Development of a Multi-Enzymatic Approach for the Modification of Biopolymers with Ferulic Acid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Pre-Treatment of Arabinoxylan
2.2.2. Enzymatic Treatment of Arabinoxylan
2.2.3. Determination of Enzymatic Activities
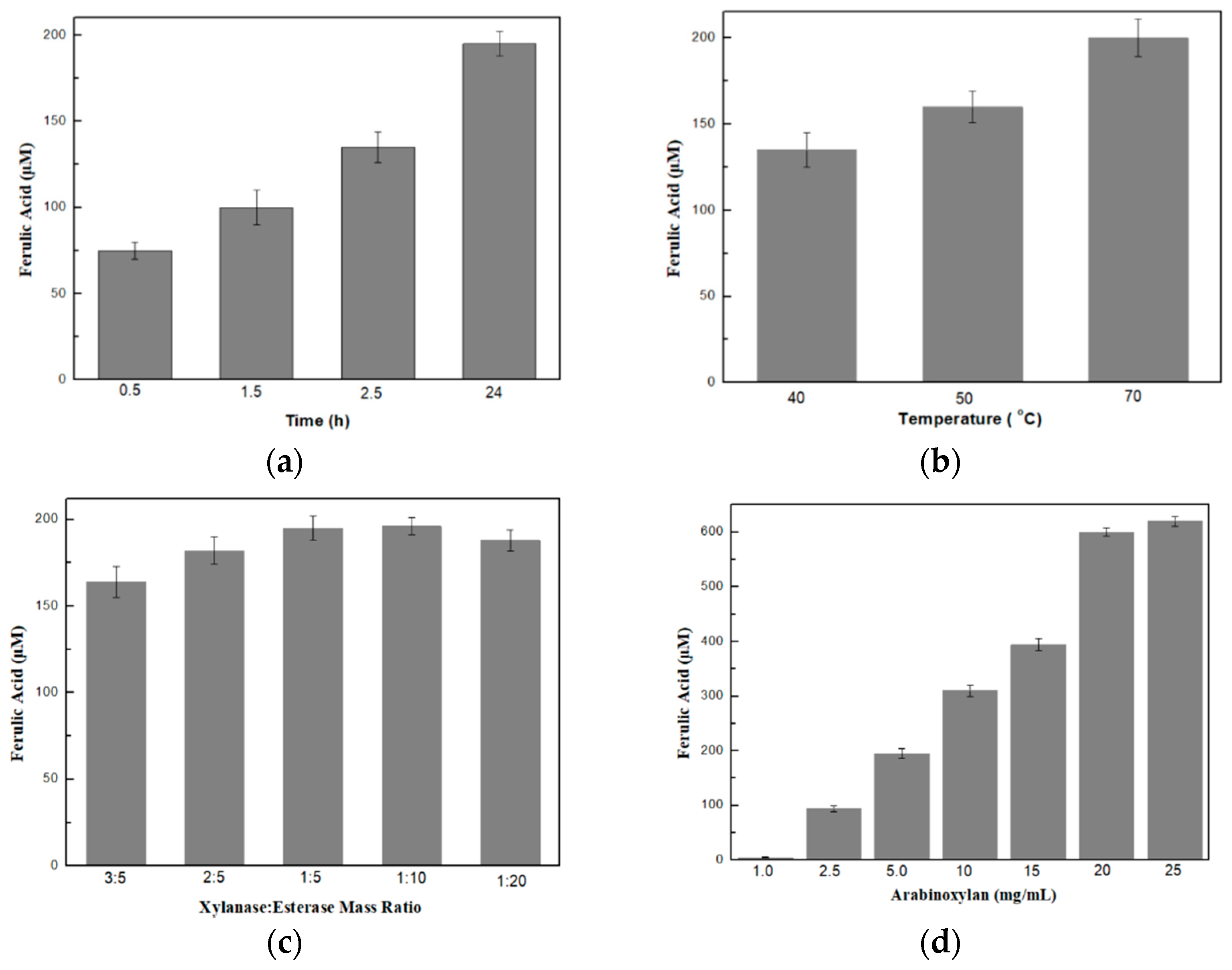
2.2.4. Optimization of the Production of Ferulic Acid
2.2.5. Synergy Studies
2.2.6. Purification of Chitosan and Preparation of the CS Solution
2.2.7. Preparation of the CS–GEL Solution
2.2.8. Preparation of the CMC Solution
2.2.9. Enzymatic Oxidation of FA and Its Grafting onto the CS, CS–GEL and CMC Films
2.2.10. Characterization of the Biopolymers and Their Enzymatically Functionalized Derivatives
Ultraviolet (UV)–Visible Spectroscopy
Attenuated Total Reflection (ATR) Spectroscopy
Scanning Electron Microscopy (SEM)
Nuclear Magnetic Resonance (NMR) Spectroscopy
2.2.11. Estimation of the Grafting Efficiency
2.2.12. Evaluation of the Antioxidant Activity of the Prepared Films and Their Derivatives
Antioxidant Activity According to the Oxidized ABTS Protocol
Antioxidant Activity According to the DPPH Protocol
2.2.13. Evaluation of the Antimicrobial Activity of the Prepared Films and Their Derivatives
3. Results and Discussion
3.1. Determination of the Enzymes’ Specific Activities
3.2. Optimization of the Bi-Enzymatic System on the Production of Ferulic Acid
3.3. Synergy Studies
3.4. Preparation of Biopolymer Films
3.5. Enzymatic Oxidation of FA and Its Grafting onto the Biopolymers
3.6. Structural Characterization of the Biopolymers and Their Enzymatically Functionalized Derivatives
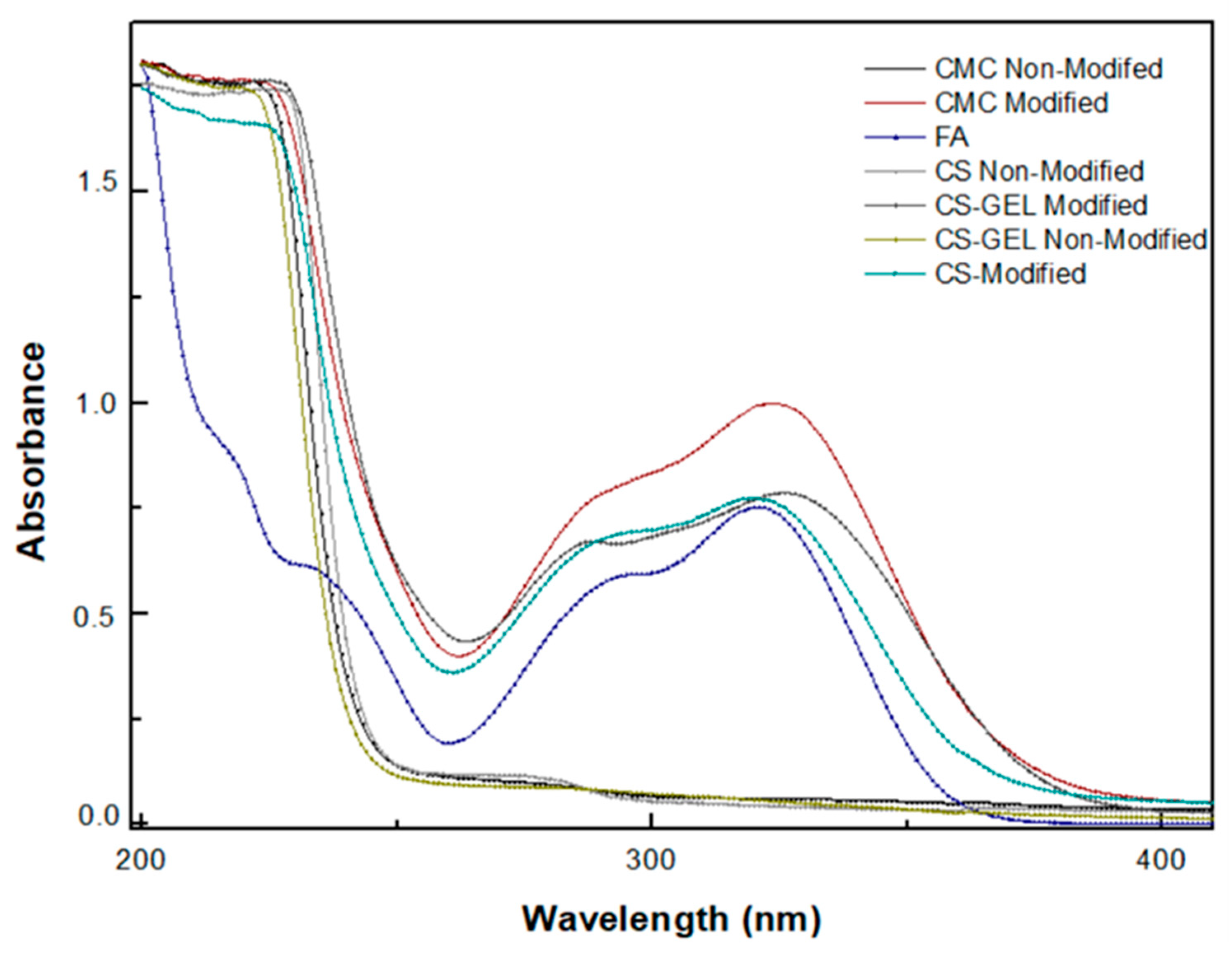
3.6.1. Ultraviolet (UV)–Visible Spectroscopy
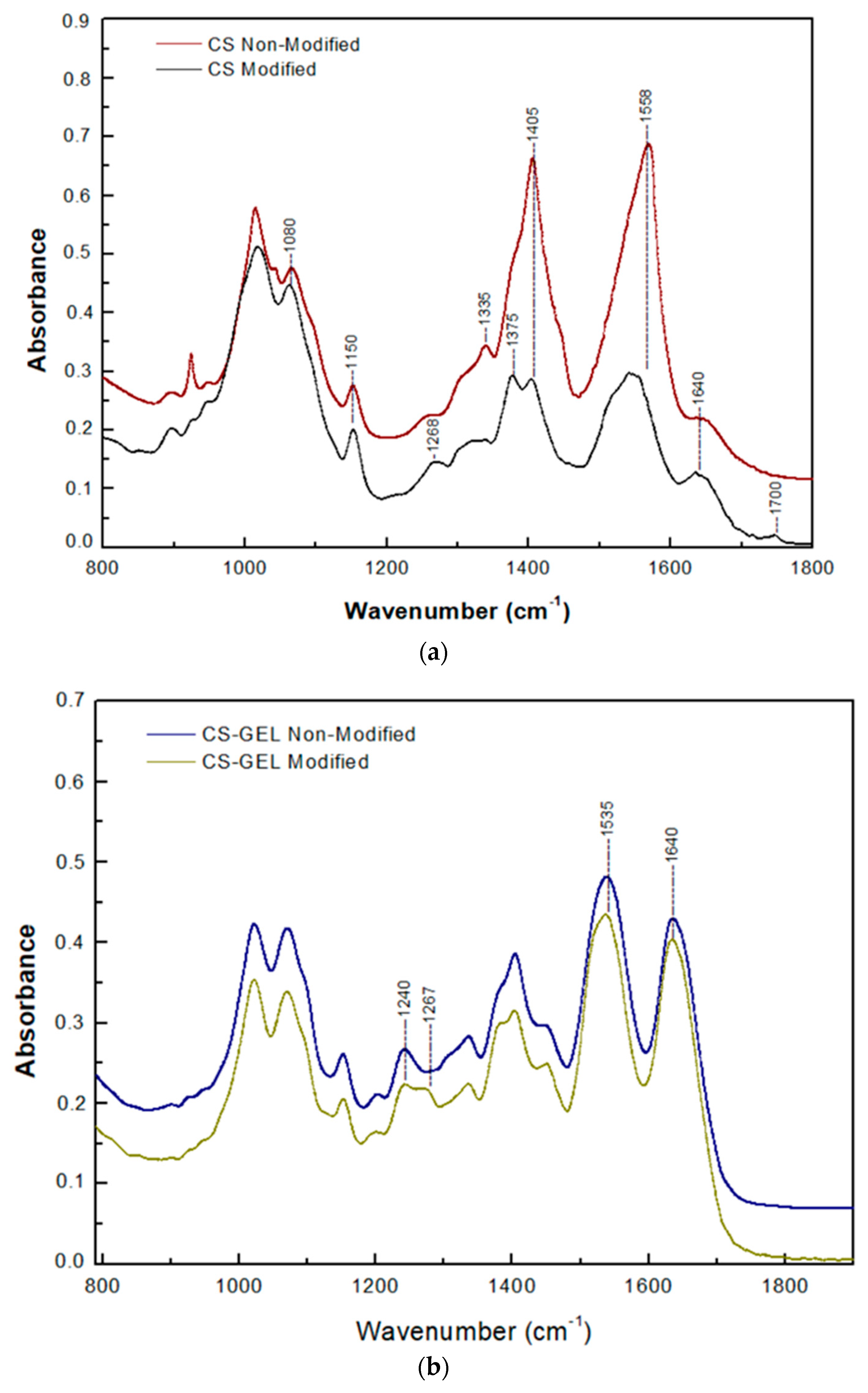
3.6.2. Attenuated Total Reflection (ATR) Spectroscopy
3.6.3. Scanning Electron Microscopy (SEM)
3.6.4. Nuclear Magnetic Resonance (NMR) Spectroscopy
3.7. Estimation of the Grafting Efficiency (Folin)
3.8. Evaluation of the Antioxidant Activity of the Biopolymers and Their Enzymatically Functionalized Derivatives
3.8.1. Antioxidant Activity According to the Oxidized ABTS Protocol
3.8.2. Antioxidant Activity According to the DPPH Protocol
3.9. Evaluation of the Antimicrobial Activity of the Biopolymers and Their Enzymatically Functionalized Derivatives
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Mkabayi, L.; Malgas, S.; Wilhelmi, B.S.; Pletschke, B.I. Evaluating Feruloyl Esterase-Xylanase Synergism for Hydroxycinnamic Acid and Xylo-Oligosaccharide Production from Untreated, Hydrothermally Pre-Treated and Dilute-Acid Pre-Treated Corn Cobs. Agronomy 2020, 10, 688. [Google Scholar] [CrossRef]
- Zhang, J.; Xia, W.; Liu, P.; Cheng, Q.; Tahirou, T.; Gu, W.; Li, B. Chitosan Modification and Pharmaceutical/Biomedical Applications. Mar. Drugs 2010, 8, 1962–1987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brasselet, C.; Pierre, G.; Dubessay, P.; Dols-Lafargue, M.; Coulon, J.; Maupeu, J.; Vallet-Courbin, A.; de Baynast, H.; Doco, T.; Michaud, P.; et al. Modification of Chitosan for the Generation of Functional Derivatives. Appl. Sci. 2019, 9, 1321. [Google Scholar] [CrossRef] [Green Version]
- Liyanage, S.; Acharya, S.; Parajuli, P.; Shamshina, J.L.; Abidi, N. Production and Surface Modification of Cellulose Bioproducts. Polymers 2021, 13, 3433. [Google Scholar] [CrossRef]
- Onwukamike, K.N.; Grelier, S.; Grau, E.; Cramail, H.; Meier, M.A.R. Critical Review on Sustainable Homogeneous Cellulose Modification: Why Renewability Is Not Enough. ACS Sustain. Chem. Eng. 2019, 7, 1826–1840. [Google Scholar] [CrossRef] [Green Version]
- Missoum, K.; Belgacem, M.N.; Bras, J. Nanofibrillated Cellulose Surface Modification: A Review. Materials 2013, 6, 1745–1766. [Google Scholar] [CrossRef] [Green Version]
- Gkantzou, E.; Chatzikonstantinou, A.V.; Fotiadou, R.; Giannakopoulou, A.; Patila, M.; Stamatis, H. Trends in the development of innovative nanobiocatalysts and their application in biocatalytic transformations. Biotechnol. Adv. 2021, 51, 107738. [Google Scholar] [CrossRef]
- Giannakopoulou, A.; Gkantzou, E.; Polydera, A.; Stamatis, H. Multienzymatic Nanoassemblies: Recent Progress and Applications. Trends Biotechnol. 2020, 38, 202–216. [Google Scholar] [CrossRef]
- Agrawal, R.; Semwal, S.; Kumar, R.; Mathur, A.; Gupta, R.P.; Tuli, D.K.; Satlewal, A. Synergistic Enzyme Cocktail to Enhance Hydrolysis of Steam Exploded Wheat Straw at Pilot Scale. Front. Energy Res. 2018, 6, 122. [Google Scholar] [CrossRef]
- Joseph, B.; Sagarika, V.K.; Sabu, C.; Kalarikkal, N.; Thomas, S. Cellulose nanocomposites: Fabrication and biomedical applications. J. Bioresour. Bioprod. 2020, 5, 223–237. [Google Scholar] [CrossRef]
- Yang, C.; Zhou, Y.; Zheng, Y.; Li, C.; Sheng, S.; Wang, J.; Wu, F. Enzymatic modification of chitosan by cinnamic acids: Antibacterial activity against Ralstonia solanacearum. Int. J. Biol. Macromol. 2016, 87, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Sun, J.; An, X.; Zheng, M.; Lu, Z.; Lu, F.; Zhang, C. Preparation of ferulic acid-grafted chitosan using recombinant bacterial laccase and its application in mango preservation. RSC Adv. 2018, 8, 6759–6767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.; Liu, Y.; Gaber, M.W.; Bumgardner, J.D.; Haggard, W.O.; Yang, Y. Development of chitosan-ellagic acid films as a local drug delivery system to induce apoptotic death of human melanoma cells. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 90, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, M.B.; Struszczyk-Swita, K.; Li, X.; Szczęsna-Antczak, M.; Daroch, M. Enzymatic Modifications of Chitin, Chitosan, and Chitooligosaccharides. Front. Bioeng. Biotechnol. 2019, 7, 243. [Google Scholar] [CrossRef] [Green Version]
- Aljawish, A.; Chevalot, I.; Piffaut, B.; Rondeau-Mouro, C.; Girardin, M.; Jasniewski, J.; Scher, J.; Muniglia, L. Functionalization of chitosan by laccase-catalyzed oxidation of ferulic acid and ethyl ferulate under heterogeneous reaction conditions. Carbohydr. Polym. 2012, 87, 537–544. [Google Scholar] [CrossRef]
- Hu, Q.; Luo, Y. Polyphenol-chitosan conjugates: Synthesis, characterization, and applications. Carbohydr. Polym. 2016, 151, 624–639. [Google Scholar] [CrossRef]
- Sousa, F.; Guebitz, G.M.; Kokol, V. Antimicrobial and antioxidant properties of chitosan enzymatically functionalized with flavonoids. Process Biochem. 2009, 44, 749–756. [Google Scholar] [CrossRef]
- Fras-Zemljič, L.; Kokol, V.; Čakara, D. Antimicrobial and antioxidant properties of chitosan-based viscose fibres enzymatically functionalized with flavonoids. Text. Res. J. 2011, 81, 1532–1540. [Google Scholar] [CrossRef]
- Morin, S.; Bockstal, L.; Jacquet, N.; Richel, A. One-step enzymatic grafting of ferulic acid with cellulose to enhance matrices–fibres compatibility in bio-composites. J. Mater. Sci. 2019, 54, 13314–13321. [Google Scholar] [CrossRef]
- Yadav, S.; Mehrotra, G.K.; Bhartiya, P.; Singh, A.; Dutta, P.K. Preparation, physicochemical and biological evaluation of quercetin based chitosan–gelatin film for food packaging. Carbohydr. Polym. 2020, 227, 115348. [Google Scholar] [CrossRef]
- Rocasalbas, G.; Francesko, A.; Touriño, S.; Fernández-Francos, X.; Guebitz, G.M.; Tzanov, T. Laccase-assisted formation of bioactive chitosan/gelatin hydrogel stabilized with plant polyphenols. Carbohydr. Polym. 2013, 92, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Underlin, E.N.; Frommhagen, M.; Dilokpimol, A.; van Erven, G.; de Vries, R.P.; Kabel, M.A. Feruloyl Esterases for Biorefineries: Subfamily Classified Specificity for Natural Substrates. Front. Bioeng. Biotechnol. 2020, 8, 332. [Google Scholar] [CrossRef]
- Nordberg Karlsson, E.; Schmitz, E.; Linares-Pastén, J.A.; Adlercreutz, P. Endo-xylanases as tools for production of substituted xylooligosaccharides with prebiotic properties. Appl. Microbiol. Biotechnol. 2018, 102, 9081–9088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lau, T.; Harbourne, N.; Oruña-Concha, M.J. Optimization of enzyme-assisted extraction of ferulic acid from sweet corn cob by response surface methodology. J. Sci. Food Agric. 2020, 100, 1479–1485. [Google Scholar] [CrossRef]
- Zduńska, K.; Dana, A.; Kolodziejczak, A.; Rotsztejn, H. Antioxidant Properties of Ferulic Acid and Its Possible Application. Skin Pharmacol. Physiol. 2018, 31, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Borges, A.; Ferreira, C.; Saavedra, M.J.; Simões, M. Antibacterial activity and mode of action of ferulic and gallic acids against pathogenic bacteria. Microb. Drug Resist. 2013, 19, 256–265. [Google Scholar] [CrossRef]
- Shi, C.; Zhang, X.; Sun, Y.; Yang, M.; Song, K.; Zheng, Z.; Chen, Y.; Liu, X.; Jia, Z.; Dong, R.; et al. Antimicrobial Activity of Ferulic Acid Against Cronobacter sakazakii and Possible Mechanism of Action. Foodborne Pathog. Dis. 2016, 13, 196–204. [Google Scholar] [CrossRef]
- Yin, Z.N.; Wu, W.J.; Sun, C.Z.; Liu, H.F.; Chen, W.B.; Zhan, Q.P.; Lei, Z.G.; Xin, X.; Ma, J.J.; Yao, K.; et al. Antioxidant and Anti-inflammatory Capacity of Ferulic Acid Released from Wheat Bran by Solid-state Fermentation of Aspergillus Niger. Biomed. Environ. Sci. 2019, 32, 11–21. [Google Scholar] [CrossRef]
- Bai, R.; Yu, Y.; Wang, Q.; Shen, J.; Yuan, J.; Fan, X. Laccase-Catalyzed polymerization of hydroquinone incorporated with chitosan oligosaccharide for enzymatic coloration of cotton. Appl. Biochem. Biotechnol. 2020, 191, 605–622. [Google Scholar] [CrossRef]
- Liu, N.; Ni, S.; Ragauskas, A.J.; Meng, X.; Hao, N.; Fu, Y. Laccase-mediated functionalization of chitosan with 4-hexyloxyphenol enhances antioxidant and hydrophobic properties of copolymer. J. Biotechnol. 2018, 269, 8–15. [Google Scholar] [CrossRef]
- Anghileri, A.; Lantto, R.; Kruus, K.; Arosio, C.; Freddi, G. Tyrosinase-catalyzed grafting of sericin peptides onto chitosan and production of protein-polysaccharide bioconjugates. J. Biotechnol. 2007, 127, 508–519. [Google Scholar] [CrossRef] [PubMed]
- Konieczna-Molenda, A.; Lai, V.M.F.; Fiedorowicz, M.; Khachatryan, G.; Tomasik, P. Polarized-light-stimulated enzymatic hydrolysis of xylan. Biotechnol. Prog. 2008, 24, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.L. Use of DinitrosaIicyIic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Chen, T.; Kumar, G.; Harris, M.T.; Smith, P.J.; Payne, G.F. Enzymatic grafting of hexyloxyphenol onto chitosan to alter surface and rheological Properties. Biotechnol. Bioeng. 2000, 70, 564–573. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant Determinations by the Use of a Stable Free Radical. Nature 1958, 181, 1199–2000. [Google Scholar] [CrossRef]
- Kumar, G.; Smith, P.J.; Payne, G.F. Enzymatic grafting of a natural product onto chitosan to confer water solubility under basic conditions. Biotechnol. Bioeng. 1999, 63, 154–165. [Google Scholar] [CrossRef]
- Payne, G.F.; Chaubal, M.V.; Barbari, T.A. Enzyme-catalysed polymer modification: Reaction of phenolic compounds with chitosan films. Polymer 1996, 37, 4643–4648. [Google Scholar] [CrossRef]
- Quideau, S.; Deffieux, D.; Pouységu, L. Oxidative Coupling of Phenols and Phenol Ethers. In Comprehensive Organic Synthesis, 2nd ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2014; Volume 3, pp. 656–740. ISBN 9780080977430. [Google Scholar]
- Kumirska, J.; Czerwicka, M.; Kaczyński, Z.; Bychowska, A.; Brzozowski, K.; Thöming, J.; Stepnowski, P. Application of spectroscopic methods for structural analysis of chitin and chitosan. Mar. Drugs 2010, 8, 1567–1636. [Google Scholar] [CrossRef] [Green Version]
- Božič, M.; Gorgieva, S.; Kokol, V. Homogeneous and heterogeneous methods for laccase-mediated functionalization of chitosan by tannic acid and quercetin. Carbohydr. Polym. 2012, 89, 854–864. [Google Scholar] [CrossRef]
- Akl, E.M.; Dacrory, S.; Abdel-Aziz, M.S.; Kamel, S.; Fahim, A.M. Preparation and characterization of novel antibacterial blended films based on modified carboxymethyl cellulose/phenolic compounds. Polym. Bull. 2021, 78, 1061–1085. [Google Scholar] [CrossRef]
- Aljawish, A.; Muniglia, L.; Klouj, A.; Jasniewski, J.; Scher, J.; Desobry, S. Characterization of films based on enzymatically modified chitosan derivatives with phenol compounds. Food Hydrocoll. 2016, 60, 551–558. [Google Scholar] [CrossRef]
- Aljawish, A.; Chevalot, I.; Jasniewski, J.; Revol-Junelles, A.M.; Scher, J.; Muniglia, L. Laccase-catalysed functionalisation of chitosan by ferulic acid and ethyl ferulate: Evaluation of physicochemical and biofunctional properties. Food Chem. 2014, 161, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, S.C.M.; Sadocco, P.; Alonso-Varona, A.; Palomares, T.; Eceiza, A.; Silvestre, A.J.D.; Mondragon, I.; Freire, C.S.R. Bioinspired antimicrobial and biocompatible bacterial cellulose membranes obtained by surface functionalization with aminoalkyl groups. ACS Appl. Mater. Interfaces 2013, 5, 3290–3297. [Google Scholar] [CrossRef] [PubMed]


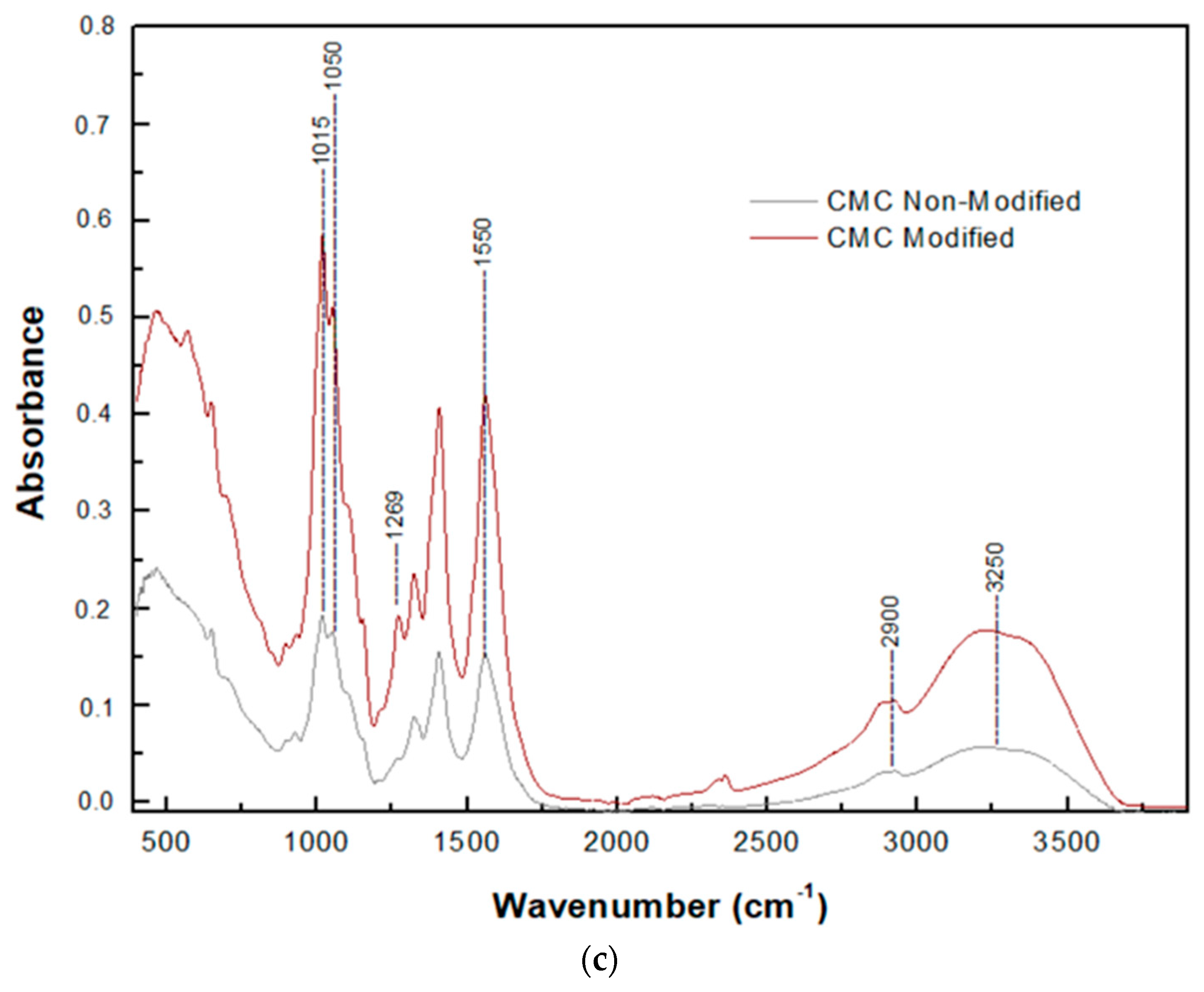

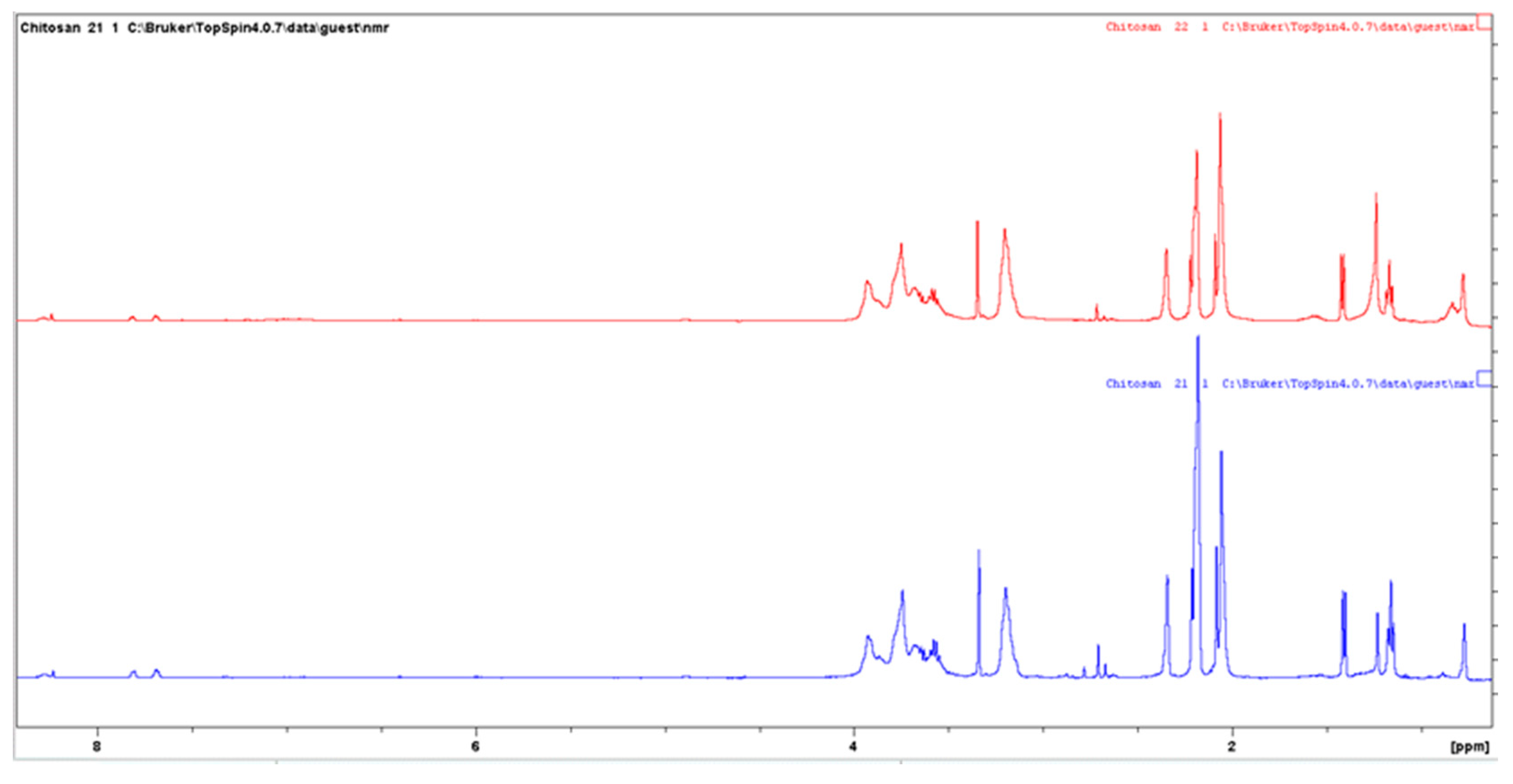

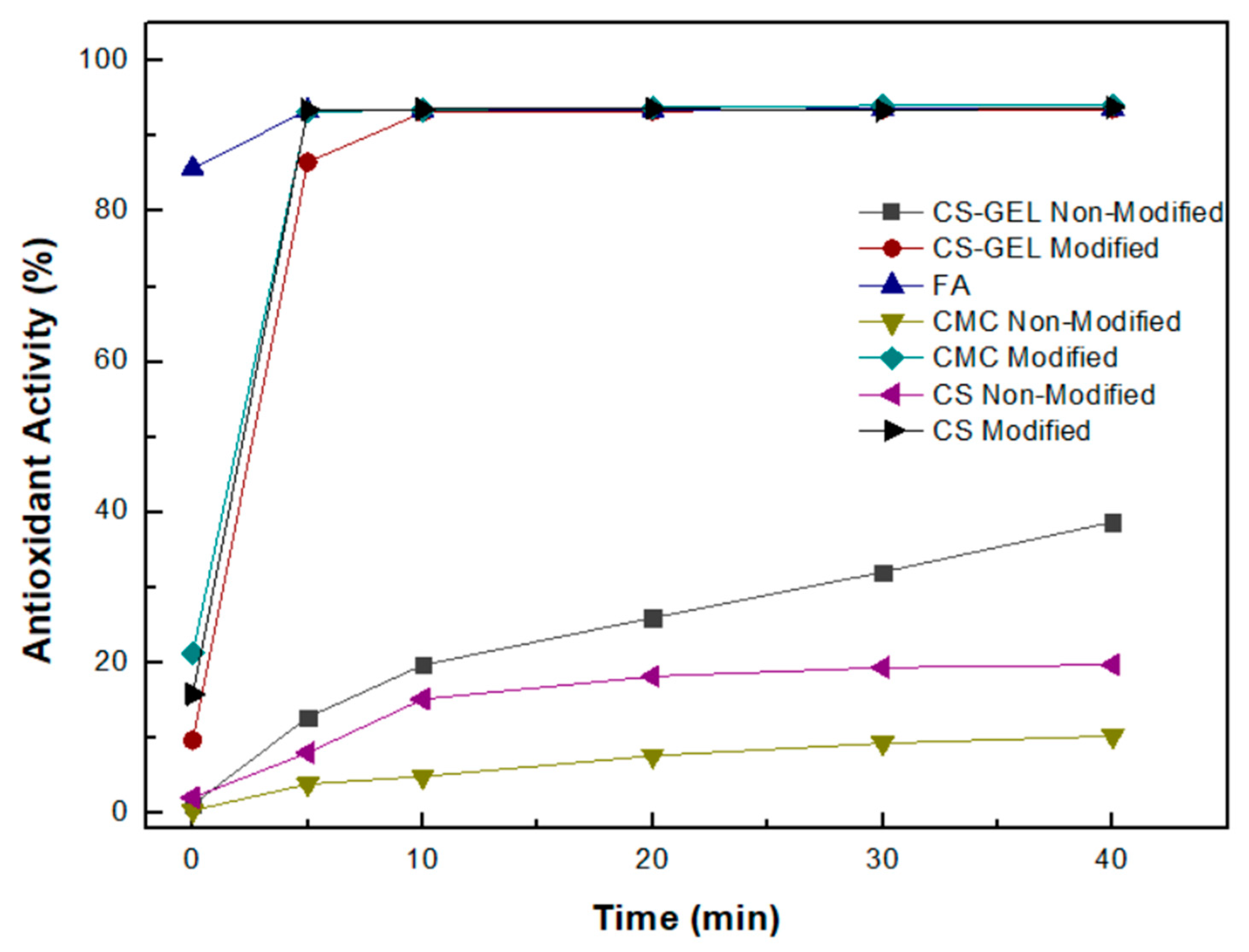
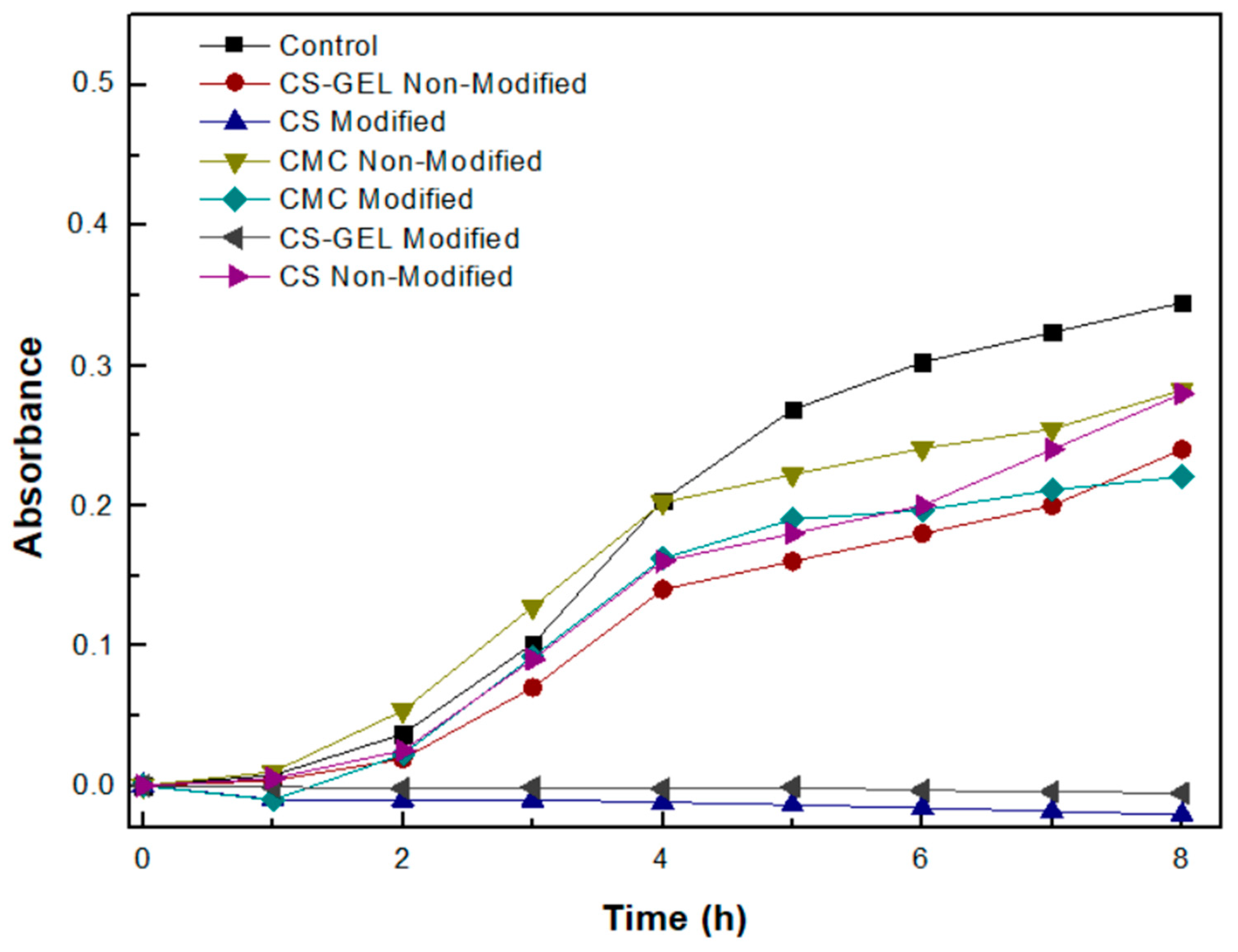
| Sample | Grafting Efficiency (%) |
|---|---|
| CS | 70 |
| CS–GEL | 95 |
| CMC | 55 |
| CMC Non-Modified | CMC Modified | CS Non-Modified | CS Modified | CS–GEL Non-Modified | CS–GEL Modified | |
|---|---|---|---|---|---|---|
| SC50 (mg/mL) | 75 | 10 | 70 | 35 | 35 | 20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giannakopoulou, A.; Tsapara, G.; Troganis, A.N.; Koralli, P.; Chochos, C.L.; Polydera, A.C.; Katapodis, P.; Barkoula, N.-M.; Stamatis, H. Development of a Multi-Enzymatic Approach for the Modification of Biopolymers with Ferulic Acid. Biomolecules 2022, 12, 992. https://doi.org/10.3390/biom12070992
Giannakopoulou A, Tsapara G, Troganis AN, Koralli P, Chochos CL, Polydera AC, Katapodis P, Barkoula N-M, Stamatis H. Development of a Multi-Enzymatic Approach for the Modification of Biopolymers with Ferulic Acid. Biomolecules. 2022; 12(7):992. https://doi.org/10.3390/biom12070992
Chicago/Turabian StyleGiannakopoulou, Archontoula, Georgia Tsapara, Anastassios N. Troganis, Panagiota Koralli, Christos L. Chochos, Angeliki C. Polydera, Petros Katapodis, Nektaria-Marianthi Barkoula, and Haralambos Stamatis. 2022. "Development of a Multi-Enzymatic Approach for the Modification of Biopolymers with Ferulic Acid" Biomolecules 12, no. 7: 992. https://doi.org/10.3390/biom12070992
APA StyleGiannakopoulou, A., Tsapara, G., Troganis, A. N., Koralli, P., Chochos, C. L., Polydera, A. C., Katapodis, P., Barkoula, N.-M., & Stamatis, H. (2022). Development of a Multi-Enzymatic Approach for the Modification of Biopolymers with Ferulic Acid. Biomolecules, 12(7), 992. https://doi.org/10.3390/biom12070992









_Stamatis.png)


