The Impact of Zinc and Zinc Homeostasis on the Intestinal Mucosal Barrier and Intestinal Diseases
Abstract
:1. Introduction
2. Zinc Homeostasis in the Intestinal Mucosal Barrier
2.1. Zinc Absorption and Excretion
2.2. Interactions between the Zinc Homeostasis Imbalance and Intestinal Mucosal Barrier Injury
3. Mechanism of Zinc Homeostasis Affecting the Intestinal Mucosal Barrier
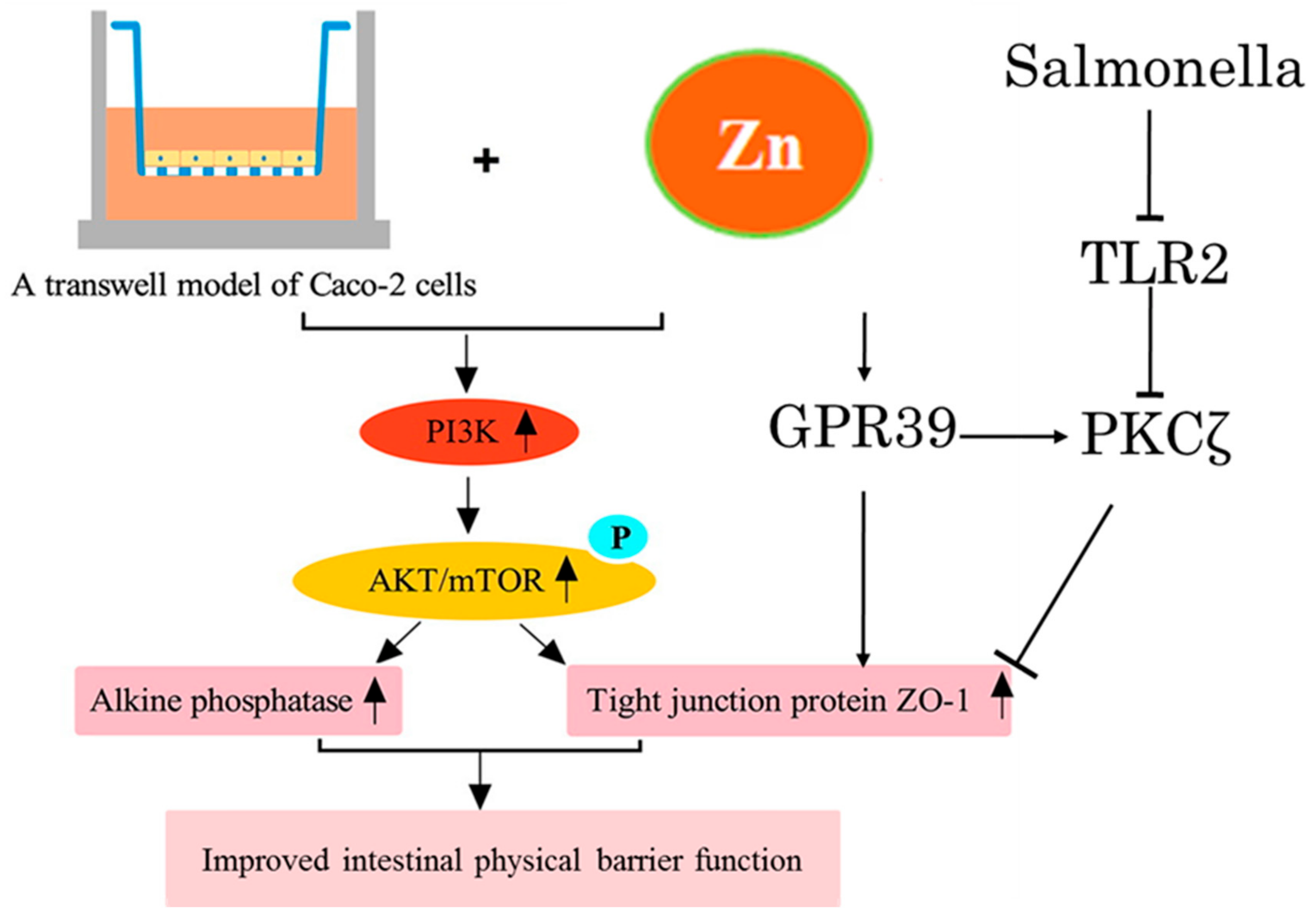
3.1. Intestinal Mucosal Physical and Chemical Barriers
3.2. Intestinal Mucosal Immune and Biological Barriers
4. Research Advances in the Relationship between Zinc Homeostasis and Gut Diseases
4.1. Inflammatory Bowel Disease
4.2. Irritable Bowel Syndrome
4.3. Colorectal Cancer
4.4. Celiac Disease
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Maret, W. Molecular aspects of human cellular zinc homeostasis: Redox control of zinc potentials and zinc signals. Biometals 2009, 22, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Vallee, B.L.; Auld, D.S. Zinc Coordination, Function, and Structure of Zinc Enzymes and Other Proteins. Biochemistry 1990, 29, 5647–5659. [Google Scholar] [CrossRef] [PubMed]
- Shankar, A.H.; Prasad, A.S. Zinc and immune function: The biological basis of altered resistance to infection. Am. J. Clin. Nutr. 1998, 68 (Suppl. S2), 447S–463S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasad, A.S. Zinc: An antioxidant and anti-inflammatory agent: Role of zinc in degenerative disorders of aging. J. Trace Elem. Med. Biol. 2014, 28, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M.; Hirano, T. Intracellular zinc homeostasis and zinc signaling. Cancer Sci. 2008, 99, 1515–1522. [Google Scholar] [CrossRef] [PubMed]
- Hirano, T.; Murakami, M.; Fukada, T.; Nishida, K.; Yamasaki, S.; Suzuki, T. Roles of zinc and zinc signaling in immunity: Zinc as an intracellular signaling molecule. Adv. Immunol. 2008, 97, 149–176. [Google Scholar] [PubMed]
- Maares, M.; Haase, H. A Guide to Human Zinc Absorption: General Overview and Recent Advances of In Vitro Intestinal Models. Nutrients 2020, 12, 762. [Google Scholar] [CrossRef] [Green Version]
- Kambe, T.; Taylor, K.M.; Fu, D. Zinc transporters and their functional integration in mammalian cells. J. Biol. Chem. 2021, 296, 100320. [Google Scholar] [CrossRef]
- Kambe, T.; Hashimoto, A.; Fujimoto, S. Current understanding of ZIP and ZnT zinc transporters in human health and diseases. Cell Mol. Life Sci. 2014, 71, 3281–3295. [Google Scholar] [CrossRef]
- Lichten, L.A.; Cousins, R.J. Mammalian Zinc Transporters: Nutritional and Physiologic Regulation. Annu. Rev. Nutr. 2009, 29, 153–176. [Google Scholar] [CrossRef]
- Kimura, T.; Kambe, T. The Functions of Metallothionein and ZIP and ZnT Transporters: An Overview and Perspective. Int. J. Mol. Sci. 2016, 17, 336. [Google Scholar] [CrossRef] [Green Version]
- Oshima, T.; Miwa, H. Gastrointestinal mucosal barrier function and diseases. J. Gastroenterol. 2016, 51, 768–778. [Google Scholar] [CrossRef]
- Dorofeyev, A.E.; Vasilenko, I.V.; Rassokhina, O.A.; Kondratiuk, R.B. Mucosal Barrier in Ulcerative Colitis and Crohn’s Disease. Gastroenterol. Res. Pract. 2013, 2013, 431231. [Google Scholar] [CrossRef]
- Gonzalez-Castro, A.M.; Martinez, C.; Salvo-Romero, E.; Fortea, M.; Pardo-Camacho, C.; Perez-Berezo, T.; Alonso-Cotoner, C.; Santos, J.; Vicario, M. Mucosal pathobiology and molecular signature of epithelial barrier dysfunction in the small intestine in irritable bowel syndrome. J. Gastroenterol. Hepatol. 2017, 32, 53–63. [Google Scholar] [CrossRef] [Green Version]
- Zhong, W.; McClain, C.J.; Cave, M.; Kang, Y.J.; Zhou, Z.X. The role of zinc deficiency in alcohol-induced intestinal barrier dysfunction. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 298, G625–G633. [Google Scholar] [CrossRef] [Green Version]
- Sturniolo, G.C.; Fries, W.; Mazzon, E.; Di Leo, V.; Barollo, M.; D’Inca, R. Effect of zinc supplementation on intestinal permeability in experimental colitis. J. Lab. Clin. Med. 2002, 139, 311–315. [Google Scholar] [CrossRef]
- Krebs, N.F. Overview of zinc absorption and excretion in the human gastrointestinal tract. J. Nutr. 2000, 130 (Suppl. S5), 1374S–1377S. [Google Scholar] [CrossRef] [Green Version]
- Menard, M.P.; Cousins, R.J. Zinc Transport by Brush-Border Membrane-Vesicles from Rat Intestine. J. Nutr. 1983, 113, 1434–1442. [Google Scholar] [CrossRef] [PubMed]
- King, J.C.; Shames, D.M.; Woodhouse, L.R. Zinc homeostasis in humans. J. Nutr. 2000, 130, 1360S–1366S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baltaci, A.K.; Yuce, K. Zinc Transporter Proteins. Neurochem. Res. 2018, 43, 517–530. [Google Scholar] [CrossRef] [PubMed]
- Hennigar, S.R.; McClung, J.P. Zinc Transport in the Mammalian Intestine. Compr. Physiol. 2018, 9, 59–74. [Google Scholar]
- Geiser, J.; Venken, K.J.; De Lisle, R.C.; Andrews, G.K. A mouse model of acrodermatitis enteropathica: Loss of intestine zinc transporter ZIP4 (Slc39a4) disrupts the stem cell niche and intestine integrity. PLoS Genet. 2012, 8, e1002766. [Google Scholar] [CrossRef] [Green Version]
- Hashimoto, A.; Nakagawa, M.; Tsujimura, N.; Miyazaki, S.; Kizu, K.; Goto, T.; Komatsu, Y.; Matsunaga, A.; Shirakawa, H.; Narita, H.; et al. Properties of Zip4 accumulation during zinc deficiency and its usefulness to evaluate zinc status: A study of the effects of zinc deficiency during lactation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 310, R459–R468. [Google Scholar] [CrossRef]
- Dufner-Beattie, J.; Kuo, Y.M.; Gitschier, J.; Andrews, G.K. The adaptive response to dietary zinc in mice involves the differential cellular localization and zinc regulation of the zinc transporters ZIP4 and ZIP5. J. Biol. Chem. 2004, 279, 49082–49090. [Google Scholar] [CrossRef] [Green Version]
- Weaver, B.P.; Dufner-Beattie, J.; Kambe, T.; Andrews, G.K. Novel zinc-responsive post-transcriptional mechanisms reciprocally regulate expression of the mouse Slc39a4 and Slc39a5 zinc transporters (Zip4 and Zip5). Biol. Chem. 2007, 388, 1301–1312. [Google Scholar] [CrossRef] [Green Version]
- Mao, X.Q.; Kim, B.E.; Wang, F.; Eide, D.J.; Pettis, M.J. A histidine-rich cluster mediates the ubiquitination and degradation of the human zinc transporter, hZIP4, and protects against zinc cytotoxicity. Biophys. J. 2007, 282, 6992–7000. [Google Scholar] [CrossRef] [Green Version]
- Guthrie, G.J.; Aydemir, T.B.; Troche, C.; Martin, A.B.; Chang, S.M.; Cousins, R.J. Influence of ZIP14 (slc39A14) on intestinal zinc processing and barrier function. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 308, G171–G178. [Google Scholar] [CrossRef] [Green Version]
- Liuzzi, J.P.; Bobo, J.A.; Cui, L.; McMahon, R.J.; Cousins, R.J. Zinc transporters 1, 2 and 4 are differentially expressed and localized in rats during pregnancy and lactation. J. Nutr. 2003, 133, 342–351. [Google Scholar] [CrossRef] [Green Version]
- Liuzzi, J.P.; Blanchard, R.K.; Cousins, R.J. Differential regulation of zinc transporter 1, 2, and 4 mRNA expression by dietary zinc in rats. J. Nutr. 2001, 131, 46–52. [Google Scholar] [CrossRef] [Green Version]
- Jou, M.Y.; Hall, A.G.; Philipps, A.F.; Kelleher, S.L.; Lonnerdal, B. Tissue-specific alterations in zinc transporter expression in intestine and liver reflect a threshold for homeostatic compensation during dietary zinc deficiency in weanling rats. J. Nutr. 2009, 139, 835–841. [Google Scholar] [CrossRef] [Green Version]
- Valentine, R.A.; Jackson, K.A.; Christie, G.R.; Mathers, J.C.; Taylor, P.M.; Ford, D. ZnT5 variant B is a bidirectional zinc transporter and mediates zinc uptake in human intestinal Caco-2 cells. J. Biol. Chem. 2007, 282, 14389–14393. [Google Scholar] [CrossRef] [Green Version]
- Fukunaka, A.; Suzuki, T.; Kurokawa, Y.; Yamazaki, T.; Fujiwara, N.; Ishihara, K.; Migaki, H.; Okumura, K.; Masuda, S.; Yamaguchi-Iwai, Y.; et al. Demonstration and characterization of the heterodimerization of ZnT5 and ZnT6 in the early secretory pathway. J. Biol. Chem. 2009, 284, 30798–30806. [Google Scholar] [CrossRef] [Green Version]
- Kirschke, C.P.; Huang, L.P. ZnT7, a novel mammalian zinc transporter, accumulates zinc in the Golgi apparatus. J. Biol. Chem. 2003, 278, 4096–4102. [Google Scholar] [CrossRef] [Green Version]
- Devergnas, S.; Chimienti, F.; Naud, N.; Pennequin, A.; Coquerel, Y.; Chantegrel, J.; Favier, A.; Seve, M. Differential regulation of zinc efflux transporters ZnT-1, ZnT-5 and ZnT-7 gene expression by zinc levels: A real-time RT-PCR study. Biochem. Pharmacol. 2004, 68, 699–709. [Google Scholar] [CrossRef]
- Cousins, R.J.; Blanchard, R.K.; Popp, M.P.; Liu, L.; Cao, J.; Moore, J.B.; Green, C.L. A global view of the selectivity of zinc deprivation and excess on genes expressed in human THP-1 mononuclear cells. Proc. Natl. Acad. Sci. USA 2003, 100, 6952–6957. [Google Scholar] [CrossRef] [Green Version]
- Overbeck, S.; Uciechowski, P.; Ackland, M.L.; Ford, D.; Rink, L. Intracellular zinc homeostasis in leukocyte subsets is regulated by different expression of zinc exporters ZnT-1 to ZnT-9. J. Leukoc. Biol. 2008, 83, 368–380. [Google Scholar] [CrossRef]
- Martin, L.; Lodemann, U.; Bondzio, A.; Gefeller, E.M.; Vahjen, W.; Aschenbach, J.R.; Zentek, J.; Pieper, R. A High Amount of Dietary Zinc Changes the Expression of Zinc Transporters and Metallothionein in Jejunal Epithelial Cells in Vitro and in Vivo but Does Not Prevent Zinc Accumulation in Jejunal Tissue of Piglets. J. Nutr. 2013, 143, 1205–1210. [Google Scholar] [CrossRef] [Green Version]
- Miyoshi, Y.; Tanabe, S.; Suzuki, T. Cellular zinc is required for intestinal epithelial barrier maintenance via the regulation of claudin-3 and occludin expression. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 311, G105–G116. [Google Scholar] [CrossRef] [Green Version]
- Camilleri, M. What is the leaky gut? Clinical considerations in humans. Curr. Opin. Clin. Nutr. Metab. Care 2021, 24, 473–482. [Google Scholar] [CrossRef]
- Zhang, M.Q.; Yi, Z.Y.; Ma, L.Y.; Li, S.S.; Han, L.; Cao, Q. Zinc Level in Pathogenesis of Inflammatory Bowel Disease A Systematic Review and Meta-analysis. Top. Clin. Nutr. 2021, 36, 319–330. [Google Scholar] [CrossRef]
- Griffin, I.J.; Kim, S.C.; Hicks, P.D.; Liang, L.K.; Abrams, S.A. Zinc metabolism in adolescents with Crohn’s disease. Pediatr. Res. 2004, 56, 235–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Main, A.N.; Hall, M.J.; Russell, R.I.; Fell, G.S.; Mills, P.R.; Shenkin, A. Clinical experience of zinc supplementation during intravenous nutrition in Crohn’s disease: Value of serum and urine zinc measurements. Gut 1982, 23, 984–991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, D.L.; Achkar, J.P.; Haritunians, T.; Jacobs, J.P.; Hui, K.Y.; D’Amato, M.; Brand, S.; Radford-Smith, G.; Halfvarson, J.; Niess, J.H.; et al. A Pleiotropic Missense Variant in SLC39A8 Is Associated with Crohn’s Disease and Human Gut Microbiome Composition. Gastroenterology 2016, 151, 724–732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, C.; Lv, H.; Chen, Z.; Wang, L.; Wu, X.; Chen, Z.; Zhang, W.; Liang, R.; Jiang, Z. Dietary Zinc Oxide Modulates Antioxidant Capacity, Small Intestine Development, and Jejunal Gene Expression in Weaned Piglets. Biol. Trace Elem. Res. 2017, 175, 331–338. [Google Scholar] [CrossRef]
- Hu, C.H.; Qian, Z.C.; Song, J.; Luan, Z.S.; Zuo, A.Y. Effects of zinc oxide-montmorillonite hybrid on growth performance, intestinal structure, and function of broiler chicken. Poult. Sci. 2013, 92, 143–150. [Google Scholar] [CrossRef]
- Wen, M.; Zhao, H.; Liu, G.M.; Chen, X.L.; Wu, B.; Tian, G.; Cai, J.Y.; Jia, G. Effect of Zinc Supplementation on Growth Performance, Intestinal Development, and Intestinal Barrier-Related Gene Expression in Pekin Ducks. Biol. Trace Elem. Res. 2018, 183, 351–360. [Google Scholar] [CrossRef]
- Pearce, S.C.; Fernandez, M.V.S.; Torrison, J.; Wilson, M.E.; Baumgard, L.H.; Gabler, N.K. Dietary organic zinc attenuates heat stress-induced changes in pig intestinal integrity and metabolism. J. Anim. Sci. 2015, 93, 4702–4713. [Google Scholar] [CrossRef]
- Liu, P.; Pieper, R.; Tedin, L.; Martin, L.; Meyer, W.; Rieger, J.; Plendl, J.; Vahjen, W.; Zentek, J. Effect of dietary zinc oxide on jejunal morphological and immunological characteristics in weaned piglets. J. Anim. Sci. 2014, 92, 5009–5018. [Google Scholar] [CrossRef]
- Maares, M.; Keil, C.; Straubing, S.; Robbe-Masselot, C.; Haase, H. Zinc Deficiency Disturbs Mucin Expression, O-Glycosylation and Secretion by Intestinal Goblet Cells. Int. J. Mol. Sci. 2020, 21, 6149. [Google Scholar] [CrossRef]
- Ohashi, W.; Kimura, S.; Iwanaga, T.; Furusawa, Y.; Irie, T.; Izumi, H.; Watanabe, T.; Hijikata, A.; Hara, T.; Ohara, O.; et al. Zinc Transporter SLC39A7/ZIP7 Promotes Intestinal Epithelial Self-Renewal by Resolving ER Stress. PLoS Genet. 2016, 12, e1006349. [Google Scholar] [CrossRef]
- Finamore, A.; Massimi, M.; Conti Devirgiliis, L.; Mengheri, E. Zinc deficiency induces membrane barrier damage and increases neutrophil transmigration in Caco-2 cells. J. Nutr. 2008, 138, 1664–1670. [Google Scholar] [CrossRef] [Green Version]
- Song, Z.H.; Xiao, K.; Ke, Y.L.; Jiao, L.F.; Hu, C.H. Zinc oxide influences mitogen-activated protein kinase and TGF-beta 1 signaling pathways, and enhances intestinal barrier integrity in weaned pigs. Innate Immun. 2015, 21, 341–348. [Google Scholar] [CrossRef]
- Song, Z.H.; Ke, Y.L.; Xiao, K.; Jiao, L.F.; Hong, Q.H.; Hu, C.H. Diosmectite-zinc oxide composite improves intestinal barrier restoration and modulates TGF-beta 1, ERK1/2, and Akt in piglets after acetic acid challenge. J. Anim. Sci. 2015, 93, 1599–1607. [Google Scholar] [CrossRef]
- Cohen, L.; Sekler, I.; Hershfinkel, M. The zinc sensing receptor, ZnR/GPR39, controls proliferation and differentiation of colonocytes and thereby tight junction formation in the colon. Cell Death Dis. 2014, 5, e1307. [Google Scholar] [CrossRef] [Green Version]
- Shao, Y.X.; Wolf, P.G.; Guo, S.S.; Guo, Y.M.; Gaskins, H.R.; Zhang, B.K. Zinc enhances intestinal epithelial barrier function through the PI3K/AKT/mTOR signaling pathway in Caco-2 cells. J. Nutr. Biochem. 2017, 43, 18–26. [Google Scholar] [CrossRef]
- Sharir, H.; Zinger, A.; Nevo, A.; Sekler, I.; Hershfinkel, M. Zinc Released from Injured Cells Is Acting via the Zn2+-sensing Receptor, ZnR, to Trigger Signaling Leading to Epithelial Repair. J. Biol. Chem. 2010, 285, 26097–26106. [Google Scholar] [CrossRef] [Green Version]
- Sunuwar, L.; Medini, M.; Cohen, L.; Sekler, I.; Hershfinkel, M. The zinc sensing receptor, ZnR/GPR39, triggers metabotropic calcium signalling in colonocytes and regulates occludin recovery in experimental colitis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2016, 371, 20150420. [Google Scholar]
- Shao, Y.X.; Lei, Z.; Wolf, P.G.; Gao, Y.; Guo, Y.M.; Zhang, B.K. Zinc Supplementation, via GPR39, Upregulates PKC zeta to Protect Intestinal Barrier Integrity in Caco-2 Cells Challenged by Salmonella enterica Serovar Typhimurium. J. Nutr. 2017, 147, 1282–1289. [Google Scholar] [CrossRef] [Green Version]
- Quarterman, J.; Jackson, F.A.; Morrison, J.N. The effect of zinc deficiency on sheep intestinal mucin. Life Sci. 1976, 19, 979–986. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Cougnon, F.B.L.; Wanniarachchi, Y.A.; Hayden, J.A.; Nolan, E.M. Reduction of Human Defensin 5 Affords a High-Affinity Zinc-Chelating Peptide. ACS Chem. Biol. 2013, 8, 1907–1911. [Google Scholar] [CrossRef] [Green Version]
- Chakraborti, S.; Chatterjee, T.; Joshi, P.; Poddar, A.; Bhattacharyya, B.; Singh, S.P.; Gupta, V.; Chakrabarti, P. Structure and Activity of Lysozyme on Binding to ZnO Nanoparticles. Langmuir 2010, 26, 3506–3513. [Google Scholar] [CrossRef]
- Podany, A.; Geletzke, A.; Lee, S.; Soybel, D.; Kelleher, S. The Zinc Transporter ZnT2 is Necessary for Stabilization of Granule Contents in Intestinal Paneth Cell Granules. FASEB J. 2015, 29, 1011.1. [Google Scholar] [CrossRef]
- Podany, A.B.; Wright, J.; Lamendella, R.; Soybel, D.I.; Kelleher, S.L. ZnT2-Mediated Zinc Import Into Paneth Cell Granules Is Necessary for Coordinated Secretion and Paneth Cell Function in Mice. Cell. Mol. Gastroenterol. Hepatol. 2016, 2, 369–383. [Google Scholar] [CrossRef] [Green Version]
- Jarosz, L.; Marek, A.; Gradzki, Z.; Kwiecien, M.; Zylinska, B.; Kaczmarek, B. Effect of feed supplementation with zinc glycine chelate and zinc sulfate on cytokine and immunoglobulin gene expression profiles in chicken intestinal tissue. Poult. Sci. 2017, 96, 4224–4235. [Google Scholar] [CrossRef]
- Pabst, O. New concepts in the generation and functions of IgA. Nat. Rev. Immunol. 2012, 12, 821–832. [Google Scholar] [CrossRef]
- Han, X.Y.; Ma, Y.F.; Lv, M.Y.; Wu, Z.P.; Qian, L.C. Chitosan-zinc chelate improves intestinal structure and mucosal function and decreases apoptosis in ileal mucosal epithelial cells in weaned pigs. Br. J. Nutr. 2014, 111, 1405–1411. [Google Scholar] [CrossRef] [Green Version]
- Lahiri, A.; Abraham, C. Activation of pattern recognition receptors up-regulates metallothioneins, thereby increasing intracellular accumulation of zinc, autophagy, and bacterial clearance by macrophages. Gastroenterology 2014, 147, 835–846. [Google Scholar] [CrossRef] [Green Version]
- Laurin, D.E.; Barnes, D.M.; Klasing, K.C. Rates of metallothionein synthesis, degradation and accretion in a chicken macrophage cell line. Proc. Soc. Exp. Biol. Med. 1990, 194, 157–164. [Google Scholar] [CrossRef]
- Kelly, P.; Feakins, R.; Domizio, P.; Murphy, J.; Bevins, C.; Wilson, J.; Mcphail, G.; Poulsom, R.; Dhaliwal, W. Paneth cell granule depletion in the human small intestine under infective and nutritional stress. Clin. Exp. Immunol. 2004, 135, 303–309. [Google Scholar] [CrossRef]
- Glahn, R.P.; Tako, E.; Reed, S.; Neuman, H.; Moscovich, S.; Koren, O. Chronic Zinc Deficiency Alters Chick Gut Microbiota Composition and Function. Nutrients 2015, 7, 9768–9784. [Google Scholar]
- Zackular, J.P.; Moore, J.L.; Jordan, A.T.; Juttukonda, L.J.; Noto, M.J.; Nicholson, M.R.; Crews, J.D.; Semler, M.W.; Zhang, Y.; Ware, L.B.; et al. Dietary zinc alters the microbiota and decreases resistance to Clostridium difficile infection. Nat. Med. 2016, 22, 1330–1334. [Google Scholar] [CrossRef] [PubMed]
- Vahjen, W.; Pieper, R.; Zentek, J. Bar-Coded Pyrosequencing of 16S rRNA Gene Amplicons Reveals Changes in Ileal Porcine Bacterial Communities Due to High Dietary Zinc Intake. Appl. Environ. Microb. 2010, 76, 6689–6691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watly, J.; Potocki, S.; Rowinska-Zyrek, M. Zinc Homeostasis at the Bacteria/Host Interface-From Coordination Chemistry to Nutritional Immunity. Chem.-Eur. J. 2016, 22, 15992–16010. [Google Scholar] [CrossRef] [PubMed]
- Kehl-Fie, T.E.; Skaar, E.P. Nutritional immunity beyond iron: A role for manganese and zinc. Curr. Opin. Chem. Biol. 2010, 14, 218–224. [Google Scholar] [CrossRef] [Green Version]
- Geboes, K.; Riddell, R.; Ost, A.; Jensfelt, B.; Persson, T.; Lofberg, R. A reproducible grading scale for histological assessment of inflammation in ulcerative colitis. Gut 2000, 47, 404–409. [Google Scholar] [CrossRef] [Green Version]
- Naftali, T.; Reshef, L.; Kovacs, A.; Porat, R.; Amir, I.; Konikoff, F.M.; Gophna, U. Distinct Microbiotas are Associated with Ileum-Restricted and Colon-Involving Crohn’s Disease. Inflamm. Bowel Dis. 2016, 22, 293–302. [Google Scholar] [CrossRef]
- Lakatos, P.L. Environmental Factors Affecting Inflammatory Bowel Disease: Have We Made Progress? Dig. Dis. 2009, 27, 215–225. [Google Scholar] [CrossRef]
- Karlinger, K.; Gyorke, T.; Mako, E.; Mester, A.; Tarjan, Z. The epidemiology and the pathogenesis of inflammatory bowel disease. Eur. J. Radiol. 2000, 35, 154–167. [Google Scholar] [CrossRef]
- Wasser, T.E.; Reed, J.F.; Moser, K.; Robson, P.; Faust, L.; Fink, L.L.; Wunderler, D. Nutritional Assessment and Disease-Activity for Patients with Inflammatory Bowel-Disease. Can. J. Gastroenterol. 1995, 9, 131–136. [Google Scholar] [CrossRef]
- Siva, S.; Rubin, D.T.; Gulotta, G.; Wroblewski, K.; Pekow, J. Zinc Deficiency is Associated with Poor Clinical Outcomes in Patients with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2017, 23, 152–157. [Google Scholar] [CrossRef] [Green Version]
- Ananthakrishnan, A.N.; Khalili, H.; Song, M.; Higuchi, L.M.; Richter, J.M.; Chan, A.T. Zinc intake and risk of Crohn’s disease and ulcerative colitis: A prospective cohort study. Int. J. Epidemiol. 2015, 44, 1995–2005. [Google Scholar] [CrossRef]
- Higashimura, Y.; Takagi, T.; Naito, Y.; Uchiyama, K.; Mizushima, K.; Tanaka, M.; Hamaguchi, M.; Itoh, Y. Zinc Deficiency Activates the IL-23/Th17 Axis to Aggravate Experimental Colitis in Mice. J. Crohns Colitis. 2020, 14, 856–866. [Google Scholar] [CrossRef]
- Maywald, M.; Wessels, I.; Rink, L. Zinc Signals and Immunity. Int. J. Mol. Sci. 2017, 18, 2222. [Google Scholar] [CrossRef] [Green Version]
- Wiegand, S.; Zakrzewski, S.S.; Eichner, M.; Schulz, E.; Gunzel, D.; Pieper, R.; Rosenthal, R.; Barmeyer, C.; Bleich, A.; Dobrindt, U.; et al. Zinc treatment is efficient against Escherichia coli alpha-haemolysin-induced intestinal leakage in mice. Sci. Rep. 2017, 7, 45649. [Google Scholar] [CrossRef]
- El-Salhy, M. Recent developments in the pathophysiology of irritable bowel syndrome. World J. Gastroenterol. 2015, 21, 7621–7636. [Google Scholar] [CrossRef]
- Ohman, L.; Simren, M. New insights into the pathogenesis and pathophysiology of irritable bowel syndrome. Dig. Liver. Dis. 2007, 39, 201–215. [Google Scholar] [CrossRef]
- Bahrami, A.; Gonoodi, K.; Khayyatzadeh, S.S.; Tayefi, M.; Darroudi, S.; Bahrami-Taghanaki, H.; Eslami, S.; Jaberi, N.; Ferns, G.A.; Farahmand, K.; et al. The association of trace elements with premenstrual syndrome, dysmenorrhea and irritable bowel syndrome in adolescents. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 233, 114–119. [Google Scholar] [CrossRef]
- Torres, M.J.; Sabate, J.M.; Bouchoucha, M.; Buscail, C.; Hercberg, S.; Julia, C. Food consumption and dietary intakes in 36,448 adults and their association with irritable bowel syndrome: Nutrinet-Sante study. Ther. Adv. Gastroenterol. 2018, 11, 1756283X17746625. [Google Scholar] [CrossRef] [Green Version]
- Rezazadegan, M.; Soheilipour, M.; Tarrahi, M.J.; Amani, R. Correlation between Zinc Nutritional Status with Serum Zonulin and Gastrointestinal Symptoms in Diarrhea-Predominant Irritable Bowel Syndrome: A Case-Control Study. Dig. Dis. Sci. 2022. [Google Scholar] [CrossRef]
- Vela, G.; Stark, P.; Socha, M.; Sauer, A.K.; Hagmeyer, S.; Grabrucker, A.M. Zinc in Gut-Brain Interaction in Autism and Neurological Disorders. Neural Plast. 2015, 2015, 972791. [Google Scholar] [CrossRef] [Green Version]
- Russo, A.J. Decreased zinc and increased copper in individuals with anxiety. Nutr. Metab. Insights 2011, 4, 1–5. [Google Scholar] [CrossRef]
- Hujoel, I.A. Nutritional status in irritable bowel syndrome: A North American population-based study. JGH Open Access J. Gastroenterol. Hepatol. 2020, 4, 656–662. [Google Scholar] [CrossRef] [Green Version]
- Russo, A.J. Increased Copper in Individuals with Autism Normalizes Post Zinc Therapy More Efficiently in Individuals with Concurrent GI Disease. Nutr. Metab. Insights 2011, 4, 49–54. [Google Scholar] [CrossRef]
- Zhang, L.; Song, J.; Hou, X.H. Mast Cells and Irritable Bowel Syndrome: From the Bench to the Bedside. J. Neurogastroenterol. 2016, 22, 181–192. [Google Scholar] [CrossRef] [Green Version]
- Ding, N.; Jiang, J.; Qin, P.P.; Wang, Q.X.; Hu, J.T.; Li, Z.G. Mast cells are important regulator of acupoint sensitization via the secretion of tryptase, 5-hydroxytryptamine, and histamine. PLoS ONE 2018, 13, e0194022. [Google Scholar] [CrossRef]
- Wouters, M.M.; Balemans, D.; Van Wanrooy, S.; Dooley, J.; Cibert-Goton, V.; Alpizar, Y.A.; Valdez-Morales, E.E.; Nasser, Y.; Van Veldhoven, P.P.; Vanbrabant, W.; et al. Histamine Receptor H1-Mediated Sensitization of TRPV1 Mediates Visceral Hypersensitivity and Symptoms in Patients With Irritable Bowel Syndrome. Gastroenterology 2016, 150, 875–887.e9. [Google Scholar] [CrossRef] [Green Version]
- Crowell, M.D.; Shetzline, M.A.; Moses, P.L.; Mawe, G.M.; Talley, N.J. Enterochromaffin cells and 5-HT signaling in the pathophysiology of disorders of gastrointestinal function. Curr. Opin. Investig. Drugs 2004, 5, 55–60. [Google Scholar]
- Keating, D.J.; Spencer, N.J. Release of 5-Hydroxytryptamine from the Mucosa Is Not Required for the Generation or Propagation of Colonic Migrating Motor Complexes. Gastroenterology 2010, 138, 659–670.E2. [Google Scholar] [CrossRef]
- Fu, R.; Chen, M.X.; Chen, Y.; Mao, G.Q.; Liu, S.Y. Expression and clinical significance of 5-HT and 5-HT3R in the intestinal mucosa of patient with diarrhea-type irritable bowel syndrome. Exp. Ther. Med. 2019, 17, 3077–3082. [Google Scholar] [CrossRef] [Green Version]
- Gunn, D.; Garsed, K.; Lam, C.; Singh, G.; Lingaya, M.; Wahl, V.; Niesler, B.; Henry, A.; Hall, I.P.; Whorwell, P.; et al. Abnormalities of mucosal serotonin metabolism and 5-HT3 receptor subunit 3C polymorphism in irritable bowel syndrome with diarrhoea predict responsiveness to ondansetron. Aliment. Pharm. Ther. 2019, 50, 538–546. [Google Scholar] [CrossRef] [Green Version]
- Pihl, E.; Gustafso, G.T. Heavy Metals in Rat Mast Cell Granules. Lab. Investig. 1967, 17, 588–598. [Google Scholar]
- Yamasaki, S.; Sakata-Sogawa, K.; Hasegawa, A.; Suzuki, T.; Kabu, K.; Sato, E.; Kurosaki, T.; Yamashita, S.; Tokunaga, M.; Nishida, K.; et al. Zinc is a novel intracellular second messenger. J. Cell Biol. 2007, 177, 637–645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kabu, K.; Yamasaki, S.; Kamimura, D.; Ito, Y.; Hasegawa, A.; Sato, E.; Kitamura, H.; Nishida, K.; Hirano, T. Zinc is required for Fc epsilon RI-mediated mast cell activation. J. Immunol. 2006, 177, 1296–1305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takeda, A.; Itoh, H.; Imano, S.; Oku, N. Impairment of GABAergic neurotransmitter system in the amygdala of young rats after 4-week zinc deprivation. Neurochem. Int. 2006, 49, 746–750. [Google Scholar] [CrossRef]
- Moloney, R.D.; O’Mahony, S.M.; Dinan, T.G.; Cryan, J.F. Stress-induced visceral pain: Toward animal models of irritable-bowel syndrome and associated comorbidities. Front. Psychiatry 2015, 6, 15. [Google Scholar] [CrossRef] [Green Version]
- Lepping, P.; Huber, M. Role of Zinc in the Pathogenesis of Attention-Deficit Hyperactivity Disorder Implications for Research and Treatment. CNS Drugs 2010, 24, 721–728. [Google Scholar]
- Toth, K. Zinc in Neurotransmission. Annu. Rev. Nutr. 2011, 31, 139–153. [Google Scholar] [CrossRef]
- Posserud, I.; Agerforz, P.; Ekman, R.; Bjornsson, E.S.; Abrahamsson, H.; Simren, M. Altered visceral perceptual and neuroendocrine response in patients with irritable bowel syndrome during mental stress. Gut 2004, 53, 1102–1108. [Google Scholar] [CrossRef]
- Galligan, J.J. 5-hydroxytryptamine, ulcerative colitis, and irritable bowel syndrome: Molecular connections. Gastroenterology 2004, 126, 1897–1899. [Google Scholar] [CrossRef]
- Zhang, C.F.; Cheng, R.Q.; Ding, J.; Li, X.J.; Niu, H.W.; Li, X. Serum Copper and Zinc Levels and Colorectal Cancer in Adults: Findings from the National Health and Nutrition Examination 2011–2016. Biol. Trace Elem. Res. 2021, 200, 2033–2039. [Google Scholar] [CrossRef]
- Khoshdel, Z.; Naghibalhossaini, F.; Abdollahi, K.; Shojaei, S.; Moradi, M.; Malekzadeh, M. Serum Copper and Zinc Levels Among Iranian Colorectal Cancer Patients. Biol. Trace Elem. Res. 2016, 170, 294–299. [Google Scholar] [CrossRef]
- Christudoss, P.; Selvakumar, R.; Pulimood, A.B.; Fleming, J.J.; Mathew, G. Zinc and Zinc Related Enzymes in Precancerous and Cancerous Tissue in the Colon of Dimethyl Hydrazine Treated Rats. Asian Pac. J. Cancer Prev. 2012, 13, 487–492. [Google Scholar] [CrossRef] [Green Version]
- Li, P.W.; Xu, J.M.; Shi, Y.; Ye, Y.; Chen, K.; Yang, J.; Wu, Y.H. Association between zinc intake and risk of digestive tract cancers: A systematic review and meta-analysis. Clin. Nutr. 2014, 33, 415–420. [Google Scholar] [CrossRef]
- He, G.Y.; Zhu, H.F.; Yao, Y.K.; Chai, H.R.; Wang, Y.Q.; Zhao, W.L.; Fu, S.Z.; Wang, Y.X. Cysteine-rich intestinal protein 1 silencing alleviates the migration and invasive capability enhancement induced by excessive zinc supplementation in colorectal cancer cells. Am. J. Transl. Res. 2019, 11, 3578–3588. [Google Scholar]
- Prasad, A.S.; Beck, F.W.J.; Bao, B.; Fitzgerald, J.T.; Snell, D.C.; Steinberg, J.D.; Cardozo, L.J. Zinc supplementation decreases incidence of infections in the elderly: Effect of zinc on generation of cytokines and oxidative stress. Am. J. Clin. Nutr. 2007, 85, 837–844. [Google Scholar] [CrossRef] [Green Version]
- Shaheen, A.A.; Elfattah, A.A.A. Effect of Dietary Zinc on Lipid-Peroxidation, Glutathione, Protein Thiols Levels and Superoxide-Dismutase Activity in Rat-Tissues. Int. J. Biochem. Cell Biol. 1995, 27, 89–95. [Google Scholar] [CrossRef]
- Zhang, Q.R.; Xue, Y.; Fu, Y.X.; Bao, B.W.; Guo, M.Y. Zinc Deficiency Aggravates Oxidative Stress Leading to Inflammation and Fibrosis in Lung of Mice. Biol. Trace Elem. Res. 2021. [Google Scholar] [CrossRef]
- Al-saran, N.; Subash-Babu, P.; Al-Nouri, D.M.; Alfawaz, H.A.; Alshatwi, A.A. Zinc enhances CDKN2A, pRb1 expression and regulates functional apoptosis via upregulation of p53 and p21 expression in human breast cancer MCF-7 cell. Environ. Toxicol. Pharmacol. 2016, 47, 19–27. [Google Scholar] [CrossRef]
- Ho, E. Zinc deficiency, DNA damage and cancer risk. J. Nutr. Biochem. 2004, 15, 572–578. [Google Scholar] [CrossRef]
- Song, Y.; Leonard, S.W.; Traber, M.G.; Ho, E. Zinc Deficiency Affects DNA Damage, Oxidative Stress, Antioxidant Defenses, and DNA Repair in Rats. J. Nutr. 2009, 139, 1626–1631. [Google Scholar] [CrossRef] [Green Version]
- Ho, E.; Ames, B.N. Low intracellular zinc induces oxidative DNA damage, disrupts p53, NF kappa B, and AP1 DNA binding, and affects DNA repair in a rat glioma cell line. Proc. Natl. Acad. Sci. USA 2002, 99, 16770–16775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheng, N.Q.; Yan, L.; You, W.Q.; Tan, G.W.; Gong, J.F.; Chen, H.Q.; Yang, Y.; Hu, L.D.; Wang, Z.G. Knockdown of SLC39A7 inhibits cell growth and induces apoptosis in human colorectal cancer cells. Acta Biochim. Biophys. Sin. 2017, 49, 926–934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barresi, V.; Valenti, G.; Spampinato, G.; Musso, N.; Castorina, S.; Rizzarelli, E.; Condorelli, D.F. Transcriptome analysis reveals an altered expression profile of zinc transporters in colorectal cancer. J. Cell. Biochem. 2018, 119, 9707–9719. [Google Scholar] [CrossRef] [PubMed]
- Cichewicz, A.B.; Mearns, E.S.; Taylor, A.; Boulanger, T.; Gerber, M.; Leffler, D.A.; Drahos, J.; Sanders, D.S.; Thomas Craig, K.J.; Lebwohl, B. Diagnosis and Treatment Patterns in Celiac Disease. Dig. Dis. Sci. 2019, 64, 2095–2106. [Google Scholar] [CrossRef]
- Ryan, M.; Grossman, S. Celiac disease: Implications for patient management. Gastroenterol. Nurs. 2011, 34, 225–228. [Google Scholar] [CrossRef]
- Fasano, A. Clinical presentation of celiac disease in the pediatric population. Gastroenterology 2005, 128 (Suppl. S1), S68–S73. [Google Scholar] [CrossRef]
- Ciclitira, P.J.; Johnson, M.W.; Dewar, D.H.; Ellis, H.J. The pathogenesis of coeliac disease. Mol. Asp. Med. 2005, 26, 421–458. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, L.; Li, L.; Du, Z. Evaluation of Serum Levels of Copper and Zinc in Patients with Celiac Disease Seropositivity: Findings from the National Health and Nutrition Examination Survey. Biol. Trace Elem. Res. 2022. [Google Scholar] [CrossRef]
- Caruso, R.; Pallone, F.; Stasi, E.; Romeo, S.; Monteleone, G. Appropriate nutrient supplementation in celiac disease. Ann. Med. 2013, 45, 522–531. [Google Scholar] [CrossRef]
- Skrovanek, S.; DiGuilio, K.; Bailey, R.; Huntington, W.; Urbas, R.; Mayilvaganan, B.; Mercogliano, G.; Mullin, J.M. Zinc and gastrointestinal disease. World J. Gastrointest. Pathophysiol. 2014, 5, 496–513. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, Y.; Zhang, B. The Impact of Zinc and Zinc Homeostasis on the Intestinal Mucosal Barrier and Intestinal Diseases. Biomolecules 2022, 12, 900. https://doi.org/10.3390/biom12070900
Wan Y, Zhang B. The Impact of Zinc and Zinc Homeostasis on the Intestinal Mucosal Barrier and Intestinal Diseases. Biomolecules. 2022; 12(7):900. https://doi.org/10.3390/biom12070900
Chicago/Turabian StyleWan, Yan, and Bingkun Zhang. 2022. "The Impact of Zinc and Zinc Homeostasis on the Intestinal Mucosal Barrier and Intestinal Diseases" Biomolecules 12, no. 7: 900. https://doi.org/10.3390/biom12070900
APA StyleWan, Y., & Zhang, B. (2022). The Impact of Zinc and Zinc Homeostasis on the Intestinal Mucosal Barrier and Intestinal Diseases. Biomolecules, 12(7), 900. https://doi.org/10.3390/biom12070900





