Abstract
Tuberculosis (TB) is a widespread infectious disease caused by Mycobacterium tuberculosis. The increasing incidence of multidrug-resistant (MDR) and extensively drug-resistant (XDR) strains has created a need for new antiTB agents with new chemical scaffolds to combat the disease. Thus, the key question is: how to search for new antiTB and where to look for them? One of the possibilities is to search among natural products (NPs). In order to search for new antiTB drugs, the detailed phytochemical study of the whole Dicranostigma franchetianum plant was performed isolating wide spectrum of isoquinoline alkaloids (IAs). The chemical structures of the isolated alkaloids were determined by a combination of MS, HRMS, 1D, and 2D NMR techniques, and by comparison with literature data. Alkaloids were screened against Mycobacterium tuberculosis H37Ra and four other mycobacterial strains (M. aurum, M. avium, M. kansasii, and M. smegmatis). Alkaloids 3 and 5 showed moderate antimycobacterial activity against all tested strains (MICs 15.625–31.25 µg/mL). Furthermore, ten semisynthetic berberine (16a–16k) derivatives were developed and tested for antimycobacterial activity. In general, the derivatization of berberine was connected with a significant increase in antimycobacterial activity against all tested strains (MICs 0.39–7.81 μg/mL). Two derivatives (16e, 16k) were identified as compounds with micromolar MICs against M. tuberculosis H37Ra (MIC 2.96 and 2.78 µM). All compounds were also evaluated for their in vitro hepatotoxicity on a hepatocellular carcinoma cell line (HepG2), exerting lower cytotoxicity profile than their MIC values, thereby potentially reaching an effective concentration without revealing toxic side effects.
1. Introduction
Dicranostigma Hooker f. & Thomson is a small genus of the Papaveraceae family with eight accepted species native to the Himalayas and western China [1,2]. Plants of this genus are also known as eastern horned poppies. Although resembling the true horned poppies of Glaucium, they have stigmas with two lobes and fruit with only traces of “horns”. These plants have been used for a long time in folk medicine to treat various diseases in China. They are effective for the treatment of tonsillitis, hepatitis, sore throat, scrofula, bald scars, and scabies [3,4,5]. Some species of Dicranostigma have been investigated in several phytochemical and pharmacological studies. So far, more than 30 isoquinoline alkaloids (IAs) of different structural scaffolds have been either identified in or isolated from the genus Dicranostigma [1,6,7,8]. Alkaloids from Dicranostigma plants have been intensively investigated for their promising activities, such as anti-inflammatory, antibacterial, antiviral, and hepatoprotective effects [9,10,11].
Dicranostigma franchetianum (Prain) Fedde is an herbaceous bushy annual plant that is native to western China. In Europe, the herb is known as a cultivated plant in botanical gardens.
So far, 13 IAs have been isolated from this species, including berberine, protopine, chelerythrine, and sanguinarine [12,13,14,15].
Tuberculosis (TB) is a widespread infectious disease caused by Mycobacterium tuberculosis (Mtb). According to the Global Tuberculosis Report 2021, issued by the World Health Organization (WHO), the latent form of Mtb has infected about a quarter of the world’s population, but only a small part (5–10%) will develop this bacterial disease. Contrary to expectations, a significant drop in newly diagnosed cases is mentioned in the global TB report 2021 (5.8 million in 2020, 7.1 million in 2019). Unfortunately, reduced access to TB treatment and diagnosis due to the coronavirus (COVID-19) pandemic (in the context of lockdowns, concerns about the risks of going to the health care facilities, the stigma associated with similarities in the symptoms related to COVID-19 and TB, etc.) has resulted in an increase in deaths caused by Mtb (1.3 million in 2020 vs. 1.2 million in 2019). Until the COVID-19 pandemic, TB was the leading cause of death from a single infectious agent, ranking above HIV/AIDS (13th leading cause worldwide in 2019). However, predictions suggest that the number of people developing TB and dying from it could be much worse in 2021 and 2022.
Although most TB forms are treatable and curable, the global treatment success rate for people treated for TB with first-line regimens was 86% in 2019 (77% among people living with HIV). Standard treatment of drug-susceptible TB comprises the administration of four first-line anti-TB drugs: isoniazid (INH), rifampicin (RIF), pyrazinamide (PZA), and ethambutol (EMB). However, resistance to all of them occurs and poses a serious public health threat; thus, the treatment of TB is becoming challenging due to the emergence of multidrug-resistant TB (MDR-TB) and extensively drug-resistant TB (XDR-TB) strains [16]. Usually, MDR-TB refers to the strains that are resistant to INH and RIF, while XDR-TB strains are resistant to all first-line anti-TB drugs and, additionally, to some second-line anti-TB drugs, including fluoroquinolones and at least one of three injectables drugs (i.e., kanamycin, amikacin, capreomycin) [17]. Testing TB drug resistance is vitally important for the treatment, including microbiological methods, rapid molecular tests, and sequencing technologies. Thus, in 2020, 71% of people with bacteriologically confirmed pulmonary TB were screened for resistance, revealing 158 thousand cases with a resistant form of TB. The success rate of treatment for MDR-TB was only 59% in 2018.
According to the WHO report from August 2021 [18], there are currently 25 drugs that are being investigated for the treatment of TB in different stages of clinical trials [18,19]. Given the increasing resistance to Mtb, searching for new anti-TB agents with new chemical scaffolds is essential to combat the disease. Despite the rise of combinatorial and medicinal chemistry approaches, natural products (NPs) still hold a valuable position as a source of novel drugs, but fundamental research on NPs has started to seem like an endangered species [20,21,22]. On the other hand, isolated NPs are often endowed with non-drug-like properties, low potency, low selectivity, and other inadequate properties; thus, their core scaffolds often require extensive structure-activity optimization while also improving their ADME profile to become valid and potent drugs [22,23]. From this perspective, semisynthesis using a NP as a starting building block for chemical modification is well established in the drug discovery process. According to this approach, several semisynthetic derivatives of NPs (i.e., sampangine, cleistopholine, berberine, and galanthamine) have been studied for their antimycobacterial potential [24,25,26,27].
The interesting biological activities of the IAs and the absence of current phytochemical study of D. franchetianum encouraged us to examine this species. As a part of our ongoing research on IAs and their semisynthetic derivatives as potential drugs, this work reports on the isolation of alkaloids from the whole plant of D. franchetianum, preparation of berberine derivatives, and their in vitro antimycobacterial activity against Mtb H37Ra, and four other mycobacterial strains (M. aurum, M. avium, M. kansasii, and M. smegmatis).
2. Materials and Methods
2.1. General Experimental Procedures
All solvents were treated using standard techniques before use. All reagents and catalysts were purchased from Sigma Aldrich, Czech Republic, and used without purification. NMR spectra were recorded in either CDCl3 or CD3OD on a VNMR S500 (Varian, Palo Alto, CA, USA) spectrometer operating at 500 MHz for proton nuclei and 125.7 MHz for carbon nuclei at ambient temperature. ESI-HRMS were obtained with a Waters Synapt G2-Si hybrid mass analyzer of a quadrupole-time-of-flight (Q-TOF) type, coupled to a Waters Acquity I-Class UHPLC system (Waters, Millford, MA, USA). The EI-MS were obtained on an Agilent 7890A GC 5975 inert MSD operating in EI mode at 70 eV (Agilent Technologies, Santa Clara, CA, USA). A DB-5 column (30 m × 0.25 mm × 0.25 μm, Agilent Technologies, USA) was used with a temperature program: 100–180 °C at 15 °C/min, 1 min hold at 180 °C, and 180–300 °C at 5 °C/min and 5 min hold at 300 °C; and detection range m/z 40–600. The injector temperature was 280 °C. The flow rate of carrier gas (helium) was 0.8 mL/min. A split ratio of 1:15 was used. Fractionation of fractions II and V was performed with a BÜCHI Sepacore flash system ×10 equipped with a BÜCHI control unit C-620, BÜCHI fraction collector C-660, UV photometer C-640, and BÜCHI pump modules C-605 (BÜCHI, Flawil, Switzerland). TLC was carried out on Merck precoated silica gel 60 F254 and Merck precoated silica gel 60 RP-18 F254 plates. Compounds on the plate were observed under UV light (254 and 366 nm) and visualized by spraying with Dragendorff’s reagent.
2.2. Plant Material
The plant material used from Dicranostigma franchetianum came from a monoculture introduced to the Garden of Medicinal Plants at the Faculty of Pharmacy, Charles University in Hr. Králové in 2014 (seeds from the Centre of Medicinal Plants of Masaryk University in Brno). Botanical identification was performed by Prof. L. Opletal. A voucher specimen is deposited in the Herbarium of the Faculty of Pharmacy in Hradec Králové under number: CUFPH-16130/AL-540.
2.3. Extraction and Isolation of Alkaloids
Finely cut and dried aerial parts of D. franchetianum (11.87 kg) were minced and sequentially extracted with 95% EtOH (500 g of material boiled with 3 L of EtOH) for 30 min. The combined extracts were evaporated to the consistency of thin syrup, to which 6 L of distilled water at 80 °C was added, and the pH adjusted to 1–1.5 by the addition of 2% H2SO4. The suspension was filtered through Celite 545. The filtrate was alkalized by 10% Na2CO3 (pH 9–10) and extracted with CHCl3 (3 × 5 L). The organic layer was evaporated to give 120 g of dark brown syrup. This alkaloid summary extract was again dissolved in 2% H2SO4 (3 L), defatted with Et2O (3 × 2 L), and alkalized to pH 9–10 with 10% Na2CO3. The water layer was extracted with EtOAc (4 × 2 L) to give 47 g of concentrated alkaloidal extract in the form of brown syrup.
The extract (47 g), adsorbed on silica gel (ratio of media to sample 2:1) and loaded onto a PP pre-column (cartridge 40 × 150 mm, Büchi), was separated by flash chromatography (Sepacore® Flash Chromatography System ×10, Büchi) on silica gel (silica gel 60, 40–63 µm, glass column 49 × 460 mm, Büchi) using a mobile phase containing light petroleum (solvent A), EtOAC (solvent B) and MeOH (solvent C). Separation of the extract was carried out for 360 min, starting with isocratic elution with solvents A and B (20% B, 15 min), followed by linear gradient elution (20% B→100% B, 150 min) and isocratic solvent elution (100% B, 15 min). Chromatography of the extract was continued by linear gradient elution with solvents B and C (100% B→100% C, 165 min), and the separation was completed by isocratic elution with solvent C (15 min). The separation flow rate was set at 110 mL/min, and UV detection was performed at 254 nm, 290 nm, 315 nm and 365 nm. A total of 208 fractions (190 mL) were collected and, based on analytical TLC, combined into 12 fractions (A–L). Separation of fractions into individual alkaloids was performed by preparative TLC on silica gel (silica gel 60 GF254). Conditions for preparative TLC, additional separation processes (e.g., crystallization) for each fraction, and isolated amount of each alkaloid were as follows:
Fraction A (250 mg, cHx:Et2NH, 97:3, 2×) gave compound 1 (140 mg).
Fraction B (190 mg, cHx:Et2NH, 95:5, 2×) gave compound 2 (77 mg).
Fraction C (434 mg, To:Et2NH, 99:1, 2×) gave compounds 4 (20 mg) and 5 (145 mg).
Fraction D (807 mg, To:cHx:EtOAc:MeOH, 45:30:35:1) gave compound 3 (149 mg).
Fraction E (2.2 g, To:cHx:Et2NH, 45:45:10) gave alkaloids 6 (720 mg) and 7 (452 mg).
Fraction F (8.9 g) was partially separated by preparative TLC (2.0 g, To:Et2NH, 96:4, 3×) into 2 zones F1 (23 mg) and F2 (1.5 g). Subsequently, the F1 subfraction was separated by additional preparative TLC (EtOAc:MeOH:H2O 100:13:10, 2×) to give 8 (8 mg). Subfraction F2 (28 mg) gave compound 9 (2 mg) after crystalization from the mixture EtOH:CHCl3.
Fraction G (890 mg, To:cHx:Et2NH, 6:3:1) gave subfractions G1–G3. Subfraction G1 (115 mg) was recrystallized from the mixture EtOH:CHCl3 (1:1) to give compound 10 (56 mg). Additional preparative TLC of subfraction G2 (78 mg, To:cHx:Et2NH, 5:4:1, 2×) gave compounds 11 (15 mg) and 12 (13 mg).
Fraction H (730 mg, EtOAc:MeOH:H2O:TFAA, 100:40:5:0.1, 2×) gave four subfractions H1–H4. Crystallization of subfraction H1 (97 mg) yielded compound 13 (34 mg). Additional preparative TLC of subfraction H3 (40 mg, EtOAc:MeOH:H2O:TFAA 100:40:5:0.1, 2×) gave compound 14 (3 mg).
Fraction J (184 mg, To:cHx:EtOAc:MeOH 45:30:35:1, 1×) gave three subfractions J1–J3. Subfraction J1 (53 mg) yielded compound 15 (11 mg), after additional preparative TLC (To:Et2NH, 95:5,2×).
Fraction L (2.3 g) was recrystallized from EtOH to give 1.1 g of 16.
Other fractions and subfractions have not been used to isolate alkaloids due to either the low amount or complex mixture of compounds.
2.4. Preparation of Berberine Derivatives
The same procedure as described previously has been used to afford the corresponding ethers. NMR and mass spectra of the synthesized derivatives (16a–16k) were in agreement with a previous report [28].
2.5. Antimycobacterial Screening
The antimycobacterial assay was performed with rapidly growing Mycolicibacterium smegmatis DSM 43465 (ATCC 607), Mycolicibacterium aurum DSM 43999 (ATCC 23366), and non-tuberculous mycobacteria, namely Mycobacterium avium DSM 44156 (ATCC 25291), and Mycobacterium kansasii DSM 44162 (ATCC 12478) from German Collection of Microorganisms and Cell Cultures (Braunschweig, Germany), and with an avirulent strain of Mtb H37Ra ITM-M006710 (ATCC 9431) from Belgian Co-ordinated Collections of Micro-organisms (Antwerp, Belgium). The technique used for activity determination was the microdilution broth panel method using 96-well microtitration plates. The culture medium was Middlebrook 7H9 broth (Sigma-Aldrich, Steinheim, Germany) enriched with 0.4% glycerol (Sigma-Aldrich, Steinheim, Germany) and 10% Middlebrook OADC growth supplement (Himedia, Mumbai, India) [29,30].
The mycobacterial strains were cultured on Middlebrook 7H9 agar and suspensions were prepared in Middlebrook 7H9 broth. The final density was adjusted to 1.0 on the McFarland scale and diluted in the ratio of either 1:20 (for rapidly growing mycobacteria) or 1:10 (for slow-growing mycobacteria) with broth.
The tested compounds were dissolved in DMSO (Sigma-Aldrich, Steinheim, Germany), then Middlebrook broth was added to obtain a concentration of 2000 µg/mL. The standards used for activity determination were INH, RIF, and CPX (Sigma-Aldrich, Steinheim, Germany). Final concentrations were reached by binary dilution and addition of mycobacterial suspension and were set as 500, 250, 125, 62.5, 31.25, 15.625, 7.81, and 3.91 µg/mL. INH was diluted in the range 500–3.91 µg/mL for screening against rapidly growing mycobacteria, 2000–15.625 µg/mL for M. avium, 50–0.39 µg/mL for M. kansasii, and 1–0.0078 µg/mL for Mtb. RIF final concentrations ranged from 50 to 0.39 µg/mL for rapidly growing mycobacteria, from 1 to 0.0078 µg/mL for M. avium, and from 0.1 to 0.00078 µg/mL for Mtb and M. kansasii. CPX was used for screening antimycobacterial activity with final concentrations of 1, 0.5, 0.25, 0.125, 0.0625, 0.0313, 0.0156, and 0.0078 µg/mL. The final concentration of DMSO did not exceed 2.5% (v/v) and did not affect the growth of any of the strains. Positive (broth, DMSO, bacteria) and negative (broth, DMSO) growth controls were included.
Plates containing slow-growing mycobacteria were sealed with polyester adhesive film and incubated in the dark at 37 °C without agitation. The addition of a 0.01% solution of resazurin sodium salt followed after 48 h of incubation for M. smegmatis, after 72 h for M. aurum, after 96 h for M. avium and M. kansasii, and after 120 h for Mtb. Microtitration panels were then incubated for a further 2.5 h to determine the activity against M. smegmatis, 4 h for M. aurum, 6 h for M. avium and M. kansasii, and 18 h for Mtb.
The antimycobacterial activity was expressed as minimal inhibition concentration (MIC) and the value was read on the basis of stain color change (blue color–active compound; pink color–inactive compound). MIC values for standards were in the range 15.625–31.25 µg/mL for INH, 12.5–25 µg/mL for RIF and 0.0625–0.125 µg/mL for CPX against M. smegmatis, 1.95–3.91 µg/mL for INH, 0.39–0.78 µg/mL for RIF, and 0.0078–0.0156 µg/mL for CPX against M. aurum, 500–1000 µg/mL for INH, 0.0625–0.125 µg/mL for RIF and 0.5 µg/mL for CPX against M. avium, 3.125–6.25 µg/mL for INH, 0.025–0.05 µg/mL for RIF and 0.25 µg/mL for CPX against M. kansasii, and 0.125–0.25 µg/mL for INH, 0.00156–0.0031 µg/mL for RIF and 0.125–0.25 for CPX against Mtb. All experiments were conducted in duplicate.
2.6. Cytotoxicity Assay
Human hepatocellular carcinoma HepG2 cells (ATCC HB-8065; passage 20–25), purchased from Health Protection Agency Culture Collections (ECACC, Salisbury, UK), were cultured in Minimum Essential Eagle Medium supplemented with 10% fetal bovine serum and 1% L-glutamine solution (Sigma-Aldrich, St. Louis, MO, USA) at 37 °C in a humidified atmosphere containing 5% CO2. For passage, the cells were treated with trypsin/EDTA (Sigma-Aldrich, St. Louis, MO, USA) at 37 °C and then harvested. For cytotoxicity evaluation, the cells treated with the test substances were used, while untreated HepG2 cells served as the control group; blank was included, as well. The cells were seeded in a 96-well plate at a density of 50,000 cells per well and incubated for 24 h. The following day, the cells were treated with each of the test compounds dissolved in DMSO (the highest DMSO concentration used was 0.5% v/v). The test substances were prepared at different concentrations in triplicates according to their solubility and incubated for 24 h in a humidified atmosphere containing 5% CO2 at 37 °C. After incubation, a solution of thiazolyl blue tetrazolium bromide (Sigma-Aldrich, St. Louis, MO, USA) in RPMI 1640 without phenol red (BioTech, Praha, Czech Republic) was added and incubated for 30 min in a humidified atmosphere containing 5% CO2 at 37 °C. Then, the formazan crystals formed were dissolved in DMSO and the absorbance of the samples was recorded at 570 nm (BioTek, Synergy Neo2 Multi-Mode Reader NEO2SMALPHAB). IC50 values were calculated by nonlinear regression from a semi-logarithmic plot of incubation concentration versus percentage of absorbance relative to untreated controls using GraphPad Prism software (version 9; GraphPad Software, Inc., La Jolla, CA, USA). The obtained results of the experiments are presented as the concentration that reduces the viability of cells from the maximal viability to 50% (IC50).
3. Results and Discussion
3.1. Isolation of Isoquinoline Alkaloids from Dicranostigma Franchetianum and Their Antimycobacterial Activity
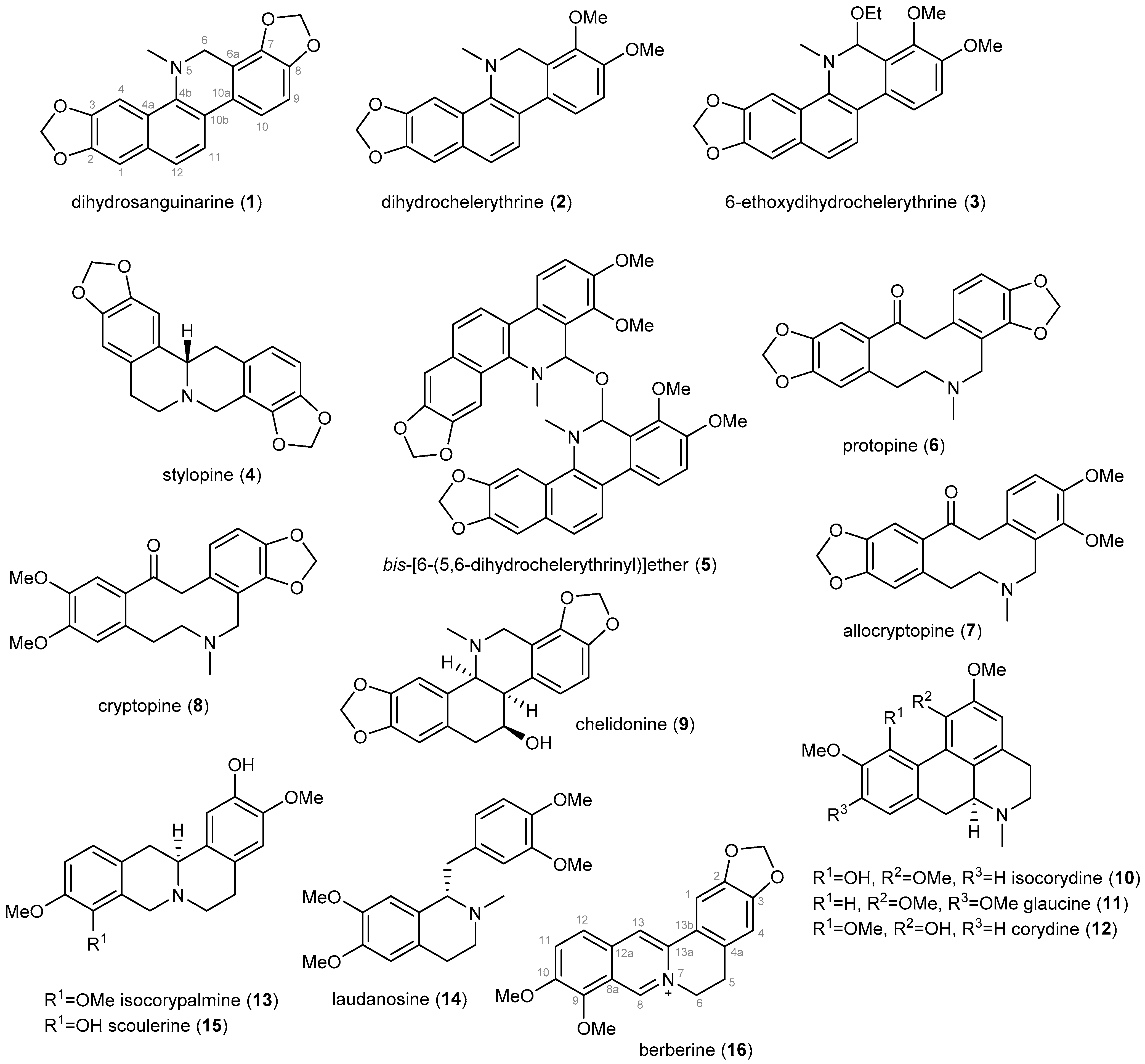
Sixteen previously described IAs (1–16, Figure 1) has been isolated from the whole plant of D. franchetianum by standard chromatographic methods, as described in the Experimental section. By a combination of MS, ESI-HRMS, 1D and 2D NMR experiments, optical rotation, and by comparison of the obtained data with the literature, the compounds were identified as dihydrosanquinarine (1) [31], dihydrochelerythrine (2) [31], 6-ethoxydihydrochelerythrine (3) [32], stylopine (4) [33], bis-[6-(5,6-dihydrochelerythrinyl)]ether (5) [34], protopine (6) [35], allocryptopine (7) [33], cryptopine (8) [35], chelidonine (9) [36], isocorydine (10) [37], glaucine (11) [38], corydine (12) [37], isocorypalmine (13) [39], laudanosine (14) [40], scoulerine (15) [41] and berberine (16) [42]. The isolated IAs belong to the benzophenanthridine (1–3, 9), protoberberine (4, 13, 15, 16), bisbenzophenanthridine (5), protopine (6–8), aporphine (10–12), and simple benzylisoquinoline (14) structural types.
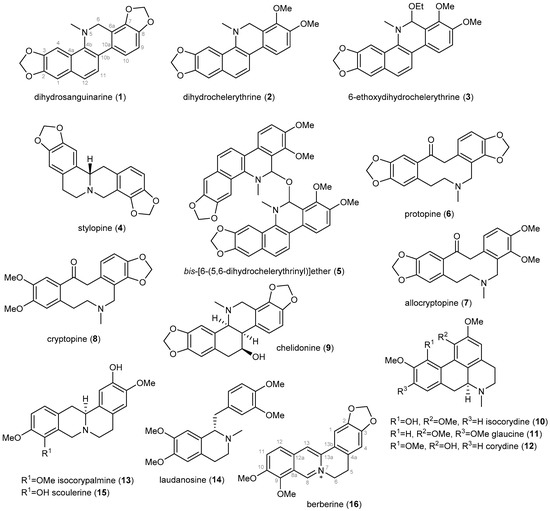
Figure 1.
Isoquinoline alkaloids isolated from the whole plant of Dicranostigma franchetianum.
Considering the anti-TB potency of alkaloids containing an isoquinoline heterocycle in their structure [43], the compounds isolated in sufficient amounts have been screened for their antimycobacterial potential against five Mycobacterium or Mycolicibacterium strains (Table 1). Most of the tested alkaloids showed either weak or no antimycobacterial potency (MIC ≥ 125 µg/mL, Table 1). Alkaloids 3 and 5, which contain a benzophenanthridine skeleton in their structure, displayed moderate activity against all the tested mycobacterial strains (MICs 15.625–31.25 µg/mL, Table 1). However, alkaloid 3 was recognized as an isolation artifact only. According to the isolation conditions, chelerythrine was unintentionally introduced to ethanol in an acidic environment at the very beginning of the phytochemical work, where the iminium electrophile was readily trapped by a nucleophile. Thus, this nucleophilic addition furnished a hemiaminal ether 3. Compound 5 is a dimer of two molecules of dihydrochelerythrine connected at position C-6 by an ether bridge and has been isolated previously from Zanthoxylum paracanthum and Z. monophyllum [44,45]. Dostál et al. have previously reported chelerythrine-like compounds in a thorough description of the dimerization. We assume that the action of the basic aqueous conditions of the phytochemical process yielded this dimer 5 [46]. Due to the induced dimerization, chelerythrine has never appeared in our final steps of isolation. Nevertheless, compound 5 exhibited strong activity against Aspergillus fumigatus (IC50 = 0.9 µM) and methicillin-resistant Staphylococcus aureus (IC50 = 1 µM) [45]. The improved antimycobacterial activity of 3 and 5, compared to dihydrochelerythrine itself (2), can be an inspiration for the preparation of more potent antimycobacterial compounds derived from the benzophenanthridine skeleton.

Table 1.
In vitro antimycobacterial activity against Mtb H37Ra, Mycolicibacterium aurum, Mycobacterium avium, Mycobacterium kansasii, and Mycolicibacterium smegmatis (MIC), cytotoxicity (IC50), selectivity index (SI), and calculated lipophilicity (ClogP) of isoquinoline alkaloids and berberine derivatives.
In previous studies, berberine (16) exerted potent antimycobacterial activity against M. intracellulare with a MIC value of 1.56 µg/mL [47], and moderate activity against Mycolicibacterium smegmatis (MIC 25 µg/mL) [48]. On the contrary, the study of Gentry et al. 1998 disclosed a weak activity of 16 against M. intracellulare (MIC 200 µg/mL). For this reason, we revised the data for berberine (16) when challenged with other Mycobacterium strains; the compound demonstrated moderate activity against M. avium and M. kansasii with MIC values of 31.25 µg/mL against both strains (Table 1). Semisynthetic derivatives of 16 are reported as compounds with strong antimycobacterial potential, e.g., 2,3,9-triethoxy-3,9-dibenzyloxy-10-methoxy-13-n-octylprotoberberine chloride displaying activity against drug-susceptible Mtb strain H37Rv with a MIC value of 0.125 µg/mL. Moreover, this berberine derivative showed an intriguing effect against RIF- and INH-resistant Mtb, which implies a new mechanism of action [27]. Because berberine (16) was isolated in quantity allowing preparation of its semisynthetic derivatives, we continued our study with the design and synthesis of C-9 substituted berberine analogues to inspect their antimycobacterial activity.
3.2. Berberine Derivatives (16a–16k) and Their Antimycobacterial Activity
Berberine is currently one of the most studied NPs, with a wide range of biological activities, including antimicrobial, hepatoprotective, antihyperlipidemic, anticancer, antidiabetic, anti-inflammatory, and antiarrhythmic effects [49,50,51,52,53]. It is available globally, mainly as a root extract, providing antimicrobial and antidiabetic effects. Recent studies have shown that berberine can enhance the inhibitory efficiency of antibiotics against clinically multi-drug resistant isolates of Staphylococcus aureus [54]. Besides, berberine administration to mice with pulmonary tuberculosis resulted in decreased lung pathology with no additive or synergistic effects on bacterial burden [55]. Berberine has low permeability through biological membranes and poor bioavailability, limiting its application and further development. Structural modification of NPs is a powerful method to improve their pharmacological properties.
For this reason, structural modifications of berberine (16) that increase its lipophilicity, mostly at the C-9 position, have been pursued to address these drawbacks [28,56]. Based on our previous report on the antimycobacterial activity of semisynthetic derivatives of Amaryllidaceae alkaloids, which showed promising activity against three Mycobacterium strains, we selected berberine (16) for the preparation of a small library of its semisynthetic derivatives, which have been screened for antimycobacterial activity against five Mycobacterium strains (Table 1). Given the necessity of long-term administration of anti-TB drugs, we have also determined their potential hepatotoxicity in vitro using a hepatocellular HepG2 cell line.
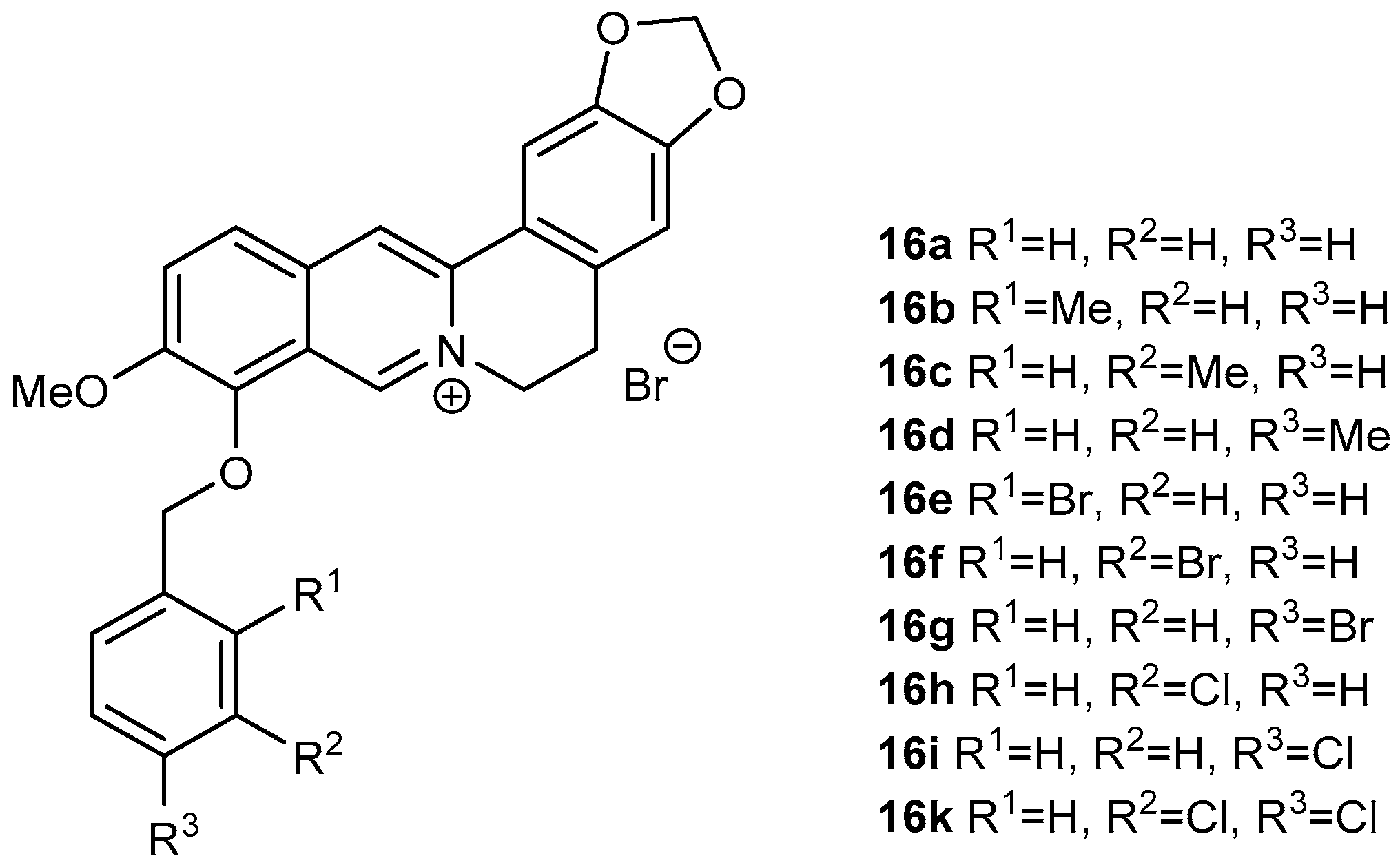
Derivatives (16a–16k; Figure 2) of berberine (16) were synthesized using the same method as reported by Sobolová et al., 2020 [28].
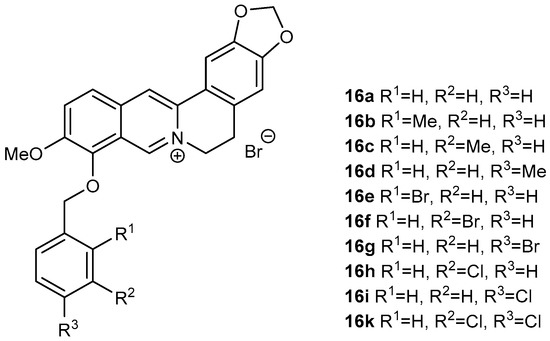
Figure 2.
Berberine derivatives from our library investigated as antitubercular agents within this study [28].
In all cases, replacing the methoxy group at position C-9 by differently substituted benzyl appendages was associated with a significant increase in antimycobacterial activity against all studied Mycobacterium strains (MIC 0.39–7.81 µg/mL, Table 1). For all the tested compounds, Mycolicibacterium smegmatis was the most sensitive strain. Among the methyl-substituted benzylberberine derivatives (16b–16d), a para position of the methyl group on the aromatic ring yielded the most active compound. Bromine substitution within the benzyl moiety resulted in better activity against all strains, generating the most active compound within the study 9-O-(2-brombenzyl)berberine (16e) (MIC 0.390–3.125 µg/mL). For this halogen substituent, the ortho position at the aromatic ring was connected with the highest activity. 3,4-Dichloro-substituted analogue 16k showed the same activity against Mtb as 16e, and either the same or slightly lower potency against the other tested strains (Figure 2, Table 1).
3.3. Lipophilicity versus Activity
Lipophilicity is one of the most important physicochemical properties of a compound that is crucially related to cell transmembrane transport. The mycobacterial cell wall is rich in mycolic acids, a distinctive feature of the mycobacterial cell wall, which efficiently prevents the penetration of drugs. Drugs with higher lipophilicity may exert better activity against Mtb [57], and thus we calculated ClogP values using the ChemBioDraw Ultra program (ver 18.1; PerkinElmer, Waltham, MA, USA). The ClogP value is correlated directly to the molecular hydrophobicity, presuming the diffusion through the biological membranes. All synthesized derivatives showed a higher ClogP value (0.99–2.30) in comparison with parent berberine (−0.77).
3.4. Cytotoxicity of Berberine Derivatives
Compounds with MIC values lower than 20 µM for Mtb H37Ra were also evaluated for in vitro cytotoxicity on hepatocellular carcinoma cells (HepG2) using an MTT assay. The HepG2 cell line serves as an in vitro model for hepatotoxicity for early drug screening. Moreover, the hepatocellular model was chosen since anti-TB drugs are known to carry the risk of hepatotoxicity, mostly ascribed to their long-term administration [58].
Evaluation of cytotoxicity allows the calculation of selectivity indexes (SI) as the ratio of IC50, HepG2 to MIC of Mtb H37Ra (Table 1; Table S1). In general, values of SI higher than 10 indicate more acceptable toxicity (analogous to the therapeutic index). The active compounds were screened at an initial concentration of 50 µM, and the IC50 values were subsequently determined. The effective cytotoxic concentrations of all tested compounds are listed in Table 1.
Among berberine derivatives, compound 16h exhibited the highest cytotoxicity with an IC50 value of 9.44 ± 0.29 µM. The most active derivatives in terms of the highest antimycobacterial potency, i.e., 16e and 16k, showed cytotoxicity, with IC50 values of 11.61 ± 0.29 µM, and 12.66 ± 2.51 µM, respectively, reaching SI values of 3.92 for 16e, and 4.55 for 16k. These values indicate a potential risk of hepatotoxicity. Thus, further research to find a balance between antimycobacterial efficacy and hepatotoxicity will be necessary to optimize the berberine scaffold.
4. Conclusions
In conclusion, the phytochemical investigation of the alkaloidal extract of the whole plant of Dicranostigma franchetianum allowed the isolation of 16 known isoquinoline alkaloids of different structural types. Compounds isolated in sufficient amounts were evaluated for their in vitro antimycobacterial activity against Mtb H37Ra and four other mycobacterial strains of clinical importance or usually used in antimycobacterial screening (Mycolicibacterium aurum, Mycobacterium avium, Mycobacterium kansasii, and Mycolicibacterium smegmatis). 6-Ethoxydihydrochelerythrine and bis-[6-(5,6-dihydrochelerythrinyl)]ether, both containing a benzophenanthridine nucleus in their structure, demonstrated moderate activity against all tested strains. The strong antimycobacterial potential of berberine, reported in previous studies, was not confirmed.
On the other hand, its semisynthetic derivatives demonstrated significant activity increment against all tested mycobacterial strains. Unfortunately, the most active berberine derivatives showed a relatively high cytotoxicity profile, as determined on HepG2 cell lines, indicating a potential hepatotoxicity risk. Therefore, further research on the berberine scaffold will focus on developing novel agents retaining high antimycobacterial profile while also reducing the cytotoxicity of the final entity. Further research warrants testing the top-ranked drug candidates in a combinatorial regimen by checkerboard studies to determine their synergistic properties with known antitubercular drugs. In vivo toxicity and in vivo efficacy will be determined using Galleria mellonella animal model. As promising antimycobacterial compounds, this pilot study presents prospective C-9 substituted berberine derivatives. Nevertheless, structure optimization needs further development and endeavor.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biom12060844/s1, Table S1: Measured data cell viability (16a–16k).
Author Contributions
Term, J.K. (Jan Korábečný), L.O., L.C.; Methodology, V.W., O.J., J.K. (Jana Křoustková), K.H.-B., J.K. (Jan Korábečný), E.K., A.H., J.K. (Jiří Kuneš), J.C., L.C., K.S.; supervision, O.J., J.K. (Jan Korábečný), L.O., L.C., K.S.; investigation, V.W., O.J., J.K. (Jana Křoustková), K.H.-B., J.K. (Jan Korá-bečný), E.K., A.H., K.K., M.Š., R.V., J.K. (Jiří Kuneš), J.C.; writing–original draft, V.W., O.J., J.K. (Jana Křoustková), K.H.-B., J.K. (Jan Korábečný), E.K., A.H., K.K., M.Š., R.V., J.K. (Jiří Kuneš), L.C., K.S.; writing–review, V.W., O.J., K.H.-B., J.K. (Jan Korábečný), E.K., A.H., K.K., M.Š., R.V., J.K. (Jiří Kuneš), L.O., J.C., L.C., K.S.; editing, V.W., O.J., J.K. (Jana Křoustková), J.K. (Jan Korábečný), E.K., A.H., K.K., M.Š., R.V., J.K. (Jiří Kuneš), L.O., J.C., L.C., K.S. All authors have read and agreed to the published version of the manuscript.
Funding
This project was supported by Charles University grants (SVV UK 260 548, Progress/UK Q42), by project, “Modernization and Extension of the Doctoral Study Field of Pharmacognosy and Toxicity of Natural Products” in the study program Pharmacy (reg. No. CZ.02.2.69/0.0/0.0/16_018/0002736), and by Ministry of Health of the Czech Republic (project No. NU21-05-00446).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors wish to thank Gerald Blunden for the critical reading of the manuscript of English corrections. We also thank Ing. Jana Vacková and Ida Dufková for technical assistance in microbiological screening.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dang, Y.; Gong, H.F.; Liu, J.X.; Yu, S.J. Alkaloid from Dicranostigma leptopodum (Maxim) Fedde. Chin. Chem. Lett. 2009, 20, 1218–1220. [Google Scholar] [CrossRef]
- Liu, D.H.; Zhang, T.C.; Liu, J.X.; Di, D.L.; Dang, Y. Chemical Constituents of Alkaloids from Dicranostigma leptopodum. Chin. Tradit. Herb Drugs 2011, 42, 1505–1509. [Google Scholar]
- Qiang, Z. Insights into Chemical Constituents of Alkaloids from Wild Medicinal Plant Dicranostigma leptodum (Maxim.) Fedde. J. Phys. Conf. Ser. 2019, 1176, 042056. [Google Scholar] [CrossRef]
- Zhang, W.-H.; Lv, M.-H.; Jun, H.; Wang, Q.-P.; Wang, Q. Dicranostigma leptopodum (Maxim.) Fedde induced apoptosis in SMMC-7721 human hepatoma cells and inhibited tumor growth in mice. Nat. Sci. 2010, 2, 457–463. [Google Scholar] [CrossRef]
- Zhong, M.; Ma, Y.-X.; Liu, J.-X.; Di, D.-L. A new quaternary protoberberine alkaloid isolated from Dicranostigma leptopodum (Maxim.) Fedde. Nat. Prod. Res. 2014, 28, 507–510. [Google Scholar] [CrossRef]
- Chen, Y.; Li, R.; Gao, R.; Yan, Q.; Zhong, M.; Liu, J.; Zhao, Q.; Di, D. Total content determination for the effective fraction of the alkaloids in Dicranostigma leptopodum (Maxim.) Fedde by HPLC and ultraviolet-visible spectrophotometry. Anal. Methods 2016, 8, 2645–2652. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, J.; Yan, Q.; Zhong, M.; Liu, J.; Di, D.; Liu, J. Simultaneous determination of the content of isoquinoline alkaloids in Dicranostigma leptopodum (Maxim.) Fedde and the effective fractionation of the alkaloids by high-performance liquid chromatography with diode array detection. J. Sep. Sci. 2015, 38, 9–17. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, X.; Liu, J.; Di, D. Three-phase solvent systems for the comprehensive separation of a wide variety of compounds from Dicranostigma leptopodum by high-speed counter-current chromatography: Liquid Chromatography. J. Sep. Sci. 2015, 38, 2038–2045. [Google Scholar] [CrossRef]
- Sun, R.; Jiang, H.; Zhang, W.; Yang, K.; Wang, C.; Fan, L.; He, Q.; Feng, J.; Du, S.; Deng, Z.; et al. Cytotoxicity of Aporphine, Protoberberine, and Protopine Alkaloids from Dicranostigma leptopodum (Maxim.) Fedde. Evid.-Based Complement. Altern. 2014, 2014, 580483. [Google Scholar] [CrossRef]
- Zhao, Q.; Han, Y.; Du, Y.P.; Wang, T.P.; Wang, Q. The effect of Dicranostigma leptopodum (Maxim.) Fedde (DLF) extraction on suppressing oxidative hemolysis of erythrocytes and its mechanism. J. Lanzhou Univ. Med. Sci. 2006, 32, 40–45. [Google Scholar]
- Tingpu, W.; Yixia, G.; Qiang, Z.; Yijun, Y.; Weichao, M.; Li, X.; Chenghui, L. Antibacterial activity and mechanism of alkaloids from Dicranostigma leptopodum (Maxim.) Fedde on Klebsiella pneumoniae. J. Tianshui Norm. Univ. 2018, 38, 24–28. [Google Scholar]
- Chelombit’ko, V.A. Dicranostigma franchetianum (Prain) Fedde: A plant promising as a source of the alkaloid isocorydine. Pharm. Chem. J. 1979, 13, 844–845. [Google Scholar] [CrossRef]
- Táborská, E.; Věžník, F.; Slavíková, L.; Slavík, J. Quaternary alkaloids of three species of Dicranostigma HOOK. f. et THOMS. Collect. Czech. Chem. Commun. 1978, 43, 1108–1112. [Google Scholar] [CrossRef]
- Slavíková, L.; Slavik, J. Alkaloide der mohngewächse (Papaveraceae) IX. Dicranostigma franchetianum (Prain) Fedde. Collect. Czech. Chem. Commun. 1959, 24, 559–563. [Google Scholar] [CrossRef]
- Manske, R.H.F. The alkaloids of Papaveraceous plants: XXXII. Stylophorum diphyllum (Michx.) Nutt., Dicranostigma franchetianum (Prain) Fedde and Glaucium serpieri Heldr. Can. J. Res. B 1942, 20, 53–56. [Google Scholar] [CrossRef]
- Udwadia, Z.F.; Amale, R.A.; Ajbani, K.K.; Rodrigues, C. Totally Drug-Resistant Tuberculosis in India. Clin. Infect. Dis. 2012, 54, 579–581. [Google Scholar] [CrossRef]
- Koul, A.; Arnoult, E.; Lounis, N.; Guillemont, J.; Andries, K. The challenge of new drug discovery for tuberculosis. Nature 2011, 469, 483–490. [Google Scholar] [CrossRef]
- Global Tuberculosis Programme. Global Tuberculosis Report 2021. Available online: https://www.who.int/publications/i/item/9789240037021 (accessed on 8 March 2022).
- Han, J.; Liu, X.; Zhang, L.; Quinn, R.J.; Feng, Y. Anti-mycobacterial natural products and mechanisms of action. Nat. Prod. Rep. 2022, 39, 77–89. [Google Scholar] [CrossRef]
- Von Nussbaum, F.; Brands, M.; Hinzen, B.; Weigand, S.; Häbich, D. Antibacterial natural products in medicinal chemistry—Exodus or revival? Angew. Chem. Int. Ed. Engl. 2006, 45, 5072–5129. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; The International Natural Product Sciences Taskforce; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Cazzaniga, G.; Mori, M.; Chiarelli, L.R.; Gelain, A.; Meneghetti, F.; Villa, S. Natural products against key Mycobacterium tuberculosis enzymatic targets: Emerging opportunities for drug discovery. Eur. J. Med. Chem. 2021, 224, 113732. [Google Scholar] [CrossRef] [PubMed]
- Maafi, N.; Mamun, A.A.; Janďourek, O.; Maříková, J.; Breiterová, K.; Diepoltová, A.; Konečná, K.; Hošťálková, A.; Hulcová, D.; Kuneš, J.; et al. Semisynthetic Derivatives of Selected Amaryllidaceae Alkaloids as a New Class of Antimycobacterial Agents. Molecules 2021, 26, 6023. [Google Scholar] [CrossRef] [PubMed]
- Claes, P.; Cappoen, D.; Mbala, B.M.; Jacobs, J.; Mertens, B.; Mathys, V.; Verschaeve, L.; Huygen, K.; De Kimpe, N. Synthesis and antimycobacterial activity of analogues of the bioactive natural products sampangine and cleistopholine. Eur. J. Med. Chem. 2013, 67, 98–110. [Google Scholar] [CrossRef]
- Li, Y.H.; Fu, H.G.; Su, F.; Gao, L.M.; Tang, S.; Bi, C.W.; Li, Y.H.; Wang, Y.X.; Song, D.Q. Synthesis and structure-activity relationship of 8-substituted protoberberine derivatives as a novel class of antitubercular agents. Chem. Cent. J. 2013, 7, 117. [Google Scholar] [CrossRef]
- Liu, Y.X.; Xiao, C.L.; Wang, Y.X.; Li, Y.H.; Yang, Y.H.; Li, Y.B.; Bi, C.W.; Gao, L.M.; Jiang, J.D.; Song, D.Q. Synthesis, structure-activity relationship and in vitro anti-mycobacterial evaluation of 13-n-octylberberine derivatives. Eur. J. Med. Chem. 2012, 52, 151–158. [Google Scholar] [CrossRef]
- Sobolová, K.; Hrabinová, M.; Hepnarová, V.; Kučera, T.; Kobrlová, T.; Benková, M.; Janočková, J.; Doležal, R.; Prchal, L.; Benek, O.; et al. Discovery of novel berberine derivatives with balanced cholinesterase and prolyl oligopeptidase inhibition profile. Eur. J. Med. Chem. 2020, 203, 112593. [Google Scholar] [CrossRef]
- Franzblau, S.G.; Witzig, R.S.; McLaughlin, J.C.; Torres, P.; Madico, G.; Hernandez, A.; Degnan, M.T.; Cook, M.B.; Quenzer, V.K.; Ferguson, R.M.; et al. Rapid, low-technology MIC determination with clinical Mycobacterium tuberculosis isolates by using the microplate Alamar Blue assay. J. Clin. Microbiol. 1998, 36, 362–366. [Google Scholar] [CrossRef]
- Schön, T.; Werngren, J.; Machado, D.; Borroni, E.; Wijkander, M.; Lina, G.; Mouton, J.; Matuschek, E.; Kahlmeter, G.; Giske, C.; et al. Antimicrobial susceptibility testing of Mycobacterium tuberculosis complex isolates—The EUCAST broth microdilution reference method for MIC determination. Clin. Microbiol. Infect. 2020, 26, 1488–1492. [Google Scholar] [CrossRef]
- Romo-Pérez, A.; Miranda, L.D.; Chávez-Blanco, A.D.; Dueñas-González, A.; Camacho-Corona, M.d.R.; Acosta-Huerta, A.; García, A. Mild C(sp3)–H functionalization of dihydrosanguinarine and dihydrochelerythrine for development of highly cytotoxic derivatives. Eur. J. Med. Chem. 2017, 138, 1–12. [Google Scholar] [CrossRef]
- Miao, F.; Yang, X.-J.; Zhou, L.; Hu, H.-J.; Zheng, F.; Ding, X.-D.; Sun, D.-M.; Zhou, C.-D.; Sun, W. Structural modification of sanguinarine and chelerythrine and their antibacterial activity. Nat. Prod. Res. 2011, 25, 863–875. [Google Scholar] [CrossRef]
- Kiryakov, H.G.; Iskrenova, E.; Daskalova, E.; Kuzmanov, B.; Evstatieva, L. Alkaloids of Corydalis slivenensis. Planta Med. 1982, 44, 168–170. [Google Scholar] [CrossRef]
- Dostál, J.; Táborská, E.; Slavík, J.; Potáček, M.; de Hoffmann, E. Structure of Chelerythrine Base. J. Nat. Prod. 1995, 58, 723–729. [Google Scholar] [CrossRef]
- Seger, C.; Sturm, S.; Strasser, E.-M.; Ellmerer, E.; Stuppner, H. 1H and 13C NMR signal assignment of benzylisoquinoline alkaloids from Fumaria officinalis L. (Papaveraceae). Magn. Reson. Chem. 2004, 42, 882–886. [Google Scholar] [CrossRef]
- Takao, N.; Kamigauchi, M.; Iwasa, K.; Morita, N.; Kuriyama, K. Stereochemie von Hydrobenzo[c]phenanthridin-Alkaloiden. Chiroptische Eigenschaften und absolute Konfiguration von (+)-14-Epicorynolin, (+)-Corynolin, (+)-Chelidonin und verwandten Verbindungen. Arch. Pharm. 1984, 317, 223–237. [Google Scholar] [CrossRef]
- Ferreira, M.L.R.; de Pascoli, I.C.; Nascimento, I.R.; Zukerman-Schpector, J.; Lopes, L.M.X. Aporphine and bisaporphine alkaloids from Aristolochia lagesiana var. intermedia. Phytochemistry 2010, 71, 469–478. [Google Scholar] [CrossRef]
- Arafa, A.; Mohamed, M.; Eldahmy, S.I. The aerial parts of yellow horn poppy (Glaucium flavum Cr.) growing in Egypt: Isoquinoline alkaloids and biological activities. J. Pharm. Sci. Res. 2016, 8, 323–332. [Google Scholar]
- Gadhiya, S.; Ponnala, S.; Harding, W.W. A divergent route to 9,10-oxygenated tetrahydroprotoberberine and 8-oxoprotoberberine alkaloids: Synthesis of (±)-isocorypalmine and oxypalmatine. Tetrahedron 2015, 71, 1227–1231. [Google Scholar] [CrossRef]
- Pacheco, J.C.O.; Lahm, G.; Opatz, T. Synthesis of Alkaloids by Stevens Rearrangement of Nitrile-Stabilized Ammonium Ylides: (±)-Laudanosine, (±)-Laudanidine, (±)-Armepavine, (±)-7-Methoxycryptopleurine, and (±)-Xylopinine. J. Org. Chem. 2013, 78, 4985–4992. [Google Scholar] [CrossRef]
- Schrittwieser, J.H.; Resch, V.; Wallner, S.; Lienhart, W.-D.; Sattler, J.H.; Resch, J.; Macheroux, P.; Kroutil, W. Biocatalytic Organic Synthesis of Optically Pure (S)-Scoulerine and Berbine and Benzylisoquinoline Alkaloids. J. Org. Chem. 2011, 76, 6703–6714. [Google Scholar] [CrossRef]
- Min, Y.D.; Yang, M.C.; Lee, K.H.; Kim, K.R.; Choi, S.U.; Lee, K.R. Protoberberine alkaloids and their reversal activity of P-gp expressed multidrug resistance (MDR) from the rhizome of Coptis japonica Makino. Arch. Pharm. Res. 2006, 29, 757–761. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.K.; Tripathi, G.; Kishore, N.; Singh, R.K.; Singh, A.; Tiwari, V.K. Drug development against tuberculosis: Impact of alkaloids. Eur. J. Med. Chem. 2017, 137, 504–544. [Google Scholar] [CrossRef]
- Omosa, L.K.; Nchiozem-Ngnitedem, V.-A.; Mukavi, J.; Atieno Okoko, B.; Nyaboke, H.O.; Hashim, I.; Matundura, J.O.; Efferth, T.; Spiteller, M. Cytotoxic alkaloids from the root of Zanthoxylum paracanthum (Mildbr.) Kokwaro. Nat. Prod. Res. 2021, 36, 2518–2525. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Guzmán, R.; Fulks, L.C.; Radwan, M.M.; Burandt, C.L.; Ross, S.A. Chemical constituents, antimicrobial and antimalarial activities of Zanthoxylum monophyllum. Planta Med. 2011, 77, 1542–1544. [Google Scholar] [CrossRef] [PubMed]
- Dostál, J.; Slavík, J.; Potáček, M.; Marek, R.; Humpa, O.; Sklenář, V.; Toušek, J.; Hoffmann, E.; Rozenberg, R. Structural Studies of Chelirubine and Chelilutine Free Bases. Collect. Czech. Chem. Commun. 1998, 63, 1045–1055. [Google Scholar] [CrossRef]
- Okunade, A.L.; Hufford, C.D.; Richardson, M.D.; Peterson, J.R.; Clark, A.M. Antimicrobial properties of alkaloids from Xanthorhiza simplicissima. J. Pharm. Sci. 1994, 83, 404–406. [Google Scholar] [CrossRef] [PubMed]
- Gentry, E.J.; Jampani, H.B.; Keshavarz-Shokri, A.; Morton, M.D.; Vander Velde, D.; Telikepalli, H.; Mitscher, L.A.; Shawar, R.; Humble, D.; Baker, W. Antitubercular Natural Products: Berberine from the Roots of Commercial Hydrastis canadensis Powder. Isolation of Inactive 8-Oxotetrahydrothalifendine, Canadine, β-Hydrastine, and Two New Quinic Acid Esters, Hycandinic Acid Esters-1 and -2. J. Nat. Prod. 1998, 61, 1187–1193. [Google Scholar] [CrossRef]
- Affuso, F.; Mercurio, V.; Fazio, V.; Fazio, S. Cardiovascular and metabolic effects of Berberine. World J. Cardiol. 2010, 2, 71–77. [Google Scholar] [CrossRef]
- Yao, L.; Wu, L.L.; Li, Q.; Hu, Q.M.; Zhang, S.Y.; Liu, K.; Jiang, J.Q. Novel berberine derivatives: Design, synthesis, antimicrobial effects, and molecular docking studies. Chin. J. Nat. Med. 2018, 16, 774–781. [Google Scholar] [CrossRef]
- Abd El-Salam, M.; Mekky, H.; El-Naggar, E.M.B.; Ghareeb, D.; El-Demellawy, M.; El-Fiky, F. Hepatoprotective properties and biotransformation of berberine and berberrubine by cell suspension cultures of Dodonaea viscosa and Ocimum basilicum. S. Afr. J. Bot. 2015, 97, 191–195. [Google Scholar] [CrossRef]
- Caliceti, C.; Franco, P.; Spinozzi, S.; Roda, A.; Cicero, A.F. Berberine: New Insights from Pharmacological Aspects to Clinical Evidences in the Management of Metabolic Disorders. Curr. Med. Chem. 2016, 23, 1460–1476. [Google Scholar] [CrossRef] [PubMed]
- Hošťálková, A.; Maříková, J.; Opletal, L.; Korábečný, J.; Hulcová, D.; Kuneš, J.; Nováková, L.; Perez, D.I.; Jun, D.; Kučera, T.; et al. Isoquinoline Alkaloids from Berberis vulgaris as Potential Lead Compounds for the Treatment of Alzheimer’s Disease. J. Nat. Prod. 2019, 82, 239–248. [Google Scholar] [CrossRef]
- Chu, M.; Zhang, M.-B.; Liu, Y.-C.; Kang, J.-R.; Chu, Z.-Y.; Yin, K.-L.; Ding, L.-Y.; Ding, R.; Xiao, R.-X.; Yin, Y.-N.; et al. Role of Berberine in the Treatment of Methicillin-Resistant Staphylococcus aureus Infections. Sci. Rep. 2016, 6, 24748. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, M.; Chia, J.E.; Hazra, R.; Saqib, M.; Maine, R.A.; Guler, R.; Suzuki, H.; Mishra, B.B.; Brombacher, F.; Parihar, S.P. Evaluation of Berberine as an Adjunct to TB Treatment. Front. Immunol. 2021, 12, 656419. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, S.J.; Lee, J.H.; Sun, W.S.; Kim, J.H. Antimicrobial activity of 9-O-acyl- and 9-O-alkylberberrubine derivatives. Planta Med. 2002, 68, 277–281. [Google Scholar] [CrossRef]
- Piccaro, G.; Poce, G.; Biava, M.; Giannoni, F.; Fattorini, L. Activity of lipophilic and hydrophilic drugs against dormant and replicating Mycobacterium tuberculosis. J. Antibiot. 2015, 68, 711–714. [Google Scholar] [CrossRef]
- Senousy, B.E.; Belal, S.I.; Draganov, P.V. Hepatotoxic effects of therapies for tuberculosis. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 543–556. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).