety triphenylphosphonium (TPP) is necessary to provide mitochondrial penetrance [116,117]. Specificity for TRAP1 over Hsp90 may also be a necessary consideration, as well-established Hsp90 ATP-competitive inhibitors cannot differentiate between the ATP-binding pockets, potentially leading to off-target toxicity [33].
5.1. Gamitrinibs
The most widely used mitochondrial Hsp90 inhibitors are gamitrinibs (G), small molecules consisting of the Hsp90 inhibitor 17-allylamino-17-demethoxygeldanamycin (17-AAG) attached to a mitochondrial-targeting moiety such as cyclic guanidinium repeats or TPP (G-G1-4 and G-TPP, respectively) [118]. These gamitrinibs have demonstrably reduced the viability of prostate [91,119,120,121,122], colon [119,123], melanoma [119,124], cervix [122,125], ovary [122], breast [118,119,121,124,125], and glioma cancers [126], particularly glioblastomas [120,124,127,128,129]. Gamitrinibs disrupt the anti-apoptotic effects of TRAP1, as evidenced by decreased mitochondrial membrane potential and increased cytochrome c release in G-TPP-treated PC3 prostate cancer cells [119]. Furthermore, the stability of the sensitive cytosolic Hsp90 client proteins Akt and phospho-Y416-Src was impacted by 17-AAG treatment, but unaffected by G-TPP in PC3 cells, demonstrating the selective targeting of gamitrinibs to the mitochondria [119]. A further consideration is the potential for resistance development, as PC3 cells continuously incubated with 17-AAG eventually became resistant to G-TPP, but not G-G4 [118,119]. This finding potentially suggests that the choice of mitochondrial-targeting moiety may be critically important and not necessarily limited simply to drug transport. Overall, selective TRAP1 inhibition with ATP-competitive gamitrinib derivatives remains a challenge. Further, these data emphasize the importance of understanding effectors of TRAP1 for the identification of potential combinatorial therapeutic targets to augment inhibition of TRAP1-mediated signaling pathways.
5.2. Purine-Scaffold Inhibitors
In addition to 17-AAG, mitochondrial targeting of the purine-scaffold Hsp90 inhibitor PU-H71 has also demonstrated efficacy against TRAP1. A TPP-conjugated derivative of PU-H71 (SMTIN-P01) showed a remarkable ability to target mitochondria over non-conjugated PU-H71 and a slight improvement in cytotoxicity over gamitrinibs [130]. Interestingly, adjustments to the length of the TPP resulted in changes in inhibitor behavior. When the TPP was modified to have a 10-length carbon chain (as opposed to the standard 6-length carbon chain), this so-called SMTIN-C10 induced structural changes to TRAP1 and demonstrated increased inhibition of TRAP1 [52]. SMTIN-C10 was found to bind to an allosteric binding site at E115 in the N-terminal domain of TRAP1, in addition to binding to the ATP pocket, resulting in TRAP1 adopting a closed formation [52]. This long linker approach was adapted for other TRAP1 inhibitors as well, including Mitoquinone. TPP-Mitoquinone has shown utility and specificity by targeting the client-binding middle domain of TRAP1 [117]. Mitoquinone has been demonstrated to have protective properties in various animal models of neurological maladies, such as traumatic brain injury [131], Huntington’s disease [132], amyotrophic lateral sclerosis (ALS) [133], and Alzheimer’s disease [134]. This finding is contradictory to the working model of TRAP1 function, especially considering that TRAP1 downregulation is observed in Alzheimer’s disease patients [135] and its overexpression is protective against oxidative stress in ALS [62]. These results highlight the need to understand the disease-specific contexts of TRAP1 function to identify appropriate disease models for the evaluation of TRAP1 inhibitors.
5.3. New Inhibitors
Since their discovery, Hsp90 inhibitors have primarily targeted the ATP-binding pocket (Figure 5). This is the mechanism of the natural product geldanamycin (GA) [136,137,138] and its derivatives, as well as the first synthetic inhibitor of TRAP1, Shepherdin [139]. Shepherdin was designed by imitating the minimal Hsp90-binding sequence of Survivin (aa 79–87), an anti-apoptotic protein that binds to the N-domain of Hsp90 [140]. Consequently, Shepherdin was also found to disrupt Hsp90-ATP binding with 13 predicted sites of hydrogen bonding in the ATP pocket [139]. Modeling studies based on the structure of Shepherdin identified the small molecule 5-aminoimidazole-4-carboxamide-1-β-D-ribofuranoside (AICAR), a previously characterized AMPK activator [141,142], as a potential Hsp90 inhibitor, though its development as a scaffold for Hsp90 inhibition has not been pursued.
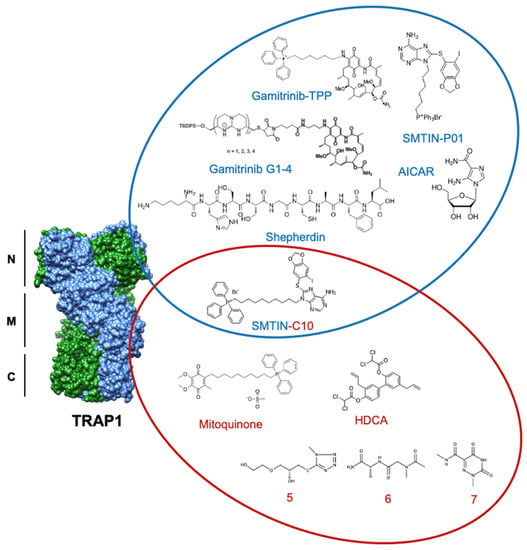
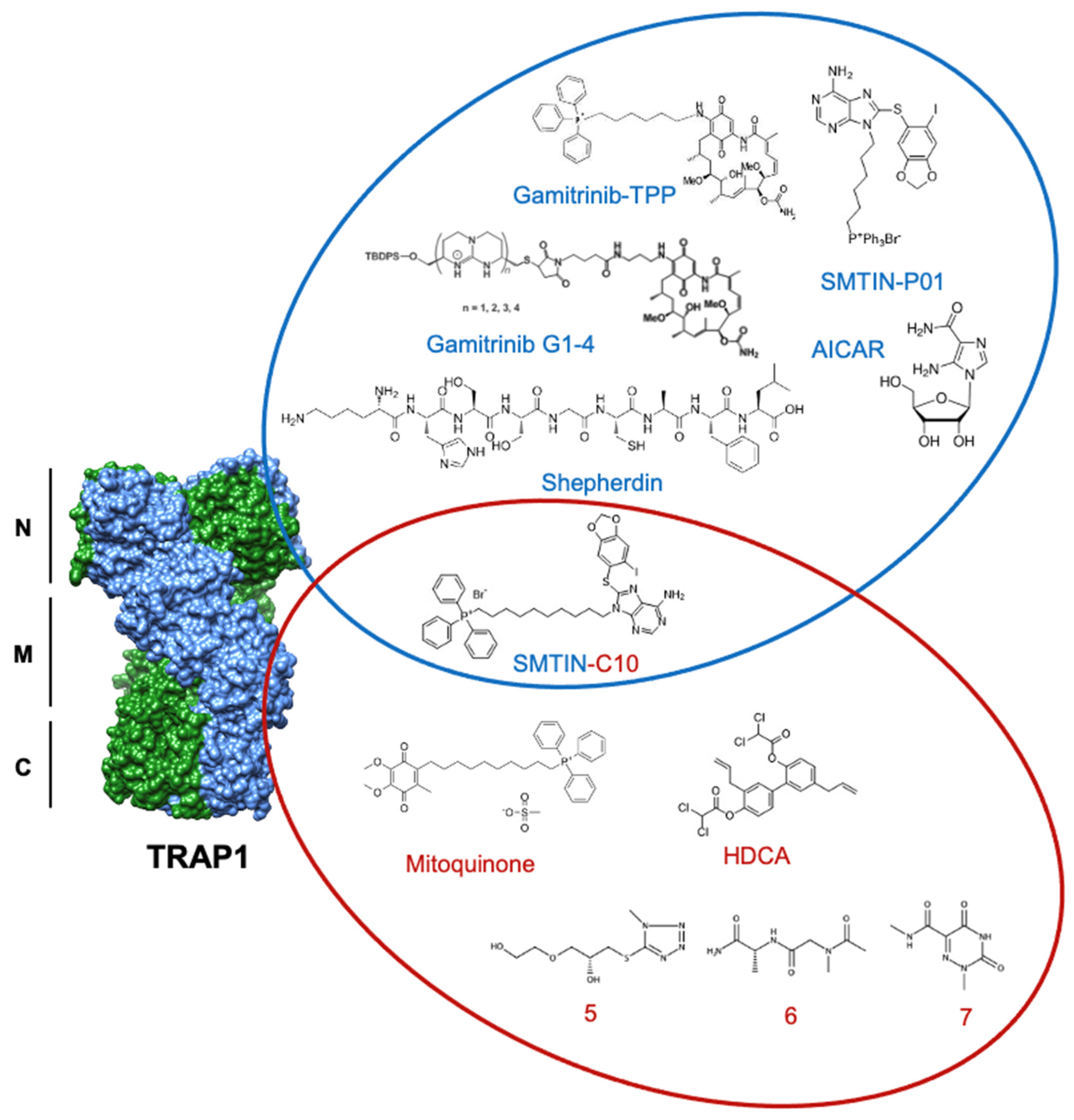
Figure 5.
Structures of discussed TRAP1 inhibitors. ATP-competitive small molecules targeting the N-domain of TRAP1 (PDB: 6xg6) are labeled blue, while allosteric inhibitors, primarily targeting the TRAP1 middle domain, are labeled red. SMTIN-C10 is a bifunctional inhibitor, with elements of both ATP-competitive and allosteric inhibition.
Though ATP-competitive Hsp90 inhibitors are still widely used, an alternative approach in hopes of achieving TRAP1 specificity over other Hsp90 family members has emerged through allosteric targeting. One example of this strategy is honokiol bis-dichloroacetate (HDCA), which is able to specifically inhibit TRAP1 by binding to an allosteric pocket within the middle domain. This pocket has a surface landscape defined by a positively charged region sandwiched between two negatively charged regions that are separated from each other by a large hydrophobic area. HDCA binds in this hydrophobic area and allosterically inhibits TRAP1 ATPase activity, but not that of Hsp90 [43].
Further, computational methods by Sanchez-Martin et al. utilized the unique asymmetry of TRAP1 to identify an allosteric pocket on the straight protomer of the TRAP1 dimer that can serve as a TRAP1-specific inhibitor binding surface [42]. Inter-domain communication is essential to the ATPase cycle of TRAP1, and previous work has shown that inhibitor-bound TRAP1 stalls in the NTD dimerized phase [143]. In agreement, the computationally identified compounds (compounds 5–7) were hypothesized to inhibit TRAP1 by reducing the ability of the ATP-binding site to communicate with the client-binding region of the middle domain. In fact, several of these small molecules were shown to decrease TRAP1 ATPase activity to a degree comparable to that of 17-AAG, while not significantly interfering with Hsp90 ATPase activity, demonstrating specificity for TRAP1 [42]. Furthermore, allosterically inhibited TRAP1 bound approximately 30% less SDHA than its control and experienced a significant increase in succinate-coenzyme-Q reductase (SQR) activity. While the tested compound did not alter cell viability, it delayed cell proliferation over a 96 h observation [42]. The successful utilization of TRAP1 asymmetry to identify unique allosteric binding pockets provides a significant starting point for future inhibitor work.
6. Future Perspectives
The function of TRAP1 as a regulator of cellular metabolic flux and mitochondrial apoptosis underscores a duality in which cell fate decisions are determined (Figure 6). Normal cells demonstrate basal TRAP1 expression, facilitating oxidative metabolism and programmed cell death. Dysregulation of TRAP1 expression manifests in noted hallmarks of cancer, including cell death resistance and deregulation of cellular energetics [144]. A thorough delineation of the mechanism of TRAP1 function in these roles is essential to combatting diseases of mitochondrial dysfunction, including cancer and neurodegeneration.
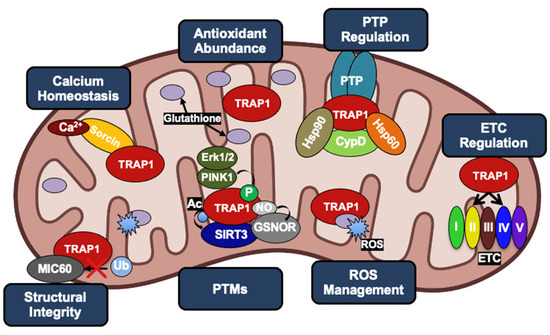
Figure 6.
The multiple roles of TRAP1 in cancer cell mitochondria, revolving around evasion of apoptosis and metabolic reprogramming. TRAP1 acts as a chaperone for the Ca2+ binding protein Sorcin as well as Complexes II and IV of the ETC. Increased TRAP1 levels are associated with calcium tolerance, increased levels of the antioxidant glutathione, reduced levels of ROS, reduced levels of MIC60 ubiquitination, and in many cases, a shift towards the Warburg effect. TRAP1, along with Hsp90 and Hsp60, can form a complex with CypD to prevent opening of the PTP. TRAP1 is post-translationally modified by PINK1, Erk1/2, GSNOR, and SIRT3.
Though our understanding of the cellular impact of TRAP1 is coming into focus, several outstanding questions remain that are essential to our comprehension of the full scope of TRAP1 biology. (1) Is TRAP1 ATPase activity, and by extension TRAP1 chaperone function, essential for its biological activity? ATP-competitive inhibitors of TRAP1 demonstrate efficacy in cell models of cancer, suggesting that TRAP1 function is coupled to its ATPase activity; however, catalytically inactive TRAP1 mutants are able to complement TRAP1 function and revert metabolic dysfunction [26]. Reconciling these disparate observations is an ongoing challenge. (2) What is the physiological impact of TRAP1 dimeric and tetrameric forms, and is transition between these states essential for its function? Cytosolic Hsp90s are well-established dimers, and though the domain architecture of TRAP1 is similar, it remains unclear whether the TRAP1 dimer is the primary biological unit. (3) Is specific targeting of TRAP1 in cancer essential? Many existing TRAP1 inhibitors are mitochondrially targeted Hsp90 inhibitors. Though strategic inhibition of cytosolic Hsp90 has yet to demonstrate clinical success, perhaps simultaneous disruption of TRAP1 and the mitochondrial Hsp90 pool will prove efficacious [145]. (4) Can TRAP1 be used as a biomarker in cancer? Previous work has demonstrated that circulating Hsp90 can potentially be used as a biomarker in certain conditions, however the presence of circulating TRAP1 has not been evaluated [146,147,148]. Similarly, TRAP1 expression and activity is dysregulated in cancer, potentially suggesting an ability to serve as a predictive indicator of disease state. (5) TRAP1 mutations have been implicated in several conditions, including congenital anomalies of the kidney and urinary tract (CAKUT), vertebral defects, anal atresia, cardiac defects, tracheo-esophageal fistula, renal anomalies, and limb abnormalities (VACTERL), Parkinson’s disease, cardiac hypertrophy, and severe autoinflammation [55,149,150,151]. What is the structural basis for the impact of these mutations on TRAP1 function? Is mutant-TRAP1 association with these diseases a consequence of its role as a more general regulator of mitochondrial dynamics [152]? (6) Can differential PTM of TRAP1 in normal and disease states predict disease-associated phenotypes? Indeed, it has been shown that PTMs modulate TRAP1, however whether this necessarily predicts TRAP1 behavior in disease states remains to be tested. (7) Do TRAP1 PTMs compensate for a lack of dedicated co-chaperones? In the case of Hsp90, a single phosphorylation can functionally replace the loss of the yeast co-chaperone Hch1 [101]. The relevance of this mechanism for TRAP1 has not yet been investigated, however the reliance of cytosolic Hsp90 on co-chaperone interaction suggests that TRAP1 PTMs can recapitulate some co-chaperone activities. (8) Can these PTMs be specifically manipulated to alter TRAP1 function? Many cancers are associated with increased TRAP1 activity, and decreased TRAP1 activity or loss-of-function mutations contribute to the pathogenesis of some neurodegenerative diseases [153]. Previous work discussed here demonstrates that PTMs play a role in the regulation of TRAP1 stability, and TRAP1 PTMs are dysregulated in disease. High-throughput methods [154] as well as the study of cytosolic Hsp90s suggest that TRAP1 function will be regulated by a constellation of PTMs with differential incidence that correlates with disease state [3].
The literature reviewed here from several experimental systems demonstrates that in cancers that overexpress TRAP1, attenuation of TRAP1 expression or activity is sufficient to slow cell growth, and in some instances, induce apoptosis. Furthermore, nuanced studies of Hsp90 have demonstrated that PTM can modulate the efficacy of Hsp90 inhibitors [3], implying a similar framework for the application of TRAP1 inhibitors. The identification of predictive indicators of response to TRAP1 inhibition and potential targets for anti-cancer therapy in combination with TRAP1 inhibitors are two essential pieces of information that can be gained from decrypting the TRAP1 chaperone code.
Author Contributions
Conceptualization, M.R.W.; writing—original draft preparation, L.A.W. and M.R.W.; writing—review and editing, L.A.W., S.J.B., D.B., M.M. and M.R.W.; visualization, L.A.W., S.J.B. and M.R.W.; supervision, M.R.W.; project administration, M.R.W.; funding acquisition, D.B., M.M. and M.R.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partly supported by funds from SUNY Upstate Medical University and The Upstate Foundation (M.M., D.B. and M.R.W.).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We would like to thank Giorgio Colombo and Len Neckers for insightful scientific discussions. The figures were created using elements from Biorender.com on an institutional license from SUNY Upstate Medical University.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
17-AAG, 17-allylamino-17-demethoxygeldanamycin; AICAR, 5-aminoimidazole-4-carboxamide-1-β-D-ribofuranoside; ALS, amyotrophic lateral sclerosis; ANT, adenosine nucleotide translocator; ATP, adenosine triphosphate; ATPB, ATP synthase subunit beta; AMPK, AMP-activated protein kinase; CAKUT, congenital anomalies of the kidney and urinary tract, CDK1, cyclin-dependent kinase 1; COXII/IV, cytochrome c oxidase subunit 2/4; CSC, cancer stem cells; CypD, cyclophilin D; Erk1/2, extracellular signal-regulated kinase 1/2; ETC, electron transport chain; G, gamitrinib; G1-4, cyclic guanidinium 1-4; GA, geldanamycin; GDH, glutamate dehydrogenase; GMB, glioblastoma; GSH, reduced glutathione; GSNOR, S-nitrosoglutathione reductase; HCC, hepatocellular carcinoma; HDCA, honokiol bis-dichloroacetate; HIF1α, hypoxia inducible factor 1α; Hsp, heat shock protein; Hsp60, heat shock protein 60; Hsp90, heat shock protein 90; HTRA2, high-temperature requirement A2; IMM, inner mitochondrial membrane; KO, knockout; MAD2, mitotic arrest deficiency 2; MICOS, mitochondrial contact site and cristae organizing system; NDUFA9, NADH:ubiquinone oxidoreductase subunit A9; NRF2, nuclear factor erythroid 2-related factor 2; NTD, N-terminal domain; OMM, outer mitochondrial membrane; PD, Parkinson’s disease; PINK1, phosphatase and tensin homolog (PTEN)-induced kinase 1; PPP, pentose phosphate pathway; PT, mitochondrial permeability transition; PTM, post-translational modification; PTP, permeability transition pore; ROS, reactive oxygen species; SDH, succinate dehydrogenase; SDHA/B, succinate dehydrogenase subunit a/b; SIRT3, sirtuin-3; SNO, S-nitrosylation; SOD2, superoxide dismutase 2; SQR, succinate-coenzyme-Q reductase; TBP7, TATA-Box binding protein 7; TCA, tricarboxylic acid cycle; TPP, triphenylphosphonium; TRAP1, tumor necrosis factor (TNF) receptor-associated protein-1; VACTERL, vertebral defects, anal atresia, cardiac defects, tracheo-esophageal fistula, renal anomalies, and limb abnormalities; VHL, Von Hippel Lindau.
References
- Prodromou, C.; Bjorklund, D.M. Advances towards Understanding the Mechanism of Action of the Hsp90 Complex. Biomolecules 2022, 12, 600. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.L. Mutations in Hsp90 Cochaperones Result in a Wide Variety of Human Disorders. Front. Mol. Biosci. 2021, 8, 787260. [Google Scholar] [CrossRef] [PubMed]
- Backe, S.J.; Sager, R.A.; Woodford, M.R.; Makedon, A.M.; Mollapour, M. Post-Translational Modifications of Hsp90 and Translating the Chaperone Code. J. Biol. Chem. 2020, 295, 11099–11117. [Google Scholar] [CrossRef] [PubMed]
- Cechetto, J.D.; Gupta, R.S. Immunoelectron Microscopy Provides Evidence That Tumor Necrosis Factor Receptor-Associated Protein 1 (TRAP-1) Is a Mitochondrial Protein Which Also Localizes at Specific Extramitochondrial Sites. Exp. Cell Res. 2000, 260, 30–39. [Google Scholar] [CrossRef]
- Pridgeon, J.W.; Olzmann, J.A.; Chin, L.-S.; Li, L. PINK1 Protects against Oxidative Stress by Phosphorylating Mitochondrial Chaperone TRAP1. PLoS Biol. 2007, 5, e172. [Google Scholar] [CrossRef]
- Yoshida, S.; Tsutsumi, S.; Muhlebach, G.; Sourbier, C.; Lee, M.-J.; Lee, S.; Vartholomaiou, E.; Tatokoro, M.; Beebe, K.; Miyajima, N.; et al. Molecular Chaperone TRAP1 Regulates a Metabolic Switch between Mitochondrial Respiration and Aerobic Glycolysis. Proc. Natl. Acad. Sci. USA 2013, 110, E1604–E1612. [Google Scholar] [CrossRef]
- Song, H.Y.; Dunbar, J.D.; Zhang, Y.X.; Guo, D.; Donner, D.B. Identification of a Protein with Homology to Hsp90 That Binds the Type 1 Tumor Necrosis Factor Receptor. J. Biol. Chem. 1995, 270, 3574–3581. [Google Scholar] [CrossRef]
- Felts, S.J.; Owen, B.A.L.; Nguyen, P.; Trepel, J.; Donner, D.B.; Toft, D.O. The Hsp90-Related Protein TRAP1 Is a Mitochondrial Protein with Distinct Functional Properties. J. Biol. Chem. 2000, 275, 3305–3312. [Google Scholar] [CrossRef]
- Cannino, G.; Ciscato, F.; Masgras, I.; Sánchez-Martín, C.; Rasola, A. Metabolic Plasticity of Tumor Cell Mitochondria. Front. Oncol. 2018, 8, 333. [Google Scholar] [CrossRef]
- Masgras, I.; Ciscato, F.; Brunati, A.M.; Tibaldi, E.; Indraccolo, S.; Curtarello, M.; Chiara, F.; Cannino, G.; Papaleo, E.; Lambrughi, M.; et al. Absence of Neurofibromin Induces an Oncogenic Metabolic Switch via Mitochondrial ERK-Mediated Phosphorylation of the Chaperone TRAP1. Cell Rep. 2017, 18, 659–672. [Google Scholar] [CrossRef]
- Masgras, I.; Laquatra, C.; Cannino, G.; Serapian, S.A.; Colombo, G.; Rasola, A. The Molecular Chaperone TRAP1 in Cancer: From the Basics of Biology to Pharmacological Targeting. Semin. Cancer Biol. 2021, 76, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Porter, G.A.; Beutner, G. Cyclophilin D, Somehow a Master Regulator of Mitochondrial Function. Biomolecules 2018, 8, 176. [Google Scholar] [CrossRef] [PubMed]
- Altieri, D.C.; Stein, G.S.; Lian, J.B.; Languino, L.R. TRAP-1, the Mitochondrial Hsp90. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Res. 2012, 1823, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Schopf, F.H.; Biebl, M.M.; Buchner, J. The HSP90 Chaperone Machinery. Nat. Rev. Mol. Cell Biol. 2017, 18, 345–360. [Google Scholar] [CrossRef] [PubMed]
- Obermann, W.M.J.; Sondermann, H.; Russo, A.A.; Pavletich, N.P.; Hartl, F.U. In Vivo Function of Hsp90 Is Dependent on ATP Binding and ATP Hydrolysis. J. Cell Biol. 1998, 143, 901–910. [Google Scholar] [CrossRef] [PubMed]
- Panaretou, B. ATP Binding and Hydrolysis Are Essential to the Function of the Hsp90 Molecular Chaperone Invivo. EMBO J. 1998, 17, 4829–4836. [Google Scholar] [CrossRef]
- Sahasrabudhe, P.; Rohrberg, J.; Biebl, M.M.; Rutz, D.A.; Buchner, J. The Plasticity of the Hsp90 Co-Chaperone System. Mol. Cell 2017, 67, 947–961.e5. [Google Scholar] [CrossRef]
- Lavery, L.A.; Partridge, J.R.; Ramelot, T.A.; Elnatan, D.; Kennedy, M.A.; Agard, D.A. Structural Asymmetry in the Closed State of Mitochondrial Hsp90 (TRAP1) Supports a Two-Step ATP Hydrolysis Mechanism. Mol. Cell 2014, 53, 330–343. [Google Scholar] [CrossRef]
- Partridge, J.R.; Lavery, L.A.; Elnatan, D.; Naber, N.; Cooke, R.; Agard, D.A. A Novel N-Terminal Extension in Mitochondrial TRAP1 Serves as a Thermal Regulator of Chaperone Activity. eLife 2014, 3, e03487. [Google Scholar] [CrossRef]
- Ali, M.M.U.; Roe, S.M.; Vaughan, C.K.; Meyer, P.; Panaretou, B.; Piper, P.W.; Prodromou, C.; Pearl, L.H. Crystal Structure of an Hsp90–Nucleotide–P23/Sba1 Closed Chaperone Complex. Nature 2006, 440, 1013–1017. [Google Scholar] [CrossRef]
- Mayer, M.P.; Le Breton, L. Hsp90: Breaking the Symmetry. Mol. Cell 2015, 58, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Mollapour, M.; Bourboulia, D.; Beebe, K.; Woodford, M.R.; Polier, S.; Hoang, A.; Chelluri, R.; Li, Y.; Guo, A.; Lee, M.-J.; et al. Asymmetric Hsp90 N Domain SUMOylation Recruits Aha1 and ATP-Competitive Inhibitors. Mol. Cell 2014, 53, 317–329. [Google Scholar] [CrossRef] [PubMed]
- Retzlaff, M.; Hagn, F.; Mitschke, L.; Hessling, M.; Gugel, F.; Kessler, H.; Richter, K.; Buchner, J. Asymmetric Activation of the Hsp90 Dimer by Its Cochaperone Aha1. Mol. Cell 2010, 37, 344–354. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, C.K.; Gohlke, U.; Sobott, F.; Good, V.M.; Ali, M.M.U.; Prodromou, C.; Robinson, C.V.; Saibil, H.R.; Pearl, L.H. Structure of an Hsp90-Cdc37-Cdk4 Complex. Mol. Cell 2006, 23, 697–707. [Google Scholar] [CrossRef]
- Elnatan, D.; Betegon, M.; Liu, Y.; Ramelot, T.; Kennedy, M.A.; Agard, D.A. Symmetry Broken and Rebroken during the ATP Hydrolysis Cycle of the Mitochondrial Hsp90 TRAP1. eLife 2017, 6, e25235. [Google Scholar] [CrossRef]
- Joshi, A.; Dai, L.; Liu, Y.; Lee, J.; Ghahhari, N.M.; Segala, G.; Beebe, K.; Jenkins, L.M.; Lyons, G.C.; Bernasconi, L.; et al. The Mitochondrial HSP90 Paralog TRAP1 Forms an OXPHOS-Regulated Tetramer and Is Involved in Mitochondrial Metabolic Homeostasis. BMC Biol. 2020, 18, 10. [Google Scholar] [CrossRef]
- Park, H.-K.; Hong, J.-H.; Oh, Y.T.; Kim, S.S.; Yin, J.; Lee, A.-J.; Chae, Y.C.; Kim, J.H.; Park, S.-H.; Park, C.-K.; et al. Interplay between TRAP1 and Sirtuin-3 Modulates Mitochondrial Respiration and Oxidative Stress to Maintain Stemness of Glioma Stem Cells. Cancer Res 2019, 79, 1369–1382. [Google Scholar] [CrossRef]
- Lisanti, S.; Garlick, D.S.; Bryant, K.G.; Tavecchio, M.; Mills, G.B.; Lu, Y.; Kossenkov, A.V.; Showe, L.C.; Languino, L.R.; Altieri, D.C. Transgenic Expression of the Mitochondrial Chaperone TNFR-Associated Protein 1 (TRAP1) Accelerates Prostate Cancer Development. J. Biol. Chem. 2016, 291, 25247–25254. [Google Scholar] [CrossRef]
- Ramkumar, B.; Dharaskar, S.P.; Mounika, G.; Paithankar, K.; Sreedhar, A.S. Mitochondrial Chaperone, TRAP1 as a Potential Pharmacological Target to Combat Cancer Metabolism. Mitochondrion 2020, 50, 42–50. [Google Scholar] [CrossRef]
- Si, T.; Yang, G.; Qiu, X.; Luo, Y.; Liu, B.; Wang, B. Expression of Tumor Necrosis Factor Receptor-Associated. Int. J. Clin. Exp. Pathol. 2015, 8, 13090–13095. [Google Scholar]
- Zhang, B.; Wang, J.; Huang, Z.; Wei, P.; Liu, Y.; Hao, J.; Zhao, L.; Zhang, F.; Tu, Y.; Wei, T. Aberrantly Upregulated TRAP1 Is Required for Tumorigenesis of Breast Cancer. Oncotarget 2015, 6, 44495–44508. [Google Scholar] [CrossRef] [PubMed]
- Xie, S. The Mitochondrial Chaperone TRAP1 as a Candidate Target of Oncotherapy. Front. Oncol. 2021, 10, 11. [Google Scholar]
- Serapian, S.A.; Sanchez-Martín, C.; Moroni, E.; Rasola, A.; Colombo, G. Targeting the Mitochondrial Chaperone TRAP1: Strategies and Therapeutic Perspectives. Trends Pharmacol. Sci. 2021, 42, 566–576. [Google Scholar] [CrossRef] [PubMed]
- Miyata, Y.; Nakamoto, H.; Neckers, L. The Therapeutic Target Hsp90 and Cancer Hallmarks. Curr. Pharm. Des. 2013, 19, 347–365. [Google Scholar] [CrossRef] [PubMed]
- Trepel, J.; Mollapour, M.; Giaccone, G.; Neckers, L. Targeting the Dynamic HSP90 Complex in Cancer. Nat. Rev. Cancer 2010, 10, 537–549. [Google Scholar] [CrossRef]
- Zong, H.; Gozman, A.; Caldas-Lopes, E.; Taldone, T.; Sturgill, E.; Brennan, S.; Ochiana, S.O.; Gomes-DaGama, E.M.; Sen, S.; Rodina, A.; et al. A Hyperactive Signalosome in Acute Myeloid Leukemia Drives Addiction to a Tumor-Specific Hsp90 Species. Cell Rep. 2015, 13, 2159–2173. [Google Scholar] [CrossRef]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg Effect: The Metabolic Requirements of Cell Proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef]
- Martínez-Reyes, I.; Chandel, N.S. Mitochondrial TCA Cycle Metabolites Control Physiology and Disease. Nat. Commun. 2020, 11, 102. [Google Scholar] [CrossRef]
- Woodford, M.R.; Baker-Williams, A.J.; Sager, R.A.; Backe, S.J.; Blanden, A.R.; Hashmi, F.; Kancherla, P.; Gori, A.; Loiselle, D.R.; Castelli, M.; et al. The Tumor Suppressor Folliculin Inhibits Lactate Dehydrogenase A and Regulates the Warburg Effect. Nat. Struct. Mol. Biol. 2021, 28, 662–670. [Google Scholar] [CrossRef]
- DeBerardinis, R.J.; Chandel, N.S. We Need to Talk about the Warburg Effect. Nat. Metab. 2020, 2, 127–129. [Google Scholar] [CrossRef]
- Warburg, O. The Metabolism of Carcinoma Cells. J. Cancer Res. 1925, 9, 148–163. [Google Scholar] [CrossRef]
- Sanchez-Martin, C.; Moroni, E.; Ferraro, M.; Laquatra, C.; Cannino, G.; Masgras, I.; Negro, A.; Quadrelli, P.; Rasola, A.; Colombo, G. Rational Design of Allosteric and Selective Inhibitors of the Molecular Chaperone TRAP1. Cell Rep. 2020, 31, 107531. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Martin, C.; Menon, D.; Moroni, E.; Ferraro, M.; Masgras, I.; Elsey, J.; Arbiser, J.L.; Colombo, G.; Rasola, A. Honokiol Bis-Dichloroacetate Is a Selective Allosteric Inhibitor of the Mitochondrial Chaperone TRAP1. Antioxid. Redox Signal. 2021, 34, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Sciacovelli, M.; Guzzo, G.; Morello, V.; Frezza, C.; Zheng, L.; Nannini, N.; Calabrese, F.; Laudiero, G.; Esposito, F.; Landriscina, M.; et al. The Mitochondrial Chaperone TRAP1 Promotes Neoplastic Growth by Inhibiting Succinate Dehydrogenase. Cell Metab. 2013, 17, 988–999. [Google Scholar] [CrossRef]
- Serapian, S.A.; Moroni, E.; Ferraro, M.; Colombo, G. Atomistic Simulations of the Mechanisms of the Poorly Catalytic Mitochondrial Chaperone Trap1: Insights into the Effects of Structural Asymmetry on Reactivity. ACS Catal. 2021, 11, 8605–8620. [Google Scholar] [CrossRef]
- Xiang, F.; Ma, S.; Lv, Y.; Zhang, D.; Song, H.; Huang, Y. Tumor Necrosis Factor Receptor-Associated Protein 1 Regulates Hypoxia-Induced Apoptosis through a Mitochondria-Dependent Pathway Mediated by Cytochrome c Oxidase Subunit II. Burn. Trauma 2019, 7, s41038-019-0154-3s41038–s019. [Google Scholar] [CrossRef]
- Xiang, F.; Ma, S.-Y.; Zhang, D.-X.; Zhang, Q.; Huang, Y.-S. Tumor Necrosis Factor Receptor-Associated Protein 1 Improves Hypoxia-Impaired Energy Production in Cardiomyocytes through Increasing Activity of Cytochrome c Oxidase Subunit II. Int. J. Biochem. Cell Biol. 2016, 79, 239–248. [Google Scholar] [CrossRef]
- Bezawork-Geleta, A.; Rohlena, J.; Dong, L.; Pacak, K.; Neuzil, J. Mitochondrial Complex II: At the Crossroads. Trends Biochem. Sci. 2017, 42, 312–325. [Google Scholar] [CrossRef]
- Liu, Y.; Elnatan, D.; Sun, M.; Myasnikov, A.G.; Agard, D.A. Cryo-EM Reveals the Dynamic Interplay between Mitochondrial Hsp90 and SdhB Folding Intermediates. bioRxiv 2020. [Google Scholar] [CrossRef]
- Agarwal, E.; Altman, B.J.; Seo, J.H.; Ghosh, J.C.; Kossenkov, A.V.; Tang, H.-Y.; Krishn, S.R.; Languino, L.R.; Gabrilovich, D.I.; Speicher, D.W.; et al. Myc-Mediated Transcriptional Regulation of the Mitochondrial Chaperone TRAP1 Controls Primary and Metastatic Tumor Growth. J. Biol. Chem. 2019, 294, 10407–10414. [Google Scholar] [CrossRef]
- Chae, Y.C.; Angelin, A.; Lisanti, S.; Kossenkov, A.V.; Speicher, K.D.; Wang, H.; Powers, J.F.; Tischler, A.S.; Pacak, K.; Fliedner, S.; et al. Landscape of the Mitochondrial Hsp90 Metabolome in Tumours. Nat. Commun. 2013, 4, 2139. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Ferraro, M.; Thomas, A.P.; Chung, J.M.; Yoon, N.G.; Seol, J.-H.; Kim, S.; Kim, H.; An, M.Y.; Ok, H.; et al. Dual Binding to Orthosteric and Allosteric Sites Enhances the Anticancer Activity of a TRAP1-Targeting Drug. J. Med. Chem. 2020, 63, 2930–2940. [Google Scholar] [CrossRef] [PubMed]
- Masgras, I.; Sanchez-Martin, C.; Colombo, G.; Rasola, A. The Chaperone TRAP1 As a Modulator of the Mitochondrial Adaptations in Cancer Cells. Front. Oncol. 2017, 7, 58. [Google Scholar] [CrossRef]
- Rizza, S.; Montagna, C.; Cardaci, S.; Maiani, E.; Di Giacomo, G.; Sanchez-Quiles, V.; Blagoev, B.; Rasola, A.; De Zio, D.; Stamler, J.S.; et al. S-Nitrosylation of the Mitochondrial Chaperone TRAP1 Sensitizes Hepatocellular Carcinoma Cells to Inhibitors of Succinate Dehydrogenase. Cancer Res. 2016, 76, 4170–4182. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, J.C.; Zimprich, A.; Carvajal Berrio, D.A.; Schindler, K.M.; Maurer, B.; Schulte, C.; Bus, C.; Hauser, A.-K.; Kübler, M.; Lewin, R.; et al. Metformin Reverses TRAP1 Mutation-Associated Alterations in Mitochondrial Function in Parkinson’s Disease. Brain 2017, 140, 2444–2459. [Google Scholar] [CrossRef]
- Guzzo, G.; Sciacovelli, M.; Bernardi, P.; Rasola, A. Inhibition of Succinate Dehydrogenase by the Mitochondrial Chaperone TRAP1 Has Anti-Oxidant and Anti-Apoptotic Effects on Tumor Cells. Oncotarget 2014, 5, 11897–11908. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-C.; Tseng, L.-M.; Lee, H.-C. Role of Mitochondrial Dysfunction in Cancer Progression. Exp. Biol. Med. 2016, 241, 1281–1295. [Google Scholar] [CrossRef] [PubMed]
- Selak, M.A.; Armour, S.M.; MacKenzie, E.D.; Boulahbel, H.; Watson, D.G.; Mansfield, K.D.; Pan, Y.; Simon, M.C.; Thompson, C.B.; Gottlieb, E. Succinate Links TCA Cycle Dysfunction to Oncogenesis by Inhibiting HIF-α Prolyl Hydroxylase. Cancer Cell 2005, 7, 77–85. [Google Scholar] [CrossRef]
- Zong, S.; Wu, M.; Gu, J.; Liu, T.; Guo, R.; Yang, M. Structure of the Intact 14-Subunit Human Cytochrome c Oxidase. Cell Res. 2018, 28, 1026–1034. [Google Scholar] [CrossRef]
- Lisanti, S.; Tavecchio, M.; Chae, Y.C.; Liu, Q.; Brice, A.K.; Thakur, M.L.; Languino, L.R.; Altieri, D.C. Deletion of the Mitochondrial Chaperone TRAP-1 Uncovers Global Reprogramming of Metabolic Networks. Cell Rep. 2014, 8, 671–677. [Google Scholar] [CrossRef]
- Sullivan, L.B.; Chandel, N.S. Mitochondrial Reactive Oxygen Species and Cancer. Cancer Metab. 2014, 2, 17. [Google Scholar] [CrossRef] [PubMed]
- Clarke, B.E.; Kalmar, B.; Greensmith, L. Enhanced Expression of TRAP1 Protects Mitochondrial Function in Motor Neurons under Conditions of Oxidative Stress. Int. J. Mol. Sci. 2022, 23, 1789. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.H.; Plescia, J.; Dohi, T.; Rosa, J.; Doxsey, S.J.; Altieri, D.C. Regulation of Tumor Cell Mitochondrial Homeostasis by an Organelle-Specific Hsp90 Chaperone Network. Cell 2007, 131, 257–270. [Google Scholar] [CrossRef] [PubMed]
- Leav, I.; Plescia, J.; Goel, H.L.; Li, J.; Jiang, Z.; Cohen, R.J.; Languino, L.R.; Altieri, D.C. Cytoprotective Mitochondrial Chaperone TRAP-1 As a Novel Molecular Target in Localized and Metastatic Prostate Cancer. Am. J. Pathol. 2010, 176, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Vartholomaiou, E.; Madon-Simon, M.; Hagmann, S.; Mühlebach, G.; Wurst, W.; Floss, T.; Picard, D. Cytosolic Hsp90α and Its Mitochondrial Isoform Trap1 Are Differentially Required in a Breast Cancer Model. Oncotarget 2017, 8, 17428–17442. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Linehan, W.M.; Schmidt, L.S.; Crooks, D.R.; Wei, D.; Srinivasan, R.; Lang, M.; Ricketts, C.J. The Metabolic Basis of Kidney Cancer. Cancer Discov. 2019, 9, 1006–1021. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. HIF-1: Upstream and Downstream of Cancer Metabolism. Curr. Opin. Genet. Dev. 2010, 20, 51–56. [Google Scholar] [CrossRef]
- Kowalik, M.A.; Guzzo, G.; Morandi, A.; Perra, A.; Menegon, S.; Masgras, I.; Trevisan, E.; Angioni, M.M.; Fornari, F.; Quagliata, L.; et al. Metabolic Reprogramming Identifies the Most Aggressive Lesions at Early Phases of Hepatic Carcinogenesis. Oncotarget 2016, 7, 32375–32393. [Google Scholar] [CrossRef]
- Iacobazzi, V.; Infantino, V. Citrate—New Functions for an Old Metabolite. Biol. Chem. 2014, 395, 387–399. [Google Scholar] [CrossRef]
- Liu, J.; Peng, Y.; Wei, W. Cell Cycle on the Crossroad of Tumorigenesis and Cancer Therapy. Trends Cell Biol. 2022, 32, 30–44. [Google Scholar] [CrossRef]
- Amoroso, M.R.; Matassa, D.S.; Laudiero, G.; Egorova, A.V.; Polishchuk, R.S.; Maddalena, F.; Piscazzi, A.; Paladino, S.; Sarnataro, D.; Garbi, C.; et al. TRAP1 and the Proteasome Regulatory Particle TBP7/Rpt3 Interact in the Endoplasmic Reticulum and Control Cellular Ubiquitination of Specific Mitochondrial Proteins. Cell Death Differ. 2012, 19, 592–604. [Google Scholar] [CrossRef] [PubMed]
- Sisinni, L.; Maddalena, F.; Condelli, V.; Pannone, G.; Simeon, V.; Li Bergolis, V.; Lopes, E.; Piscazzi, A.; Matassa, D.S.; Mazzoccoli, C.; et al. TRAP1 Controls Cell Cycle G2-M Transition through the Regulation of CDK1 and MAD2 Expression/Ubiquitination: TRAP1 Regulates Mitotic Entry through CDK1 Quality Control. J. Pathol. 2017, 243, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Kim, C.N.; Yang, J.; Jemmerson, R.; Wang, X. Induction of Apoptotic Program in Cell-Free Extracts: Requirement for DATP and Cytochrome c. Cell 1996, 86, 147–157. [Google Scholar] [CrossRef]
- Li, P.; Nijhawan, D.; Budihardjo, I.; Srinivasula, S.M.; Ahmad, M.; Alnemri, E.S.; Wang, X. Cytochrome c and DATP-Dependent Formation of Apaf-1/Caspase-9 Complex Initiates an Apoptotic Protease Cascade. Cell 1997, 91, 479–489. [Google Scholar] [CrossRef]
- Javadov, S.; Chapa-Dubocq, X.; Makarov, V. Different Approaches to Modeling Analysis of Mitochondrial Swelling. Mitochondrion 2018, 38, 58–70. [Google Scholar] [CrossRef]
- Orrenius, S.; Gogvadze, V.; Zhivotovsky, B. Calcium and Mitochondria in the Regulation of Cell Death. Biochem. Biophys. Res. Commun. 2015, 460, 72–81. [Google Scholar] [CrossRef]
- Bernardi, P.; Rasola, A.; Forte, M.; Lippe, G. The Mitochondrial Permeability Transition Pore: Channel Formation by F-ATP Synthase, Integration in Signal Transduction, and Role in Pathophysiology. Physiol. Rev. 2015, 95, 1111–1155. [Google Scholar] [CrossRef]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of Apoptosis Signalling Pathways by Reactive Oxygen Species. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Res. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Hua, G.; Zhang, Q.; Fan, Z. Heat Shock Protein 75 (TRAP1) Antagonizes Reactive Oxygen Species Generation and Protects Cells from Granzyme M-Mediated Apoptosis. J. Biol. Chem. 2007, 282, 20553–20560. [Google Scholar] [CrossRef]
- Im, C.-N.; Lee, J.-S.; Zheng, Y.; Seo, J.-S. Iron Chelation Study in a Normal Human Hepatocyte Cell Line Suggests That Tumor Necrosis Factor Receptor-Associated Protein 1 (TRAP1) Regulates Production of Reactive Oxygen Species. J. Cell. Biochem. 2007, 100, 474–486. [Google Scholar] [CrossRef]
- Costantino, E.; Maddalena, F.; Calise, S.; Piscazzi, A.; Tirino, V.; Fersini, A.; Ambrosi, A.; Neri, V.; Esposito, F.; Landriscina, M. TRAP1, a Novel Mitochondrial Chaperone Responsible for Multi-Drug Resistance and Protection from Apoptotis in Human Colorectal Carcinoma Cells. Cancer Lett. 2009, 279, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Landriscina, M.; Laudiero, G.; Maddalena, F.; Amoroso, M.R.; Piscazzi, A.; Cozzolino, F.; Monti, M.; Garbi, C.; Fersini, A.; Pucci, P.; et al. Mitochondrial Chaperone Trap1 and the Calcium Binding Protein Sorcin Interact and Protect Cells against Apoptosis Induced by Antiblastic Agents. Cancer Res. 2010, 70, 6577–6586. [Google Scholar] [CrossRef] [PubMed]
- Suarez, J.; McDonough, P.M.; Scott, B.T.; Suarez-Ramirez, A.; Wang, H.; Fricovsky, E.S.; Dillmann, W.H. Sorcin Modulates Mitochondrial Ca2+ Handling and Reduces Apoptosis in Neonatal Rat Cardiac Myocytes. Am. J. Physiol. -Cell Physiol. 2013, 304, C248–C256. [Google Scholar] [CrossRef] [PubMed]
- Elnatan, D.; Agard, D.A. Calcium Binding to a Remote Site Can Replace Magnesium as Cofactor for Mitochondrial Hsp90 (TRAP1) ATPase Activity. J. Biol. Chem. 2018, 293, 13717–13724. [Google Scholar] [CrossRef]
- Zhang, L.; Su, N.; Luo, Y.; Chen, S.; Zhao, T. TRAP1 Inhibits MIC60 Ubiquitination to Mitigate the Injury of Cardiomyocytes and Protect Mitochondria in Extracellular Acidosis. Cell Death Discov. 2021, 7, 389. [Google Scholar] [CrossRef]
- Friedman, J.R.; Mourier, A.; Yamada, J.; McCaffery, J.M.; Nunnari, J. MICOS Coordinates with Respiratory Complexes and Lipids to Establish Mitochondrial Inner Membrane Architecture. eLife 2015, 4, e07739. [Google Scholar] [CrossRef]
- Rampelt, H.; Zerbes, R.M.; van der Laan, M.; Pfanner, N. Role of the Mitochondrial Contact Site and Cristae Organizing System in Membrane Architecture and Dynamics. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Res. 2017, 1864, 737–746. [Google Scholar] [CrossRef]
- Baines, C.P.; Gutiérrez-Aguilar, M. The Mitochondrial Permeability Transition Pore: Is It Formed by the ATP Synthase, Adenine Nucleotide Translocators or Both? Biochim. Et Biophys. Acta (BBA)-Bioenerg. 2020, 1861, 148249. [Google Scholar] [CrossRef]
- Carrer, A.; Tommasin, L.; Šileikytė, J.; Ciscato, F.; Filadi, R.; Urbani, A.; Forte, M.; Rasola, A.; Szabò, I.; Carraro, M.; et al. Defining the Molecular Mechanisms of the Mitochondrial Permeability Transition through Genetic Manipulation of F-ATP Synthase. Nat. Commun. 2021, 12, 4835. [Google Scholar] [CrossRef]
- Giorgio, V.; von Stockum, S.; Antoniel, M.; Fabbro, A.; Fogolari, F.; Forte, M.; Glick, G.D.; Petronilli, V.; Zoratti, M.; Szabó, I.; et al. Dimers of Mitochondrial ATP Synthase Form the Permeability Transition Pore. Proc. Natl. Acad. Sci. USA 2013, 110, 5887–5892. [Google Scholar] [CrossRef]
- Kang, B.H.; Tavecchio, M.; Goel, H.L.; Hsieh, C.-C.; Garlick, D.S.; Raskett, C.M.; Lian, J.B.; Stein, G.S.; Languino, L.R.; Altieri, D.C. Targeted Inhibition of Mitochondrial Hsp90 Suppresses Localised and Metastatic Prostate Cancer Growth in a Genetic Mouse Model of Disease. Br. J. Cancer 2011, 104, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Baines, C.P.; Kaiser, R.A.; Purcell, N.H.; Blair, N.S.; Osinska, H.; Hambleton, M.A.; Brunskill, E.W.; Sayen, M.R.; Gottlieb, R.A.; Dorn, G.W.; et al. Loss of Cyclophilin D Reveals a Critical Role for Mitochondrial Permeability Transition in Cell Death. Nature 2005, 434, 658–662. [Google Scholar] [CrossRef] [PubMed]
- Matassa, D.S.; Amoroso, M.R.; Maddalena, F.; Landriscina, M. New Insights into TRAP1 Pathway. Am. J. Cancer Res. 2012, 2, 235–248. [Google Scholar] [PubMed]
- Ghosh, J.C.; Siegelin, M.D.; Dohi, T.; Altieri, D.C. Heat Shock Protein 60 Regulation of the Mitochondrial Permeability Transition Pore in Tumor Cells. Cancer Res. 2010, 70, 8988–8993. [Google Scholar] [CrossRef] [PubMed]
- Lebedev, I.; Nemajerova, A.; Foda, Z.H.; Kornaj, M.; Tong, M.; Moll, U.M.; Seeliger, M.A. A Novel In Vitro CypD-Mediated P53 Aggregation Assay Suggests a Model for Mitochondrial Permeability Transition by Chaperone Systems. J. Mol. Biol. 2016, 428, 4154–4167. [Google Scholar] [CrossRef] [PubMed]
- Sinha, D.; D’Silva, P. Chaperoning Mitochondrial Permeability Transition: Regulation of Transition Pore Complex by a J-Protein, DnaJC15. Cell Death Dis. 2014, 5, e1101. [Google Scholar] [CrossRef]
- Niemi, N.M.; Pagliarini, D.J. The Extensive and Functionally Uncharacterized Mitochondrial Phosphoproteome. J. Biol. Chem. 2021, 297, 100880. [Google Scholar] [CrossRef]
- Faienza, F.; Lambrughi, M.; Rizza, S.; Pecorari, C.; Giglio, P.; Salamanca Viloria, J.; Allega, M.F.; Chiappetta, G.; Vinh, J.; Pacello, F.; et al. S-Nitrosylation Affects TRAP1 Structure and ATPase Activity and Modulates Cell Response to Apoptotic Stimuli. Biochem. Pharmacol. 2020, 176, 113869. [Google Scholar] [CrossRef]
- Rasola, A.; Neckers, L.; Picard, D. Mitochondrial Oxidative Phosphorylation TRAP(1)Ped in Tumor Cells. Trends Cell Biol. 2014, 24, 455–463. [Google Scholar] [CrossRef]
- Cloutier, P.; Coulombe, B. Regulation of Molecular Chaperones through Post-Translational Modifications: Decrypting the Chaperone Code. Biochim. Biophys. Acta (BBA)-Gene Regul. Mech. 2013, 1829, 443–454. [Google Scholar] [CrossRef]
- Zuehlke, A.D.; Reidy, M.; Lin, C.; LaPointe, P.; Alsomairy, S.; Lee, D.J.; Rivera-Marquez, G.M.; Beebe, K.; Prince, T.; Lee, S.; et al. An Hsp90 Co-Chaperone Protein in Yeast Is Functionally Replaced by Site-Specific Posttranslational Modification in Humans. Nat. Commun. 2017, 8, 15328. [Google Scholar] [CrossRef] [PubMed]
- Quinn, P.M.J.; Moreira, P.I.; Ambrósio, A.F.; Alves, C.H. PINK1/PARKIN Signalling in Neurodegeneration and Neuroinflammation. Acta Neuropathol. Commun. 2020, 8, 189. [Google Scholar] [CrossRef] [PubMed]
- Arena, G.; Gelmetti, V.; Torosantucci, L.; Vignone, D.; Lamorte, G.; De Rosa, P.; Cilia, E.; Jonas, E.A.; Valente, E.M. PINK1 Protects against Cell Death Induced by Mitochondrial Depolarization, by Phosphorylating Bcl-XL and Impairing Its pro-Apoptotic Cleavage. Cell Death Differ. 2013, 20, 920–930. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.C.; Loh, S.H.Y.; Martins, L.M. Drosophila Trap1 Protects against Mitochondrial Dysfunction in a PINK1/Parkin Model of Parkinson’s Disease. Cell Death Dis. 2013, 4, e467. [Google Scholar] [CrossRef]
- Zhang, L.; Karsten, P.; Hamm, S.; Pogson, J.H.; Müller-Rischart, A.K.; Exner, N.; Haass, C.; Whitworth, A.J.; Winklhofer, K.F.; Schulz, J.B.; et al. TRAP1 Rescues PINK1 Loss-of-Function Phenotypes. Hum. Mol. Genet. 2013, 22, 2829–2841. [Google Scholar] [CrossRef]
- Fiesel, F.C.; James, E.D.; Hudec, R.; Springer, W. Mitochondrial Targeted HSP90 Inhibitor Gamitrinib-TPP (G-TPP) Induces PINK1/Parkin-Dependent Mitophagy. Oncotarget 2017, 8, 106233–106248. [Google Scholar] [CrossRef]
- Rasola, A.; Sciacovelli, M.; Chiara, F.; Pantic, B.; Brusilow, W.S.; Bernardi, P. Activation of Mitochondrial ERK Protects Cancer Cells from Death through Inhibition of the Permeability Transition. Proc. Natl. Acad. Sci. USA 2010, 107, 726–731. [Google Scholar] [CrossRef]
- Miyazaki, T.; Neff, L.; Tanaka, S.; Horne, W.C.; Baron, R. Regulation of Cytochrome c Oxidase Activity by C-Src in Osteoclasts. J. Cell Biol. 2003, 160, 709–718. [Google Scholar] [CrossRef]
- Ogura, M.; Yamaki, J.; Homma, M.K.; Homma, Y. Mitochondrial C-Src Regulates Cell Survival through Phosphorylation of Respiratory Chain Components. Biochem. J. 2012, 447, 281–289. [Google Scholar] [CrossRef]
- George, J.; Ahmad, N. Mitochondrial Sirtuins in Cancer: Emerging Roles and Therapeutic Potential. Cancer Res. 2016, 76, 2500–2506. [Google Scholar] [CrossRef]
- Stomberski, C.T.; Hess, D.T.; Stamler, J.S. Protein S-Nitrosylation: Determinants of Specificity and Enzymatic Regulation of S-Nitrosothiol-Based Signaling. Antioxid. Redox Signal. 2019, 30, 1331–1351. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Hausladen, A.; Zeng, M.; Que, L.; Heitman, J.; Stamler, J.S. A Metabolic Enzyme for S-Nitrosothiol Conserved from Bacteria to Humans. Nature 2001, 410, 490–494. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Yang, Z.; Tang, C.-H.; Liu, L. Targeted Deletion of GSNOR in Hepatocytes of Mice Causes Nitrosative Inactivation of O6-Alkylguanine-DNA Alkyltransferase and Increased Sensitivity to Genotoxic Diethylnitrosamine. Carcinogenesis 2011, 32, 973–977. [Google Scholar] [CrossRef] [PubMed]
- Forrester, M.T.; Thompson, J.W.; Foster, M.W.; Nogueira, L.; Moseley, M.A.; Stamler, J.S. Proteomic Analysis of S-Nitrosylation and Denitrosylation by Resin-Assisted Capture. Nat. Biotechnol. 2009, 27, 557–559. [Google Scholar] [CrossRef]
- Stine, Z.E.; Schug, Z.T.; Salvino, J.M.; Dang, C.V. Targeting Cancer Metabolism in the Era of Precision Oncology. Nat. Rev. Drug Discov. 2022, 21, 141–162. [Google Scholar] [CrossRef]
- Bryant, K.G.; Chae, Y.C.; Martinez, R.L.; Gordon, J.C.; Elokely, K.M.; Kossenkov, A.V.; Grant, S.; Childers, W.E.; Abou-Gharbia, M.; Altieri, D.C. A Mitochondrial-Targeted Purine-Based HSP90 Antagonist for Leukemia Therapy. Oncotarget 2017, 8, 112184–112198. [Google Scholar] [CrossRef]
- Yoon, N.G.; Lee, H.; Kim, S.-Y.; Hu, S.; Kim, D.; Yang, S.; Hong, K.B.; Lee, J.H.; Kang, S.; Kim, B.-G.; et al. Mitoquinone Inactivates Mitochondrial Chaperone TRAP1 by Blocking the Client Binding Site. J. Am. Chem. Soc. 2021, 143, 19684–19696. [Google Scholar] [CrossRef]
- Kang, B.H.; Plescia, J.; Song, H.Y.; Meli, M.; Colombo, G.; Beebe, K.; Scroggins, B.; Neckers, L.; Altieri, D.C. Combinatorial Drug Design Targeting Multiple Cancer Signaling Networks Controlled by Mitochondrial Hsp90. J. Clin. Investig. 2009, 119, 454–464. [Google Scholar] [CrossRef]
- Kang, B.H.; Siegelin, M.D.; Plescia, J.; Raskett, C.M.; Garlick, D.S.; Dohi, T.; Lian, J.B.; Stein, G.S.; Languino, L.R.; Altieri, D.C. Preclinical Characterization of Mitochondria-Targeted Small Molecule Hsp90 Inhibitors, Gamitrinibs, in Advanced Prostate Cancer. Clin. Cancer Res. 2010, 16, 4779–4788. [Google Scholar] [CrossRef]
- Chae, Y.C.; Caino, M.C.; Lisanti, S.; Ghosh, J.C.; Dohi, T.; Danial, N.N.; Villanueva, J.; Ferrero, S.; Vaira, V.; Santambrogio, L.; et al. Control of Tumor Bioenergetics and Survival Stress Signaling by Mitochondrial HSP90s. Cancer Cell 2012, 22, 331–344. [Google Scholar] [CrossRef]
- Caino, M.C.; Chae, Y.C.; Vaira, V.; Ferrero, S.; Nosotti, M.; Martin, N.M.; Weeraratna, A.; O’Connell, M.; Jernigan, D.; Fatatis, A.; et al. Metabolic Stress Regulates Cytoskeletal Dynamics and Metastasis of Cancer Cells. J. Clin. Investig. 2013, 123, 2907–2920. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-K.; Lee, J.-E.; Lim, J.; Jo, D.-E.; Park, S.-A.; Suh, P.-G.; Kang, B.H. Combination Treatment with Doxorubicin and Gamitrinib Synergistically Augments Anticancer Activity through Enhanced Activation of Bim. BMC Cancer 2014, 14, 431. [Google Scholar] [CrossRef] [PubMed]
- Condelli, V.; Maddalena, F.; Sisinni, L.; Lettini, G.; Matassa, D.S.; Piscazzi, A.; Palladino, G.; Amoroso, M.R.; Esposito, F.; Landriscina, M. Targeting TRAP1 as a Downstream Effector of BRAF Cytoprotective Pathway: A Novel Strategy for Human BRAF-Driven Colorectal Carcinoma. Oncotarget 2015, 6, 22298–22309. [Google Scholar] [CrossRef]
- Karpel-Massler, G.; Ishida, C.T.; Bianchetti, E.; Shu, C.; Perez-Lorenzo, R.; Horst, B.; Banu, M.; Roth, K.A.; Bruce, J.N.; Canoll, P.; et al. Inhibition of Mitochondrial Matrix Chaperones and Antiapoptotic Bcl-2 Family Proteins Empower Antitumor Therapeutic Responses. Cancer Res. 2017, 77, 3513–3526. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Yang, J.; Kim, M.J.; Choi, S.; Chung, J.-R.; Kim, J.-M.; Yoo, Y.H.; Chung, J.; Koh, H. Tumor Necrosis Factor Receptor-Associated Protein 1 (TRAP1) Mutation and TRAP1 Inhibitor Gamitrinib-Triphenylphosphonium (G-TPP) Induce a Forkhead Box O (FOXO)-Dependent Cell Protective Signal from Mitochondria. J. Biol. Chem. 2016, 291, 1841–1853. [Google Scholar] [CrossRef]
- Wei, S.; Yin, D.; Yu, S.; Lin, X.; Savani, M.R.; Du, K.; Ku, Y.; Wu, D.; Li, S.; Liu, H.; et al. Anti-Tumor Activity of a Mitochondrial Targeted HSP90 Inhibitor in Gliomas. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2022, 28, 2180–2195. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.T.; Ishida, C.T.; Shang, E.; Shu, C.; Bianchetti, E.; Karpel-Massler, G.; Siegelin, M.D. Activation of LXR Receptors and Inhibition of TRAP1 Causes Synthetic Lethality in Solid Tumors. Cancers 2019, 11, 788. [Google Scholar] [CrossRef]
- Nguyen, T.T.T.; Zhang, Y.; Shang, E.; Shu, C.; Quinzii, C.M.; Westhoff, M.-A.; Karpel-Massler, G.; Siegelin, M.D. Inhibition of HDAC1/2 Along with TRAP1 Causes Synthetic Lethality in Glioblastoma Model Systems. Cells 2020, 9, 1661. [Google Scholar] [CrossRef]
- Wang, N.; Zhu, P.; Huang, R.; Sun, L.; Dong, D.; Gao, Y. Suppressing TRAP1 Sensitizes Glioblastoma Multiforme Cells to Temozolomide. Exp. Ther. Med. 2021, 22, 1246. [Google Scholar] [CrossRef]
- Lee, C.; Park, H.-K.; Jeong, H.; Lim, J.; Lee, A.-J.; Cheon, K.Y.; Kim, C.-S.; Thomas, A.P.; Bae, B.; Kim, N.D.; et al. Development of a Mitochondria-Targeted Hsp90 Inhibitor Based on the Crystal Structures of Human TRAP1. J. Am. Chem. Soc. 2015, 137, 4358–4367. [Google Scholar] [CrossRef]
- Haidar, M.A.; Shakkour, Z.; Barsa, C.; Tabet, M.; Mekhjian, S.; Darwish, H.; Goli, M.; Shear, D.; Pandya, J.D.; Mechref, Y.; et al. Mitoquinone Helps Combat the Neurological, Cognitive, and Molecular Consequences of Open Head Traumatic Brain Injury at Chronic Time Point. Biomedicines 2022, 10, 250. [Google Scholar] [CrossRef] [PubMed]
- Pinho, B.R.; Duarte, A.I.; Canas, P.M.; Moreira, P.I.; Murphy, M.P.; Oliveira, J.M.A. The Interplay between Redox Signalling and Proteostasis in Neurodegeneration: In Vivo Effects of a Mitochondria-Targeted Antioxidant in Huntington’s Disease Mice. Free Radic. Biol. Med. 2020, 146, 372–382. [Google Scholar] [CrossRef] [PubMed]
- Miquel, E.; Cassina, A.; Martínez-Palma, L.; Souza, J.M.; Bolatto, C.; Rodríguez-Bottero, S.; Logan, A.; Smith, R.A.J.; Murphy, M.P.; Barbeito, L.; et al. Neuroprotective Effects of the Mitochondria-Targeted Antioxidant MitoQ in a Model of Inherited Amyotrophic Lateral Sclerosis. Free Radic. Biol. Med. 2014, 70, 204–213. [Google Scholar] [CrossRef] [PubMed]
- McManus, M.J.; Murphy, M.P.; Franklin, J.L. The Mitochondria-Targeted Antioxidant MitoQ Prevents Loss of Spatial Memory Retention and Early Neuropathology in a Transgenic Mouse Model of Alzheimer’s Disease. J. Neurosci. 2011, 31, 15703–15715. [Google Scholar] [CrossRef] [PubMed]
- Koopman, M.B.; Rüdiger, S.G.D. Alzheimer Cells on Their Way to Derailment Show Selective Changes in Protein Quality Control Network. Front. Mol. Biosci. 2020, 7, 214. [Google Scholar] [CrossRef]
- DeBoer, C.; Meulman, P.A.; Wnuk, R.J.; Peterson, D.H. Geldanamycin, a New Antibiotic. J. Antibiot. 2006, 23, 442–447. [Google Scholar] [CrossRef]
- Grenert, J.P.; Sullivan, W.P.; Fadden, P.; Haystead, T.A.J.; Clark, J.; Mimnaugh, E.; Krutzsch, H.; Ochel, H.-J.; Schulte, T.W.; Sausville, E.; et al. The Amino-Terminal Domain of Heat Shock Protein 90 (Hsp90) That Binds Geldanamycin Is an ATP/ADP Switch Domain That Regulates Hsp90 Conformation*. J. Biol. Chem. 1997, 272, 23843–23850. [Google Scholar] [CrossRef]
- Prodromou, C.; Roe, S.M.; O’Brien, R.; Ladbury, J.E.; Piper, P.W.; Pearl, L.H. Identification and Structural Characterization of the ATP/ADP-Binding Site in the Hsp90 Molecular Chaperone. Cell 1997, 90, 65–75. [Google Scholar] [CrossRef]
- Plescia, J.; Salz, W.; Xia, F.; Pennati, M.; Zaffaroni, N.; Daidone, M.G.; Meli, M.; Dohi, T.; Fortugno, P.; Nefedova, Y.; et al. Rational Design of Shepherdin, a Novel Anticancer Agent. Cancer Cell 2005, 7, 457–468. [Google Scholar] [CrossRef]
- Fortugno, P.; Beltrami, E.; Plescia, J.; Fontana‡, J.; Pradhan§, D.; Marchisio, P.C.; Sessa, W.C.; Altieri, D.C. Regulation of Survivin Function by Hsp90. Proc. Natl. Acad. Sci. USA 2003, 100, 13791–13796. [Google Scholar] [CrossRef]
- Meli, M.; Pennati, M.; Curto, M.; Daidone, M.G.; Plescia, J.; Toba, S.; Altieri, D.C.; Zaffaroni, N.; Colombo, G. Small-Molecule Targeting of Heat Shock Protein 90 Chaperone Function: Rational Identification of a New Anticancer Lead. J. Med. Chem. 2006, 49, 7721–7730. [Google Scholar] [CrossRef] [PubMed]
- Tomaselli, S.; Meli, M.; Plescia, J.; Zetta, L.; Altieri, D.C.; Colombo, G.; Ragona, L. Combined in Silico and Experimental Approach for Drug Design: The Binding Mode of Peptidic and Non-Peptidic Inhibitors to Hsp90 N-Terminal Domain: NMR and MD Studies of Hsp90 Inhibitors. Chem. Biol. Drug Des. 2010, 76, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Moroni, E.; Agard, D.A.; Colombo, G. The Structural Asymmetry of Mitochondrial Hsp90 (Trap1) Determines Fine Tuning of Functional Dynamics. J. Chem. Theory Comput. 2018, 14, 1033–1044. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Neckers, L.; Kern, A.; Tsutsumi, S. Hsp90 Inhibitors Disrupt Mitochondrial Homeostasis in Cancer Cells. Chem. Biol. 2007, 14, 1204–1206. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Xu, X.; Huang, D.; Cui, D.; Liu, L.; Liu, J.; He, Z.; Liu, J.; Zheng, S.; Luo, Y. Plasma Heat Shock Protein 90alpha as a Biomarker for the Diagnosis of Liver Cancer: An Official, Large-Scale, and Multicenter Clinical Trial. EBioMedicine 2017, 24, 56–63. [Google Scholar] [CrossRef]
- Ocaña, G.J.; Sims, E.K.; Watkins, R.A.; Ragg, S.; Mather, K.J.; Oram, R.A.; Mirmira, R.G.; DiMeglio, L.A.; Blum, J.S.; Evans-Molina, C. Analysis of Serum Hsp90 as a Potential Biomarker of β Cell Autoimmunity in Type 1 Diabetes. PLoS ONE 2019, 14, e0208456. [Google Scholar] [CrossRef]
- Štorkánová, H.; Oreská, S.; Špiritović, M.; Heřmánková, B.; Bubová, K.; Komarc, M.; Pavelka, K.; Vencovský, J.; Distler, J.H.W.; Šenolt, L.; et al. Plasma Hsp90 Levels in Patients with Systemic Sclerosis and Relation to Lung and Skin Involvement: A Cross-Sectional and Longitudinal Study. Sci. Rep. 2021, 11, 1. [Google Scholar] [CrossRef]
- Saisawat, P.; Kohl, S.; Hilger, A.C.; Hwang, D.-Y.; Yung Gee, H.; Dworschak, G.C.; Tasic, V.; Pennimpede, T.; Natarajan, S.; Sperry, E.; et al. Whole-Exome Resequencing Reveals Recessive Mutations in TRAP1 in Individuals with CAKUT and VACTERL Association. Kidney Int. 2014, 85, 1310–1317. [Google Scholar] [CrossRef]
- Standing, A.S.; Hong, Y.; Paisan-Ruiz, C.; Omoyinmi, E.; Medlar, A.; Stanescu, H.; Kleta, R.; Rowcenzio, D.; Hawkins, P.; Lachmann, H.; et al. TRAP1 Chaperone Protein Mutations and Autoinflammation. Life Sci. Alliance 2020, 3, e201900376. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, D.-S.; Yan, L.; Cheng, K.-J.; Bian, Z.-Y.; Lin, G.-S. HSP75 Protects against Cardiac Hypertrophy and Fibrosis. J. Cell. Biochem. 2011, 112, 1787–1794. [Google Scholar] [CrossRef] [PubMed]
- Takamura, H.; Koyama, Y.; Matsuzaki, S.; Yamada, K.; Hattori, T.; Miyata, S.; Takemoto, K.; Tohyama, M.; Katayama, T. TRAP1 Controls Mitochondrial Fusion/Fission Balance through Drp1 and Mff Expression. PLoS ONE 2012, 7, e51912. [Google Scholar] [CrossRef] [PubMed]
- Ramos Rego, I.; Santos Cruz, B.; Ambrósio, A.F.; Alves, C.H. TRAP1 in Oxidative Stress and Neurodegeneration. Antioxidants 2021, 10, 1829. [Google Scholar] [CrossRef] [PubMed]
- Hornbeck, P.V.; Zhang, B.; Murray, B.; Kornhauser, J.M.; Latham, V.; Skrzypek, E. PhosphoSitePlus, 2014: Mutations, PTMs and Recalibrations. Nucleic Acids Res. 2015, 43, D512–D520. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).