Improvement of Biomass and Phycoerythrin Production by a Strain of Rhodomonas sp. Isolated from the Tunisian Coast of Sidi Mansour
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation of Axenic Monoclonal Cultures
2.2. DNA Extraction, PCR Amplification, Sequencing and Phylogenetic Analysis
2.3. Growth and Phycoerythrin Accumulation
2.4. Experimental Design and Data Analysis
2.4.1. Screening of Factors Influencing Phycoerythrin Production
2.4.2. Optimization of Growth and Phycoerythrin Production Using a Box–Behnken Model
2.5. Gene Expression Analysis
2.6. Statistical Analysis
3. Results
3.1. Isolation and Identification of the Microalgae Strain
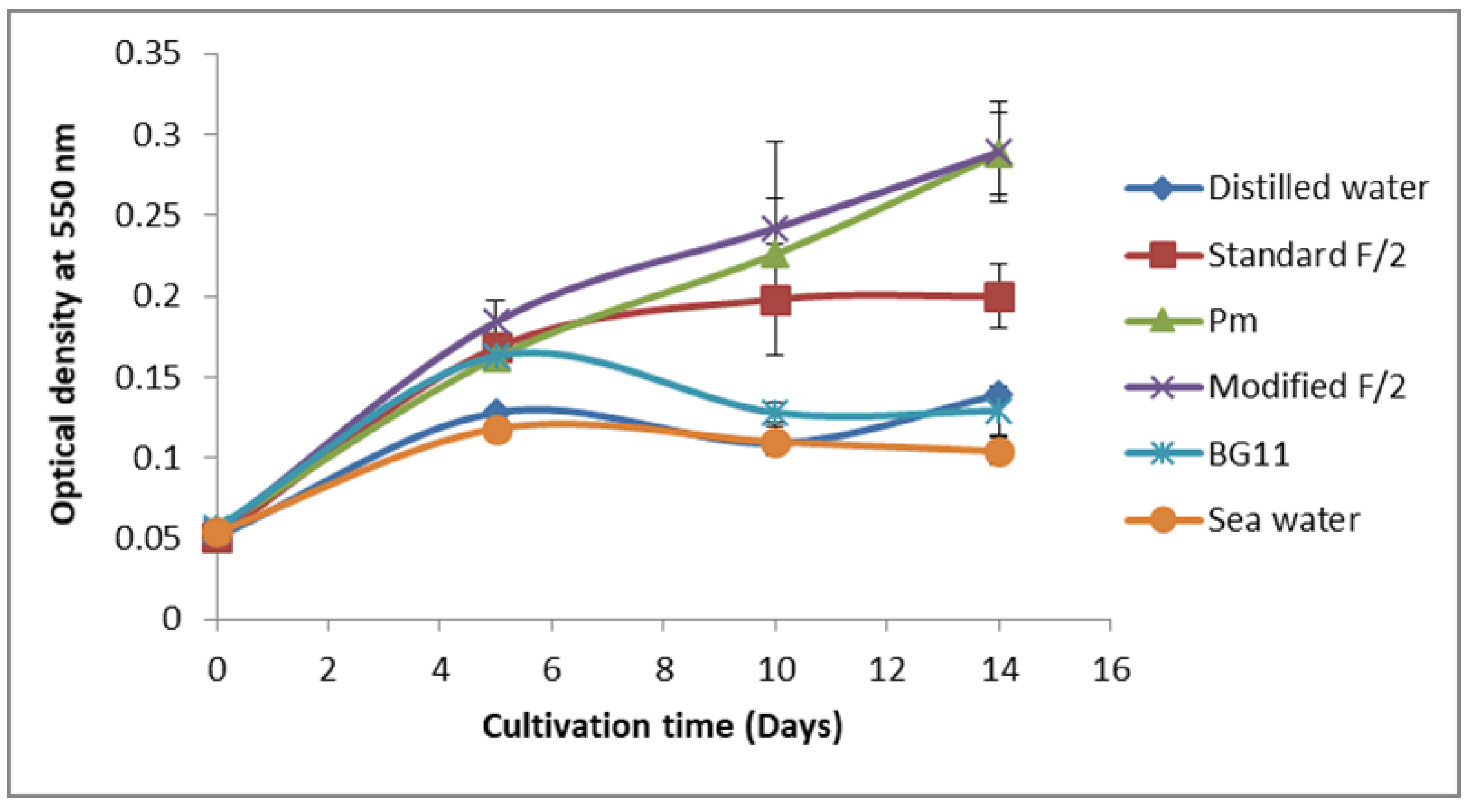
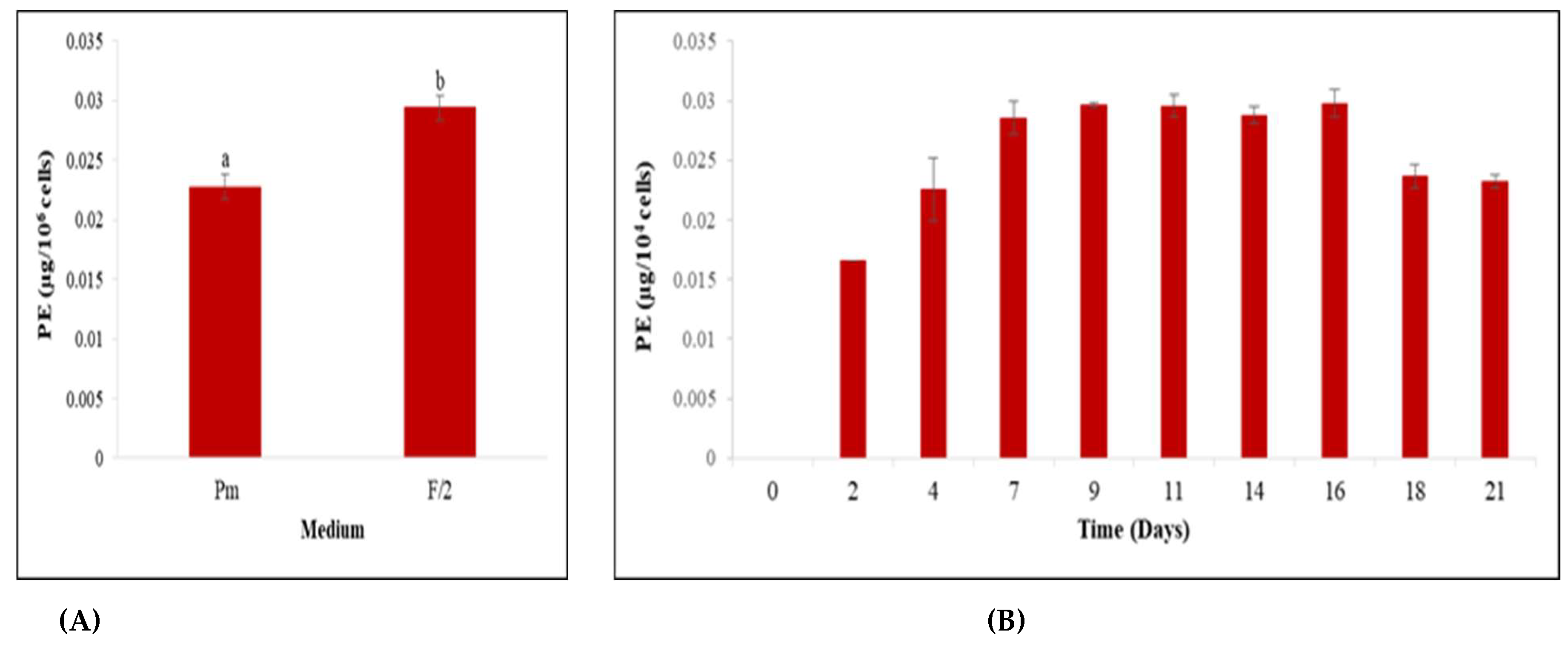
3.2. Screening for Culture Media Suitable for Cell Growth and Phycoerythrin Production
3.3. Screening of Factors Influencing Phycoerythrin Production
3.4. Combined Effects of Modified F/2 Medium Components on Biomass and Phycoerythrin Production
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dammak, M.; Haase, S.M.; Miladi, R.; Ben Amor, F.; Barkallah, M.; Gosset, D.; Pichon, C.; Huchzermeyer, B.; Fendri, I.; Denis, M.; et al. Enhanced lipid and biomass production by a newly isolated and identified marine microalga. Lipids Health Dis. 2016, 15, 209. [Google Scholar] [CrossRef] [PubMed]
- Delattre, C.; Pierre, G.; Laroche, C.; Michaud, P. Production, extraction and characterization of microalgal and cyanobacterial exopolysaccharides. Biotechnol. Adv. 2016, 34, 1159–1179. [Google Scholar] [CrossRef] [PubMed]
- Ben Amor, F.; Barkallah, M.; Elleuch, F.; Karkouch, N.; Dammak, M.; Baréa, B.; Villeneuve, P.; Abdelkafi, S.; Fendri, I. Cyanobacteria as source of marine bioactive compounds: Molecular specific detection based on Δ9 desaturase gene. Int. J. Biol. Macromol. 2017, 105, 1440–1445. [Google Scholar] [CrossRef] [PubMed]
- Ben Amor, F.; Elleuch, F.; Ben Hlima, H.; Garnier, M.; Saint-Jean, B.; Barkallah, M.; Pichon, C.; Abdelkafi, S.; Fendri, I. Proteomic analysis of the Chlorophyta Dunaliella new strain AL-1 revealed global changes of metabolism during high carotenoid production. Mar. Drugs 2017, 15, 293. [Google Scholar] [CrossRef] [PubMed]
- Ben Hlima, H.; Dammak, M.; Karkouch, N.; Hentati, F.; Laroche, C.; Michaud, P.; Fendri, I.; Abdelkafi, S. Optimal cultivation towards enhanced biomass and floridean starch production by Porphyridium marinum. Int. J. Biol. Macromol. 2019, 129, 152–161. [Google Scholar] [CrossRef]
- Borowitzka, M.A. Microalgae as sources of pharmaceuticals and other biologically active compounds. J. Appl. Phycol. 1995, 7, 3–15. [Google Scholar] [CrossRef]
- Glazer, A.N. Phycobiliproteins—A family of valuable, widely used fluorophores. J. Appl. Phycol. 1994, 6, 105–112. [Google Scholar] [CrossRef]
- Hu, L.; Huang, B.; Zuo, M.; Guo, R.-Y.; Wei, H. Preparation of the phycoerythrin subunit liposome in a photodynamic experiment on liver cancer cells. Acta Pharmacol. Sin. 2008, 29, 1539–1546. [Google Scholar] [CrossRef][Green Version]
- Deng, R.; Chow, T.J. Hypolipidemic, antioxidant, and antiinflammatory activities of microalgae Spirulina: Hypolipidemic, antioxidant, and anti-inflammatory activities of microalgae Spirulina. Cardiovasc. Ther. 2010, 28, e33–e45. [Google Scholar] [CrossRef]
- Ibáñez-González, M.J.; Mazzuca-Sobczuk, T.; Redondo-Miranda, R.M.; Molina Grima, E.; Cooney, C.L. A novel vortex flow reactor for the purification of B-phycoerythrin from Porphyridium cruentum. Chem. Eng. Res. Des. 2016, 111, 24–33. [Google Scholar] [CrossRef]
- Roy, S.; Llewellyn, C.A.; Egeland, E.S.; Johnsen, G. Phytoplankton Pigments: Characterization, Chemotaxonomy and Applications in Oceanography; Cambridge University Press: Cambridge, UK, 2011. [Google Scholar]
- Chaloub, R.M.; Motta, N.M.S.; de Araujo, S.P.; de Aguiar, P.F.; da Silva, A.F. Combined effects of irradiance, temperature and nitrate concentration on phycoerythrin content in the microalga Rhodomonas sp. (Cryptophyceae). Algal. Res. 2015, 8, 89–94. [Google Scholar] [CrossRef]
- Glazer, A.N. Light harvesting by phycobilisomes. Annu. Rev. Biophys. 1985, 14, 47–77. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Chen, S.; Wen, Z. Effects of light intensity on the production of phycoerythrin and polyunsaturated fatty acid by microalga Rhodomonas salina. Algal. Res. 2021, 58, 102397. [Google Scholar] [CrossRef]
- Chang, W.; Jiang, T.; Wan, Z.; Zhang, J.-P.; Yang, Z.-X.; Liang, D.-C. Crystal Structure of R-phycoerythrin from Polysiphonia urceolata at 2.8 Å Resolution. J. Mol. Biol. 1996, 262, 721–722. [Google Scholar] [CrossRef] [PubMed]
- Gantt, E.; Edwards, M.R.; Provasoli, L. Chloroplast Structure of the Cryptophyceae. Int. J. Cell Biol. 1971, 48, 280–290. [Google Scholar] [CrossRef]
- Vesk, M.; Dwarte, D.; Fowler, S.; Hiller, R.G. Freeze fracture immunocytochemistry of light-harvesting pigment complexes in a cryptophyte. Protoplasma 1992, 170, 166–176. [Google Scholar] [CrossRef]
- Kumar, G.; Shekh, A.; Jakhu, S.; Sharma, Y.; Kapoor, R.; Sharma, T.R. Bioengineering of microalgae: Recent advances, perspectives, and regulatory challenges for industrial application. Front. Bioeng. Biotechnol. 2020, 8, 914. [Google Scholar] [CrossRef]
- Huang, X.; Zang, X.; Wu, F.; Jin, Y.; Wang, H.; Liu, C.; Ding, Y.; He, B.; Xiao, D.; Song, X.; et al. Transcriptome sequencing of Gracilariopsis lemaneiformis to analyze the genes related to optically active phycoerythrin synthesis. PLoS ONE 2017, 12, e0170855. [Google Scholar] [CrossRef]
- Gargouch, N.; Karkouch, I.; Elleuch, J.; Elkahoui, S.; Michaud, P.; Abdelkafi, S.; Laroche, C.; Fendri, I. Enhanced B-phycoerythrin production by the red microalga Porphyridium marinum: A powerful agent in industrial applications. Int. J. Biol. Macromol. 2018, 120, 2106–2114. [Google Scholar] [CrossRef]
- Lawrenz, E.; Richardson, T.L. Differential effects of changes in spectral irradiance on photoacclimation, primary productivity and growth in Rhodomonas salina (Cryptophyceae) and Skeletonema costatum (Bacillariophyceae) in simulated blackwater environments. J. Phycol. 2017, 53, 1241–1254. [Google Scholar] [CrossRef]
- Bartual, A.; Lubián, L.M.; Gálvez, J.A.; Niell, F.X. Effect of irradiance on growth, photosynthesis, pigment content and nutrient consumption in dense cultures of Rhodomonas salina (Wislouch) (Cryptophyceae). Ciencias Marinas 2002, 28, 381–392. [Google Scholar] [CrossRef]
- Silva, A.F.; Lourenço, S.O.; Chaloub, R.M. Effects of nitrogen starvation on the photosynthetic physiology of a tropical marine microalga Rhodomonas sp. (Cryptophyceae). Aquat. Bot. 2009, 4, 291–297. [Google Scholar] [CrossRef]
- Latsos, C.; van Houcke, J.; Blommaert, L.; Verbeeke, G.P.; Kromkamp, J.; Timmermans, K.R. Effect of light quality and quantity on productivity and phycoerythrin concentration in the cryptophyte Rhodomonas sp. J. Appl. Phycol. 2021, 33, 729–741. [Google Scholar] [CrossRef]
- Wilk, K.E.; Harrop, S.J.; Jankova, L.; Edler, D.; Keenan, G.; Sharples, F.; Hiller, R.G.; Curmi, P.M.G. Evolution of a light-harvesting protein by addition of new subunits and rearrangement of conserved elements: Crystal structure of a cryptophyte phycoerythrin at 1.63-Å resolution. Proc. Natl. Acad. Sci. USA 1999, 96, 8901–8906. [Google Scholar] [CrossRef] [PubMed]
- Kronfel, C.M.; Biswas, A.; Frick, J.P.; Gutu, A.; Blensdorf, T.; Karty, J.A.; Kehoe, D.M.; Schluchter, W.M. The roles of the chaperone-like protein CpeZ and the phycoerythrobilin lyase CpeY in phycoerythrin biogenesis. Biochim. Biophys. Acta 2019, 1860, 549–561. [Google Scholar] [CrossRef]
- Guillard, R.R.L. Culture of Phytoplankton for Feeding Marine Invertebrates. In Culture of Marine Invertebrate Animals; Smith, W.L., Chanley, M.H., Eds.; Springer: Boston, MA, USA, 1975; pp. 29–60. [Google Scholar]
- Soanen, N.; Da Silva, E.; Gardarin, C.; Michaud, P.; Laroche, C. Improvement of exopolysaccharide production by Porphyridium marinum. Bioresour. Technol. 2016, 213, 231–238. [Google Scholar] [CrossRef]
- Chtourou, H.; Dahmen, I.; Hassairi, I.; Abdelkafi, S.; Sayadi, S.; Dhouib, A. Dunaliella sp. a wild algal strain isolated from the Sfax-Tunisia solar evaporating salt-ponds, a high potential for biofuel production purposes. J. Biobased Mater. Bioenergy 2014, 8, 27–34. [Google Scholar] [CrossRef]
- Fendri, I.; Chaari, A.; Dhouib, A.; Abdelkafi, S.; Sayadi, S.; Dhouib, A. Isolation, identification and characterization of a new lipolytic Pseudomonas sp., strain AHD-1, from Tunisian soil. Environ. Technol. 2010, 31, 87–95. [Google Scholar] [CrossRef]
- Miladi, R.; Manghisi, A.; Armeli Mincante, S.; Genovese, G.; Abdelkafi, S.; Morabito, M. A DNA barcoding survey of Ulva (Chlorophyta) in Tunisia and Italy reveals the presence of the overlooked alien Ulva ohnoi. Crypt. Algol. 2018, 39, 85–107. [Google Scholar] [CrossRef]
- Allen, M.M. Simple conditions for growth of unicellular blue-green algae on plates. J. Phycol. 1968, 4, 1–4. [Google Scholar] [CrossRef]
- Ben Mohamed, J.; Elleuch, J.; Drira, M.; Esteban, M.Á.; Michaud, P.; Abdelkafi, S.; Fendri, I. Characterization and biotechnological potential of two native marine microalgae isolated from the Tunisian coast. Appl. Sci. 2021, 11, 5295. [Google Scholar] [CrossRef]
- Lawrenz, E.; Fedewa, E.J.; Richardson, T.L. Extraction protocols for the quantification of phycobilins in aqueous phytoplankton extracts. J. Appl. Phycol. 2011, 23, 865–871. [Google Scholar] [CrossRef]
- Elleuch, J.; Hadj Kacem, F.; Ben Amor, F.; Hadrich, B.; Michaud, P.; Fendri, I.; Abdelkafi, S. Extracellular neutral protease from Arthrospira platensis: Production, optimization and partial characterization. Int. J. Biol. Macro. 2021, 167, 1491–1498. [Google Scholar] [CrossRef] [PubMed]
- Elleuch, J.; Ben Amor, F.; Chaaben, Z.; Frikha, F.; Michaud, P.; Fendri, I.; Abdelkafi, S. Zinc biosorption by Dunaliella sp. AL-1: Mechanism and effects on cell metabolism. Sci. Total Environ. 2021, 773, 145024. [Google Scholar] [CrossRef]
- Meng, L.; Feldman, L. A rapid TRIzol-based two-step method for DNA-free RNA extraction from Arabidopsis siliques and dry seeds. Biotechnol. J. 2010, 5, 183–186. [Google Scholar] [CrossRef]
- Elleuch, J.; Zribi, R.Z.; Ben Fguira, I.; Lacroix, M.N.; Suissi, J.; Chandre, F.; Tounsi, S.; Jaoua, S. Effects of the P20 protein from Bacillus thuringiensis israelensis on insecticidal crystal protein Cry4Ba. Int. J. Biol. Macro. 2015, 79, 174–179. [Google Scholar] [CrossRef]
- Elleuch, J.; Ben Amor, F.; Barkallah, M.; Haj Salah, J.; Smith, K.F.; Aleya, L.; Fendri, I.; Abdelkafi, S. q-PCR-based assay for the toxic dinoflagellate Karenia selliformis monitoring along the Tunisian coasts. Environ. Sci. Pollut. Res. 2021, 28, 57486–57498. [Google Scholar] [CrossRef]
- Toldrà, A.; Andree, K.B.; Fernández-Tejedor, M.; Salah, J.H.; Smith, K.F.; Aleya, L.; Fendri, I.; Abdelkafi, S. Dual quantitative PCR assay for identification and enumeration of Karlodinium veneficum and Karlodinium armiger combined with a simple and rapid DNA extraction method. J. Appl. Phycol. 2018, 28, 57486–57498. [Google Scholar] [CrossRef]
- Barkallah, M.; Elleuch, J.; Smith, K.F.; Chaari, S.; Ben Neila, I.; Fendri, I.; Michaud, P.; Abdelkafi, S. Development and application of a real-time PCR assay for the sensitive detection of diarrheic toxin producer Prorocentrum lima. J. Microbiol. Methods 2020, 178, 106081. [Google Scholar] [CrossRef]
- Elleuch, J.; Hmani, R.; Drira, M.; Chaari, S.; Ben Neila, I.; Fendri, I.; Michaud, P.; Abdelkafi, S. Potential of three local marine microalgae from Tunisian coasts for cadmium, lead and chromium removals. Sci. Total Environ. 2021, 799, 149464. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Marraskuranto, E.; Raharjo, T.J.; Kasiamdari, R.S.; Nuringtyas, T.R. Influence of salinity on growth and phycoerythrin production of Rhodomonas salina. Squalen. Bull. Mar. Fish. Postharvest Biotechnol. 2018, 13, 109. [Google Scholar] [CrossRef]
- Latsos, C.; Bakratsas, G.; Moerdijk, T.; Verbeeke, G.P.; Kromkamp, J.; Timmermans, K.R. Effect of salinity and pH on growth, phycoerythrin, and non-volatile umami taste active compound concentration of Rhodomonas salina using a D-optimal design approach. J. Appl. Phycol. 2021, 33, 3591–3602. [Google Scholar] [CrossRef]
- Oostlander, P.C.; van Houcke, J.; Wijffels, R.H.; Barbosa, M.J. Optimization of Rhodomonas sp. under continuous cultivation for industrial applications in aquaculture. Algal Res. 2020, 47, 101889. [Google Scholar] [CrossRef]
- Reed, J.L.; Motes, D.; Muller, L.; Perez, C.; Hennessey, K. Got calcium? The effects of varying concentrations of calcium on algal growth. J. Introd. Biol. Investig. 2017, 7, 2. [Google Scholar]
- Bezy, R.P.; Wiltbank, L.; Kehoe, D.M. Light-dependent attenuation of phycoerythrin gene expression reveals convergent evolution of green light sensing in cyanobacteria. Proc. Natl. Acad. Sci. USA 2011, 108, 18542–18547. [Google Scholar] [CrossRef]
- Bryant, D. The Molecular Biology of Cyanobacteria; Kluwer Academic Publishers: Alphen aan den Rijn, The Netherlands, 1994. [Google Scholar]
- Stern, D.; Witman, G. The Chlamydomonas Sourcebook, 2nd ed.; Academic Press: Amsterdam, Boston, 2009. [Google Scholar]
- He, B.; Hou, L.; Dong, M.; Shi, J.; Huang, X.; Ding, Y.; Cong, X.; Zhang, F.; Zhang, X.; Zang, X. Transcriptome analysis in Haematococcus pluvialis: Astaxanthin induction by high light with acetate and Fe2+. Int. J. Mol. Sci. 2018, 19, 175. [Google Scholar] [CrossRef]
- Talarico, L.; Cortese, A. Response of Audouinella saviana Woelkerling cultures to monochromatic light. Hydrobiologia 1993, 260261, 477–484. [Google Scholar] [CrossRef]
- Franklin, L.; Kräbs, G.; Kuhlenkamp, R. Blue light and UV-A radiation control the synthesis of mycosporine-like amino acids in Chondrus Crispus (Florideophyceae). J. Phycol. 2001, 3, 257–270. [Google Scholar] [CrossRef]
- Godínez-Ortega, J.L.; Snoeijs, P.; Robledo, D.; Freile-Pelegrin, Y.; Pedersén, M. Growth and pigment composition in the red alga Halymenia floresii cultured under different light qualities. J. Appl. Phycol. 2008, 3, 253–260. [Google Scholar] [CrossRef]
- Thien, V.Y.; Rodrigues, K.F.; Voo, C.L.Y.; Wong, C.; Yong, W. Comparative transcriptome profiling of Kappaphycus alvarezii (Rhodophyta, Solieriaceae) in response to light of different wavelengths and carbon dioxide enrichment. Plants 2021, 10, 1236. [Google Scholar] [CrossRef] [PubMed]


| Gene Abbreviation | Description | Primer Sequence 1 |
|---|---|---|
| α-tub | α-tubulin | F: 5′-AGATCACGAACGCCACCTTC-3′ |
| R: 5′-GATGGTGCGCTTCGTCTTGA -3′ | ||
| cpeB | phycoerythrin beta chain | F: 5′-GGTGGTGCAGATCTACAAGC-3′ |
| R: 5′-CATGCAGCCATACGACGGTT-3′ | ||
| 18S | Ribosomal RNA 18 S | F: 5′-AACCTGGTTGATCCTGCCAGT-3′ |
| R: 5′-TGATCCTTCTGCAGGTTCACCTAC-3′ |
| Coded Factors | Factors | Low Level (−1) | High Level (+1) |
|---|---|---|---|
| X1 | NaCl (g/L) | 17 | 37 |
| X2 | MgCl2 (g/L) | 2 | 9 |
| X3 | CaCl2 (g/L) | 0 | 2 |
| X4 | KNO3 (g/L) | 0 | 2 |
| X5 | KH2PO4 (g/L) | 0 | 0.12 |
| X6 | NaHCO3 (g/L) | 0 | 0.1 |
| X7 | MgSO4 (g/L) | 1 | 5.5 |
| X8 | NaNO3 (g/L) | 0.5 | 1.5 |
| X9 | NaH2PO4 (g/L) | 0.5 | 1.5 |
| X10 | Metal Solution (mL/L) | 0.5 | 1.5 |
| X11 | Vitamin Solution (mL/L) | 0 | 1 |
| X12 | Light intensity (µmol/m2/s) | 27 | 110 |
| X13 | pH | 5 | 9 |
| Coded Factors | Factors | −1 | 0 | +1 |
|---|---|---|---|---|
| X1 | CaCl2 (g/L) | 0.25 | 1.125 | 2 |
| X2 | Metal solution (mL/L) | 0.5 | 1 | 1.5 |
| X3 | pH | 5 | 7 | 9 |
| X4 | Light intensity (µmol/m2/s) | 43 | 92 | 141 |
| Source of Variation | DF | Seq SS | Adj SS | Adj MS | F-Value | p-Value |
|---|---|---|---|---|---|---|
| Main Effects | 13 | 3129.08 | 3129.08 | 240.698 | 6.95 | 0.000 |
| Residual Error | 26 | 900.06 | 900.06 | 34.618 | ||
| Lack of fit | 6 | 868.05 | 868.05 | 144.675 | 90.39 | 0.000 |
| Pure error | 20 | 32.01 | 32.01 | 1.601 | ||
| Total | 39 | 4029.14 |
| Terms | Coefficient | p-Value |
|---|---|---|
| Constant | 4.565 | 0.000 |
| NaCl | −2.612 | 0.009 * |
| MgCl2 | −2.026 | 0.039 * |
| CaCl2 | 4.224 | 0.000 ** |
| KNO3 | 2.313 | 0.020 * |
| KH2PO4 | −0.486 | 0.606 |
| NaHCO3 | 1.535 | 0.111 |
| MgSO4 | 1.668 | 0.085 |
| NaNO3 | −0.637 | 0.500 |
| NaH2PO4 | −1.693 | 0.080 |
| Metal solution | −3.430 | 0.001 ** |
| Vitamin solution | −0.877 | 0.355 |
| Light intensity | 3.396 | 0.001 ** |
| pH | −3.376 | 0.001 ** |
| Source of Variation | Sum Squares | Degrees of Freedom | Mean Square | Ratio | Significance (%) |
|---|---|---|---|---|---|
| Regression | 3.4357 | 14 | 0.9597 | 5.0189 | ** |
| Residual | 2.1034 | 11 | 0.1912 | ||
| Lack of fit | 2.0855 | 10 | 0.2086 | 11.6767 | 22.8% |
| Error | 0.0179 | 1 | 0.0179 | ||
| Total | 5.5391 | 25 |
| Source of Variation | Sum of Squares | Degrees of Freedom | Mean Square | Ratio | Significance (%) |
|---|---|---|---|---|---|
| Regression | 3.27605E + 0005 | 14 | 2.34003E + 0004 | 4.3819 | ** |
| Residual | 6.40829E + 0004 | 12 | 5.34024E + 0003 | ||
| Lack of fit | 5.33538E + 0004 | 10 | 5.33538E + 0003 | 0.9946 | 59.8% |
| Error | 1.07291E + 0004 | 2 | 5.36458E + 0003 | ||
| Total | 3.91688E + 0005 | 26 |
| Factor | Coefficient | F. Inflation | Ecart-Type | t.exp. | Significance. % |
|---|---|---|---|---|---|
| X0 | 0.8115 | 0.3092 | 2.62 | * | |
| X1 | 0.2565 | 1.00 | 0.1262 | 2.03 | 6.5% |
| X2 | −0.0699 | 1.00 | 0.1262 | −0.55 | 59.6% |
| X3 | 0.0367 | 1.00 | 0.1262 | 0.29 | 77.3% |
| X4 | 0.7468 | 1.00 | 0.1262 | 5.92 | *** |
| X1 2 | 0.3367 | 1.48 | 0.2093 | 1.61 | 13.3% |
| X2 2 | 0.0358 | 1.48 | 0.2093 | 0.17 | 86.2% |
| X3 2 | −0.2579 | 1.48 | 0.2093 | −1.23 | 24.3% |
| X4 2 | 0.4625 | 1.48 | 0.2093 | 2.21 | * |
| X1X2 | 0.0445 | 1.00 | 0.2186 | 0.20 | 83.6% |
| X1X3 | −0.1605 | 1.00 | 0.2186 | −0.73 | 48.4% |
| X2X3 | −0.3578 | 1.00 | 0.2186 | −1.64 | 12.7% |
| X1X4 | 0.7845 | 1.00 | 0.2186 | 3.59 | ** |
| X2X4 | 0.1285 | 1.00 | 0.2186 | 0.59 | 57.4% |
| Factor | Coefficient | F. Inflation | Ecart-Type | t.exp. | Significance (%) |
|---|---|---|---|---|---|
| X0 | 283.33333 | 42.19101 | 6.72 | *** | |
| X1 | 67.70833 | 1.00 | 21.09550 | 3.21 | ** |
| X2 | 1.04167 | 1.00 | 21.09550 | 0.05 | 96.0% |
| X3 | 6.29167 | 1.00 | 21.09550 | 0.30 | 76.7% |
| X4 | 120.87500 | 1.00 | 21.09550 | 5.73 | *** |
| X1 2 | −62.47917 | 1.25 | 31.64325 | −1.97 | 6.9% |
| X2 2 | −34.35417 | 1.25 | 31.64325 | −1.09 | 30.0% |
| X3 2 | −79.72917 | 1.25 | 31.64325 | −2.52 | * |
| X4 2 | −39.10417 | 1.25 | 31.64325 | −1.24 | 23.9% |
| X1X2 | 21.87500 | 1.00 | 36.53848 | 0.60 | 56.7% |
| X1X3 | −15.62500 | 1.00 | 36.53848 | −0.43 | 67.9% |
| X2X3 | −28.12500 | 1.00 | 36.53848 | −0.77 | 46.2% |
| X1X4 | 96.87500 | 1.00 | 36.53848 | 2.65 | * |
| X2X4 | 53.12500 | 1.00 | 36.53848 | 1.45 | 16.9% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Derbel, H.; Elleuch, J.; Tounsi, L.; Nicolo, M.S.; Rizzo, M.G.; Michaud, P.; Fendri, I.; Abdelkafi, S. Improvement of Biomass and Phycoerythrin Production by a Strain of Rhodomonas sp. Isolated from the Tunisian Coast of Sidi Mansour. Biomolecules 2022, 12, 885. https://doi.org/10.3390/biom12070885
Derbel H, Elleuch J, Tounsi L, Nicolo MS, Rizzo MG, Michaud P, Fendri I, Abdelkafi S. Improvement of Biomass and Phycoerythrin Production by a Strain of Rhodomonas sp. Isolated from the Tunisian Coast of Sidi Mansour. Biomolecules. 2022; 12(7):885. https://doi.org/10.3390/biom12070885
Chicago/Turabian StyleDerbel, Hana, Jihen Elleuch, Latifa Tounsi, Marco Sebastiano Nicolo, Maria Giovanna Rizzo, Philippe Michaud, Imen Fendri, and Slim Abdelkafi. 2022. "Improvement of Biomass and Phycoerythrin Production by a Strain of Rhodomonas sp. Isolated from the Tunisian Coast of Sidi Mansour" Biomolecules 12, no. 7: 885. https://doi.org/10.3390/biom12070885
APA StyleDerbel, H., Elleuch, J., Tounsi, L., Nicolo, M. S., Rizzo, M. G., Michaud, P., Fendri, I., & Abdelkafi, S. (2022). Improvement of Biomass and Phycoerythrin Production by a Strain of Rhodomonas sp. Isolated from the Tunisian Coast of Sidi Mansour. Biomolecules, 12(7), 885. https://doi.org/10.3390/biom12070885









