Blood Cell-Derived Microvesicles in Hematological Diseases and beyond
Abstract
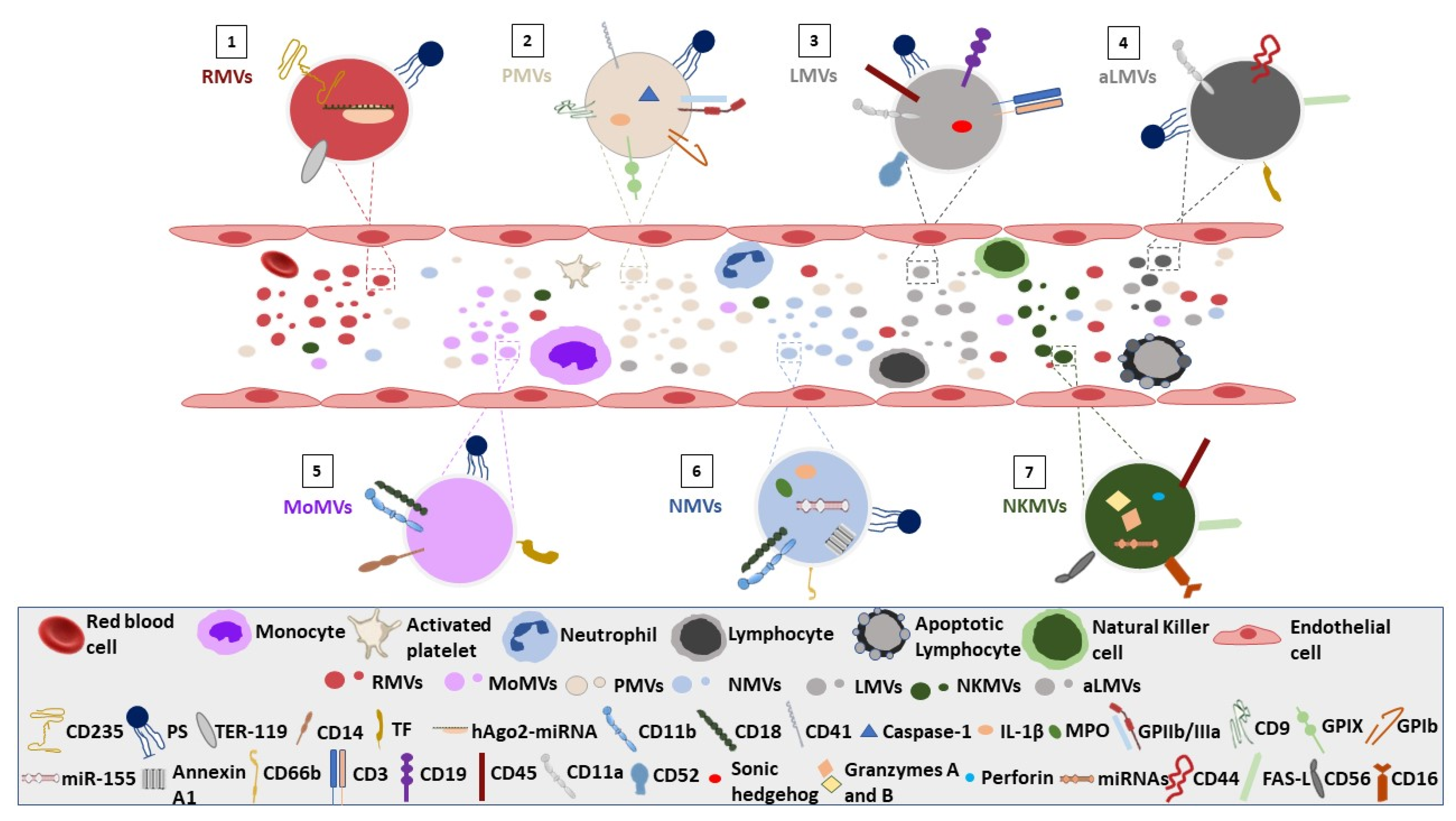
:1. Introduction
2. White Blood Cell-Derived Vesicles
2.1. General Characteristics
2.2. Neutrophil-Derived MVs
2.3. Lymphocyte-Derived MVs
2.4. Monocyte-Derived MVs
2.5. White Blood Cells Microvesiculation in Storage Conditions
3. Red Blood Cell Vesiculation
3.1. RBC Vesiculation in Disease
3.2. Red Blood Cell Microvesiculation in Storage Conditions
4. Platelet Vesiculation
4.1. PLT Vesiculation in Disease
4.2. Platelet Microvesiculation under PLT Storage Conditions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Jimenez, A.J.; Maiuri, P.; Lafaurie-Janvore, J.; Divoux, S.; Piel, M.; Perez, F. ESCRT Machinery Is Required for Plasma Membrane Repair. Science 2014, 343, 1247136. [Google Scholar] [CrossRef] [PubMed]
- Cocucci, E.; Meldolesi, J. Ectosomes and exosomes: Shedding the confusion between extracellular vesicles. Trends Cell Biol. 2015, 25, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Pollet, H.; Conrard, L.; Cloos, A.S.; Tyteca, D. Plasma Membrane Lipid Domains as Platforms for Vesicle Biogenesis and Shedding? Biomolecules 2018, 8, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ihara, T.; Yamamoto, T.; Sugamata, M.; Okumura, H.; Ueno, Y. The process of ultrastructural changes from nuclei to apoptotic body. Virchows Arch. 1998, 433, 443–447. [Google Scholar] [CrossRef]
- Doyle, L.; Wang, M. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef] [Green Version]
- Jeppesen, D.K.; Franklin, J.L.; Higginbotham, J.N.; Zhang, Q.; Coffey, R.J. Abstract 1985: Reassessment of exosome composition. 2019. Cancer Res. 2019, 79, 1985. [Google Scholar] [CrossRef]
- Kugeratski, F.G.; Hodge, K.; Lilla, S.; McAndrews, K.M.; Zhou, X.; Hwang, R.F.; Zanivan, S.; Kalluri, R. Quantitative proteomics identifies the core proteome of exosomes with syntenin-1 as the highest abundant protein and a putative universal biomarker. Nat. Cell Biol. 2021, 23, 631–641. [Google Scholar] [CrossRef]
- Nabhan, J.F.; Hu, R.; Oh, R.S.; Cohen, S.N.; Lu, Q. Formation and release of arrestin domain-containing protein 1-mediated microvesicles (ARMMs) at plasma membrane by recruitment of TSG101 protein. Proc. Natl. Acad. Sci. USA 2012, 109, 4146–4151. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Lu, Q. Plasma membrane-derived extracellular microvesicles mediate non-canonical intercellular NOTCH signaling. Nat. Commun. 2017, 8, 1–9. [Google Scholar] [CrossRef]
- Kowal, J.; Arras, G.; Colombo, M.; Jouve, M.; Morath, J.P.; Primdal-Bengtson, B.; Dingli, F.; Loew, D.; Tkach, M.; Théry, C. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc. Natl. Acad. Sci. USA 2016, 113, E968–E977. [Google Scholar] [CrossRef] [Green Version]
- Guan, F.; Xiang, X.; Xie, Y.; Li, H.; Zhang, W.; Shu, Y.; Wang, J.; Qin, W. Simultaneous metabolomics and proteomics analysis of plasma-derived extracellular vesicles. Anal. Methods 2021, 13, 1930–1938. [Google Scholar] [CrossRef]
- Luxmi, R.; King, S.M. Cilia-derived vesicles: An ancient route for intercellular communication. Semin. Cell Dev. Biol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Freitas, D.; Kim, H.S.; Fabijanic, K.; Li, Z.; Chen, H.; Mark, M.T.; Molina, H.; Martin, A.B.; Bojmar, L.; et al. Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation. Nat. Cell Biol. 2018, 20, 332–343. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Higginbotham, J.N.; Jeppesen, D.; Yang, Y.-P.; Li, W.; McKinley, E.T.; Graves-Deal, R.; Ping, J.; Britain, C.M.; Dorsett, K.A.; et al. Transfer of Functional Cargo in Exomeres. Cell Rep. 2019, 27, 940–954.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathieu, M.; Nevo, N.; Jouve, M.; Valenzuela, J.I.; Maurin, M.; Verweij, F.J.; Palmulli, R.; Lankar, D.; Dingli, F.; Loew, D.; et al. Specificities of exosome versus small ectosome secretion revealed by live intracellular tracking of CD63 and CD9. Nat. Commun. 2021, 12, 4389. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carayon, K.; Chaoui, K.; Ronzier, E.; Lazar, I.; Bertrand-Michel, J.; Roques, V.; Balor, S.; Terce, F.; Lopez, A.; Salomé, L.; et al. Proteolipidic Composition of Exosomes Changes during Reticulocyte Maturation*. J. Biol. Chem. 2011, 286, 34426–34439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thangaraju, K.; Neerukonda, S.N.; Katneni, U.; Buehler, P.W. Extracellular Vesicles from Red Blood Cells and Their Evolving Roles in Health, Coagulopathy and Therapy. Int. J. Mol. Sci. 2020, 22, 153. [Google Scholar] [CrossRef]
- Hijmans, J.G.; Stockelman, K.A.; Garcia, V.; Levy, M.V.; Brewster, L.M.; Bammert, T.D.; Greiner, J.J.; Stauffer, B.L.; Connick, E.; DeSouza, C.A. Circulating Microparticles Are Elevated in Treated HIV-1 Infection and Are Deleterious to Endothelial Cell Function. J. Am. Heart Assoc. 2019, 8, e011134. [Google Scholar] [CrossRef]
- Prakash, P.S.; Caldwell, C.C.; Lentsch, A.B.; Pritts, T.A.; Robinson, B.R. Human microparticles generated during sepsis in patients with critical illness are neutrophil-derived and modulate the immune response. J. Trauma Acute Care Surg. 2012, 73, 401–406. [Google Scholar] [CrossRef] [Green Version]
- Pantaleo, A.; Ferru, E.; Carta, F.; Mannu, F.; Simula, L.F.; Khadjavi, A.; Pippia, P.; Turrini, F. Irreversible AE1 Tyrosine Phosphorylation Leads to Membrane Vesiculation in G6PD Deficient Red Cells. PLoS ONE 2011, 6, e15847. [Google Scholar] [CrossRef] [Green Version]
- Westerman, M.; Pizzey, A.; Hirschman, J.; Cerino, M.; Weil-Weiner, Y.; Ramotar, P.; Eze, A.; Lawrie, A.; Purdy, G.; Mackie, I.; et al. Microvesicles in haemoglobinopathies offer insights into mechanisms of hypercoagulability, haemolysis and the effects of therapy. Br. J. Haematol. 2008, 142, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Enjeti, A.K.; Ariyarajah, A.; D’Crus, A.; Riveros, C.; Seldon, M.; Lincz, L.F. Circulating microvesicles are less procoagulant and carry different miRNA cargo in myelodysplasia. Blood Cells Mol. Dis. 2018, 74, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Sims, P.J.; Wiedmer, T.; Esmon, C.T.; Weiss, H.J.; Shattil, S.J. Assembly of the platelet prothrombinase complex is linked to vesiculation of the platelet plasma membrane. Studies in Scott syndrome: An isolated defect in platelet procoagulant activity. J. Biol. Chem. 1989, 264, 17049–17057. [Google Scholar] [CrossRef]
- Sproviero, D.; La Salvia, S.; Colombo, F.; Zucca, S.; Pansarasa, O.; Diamanti, L.; Costa, A.; Lova, L.; Giannini, M.; Gagliardi, S.; et al. Leukocyte Derived Microvesicles as Disease Progression Biomarkers in Slow Progressing Amyotrophic Lateral Sclerosis Patients. Front. Neurosci. 2019, 13, 344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guervilly, C.; Lacroix, R.; Forel, J.-M.; Roch, A.; Camoin-Jau, L.; Papazian, L.; Dignat-George, F. High levels of circulating leukocyte microparticles are associated with better outcome in acute respiratory distress syndrome. Crit. Care 2011, 15, R31. [Google Scholar] [CrossRef] [Green Version]
- Chironi, G.; Simon, A.; Hugel, B.; Del Pino, M.; Gariepy, J.; Freyssinet, J.-M.; Tedgui, A. Circulating Leukocyte-Derived Microparticles Predict Subclinical Atherosclerosis Burden in Asymptomatic Subjects. Arter. Thromb. Vasc. Biol. 2006, 26, 2775–2780. [Google Scholar] [CrossRef] [Green Version]
- Puga, M.L.; Menegueti, M.G.; Silvestrini, M.M.A.; Santos, L.J.D.S.; Ferreira-Nogueira, R.; Basile-Filho, A.; Teixeira-Carvalho, A.; Martins-Filho, O.A.; Auxiliadora-Martins, M. Performance of microvesicles as biomarkers of clinical outcome in sepsis and trauma: A pilot study. Biomed. Pharmacother. 2021, 146, 112490. [Google Scholar] [CrossRef]
- Aswad, M.H.; Kissová, J.; Říhová, L.; Zavřelová, J.; Ovesná, P.; Penka, M. High Level of Circulating Microparticles in Patients with BCR/ ABL Negative Myeloproliferative Neoplasm—A Pilot Study. Klin. Onkol. 2019, 32, 109–116. [Google Scholar] [CrossRef]
- Tantawy, A.A.G.; Adly, A.A.M.; Ismail, E.A.R.; Habeeb, N.M.; Farouk, A. Circulating platelet and erythrocyte microparticles in young children and adolescents with sickle cell disease: Relation to cardiovascular complications. Platelets 2012, 24, 605–614. [Google Scholar] [CrossRef]
- Willms, E.; Cabañas, C.; Mäger, I.; Wood, M.J.A.; Vader, P. Extracellular Vesicle Heterogeneity: Subpopulations, Isolation Techniques, and Diverse Functions in Cancer Progression. Front. Immunol. 2018, 9, 738. [Google Scholar] [CrossRef] [Green Version]
- Nigro, A.; Finardi, A.; Ferraro, M.M.; Manno, D.E.; Quattrini, A.; Furlan, R.; Romano, A. Selective loss of microvesicles is a major issue of the differential centrifugation isolation protocols. Sci. Rep. 2021, 11, 3589. [Google Scholar] [CrossRef]
- Kugeratski, F.G.; Atkinson, S.J.; Neilson, L.J.; Lilla, S.; Knight, J.R.P.; Serneels, J.; Juin, A.; Ismail, S.; Bryant, D.M.; Markert, E.K.; et al. Hypoxic cancer-associated fibroblasts increase NCBP2-AS2/HIAR to promote endothelial sprouting through enhanced VEGF signaling. Sci. Signal. 2019, 12, eaan8247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stam, J.; Bartel, S.; Bischoff, R.; Wolters, J.C. Isolation of extracellular vesicles with combined enrichment methods. J. Chromatogr. B 2021, 1169, 122604. [Google Scholar] [CrossRef] [PubMed]
- Menck, K.; Bleckmann, A.; Schulz, M.; Ries, L.; Binder, C. Isolation and Characterization of Microvesicles from Peripheral Blood. J. Vis. Exp. 2017, e55057. [Google Scholar] [CrossRef] [Green Version]
- Gasser, O.; Schifferli, J.A. Microparticles released by human neutrophils adhere to erythrocytes in the presence of complement. Exp. Cell Res. 2005, 307, 381–387. [Google Scholar] [CrossRef]
- Pluskota, E.; Woody, N.M.; Szpak, D.; Ballantyne, C.M.; Soloviev, D.A.; Simon, D.I.; Plow, E.F. Expression, activation, and function of integrin alphaMbeta2 (Mac-1) on neutrophil-derived microparticles. Blood 2008, 112, 2327–2335. [Google Scholar] [CrossRef] [Green Version]
- Thom, S.R.; Bhopale, V.M.; Yu, K.; Huang, W.; Kane, M.A.; Margolis, D.J. Neutrophil microparticle production and inflammasome activation by hyperglycemia due to cytoskeletal instability. J. Biol. Chem. 2017, 292, 18312–18324. [Google Scholar] [CrossRef] [Green Version]
- Mesri, M.; Altieri, D.C. Endothelial cell activation by leukocyte microparticles. J. Immunol. 1998, 161, 4382–4387. [Google Scholar]
- Mesri, M.; Altieri, D.C. Leukocyte Microparticles Stimulate Endothelial Cell Cytokine Release and Tissue Factor Induction in a JNK1 Signaling Pathway. J. Biol. Chem. 1999, 274, 23111–23118. [Google Scholar] [CrossRef] [Green Version]
- Gomez, I.; Ward, B.; Souilhol, C.; Recarti, C.; Ariaans, M.; Johnston, J.; Burnett, A.; Mahmoud, M.; Luong, L.A.; West, L.; et al. Neutrophil microvesicles drive atherosclerosis by delivering miR-155 to atheroprone endothelium. Nat. Commun. 2020, 11, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Pitanga, T.N.; França, L.D.A.; Rocha, V.C.J.; Meirelles, T.; Borges, V.M.; Gonçalves, M.S.; Pontes-De-Carvalho, L.C.; Noronha-Dutra, A.A.; Dos-Santos, W.L.C. Neutrophil-derived microparticles induce myeloperoxidase-mediated damage of vascular endothelial cells. BMC Cell Biol. 2014, 15, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.-P.; Wang, X.-Y.; Pan, X.-Y.; Hu, W.-W.; Cai, S.-T.; Joshi, K.; Deng, L.-H.; Ma, D. Circulating Neutrophil-Derived Microparticles Associated with the Prognosis of Patients with Sepsis. J. Inflamm. Res. 2020, ume 13, 1113–1124. [Google Scholar] [CrossRef]
- Boscolo, A.; Campello, E.; Bertini, D.; Spiezia, L.; Lucchetta, V.; Piasentini, E.; Radu, C.M.; Manesso, L.; Ori, C.; Simioni, P. Levels of circulating microparticles in septic shock and sepsis-related complications: A case-control study. Minerva Anestesiol. 2019, 85, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Wang, J.; Li, T.; Cui, P.; Hou, B.; Zhuang, C.; Wei, G.; Zhang, S.; Li, H.; Hu, Y. Predicting disease progression in advanced non-small cell lung cancer with circulating neutrophil-derived and platelet-derived microparticles. BMC Cancer 2021, 21, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Liu, C.; Bi, Z.-Y.; Zhou, Q.; Zhang, H.; Li, L.-L.; Zhang, J.; Song, Y.-Y.; Zhang, F.; Yang, H.-M.; et al. Comprehensive landscape of extracellular vesicle-derived RNAs in cancer initiation, progression, metastasis and cancer immunology. Mol. Cancer 2020, 19, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Leal, A.C.; Mizurini, D.M.; Gomes, T.; Rochael, N.C.; Saraiva, E.M.; Dias, M.S.; Werneck, C.C.; Sielski, M.S.; Vicente, C.P.; Monteiro, R.Q. Tumor-Derived Exosomes Induce the Formation of Neutrophil Extracellular Traps: Implications For The Establishment of Cancer-Associated Thrombosis. Sci. Rep. 2017, 7, 6438. [Google Scholar] [CrossRef]
- Chiva-Blanch, G.; Padró, T.; Alonso, R.; Crespo, J.; de Isla, L.P.; Mata, P.; Badimon, L. Liquid Biopsy of Extracellular Microvesicles Maps Coronary Calcification and Atherosclerotic Plaque in Asymptomatic Patients With Familial Hypercholesterolemia. Arter. Thromb. Vasc. Biol. 2019, 39, 945–955. [Google Scholar] [CrossRef] [Green Version]
- Eken, C.; Sadallah, S.; Martin, P.J.; Treves, S.; Schifferli, J.A. Ectosomes of polymorphonuclear neutrophils activate multiple signaling pathways in macrophages. Immunobiology 2012, 218, 382–392. [Google Scholar] [CrossRef]
- Rhys, H.I.; Dell’Accio, F.; Pitzalis, C.; Moore, A.; Norling, L.V.; Perretti, M. Neutrophil Microvesicles from Healthy Control and Rheumatoid Arthritis Patients Prevent the Inflammatory Activation of Macrophages. EBioMedicine 2018, 29, 60–69. [Google Scholar] [CrossRef] [Green Version]
- Guimaraes Junior, M.H.; Ferrari, T.C.A.; Teixeira-Carvalho, A.; Moreira, M.L.; de Souza Santos, L.J.; Costa-Silva, M.F.; Coelho, R.M.P.; Pinto, P.; Ris, T.H.; Salles, J.T.; et al. Cell-derived microvesicles in infective endocarditis: Role in diagnosis and potential for risk stratification at hospital admission. J. Infect. 2019, 79, 101–107. [Google Scholar] [CrossRef]
- Baka, Z.; Senolt, L.; Vencovsky, J.; Mann, H.; Simon, P.S.; Kittel, Á.; Buzás, E.; Nagy, G. Increased serum concentration of immune cell derived microparticles in polymyositis/dermatomyositis. Immunol. Lett. 2010, 128, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Suades, R.; Padro, T.; Alonso, R.; Lopez-Miranda, J.; Mata, P.; Badimon, L. Circulating CD45+/CD3+ lymphocyte-derived microparticles map lipid-rich atherosclerotic plaques in familial hypercholesterolaemia patients. Thromb Haemost. 2014, 111, 111–121. [Google Scholar] [CrossRef]
- Benameur, T.; Soleti, R.; Porro, C.; Andriantsitohaina, R.; Martínez, M.C. Microparticles Carrying Sonic Hedgehog Favor Neovascularization through the Activation of Nitric Oxide Pathway in Mice. PLoS ONE 2010, 5, e12688. [Google Scholar] [CrossRef] [Green Version]
- Meziani, F.; Tesse, A.; David, E.; Martinez, M.C.; Wangesteen, R.; Schneider, F.; Andriantsitohaina, R. Shed Membrane Particles from Preeclamptic Women Generate Vascular Wall Inflammation and Blunt Vascular Contractility. Am. J. Pathol. 2006, 169, 1473–1483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiva-Blanch, G.; Suades, R.; Crespo, J.; Vilahur, G.; Arderiu, G.; Padro, T.; Corella, D.; Salas-Salvado, J.; Aros, F.; Martinez-Gonzalez, M.A.; et al. CD3(+)/CD45(+) and SMA-alpha(+) circulating microparticles are increased in individuals at high cardiovascular risk who will develop a major cardiovascular event. Int. J. Cardiol. 2016, 208, 147–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boysen, J.; Nelson, M.; Magzoub, G.; Maiti, G.P.; Sinha, S.; Goswami, M.; Vesely, S.K.; Shanafelt, T.D.; Kay, N.E.; Ghosh, A.K. Dynamics of microvesicle generation in B-cell chronic lymphocytic leukemia: Implication in disease progression. Leukemia 2016, 31, 350–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frleta, D.; Ochoa, C.E.; Kramer, H.B.; Khan, S.A.; Stacey, A.R.; Borrow, P.; Kessler, B.M.; Haynes, B.F.; Bhardwaj, N. HIV-1 infection–induced apoptotic microparticles inhibit human DCs via CD44. J. Clin. Investig. 2012, 122, 4685–4697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tesse, A.; Martinez, M.C.; Hugel, B.; Chalupsky, K.; Muller, C.D.; Meziani, F.; Mitolo-Chieppa, D.; Freyssinet, J.M.; Andriantsitohaina, R. Upregulation of proinflammatory proteins through NF-kappaB pathway by shed membrane microparticles results in vascular hyporeactivity. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 2522–2527. [Google Scholar] [CrossRef] [Green Version]
- Mostefai, H.A.; Agouni, A.; Carusio, N.; Mastronardi, M.L.; Heymes, C.; Henrion, D.; Andriantsitohaina, R.; Martinez, M.C. Phosphatidylinositol 3-kinase and xanthine oxidase regulate nitric oxide and reactive oxygen species productions by apoptotic lymphocyte microparticles in endothelial cells. J. Immunol. 2008, 180, 5028–5035. [Google Scholar] [CrossRef] [Green Version]
- Mallat, Z.; Hugel, B.; Ohan, J.; Leseche, G.; Freyssinet, J.M.; Tedgui, A. Shed membrane microparticles with procoagulant potential in human atherosclerotic plaques: A role for apoptosis in plaque thrombogenicity. Circulation 1999, 99, 348–353. [Google Scholar] [CrossRef] [Green Version]
- Cai, C.; Tahiri, H.; Fortin, C.; Ortiz, C.; Sintjago, H.; Yang, C.; Hardy, P. Lymphocytic microparticles suppress retinal angiogenesis via targeting Müller cells in the ischemic retinopathy mouse model. Exp. Cell Res. 2021, 399, 112470. [Google Scholar] [CrossRef] [PubMed]
- Cochran, A.M.; Kornbluth, J. Extracellular Vesicles From the Human Natural Killer Cell Line NK3.3 Have Broad and Potent Anti-Tumor Activity. Front. Cell Dev. Biol. 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-H.; Li, J.; Li, L.; Sun, J.; Fabbri, M.; Wayne, A.S.; Seeger, R.C.; Jong, A.Y. Extracellular vesicles derived from natural killer cells use multiple cytotoxic proteins and killing mechanisms to target cancer cells. J. Extracell. Vesicles 2019, 8, 1588538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mikhailova, V.A.; Ovchinnikova, O.M.; Zainulina, M.S.; Sokolov, D.I.; Sel’Kov, S.A. Detection of Microparticles of Leukocytic Origin in the Peripheral Blood in Normal Pregnancy and Preeclampsia. Bull. Exp. Biol. Med. 2014, 157, 751–756. [Google Scholar] [CrossRef] [PubMed]
- Tamari, T.; Brenner, B.; Aharon, A. Monocyte-derived microparticles and exosomes induce procoagulant and apoptotic effects on endothelial cells. Thromb. Haemost. 2008, 100, 878–885. [Google Scholar] [CrossRef]
- Shet, A.; Aras, O.; Gupta, K.; Hass, M.J.; Rausch, D.J.; Saba, N.; Koopmeiners, L.; Key, N.S.; Hebbel, R.P. Sickle blood contains tissue factor–positive microparticles derived from endothelial cells and monocytes. Blood 2003, 102, 2678–2683. [Google Scholar] [CrossRef] [Green Version]
- Lok, C.A.R.; Van Der Post, J.A.M.; Sargent, I.L.; Hau, C.M.; Sturk, A.; Boer, K.; Nieuwland, R. Changes in Microparticle Numbers and Cellular Origin During Pregnancy and Preeclampsia. Hypertens. Pregnancy 2008, 27, 344–360. [Google Scholar] [CrossRef]
- Tőkés-Füzesi, M.; Ruzsics, I.; Rideg, O.; Kustán, P.; Kovács, G.L.; Molnár, T. Role of microparticles derived from monocytes, endothelial cells and platelets in the exacerbation of COPD. Int. J. Chronic Obstr. Pulm. Dis. 2018, ume 13, 3749–3757. [Google Scholar] [CrossRef] [Green Version]
- Nomura, S.; Kanazawa, S.; Fukuhara, S. Effects of efonidipine on platelet and monocyte activation markers in hypertensive patients with and without type 2 diabetes mellitus. J. Hum. Hypertens. 2002, 16, 539–547. [Google Scholar] [CrossRef] [Green Version]
- Omoto, S.; Nomura, S.; Shouzu, A.; Nishikawa, M.; Fukuhara, S.; Iwasaka, T. Detection of monocyte-derived microparticles in patients with Type II diabetes mellitus. Diabetologia 2002, 45, 550–555. [Google Scholar] [CrossRef] [Green Version]
- Ogata, N.; Nomura, S.; Shouzu, A.; Imaizumi, M.; Arichi, M.; Matsumura, M. Elevation of monocyte-derived microparticles in patients with diabetic retinopathy. Diabetes Res. Clin. Pract. 2006, 73, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Holvoet, P.; Vanhaverbeke, M.; Bloch, K.; Baatsen, P.; Sinnaeve, P.; Janssens, S. Low MT-CO1 in Monocytes and Microvesicles Is Associated With Outcome in Patients With Coronary Artery Disease. J. Am. Heart Assoc. 2016, 5. [Google Scholar] [CrossRef] [PubMed]
- Gerotziafas, G.T.; Van Dreden, P.; Chaari, M.; Galea, V.; Khaterchi, A.; Lionnet, F.; Stankovic-Stojanovic, K.; Blanc-Brude, O.; Woodhams, B.; Maier-Redelsperger, M.; et al. The acceleration of the propagation phase of thrombin generation in patients with steady-state sickle cell disease is associated with circulating erythrocyte-derived microparticles. Thromb. Haemost. 2012, 107, 1044–1052. [Google Scholar] [CrossRef] [PubMed]
- Camus, S.M.; Gausserès, B.; Bonnin, P.; Loufrani, L.; Grimaud, L.; Charue, D.; De Moraes, J.A.; Renard, J.-M.; Tedgui, A.; Boulanger, C.M.; et al. Erythrocyte microparticles can induce kidney vaso-occlusions in a murine model of sickle cell disease. Blood 2012, 120, 5050–5058. [Google Scholar] [CrossRef] [Green Version]
- Freitas Leal, J.K.; Preijers, F.; Brock, R.; Adjobo-Hermans, M.; Bosman, G. Red Blood Cell Homeostasis and Altered Vesicle Formation in Patients With Paroxysmal Nocturnal Hemoglobinuria. Front. Physiol. 2019, 10, 578. [Google Scholar] [CrossRef] [Green Version]
- Nantakomol, D.; Palasuwan, A.; Chaowanathikhom, M.; Soogarun, S.; Imwong, M. Red cell and platelet-derived microparticles are increased in G6PD-deficient subjects. Eur. J. Haematol. 2012, 89, 423–429. [Google Scholar] [CrossRef]
- Peng, L.; Li, Y.; Li, X.; Du, Y.; Li, L.; Hu, C.; Zhang, J.; Qin, Y.; Wei, Y.; Zhang, H. Extracellular Vesicles Derived from Intermittent Hypoxia–Treated Red Blood Cells Impair Endothelial Function Through Regulating eNOS Phosphorylation and ET-1 Expression. Cardiovasc. Drugs Ther. 2020, 35, 901–913. [Google Scholar] [CrossRef]
- Khalyfa, A.; Sanz-Rubio, D. The Mystery of Red Blood Cells Extracellular Vesicles in Sleep Apnea with Metabolic Dysfunction. Int. J. Mol. Sci. 2021, 22, 4301. [Google Scholar] [CrossRef]
- Giannopoulos, G.; Oudatzis, G.; Paterakis, G.; Synetos, A.; Tampaki, E.; Bouras, G.; Hahalis, G.; Alexopoulos, D.; Tousoulis, D.; Cleman, M.W.; et al. Red blood cell and platelet microparticles in myocardial infarction patients treated with primary angioplasty. Int. J. Cardiol. 2014, 176, 145–150. [Google Scholar] [CrossRef]
- Yuan, Y.; Maitusong, M.; Muyesai, N. Association of endothelial and red blood cell microparticles with acute myocardial infarction in Chinese: A retrospective study. Ann. Palliat. Med. 2020, 9, 1564–1570. [Google Scholar] [CrossRef]
- Mantel, P.-Y.; Hoang, A.N.; Goldowitz, I.; Potashnikova, D.; Hamza, B.; Vorobjev, I.; Ghiran, I.; Toner, M.; Irimia, D.; Ivanov, A.R.; et al. Malaria-Infected Erythrocyte-Derived Microvesicles Mediate Cellular Communication within the Parasite Population and with the Host Immune System. Cell Host Microbe 2013, 13, 521–534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bebesi, T.; Kitka, D.; Gaál, A.; Szigyártó, I.C.; Deák, R.; Beke-Somfai, T.; Koprivanacz, K.; Juhász, T.; Bóta, A.; Varga, Z.; et al. Storage conditions determine the characteristics of red blood cell derived extracellular vesicles. Sci. Rep. 2022, 12, 977. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xi, J.; Hao, X.; Deng, W.; Liu, J.; Wei, C.; Gao, Y.; Zhang, L.; Wang, H. Red blood cells release microparticles containing human argonaute 2 and miRNAs to target genes of Plasmodium falciparum. Emerg. Microbes Infect. 2017, 6, e75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, M.; Wang, Y.; Zhu, Q.; Zhao, J.; Wang, Y.; Shang, M.; Liu, M.; Wu, Y.; Song, J.; Liu, Y. Protective effects of circulating microvesicles derived from ischemic preconditioning on myocardial ischemia/reperfusion injury in rats by inhibiting endoplasmic reticulum stress. Apoptosis 2018, 23, 436–448. [Google Scholar] [CrossRef] [PubMed]
- Lamontagne-Proulx, J.; St-Amour, I.; Labib, R.; Pilon, J.; Denis, H.L.; Cloutier, N.; Roux-Dalvai, F.; Vincent, A.T.; Mason, S.L.; Williams-Gray, C.; et al. Portrait of blood-derived extracellular vesicles in patients with Parkinson’s disease. Neurobiol. Dis. 2018, 124, 163–175. [Google Scholar] [CrossRef] [Green Version]
- Sheng, L.; Stewart, T.; Yang, D.; Thorland, E.; Soltys, D.; Aro, P.; Khrisat, T.; Xie, Z.; Li, N.; Liu, Z.; et al. Erythrocytic α-synuclein contained in microvesicles regulates astrocytic glutamate homeostasis: A new perspective on Parkinson’s disease pathogenesis. Acta Neuropathol. Commun. 2020, 8, 1–22. [Google Scholar] [CrossRef]
- Helal, O.; Defoort, C.; Robert, S.; Marin, C.; Lesavre, N.; Lopez-Miranda, J.; Risérus, U.; Basu, S.; Lovegrove, J.; McMonagle, J.; et al. Increased levels of microparticles originating from endothelial cells, platelets and erythrocytes in subjects with metabolic syndrome: Relationship with oxidative stress. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 665–671. [Google Scholar] [CrossRef]
- Zwaal, R.F.; Comfurius, P.; Bevers, E.M. Scott syndrome, a bleeding disorder caused by defective scrambling of membrane phospholipids. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2004, 1636, 119–128. [Google Scholar] [CrossRef]
- Tantawy, A.A.G.; Matter, R.M.; Hamed, A.A.; Telbany, M.A.S.E.D.E. Platelet microparticles in immune thrombocytopenic purpura in pediatrics. Pediatr. Hematol. Oncol. 2010, 27, 283–296. [Google Scholar] [CrossRef]
- Galli, M.; Grassi, A.; Barbui, T. Platelet-derived microvesicles in thrombotic thrombocytopenic purpura and hemolytic uremic syndrome. Thromb. Haemost. 1996, 75, 427–431. [Google Scholar] [CrossRef]
- Sewify, E.M.; Sayed, D.; Aal, R.F.A.; Ahmad, H.M.; Abdou, M.A. Increased circulating red cell microparticles (RMP) and platelet microparticles (PMP) in immune thrombocytopenic purpura. Thromb. Res. 2013, 131, e59–e63. [Google Scholar] [CrossRef] [PubMed]
- Catani, L.; Cavo, M.; Palandri, F. The Power of Extracellular Vesicles in Myeloproliferative Neoplasms: “Crafting” a Microenvironment That Matters. Cells 2021, 10, 2316. [Google Scholar] [CrossRef] [PubMed]
- Tahmasbi, L.; Karimi, M.; Kafiabadi, S.A.; Nikougoftar, M.; Haghpanah, S.; Ranjbaran, R.; Moghadam, M. Evaluation of Plasma Platelet Microparticles in Thrombotic Thrombocytopenic Purpura. Ann. Clin. Lab. Sci. 2017, 47, 62–67. [Google Scholar] [PubMed]
- Campello, E.; Spiezia, L.; Radu, C.M.; Bulato, C.; Gavasso, S.; Tormene, D.; Woodhams, B.; Valle, F.D.; Simioni, P. Circulating microparticles and the risk of thrombosis in inherited deficiencies of antithrombin, protein C and protein S. Thromb. Haemost. 2016, 115, 81–88. [Google Scholar] [CrossRef] [Green Version]
- Lincz, L.F.; Scorgie, F.; Enjeti, A.; Seldon, M. Variable plasma levels of Factor V Leiden correlate with circulating platelet microparticles in carriers of Factor V Leiden. Thromb. Res. 2012, 129, 192–196. [Google Scholar] [CrossRef]
- Trappenburg, M.C.; Van Schilfgaarde, M.; Marchetti, M.; Spronk, H.M.; ten Cate, H.; Leyte, A.; Terpstra, W.E.; Falanga, A. Elevated procoagulant microparticles expressing endothelial and platelet markers in essential thrombocythemia. Haematologica 2009, 94, 911–918. [Google Scholar] [CrossRef] [Green Version]
- Nebor, D.; Bowers, A.; Connes, P.; Hardy-Dessources, M.-D.; Knight-Madden, J.; Cumming, V.; Reid, M.; Romana, M. Plasma Concentration of Platelet-Derived Microparticles Is Related to Painful Vaso-Occlusive Phenotype Severity in Sickle Cell Anemia. PLoS ONE 2014, 9, e87243. [Google Scholar] [CrossRef]
- Vats, R.; Brzoska, T.; Bennewitz, M.F.; Jimenez, M.A.; Pradhan-Sundd, T.; Tutuncuoglu, E.; Jonassaint, J.; Gutierrez, E.; Watkins, S.C.; Shiva, S.; et al. Platelet Extracellular Vesicles Drive Inflammasome-IL-1beta-Dependent Lung Injury in Sickle Cell Disease. Am. J. Respir. Crit Care Med. 2020, 201, 33–46. [Google Scholar] [CrossRef]
- Namba, M.; Tanaka, A.; Shimada, K.; Ozeki, Y.; Uehata, S.; Sakamoto, T.; Nishida, Y.; Nomura, S.; Yoshikawa, J. Circulating platelet-derived microparticles are associated with atherothrombotic events: A marker for vulnerable blood. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 255–256. [Google Scholar] [CrossRef]
- Gkaliagkousi, E.; Nikolaidou, B.; Gavriilaki, E.; Lazaridis, A.; Yiannaki, E.; Anyfanti, P.; Zografou, I.; Markala, D.; Douma, S. Increased erythrocyte- and platelet-derived microvesicles in newly diagnosed type 2 diabetes mellitus. Diabetes Vasc. Dis. Res. 2019, 16, 458–465. [Google Scholar] [CrossRef]
- Rodrigues, K.F.; Pietrani, N.T.; Fernandes, A.P.; Bosco, A.A.; de Sousa, M.C.R.; Silva, I.D.F.O.; Silveira, J.N.; Campos, F.M.F.; Gomes, K.B. Circulating microparticles levels are increased in patients with diabetic kidney disease: A case-control research. Clin. Chim. Acta 2018, 479, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, K.; Morishita, E.; Sekiya, A.; Omote, M.; Kadono, T.; Asakura, H.; Hashimoto, M.; Kobayashi, M.; Nakatsumi, Y.; Takada, S.; et al. Plasma Levels of Platelet-Derived Microparticles in Patients with Obstructive Sleep Apnea Syndrome. J. Atheroscler. Thromb. 2012, 19, 98–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, J.; Xing, H.; Sha, S.; Li, J.; Miao, Y.; Zhang, Q. Analysis of Circulating Microvesicles Levels and Effects of Associated Factors in Elderly Patients With Obstructive Sleep Apnea. Front. Aging Neurosci. 2021, 13, 48. [Google Scholar] [CrossRef] [PubMed]
- Ando, M.; Iwata, A.; Ozeki, Y.; Tsuchiya, K.; Akiba, T.; Nihei, H. Circulating platelet-derived microparticles with procoagulant activity may be a potential cause of thrombosis in uremic patients. Kidney Int. 2002, 62, 1757–1763. [Google Scholar] [CrossRef] [Green Version]
- Burton, J.; Hamali, H.A.; Singh, R.; Abbasian, N.; Parsons, R.; Patel, A.; Goodall, A.; Brunskill, N.J. Elevated Levels of Procoagulant Plasma Microvesicles in Dialysis Patients. PLoS ONE 2013, 8, e72663. [Google Scholar] [CrossRef] [Green Version]
- Duchez, A.-C.; Boudreau, L.H.; Naika, G.S.; Bollinger, J.; Belleannée, C.; Cloutier, N.; Laffont, B.; Mendoza-Villarroel, R.E.; Levesque, T.; Rollet-Labelle, E.; et al. Platelet microparticles are internalized in neutrophils via the concerted activity of 12-lipoxygenase and secreted phospholipase A2-IIA. Proc. Natl. Acad. Sci. USA 2015, 112, E3564–E3573. [Google Scholar] [CrossRef] [Green Version]
- Tessandier, N.; Melki, I.; Cloutier, N.; Allaeys, I.; Miszta, A.; Tan, S.; Milasan, A.; Michel, S.; Benmoussa, A.; Lévesque, T.; et al. Platelets Disseminate Extracellular Vesicles in Lymph in Rheumatoid Arthritis. Arter. Thromb. Vasc. Biol. 2020, 40, 929–942. [Google Scholar] [CrossRef]
- Trappenburg, M.C.; Van Schilfgaarde, M.; Frerichs, F.C.; Spronk, H.M.; Cate, H.T.; De Fijter, C.W.; Terpstra, W.E.; Leyte, A. Chronic renal failure is accompanied by endothelial activation and a large increase in microparticle numbers with reduced procoagulant capacity. Nephrol. Dial. Transplant. 2011, 27, 1446–1453. [Google Scholar] [CrossRef] [Green Version]
- Szilágyi, B.; Fejes, Z.; Rusznyák, Á.; Fenyvesi, F.; Pócsi, M.; Halmi, S.; Griger, Z.; Kunapuli, S.P.; Kappelmayer, J.; Nagy, B.J. Platelet Microparticles Enriched in miR-223 Reduce ICAM-1-Dependent Vascular Inflammation in Septic Conditions. Front. Physiol. 2021, 12, 691. [Google Scholar] [CrossRef]
- Soriano, S.; Carmona, A.; Triviño, F.; Rodriguez, M.; Alvarez-Benito, M.; Martín-Malo, A.; Alvarez-Lara, M.-A.; Ramírez, R.; Aljama, P.; Carracedo, J. Endothelial damage and vascular calcification in patients with chronic kidney disease. Am. J. Physiol. Physiol. 2014, 307, F1302–F1311. [Google Scholar] [CrossRef] [Green Version]
- Brill, A.; Dashevsky, O.; Rivo, J.; Gozal, Y.; Varon, D. Platelet-derived microparticles induce angiogenesis and stimulate post-ischemic revascularization. Cardiovasc. Res. 2005, 67, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Dow, R.; Ridger, V. Neutrophil microvesicles and their role in disease. Int. J. Biochem. Cell Biol. 2021, 141, 106097. [Google Scholar] [CrossRef] [PubMed]
- Zhan, D.; McConachie, E.; Edwards, S.; Wright, H.; Moots, R.; Honsawek, S. Isolation of Microvesicles from Human Circulating Neutrophils. BIO-PROTOCOL 2021, 11. [Google Scholar] [CrossRef]
- Sarkar, A.; Mitra, S.; Mehta, S.; Raices, R.; Wewers, M.D. Monocyte Derived Microvesicles Deliver a Cell Death Message via Encapsulated Caspase-1. PLoS ONE 2009, 4, e7140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aupeix, K.; Hugel, B.; Martin, T.; Bischoff, P.; Lill, H.; Pasquali, J.L.; Freyssinet, J.M. The significance of shed membrane particles during programmed cell death in vitro, and in vivo, in HIV-1 infection. J. Clin. Investig. 1997, 99, 1546–1554. [Google Scholar] [CrossRef] [PubMed]
- Shefler, I.; Pasmanik-Chor, M.; Kidron, D.; Mekori, Y.A.; Hershko, A.Y. T cell–derived microvesicles induce mast cell production of IL-24: Relevance to inflammatory skin diseases. J. Allergy Clin. Immunol. 2014, 133, 217–224.e3. [Google Scholar] [CrossRef]
- Del Conde, I.; Shrimpton, C.N.; Thiagarajan, P.; Lopez, J.A. Tissue-factor-bearing microvesicles arise from lipid rafts and fuse with activated platelets to initiate coagulation. Blood 2005, 106, 1604–1611. [Google Scholar] [CrossRef]
- Pelissier Vatter, F.A.; Cioffi, M.; Hanna, S.J.; Castarede, I.; Caielli, S.; Pascual, V.; Matei, I.; Lyden, D. Extracellular vesicle- and particle-mediated communication shapes innate and adaptive immune responses. J. Exp. Med. 2021, 218, e20202579. [Google Scholar] [CrossRef]
- Wang, N.-F.; Zhao, C.-S.; You, Y.-M.; Wang, Y.-J.; Liu, F.; Cai, F.-F.; Zhang, D.-D. Relationship between Leukocytes Derived Microparticles and Minimal Residual Disease and Prognosis of Acute Myeloid Leukemia. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2021, 29, 325–348. [Google Scholar]
- Lehner, G.F.; Harler, U.; Haller, V.M.; Feistritzer, C.; Hasslacher, J.; Dunzendorfer, S.; Bellmann, R.; Joannidis, M. Characterization of Microvesicles in Septic Shock Using High-Sensitivity Flow Cytometry. Shock 2016, 46, 373–381. [Google Scholar] [CrossRef] [Green Version]
- Priou, P.; Gagnadoux, F.; Tesse, A.; Mastronardi, M.L.; Agouni, A.; Meslier, N.; Racineux, J.-L.; Martinez, M.C.; Trzepizur, W.; Andriantsitohaina, R. Endothelial Dysfunction and Circulating Microparticles from Patients with Obstructive Sleep Apnea. Am. J. Pathol. 2010, 177, 974–983. [Google Scholar] [CrossRef] [PubMed]
- Trzepizur, W.; Martinez, M.C.; Priou, P.; Andriantsitohaina, R.; Gagnadoux, F. Microparticles and vascular dysfunction in obstructive sleep apnoea. Eur. Respir. J. 2014, 44, 207–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayers, L.; Stoewhas, A.-C.; Ferry, B.; Latshang, T.D.; Cascio, C.M.L.; Sadler, R.; Stadelmann, K.; Tesler, N.; Huber, R.; Achermann, P.; et al. Circulating levels of cell-derived microparticles are reduced by mild hypobaric hypoxia: Data from a randomised controlled trial. Eur. J. Appl. Physiol. 2014, 114, 1067–1073. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, C.; Wei, Y.; Yang, S.; Cui, C.; Yang, J.; Zhang, J.; Qiao, R. Increased circulating microparticles in women with preeclampsia. Int. J. Lab. Hematol. 2018, 40, 352–358. [Google Scholar] [CrossRef]
- Gasser, O.; Hess, C.; Miot, S.; Deon, C.; Sanchez, J.-C.; Schifferli, J.A. Characterisation and properties of ectosomes released by human polymorphonuclear neutrophils. Exp. Cell Res. 2003, 285, 243–257. [Google Scholar] [CrossRef]
- Dalli, J.; Norling, L.V.; Renshaw, D.; Cooper, D.; Leung, K.-Y.; Perretti, M. Annexin 1 mediates the rapid anti-inflammatory effects of neutrophil-derived microparticles. Blood 2008, 112, 2512–2519. [Google Scholar] [CrossRef] [Green Version]
- Kolonics, F.; Kajdácsi, E.; Farkas, V.J.; Veres, D.S.; Khamari, D.; Kittel, Á.; Merchant, M.L.; McLeish, K.R.; Lőrincz, M.; Ligeti, E. Neutrophils produce proinflammatory or anti-inflammatory extracellular vesicles depending on the environmental conditions. J. Leukoc. Biol. 2020, 109, 793–806. [Google Scholar] [CrossRef]
- Timar, C.; Lőrincz, Á.M.; Ligeti, E. Changing world of neutrophils. Pflügers Arch.-Eur. J. Physiol. 2013, 465, 1521–1533. [Google Scholar] [CrossRef]
- Ajikumar, A.; Long, M.B.; Heath, P.R.; Wharton, S.B.; Ince, P.G.; Ridger, V.C.; Simpson, J.E. Neutrophil-Derived Microvesicle Induced Dysfunction of Brain Microvascular Endothelial Cells In Vitro. Int. J. Mol. Sci. 2019, 20, 5227. [Google Scholar] [CrossRef] [Green Version]
- El Habhab, A.; Altamimy, R.; Abbas, M.; Kassem, M.; Amoura, L.; Qureshi, A.W.; El Itawi, H.; Kreutter, G.; Khemais-Benkhiat, S.; Zobairi, F.; et al. Significance of neutrophil microparticles in ischaemia-reperfusion: Pro-inflammatory effectors of endothelial senescence and vascular dysfunction. J. Cell Mol. Med. 2020, 24, 7266–7281. [Google Scholar] [CrossRef]
- Slater, T.W.; Finkielsztein, A.; Mascarenhas, L.A.; Mehl, L.C.; Butin-Israeli, V.; Sumagin, R. Neutrophil Microparticles Deliver Active Myeloperoxidase to Injured Mucosa To Inhibit Epithelial Wound Healing. J. Immunol. 2017, 198, 2886–2897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Headland, S.E.; Jones, H.R.; Norling, L.V.; Kim, A.; Souza, P.R.; Corsiero, E.; Gil, C.D.; Nerviani, A.; Dell’Accio, F.; Pitzalis, C.; et al. Neutrophil-derived microvesicles enter cartilage and protect the joint in inflammatory arthritis. Sci. Transl. Med. 2015, 7, 315ra190. [Google Scholar] [CrossRef] [PubMed]
- Cointe, S.; Vallier, L.; Esnault, P.; Dacos, M.; Bonifay, A.; Macagno, N.; Souab, K.H.; Chareyre, C.; Judicone, C.; Frankel, D.; et al. Granulocyte microvesicles with a high plasmin generation capacity promote clot lysis and improve outcome in septic shock. Blood 2022, 139, 2377–2391. [Google Scholar] [CrossRef] [PubMed]
- Kalina, T.; Fišer, K.; Pérez-Andrés, M.; Kuzílková, D.; Cuenca, M.; Bartol, S.J.W.; Blanco, E.; Engel, P.; Van Zelm, M.C. CD Maps—Dynamic Profiling of CD1–CD100 Surface Expression on Human Leukocyte and Lymphocyte Subsets. Front. Immunol. 2019, 10, 2434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, Q.; Xiong, W.; Yang, C.; Gagnon, C.; Hardy, P. Lymphocyte-derived microparticles induce bronchial epithelial cells’ pro-inflammatory cytokine production and apoptosis. Mol. Immunol. 2013, 55, 220–230. [Google Scholar] [CrossRef]
- De Luca, L.; D’Arena, G.; Simeon, V.; Trino, S.; Laurenzana, I.; Caivano, A.; La Rocca, F.; Villani, O.; Mansueto, G.; Deaglio, S.; et al. Characterization and prognostic relevance of circulating microvesicles in chronic lymphocytic leukemia. Leuk. Lymphoma. 2017, 58, 1424–1432. [Google Scholar] [CrossRef]
- Scanu, A.; Molnarfi, N.; Brandt, K.J.; Gruaz, L.; Dayer, J.M.; Burger, D. Stimulated T cells generate microparticles, which mimic cellular contact activation of human monocytes: Differential regulation of pro- and anti-inflammatory cytokine production by high-density lipoproteins. J. Leukoc. Biol. 2008, 83, 921–927. [Google Scholar] [CrossRef] [Green Version]
- Angelillo-Scherrer, A. Leukocyte-Derived Microparticles in Vascular Homeostasis. Circ. Res. 2012, 110, 356–369. [Google Scholar] [CrossRef] [Green Version]
- AgouTii, A.; Mostefai, H.A.; Porro, C.; Carusio, N.; Favre, J.; Richard, V.; Henrion, D.; Martinez, M.C.; Andriantsitohaina, R. Sonic hedgehog carried by microparticles corrects endothelial injury through nitric oxide release. FASEB J. 2007, 21, 2735–2741. [Google Scholar] [CrossRef] [Green Version]
- Martínez, M.C.; Larbret, F.; Zobairi, F.; Coulombe, J.; Debili, N.; Vainchenker, W.; Ruat, M.; Freyssinet, J.-M. Transfer of differentiation signal by membrane microvesicles harboring hedgehog morphogens. Blood 2006, 108, 3012–3020. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.; Mwaikambo, B.R.; Zhu, T.; Gagnon, C.; LaFleur, J.; Seshadri, S.; Lachapelle, P.; Lavoie, J.-C.; Chemtob, S.; Hardy, P. Lymphocytic microparticles inhibit angiogenesis by stimulating oxidative stress and negatively regulating VEGF-induced pathways. Am. J. Physiol. Integr. Comp. Physiol. 2008, 294, R467–R476. [Google Scholar] [CrossRef] [PubMed]
- VanWijk, M.J.; Svedas, E.; Boer, K.; Nieuwland, R.; van Bavel, E.; Kublickiene, K. Isolated microparticles, but not whole plasma, from women with preeclampsia impair endothelium-dependent relaxation in isolated myometrial arteries from healthy pregnant women. Am. J. Obstet. Gynecol. 2002, 187, 1686–1693. [Google Scholar] [CrossRef] [PubMed]
- Federici, C.; Shahaj, E.; Cecchetti, S.; Camerini, S.; Casella, M.; Iessi, E.; Camisaschi, C.; Paolino, G.; Calvieri, S.; Ferro, S.; et al. Natural-killer-derived extracellular vesicles: Immune sensors and interactors. Front. Immunol. 2020, 11, 262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Markova, K.; Mikhailova, V.; Milyutina, Y.; Korenevsky, A.; Sirotskaya, A.; Rodygina, V.; Tyshchuk, E.; Grebenkina, P.; Simbirtsev, A.; Selkov, S.; et al. Effects of Microvesicles Derived from NK Cells Stimulated with IL-1beta on the Phenotype and Functional Activity of Endothelial Cells. Int. J. Mol. Sci. 2021, 22, 13663. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Xie, M.; Hun, M.; She, Z.; Li, C.; Luo, S.; Chen, X.; Wan, W.; Wen, C.; Tian, J. Natural Killer Cell-Derived Extracellular Vesicles: Novel Players in Cancer Immunotherapy. Front. Immunol. 2021, 12, 1970. [Google Scholar] [CrossRef]
- Satta, N.; Freyssinet, J.; Toti, F. The significance of human monocyte thrombomodulin during membrane vesiculation and after stimulation by lipopolysaccharide. Br. J. Haematol. 1997, 96, 534–542. [Google Scholar] [CrossRef]
- Aleman, M.M.; Gardiner, C.; Harrison, P.; Wolberg, A.S. Differential contributions of monocyte- and platelet-derived microparticles towards thrombin generation and fibrin formation and stability. J. Thromb. Haemost. 2011, 9, 2251–2261. [Google Scholar] [CrossRef] [Green Version]
- Rauch, U.; Bonderman, D.; Bohrmann, B.; Badimon, J.J.; Himber, J.; Riederer, M.A.; Nemerson, Y. Transfer of tissue factor from leukocytes to platelets is mediated by CD15 and tissue factor. Blood 2000, 96, 170–175. [Google Scholar] [CrossRef]
- Poitevin, S.; Cochery-Nouvellon, E.; Dupont, A.; Nguyen, P. Monocyte IL-10 produced in response to lipopolysaccharide modulates thrombin generation by inhibiting tissue factor expression and release of active tissue factor-bound microparticles. Thromb. Haemost. 2007, 97, 598–607. [Google Scholar] [CrossRef]
- Pérez-Casal, M.; Downey, C.; Fukudome, K.; Marx, G.; Toh, C.H. Activated protein C induces the release of microparticle-associated endothelial protein C receptor. Blood 2005, 105, 1515–1522. [Google Scholar] [CrossRef]
- Satta, N.; Toti, F.; Feugeas, O.; Bohbot, A.; Dachary-Prigent, J.; Eschwège, V.; Hedman, H.; Freyssinet, J.M. Monocyte vesiculation is a possible mechanism for dissemination of membrane-associated procoagulant activities and adhesion molecules after stimulation by lipopolysaccharide. J. Immunol. 1994, 153, 3245–3255. [Google Scholar] [PubMed]
- Falati, S.; Liu, Q.; Gross, P.; Merrill-Skoloff, G.; Chou, J.; Vandendries, E.; Celi, A.; Croce, K.; Furie, B.C.; Furie, B. Accumulation of Tissue Factor into Developing Thrombi In Vivo Is Dependent upon Microparticle P-Selectin Glycoprotein Ligand 1 and Platelet P-Selectin. J. Exp. Med. 2003, 197, 1585–1598. [Google Scholar] [CrossRef] [PubMed]
- Chou, J.; Mackman, N.; Merrill-Skoloff, G.; Pedersen, B.; Furie, B.C.; Furie, B. Hematopoietic cell-derived microparticle tissue factor contributes to fibrin formation during thrombus propagation. Blood 2004, 104, 3190–3197. [Google Scholar] [CrossRef] [Green Version]
- Steppich, B.; Mattisek, C.; Sobczyk, D.; Kastrati, A.; Schömig, A.; Ott, I. Tissue factor pathway inhibitor on circulating microparticles in acute myocardial infarction. Thromb. Haemost. 2005, 93, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.G.; Williams, J.C.; Davis, B.K.; Jacobson, K.; Doerschuk, C.M.; Ting, J.P.; Mackman, N. Monocytic microparticles activate endothelial cells in an IL-1beta-dependent manner. Blood 2011, 118, 2366–2374. [Google Scholar] [CrossRef] [Green Version]
- Eyre, J.; Burton, J.O.; Saleem, M.A.; Mathieson, P.W.; Topham, P.S.; Brunskill, N.J. Monocyte- and Endothelial-Derived Microparticles Induce an Inflammatory Phenotype in Human Podocytes. Nephron 2011, 119, e58–e66. [Google Scholar] [CrossRef]
- Carmona, A.; Guerrero, F.; Jimenez, M.J.; Ariza, F.; Agüera, M.L.; Obrero, T.; Noci, V.; Muñoz-Castañeda, J.R.; Rodríguez, M.; Soriano, S.; et al. Inflammation, Senescence and MicroRNAs in Chronic Kidney Disease. Front. Cell Dev. Biol. 2020, 8, 739. [Google Scholar] [CrossRef]
- Nomura, S.; Takahashi, N.; Inami, N.; Kajiura, T.; Yamada, K.; Nakamori, H.; Tsuda, N. Probucol and ticlopidine: Effect on platelet and monocyte activation markers in hyperlipidemic patients with and without type 2 diabetes. Atherosclerosis 2004, 174, 329–335. [Google Scholar] [CrossRef]
- Li, S.; Wei, J.; Zhang, C.; Li, X.; Meng, W.; Mo, X.; Zhang, Q.; Liu, Q.; Ren, K.; Du, R.; et al. Cell-Derived Microparticles in Patients with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Cell. Physiol. Biochem. 2016, 39, 2439–2450. [Google Scholar] [CrossRef]
- Keating, F.K.; Butenas, S.; Fung, M.K.; Schneider, D.J. Platelet-white blood cell (WBC) interaction, WBC apoptosis, and procoagulant activity in stored red blood cells. Transfusion 2010, 51, 1086–1095. [Google Scholar] [CrossRef]
- Pinheiro, M.K.; Tamagne, M.; Elayeb, R.; Andrieu, M.; Pirenne, F.; Vingert, B. Blood microparticles are a component of immune modulation in red blood cell transfusion. Eur. J. Immunol. 2020, 50, 1237–1240. [Google Scholar] [CrossRef] [PubMed]
- Bosman, G.J.; Lasonder, E.; Luten, M.; Roerdinkholder-Stoelwinder, B.; Novotny, V.M.; Bos, H.; De Grip, W.J. The proteome of red cell membranes and vesicles during storage in blood bank conditions. Transfusion 2008, 48, 827–835. [Google Scholar] [PubMed]
- Berckmans, R.J.; Nieuwland, R.; Böing, A.N.; Romijn, F.P.; Hack, C.E.; Sturk, A. Cell-derived microparticles circulate in healthy humans and support low grade thrombin generation. Thromb. Haemost. 2001, 85, 639–649. [Google Scholar] [PubMed]
- Koshiar, R.L.; Somajo, S.; Norström, E.; Dahlbäck, B. Erythrocyte-Derived Microparticles Supporting Activated Protein C-Mediated Regulation of Blood Coagulation. PLoS ONE 2014, 9, e104200. [Google Scholar] [CrossRef] [Green Version]
- Johansen, M.E.; Bidot, C.; Horstman, L.L.; Ahn, Y.S.; Jy, W. Red cell-derived microparticles (RMP) as haemostatic agent. Thromb. Haemost. 2013, 110, 751–760. [Google Scholar] [CrossRef] [Green Version]
- Van Der Meijden, P.E.; Van Schilfgaarde, M.; Van Oerle, R.; Renne, T.; ten Cate, H.; Spronk, H.M. Platelet- and erythrocyte-derived microparticles trigger thrombin generation via factor XIIa. J. Thromb. Haemost. 2012, 10, 1355–1362. [Google Scholar]
- Biro, E.; Sturk-Maquelin, K.N.; Vogel, G.M.T.; Meuleman, D.G.; Smit, M.J.; Hack, C.E.; Sturk, A.; Nieuwland, R. Human cell-derived microparticles promote thrombus formation in vivo in a tissue factor-dependent manner. J. Thromb. Haemost. 2003, 1, 2561–2568. [Google Scholar] [CrossRef] [Green Version]
- Bouchard, B.; Orfeo, T.; Keith, H.N.; Lavoie, E.M.; Gissel, M.; Fung, M.; Mann, K.G. Microparticles formed during storage of red blood cell units support thrombin generation. J. Trauma Acute Care Surg. 2018, 84, 598–605. [Google Scholar] [CrossRef]
- Donadee, C.; Raat, N.; Kanias, T.; Tejero, J.; Lee, J.; Kelley, E.; Zhao, X.; Liu, C.; Reynolds, H.; Azarov, I.; et al. Nitric Oxide Scavenging by Red Blood Cell Microparticles and Cell-Free Hemoglobin as a Mechanism for the Red Cell Storage Lesion. Circulation 2011, 124, 465–476. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, W.; Christ, G.J.; Gladwin, M.T.; Kim-Shapiro, D.B. Nitric oxide scavenging by red cell microparticles. Free Radic. Biol. Med. 2013, 65, 1164–1173. [Google Scholar] [CrossRef] [Green Version]
- Nader, E.; Romana, M.; Guillot, N.; Fort, R.; Stauffer, E.; Lemonne, N.; Garnier, Y.; Skinner, S.C.; Etienne-Julan, M.; Robert, M.; et al. Association Between Nitric Oxide, Oxidative Stress, Eryptosis, Red Blood Cell Microparticles, and Vascular Function in Sickle Cell Anemia. Front. Immunol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Merle, N.S.; Grunenwald, A.; Rajaratnam, H.; Gnemmi, V.; Frimat, M.; Figueres, M.-L.; Knockaert, S.; Bouzekri, S.; Charue, D.; Noe, R.; et al. Intravascular hemolysis activates complement via cell-free heme and heme-loaded microvesicles. JCI Insight 2018, 3, e96910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaichompoo, P.; Kumya, P.; Khowawisetsut, L.; Chiangjong, W.; Chaiyarit, S.; Pongsakul, N.; Sirithanaratanakul, N.; Fucharoen, S.; Thongboonkerd, V.; Pattanapanyasat, K. Characterizations and proteome analysis of platelet-free plasma-derived microparticles in beta-thalassemia/hemoglobin E patients. J. Proteomics. 2012, 76, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Fernandez Bello, I.; Jimenez-Yuste, V.; de Paz, R.; Martin Salces, M.; Justo Sanz, R.; Monzon Manzano, E.; Arias-Salgado, E.G.; Alvarez Roman, M.T.; Rivas Pollmar, M.I.; Goyanes, I.; et al. Factors Involved in Maintaining Haemostasis in Patients with Myelodysplastic Syndrome. Thromb. Haemost. 2018, 118, 734–744. [Google Scholar] [CrossRef]
- Delvasto-Nunez, L.; Roem, D.; Bakhtiari, K.; van Mierlo, G.; Meijers, J.C.M.; Jongerius, I.; Zeerleder, S.S. Iron-Driven Alterations on Red Blood Cell-Derived Microvesicles Amplify Coagulation during Hemolysis via the Intrinsic Tenase Complex. Thromb. Haemost. 2021, 122, 80–91. [Google Scholar]
- Said, A.S.; Rogers, S.; Doctor, A. Physiologic Impact of Circulating RBC Microparticles upon Blood-Vascular Interactions. Front. Physiol. 2018, 8, 1120. [Google Scholar] [CrossRef]
- Zecher, D.; Cumpelik, A.; Schifferli, J.A. Erythrocyte-Derived Microvesicles Amplify Systemic Inflammation by Thrombin-Dependent Activation of Complement. Arter. Thromb. Vasc. Biol. 2014, 34, 313–320. [Google Scholar] [CrossRef] [Green Version]
- Perrotta, S.; Gallagher, P.G.; Mohandas, N. Hereditary spherocytosis. Lancet 2008, 372, 1411–1426. [Google Scholar] [CrossRef]
- Awojoodu, A.O.; Keegan, P.M.; Lane, A.R.; Zhang, Y.; Lynch, K.R.; Platt, M.O.; Botchwey, E.A. Acid sphingomyelinase is activated in sickle cell erythrocytes and contributes to inflammatory microparticle generation in SCD. Blood 2014, 124, 1941–1950. [Google Scholar] [CrossRef] [Green Version]
- Neidlinger, N.A.; Larkin, S.K.; Bhagat, A.; Victorino, G.P.; Kuypers, F.A. Hydrolysis of Phosphatidylserine-exposing Red Blood Cells by Secretory Phospholipase A2 Generates Lysophosphatidic Acid and Results in Vascular Dysfunction. J. Biol. Chem. 2006, 281, 775–781. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, J.; Stewart, T.; Sheng, L.; Li, N.; Bullock, K.; Song, N.; Shi, M.; Banks, W.A.; Zhang, J. Transmission of alpha-synuclein-containing erythrocyte-derived extracellular vesicles across the blood-brain barrier via adsorptive mediated transcytosis: Another mechanism for initiation and progression of Parkinson’s disease? Acta Neuropathol. Commun. 2017, 5, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borgheti-Cardoso, L.N.; Kooijmans, S.A.; Chamorro, L.G.; Biosca, A.; Lantero, E.; Ramírez, M.; Avalos-Padilla, Y.; Crespo, I.; Fernández, I.; Fernandez-Becerra, C.; et al. Extracellular vesicles derived from Plasmodium-infected and non-infected red blood cells as targeted drug delivery vehicles. Int. J. Pharm. 2020, 587, 119627. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, S.; Dumoga, S.; Singh, N. Red blood cells membrane-derived nanoparticles: Applications and key challenges in their clinical translation. WIREs Nanomed. Nanobiotechnol. 2022, 14, e1776. [Google Scholar] [CrossRef] [PubMed]
- Tzounakas, V.L.; Anastasiadi, A.T.; Lekka, M.E.; Papageorgiou, E.G.; Stamoulis, K.; Papassideri, I.S.; Kriebardis, A.G.; Antonelou, M.H. Deciphering the Relationship Between Free and Vesicular Hemoglobin in Stored Red Blood Cell Units. Front. Physiol. 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Tzounakas, V.L.; Stamoulis, K.E.; Anastasiadi, A.T.; Papassideri, I.S.; Kriebardis, A.G.; Rinalducci, S.; Antonelou, M.H. Leukoreduction makes a difference: A pair proteomics study of extracellular vesicles in red blood cell units. Transfus. Apher. Sci. 2021, 60, 103166. [Google Scholar] [CrossRef] [PubMed]
- Levin, G.; Sukhareva, E. The influence of thermal trauma on pro- and anticoagulant activity of erythrocyte-derived microvesicles. Burns 2016, 42, 1528–1533. [Google Scholar] [CrossRef]
- Levin, G.Y.; Sukhareva, E.G. Antithrombin Activity of Erythrocyte Microvesicles. Bull. Exp. Biol. Med. 2017, 162, 718–721. [Google Scholar] [CrossRef]
- Noubouossie, D.F.; Henderson, M.W.; Mooberry, M.; Ilich, A.; Ellsworth, P.; Piegore, M.; Skinner, S.C.; Pawlinski, R.; Welsby, I.; Renné, T.; et al. Red blood cell microvesicles activate the contact system, leading to factor IX activation via 2 independent pathways. Blood 2020, 135, 755–765. [Google Scholar] [CrossRef]
- Hashemi Tayer, A.; Amirizadeh, N.; Ahmadinejad, M.; Nikougoftar, M.; Deyhim, M.R.; Zolfaghari, S. Procoagulant Activity of Red Blood Cell-Derived Microvesicles during Red Cell Storage. Transfus. Med. Hemother. 2019, 46, 224–230. [Google Scholar] [CrossRef]
- Said, A.S.; Doctor, A. Influence of red blood cell-derived microparticles upon vasoregulation. Blood Transfus. 2017, 15, 522. [Google Scholar] [CrossRef]
- Gao, Y.; Jin, H.; Tan, H.; Cai, X.; Sun, Y. Erythrocyte-derived extracellular vesicles aggravate inflammation by promoting the proinflammatory macrophage phenotype through TLR4-MyD88-NF-kappaB-MAPK pathway. J. Leukoc. Biol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Jank, H.; Salzer, U. Vesicles generated during storage of red blood cells enhance the generation of radical oxygen species in activated neutrophils. Sci. World J. 2011, 11, 173–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sloand, E.M.; Mainwaring, L.; Keyvanfar, K.; Chen, J.; Maciejewski, J.; Klein, H.G.; Young, N.S. Transfer of glycosylphosphatidylinositol-anchored proteins to deficient cells after erythrocyte transfusion in paroxysmal nocturnal hemoglobinuria. Blood 2004, 104, 3782–3788. [Google Scholar] [CrossRef] [PubMed]
- Dahlbaeck, B.; Wiedmer, T.; Sims, P.J. Binding of anticoagulant vitamin K-dependent protein S to platelet-derived microparticles. Biochemistry 1992, 31, 12769–12777. [Google Scholar] [CrossRef]
- Lindemann, S.; Tolley, N.D.; Dixon, D.A.; McIntyre, T.M.; Prescott, S.M.; Zimmerman, G.A.; Weyrich, A.S. Activated platelets mediate inflammatory signaling by regulated interleukin 1beta synthesis. J. Cell Biol. 2001, 154, 485–490. [Google Scholar] [CrossRef] [Green Version]
- Boudreau, L.H.; Duchez, A.-C.; Cloutier, N.; Soulet, D.; Martin, N.; Bollinger, J.; Paré, A.; Rousseau, M.; Naika, G.S.; Lévesque, T.; et al. Platelets release mitochondria serving as substrate for bactericidal group IIA-secreted phospholipase A2 to promote inflammation. Blood 2014, 124, 2173–2183. [Google Scholar] [CrossRef] [Green Version]
- Dinkla, S.; Van Cranenbroek, B.; Van Der Heijden, W.A.; He, X.; Wallbrecher, R.; Dumitriu, I.E.; Van Der Ven, A.J.; Bosman, G.J.C.G.M.; Koenen, H.J.P.M.; Joosten, I. Platelet microparticles inhibit IL-17 production by regulatory T cells through P-selectin. Blood 2016, 127, 1976–1986. [Google Scholar] [CrossRef] [Green Version]
- Badimon, L.; Suades, R.; Fuentes, E.; Palomo, I.; Padró, T. Role of Platelet-Derived Microvesicles As Crosstalk Mediators in Atherothrombosis and Future Pharmacology Targets: A Link between Inflammation, Atherosclerosis, and Thrombosis. Front. Pharmacol. 2016, 7, 293. [Google Scholar] [CrossRef] [Green Version]
- György, B.; Pálóczi, K.; Kovács, A.; Barabás, E.; Bekő, G.; Várnai, K.; Pállinger, É.; Szabó-Taylor, K.; Szabó, T.G.; Kiss, A.A.; et al. Improved circulating microparticle analysis in acid-citrate dextrose (ACD) anticoagulant tube. Thromb. Res. 2014, 133, 285–292. [Google Scholar] [CrossRef] [Green Version]
- Palviainen, M.; Saraswat, M.; Varga, Z.; Kitka, D.; Neuvonen, M.; Puhka, M.; Joenväärä, S.; Renkonen, R.; Nieuwland, R.; Takatalo, M.; et al. Extracellular vesicles from human plasma and serum are carriers of extravesicular cargo—Implications for biomarker discovery. PLoS ONE 2020, 15, e0236439. [Google Scholar] [CrossRef]
- Burgelman, M.; Vandendriessche, C.; Vandenbroucke, R.E. Extracellular Vesicles: A Double-Edged Sword in Sepsis. Pharmaceuticals 2021, 14, 829. [Google Scholar] [CrossRef] [PubMed]
- Mause, S.F.; Ritzel, E.; Liehn, E.A.; Hristov, M.; Bidzhekov, K.; Muller-Newen, G.; Soehnlein, O.; Weber, C. Platelet Microparticles Enhance the Vasoregenerative Potential of Angiogenic Early Outgrowth Cells After Vascular Injury. Circulation 2010, 122, 495–506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kailashiya, J.; Gupta, V.; Dash, D.; Zhang, C.R.; Cristino, A.S.; Grady, J.P.; Fink, J.L.; Moore, A.S. Engineered human platelet-derived microparticles as natural vectors for targeted drug delivery. Oncotarget 2019, 10, 5835–5846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tripisciano, C.; Weiss, R.; Karuthedom George, S.; Fischer, M.B.; Weber, V. Extracellular Vesicles Derived From Platelets, Red Blood Cells, and Monocyte-Like Cells Differ Regarding Their Ability to Induce Factor XII-Dependent Thrombin Generation. Front. Cell Dev. Biol. 2020, 8, 298. [Google Scholar] [CrossRef] [PubMed]
- Keuren, J.F.; Magdeleyns, E.J.; Govers-Riemslag, J.W.; Lindhout, T.; Curvers, J. Effects of storage-induced platelet microparticles on the initiation and propagation phase of blood coagulation. Br. J. Haematol. 2006, 134, 307–313. [Google Scholar] [CrossRef] [PubMed]
| White Blood Cell-Derived MVs | |||||||
|---|---|---|---|---|---|---|---|
| MVs Type | MVs Source | Isolation/Characterization Method | MVs Phenotype | Function/Role | Disease | Effects/Relation to Disease | References |
| Neutrophil-derived MVs (NMVs) | Stimulation of isolated PMNs (cell cultures) | Centrifugation/Flow Cytometry | PS+ NMVs | Classic pathway of complement activation | Systemic inflammation diseases | [36] | |
| Stimulation of isolated PMNs (cell cultures) | Centrifugation/Flow Cytometry | CD11b+ CD18+ MVs | Platelet activation | Atherosclerosis, Chronic Prothrombotic States, Cardiovascular Diseases | Pro-thrombotic effects | [37] | |
| Stimulation of isolated PMNs (cell cultures) | Centrifugation/Flow Cytometry | IL-1β+ NMVs | Immunomodulatory roles | Hyperglycemia | [38] | ||
| Stimulation of isolated PMNs (cell cultures) | Centrifugation/Flow Cytometry | miR-155 enriched NMVs | Induce cytokine release by ECs and stress signaling pathways | Vascular Inflammation, Atherosclerosis | Endothelial dysfunction, Atherosclerotic plaque development | [39,40,41] | |
| Stimulation of isolated PMNs (cell cultures) | Differential centrifugation/Flow Cytometry | MPO+ NMVs | ECs damage and vascular dysfunction | Vasculitis, Sepsis | [42] | ||
| Blood plasma | Differential centrifugation/Flow Cytometry | CD66b+ MVs accumulation | Pro-inflammatory effects | Sepsis, Trauma, Non-Small Cell Lung Cancer | Clinical outcome, Disease progression | [28,43,44,45,46,47] | |
| Blood plasma | Differential centrifugation/Flow Cytometry | CD11b+ CD66+ PS+ MVs accumulation | Familial Hypercholesterolemia | Cardiovascular risk and coronary calcification and atherosclerotic plaque burden biomarker | [48] | ||
| Stimulation of isolated PMNs (cell cultures) | Centrifugation/Flow cytometry | Annexin A1+ NMVs | Anti-inflammatory cytokines release | Rheumatoid Arthritis | Protective role | [49,50] | |
| Blood plasma | Differential centrifugation/Flow Cytometry | CD66b+ MVs accumulation | Infectious Endocarditis | Independent predictor of mortality | [51] | ||
| Lymphocyte-derived MVs (LMVs) | Blood plasma | Differential centrifugation/Flow Cytometry & TEM | CD3+ and CD19+ MVs accumulation | Pro-inflammatory role | Polymyositis/Dermatomyositis (PM/DM) | Possible role in the pathogenesis of PM/DM | [52] |
| Blood plasma | Differential centrifugation/Flow Cytometry | CD45+ CD3+ PS+ MVs accumulation | Familial Hypercholesterolaemia Patients | Markers of lipid-rich atherosclerotic plaques | [53] | ||
| Human lymphoid CEM T cell line (cell cultures) | Differential centrifugation/Immunoblot analysis | Sonic hedgehog+ LMVs | Endothelial damage repair, neovascularization | Ischemic Cardiovascular Diseases | [54] | ||
| Blood plasma | Differential centrifugation/Flow Cytometry & Elisa kit | CD11a+ MVs accumulation | Pro-inflammatory effects on vessels, endothelial dysfunction in arteries | Pre-eclampsia | [55] | ||
| Blood plasma | Differential centrifugation/Flow Cytometry & Elisa kit | CD45+ CD3+ PS+ MVs accumulation | Cardiovascular Diseases (CVD) | Potential prognostic biomarkers of incident CVD | [56] | ||
| Primary CLL B-cells | Differential centrifugation/Flow Cytometry | CD52+ MVs accumulation | Chronic Lymphocytic Leukemia | Disease progression | [57] | ||
| Apoptotic Lymphocyte-derived MVs (aLMVs) | Apoptotic human lymphoid CEM T cell line (cell cultures) | Differential centrifugation/Flow Cytometry | CD44+ aLMVs accumulation | Inhibition of dendritic cells function | HIV-1 | Promising therapeutic targets | [58] |
| Apoptotic human lymphoid CEM T cell line (cell cultures) & Blood plasma | Differential centrifugation/Prothrombinase assay & Immunostaining | Fas-L+ aLMVs accumulation | Endothelial function impairment, vascular hyporeactivity induction | Diabetes, Inflammatory diseases | [59,60] | ||
| Atherosclerotic plaque-derived aLMVs | Differential centrifugation/Prothrombinase assay & Elisa assay | CD11a+ PS+ and TF+ aLMVs | Procoagulant activity | Atherosclerosis | Plaque thrombogenicity determinants | [61] | |
| Apoptotic human CEM T lymphocytes line (cell cultures) | Differential centrifugation/Flow Cytometry | PS+ aLMVs | Retinal angiogenesis suppression | Ischemic retinopathy | Promising therapeutic approach | [62] | |
| Natural Killer-derived MVs (NKMVs) | Stimulation of NK Cell Line | Differential centrifugation/DLS & Immunoblot analysis | Perforin, granzymes A and B, granulysin, FasL and miRNAs enriched NKMVs | Inhibit proliferation and induce apoptosis of tumor cell lines | Potentially effective, safe, and universal immunotherapeutic agents | [63,64] | |
| Blood samples | Differential centrifugation/Flow Cytometry | Low levels of CD45+ CD16+ CD56+ MVs | Defect in active NK cell death induction | Pre-eclampsia | [65] | ||
| Monocyte-derived MVs (MoMVs) | Stimulation of monocyte cell line (THP-1) | Centrifugation/Flow Cytometry | CD18+ CD14+ PS+ TF+ MVs | Induction of endothelial thrombogenicity and apoptosis | Inflammatory diseases and hypercoagulable states | Endothelial cell dysfunction | [66] |
| Blood plasma | Differential centrifugation/Flow Cytometry | CD14+ PS+ TF+ accumulation in sickle crisis | Procoagulant activity | Sickle Cell Disease | Contribution to thrombotic occlusive events (e.g., stroke) | [67] | |
| Blood plasma | Differential centrifugation/Flow Cytometry | CD11b+ and CD14+ MVs accumulation | Pre-eclampsia | Probably a systemic inflammatory response marker | [68] | ||
| Blood plasma | Differential centrifugation/Flow Cytometry | CD14+ MVs accumulation | Contribute to Chronic obstructive pulmonary disease exacerbations (unknown mechanism) | Chronic obstructive pulmonary disease | Potential predictive biomarker | [69] | |
| Blood plasma | Differential centrifugation/Flow Cytometry | CD14+ MVs accumulation | Cardiovascular complications | Hypertension, hyperlipidemia with type II diabetes | Potential therapeutic target | [70] | |
| Blood plasma | Differential centrifugation/Flow Cytometry | CD14+ PS+ MVs accumulation | Vascular endothelial damage | Type II diabetes mellitus | Potential biomarker of CVD complication | [71] | |
| Blood plasma | Centrifugation/Flow Cytometry | CD14+ PS+ MVs accumulation | Enhancement the procoagulant activity, Adhesion molecules activation by ECs | Diabetic Retinopathy | Microvascular occlusions development, Potential biomarker of diabetic retinopathy progression | [72] | |
| Blood plasma | Centrifugation and Magnetic beads/NTA, TEM, PCR | CD14+ MVs expressing low levels of mitochondrial cytochrome oxidase, subunit I (MT-COI) | Coronary Artery Disease | Predictive marker for CVD risk | [73] | ||
| Red Blood Cell-Derived MVs | ||||||
|---|---|---|---|---|---|---|
| MVs Source | Isolation/Characterization Method | MVs Phenotype | Function/Role | Disease | Effects/Relation to Disease | References |
| Blood plasma | Differential centrifugation/Flow Cytometry | CD235+ PS+ MVs accumulation | Thrombin activation, Nitric Oxide bioavailability disruption | Sickle Cell Disease and Thalassaemia Intermedia | Potential pro-thrombotic marker | [22,74] |
| Blood plasma | Differential centrifugation/Flow Cytometry | CD235a+ PS+ MVs accumulation | Myeloproliferative neoplasms | [29] | ||
| Blood plasma | Differential centrifugation/Flow Cytometry | CD235a+ MVs accumulation | Positive correlation with aortic stiffness, pulmonary artery pressure, and tricuspid regurgitant velocity | Sickle Cell Disease | Potential biomarker for vascular dysfunction and disease severity | [30] |
| TSP-1 stimulated RBCs, isolated from blood | Centrifugation/Flow Cytometry | CD235a+ PS+ MVs accumulation | Endothelial cell damage, vascular dysfunction, renal vaso-occlusion | Sickle Cell Disease (mice) | [75] | |
| Blood plasma | Differential centrifugation/Flow Cytometry | CD235a+ PS- and CD235a+ CD59- MVs accumulation | Disturbed contribution to hemostasis and thrombosis | Paroxysmal Nocturnal Hemoglobinuria | [76] | |
| Blood plasma | Differential centrifugation/Flow Cytometry, Atomic Force Microscopy | CD235a+ PS+ MVs accumulation | Positive correlation with G6PD enzyme activity | G6PD deficiency | Potential biomarker of G6PD deficiency severity | [77] |
| Stimulated RBCs, isolated from blood | Ultracentrifugation/NTA, Immunoblotting analysis and TEM | CD235a+ MVs accumulation | Disturbed the homeostasis of vascular tone, hypertension induction | Obstructive Sleep Apnea | Endothelial dysfunction marker, Obstructive Sleep Apnea severity | [78,79] |
| Blood plasma | Differential centrifugation/Flow Cytometry and TEM | CD235a+ PS+ MVs accumulation | correlation with several coronary artery diseases and adverse clinical events | Myocardial Infarction | [80,81] | |
| RBCs infected with P. falciparum (cell cultures) | Differential centrifugation, filtration, 60% sucrose cushion/Flow cytometry, TEM and Immunoblotting analysis | RMVs-derived from Malaria Infected RBCs | Immunomodulatory properties, Transmission stage parasite development stimulation | Malaria Infection | [82] | |
| RBCs infected with P. falciparum (cell cultures) | Differential centrifugation/Flow Cytometry, PCR, Immunoblotting analysis | Enriched in hAgo2-miRNA complexes RMVs | innate resistance of RBCs to malaria infection | Malaria Infection | Therapeutic potential as drug delivery systems | [83,84] |
| Blood plasma | Differential centrifugation/Flow Cytometry, TEM | TER-119+ MVs accumulation | Cardioprotective effects | Ischemic preconditioning | [85] | |
| Blood plasma | Immuno-capture method/NTA, Immunoblotting analysis and TEM | Enriched in a-synuclein CD235a+ MVs | Rapid crossing of the blood-brain barrier | Parkinson Disease | Progression or initiation disease biomarker | [86,87] |
| Blood plasma | Differential centrifugation/Flow Cytometry | PS+ CD235a+ MVs accumulation | Association with hyperlipidemia | Metabolic Syndrome | [88] | |
| Platelet-Derived MVs | ||||||
|---|---|---|---|---|---|---|
| MVs Source | Isolation/Characterization Method | MVs Phenotype | Function/Role | Disease | Effects/Relation to Disease | References |
| Blood plasma | Differential centrifugation/Flow Cytometry | CD41+ MVs accumulation | Positive correlation with aortic stiffness, pulmonary artery pressure, and tricuspid regurgitant velocity | Sickle Cell Disease | Potential biomarker for vascular dysfunction and disease severity biomarker | [30] |
| Blood plasma/In vitro stimulation of platelets | Differential centrifugation/NTA & Flow Cytometry | Low levels of CD41+ MVs | Reduced or defected procoagulant activity | Scott syndrome, myelodysplastic syndromes | High bleeding risk | [23,24,89] |
| Blood plasma | Differential centrifugation/Flow Cytometry | CD41+ MVs accumulation | Hypercoagulability state and thrombotic events, Pro-thrombotic tendency | Immune thrombocytopenic purpura, Thrombocytopenia, Myeloproliferative Neoplasms | Protection against bleeding events | [90,91,92,93] |
| Blood plasma | Differential centrifugation/DLS, Flow Cytometry | CD41+ MVs accumulation | Thrombotic events | Thrombotic Thrombocytopenic Purpura | [94] | |
| Blood plasma | Differential centrifugation/Flow Cytometry | CD41+ MVs accumulation | Pro-thrombotic potential | Thrombocythemia | Risk factors for thrombosis | [95,96,97] |
| Blood plasma | Differential centrifugation/Flow Cytometry | CD41+ PS+ MVs accumulation in severe SCD | Vaso-occlusive events | Sickle Cell Disease | Disease severity biomarker | [98] |
| Blood plasma | Differential centrifugation/NTA, Elisa kit, Immunoblotting analysis | IL-1β+ and caspase-1+ PMVs accumulation | Lung vaso-occlusion | Sickle Cell Disease | Potential therapeutic targeting | [99] |
| Blood plasma | Differential centrifugation/Elisa kit | -GPIb+ GPIIb/IIIa+ GPIX+ CD9+ PMVs accumulation (Elisa kit) | Correlation with calcification | Acute Coronary Syndrome | Independent predictor for thrombotic events, atherothrombotic events risk | [100] |
| Blood plasma | Differential centrifugation/Flow Cytometry | CD42a+ PS+ MVs accumulation | Association with glycemic profile | Newly diagnosed diabetes mellitus type 2 | Early markers of thrombosis | [101] |
| Blood plasma | Differential centrifugation/Flow Cytometry | CD41+ PS+ MVs accumulation | Correlation with glycemic markers | Diabets Mellitus type 2 | Potential biomarkers for Diabets Mellitus type 2 | [102] |
| Blood plasma | Differential centrifugation/Elisa kit | GPIb+ GPIIb/IIIa+ GPIX+ CD9+ PMVs accumulation (Elisa kit) | Correlation with apneahypopnea index | Obstructive Sleep Apnea | Potential biomarker of increased cardiovascular risk | [103] |
| Blood plasma | Differential centrifugation/Flow Cytometry | CD41a+ PS+ MVs accumulation | Correlation with OSA severity indicators | Obstructive Sleep Apnea | Potential OSA severity biomarker | [104] |
| Blood plasma | Differential centrifugation/Flow Cytometry | CD41a+ PS+ MVs accumulation | Association with thrombotic events | End Stage Renal Disease | Potential triggering factor for thrombotic events | [105] |
| Blood plasma | Differential centrifugation/TEM, NTA, Immunoblotting analysis | CD42b+ MVs accumulation | Procoagulant activity | End Stage Renal Disease | Potential mediators or predictors of occlusive cardiovascular events | [106] |
| Blood plasma | Differential centrifugation/Flow Cytometry | GpΙΙb/ΙΙΙa+ PS+ MVs accumulation | Correlation with glycemia and oxidative stress markers | Metabolic Syndrome | Potential marker of predisposal for diabetes | [88] |
| In vitro stimulation of platelets | Differential centrifugation/Flow Cytometry | Internalization of CD41+ MVs by neutrophils | Inflammatory phenotype of neutrophils induction | Rheumatoid Arthritis | Implication in rheumatoid arthritis pathogenesis | [107,108] |
| Blood plasma | Differential centrifugation/Flow Cytometry | CD41+ PS+ MVs accumulation | Low procoagulant activity | CKD stage 4 | [109] | |
| Blood plasma | Differential centrifugation/Flow Cytometry, PCR | CD41a+ PS+ MVs containing miR-223 accumulation | miR-223-mediated anti-inflammatory effects | Sepsis | Protective role against sepsis-related vascular inflammation | [110] |
| Blood plasma | Differential centrifugation/Flow Cytometry | Low CD41+ MVs levels | Disseminated Intravascular Coagulation (in sepsis) | Better disease outcome | [111] | |
| Blood plasma | Differential centrifugation/Flow Cytometry | CD41+ MVs accumulation | Angiogenic activity | Chronic Ischemia | [112] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Georgatzakou, H.T.; Fortis, S.P.; Papageorgiou, E.G.; Antonelou, M.H.; Kriebardis, A.G. Blood Cell-Derived Microvesicles in Hematological Diseases and beyond. Biomolecules 2022, 12, 803. https://doi.org/10.3390/biom12060803
Georgatzakou HT, Fortis SP, Papageorgiou EG, Antonelou MH, Kriebardis AG. Blood Cell-Derived Microvesicles in Hematological Diseases and beyond. Biomolecules. 2022; 12(6):803. https://doi.org/10.3390/biom12060803
Chicago/Turabian StyleGeorgatzakou, Hara T., Sotirios P. Fortis, Effie G. Papageorgiou, Marianna H. Antonelou, and Anastasios G. Kriebardis. 2022. "Blood Cell-Derived Microvesicles in Hematological Diseases and beyond" Biomolecules 12, no. 6: 803. https://doi.org/10.3390/biom12060803








