Exploration of Risk Factors for Poor Prognosis of Non-Traumatic Non-Aneurysmal Subarachnoid Hemorrhage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Clinical Factors
2.3. Statistical Analyses
3. Result
3.1. Clinical Data
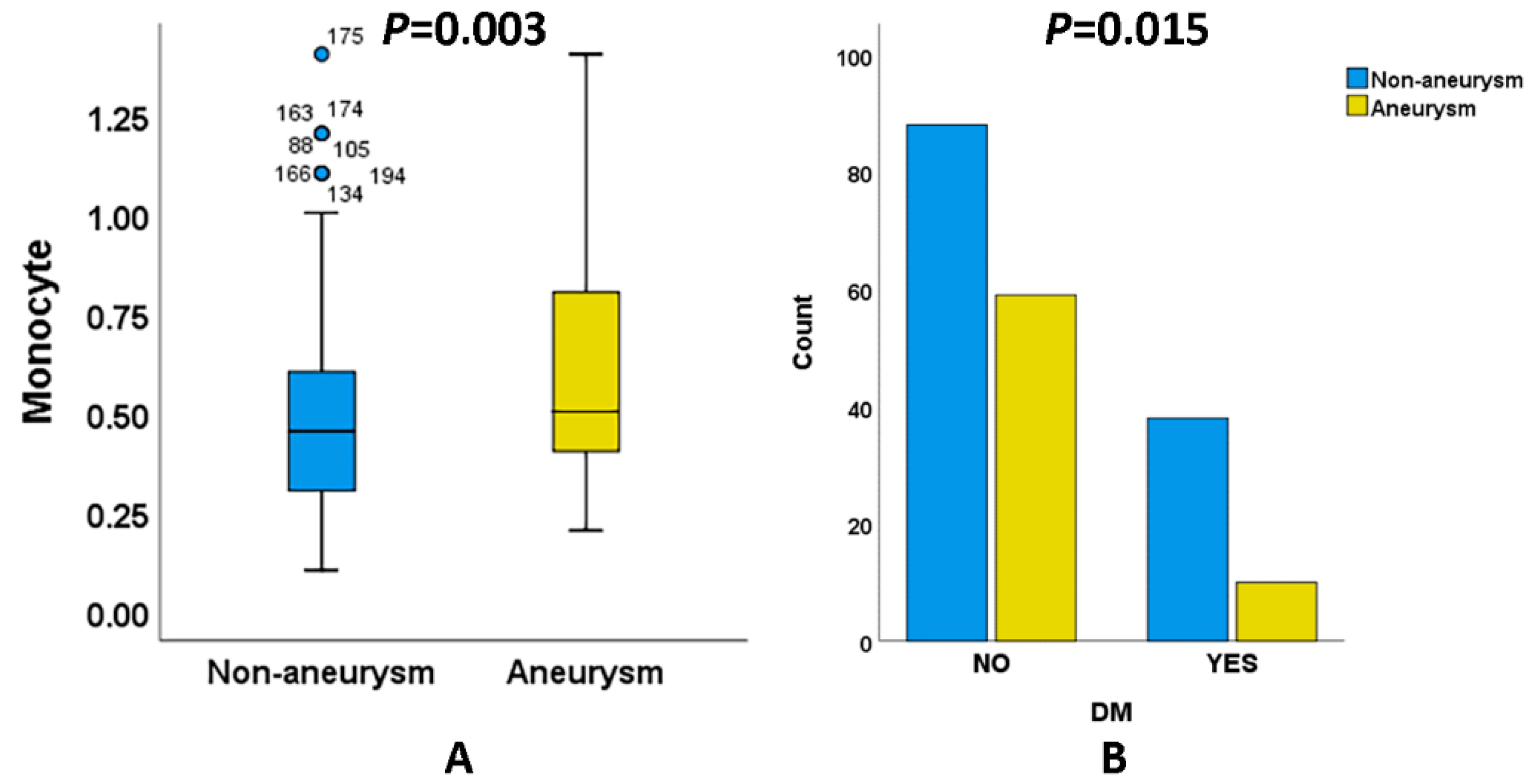
3.2. Comparison between Patients with aSAH and naSAH
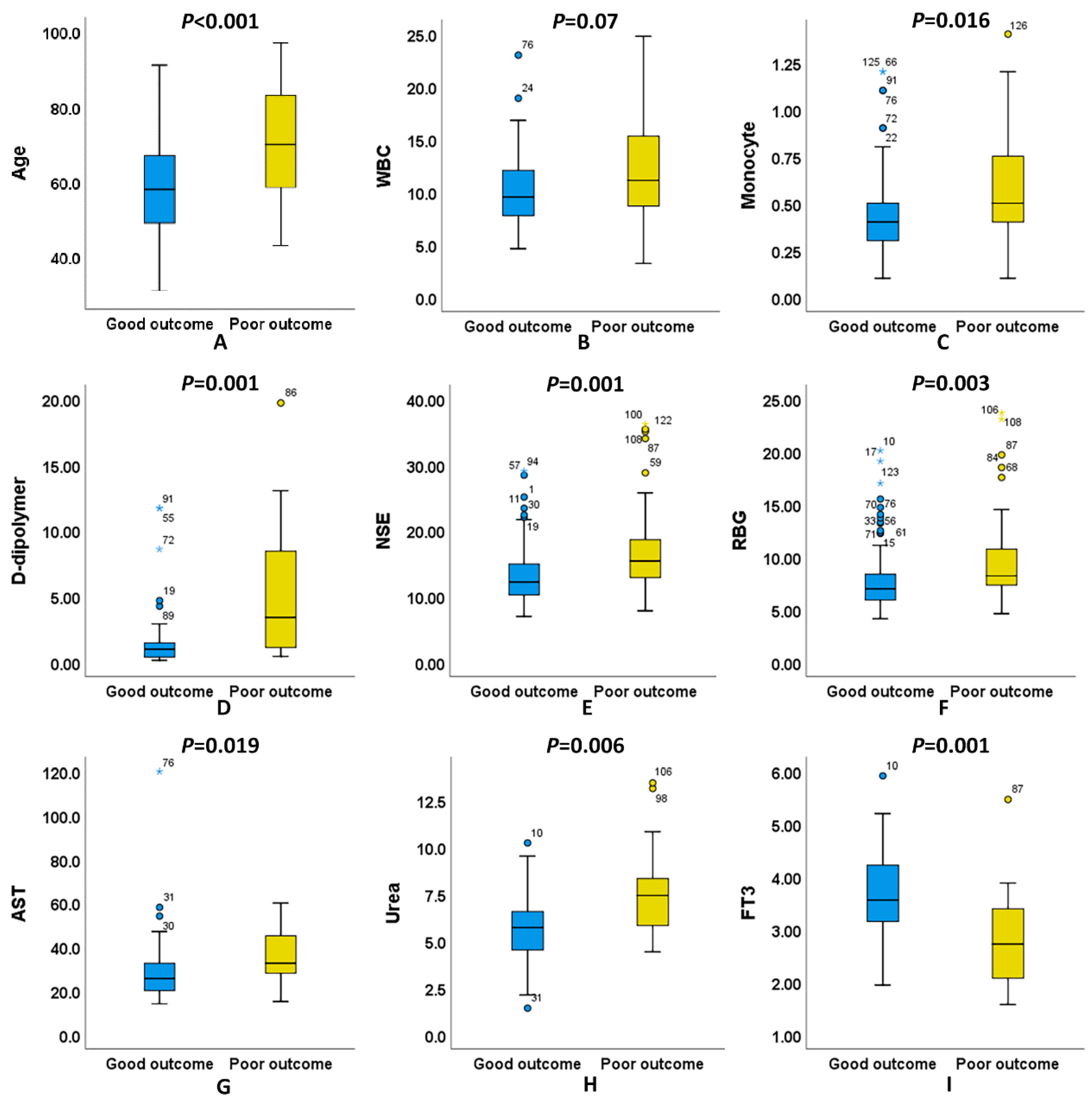
3.3. Differences in Age and Hematological Indicators between Good Outcome and Poor Outcome of naSAH Patients
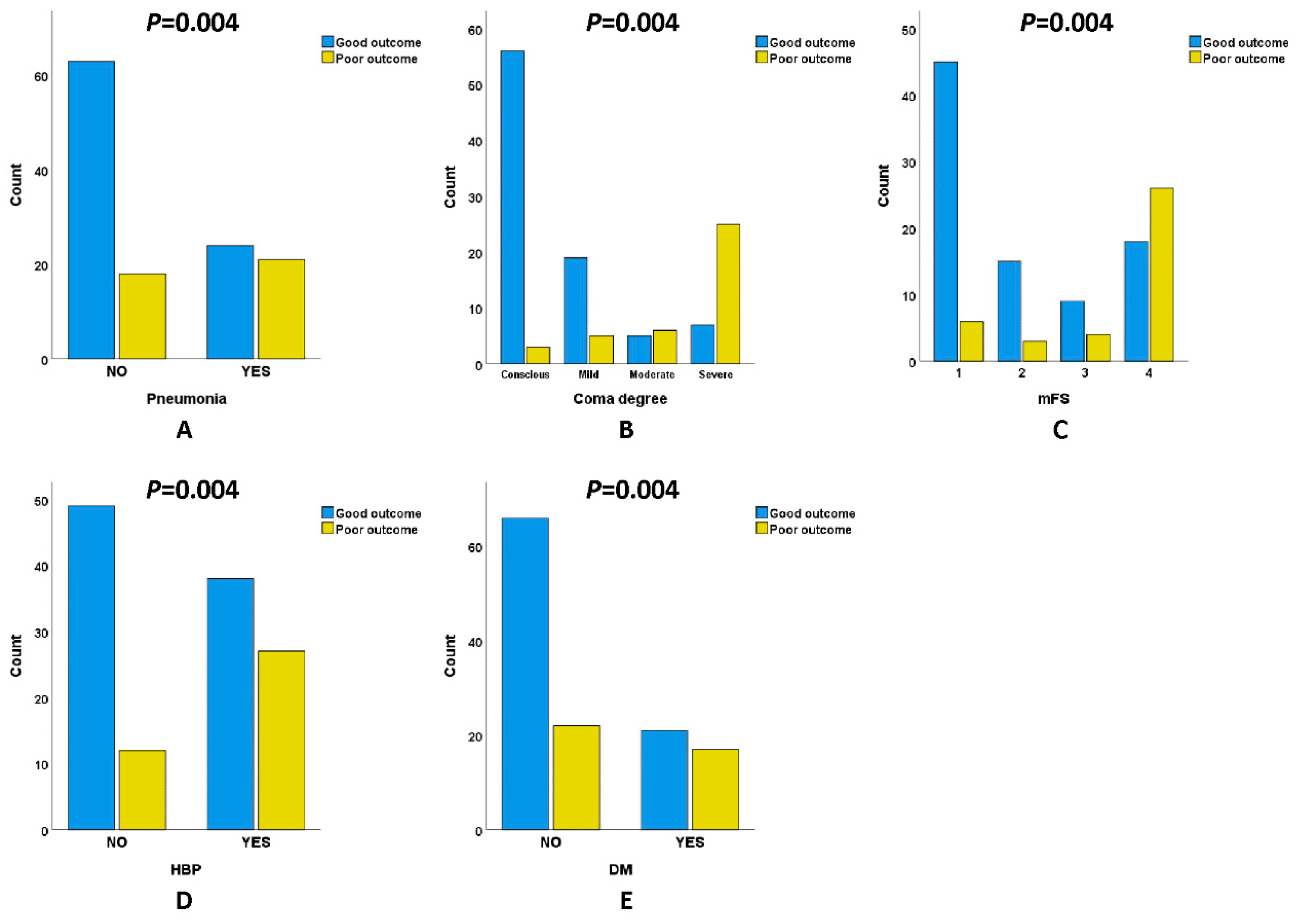
3.4. Comparison of Pneumonia Incidence, Clinical Scale, and Previous Disease History in Patients with naSAH
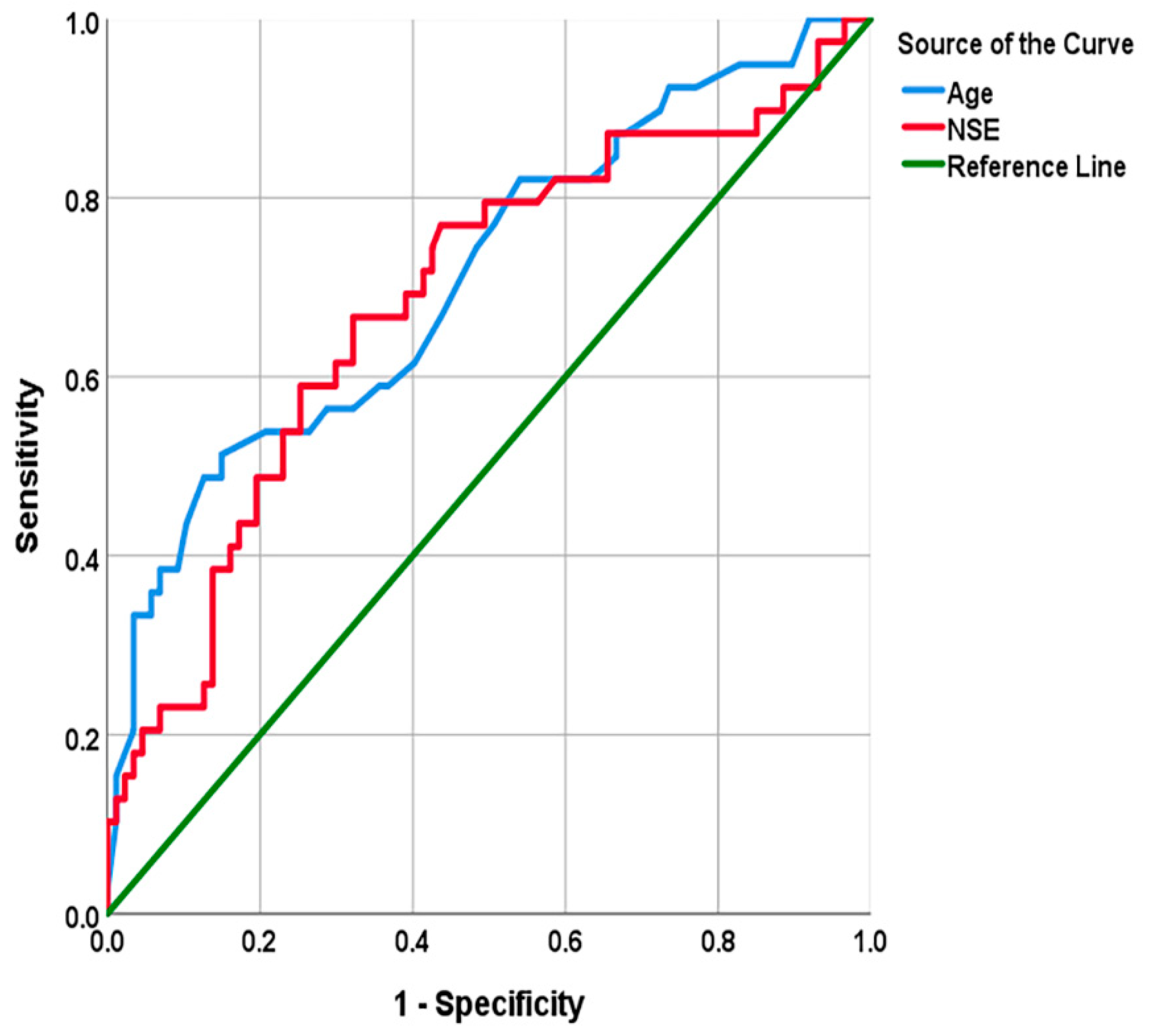
3.5. Screening of Independent Predictors of Adverse Prognosis of naSAH and Evaluation of Their Predictive Ability
3.6. The Multi-Factor Discriminant Model Can Make up for the Poor Goodness of Fit of the Logical Regression Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hop, J.W.; Rinkel, G.J.; Algra, A.; Van Gijn, J. Case-fatality rates and functional outcome after subarachnoid hemorrhage: A systematic review. Stroke 1997, 28, 660–664. [Google Scholar] [CrossRef]
- Achrén, A.; Raj, R.; Siironen, J.; Laakso, A.; Marjamaa, J. Spontaneous angiogram-negative subarachnoid hemorrhage: A retrospective single center cohort study. Acta Neurochir. 2022, 164, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhao, Y.; Liu, Y.; Wang, X.; Xu, H.; Fang, Y.; Zhang, A.; Lenahan, C.; Luo, Y.; Chen, S. Effect of stress-induced hyperglycemia after non-traumatic non-aneurysmal subarachnoid hemorrhage on clinical complications and functional outcomes. CNS Neurosci. Ther. 2022, 28, 942–952. [Google Scholar] [CrossRef]
- Kapadia, A.; Schweizer, T.A.; Spears, J.; Cusimano, M.; Macdonald, R.L. Nonaneurysmal Perimesencephalic Subarachnoid Hemorrhage: Diagnosis, Pathophysiology, Clinical Characteristics, and Long-Term Outcome. World Neurosurg. 2014, 82, 1131–1143. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, M.; Gandhi, C.; Dragonette, J.; Schmidt, M.; Cole, C.; Santarelli, J.; Lehrer, R.; Al-Mufti, F.; Bowers, C.A. Increasing Frailty Predicts Worse Outcomes and Increased Complications After Angiogram-Negative Subarachnoid Hemorrhages. World Neurosurg. 2020, 134, e181–e188. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, M.K.; Gandhi, C.; Dragonette, J.; Schmidt, M.; Cole, C.; Santarelli, J.; Lehrer, R.; Al-Mufti, F.; Bowers, C.A. A comparison of frailty indices in predicting length of inpatient stay and discharge destination following angiogram-negative subarachnoid hemorrhage. Br. J. Neurosurg. 2021, 35, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Zhang, Z.; Zhang, W.B.; Wang, X.; Lenahan, C.; Fang, Y.; Luo, Y.; Liu, Y.; Mei, S.; Chen, S.; et al. Development of a nomogram for predicting clinical outcome in patients with angiogram-negative subarachnoid hemorrhage. CNS Neurosci. Ther. 2021, 27, 1339–1347. [Google Scholar] [CrossRef]
- Gusdon, A.M.; Savarraj, J.P.J.; Shihabeddin, E.; Paz, A.; Assing, A.; Ko, S.-B.; McCullough, L.D.; Choi, H.A. Time Course of Peripheral Leukocytosis and Clinical Outcomes After Aneurysmal Subarachnoid Hemorrhage. Front. Neurol. 2021, 12, 694996. [Google Scholar] [CrossRef]
- Muhammad, S.; Hänggi, D. Inflammation and Anti-Inflammatory Targets after Aneurysmal Subarachnoid Hemorrhage. Int. J. Mol. Sci. 2021, 22, 7355. [Google Scholar] [CrossRef]
- Mohme, M.; Sauvigny, T.; Mader, M.M.-D.; Schweingruber, N.; Maire, C.L.; Rünger, A.; Ricklefs, F.; Regelsberger, J.; Schmidt, N.O.; Westphal, M.; et al. Immune Characterization in Aneurysmal Subarachnoid Hemorrhage Reveals Distinct Monocytic Activation and Chemokine Patterns. Transl. Stroke Res. 2020, 11, 1348–1361. [Google Scholar] [CrossRef]
- Feghali, J.; Kim, J.; Gami, A.; Rapaport, S.; Caplan, J.M.; McDougall, C.G.; Huang, J.; Tamargo, R.J.; Jackson, C.M. Monocyte-based inflammatory indices predict outcomes following aneurysmal subarachnoid hemorrhage. Neurosurg. Rev. 2021, 44, 3499–3507. [Google Scholar] [CrossRef] [PubMed]
- Cahill, J.; Calvert, J.W.; Zhang, J.H. Mechanisms of early brain injury after subarachnoid hemorrhage. J. Cereb. Blood Flow Metab. 2006, 26, 1341–1353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrod, C.G.; Bendok, B.R.; Batjer, H.H. Prediction of Cerebral Vasospasm in Patients Presenting with Aneurysmal Subarachnoid Hemorrhage: A Review. Neurosurgery 2005, 56, 633–654. [Google Scholar] [CrossRef]
- Xu, Z.; Shi, W.-H.; Xu, L.-B.; Shao, M.-F.; Chen, Z.-P.; Zhu, G.-C.; Hou, Q. Resident Microglia Activate before Peripheral Monocyte Infiltration and p75NTR Blockade Reduces Microglial Activation and Early Brain Injury after Subarachnoid Hemorrhage. ACS Chem. Neurosci. 2019, 10, 412–423. [Google Scholar] [CrossRef]
- Korja, M.; Thorn, L.M.; Hägg, S.; Putaala, J.; Liebkind, R.; Harjutsalo, V.; Forsblom, C.M.; Gordin, D.; Tatlisumak, T.; Groop, P.-H.; et al. Subarachnoid hemorrhage in type 1 diabetes: A prospective cohort study of 4083 patients with diabetes. Diabetes Care 2013, 36, 3754–3758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Rooij, N.K.; Linn, F.H.H.; Van Der Plas, J.A.; Algra, A.; Rinkel, G.J.E. Incidence of subarachnoid haemorrhage: A systematic review with emphasis on region, age, gender and time trends. J. Neurol. Neurosurg. Psychiatry 2007, 78, 1365–1372. [Google Scholar] [CrossRef]
- Ikawa, F.; Ichihara, N.; Uno, M.; Shiokawa, Y.; Toyoda, K.; Minematsu, K.; Kobayashi, S.; Yamaguchi, S.; Kurisu, K. Visualisation of the non-linear correlation between age and poor outcome in patients with aneurysmal subarachnoid haemorrhage. J. Neurol. Neurosurg. Psychiatry 2021, 92, 1173–1180. [Google Scholar] [CrossRef]
- van Donkelaar, C.E.; Bakker, N.A.; Birks, J.; Veeger, N.J.; Metzemaekers, J.D.; Molyneux, A.J.; Groen, R.J.M.; van Dijk, J.M.C. Prediction of Outcome After Aneurysmal Subarachnoid Hemorrhage. Stroke 2019, 50, 837–844. [Google Scholar] [CrossRef]
- Macdonald, R.L. Age and outcome after aneurysmal subarachnoid haemorrhage. J. Neurol. Neurosurg. Psychiatry 2021, 92, 1143. [Google Scholar] [CrossRef]
- Jabbarli, R.; Reinhard, M.; Roelz, R.; Shah, M.; Niesen, W.D.; Kaier, K.; Taschner, C.; Weyerbrock, A.; Van Velthoven, V. The predictors and clinical impact of intraventricular hemorrhage in patients with aneurysmal subarachnoid hemorrhage. Int. J. Stroke 2016, 11, 68–76. [Google Scholar] [CrossRef]
- Arca, G.; Arnaez, J.; Agut, T.; Núñez, C.; Stephan-Otto, C.; Valls, A.; García-Alix, A. Neuron-specific enolase is correlated with lesion topology, relative infarct volume and outcome of symptomatic NAIS. Arch. Dis. Child. Fetal Neonatal Ed. 2020, 105, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Kirkova, J.; Walsh, D.; Aktas, A.; Davis, M.P. Cancer Symptom Clusters: Old Concept But New Data. Am. J. Hosp. Palliat. Med. 2010, 27, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Kacira, T.; Kemerdere, R.; Atukeren, P.; Hanimoglu, H.; Sanus, G.Z.; Kucur, M.; Tanriverdi, T.; Gumustas, K.; Kaynar, M.Y. Detection Of Caspase-3, Neuron Specific Enolase, And High-Sensitivity C-Reactive Protein Levels In Both Cerebrospinal Fluid And Serum Of Patients After Aneurysmal Subarachnoid Hemorrhage. Neurosurgery 2007, 60, 674–680. [Google Scholar] [CrossRef] [PubMed]
- Quintard, H.; Leduc, S.; Ferrari, P.; Petit, I.; Ichai, C. Early and persistent high level of PS 100beta is associated with increased poor neurological outcome in patients with SAH: Is there a PS 100beta threshold for SAH prognosis? Crit. Care 2016, 20, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takeuchi, S.; Kumagai, K.; Toyooka, T.; Otani, N.; Wada, K.; Mori, K. Intravenous Hydrogen Therapy With Intracisternal Magnesium Sulfate Infusion in Severe Aneurysmal Subarachnoid Hemorrhage. Stroke 2021, 52, 20–27. [Google Scholar] [CrossRef]
- Fukuda, H.; Lo, B.; Yamamoto, Y.; Handa, A.; Yamamoto, Y.; Kurosaki, Y.; Yamagata, S. Plasma D-dimer may predict poor functional outcomes through systemic complications after aneurysmal subarachnoid hemorrhage. J. Neurosurg. 2017, 127, 284–290. [Google Scholar] [CrossRef] [Green Version]
- Fukuda, H.; Handa, A.; Koyanagi, M.; Lo, B.; Yamagata, S. Association of plasma D-dimer level with thromboembolic events after endovascular coil treatment of ruptured cerebral aneurysms. J. Neurosurg. 2018, 130, 509–516. [Google Scholar] [CrossRef] [Green Version]
- Kreitschmann-Andermahr, I.; Hoff, C.; Saller, B.; Niggemeier, S.; Pruemper, S.; Hutter, B.O.; Rohde, V.; Gressner, A.; Matern, S.; Gilsbach, J.M. Prevalence of Pituitary Deficiency in Patients after Aneurysmal Subarachnoid Hemorrhage. J. Clin. Endocrinol. Metab. 2004, 89, 4986–4992. [Google Scholar] [CrossRef] [Green Version]
- Parenti, G.; Cecchi, P.C.; Ragghianti, B.; Schwarz, A.; Ammannati, F.; Mennonna, P.; Di Rita, A.; Gallina, P.; Di Lorenzo, N.; Innocenti, P.; et al. Evaluation of the anterior pituitary function in the acute phase after spontaneous subarachnoid hemorrhage. J. Endocrinol. Investig. 2011, 34, 361–365. [Google Scholar] [CrossRef]

| Test Result Variables | Non-Aneurysm | Aneurysm | p-Value |
|---|---|---|---|
| Number | 126 | 69 | |
| Age | 62.02 ± 14.23 | 61.94 ± 14.41 | 0.973 a |
| Hospitalization days | 15.00 (9.50) | 17.00 (9.00) | 0.052 b |
| WBC | 10.76 ± 4.12 | 10.99 ± 3.77 | 0.706 a |
| Lymphocyte | 1.40 (1.00) | 1.30 (0.90) | 0.390 b |
| Monocyte | 0.50 ± 0.26 | 0.60 ± 0.27 | 0.003 a |
| Neutrophil | 8.54 ± 4.16 | 8.70 ± 3.90 | 0.798 a |
| NLR | 5.51 (8.71) | 7.08 (7.27) | 0.502 b |
| PT | 11.35 (1.30) | 11.10 (1.30) | 0.056 b |
| APTT | 25.04 ± 3.94 | 24.25 ± 3.45 | 0.163 a |
| D-dipolymer | 1.12 (1.98) | 1.56 (2.80) | 0.208 b |
| NSE | 13.02 (6.13) | 13.67 (5.31) | 0.082 b |
| RBG | 7.62 (3.25) | 7.50 (3.10) | 0.864 b |
| TC | 4.87 ± 1.20 | 4.72 ± 1.03 | 0.358 a |
| TG | 1.37 (0.70) | 1.24 (0.75) | 0.359 b |
| HDL | 1.26 ± 0.37 | 1.31 ± 0.37 | 0.391 a |
| LDL | 3.00 ± 1.06 | 2.91 ± 0.81 | 0.475 a |
| Homocysteine | 12.59 (6.22) | 14.30 (9.01) | 0.564 b |
| AST | 27.00 (12.50) | 30.50 (8.80) | 0.292 b |
| ALT | 30.50 (21.30) | 29.00 (9.80) | 0.411 b |
| Urea | 5.80 (2.30) | 5.75 (3.80) | 0.779 b |
| Cr | 60.00 (24.50) | 64.50 (27.00) | 0.680 b |
| UA | 305.18 ± 88.63 | 299.89 ± 101.72 | 0.797 a |
| FT3 | 3.50 ± 0.92 | 3.43 ± 0.74 | 0.724 a |
| FT4 | 16.02 ± 2.57 | 16.35 ± 2.19 | 0.537 a |
| TSH | 0.76 (0.90) | 0.59 (0.98) | 0.207 b |
| Gender | 0.220 c | ||
| Male | 70 (55.6) | 32 (46.4) | |
| Female | 56 (44.4) | 37 (53.6) | |
| Pneumonia | 45 (35.7) | 25 (36.2) | 0.943 c |
| GCS | 0.169 d | ||
| 15 | 59 (46.8) | 23 (33.3) | |
| 13~14 | 24 (19.0) | 10 (14.5) | |
| 9~12 | 11 (8.7) | 15 (21.7) | |
| 3~8 | 32 (25.4) | 21 (30.4) | |
| mFS | 0.164 d | ||
| 1 | 51 (40.5) | 17 (24.6) | |
| 2 | 18 (14.3) | 16 (23.2) | |
| 3 | 13 (10.3) | 9 (13.0) | |
| 4 | 44 (34.9) | 27 (39.1) | |
| HBP | 65 (51.6) | 43 (62.3) | 0.149 c |
| DM | 38 (30.2) | 10 (14.5) | 0.015 c |
| Smoking history | 20 (15.9) | 10 (14.5) | 0.798 c |
| Drinking history | 14 (11.1) | 6 (8.7) | 0.595 c |
| Test Result Variables | All Patients | 90-Day mRS:0–2 | 90-Day mRS:3–6 | p-Value |
|---|---|---|---|---|
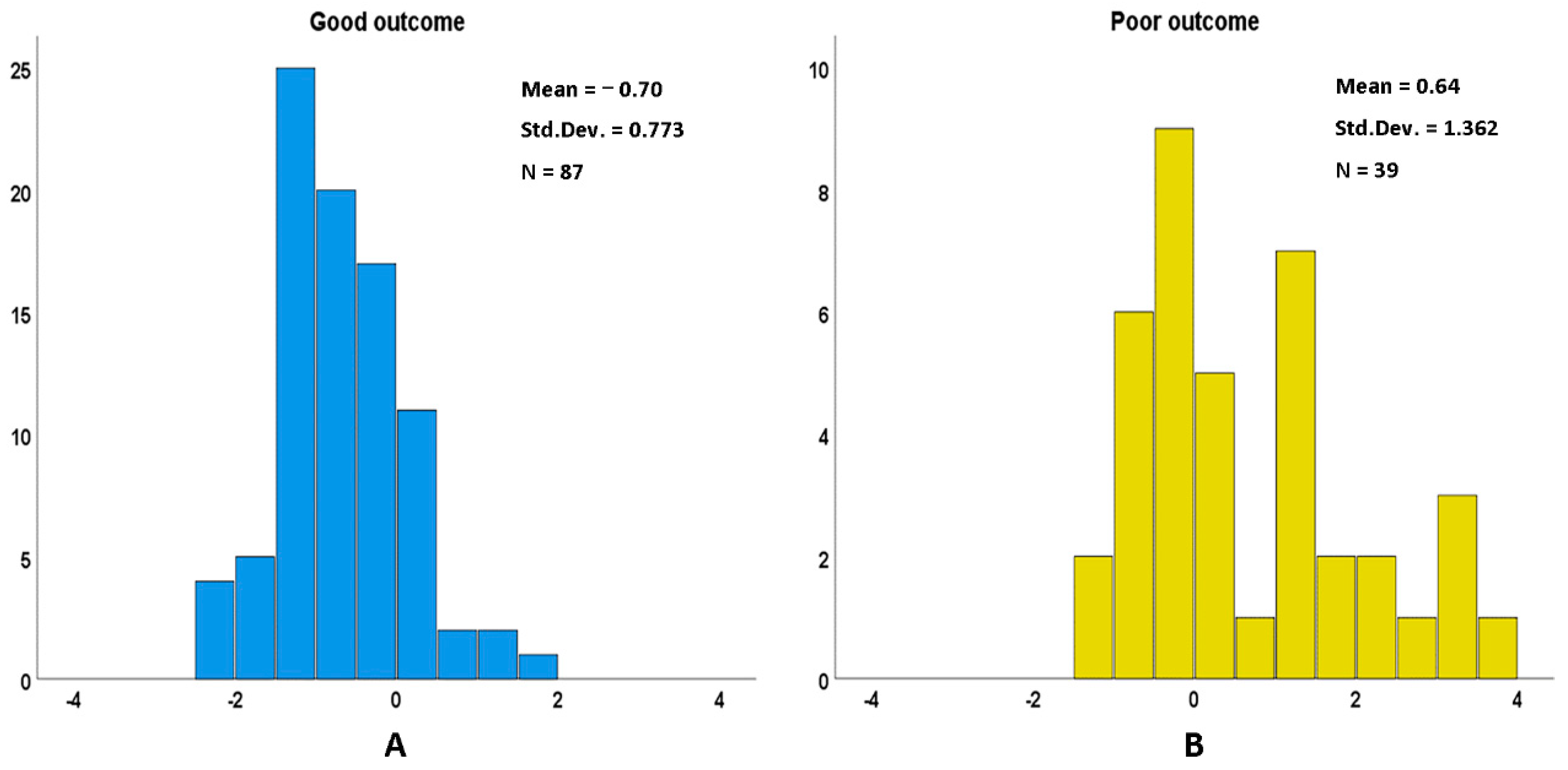
| Number | 126 | 87 (69.0) | 39 (31.0) | |
| Age | 62.02 ± 14.23 | 58.45 ± 12.26 | 70.03 ± 15.17 | <0.001 a |
| Hospitalization days | 15.00 (9.50) | 15.00 (10.00) | 13.00 (15.00) | 0.058 b |
| WBC | 10.76 ± 4.12 | 10.16 ± 3.37 | 12.11 ± 5.24 | 0.037 a |
| Lymphocyte | 1.40 (1.00) | 1.30 (1.10) | 1.40 (1.10) | 0.696 b |
| Monocyte | 0.50 ± 0.26 | 0.46 ± 0.22 | 0.59 ± 0.31 | 0.016 a |
| Neutrophil | 8.54 ± 4.16 | 8.07 ± 3.68 | 9.60 ± 4.95 | 0.089 a |
| NLR | 5.51 (8.71) | 5.33 (8.36) | 6.41 (10.57) | 0.458 b |
| PT | 11.35 (1.30) | 11.30 (1.20) | 11.40 (1.30) | 0.937 b |
| APTT | 25.04 ± 3.94 | 25.23 ± 3.90 | 25.64 ± 4.04 | 0.439 a |
| D-dipolymer | 1.12 (1.98) | 0.97 (1.11) | 3.40 (7.38) | 0.001 b |
| NSE | 13.02 (6.13) | 12.18 (5.16) | 15.38 (6.27) | 0.001 b |
| RBG | 7.62 (3.25) | 6.96 (2.51) | 8.20 (3.58) | 0.003 b |
| TC | 4.87 ± 1.20 | 4.99 ± 1.26 | 4.62 ± 1.02 | 0.117 a |
| TG | 1.37 (0.70) | 1.41 (0.72) | 1.36 (0.67) | 0.409 b |
| HDL | 1.26 ± 0.37 | 1.27 ± 0.39 | 1.24 ± 0.34 | 0.764 a |
| LDL | 3.00 ± 1.06 | 3.13 ± 1.09 | 2.73 ± 0.96 | 0.055 a |
| Homocysteine | 12.59 (6.22) | 12.56 (6.09) | 14.16 (13.63) | 0.293 b |
| AST | 27.00 (12.50) | 24.50 (12.80) | 32.50 (19.00) | 0.019 b |
| ALT | 30.50 (21.30) | 31.50 (20.00) | 28.50 (26.80) | 0.830 b |
| Urea | 5.80 (2.30) | 5.70 (2.10) | 7.40 (3.30) | 0.006 b |
| Cr | 60.00 (24.50) | 59.50 (24.80) | 63.50 (33.00) | 0.122 b |
| UA | 305.18 ± 88.63 | 296.85 ± 87.68 | 340.86 ± 86.78 | 0.095 a |
| FT3 | 3.50 ± 0.92 | 3.68 ± 0.81 | 2.84 ± 1.03 | 0.001 a |
| FT4 | 16.02 ± 2.57 | 15.94 ± 2.42 | 16.30 ± 3.14 | 0.622 a |
| TSH | 0.76 (0.90) | 0.82 (0.94) | 0.75 (0.58) | 0.331 b |
| Gender | 0.518 c | |||
| Male | 70 (55.6) | 50 (57.5) | 20 (51.3) | |
| Female | 56 (44.4) | 37 (42.5) | 19 (48.7) | |
| Pneumonia | 45 (35.7) | 24 (27.6) | 21 (53.8) | 0.004 c |
| GCS | <0.001 d | |||
| 15 | 59 (46.8) | 56 (64.4) | 3 (7.7) | |
| 13~14 | 24 (19.0) | 19 (21.8) | 5 (12.8) | |
| 9~12 | 11 (8.7) | 5 (5.7) | 6 (15.4) | |
| 3~8 | 32 (25.4) | 7 (8.0) | 25 (64.1) | |
| mFS | <0.001 d | |||
| 1 | 51 (40.5) | 45 (51.7) | 6 (15.4) | |
| 2 | 18 (14.3) | 15 (17.2) | 3 (7.7) | |
| 3 | 13 (10.3) | 9 (10.3) | 4 (10.3) | |
| 4 | 44 (34.9) | 18 (20.7) | 26 (66.7) | |
| HBP | 65 (51.6) | 38 (43.7) | 27 (69.2) | 0.008 c |
| DM | 38 (30.2) | 21 (24.1) | 17 (43.6) | 0.028 c |
| Smoking history | 20 (15.9) | 16 (18.4) | 4 (10.3) | 0.248 c |
| Drinking history | 14 (11.1) | 12 (13.8) | 2 (5.1) | 0.261 e |
| Test Result Variables | Coefficient | OR | 95%CI for OR | p-Value |
|---|---|---|---|---|
| Age | 0.11 | 1.11 | [1.01, 1.23] | 0.035 |
| D-dipolymer | 0.21 | 1.23 | [0.96, 1.58] | 0.098 |
| NSE | 0.22 | 1.25 | [1.03, 1.51] | 0.024 |
| FT3 | −1.34 | 0.26 | [0.06, 1.10] | 0.067 |
| Constant | −9.02 | 0.069 |
| Test Result Variables | AUC | 95%CI | p-Value | Cut-Off Value | Se | Sp | J |
|---|---|---|---|---|---|---|---|
| Age | 0.71 | [0.61, 0.81] | 0.000 | 69.00 | 0.51 | 0.85 | 0.36 |
| NSE | 0.68 | [0.58, 0.79] | 0.001 | 13.75 | 0.67 | 0.68 | 0.35 |
| Parameters | Coefficients | Canonical Correlation | p-Value |
|---|---|---|---|
| Age | 0.028 | 0.775 | <0.001 |
| D-dipolymer | 0.143 | ||
| NSE | 0.082 | ||
| RBG | 0.054 | ||
| Urea | 0.115 | ||
| FT3 | −0.466 | ||
| (Constant) | −3.157 |
| Test Result Variables | All Patients (N = 126) | Age ≤ 69 Years (N = 93) | Age > 69 Years (N = 33) | |||
|---|---|---|---|---|---|---|
| R | p-Value | R | p-Value | R | p-Value | |
| D-dipolymer | 0.4 | 0.001 | 0.3 | 0.018 | 0.3 | 0.267 |
| NSE | 0.3 | 0.003 | 0.2 | 0.078 | 0.3 | 0.078 |
| RBG | 0.3 | 0.001 | 0.2 | 0.109 | 0.6 | <0.001 |
| Urea | 0.3 | 0.008 | 0 | 0.820 | 0.6 | 0.004 |
| FT3 | −0.4 | 0.001 | −0.2 | 0.179 | −0.5 | 0.026 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, Y.; Chen, J.; Zhang, Y.; Zhao, F.; Zhai, Y.; Xu, X.; Xue, L.; Zhao, Y.; Wang, H. Exploration of Risk Factors for Poor Prognosis of Non-Traumatic Non-Aneurysmal Subarachnoid Hemorrhage. Biomolecules 2022, 12, 948. https://doi.org/10.3390/biom12070948
Yuan Y, Chen J, Zhang Y, Zhao F, Zhai Y, Xu X, Xue L, Zhao Y, Wang H. Exploration of Risk Factors for Poor Prognosis of Non-Traumatic Non-Aneurysmal Subarachnoid Hemorrhage. Biomolecules. 2022; 12(7):948. https://doi.org/10.3390/biom12070948
Chicago/Turabian StyleYuan, Yuan, Jingjiong Chen, Yaxuan Zhang, Fei Zhao, Yanyu Zhai, Xiaofeng Xu, Lixia Xue, Yuwu Zhao, and Hongmei Wang. 2022. "Exploration of Risk Factors for Poor Prognosis of Non-Traumatic Non-Aneurysmal Subarachnoid Hemorrhage" Biomolecules 12, no. 7: 948. https://doi.org/10.3390/biom12070948
APA StyleYuan, Y., Chen, J., Zhang, Y., Zhao, F., Zhai, Y., Xu, X., Xue, L., Zhao, Y., & Wang, H. (2022). Exploration of Risk Factors for Poor Prognosis of Non-Traumatic Non-Aneurysmal Subarachnoid Hemorrhage. Biomolecules, 12(7), 948. https://doi.org/10.3390/biom12070948







