Abstract
In recent years, the study of metal complexes and metal-based nanomaterials has aroused particular interest, leading to the promotion of new effective systems for the abatement of various viral diseases. Starting from the analysis of chemical properties, this review focuses on the employment of metal-based nanoparticles as antiviral drugs and how this interaction leads to a substantial enhancement in antiviral activity. The use of metal-based antiviral drugs has also spread for the formulation of antiviral vaccines, thanks especially to the remarkable adjuvant activities of some of the metal complexes. In particular, the small size and inert nature of Au- and Ag-based nanoparticles have been exploited for the design of systems for antiviral drug delivery, leading to the development of specific and safe therapies that lead to a decrease in side effects.
1. Introduction
As it is well known, metal ions are important components of life and are involved in many vital functions in biological systems [1,2]. Just to mention a few, they are essential cofactors of many enzymes and contribute to regulate the electrolyte balance, electron transfer, and transport of oxygen. They are also implicated in regulating both the immune system and host defense against invading pathogens. The broad spectrum of properties of metals, such as different coordination numbers, geometries, redox states, but also reactivity and thermodynamic behavior, has determined their important role in medicine and extensive use in the treatment and diagnosis of many diseases.
Throughout history, as far back as ancient Egypt, Greece, and Rome, as well as in the ancient Aztec civilization, many metals and metal compounds have been used for the treatment of disease. Copper has been used as a sterilization agent for drinking water but also for headaches and for leg ulcers that are associated with varicose veins, zinc as an antiseptic, mercurous chloride as a diuretic, and arsenic compounds for the treatment of syphilis. In 1965, the discovery by Rosemberg [3] of cisplatinum as an anticancer drug led to the development of numerous metallic drugs and many metal compounds have been designed and explored for both therapy and diagnosis [4].
Recently, continuous advances in the nanotechnology sector have led to significant progress in the diagnosis but also in the prevention and pharmacological treatment of many diseases [5,6,7]. The use of nanomaterials has made it possible to overcome some of the problems related to traditional metallodrugs, such as the poor solubility, toxicity, bioavailability, and stability of drugs, increasing at the same time the selectivity for viruses and efficacy.
In the last two years, the pandemic caused by the coronavirus SARS-CoV-2, that reached more than 50 million official cases, has focused the attention of many researchers on the study of systems useful for diagnostic and curative purposes as well as vaccines. Although the attention towards this virus has globally dominated the research in the last period, we must also remember the numerous studies that were conducted to fight other important viruses such as Dengue (DNV), Zika (ZIKV), Chikungunya (CHIKV), human immunodeficiency (HIV), Herpes simplex (HSV), Hepatitis C virus (HCV), yellow fever, Measles viruses, and also Influenza A (e.g., H1N1, H5N1).
Metal compounds play an important role acting in many ways: they can interrupt infections, alleviate symptoms, regulate the human immune system, inhibit critical enzymes in the process of virus replication, or as act adjuvants to enhance the efficacy of antiviral drugs and vaccines [8,9], as shown in Figure 1.
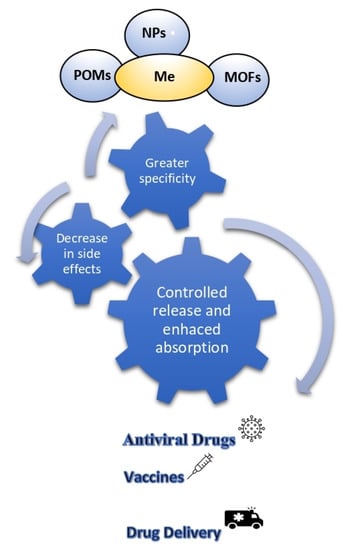
Figure 1.
Schematic representation of the main properties and applications of metal-based compounds.
In this review we report an analysis of the recent progress in the use of metal compounds as antiviral drugs, starting from the study of the chemical properties of these metal compounds with particular attention to thermodynamic properties, speciation, and structural characteristics. To give a complete picture of the importance of metal complexes in combating viruses, attention was paid not only on the most recent advances of metal complexes as antiviral drugs, but also on their use in vaccines and drug delivery systems.
2. Chemical Properties of Metal Drugs
Antiviral activity of metal complex species could be affected, first of all, by their own structural characteristics, speciation, nature of metal ions, as well as by the free functional groups in the ligands. These moieties are not only involved in the recognition processes, but also improve the solubility and effectiveness of the complexes. On the other hand, the selective recognition and interactions of a ligand towards biomolecules also depend on the size and nature of its substituents and molecular structure, which affect the lipophilicity, stereochemistry, kinetic, and thermodynamic stability [10].
Particular attention must be paid to the thermodynamic properties, such as acid-base behavior and solubility in aqueous solutions. Drug molecules generally contain one or more ionizable sites. The knowledge of the dissociation constants of pharmacologically active molecules is necessary to define the state of ionization which influences their characteristics, such as aqueous solubility, permeability, partition coefficients, and pharmacokinetic properties [11]. The solubility of a drug is a key factor for purification processes, pharmaceutical development, crystal form screening, and production control.
Another important property is the lipophilicity that enhances the permeability through the lipid layers of the cell membrane. The coordination of compounds with metal ions influences the lipophilicity, as it decreases the polarity of the ions due to the partial sharing of the metal ion with the donor groups of the compound [12,13].
Furthermore, it is known that often the complexation with metal ions enhances the antiviral activities of drugs [14], in concordance with the importance of metal ions in biological systems [15]. More precisely, many compounds employed as drugs exhibit modified pharmacological and toxicological profiles when they are administered in their metal-based form. For these reasons, in the literature there are several papers on the metal ion complexation of antiviral drugs, such as valacyclovir, acylhydrazones, ribavirin active against HCV, 2-hydroxybenzamides-base compounds used as anti-influenza virus, adefovir, biguanide derivatives active against herpes, cidofovir employed for the treatment of cytomegalovirus retinitis, and polyoxovanadates.
In particular, the protonation constants of valacyclovir (L-valine, 2-[(2-amino-1,6-dihydro-6-oxo-9-H-purin-9-yl)methoxy]ethyl ester), an antiviral molecule used against HSV, was reported. The complexation behavior of valacyclovir with Cu(II) was also investigated, evidencing the formation of 2:1 and 2:2 complexes. The techniques that were employed were potentiometry, UV-Vis, IR, MS, magneto-chemical, thermogravimetric, atomic absorption, conductivity, and elemental analysis data [16]. The biological activity of valacyclovir and its Cu(II) complexes have also been investigated, and the results highlighted the complexation with the metal ion enhances the biological activity. This can be explained by the chelation theory, i.e., the polarity of the metal ion decreases due to the partial sharing of the charge with the donor groups and to the delocalization of the π electron on the chelated ring. In this way, the metal ion lipophilic character increases, favoring its permanence through the lipid layer of the cell membrane [16].
A recent paper reported the acid-base and coordinating behavior towards Cu(II), Mn(II) and Mg(II) of three 2-hydroxy-3-methoxyphenyl acylhydrazones (HL1, HL2, and H2L3), that show activities against HSV and vaccinia virus [17]. A potentiometric and microcalorimetric study was conducted to reveal the behavior of the three acylhydrazones compounds in a mixed solvent methanol/water = 9/1. The metal complexing abilities of acylhydrazones are responsible for the biological activity of these compounds. The results proved the coordinating behavior of acylhydrazone molecules towards Mg(II), Mn(II), and Cu(II) is different depending on the R group. The three ligands, and in particular H2L3, showed a greater complexing capacity towards Cu(II). A significant correlation between the high complexing capacity and the good antiviral activity against HSV and vaccinia virus (EC50 ~ 1.5 µM, minimal cytotoxic concentration = 60 µM, selectivity index = 40) was evidenced and should be further investigated [17].
The complexation of molecules that are employed against HCV, such as ribavirin (1,β-D-ribofuranosyl-1,2,4-triazole-3-carboxamide, RBV), was also investigated. In more detail, complexes of RBV with Cu(II),whose hepatic and serum concentration significantly increases due to a HCV infection, were studied [18]. The coordination, elucidated by spectroscopic studies and DFT calculations, occurs via triazole N4 and amide oxygen atoms [18].
A total of three synthetized 2-hydroxybenzamides-based compounds, that are potentially used as anti-influenza virus therapeutics, were investigated in terms of acid-base behavior, complexing ability towards Mg(II), and biological characteristics. It was proven that all three ligands (indicated as HL1, H2L2, and HL3) are able to bind Mg(II) with different coordination abilities. Potentiometric investigations evidenced that, under physiological pH, the H2L2 form is the most efficient [19].
For the acyclic nucleoside phosphonate 9-[2-(phosphonomethoxy)ethyl]guanine (PMEG), active as anticancer and antiviral drug, the acidity constants as well as the stability constants of the Cu(II) complexes were obtained by potentiometric titrations in aqueous solutions at t = 25 °C, I = 0.1 M in NaNO3 [15].
The dissociation constants of adefovir (PMEA, 9-[2-(phosphonomethoxy)ethyl]adenine) and cidofovir (HPMPC, phosphoric acid) were investigated by potentiometric titrations in NaCl under different experimental conditions [20]. The solvent effects on the dissociation constants of the ligands were also studied as increasing of the ethyl alcohol percentage. PMEA is an active compound against a series of viruses including herpes. HPMPC is employed in AIDS patients for the treatment of cytomegalovirus retinitis [2]. Stability constants of complex species of PMEA and HPMPC with Cu(II), Ni(II), Zn(II), Co(II), Ca(II), and Mg(II) were also obtained [20].
Several complexes between biguanide derivatives NRR’(NH)CNHC(NH)NH2 ((RR’bigH: R, R’ = Bui, H; Pr, H; -CH2CH2OCH2CH2-) and Co(II) or Co(III) ions were obtained and characterized by X-ray diffraction analysis. Both compounds were active against the second type of virus herpes [21].
A very interesting correlation between the structural stability of metal drugs and their biological activity was reported in the paper of Sigel et al. [22]. Protonation constants of 9-[2-(2-phosphonoethoxy)ethyl]adenine, PEEA and of (2-phosphonoethoxy)ethane, PEE, and the stability constants of the complexes with different divalent cations were determined. Cations under study were Mg(II), Ca(II), Sr(II), Ba(II), Mn(II), Co(II), Ni(II), Cu(II), Zn(II), and Cd(II). The results showed that some of these species form six-membered chelates. Comparisons with complexes that are formed by PMEA (9-[2-(phosphonomethoxy)ethyl]adenine) with divalent cations revealed that five-membered chelates are considerably more stable than the corresponding six-membered ones. This evidence provides an explanation for the excellent antiviral properties of PMEA as opposed to PEEA [22].
Finally, polyoxovanadates (POVs), compounds with anticancer, antimicrobial, and antiviral properties, have been investigated with different techniques, as potentiometry, 51V NMR, FT-IR, EPR, and UV-Vis spectroscopy as well as X-ray crystallography. The speciation model referring to vanadate solutions was obtained by 51V NMR and potentiometric investigations. It indicates, at pH > 6, the presence of polymers containing from one to five vanadium centers, characterized by individual protonation steps. These species, as tetramer or dimer, are in rapid equilibria and can present selective inhibition of enzyme activities [23].
Finally, another important propriety to investigate for antiviral drugs is the polymorphism, i.e., the ability of a solid compound to present more than one crystal structure [24]. Each polymorph shows different physicochemical properties, such as solubility, melting point, and density, resulting in a significant influence on the efficiency of the drug produced. However, to our knowledge, in the literature there are no reports on this property inherent antiviral metal drugs.
6. Perspectives and Conclusions
This review aims to highlight that the use of drug-metal antiviral complexes and metal-based nanostructured materials has not only allowed to significantly increase the antiviral action of pre-existing drugs but has also led to the formulation of new effective antiviral therapies. To understand the behavior and mechanism of action of these metal complexes, it is essential to define their chemical characteristics, paying particular attention to their thermodynamic and structural properties. Certainly, these aspects are required to be further investigated and more studies are needed to accurately define some thermodynamic properties, such as acid-base behavior and solubility in aqueous solutions, but also some other important characteristics, such as lipophilicity and polymorphism. A greater awareness of some of these properties has also led to the development of new vaccine therapies that are characterized by higher and longer lasting humoral immune responses. Indeed, the use of MeNPs as adjuvant agents has been described to lead to potential benefits in current vaccines and has facilitated the formulation of next-generation ones against various infectious diseases. The development of nanotechnologies has also made it possible to consider MeNPs, in particular AuNP and AgNP, excellent candidates for the transport and controlled release of antiviral drugs, thanks above all to their inert nature and high ability to bind drugs if they are properly functionalized. Although there are several papers attesting to the effectiveness of these systems, others are still in progress and others need to be performed in order to obtain safer and more easily administered formulations.
Author Contributions
Writing—Original Draft Preparation, C.A., F.C., C.F. and O.G.; Writing—Review and Editing, C.A., F.C., C.F. and O.G.; Supervision, C.F. and O.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Atabey, H.; Sari, H. Study of the Ionic Equilibriums in Aqueous Solutions and Coordination Properties of Adefovir and Cidofovir Used as Antiviral Drug. Fluid Phase Equilibria 2013, 356, 201–208. [Google Scholar] [CrossRef]
- Crichton, R.R. An overview of the role of metals in biology. In Practical Approaches to Biological Inorganic Chemistry; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–16. [Google Scholar]
- Rosenberg, B.; Vancamp, L.; Krigas, T. Inhibition of Cell Division in Escherichia coli by Electrolysis Products from a Platinum Electrode. Nature 1965, 205, 698–699. [Google Scholar] [CrossRef] [PubMed]
- Yousuf, I.; Bashir, M.; Arjmand, F.; Tabassum, S. Advancement of metal compounds as therapeutic and diagnostic metallodrugs: Current frontiers and future perspectives. Coord. Chem. Rev. 2021, 445, 214104. [Google Scholar] [CrossRef]
- Chakravarty, M.; Vora, A. Nanotechnology-based antiviral therapeutics. Drug Deliv. Transl. Res. 2021, 11, 748–787. [Google Scholar] [CrossRef] [PubMed]
- Roome, T.; Razzak, A. Clinical implications of metals-based drug-delivery systems. In Metal Nanoparticles for Drug Delivery and Diagnostic Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 237–258. [Google Scholar]
- Yang, J.; Yue, L.; Yang, Z.; Miao, Y.; Ouyang, R.; Hu, Y. Metal-Based Nanomaterials: Work as Drugs and Carriers against Viral Infections. Nanomaterials 2021, 11, 2129. [Google Scholar] [CrossRef]
- De Paiva, R.E.F.; Neto, A.M.; Santos, I.A.; Jardim, A.C.G.; Corbi, P.P.; Bergamini, F.R.G. What is holding back the development of antiviral metallodrugs? A literature overview and implications for SARS-CoV-2 therapeutics and future viral outbreaks. Dalton Trans. 2020, 49, 16004–16033. [Google Scholar] [CrossRef]
- Ni, Y.; Zeng, H.; Song, X.; Zheng, J.; Wu, H.; Liu, C.; Zhang, Y. Potential metal-related strategies for prevention and treatment of COVID-19. Rare Met. 2022, 41, 1129–1141. [Google Scholar] [CrossRef]
- Poole, S.K.; Patel, S.; Dehring, K.; Workman, H.; Poole, C.F. Determination of Acid Dissociation Constants by Capillary Electrophoresis. J. Chromatogr. A 2004, 1037, 445–454. [Google Scholar] [CrossRef]
- Tehan, B.G.; Lloyd, E.J.; Wong, R.P.; Pitt, W.R.; Montana, J.G.; Manallack, D.T.; Gancia, E. Estimation of Pka Using Semiempirical Molecular Orbital Methods. Part 1: Application to Phenols and Carboxylic Acids. Quant. Struct. Act. Relat. 2002, 21, 457–472. [Google Scholar] [CrossRef]
- Kalinowska, M.; Sienkiewicz-Gromiuk, J.; Świderski, G.; Pietryczuk, A.; Cudowski, A.; Lewandowski, W. Zn(II) Complex of Plant Phenolic Chlorogenic Acid: Antioxidant, Antimicrobial and Structural Studies. Materials 2020, 13, 3745. [Google Scholar] [CrossRef]
- Kowalczyk, M.; Golonko, A.; Świsłocka, R.; Kalinowska, M.; Parcheta, M.; Swiergiel, A.; Lewandowski, W. Drug Design Strategies for the Treatment of Viral Disease. Plant Phenolic Compounds and Their Derivatives. Front. Pharmacol. 2021, 12, 709104. [Google Scholar] [CrossRef]
- Nourian, A.; Khalili, H. Sofosbuvir as a Potential Option for the Treatment of COVID-19. Acta Biomed. 2020, 91, 239–241. [Google Scholar]
- Blindauer, C.A.; Sigel, A.; Operschall, B.P.; Griesser, R.; Holy, A.; Sigel, A. Extent of Intramolecular π-stacks in Aqueous Solution in Mixed-Ligand Copper(II) Complexes Formed by Heteroaromatic Amines and the Anticancer and Antivirally Active 9-[2-(Phosphonomethoxy)Ethyl]Guanine (Pmeg). A Comparison with Related Acyclic Nucleotide Analogues. Polyhedron 2016, 103, 248–260. [Google Scholar]
- Golcu, A.; Dolaz, M.; Demirelli, H.; Diðrak, M.; Serin, S. Spectroscopic and analytic properties of new copper(II) complex of antiviral drug valacyclovir. Transit. Met. Chem. 2006, 31, 658–665. [Google Scholar] [CrossRef]
- Carcelli, M. Antiviral activity and metal ion-binding properties of some 2-hydroxy-3-methoxyphenyl acylhydrazones. Biometals 2018, 31, 81–89. [Google Scholar] [CrossRef]
- Nagaj, J.; Starosta, R.; Jeżowska-Bojczuk, M. Acid–base characterization, coordination properties towards copper(II) ions and DNA interaction studies of ribavirin, an antiviral drug. J. Inorg. Biochem. 2015, 142, 68–74. [Google Scholar] [CrossRef]
- Carcelli, M.; Rogolino, D.; Bacchi, A.; Rispoli, G.; Fisicaro, E.; Compari, C.; Sechi, M.; Stevaert, A.; Naesens, L. Metal-Chelating 2 Hydroxyphenyl Amide Pharmacophore for Inhibition of Influenza Virus Endonuclease. J. Mol. Pharm. 2014, 11, 304–316. [Google Scholar] [CrossRef]
- Wanga, C.; Zhanga, R.; Weia, X.; Lva, M.; Jiang, Z. Metalloimmunology: The metal ion-controlled immunity. Adv. Immunol. 2020, 145, 187–241. [Google Scholar]
- Kirin, V.P.; Demkin, A.G.; Sukhikh, T.S.; Ilyicheva, T.N.; Maksakov, V.A. Cobalt complexes with biguanide derivatives—Synthesis, structure and antiviral activity. J. Mol. Struct. 2022, 1250, 131486. [Google Scholar] [CrossRef]
- Fernández-Botello, A.; Griesser, R.; Holy, A.; Moreno, V.; Sigel, H. Acid-Base and Metal-Ion-Binding Properties of 9-[2-(2-Phosphonoethoxy)Ethyl]Adenine (Peea), a Relative of the Antiviral Nucleotide Analogue 9-[2-(Phosphonomethoxy)Ethyl]Adenine (Pmea). An Exercise on the Quantification of Isomeric Complex Equilibria in Solution. Inorg. Chem. 2005, 44, 5104–5117. [Google Scholar]
- Aureliano, M.; Gumerova, N.I.; Sciortino, G.; Garribba, E.; Rompel, A.; Crans, D.C. Polyoxovanadates with emerging biomedical activities. Coord. Chem. Rev. 2021, 447, 214143. [Google Scholar] [CrossRef]
- Zvoníček, V.; Skořepová, E.; Dušek, M.; Žvátora, P.; Šoóš, M. Ibrutinib Polymorphs: Crystallographic Study. Cryst. Growth Des. 2018, 18, 1315–1326. [Google Scholar] [CrossRef]
- Esquezaro, P.G.; Manzano, C.M.; Nakahata, D.H.; ISantos, I.A.; Ruiz, U.E.; Santiago, M.B.; Silva, N.B.; Martins, C.H.; Pereira, D.H.; Bergamini, F.R.G.; et al. Synthesis, spectroscopic characterization and in vitro antibacterial and antiviral activities of novel silver(I) complexes with mafenide and ethyl-mafenide. J. Mol. Struct. 2021, 1246, 131261. [Google Scholar] [CrossRef]
- Cirri, D.; Pratesi, A.; Marzo, T.; Messori, L. Metallo therapeutics for COVID-19. Exploiting metal-based compounds for the discovery of new antiviral drugs. Expert Opin. Drug Discov. 2020, 16, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Pal, M.; Musib, D.; Roy, M. Transition metal complexes as potential tools against SARS-CoV-2: An in silico approach. New J. Chem. 2021, 45, 1924. [Google Scholar] [CrossRef]
- Maldonado, N.; Amo-Ochoa, P. The role of coordination compounds in virus research. Different approaches and trends. Dalton Trans. 2021, 50, 2310–2323. [Google Scholar] [CrossRef] [PubMed]
- Tortella, G.R.; Pieretti, J.C.; Rubilar, O.; Fernández-Baldo, M.; BenavidesMendoza, A.; Diez, M.C.; Seabra, A.B. Silver, copper and copper oxide nanoparticles in the fight against human viruses: Progress and perspectives, Critical Reviews in Biotechnology. Rev. Biotechnol. 2021, 42, 431–449. [Google Scholar] [CrossRef]
- De Castro, F.; Luca, E.; Benedetti, M.; Fanizzi, F.P. compounds as potential antiviral agents. Coord. Chem. 2022, 451, 214276. [Google Scholar] [CrossRef]
- Zoppi, C.; Messori, L.; Pratesi, A. ESI MS studies highlight the selective interaction of Auranofin with protein free thiols. Dalton Trans. 2020, 49, 5906–5916. [Google Scholar] [CrossRef]
- Marzo, T.; Messori, L. A Role for Metal-Based Drugs in Fighting COVID-19 Infection? The Case of Auranofin. ACS Med. Chem. Lett. 2020, 11, 1067–1068. [Google Scholar] [CrossRef]
- Rothan, H.A.; Stone, S.; Natekar, J.; Kumari, P.; Arora, K.; Kumar, M. The FDA-approved gold drug auranofin inhibits novel coronavirus (SARS-CoV-2) replication and attenuates inflammation in human cells. Virology 2020, 547, 7–11. [Google Scholar] [CrossRef]
- Singh, A.; Kaushik, A.; Dhau, J.S.; Kumar, R. Exploring coordination preferences and biological applications of pyridyl-based organochalcogen (Se, Te) ligands. Cordination Chem. Rev. 2022, 450, 214254. [Google Scholar] [CrossRef]
- Ghanghas, P.; Choudhary, A.; Kumar, D.; Poonia, K. Coordination metal complexes with Schiff bases: Useful pharmacophores with comprehensive biological applications. Inorg. Chem. Commun. 2021, 130, 108710. [Google Scholar] [CrossRef]
- Hossain, A.M.S.; Méndez-Arriaga, J.M.; Xia, C.; Xie, J.; Gómez-Ruiz, S. Metal complexes with ONS donor Schiff bases. Polyhedron 2022, 217, 115692. [Google Scholar] [CrossRef]
- Pettinari, C.; Pettinari, R.; Di Nicola, C.; Tombesi, A.; Scuri, S.; Marchetti, F. Antimicrobial MOFs. Coord. Chem. Rev. 2021, 446, 214121. [Google Scholar] [CrossRef]
- Li, H.; Dang, C.; Tai, X.; Xue, L.; Meng, Y.; Ma, S.; Zhang, J. VALD 3, a Schif base ligand synthesized from o vanillin derivatives, induces cell cycle arrest and apoptosis in breast cancer cells by inhibiting theWnt/β catenin pathway. Sci. Rep. 2021, 11, 14985. [Google Scholar] [CrossRef]
- Kar, K.K.; Ghosh, D.; Kabi, B.; Chandra, A. A concise review on cobalt Schiff base complexes as anticancer agents. Polyhedron 2022, 222, 115890. [Google Scholar]
- Hasan, M.N.; Bera, A.; Maji, T.K.; Pal, S.K. Sensitization of nontoxic MOF for their potential drug delivery application against microbial infection. Inorg. Chim. Acta 2021, 523, 120381. [Google Scholar] [CrossRef]
- Jaros, S.W.; Król, J.; Bażanów, B.; Poradowski, D.; Chrószcz, A.; Nesterov, D.S.; Kirillov, A.M.; Smoleński, P. Antiviral, Antibacterial, Antifungal, and Cytotoxic Silver(I) BioMOF Assembled from 1,3,5-Triaza-7-Phoshaadamantane and Pyromellitic Acid. Molecules 2020, 25, 2119. [Google Scholar] [CrossRef]
- Shakeri, A.; Panahi, Y.; Johnston, T.P.; Sahebkar, A. Biological properties of metal complexes of curcumin. BioFactors 2019, 45, 304–317. [Google Scholar] [CrossRef]
- Chen, L.; Chen, J.; Zhu, L. Two Cu(II) coordination polymers: Photocatalytic Cr(VI) reduction and treatment activity on influenza A virus infection by inducing IFITM expression. Arab. J. Chem. 2020, 13, 6662–6671. [Google Scholar] [CrossRef]
- Miyauchi, M.; Sunada, K.; Hashimoto, K. Antiviral Effect pf Visible Light-Sensitive CuxO/TiO2 Photocatalyst. Catalysts 2020, 10, 1093. [Google Scholar] [CrossRef]
- Ioannou, K.; Vlasiou, M.C. Metal based complexes against SARS-CoV-2. Biometals 2022, 24, 1–14. [Google Scholar] [CrossRef]
- Shu, T.; Huang, M.; Wu, D.; Ren, Y.; Zhang, X.; Han, Y.; Mu, J.; Wang, R.; Qui, Y.; Zhang, D.; et al. SARS-coronavirus-2 Nsp13 possesses NTPase and RNA helicase activities that can be inhibited by bismuth salts. Virol. Sin. 2020, 35, 321–329. [Google Scholar] [CrossRef]
- Talebian, S.; Wallace, G.G.; Schroeder, A.; Stellacci, F.; Conde, J. Nanotechnology-based disinfectants and sensors for SARS-CoV-2. Nat. Nanotechnol. 2020, 15, 618–621. [Google Scholar] [CrossRef]
- Mohamed, G.G.; El-Sherif, A.A.; Saad, M.A.; El-Sawy, S.E.A.; Morgan, S.M. Mixed-ligand complex formation of tenoxicam drug with some transition metal ions in presence of valine: Synthesis, characterization, molecular docking, potentiometric and evaluation of the humeral immune response of calves. J. Mol. Liq. 2016, 223, 1311–1332. [Google Scholar] [CrossRef]
- Sonbati, A.Z.; Diab, M.A.; Mohamed, G.G.; Saad, M.A.; Morgan, S.M.; El-Sawy, S.E.A. Polymer complexes. LXXVII. Synthesis, characterization, spectroscopic studies and immune response in cattle of quinoline polymer complexes. Appl. Organometal. Chem. 2019, 33, e4973. [Google Scholar] [CrossRef]
- Sengupta, A.; Azharuddin, M.; Al-Otaibi, N.; Hinkula, J. Efficacy and Immune Response Elicited by Gold Nanoparticle- Based Nanovaccines against Infectious Diseases. Vaccines 2022, 10, 505. [Google Scholar] [CrossRef]
- Niikura, K.; Matsunaga, T.; Suzuki, T.; Kobayashi, S.; Yamaguchi, H.; Orba, Y.; Kawaguchi, A.; Hasegawa, H.; Kajino, K.; Ninomiya, T.; et al. Gold Nanoparticles as a Vaccine Platform: Influence of Size and Shape on Immunological Responses In Vitro and In Vivo. ACSNano 2013, 7, 3926–3938. [Google Scholar] [CrossRef]
- Staroverov, S.A.; Vidyasheva, I.V.; Gabalov, K.P.; Vasilenko, O.A.; Laskavyi, V.N.; Dykman, L.A. Immunostimulatory Effect of Gold Nanoparticles Conjugated with Transmissible Gastroenteritis Virus. Immunol. Microbiol. 2011, 151, 1350–1358. [Google Scholar] [CrossRef]
- Farfán-Castro, S.; García-Soto, M.J.; Comas-García, M.; Arévalo-Villalobos, J.-I.; Palestino, G.; González-Ortega, O.; Rosales-Mendoza, S. Synthesis and immunogenicity assesment of a gold nanoparticle conjugate for the deivery of a peptide from SARS-CoV-2. Nanomedicine 2021, 34, 102372. [Google Scholar] [CrossRef] [PubMed]
- Sekimukai, H.; Iwata-Yoshikawa, N.; Fukushi, S.; Tani, H.; Kataoka, M.; Suzuki, T.; Hasegawa, H.; Niikura, K.; Arai, K.; Nagata, N. Gold nanoparticle-adiuvanted S protein induces a strong antigen-specific-related coronavirus infection, but fails to induce protective antibodies and limit eosinophilic infiltration in lungs. Microbiol. Immunol. 2020, 64, 33–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garrido, C.; Simpson, C.A.; Dahl, N.P.; Bresee, J.; Whitehead, D.C.; Lindsey, E.A.; Harris, T.; Smith, C.; Carter, C.J.; Feldheim, D.; et al. Gold nanoparticles to improve HIV drug delivery. Future Med. Chem. 2015, 7, 1097–1107. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Hung, Y.; Lin, W.; Huang, G.S. Assessment of gold nanoparticles as a size-dependent vaccine carrier for enhancing the antibody response against synthetic foot-and-mouth disease virus peptide. Nanotechnology 2010, 21, 195101. [Google Scholar] [CrossRef] [Green Version]
- Zazo, H.; Colino, C.I.; Warzecha, K.T.; Hoss, M.; Gbureck, U.; Trautwein, C.; Tacke, F.; Lanao, J.M.; Bartneck, M. Gold Nanocarriers for Macrophage-Targeted Therapy of Human Immunodeficiency Virus. Macromol. Biosci. 2016, 17, 1600359. [Google Scholar] [CrossRef]
- Paul, A.M.; Shi, Y.; Acharya, D.; Douglas, J.R.; Cooley, A.; Anderson, J.F.; Huang, F.; Bai, F. Delivery of antiviral small interfering RNA with gold nanoparticles inhibits dengue virus infection in vitro. J. Gen. Virol. 2014, 95, 1712–1722. [Google Scholar] [CrossRef] [Green Version]
- Horcajada, P.; Chalati, T.; Serre, C.; Gillet, B.; Sebrie, C.; Baati, T.; Eubank, J.F.; Heurtaux, D.; Clayette, P.; Kreuz, C.; et al. Porous metal–organic-framework nanoscale carriers as a potential platform for drug delivery and imaging. Nat. Mater. 2010, 9, 1683–1695. [Google Scholar] [CrossRef]
- Yan, W.; Jain, A.; O’Carra, R.; Woodward, J.G.; Li, W.; Li, G.; Nath, A.; Mumper, R.J. Lipid nanoparticles with accessible nickel as a vaccine delivery system for single and multiple his-tagged HIV antigens. Res. Palliat. Care 2009, 1, 1–11. [Google Scholar]
- Zachar, O. Nanomedicine formulations for respiratory infections by inhalation delivery: COVID-19 and beyond. Med. Hypotheses 2022, 159, 110753. [Google Scholar] [CrossRef]
- Reed, S.; Orr, M.; Fox, C. Key roles of adjuvants in modern vaccines. Nat. Med. 2013, 19, 1597–1608. [Google Scholar] [CrossRef]
- Behzadi, M.; Vakili, B.; Ebrahiminezhad, A.; Nezafat, N. Iron nanoparticles as novel vaccine adjuvants. Eur. J. Pharm. Sci. 2021, 159, 105718. [Google Scholar] [CrossRef]
- Dykman, L.A. Gold nanoparticles for preparation of antibodies and vaccines against infectious diseases. Expert Rev. Vaccines 2020, 19, 465–477. [Google Scholar] [CrossRef] [Green Version]
- Dykman, L.A.; Khlebtsov, N.G. Immunological properties of gold nanoparticles. Chem. Sci. 2021, 8, 1719–1735. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Jin, Q.; Ding, P.; Zhou, W.; Chai, Y.; Li, X.; Wang, Y.; Zhang, G. Gold nanoparticles enhance immune responses in mice against recombinant classical swine fever virus E2 protein. Biotechnol. Lett. 2020, 42, 1169–1180. [Google Scholar] [CrossRef]
- Neto, L.M.M.; Kipnis, A.; Junqueira-Kipnis, A.P. Role of Metallic Nanoparticles in Vaccinology: Implications for Infectious Disease Vaccine Development. Front. Immunol. 2017, 8, 239. [Google Scholar] [CrossRef] [Green Version]
- Teng, Z.; Sun, S.; Chen, H.; Huang, J.; Du, P.; Dong, H.; Xu, X.; Mu, S.; Zhang, Z.; Guo, H. Golden-star nanoparticles as adjuvant effectively promotes immune response to foot-and-mouth disease virus-like particles vaccine. Vaccines 2018, 36, 6752–6760. [Google Scholar] [CrossRef]
- Fischer, N.O.; Blanchette, C.D.; Chromy, B.A.; Kuhn, E.A.; Segelke, B.W.; Corzett, M.; Bench, G.; Mason, P.W.; Hoeprich, P.D. Immobilization of His-tagged proteins on nickel-chelating nanolipoprotein particles. Bioconjugate Chem. 2009, 20, 460–465. [Google Scholar] [CrossRef]
- Sarkar, J.; Das, S.; Aich, S.; Bhattacharyya, P.; Acharya, K. Antiviral potential of nanoparticles for the treatment of Coronavirus infections. J. Trace Elem. Med. Biol. 2022, 72, 126977. [Google Scholar] [CrossRef]
- Maduray, K.; Parboosing, R. Metal Nanoparticles: A Promising Treatment for Viral and Arboviral Infections. Biol. Trace Elem. Res. 2021, 199, 3159–3176. [Google Scholar] [CrossRef]
- Tortella, G.; Rubilar, O.; Fincheira, P.; Pieretti, J.C.; Duran, P.; Lourenço, I.; Seabra, A.B. Bactericidal and Virucidal Activities of Biogenic Metal-Based Nanoparticles: Advances and Perspectives. Antibiotics 2021, 10, 783. [Google Scholar] [CrossRef]
- Rai, M.M.; Ingle, A.P.; Gupta, I.; Brandelli, A. Bioactivity of noble metal nanoparticles decorated with biopolymers and their application in drug delivery. Int. J. Pharm. 2015, 496, 159–172. [Google Scholar] [CrossRef]
- Bibi, S.; Urrehman, S.; Khalid, L.; Yaseen, M.; Khan, A.Q.; Jia, R. Metal doped fullerene complexes as promising drug delivery materials against COVID-19. Chem. Pap. 2021, 75, 6487–6497. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).