1. Introduction
Transient receptor potential (TRP) channels constitute a superfamily of non-selective cationic channels that exhibit a common structure. They are composed of N- and C-terminal regions containing protein interaction motifs and six transmembrane domains (TM1-TM6). The putative ion conducting pore is located between the fifth and sixth TM domains. According to their activation stimuli and the presence of regulatory domains on the cytosolic N- and C-termini, the TRP superfamily is subdivided into seven main subfamilies: TRPC1-7 (Canonical), TRPV1-6 (Vanilloid), TRPM1-8 (Melastatin), TRPP1-4 (Polycystin), TRPML1-3 (Mucolipin), TRPA1 (Ankyrin), and TRPN (no mechanoreceptor potential C, also named NOMPC). Functional TRPs are composed of four homo- or heteromultimeric subunits.
Among TRPV channels, TRPV4 is a Ca
2+-permeable channel whose expression in mammals is widespread throughout the body: ear, eye, skin, cardiovascular, digestive, immune, musculoskeletal, nervous, reproductive, and respiratory systems (for review see [
1]). Due to its Ca
2+ permeability (PCa/PNa ≈ 7 in HEK293 cells transiently transfected with wild type TRPV4, [
2]), activation of TRPV4 induces an increase in cytosolic Ca
2+ concentration ([Ca
2+]
c) which plays a key role in fundamental cellular processes such as apoptosis, contraction, migration, and proliferation in many cell types. Thus, owing to its ubiquitous expression and its role in mediating Ca
2+ influx, it is now recognized that TRPV4 supports a great number of roles in various physiological and disease states [
3].
A wide variety of stimuli, including physical (cell swelling and shear stress), thermal (moderate heat), and chemical (endogenous and exogenous ligands) stimuli such as the arachidonic acid metabolite epoxyeicosatrienoic acid and the synthetic agonist GSK-1016790A, in particular [
4], activate TRPV4. In addition, other modulatory intermediates affect the channel function. Indeed, a range of phosphorylations or protein–protein interactions affect the sensitivity to gating stimuli or channel trafficking. For example, in TRPV4 transfected HEK293 cells, the serine/threonine protein kinases A and C (PKA and PKC) modulate the gating of the channel by cell swelling [
5]. In the same cellular model (neurons), plasma membrane localization of TRPV4 is also modulated by the cytoskeletal protein kinase C and casein kinase substrate (PACSIN 3) [
6], the ubiquitin ligase AIP4 [
7], the ubiquitously expressed endoplasmic reticulum-associated protein OS-9 [
8], the stromal interaction molecule 1 (STIM1) [
9], or the phosphatidylinositol 3-kinase (PI3K) [
10]. Moreover, several studies show a modulatory role of hypoxia on TRPV4 channel function, which occurs, not only in a variety of chronic lung diseases [
11], but also in other organ systems and related diseases [
12]. Indeed, TRPV4 activity is increased by hypoxic stimuli in rat pulmonary arterial smooth muscle cells [
13,
14,
15], human umbilical vascular endothelial cells [
16], rat hippocampal astrocytes [
17], human SH-SY5Y neuroblastoma cell line [
18], rat heart tissue-derived H9C2 cardiac myoblast cell line, and neonatal rat ventricle myocytes [
19].
Whereas TRPV4 is obviously mechanosensitive [
20], there is additional evidence that parameters such as confluence for adherent cells affect whole-cell mechanical behaviour, e.g., confluent fibroblastic cells were 50% stiffer than non-confluent ones [
21]. Therefore, culture conditions under which the cell seeding is performed may modulate the functionality of the channel. Thus, in the present study, we investigated the influence of cell density on the effect of hypoxia on TRPV4 activity. We sought to compare TRPV4-induced cytosolic Ca
2+ responses (Ca
2+ imaging and microspectrofluorimetric assay) and single channel activity (patch-clamp technique in cell-attached configuration) in transiently TRPV4-transfected HEK293T cells seeded at low (non-confluent cells) and high (confluent cells) densities, and cultured under in vitro normoxia (21% O
2) or hypoxia (1% O
2) for 48 h. Note that in this study, the terms “normoxia” and “hypoxia” are used to distinguish between the various levels of oxygenation in the incubator. Thus, “normoxia” corresponds to atmospheric oxygen pressure, the commonly used oxygen pressure for cell cultures, but not to “tissue normoxia” also called “physioxia” which is characteristic of each tissue [
22].
Direct Ca
2+ influx through the channel was measured using an intramolecular Bioluminescence Resonance Energy Transfer (BRET) probe in which the TRPV4 channel was fused to a BRET sensor with high affinity for Ca
2+ (Calflux-VTN) [
23]. TRPV4 plasma membrane translocation was studied using confocal microscopy, cell surface biotinylation of proteins, and bystander BRET in which TRPV4 channel membrane density was monitored [
24]. Our results show that hypoxia exposure has a differential effect on TRPV4 activation depending on cell confluence. At low confluence levels, hypoxia increases TRPV4 response, whereas at high confluence levels, TRPV4 response is strongly inhibited, due to channel internalization. Thus, cell density appears to be a crucial parameter for TRPV4 channel activity.
2. Materials and Methods
2.1. Cell Culture
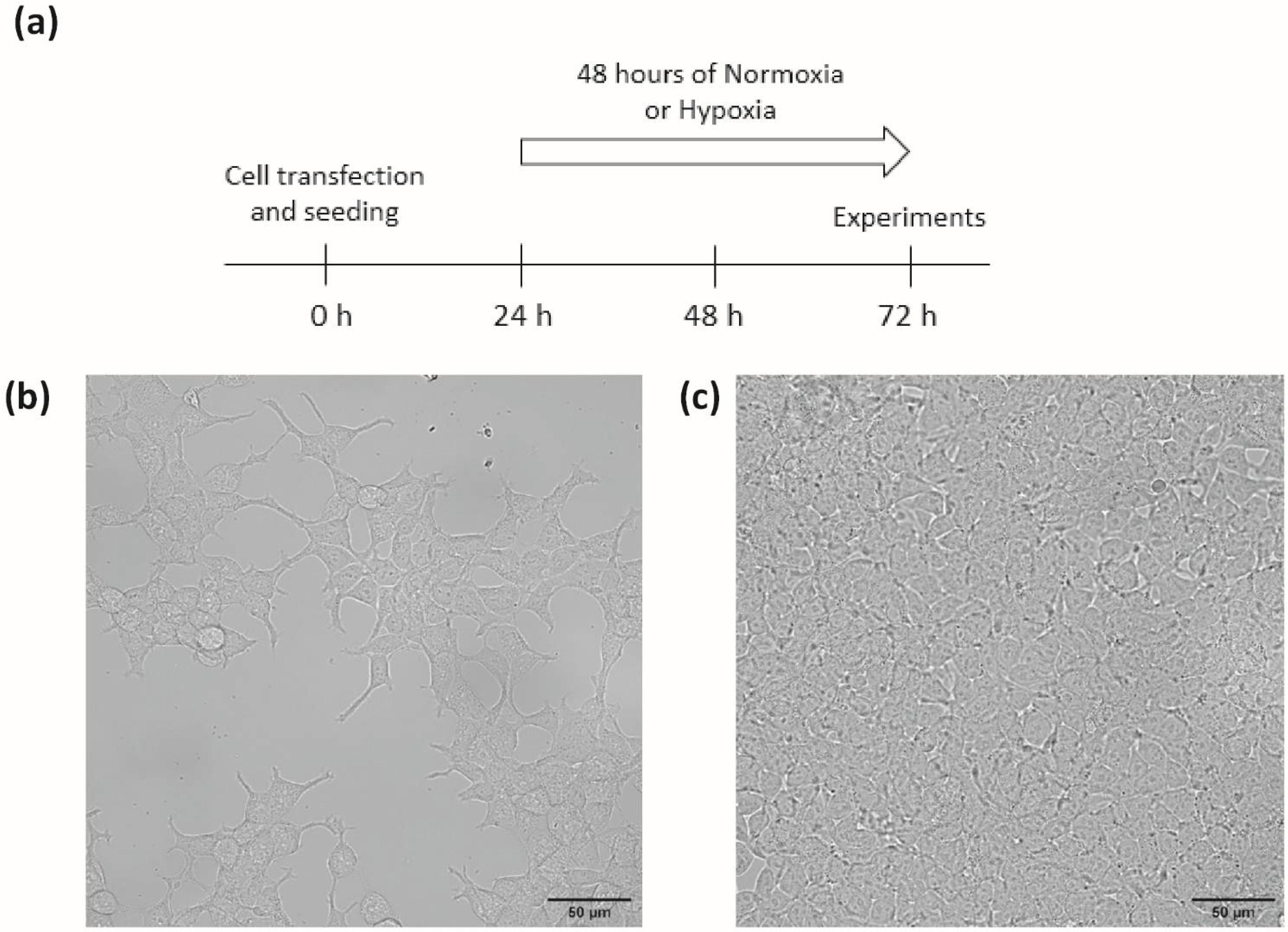
Transiently transfected HEK293T were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 1% penicillin-streptomycin, 1% nonessential amino acids, and 10% foetal bovine serum (FBS). In order to test the influence of cell density, cells were seeded on adequate support, either at a density of 1 × 10
4 cells/cm
2, referred to as the low-density (LD) condition, or at a density of 3 × 10
5 cells/cm
2, referred to as the high-density (HD) condition, corresponding to non-confluent or confluent cells, respectively, on the day of experiments, after 72 h of culture: 24 h of transfection plus 48 h of normoxia or hypoxia (
Figure 1). Cells were maintained at 37 °C in a humidified atmosphere gassed with 5% CO
2. HEK293T from control group (“normoxia”) were exposed to a gas mixture containing 21% O
2, 74% N
2, and 5% CO
2. Cells from the hypoxia group were exposed to a gas mixture containing 1% O
2, 94% N
2, and 5% CO
2 (“hypoxia”) for 48 h in a tri-gas incubator (Heracell 150i, ThermoScientific, Saint Herblain, France).
2.2. Plasmids and Transfection
To generate the BRET constructs, mNeonGreen (mNeonG) and nanoluciferase (nLuc) were mainly used to improve the brightness of the assay and are referred to as mNeonG and nLuc in the rest of this paper. For confocal microscopy, TRPV4-YFP and non-tagged TRPV4 were used in biotinylation and calcium assays, respectively in order to keep native structure and avoid signal interference of the fluorophore with the Fura emitting wavelength during the calcium test.
Mammalian expression vectors for the mNeonG-TRPV4-nLuc has been previously described in [
25]. The mammalian expression vector encoding the mNeonG-Calflux-nLuc-TRPV4 fusion protein was constructed using cDNA “bricks”, obtained by gene synthesis (Genescript, Leiden, The Netherlands), that contains the cDNA sequences for mNeonG (bricks 1.1), the calflux calcium ion sensor (Calflux, brick 1.2), nLuc (brick 1.3) and TRPV4 ion channel (brick 2 and 3) as indicated in
Supplementary Figure S1. The corresponding sequences are given in
Supplementary Table S1.
A similar strategy was used to construct TRPV4-YFP, TRPV4-nLuc, and non-tagged TRPV4 with cDNA bricks that encode empty cDNA bricks (brick 1), to rebuild the Kozak sequence before ATG and TRPV4 ion channel (bricks 2 and 3) and YFP, nLuc, or Stop (brick 4) (
Supplementary Figure S1 and Supplementary Table S1).
To construct vectors encoding mNeonG-CAAX fusion proteins, in order to evaluate TRPV4 endocytosis by measuring the decrease in the non-specific BRET (bystander BRET), we used cDNA bricks containing mNeonGreen (brick 1), and CAAX (brick 2). All brick sequences are given in
Supplementary Table S1 and were provided as cDNA cloned into a pUC-Kana vector devoid of BsmBI restriction sites (Genescript).
Using the BsmB1 type IIS enzyme and T4 DNA ligase, the cDNA bricks were assembled in frame and in the right order into pcDNA3.1(+)-Lac Z vector, which allowed direct visualization of the assembly efficiency using a colorimetric test based on alpha complementation. Briefly, a ligation mix containing 2 µL of T4 DNA ligase buffer 10X, 1 U of BsmBI enzyme, 1 U of T4 DNA ligase, 0.4 mM ATP, 4 mM dithiothreitol, 200 ng of pcDNA3.1(+)-Lac Z vector, and 100 ng of each brick needed to obtain the cited final construct. Then the mix was subjected to 35–40 cycles alternating two steps (step 1: 37 °C for 1 min, step 2: 16 °C for 1 min) before the enzymes were inactivated for 5 min at 55 °C. The ligation mix was then used to transform Escherichia coli DH5α by thermal shock to amplify the plasmids.
The day of transfection, cells were trypsinized and transient transfections were performed on suspended cells using polyethylenimine (PEI, linear, Mr 25,000; Polysciences, Warrington, PA, USA) with a PEI/DNA ratio of 4:1, as already described in [
26]. For patch-clamp and Ca
2+ assays, TRPV4 WT or other BRET probes were transfected with 0.1 µg of the corresponding expression vector and 1.9 µg of empty pcDNA3.1(+) vector for a 6-well plate equivalent. For confocal imaging, TRPV4-YFP was transfected with 0.25 µg of the corresponding expression vector and 1.75 µg of empty pcDNA3.1(+) vector for a 6-well plate equivalent. When intramolecular BRET assays were performed using Calflux BRET sensor N-terminally fused to TRPV4, HEK293T cells were transfected with 0.01 µg of the corresponding expression vector and 2 µg of empty pcDNA3.1(+) vector. Alternatively, HEK293T cells were transfected with 0.01 µg of TRPV4-nLuc expression vector and 2 µg of mNeonG-CAAX expression vector for intermolecular BRET assays. Resuspended cells were seeded on adequate support (glass coverslips, Petri dishes, or 96-well plates) at low or high densities.
2.3. Cytoplasmic Ca2+ Measurements
Variations in [Ca2+]c were determined using the Fura-2-LR/AM and Fluo-4/AM dyes. Briefly, for single cell measurements, cells seeded on glass coverslips were incubated with 5 µM Fura-2-LR/AM in Krebs-HEPES solution (see composition below) at 37 °C for 30 min, then washed and maintained in the same saline solution before fluorescence measurements. Cells were placed in a chamber of an inverted epifluorescence microscope (Nikon, Eclipse Ti2-U) equipped with a Nikon Apo Plan x40/1.3 NA oil immersion objective. Digital images were sampled at 16-bit resolution by a fast-scan digital camera (Hamamatsu, ORCA-Flash4.0 LT+). Fura-2-LR was alternately excited at 340 and 380 nm using a pE-340 Fura illuminator (CoolLED). The ratio R340/380 of the fluorescence intensity emitted at 510 nm at the two excitation wavelengths was calculated. Regions of interest were drawn around each cell to detect cellular ratio variations. The amplitude of the calcium rise was determined as the maximum ΔR340/380 reached during the recording time, i.e., between 30 and 420 s (corresponding to time after GSK1016790A application), depending on the cells. Imaging was controlled by NIS-Elements AR 5.3 imaging software (Nikon, Champigny sur Marne, France). All the images were background subtracted. These measurements allowed determining the percentage of responding cells, and then, their selection for analyses.
For cell population measurements, cells seeded in 96-well plates were incubated with 2 µM Fluo-4/AM in Krebs-HEPES solution at 37 °C for 30 min, then washed and maintained in the same saline solution before the fluorescence measurements. The Fluo-4/AM fluorescence intensity was measured at 485/520 nm (excitation/emission) using a microplate spectrophotometer reader (SPECTROstarNano2.10, BMG Labtech, Champigny sur Marne, France), in the same conditions as BRET measurements. Δ(F/F0) represents the difference between F/F0 and the basal F/F0 measured without GSK.
When mentioned, the TRPV4 inhibitor, HC067047, was added at 1 µM final concentration to the extracellular solution 30 min before the ejection of TRPV4 agonist, GSK1016790A, in the bathing solution at concentrations mentioned in the text.
2.4. Electrophysiological Recordings
Single-channel activity was recorded from cell-attached patches using the technique described by Hamill et al. [
27]. The electrodes were pulled on a DMZ-Universal puller (Zeitz Instruments) in two stages from borosilicate glass capillaries (1.5 mm OD, 1.16 mm ID, Harvard Apparatus). The pipettes had a mean resistance of 5–7 MΩ when measured in standard recording conditions. Cells seeded on Petri dishes were viewed under phase contrast with a Nikon Diaphot inverted microscope. A RK 400 patch amplifier (Biologic) was used for cell-attached recordings. Channel activity was recorded over 60 s (one series of 60 sweeps of 1 s). Stimulus control, data acquisition, and processing were carried out on a computer fitted with a Digidata 1550 interface, using Clampex v11 software (Molecular Devices, Foster City, CA, USA). Current records were filtered with a HumSilencer noise eliminator (Molecular Devices). Data were analysed using Clampfit v11 software (Molecular Devices). GSK1016790A was applied to the recorded cell via the recording pipette in absence or presence of HC067047 in the pipette solution and bathing solution.
2.5. Confocal Imaging
TRPV4-YFP and mNeonG-CAAX transfected HEK293T seeded in 96-well plates were observed as “living cells” under a Nikon D-Eclipse C1 confocal scanning microscope using a Nikon Apo Plan x60/1.4 NA oil immersion objective (excitation wavelength 488 nm; emitted fluorescence recorded at 500–530 nm).
2.6. Cell Surface Biotinylation
Cells, seeded on 6-well plates coated with polylysine (Sigma, Saint Quentin Fallavier, France), were detached with accutase (400–600 units/mL from Sigma) and resuspended after centrifugation in ice-cold phosphate-buffered saline (PBS) supplemented with 2 mM CaCl2 and 2 mM MgCl2. Centrifugations were performed between each step to pellet cells. They were labelled for 30 min at 4 °C with membrane-impermeable EZ-link NHS-SS-biotin (0.5 mg/mL, Pierce, 21331) to biotinylate surface proteins. Afterward, the unconjugated biotin was quenched for 20 min at 4 °C with 50 mM Tris·HCl in PBS, and the cells were lysed in RIPA lysis buffer system supplemented with protease inhibitor cocktail (1:100), sodium orthovanadate (1:100), and phenylmethanesulfonyl fluoride (PMSF) (1:100) (Santa Cruz Biotechnology, Sc-24948) for 30 min at 4 °C. Insoluble materials were then removed by centrifugation at 15,000× g for 10 min at 4 °C, and the amount of protein was assessed by the Lowry method (Bio-Rad, 5000111, Marnes-la-Coquette, France). Equal amounts of proteins were incubated with streptavidin-agarose beads (Pierce, 20353, Rockford, IL, USA) at 4 °C overnight. The biotinylated proteins were then eluted by boiling for 10 min at 95 °C in Laemmli sample buffer (Bio-Rad, 1610747) supplemented with β-mercaptoethanol. Cell lysates of 30 μg total or of 350 μg biotinylated proteins were used for Western blot analysis.
Proteins were loaded onto a 4–20% acrylamide gel and resolved by electrophoresis. The proteins were then transferred onto nitrocellulose membranes using the Trans-Blot Turbo Transfer System. After saturation for 1 h using 0.1% TBS-Tween and 5% nonfat milk, membranes were incubated overnight at 4 °C with the primary antibody anti-TRPV4, (AB191580, 1:1000, Abcam, Paris, France). The membranes were then treated with the corresponding horseradish peroxidase-linked secondary antibodies (Pl1000, 1:10,000, Vector) for 1 h at room temperature. Membranes were processed for chemiluminescent detection using horseradish peroxidase substrate (Millipore, Molsheim, France) according to the manufacturer’s instructions. Immunoblots were then revealed and acquired using a Bio-Rad ChemiDoc XRS+ System. Images were quantified using Image Laboratory software (Bio-Rad). Equal protein loading was verified using the stain-free method.
2.7. BRET Measurements
2.7.1. Filter-Based BRET Assays
BRET signals emitted by the total cell population in each well of 96-well plates were measured using a multidetector TriStar2 LB942 microplate reader (Berthold Technologies, Bad Wildbad, Germany) with emission filters centred at 460 ± 20 nm for nLuc (energy donor) and 510 ± 40 nm for mNeonG (energy acceptor). The BRET signal was determined by calculating the ratio of the emission intensity measured in the acceptor window (Iacceptor) over the emission intensity measured in the donor window (Idonor), according to “BRET” = Iacceptor/Idonor.
Due to the overlapping emission spectra of nLuc and mNeonG, a fraction of the light detected in the mNeonG filter originates from the nLuc emission, resulting in a contaminating signal [
28]. In that configuration, the net BRET was therefore defined as the BRET ratio of cells co-expressing nLuc and mNeonG constructs minus the BRET ratio of cells expressing only the nLuc construct in the same experiment.
To assess the functionality of TRPV4 channel BRET-based probes, Furimazine, the substrate for BRET reaction, was added (diluted at 1/500 in the well) and incubated for 30 min before TRPV4 agonist was injected in the presence or absence of TRPV4 inhibitor. BRET signals were measured in DMEM w/o red phenol, 5 min after the addition of GSK1016790A, a TRPV4 agonist.
2.7.2. Spectral BRET Assays
Full BRET spectra were acquired using an optical fibre linked to a spectrometer (IsoPlane SCT320, TELEDYNE, Lisse, France) equipped with a liquid-nitrogen-cooled charge-coupled device camera for recording the full visible spectrum (BLAZE 400, TELEDYNE). The bioluminescent signal was recorded from transfected cells seeded on glass coverslips and placed into an opaque home-made measurement chamber. Using the LabView programming language (National Instruments), an interface was developed to acquire the bioluminescent spectra and perform real-time spectral decomposition of the BRET signal into its various components, as described in [
29].
2.8. Recording Solutions
The standard Krebs-HEPES extracellular solution contained (in mM): 118.4 NaCl, 4.7 KCl, 2 CaCl2, 1.2 MgSO4, 4 NaHCO3, 1.2 KH2PO4, 6 D-glucose, and 10 N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES). The osmolality (measured with a cryoosmometer type 15 Löser/Fisher Scientific, Illkirch, France) of the external salt solution was adjusted to 310 mosm/kg with mannitol, and pH adjusted to 7.4 with NaOH.
For the cell-attached patch clamp recording, the bathing solution had the following composition (in mM): 140 KCl, 2.2 CaCl2, 1.2 MgCl2, 14 D-glucose, and 10 HEPES (osmolality: 310 mosm/kg; pH = 7.4, adjusted with KOH). The recording pipette was filled with a solution containing (in mM): 140 NaCl, 2.2 CaCl2, 1.2 MgCl2, 14 D-glucose, 10 HEPES, and 10 tetraethylammonium chloride (TEA), (osmolality: 310 mosm/kg; pH = 7.4).
2.9. Reagents
General salts were from VWR. All other chemicals were purchased from Sigma, except FBS (Eurobio, Les Ulis, France), and furimazine which was obtained from Promega. GSK1016790A and HC067047 were dissolved in DMSO and diluted 1:2000 in the bath to the final mentioned concentrations. At this dilution, the solvent (DMSO) alone had no effect on channel activity.
2.10. Data and Statistical Analysis
Results are expressed as mean ± standard deviation (SD). The n number of responsive cells (
Figure 2 and
Figure 3) is indicated in each bar graph figure. A Mann–Whitney test was used to determine statistical significance between two groups. A Kruskal–Wallis followed by Dunn test was used to compare multiple groups. Comparison of cell response proportions was performed with a chi-squared test. Differences with
p < 0.05 were considered significant. Statistical analysis was performed with the Prism 5 software (GraphPad, La Jolla, CA, USA).
4. Discussion
In previous studies, in PASMC from chronically hypoxic rats naturally expressing TRPV4, we and others have already described that in vivo chronic hypoxia increases TRPV4 channel activity [
13,
14]. We have also shown that culturing PASMC from normoxic rats under in vitro hypoxia (1% O
2 for 48 h) produces the same effects on TRPV4, increasing its Ca
2+ response and channel activity [
15]. In another article, the effect of hypoxia on TRPV4 was also assessed on cells without native expression of the channel [
18]. In that study, TRPV4-dependent Ca
2+ influx was reported to be increased in TRPV4-transfected HEK293T cells (cultured at 4 × 10
4 cells/cm
2, corresponding to LD in our study) treated with cobalt chloride, an experimental condition known to mimic hypoxia [
18]. In the present study, we have shown that TRPV4-transfected HEK293T cells cultured at LD under hypoxia (1% O
2 for 48 h) display higher TRPV4 responses to GSK stimulation.
Indeed, both Ca
2+ responses and TRPV4 channel activity were increased in the hypoxic condition, confirming the effect of hypoxia on TRPV4 in vitro. How TRPV4 is modulated by hypoxia is still unclear, but there is evidence suggesting a specific effect of hypoxia on binding sites of the TRPV1 channel, affecting its conformation [
32]. Thus, since TRPV channel members share high sequence identity (for example TRPV1 and TRPV4 share 45% sequence identity) [
33], TRPV4 may also be modulated by hypoxia through conformational changes via a specific effect of hypoxia on amino acid residues. These changes could arise from post-translational modifications such as hydroxylation. Indeed, it was previously shown that TRPV3, another member of the TRPV family, was hydroxylated by FIH (factor inhibiting hypoxia inducible factors) on asparagine 242 in intracellular ankyrin repeat domains [
34]. Hypoxia, inhibitors of FIH or mutation on asparagine 242 resulted in potentiation of TRPV3-mediated currents suggesting that oxygen-dependent hydroxylation can modulate ion channel activity [
34].
In addition, our study reveals an impact of cell density on TRPV4 channel activity and Ca
2+ response modulation by hypoxia. Our results show that hypoxia had a differential effect in TRPV4-transfected HEK293T cells cultured at either LD or HD. Surprisingly, agonist-induced Ca
2+ responses and channel activity were decreased in hypoxic cells at HD. Several studies previously reported that cell density affects various physiological processes. Accordingly, it was found that confluent osteoblastic cells had a higher basal Ca
2+ level than non-confluent ones and that Ca
2+ responses to ionomycin, thapsigargine, ATP, and caffeine were reduced at high density [
35]. This decrease was dependent on the formation of cell junctions, diminution of the inositol 1,4,5-trisphosphate (IP
3) receptor and smooth-surfaced endoplasmic reticulum expression [
35]. Additionally, in rat aortic smooth muscle cells, there is evidence that cell confluence affects β-adrenergic receptor signalling. Indeed, at HD, isoprenaline response involved both β1- and β2-adrenergic receptors, whereas at LD, the response only involved β2-adrenergic receptor and the 3′,5′-cyclic adenosine monophosphate (cAMP) rise was reduced in comparison with cells grown at HD [
36]. In smooth muscle cells, the modification of this signalling pathway could alter various physiological responses such as contraction, migration or proliferation. Modulation of β-adrenergic receptor signalling by cell confluence was reported in HEK293 cells stably overexpressing β2-adrenergic receptor. According to cell density, the transduction signalling pathway selectivity of cells’ β2-adrenergic receptor ligands was different. As a consequence, some ligands induced an increase in intracellular cAMP without activating extracellular signal-regulated kinase (ERK) only in the HD condition, whereas at LD, ERK was phosphorylated following cAMP increase [
37].
An important feature of cell density that can explain the modulation of cell responses is the modification of the tension in the plasma membrane with confluence inducing a change in cell mechanical behaviour. Indeed, it was shown that the rigidity of osteoblastic and fibroblastic cells was altered with culture conditions [
21]. Confluent cells were stiffer than non-confluent ones and formed aligned stress fibres in response to mechanical stress, affecting cytoskeleton microstructure. Moreover, the increased formation of cell–cell junctions in confluent cells also increased the rigidity of the cytoskeleton [
21]. Moreover, hypoxia was shown to induce cytoskeleton reorganization in PASMC where actin filament and intermediate filament networks were more abundant [
15]. Since TRPV4 directly interacts with the cytoskeleton [
38], cell contacts can impair cytoskeleton organization and reorganization in response to hypoxia, and consequently TRPV4 trafficking or conformational change. Indeed, as reported, confluence increased claudin-2 expression, a component of tight junctions, controlled by zonula occludens-1 and -2 and Rac [
39]. These cell contacts also inhibited proliferation through the reduction of the formation of F-stress fibres induced by inhibition of YAP/TAZ (co-transcriptional regulators) signalling that impaired autophagosome formation [
40]. This study suggests that modifications of cell confluence and matrix stiffness regulate YAP/TAZ activity, actin dynamics, and autophagy that are involved especially in proliferation and sensitivity to hypoxia via altered Hippo signalling. This pathway is regulated by AMP-activated protein kinase and mechanistic target of rapamycin (mTOR), determining cell proliferation [
40,
41]. The Hippo pathway is also known to be affected by hypoxia since reactive oxygen species (ROS) production induces transcriptional changes through the MST/YAP/FoxO pathway and leads to the activation of pro-apoptotic genes [
41]. The fact that both cell density and matrix stiffness, as well as hypoxia, can affect cell signalling corroborates the previous assumption that TRPV4, as a mechanosensitive channel, may be particularly affected by these parameters.
Thus, the loss of TRPV4 response under hypoxia at HD could be explained by a relocation of the channel. Indeed, our present results suggest TRPV4 internalization in these conditions. First, in confocal imaging, TRPV4 was expressed in the vicinity of the plasma membrane at LD under both normoxia and hypoxia but only in normoxia for cells grown at HD. However, at HD under hypoxia, TRPV4 staining was diffuse in the cells suggesting a cytosolic expression. Moreover, cell surface biotinylation showed that TRPV4 was expressed at the plasma membrane at LD under both normoxia and hypoxia and in normoxia for cells grown at HD. But at HD under hypoxia, TRPV4 membrane addressing was reduced. To further analyse TRPV4 membrane expression, we built on a bystander BRET assay initially described to quantitatively monitor G protein-coupled receptors (GPCRs) and β-arrestin trafficking [
24]. We anticipated that such an assay could specifically assess TRPV4 endocytosis by measuring the decrease in the non-specific BRET (bystander BRET) occurring in the plasma membrane plane between TRPV4 fused to the bioluminescent donor nLuc (TRPV4-nLuc) and a fluorescent acceptor fused to a plasma membrane-anchoring peptide such as the CAAX peptide [
24]. As shown in
Figure 9, this assay confirmed the observation of a former study [
10] indicating that GSK triggers a rapid endocytosis of TRPV4, as shown by the progressive decrease in the BRET value between TRPV4-nLuc and mNeonG-CAAX following GSK challenging. Moreover, we could confirm that, in hypoxic cells grown at HD, TRPV4 expression at the plasma membrane was highly reduced and TRPV4 did not respond anymore to a further activation by GSK. This indicates that TRPV4 activation was impaired in hypoxia at HD due to an already internalized channel in this condition. The study from Baratchi et al. reported that TRPV4 translocated to the recycling endosomes after GSK stimulation and that the density of TRPV4 at the plasma membrane was controlled through both complete and partial vesicular fusion [
10]. It is noteworthy that shear stress stimulation can also modify TRPV4 trafficking in endothelial cells where TRPV4 co-localized with β-catenin, an adapter protein of the adherens junctions at the point of cell–cell contact, and relocalised after shear stress, losing their interaction with β-catenin [
42].
The endocytosis process is known to be mechanosensitive and can thus be regulated by membrane tension and rigidity in association with changes in membrane composition. It was shown that increased membrane tension and rigidity decrease global endocytosis [
43,
44] whereas hypoxia promotes endocytosis (e.g., of the Na
+/K
+-ATPase and alveolar epithelial sodium channel (ENaC) [
44].
Overall, the combination of high cell density and hypoxia might change cell rigidity and lipid membrane composition, modulating TRPV4 activation. Such modifications could affect TRPV4 localisation through impairment of TRPV4 addressing to membrane or internalization following desensitisation of the channel. Even though our data show that TRPV4 is clearly sensitive to cell density, it is highly probable that many other channels can also be affected by cell density. Indeed, mechanosensitivity is very important in many physiological processes. A wide range of channels (SAC, stretch-activated channels) act as mechanotransducers by converting physical forces into biological signals [
20]. Thus, the loss of TRPV4 expression to the membrane could be compensated by the action of other SAC. Indeed, such a compensatory mechanism was demonstrated for filamins in cultured podocytes exposed to mechanical stress where the knock-out of filamin A was compensated by the expression of filamin B [
45]. A similar effect was reported in smooth muscle cells in which an increase in TRPC3 expression was observed when TRPC6 was invalidated [
46].
Indeed, the present study shows how experimental conditions can be important parameters when evaluating the effect of hypoxia on TRPV4 channel, and more widely on ionic channels, considering the modification of their expression, trafficking and activity. This also raises awareness of the choice of models used in experimentation. Here, we compared cell confluence in a two dimensional (2D) cell culture model, but it would be interesting to study how TRPV4 responds to hypoxia in a three dimensional (3D) cell culture model, more relevant to physiological conditions, where cells undergo important mechanical tension since they have more cell-to-cell interactions and interact with the extracellular matrix [
47,
48]. Interestingly, in a 3D model, TRPV4 displayed a diffuse cytoplasmic localization in three types of cancer cells [
49], suggesting that TRPV4 localisation is very sensitive to mechanical forces. In addition, another study demonstrated that osteogenic differentiation is regulated by reciprocal feedback between cell volume expansion and TRPV4 activation in mesenchymal stem cells cultured in 3D matrices [
50]. These data support the idea that TRPV4 is not only sensitive to cell density in conventional 2D models but can also be affected by mechanical stress occurring in 3D models.
In conclusion, our data highlight the importance of a specific culture condition, namely cell confluence, which can influence many cellular processes. We show that HD alters TRPV4 addressing to the plasma membrane and, thus, GSK-induced Ca2+ responses in cells exposed to hypoxia. Accordingly, cell confluence should be taken into account when using cell culture to study various pathophysiological pathways, especially considering the differential impact of hypoxia on TRPV4 channel signalling. These results also raise awareness of the impact of techniques and methods of investigation on physiological cell responses.