Role of Regulatory T Cells in Skeletal Muscle Regeneration: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Review Questions
2.2. Search Strategy
2.3. Selection
2.4. Bias Analysis
3. Results
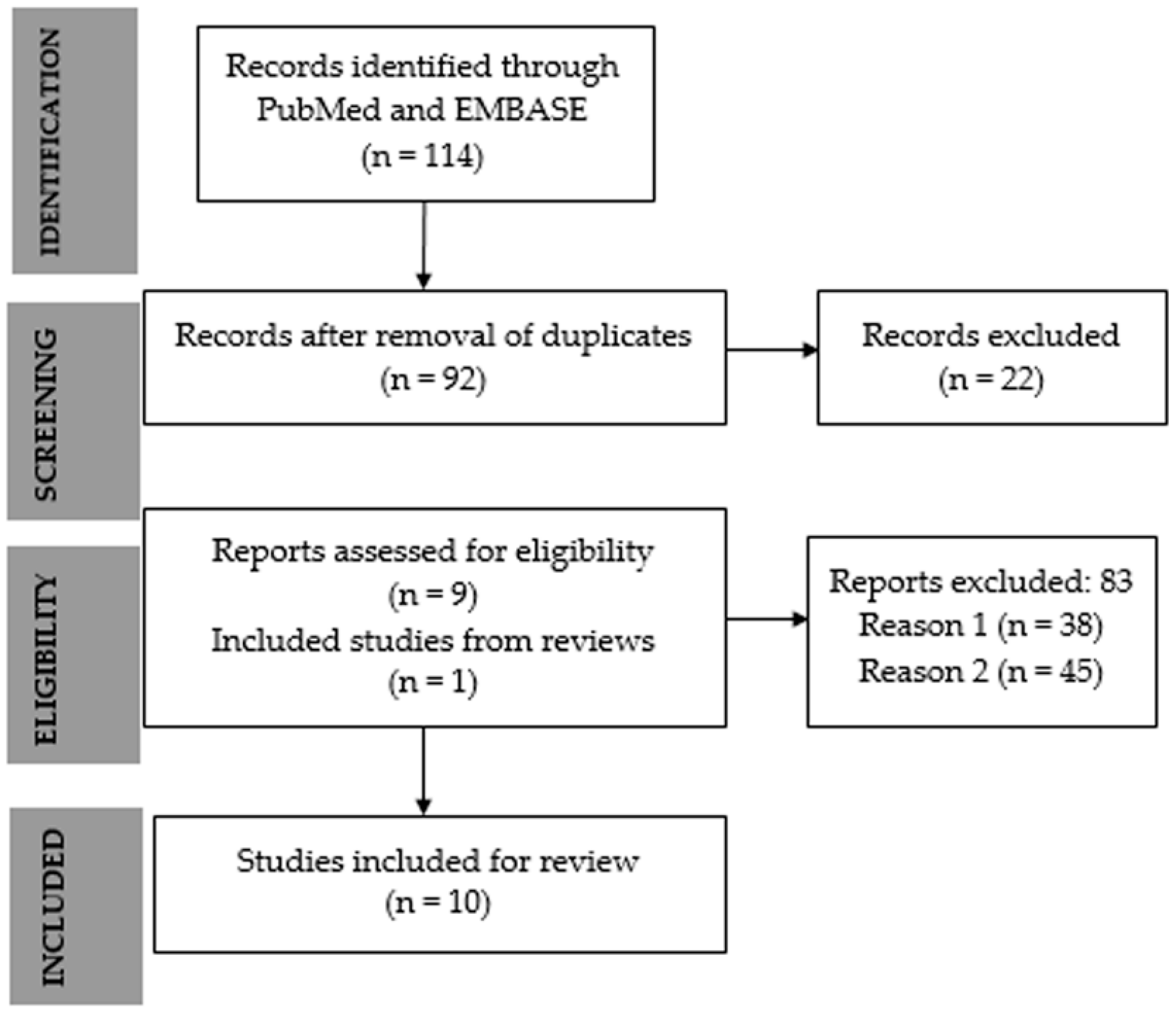
3.1. Search Results
3.2. Quality Assessment
3.3. Study Characteristics
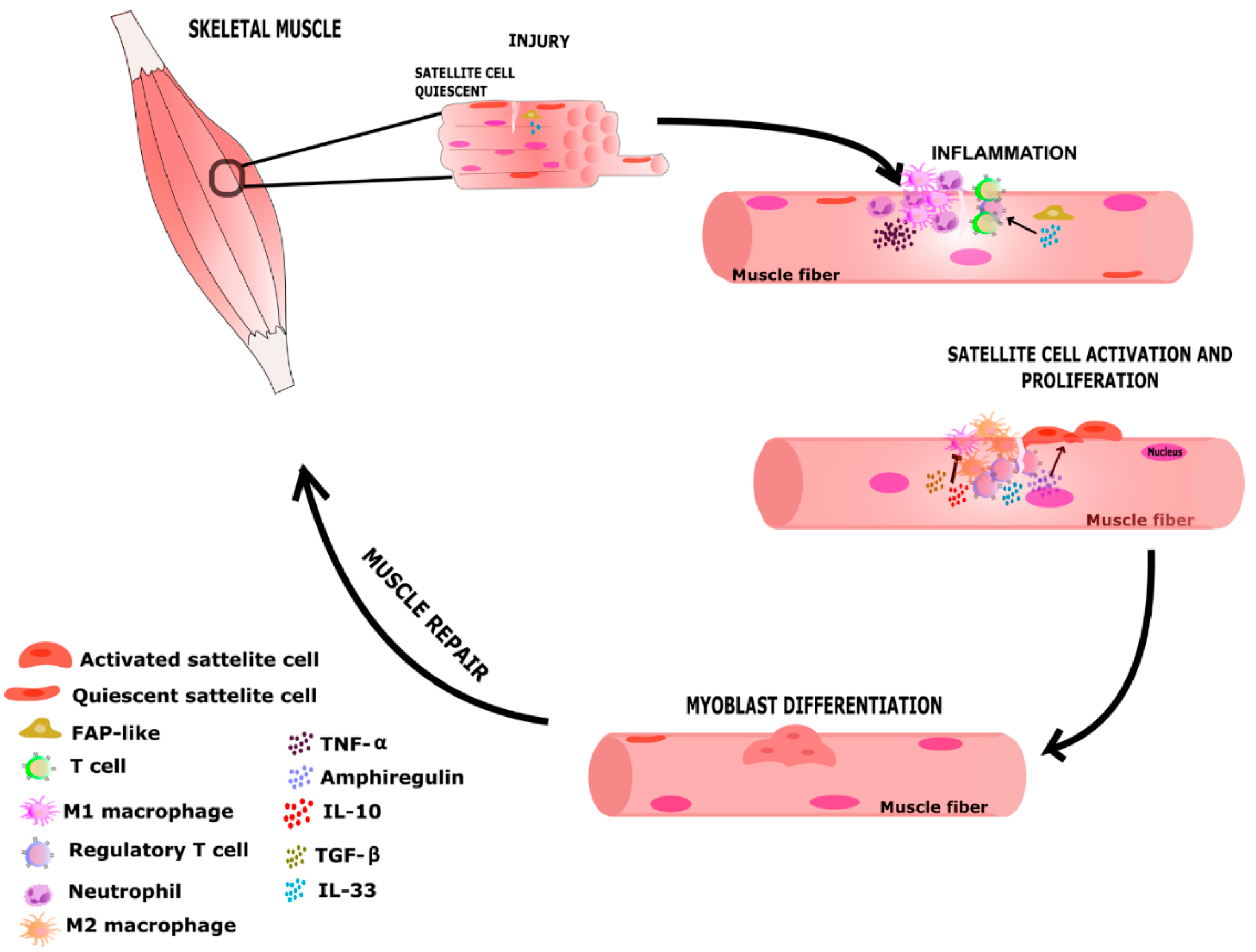
3.4. Treg Is Increased in Injured Muscle
3.5. Do Treg Cells Modify Muscle Repair?
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Frontera, W.R.; Ochala, J. Skeletal Muscle: A Brief Review of Structure and Function. Calcif. Tissue Int. 2015, 96, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Mauro, A. Satellite Cell of Skeletal Muscle Fibers. J. Biophys. Biochem. Cytol. 1961, 9, 493–495. [Google Scholar] [CrossRef]
- Collins, C.A.; Olsen, I.; Zammit, P.S.; Heslop, L.; Petrie, A.; Partridge, T.A.; Morgan, J.E. Stem Cell Function, Self-Renewal, and Behavioral Heterogeneity of Cells from the Adult Muscle Satellite Cell Niche. Cell 2005, 122, 289–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baghdadi, M.B.; Tajbakhsh, S. Regulation and Phylogeny of Skeletal Muscle Regeneration. Dev. Biol. 2018, 433, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Dumont, N.A.; Wang, Y.X.; Rudnicki, M.A. Intrinsic and Extrinsic Mechanisms Regulating Satellite Cell Function. Development 2015, 142, 1572–1581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Relaix, F.; Bencze, M.; Borok, M.J.; Der Vartanian, A.; Gattazzo, F.; Mademtzoglou, D.; Perez-Diaz, S.; Prola, A.; Reyes-Fernandez, P.C.; Rotini, A.; et al. Perspectives on Skeletal Muscle Stem Cells. Nat. Commun. 2021, 12, 692. [Google Scholar] [CrossRef] [PubMed]
- Greising, S.M.; Corona, B.T.; Call, J.A. Musculoskeletal Regeneration, Rehabilitation, and Plasticity Following Traumatic Injury. Int. J. Sports Med. 2020, 41, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Heslop, L.; Morgan, J.E.; Partridge, T.A. Evidence for a Myogenic Stem Cell That Is Exhausted in Dystrophic Muscle. J. Cell Sci. 2000, 113, 2299–2308. [Google Scholar] [CrossRef] [PubMed]
- Wallace, G.Q.; McNally, E.M. Mechanisms of Muscle Degeneration, Regeneration, and Repair in the Muscular Dystrophies. Annu. Rev. Physiol. 2009, 71, 37–57. [Google Scholar] [CrossRef] [PubMed]
- Tidball, J.G. Mechanisms of Muscle Injury, Repair, and Regeneration. Compr. Physiol. 2011, 1, 2029–2062. [Google Scholar] [CrossRef] [PubMed]
- Chargé, S.B.P.; Rudnicki, M.A. Cellular and Molecular Regulation of Muscle Regeneration. Physiol. Rev. 2004, 84, 209–238. [Google Scholar] [CrossRef] [PubMed]
- Forcina, L.; Cosentino, M.; Musarò, A. Mechanisms Regulating Muscle Regeneration: Insights into the Interrelated and Time-Dependent Phases of Tissue Healing. Cells 2020, 9, 1297. [Google Scholar] [CrossRef] [PubMed]
- Tidball, J.G.; Villalta, S.A. Regulatory Interactions between Muscle and the Immune System during Muscle Regeneration. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 298, R1173–R1187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juban, G.; Chazaud, B. Metabolic Regulation of Macrophages during Tissue Repair: Insights from Skeletal Muscle Regeneration. FEBS Lett. 2017, 591, 3007–3021. [Google Scholar] [CrossRef] [Green Version]
- Arnold, L.; Henry, A.; Poron, F.; Baba-Amer, Y.; van Rooijen, N.; Plonquet, A.; Gherardi, R.K.; Chazaud, B. Inflammatory Monocytes Recruited after Skeletal Muscle Injury Switch into Antiinflammatory Macrophages to Support Myogenesis. J. Exp. Med. 2007, 204, 1057–1069. [Google Scholar] [CrossRef] [Green Version]
- Klar, A.A.; Michalak-Micka, k.; Biedermann, T.; Simmen-Meuli, C.; Reichmann, E.; Meuli, M. Characterization of M1 and M2 Polarization of Macrophages in Vascularized Human Dermo-Epidermal Skin Substitutes in Vivo. Pediatr. Surg. Int. 2018, 34, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Villalta, S.A.; Nguyen, H.X.; Deng, B.; Gotoh, T.; Tidball, J.G. Shifts in Macrophage Phenotypes and Macrophage Competition for Arginine Metabolism Affect the Severity of Muscle Pathology in Muscular Dystrophy. Hum. Mol. Genet. 2009, 18, 482–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dambuza, I.M.; He, C.; Choi, J.K.; Yu, C.-R.; Wang, R.; Mattapallil, M.J.; Wingfield, P.T.; Caspi, R.R.; Egwuagu, C.E. IL-12p35 Induces Expansion of IL-10 and IL-35-Expressing Regulatory B Cells and Ameliorates Autoimmune Disease. Nat. Commun. 2017, 8, 719. [Google Scholar] [CrossRef] [PubMed]
- Villalta, S.A.; Rinaldi, C.; Deng, B.; Liu, G.; Fedor, B.; Tidball, J.G. Interleukin-10 Reduces the Pathology of Mdx Muscular Dystrophy by Deactivating M1 Macrophages and Modulating Macrophage Phenotype. Hum. Mol. Genet. 2011, 20, 790–805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ziemkiewicz, N.; Hilliard, G.; Pullen, N.A.; Garg, K. The Role of Innate and Adaptive Immune Cells in Skeletal Muscle Regeneration. Int. J. Mol. Sci. 2021, 22, 3265. [Google Scholar] [CrossRef] [PubMed]
- Dumke, B.R.; Lees, S.J. Age-Related Impairment of T Cell-Induced Skeletal Muscle Precursor Cell Function. Am. J. Physiol. Cell Physiol. 2011, 300, C1226–C1233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, X.; Xiao, J.; Wei, Y.; Li, S.; Liu, Y.; Yin, J.; Sun, K.; Sun, H.; Wang, H.; Zhang, Z.; et al. Combination of Inflammation-Related Cytokines Promotes Long-Term Muscle Stem Cell Expansion. Cell Res. 2015, 25, 1082–1083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Xiao, Z.; Qu, C.; Cui, W.; Wang, X.; Du, J. CD8 T Cells Are Involved in Skeletal Muscle Regeneration through Facilitating MCP-1 Secretion and Gr1(High) Macrophage Infiltration. J. Immunol. 2014, 193, 5149–5160. [Google Scholar] [CrossRef] [Green Version]
- Sugiura, T.; Murakawa, Y.; Nagai, A.; Kondo, M.; Kobayashi, S. Fas and Fas Ligand Interaction Induces Apoptosis in Inflammatory Myopathies: CD4+ T Cells Cause Muscle Cell Injury Directly in Polymyositis. Arthritis Rheum. 1999, 42, 291–298. [Google Scholar] [CrossRef]
- Targoff, I.N. Immune Manifestations of Inflammatory Muscle Disease. Rheum. Dis. Clin. N. Am. 1994, 20, 857–880. [Google Scholar] [CrossRef]
- Spencer, M.J.; Montecino-Rodriguez, E.; Dorshkind, K.; Tidball, J.G. Helper (CD4(+)) and Cytotoxic (CD8(+)) T Cells Promote the Pathology of Dystrophin-Deficient Muscle. Clin. Immunol. 2001, 98, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Morrison, J.; Lu, Q.L.; Pastoret, C.; Partridge, T.; Bou-Gharios, G. T-Cell-Dependent Fibrosis in the Mdx Dystrophic Mouse. Lab. Investig. 2000, 80, 881–891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farini, A.; Meregalli, M.; Belicchi, M.; Battistelli, M.; Parolini, D.; D’Antona, G.; Gavina, M.; Ottoboni, L.; Constantin, G.; Bottinelli, R.; et al. T and B Lymphocyte Depletion Has a Marked Effect on the Fibrosis of Dystrophic Skeletal Muscles in the Scid/Mdx Mouse. J. Pathol. 2007, 213, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Gussoni, E.; Pavlath, G.K.; Miller, R.G.; Panzara, M.A.; Powell, M.; Blau, H.M.; Steinman, L. Specific T Cell Receptor Gene Rearrangements at the Site of Muscle Degeneration in Duchenne Muscular Dystrophy. J. Immunol. 1994, 153, 4798–4805. [Google Scholar] [PubMed]
- Vetrone, S.A.; Montecino-Rodriguez, E.; Kudryashova, E.; Kramerova, I.; Hoffman, E.P.; Liu, S.D.; Miceli, M.C.; Spencer, M.J. Osteopontin Promotes Fibrosis in Dystrophic Mouse Muscle by Modulating Immune Cell Subsets and Intramuscular TGF-β. J. Clin. Investig. 2009, 119, 1583–1594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lagrota-Candido, J.; Vasconcellos, R.; Cavalcanti, M.; Bozza, M.; Savino, W.; Quirico-Santos, T. Resolution of Skeletal Muscle Inflammation in Mdx Dystrophic Mouse Is Accompanied by Increased Immunoglobulin and Interferon-Gamma Production. Int. J. Exp. Pathol. 2002, 83, 121–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eghtesad, S.; Jhunjhunwala, S.; Little, S.R.; Clemens, P.R. Rapamycin Ameliorates Dystrophic Phenotype in Mdx Mouse Skeletal Muscle. Mol. Med. 2011, 17, 917–924. [Google Scholar] [CrossRef]
- Burzyn, D.; Kuswanto, W.; Kolodin, D.; Shadrach, J.L.; Cerletti, M.; Jang, Y.; Sefik, E.; Tan, T.G.; Wagers, A.J.; Benoist, C.; et al. A Special Population of Regulatory T Cells Potentiates Muscle Repair. Cell 2013, 155, 1282–1295. [Google Scholar] [CrossRef] [Green Version]
- Jin, R.M.; Warunek, J.; Wohlfert, E.A. Therapeutic Administration of IL-10 and Amphiregulin Alleviates Chronic Skeletal Muscle Inflammation and Damage Induced by Infection. Immunohorizons 2018, 2, 142–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hooijmans, C.R.; Rovers, M.M.; de Vries, R.B.M.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s Risk of Bias Tool for Animal Studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef] [Green Version]
- Cho, J.; Kuswanto, W.; Benoist, C.; Mathis, D. T Cell Receptor Specificity Drives Accumulation of a Reparative Population of Regulatory T Cells within Acutely Injured Skeletal Muscle. Proc. Natl. Acad. Sci. USA 2019, 116, 26727–26733. [Google Scholar] [CrossRef]
- Kwee, B.J.; Budina, E.; Najibi, A.J.; Mooney, D.J. CD4 T-Cells Regulate Angiogenesis and Myogenesis. Biomaterials 2018, 178, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Kuswanto, W.; Burzyn, D.; Panduro, M.; Wang, K.K.; Jang, Y.C.; Wagers, A.J.; Benoist, C.; Mathis, D. Poor Repair of Skeletal Muscle in Aging Mice Reflects a Defect in Local, Interleukin-33-Dependent Accumulation of Regulatory T Cells. Immunity 2016, 44, 355–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villalta, S.A.; Rosenthal, W.; Martinez, L.; Kaur, A.; Sparwasser, T.; Tidball, J.G.; Margeta, M.; Spencer, M.J.; Bluestone, J.A. Regulatory T Cells Suppress Muscle Inflammation and Injury in Muscular Dystrophy. Sci. Transl. Med. 2014, 6, 258ra142. [Google Scholar] [CrossRef] [PubMed]
- Castiglioni, A.; Corna, G.; Rigamonti, E.; Basso, V.; Vezzoli, M.; Monno, A.; Almada, A.E.; Mondino, A.; Wagers, A.J.; Manfredi, A.A.; et al. FOXP3+ T Cells Recruited to Sites of Sterile Skeletal Muscle Injury Regulate the Fate of Satellite Cells and Guide Effective Tissue Regeneration. PLoS ONE 2015, 10, e0128094. [Google Scholar] [CrossRef] [PubMed]
- Gazzerro, E.; Baldassari, S.; Assereto, S.; Fruscione, F.; Pistorio, A.; Panicucci, C.; Volpi, S.; Perruzza, L.; Fiorillo, C.; Minetti, C.; et al. Enhancement of Muscle T Regulatory Cells and Improvement of Muscular Dystrophic Process in Mdx Mice by Blockade of Extracellular ATP/P2X Axis. Am. J. Pathol. 2015, 185, 3349–3360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shou, J.; Shi, X.; Liu, X.; Chen, Y.; Chen, P.; Xiao, W. Programmed Death-1 Promotes Contused Skeletal Muscle Regeneration by Regulating Treg Cells and Macrophages. Lab. Investig. 2021, 101, 719–732. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Qiao, Y.; Huang, L.; Li, F.; Zhang, Z.; Ping, Y.; Shen, Z.; Lian, J.; Li, F.; Zhao, L.; et al. Regulatory T Cells Were Recruited by CCL3 to Promote Cryo-Injured Muscle Repair. Immunol. Lett. 2018, 204, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Panduro, M.; Benoist, C.; Mathis, D. Treg Cells Limit IFN-γ Production to Control Macrophage Accrual and Phenotype during Skeletal Muscle Regeneration. Proc. Natl. Acad. Sci. USA 2018, 115, E2585–E2593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.M.; Rasmussen, J.P.; Rudensky, A.Y. Regulatory T Cells Prevent Catastrophic Autoimmunity throughout the Lifespan of Mice. Nat. Immunol. 2007, 8, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Lahl, K.; Sparwasser, T. In Vivo Depletion of FoxP3+ Tregs Using the DEREG Mouse Model. Methods Mol. Biol. 2011, 707, 157–172. [Google Scholar] [CrossRef]
- Ono, M.; Shimizu, J.; Miyachi, Y.; Sakaguchi, S. Control of Autoimmune Myocarditis and Multiorgan Inflammation by Glucocorticoid-Induced TNF Receptor Family-Related Protein(High), Foxp3-Expressing CD25+ and CD25- Regulatory T Cells. J. Immunol. 2006, 176, 4748–4756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Milani, M.; Ostlie, N.; Okita, D.; Agarwal, R.K.; Caspi, R.R.; Caspi, R.; Conti-Fine, B.M. C57BL/6 Mice Genetically Deficient in IL-12/IL-23 and IFN-Gamma Are Susceptible to Experimental Autoimmune Myasthenia Gravis, Suggesting a Pathogenic Role of Non-Th1 Cells. J. Immunol. 2007, 178, 7072–7080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, R.; Hao, J.; Dayao, C.S.; Shi, F.-D.; Campagnolo, D.I. T-Bet Deficiency Decreases Susceptibility to Experimental Myasthenia Gravis. Exp. Neurol. 2009, 220, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Allenbach, Y.; Solly, S.; Grégoire, S.; Dubourg, O.; Salomon, B.; Butler-Browne, G.; Musset, L.; Herson, S.; Klatzmann, D.; Benveniste, O. Role of Regulatory T Cells in a New Mouse Model of Experimental Autoimmune Myositis. Am. J. Pathol. 2009, 174, 989–998. [Google Scholar] [CrossRef] [Green Version]
- Sakaguchi, S.; Sakaguchi, N.; Asano, M.; Itoh, M.; Toda, M. Immunologic Self-Tolerance Maintained by Activated T Cells Expressing IL-2 Receptor Alpha-Chains (CD25). Breakdown of a Single Mechanism of Self-Tolerance Causes Various Autoimmune Diseases. J. Immunol. 1995, 155, 1151–1164. [Google Scholar] [PubMed]
- Fontenot, J.D.; Gavin, M.A.; Rudensky, A.Y. Foxp3 Programs the Development and Function of CD4+CD25+ Regulatory T Cells. Nat. Immunol. 2003, 4, 330–336. [Google Scholar] [CrossRef] [PubMed]
- De Pasquale, L.; D’Amico, A.; Verardo, M.; Petrini, S.; Bertini, E.; De Benedetti, F. Increased Muscle Expression of Interleukin-17 in Duchenne Muscular Dystrophy. Neurology 2012, 78, 1309–1314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hardy, D.; Besnard, A.; Latil, M.; Jouvion, G.; Briand, D.; Thépenier, C.; Pascal, Q.; Guguin, A.; Gayraud-Morel, B.; Cavaillon, J.-M.; et al. Comparative Study of Injury Models for Studying Muscle Regeneration in Mice. PLoS ONE 2016, 11, e0147198. [Google Scholar] [CrossRef] [PubMed]
- Guardiola, O.; Andolfi, G.; Tirone, M.; Iavarone, F.; Brunelli, S.; Minchiotti, G. Induction of Acute Skeletal Muscle Regeneration by Cardiotoxin Injection. J. Vis. Exp. 2017, 119, e54515. [Google Scholar] [CrossRef]
- Le, G.; Lowe, D.A.; Kyba, M. Freeze Injury of the Tibialis Anterior Muscle. Methods Mol. Biol. 2016, 1460, 33–41. [Google Scholar] [CrossRef] [Green Version]
- Zaynitdinova, M.I.; Lavrov, A.V.; Smirnikhina, S.A. Animal Models for Researching Approaches to Therapy of Duchenne Muscular Dystrophy. Transgenic Res. 2021, 30, 709–725. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, A.; Takeda, S. Mammalian Models of Duchenne Muscular Dystrophy: Pathological Characteristics and Therapeutic Applications. J. Biomed. Biotechnol. 2011, 2011, 184393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Li, L.; Feng, K.; Fan, D.; Xue, W.; Lu, J. “Repair” Treg Cells in Tissue Injury. Cell. Physiol. Biochem. Int.. 2017, 43, 2155–2169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DiSpirito, J.R.; Zemmour, D.; Ramanan, D.; Cho, J.; Zilionis, R.; Klein, A.M.; Benoist, C.; Mathis, D. Molecular Diversification of Regulatory T Cells in Nonlymphoid Tissues. Sci. Immunol. 2018, 3, eaat5861. [Google Scholar] [CrossRef] [PubMed]
- Cipolletta, D.; Feuerer, M.; Li, A.; Kamei, N.; Lee, J.; Shoelson, S.E.; Benoist, C.; Mathis, D. PPAR-γ Is a Major Driver of the Accumulation and Phenotype of Adipose Tissue Treg Cells. Nature 2012, 486, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Harb, H.; Benamar, M.; Lai, P.S.; Contini, P.; Griffith, J.W.; Crestani, E.; Schmitz-Abe, K.; Chen, Q.; Fong, J.; Marri, L.; et al. Notch4 Signaling Limits Regulatory T-Cell-Mediated Tissue Repair and Promotes Severe Lung Inflammation in Viral Infections. Immunity 2021, 54, 1186.e7–1199.e7. [Google Scholar] [CrossRef] [PubMed]
- Mock, J.R.; Garibaldi, B.T.; Aggarwal, N.R.; Jenkins, J.; Limjunyawong, N.; Singer, B.D.; Chau, E.; Rabold, R.; Files, D.C.; Sidhaye, V.; et al. Foxp3+ Regulatory T Cells Promote Lung Epithelial Proliferation. Mucosal. Immunol. 2014, 7, 1440–1451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nosbaum, A.; Prevel, N.; Truong, H.-A.; Mehta, P.; Ettinger, M.; Scharschmidt, T.C.; Ali, N.H.; Pauli, M.L.; Abbas, A.K.; Rosenblum, M.D. Regulatory T Cells Facilitate Cutaneous Wound Healing. J. Immunol. 2016, 196, 2010–2014. [Google Scholar] [CrossRef] [PubMed]
- Raffin, C.; Vo, L.T.; Bluestone, J.A. Treg Cell-Based Therapies: Challenges and Perspectives. Nat. Rev. Immunol. 2020, 20, 158–172. [Google Scholar] [CrossRef] [PubMed]
- Tarbell, K.V.; Yamazaki, S.; Olson, K.; Toy, P.; Steinman, R.M. CD25+ CD4+ T Cells, Expanded with Dendritic Cells Presenting a Single Autoantigenic Peptide, Suppress Autoimmune Diabetes. J. Exp. Med. 2004, 199, 1467–1477. [Google Scholar] [CrossRef] [PubMed]
- Stephens, L.A.; Malpass, K.H.; Anderton, S.M. Curing CNS Autoimmune Disease with Myelin-Reactive Foxp3+ Treg. Eur. J. Immunol. 2009, 39, 1108–1117. [Google Scholar] [CrossRef] [PubMed]
- Saadoun, D.; Rosenzwajg, M.; Joly, F.; Six, A.; Carrat, F.; Thibault, V.; Sene, D.; Cacoub, P.; Klatzmann, D. Regulatory T-Cell Responses to Low-Dose Interleukin-2 in HCV-Induced Vasculitis. N. Engl. J. Med. 2011, 365, 2067–2077. [Google Scholar] [CrossRef] [PubMed]

| PubMed | Number of Results |
|---|---|
| #1) (‘‘T Lymphocytes, Regulatory’’ OR ‘‘Regulatory T-Lymphocyte’’ OR ‘‘Regulatory T Lymphocyte’’ OR ‘‘T-Lymphocyte, Regulatory’’ OR ‘‘Treg Cell’’ OR ‘‘Cell, Treg’’ OR ‘‘Cells, Treg’’ OR ‘‘Regulatory T-Lymphocytes’’ OR ‘‘Regulatory T Lymphocytes’’ OR ‘‘T-Cells, Regulatory’’ OR ‘‘Treg Cells’’ OR ‘‘Regulatory T-Cells’’ OR ‘‘Regulatory T Cell’’ OR ‘‘Cell, Regulatory T’’ OR ‘‘Cells, Regulatory T’’ OR ‘‘Regulatory T Cells’’ OR ‘‘T Cell, Regulatory’’ OR ‘‘T Cells, Regulatory’’ OR ‘‘Regulatory T-Cell’’ OR ‘‘Th3 Cells’’ OR ‘‘Cell, Th3’’ OR ‘‘Cells, Th3’’ OR ‘‘Th3 Cell’’ OR ‘‘Suppressor T-Lymphocytes, Naturally-Occurring’’ OR ‘‘Naturally-Occurring Suppressor T-Lymphocyte’’ OR ‘‘Naturally-Occurring Suppressor T-Lymphocytes’’ OR ‘‘Suppressor T Lymphocytes, Naturally Occurring’’ OR ‘‘Suppressor T-Lymphocyte, Naturally-Occurring’’ OR ‘‘Suppressor T-Cells, Naturally-Occurring’’ OR ‘‘Suppressor T-Cell’’ OR ‘‘Naturally-Occurring Suppressor T-Cells’’ OR ‘‘Suppressor T Cells, Naturally Occurring’’ OR ‘‘Suppressor T-Cell, Naturally-Occurring’’ OR ‘‘T-Cell, Naturally-Occurring Suppressor’’ OR ‘‘T-Cells, Naturally-Occurring Suppressor’’ OR ‘‘Tr1 Cells’’ OR ‘‘Tr1 Cell’’ OR ‘‘Foxp3’’) | 78,222 |
| #2) (‘‘muscle injury’’ OR ‘‘muscle diseases’’ OR ‘‘Muscles, Skeletal’’ OR ‘‘Skeletal Muscles’’ OR ‘‘Muscle, Voluntary’’ OR ‘‘Muscles, Voluntary’’ OR ‘‘Voluntary Muscle’’ OR ‘‘Voluntary Muscles’’ OR ‘‘Skeletal Muscle’’ OR ‘‘Soleus Muscle’’ OR ‘‘Muscle, Soleus’’ OR ‘‘Plantaris Muscle’’ OR ‘‘Muscle, Plantaris’’ OR ‘‘Anterior Tibial Muscle’’ OR ‘‘Muscle, Anterior Tibial’’ OR ‘‘Tibial Muscle, Anterior’’ OR ‘‘Gastrocnemius Muscle’’ OR ‘‘Muscle, Gastrocnemius’’)) | 610,824 |
| #3) (Repair OR Regenerations OR ‘‘Endogenous Regeneration’’ OR ‘‘Regeneration, Endogenous’’ OR Reparation | 738,780 |
| #4) (#1) AND (#2) AND (#3) | 81 |
| Embase ® | |
| (‘muscle injury’/exp OR ‘injury, muscle’ OR ‘muscle damage’ OR ‘muscle injury’ OR ‘muscle lesion’ OR ‘muscle trauma’ OR ‘muscular injury’ OR ‘skeletal muscle damage’ OR ‘trauma, muscle’ OR ‘muscle disease’/exp OR ‘fibromuscular disease’ OR ‘muscle defect’ OR ‘muscle disease’ OR ‘muscle disorder’ OR ‘muscle pathology’ OR ‘muscular disease’ OR ‘muscular diseases’ OR ‘muscular disorder’ OR ‘neuromuscular manifestations’) AND (‘regulatory t lymphocyte’/exp OR ‘t lymphocytes, regulatory’ OR ‘t regulatory cell’ OR ‘t regulatory cells’ OR ‘t regulatory lymphocyte’ OR ‘t-lymphocytes, regulatory’ OR ‘tr1 cell’ OR ‘tr1 cells’ OR ‘treg’ OR ‘tregs’ OR ‘immunoregulatory t cell’ OR ‘immunoregulatory t cells’ OR ‘immunoregulatory t lymphocyte’ OR ‘regulatory t cell’ OR ‘regulatory t cells’ OR ‘regulatory t lymphocyte’ OR ‘regulatory t lymphocytes’ OR ‘regulatory t-lymphocytes’ OR ‘transcription factor foxp3’/exp OR ‘foxp3 protein’ OR ‘foxp3 transcription factor’ OR ‘forkhead box p3 protein’ OR ‘forkhead box protein p3’ OR ‘protein foxp 3’ OR ‘protein foxp3’ OR ‘scurfin’ OR ‘transcription factor foxp3’ OR ‘foxp3 regulatory t lymphocyte’/exp OR ‘foxp3 t lymphocyte’/exp OR ‘foxp3 regulatory t cell’/exp OR foxp3) AND (‘muscle regeneration’/exp OR ‘muscle regeneration’ OR ‘muscular regeneration’ OR ‘regeneration, muscle’ OR ‘muscle repair’/exp) | 33 |
| Author | Species | Injury Model | Treg | Treg Characteristics |
|---|---|---|---|---|
| Burzyn D. (2013) [33] | Mouse | Cardiotoxin | Increased | Clonal expansion, increase in proliferation, and muscle Treg after 4 days post-injury. Muscle Treg cells released Amphiregulin growth factor, which acts directly on muscle MuSC in vitro and improved muscle repair in vivo |
| Cryo-injury | Increased | Clonal expansion, increase in proliferation, and muscular Treg after 8 days post-injury | ||
| mdx | Increased | Frequency of muscle Treg cells was increased at 4 and 12 week-old-mice compared to control mice. It was also observed clonal expansion of Treg | ||
| Villalta S.A. (2014) [39] | Human | DMD/BMD | Increased | Elevated numbers of Foxp3+ cells coincided with increased IL-10 expression |
| Mouse | mdx | Increased | Treg was increased mainly in lesion areas in 4- and 12-week-old mice. Treg with activated phenotype and IL-10 production | |
| Castiglioni A. (2015) [40] | Mouse | Cardiotoxin | Increased | Treg was increased since the first day post-lesion. CD3+CD4+cells have high expression of CD69 and CD25 |
| Gazzerro E. (2015) [41] | - | - | - | Not applicable |
| Kuswanto W. (2016) [38] | Mouse | Cardiotoxin | Increased | Treg increases 6 days after muscle injury in young mice (2 months) but not in aged mice (6 months) |
| Kwee B. J. (2018) [37] | - | - | - | Not applicable |
| Panduro M. (2018) [44] | Mouse | Cardiotoxin | No determined | Treg cells co-localized with macrophages in the regenerating areas of injured muscle |
| Zhang C. (2018) [43] | Mouse | Cryo-injury | Increased | Treg cells were increased in the muscle on day 4 after lesion. Muscle Treg expresses amphiregulin, IL-10, tumor growth factor (TGF)-β, and chemokine receptors (CCR)1 and CCR5. Tregs were attracted to injured muscle by Chemokine (C-C motif) ligand (CCL)3 |
| Cho J. (2019) [36] | Mouse | Cardiotoxin | Increased | Enhanced accumulation of muscle Tregs in both muscle-Treg TCR-Tg mice or in muscle Treg adoptive-transfer systems in RAG- mouse. The definitive muscle-Treg transcriptome was established only after Tregs migrated into the muscle |
| Shou J. (2021) [42] | Mouse | Contusion | Increased | Treg accumulation 3–7 days after injury |
| Author | Specie | Injury Model | Intervention | Treg | Outcomes |
|---|---|---|---|---|---|
| Burzyn D. 2013 [33] | Mouse | Cardiotoxin | TD injection in DTR-Foxp3 model | Decreased | Decreased frequency of CD45+ cells and increased inflammatory infiltrate and fibrosis |
| Cryo-injury | TD injection in DTR-Foxp3 model | Decreased | Decreased centrally nucleated fibers | ||
| mdx | anti-CD25 antibody | Decreased | Increased CK | ||
| anti-IL2/IL2 complex | Increased | Decreased CK | |||
| Villalta S. A. (2014) [39] | Mouse | mdx | anti-CD25 antibody | Decreased | Increased muscular lesion, inflammatory infiltration, and IFN-γ expression |
| mdx DEREG | TD injection in DTR-Foxp3 model | Decreased | Increase in IFN-γ expression and M1 macrophages | ||
| mdx | anti-IL2/IL2 complex | Increased | Increase in IL-10 production. Decrease in inflammatory infiltrate and myofiber lesion (evidenced by detection of albumin and CK) | ||
| Castiglioni A. (2015) [40] | - | - | - | No treatment experiments | |
| Gazzerro E. (2015) [41] | Mouse | mdx 4Cv | oATP | Increased | Reduced inflammatory infiltration associated with an increase in strength and reduced necrosis (CK decreased). Decrease in IL-6 and TGF-β expression. Increase in the number of regenerative cells evidenced by eMHC and myogenin expression. oATP can act directly on other cells of the inflammatory response, not only on Treg |
| Kuswanto W. (2016) [38] | Mouse | Cryo-injury | Mice deficient in ST2 (IL-33 receptor) on Tregs | Decreased | Increased muscle infiltrate. Decreased mean cross-sectional area of regenerating (centrally nucleated) myofibers in mice lacking ST2, specifically on Treg cells |
| Restored Treg levels in the muscle of aged mice by intramuscular injection of IL-33 | Increased | Histologic analysis evidenced improvement in regeneration in old mice supplemented with IL-33. There were elevated numbers of regenerating, centrally nucleated myofibers with a higher average myofibril cross-sectional area | |||
| Kwee B. J. (2018) [37] | Mouse | Ischemia in BL.CD4 KD | Intramuscular injection of alginate hydrogel with conditioned medium from conventional Treg induced in vitro | Not detected | No effect on angiogenesis and myogenesis in vivo |
| Panduro M. (2018) [44] | Mouse | Cardiotoxin | TD injection in DTR-Foxp3 model | Decreased | Induced an increase in IFN-γ production by NK and effector T cells. Induced macrophage dysregulation. The impact on IFN-γ production was high when Treg cells were depleted early after injury. IFN-γ inoculation mimicked Treg depletion, with increased fibrosis and inflammation |
| Zhang C. (2018) [43] | - | - | - | No treatment experiments | |
| Cho J. (2019) [36] | Mouse | mdx | mdx mice backcrossed with muscle-Treg TCR-Tg mice | Increased | Leukocyte infiltration and macrophage phenotypes in muscular injury were not significantly different in Tg+ and Tg− mdx littermates at 4 and 12 weeks. However, 12-week-old Tg+ mdx mice showed an increase in regenerating myofibers by histological analysis |
| Shou J. (2021) [42] | Mouse | Contusion | Knockout PD-1 Mice | Decreased | Reduction of the macrophage pro-inflammatory-to-anti-inflammatory switching. Downregulation of contused skeletal muscle regeneration with mitigation of muscle regeneration factors, prolonged inflammatory response period, and exacerbated oxidative stress |
| Author | In Vitro Analysis |
|---|---|
| Burzyn D. (2013) [33] | MuSCs of cardiotoxin-injured muscle from DT-treated DTR mice had decreased colony-forming capacity |
| Villalta S. A. (2014) [39] | No experiments |
| Castiglioni A. (2015) [40] | iTreg from lymph nodes induced increased MuSCs numbers and a delay in differentiation in myotubes |
| Gazzerro E. (2015) [41] | No experiments |
| Kuswanto W. (2016) [38] | No experiments |
| Kwee B. J. (2018) [37] | Conditioned medium from induced Treg showed no effect or no substantial effect on angiogenesis and myoblast proliferation and differentiation |
| Panduro M. (2018) [44] | No experiments |
| Zhang C. (2018) [43] | CCL3, a chemokine highly expressed in muscle after cryo-injury, attracted conventional Tregs in transwell plates. Anti-CCR1 antibody inhibited Treg recruitment significantly by CCL3 |
| Cho J. (2019) [36] | No experiments |
| Shou J. (2021) [42] | No experiments |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gama, J.F.G.; Romualdo, R.D.; de Assis, M.L.; de Oliveira, L.M.; Quírico-Santos, T.; Alves, L.A.; Lagrota-Candido, J. Role of Regulatory T Cells in Skeletal Muscle Regeneration: A Systematic Review. Biomolecules 2022, 12, 817. https://doi.org/10.3390/biom12060817
Gama JFG, Romualdo RD, de Assis ML, de Oliveira LM, Quírico-Santos T, Alves LA, Lagrota-Candido J. Role of Regulatory T Cells in Skeletal Muscle Regeneration: A Systematic Review. Biomolecules. 2022; 12(6):817. https://doi.org/10.3390/biom12060817
Chicago/Turabian StyleGama, Jaciara F. G., Rayza D. Romualdo, Mayara L. de Assis, Luana M. de Oliveira, Thereza Quírico-Santos, Luiz A. Alves, and Jussara Lagrota-Candido. 2022. "Role of Regulatory T Cells in Skeletal Muscle Regeneration: A Systematic Review" Biomolecules 12, no. 6: 817. https://doi.org/10.3390/biom12060817






