Association between Galectin Levels and Neurodegenerative Diseases: Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
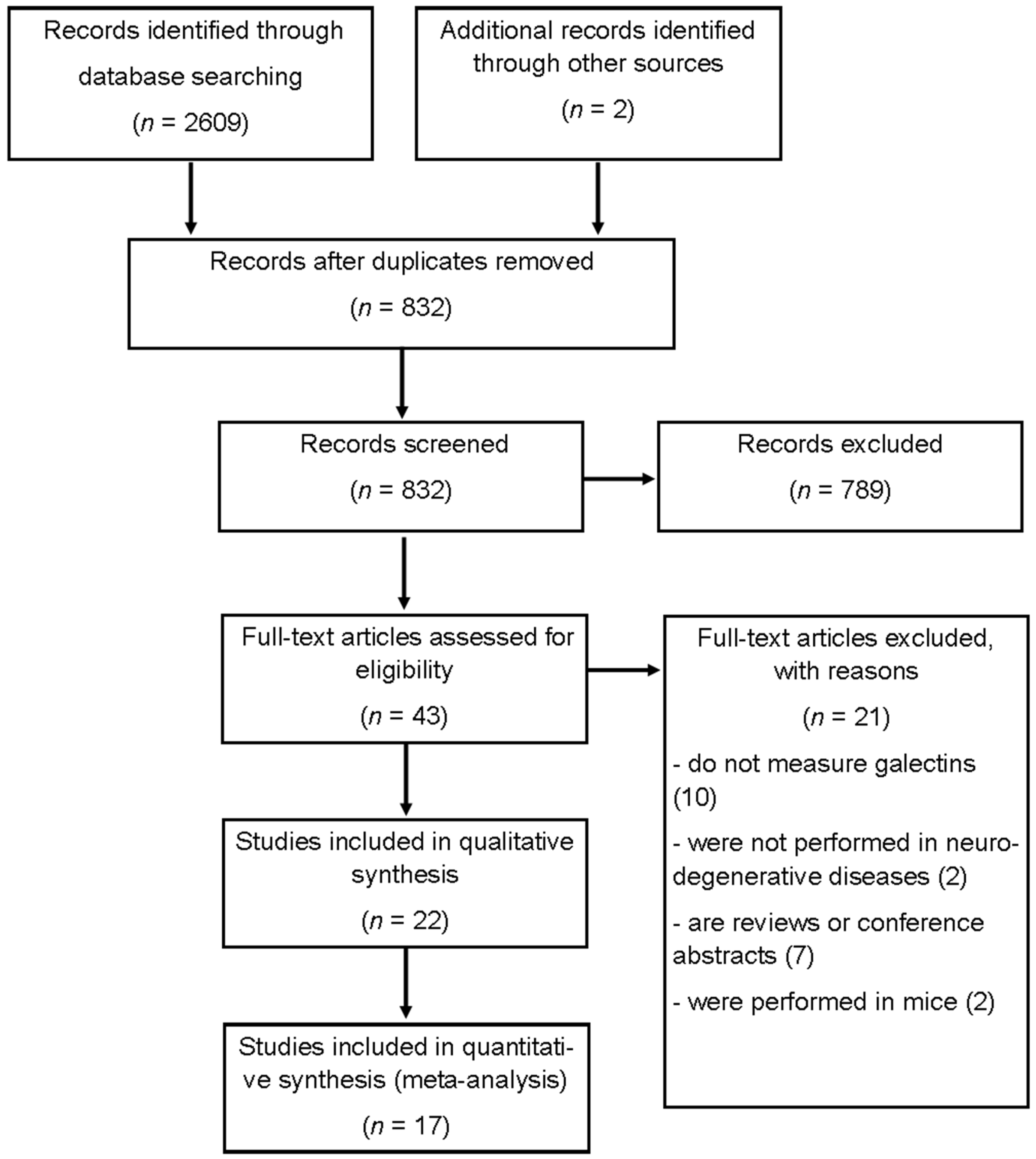
2.1. Identification of Relevant Literature
2.2. Inclusion and Exclusion Criteria
2.3. Quality Evaluation for Studies
2.4. Data Extraction
2.5. Statistical Analysis
3. Results
3.1. Characteristics of the Selected Studies and Qualitative Analysis
3.2. Galectin Expression Levels in Neurodegenerative Diseases
3.3. Heterogeneity Analysis
3.4. Sensitivity Analyses and Publication Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, M.-M.; Zhou, M.-T.; Li, S.-W.; Zhen, X.-C.; Yang, S. Glycoproteins as Diagnostic and Prognostic Biomarkers for Neurodegenerative Diseases: A Glycoproteomic Approach. J. Neurosci. Res. 2021, 99, 1308–1324. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.-P.; Xie, Y.; Meng, X.-Y.; Kang, J.-S. History and Progress of Hypotheses and Clinical Trials for Alzheimer’s Disease. Signal Transduct. Target. Ther. 2019, 4, 29. [Google Scholar] [CrossRef] [PubMed]
- Delacour, D.; Koch, A.; Jacob, R. The Role of Galectins in Protein Trafficking. Traffic 2009, 10, 1405–1413. [Google Scholar] [CrossRef] [PubMed]
- Funasaka, T.; Raz, A.; Nangia-Makker, P. Nuclear Transport of Galectin-3 and Its Therapeutic Implications. Semin. Cancer Biol. 2014, 27, 30–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nio-Kobayashi, J. Tissue- and Cell-Specific Localization of Galectins, β-Galactose-Binding Animal Lectins, and Their Potential Functions in Health and Disease. Anat. Sci. Int. 2017, 92, 25–36. [Google Scholar] [CrossRef]
- Ramos-Martínez, J.C.; Altamirano-Gómez, G.; Ramos-Martínez, I.; Valencia, J.; Hernández-Zimbrón, L.; Hernández-Juárez, J.; Echeverría-Vásquez, P.; Hernández-González, L.L.; Pérez-Campos, E.; Pérez-Campos Mayoral, L.; et al. Prognostic Value of Galectin Expression in Patients with Breast Cancer: Systematic Review and Meta-Analysis. Clin. Breast Cancer 2021. [Google Scholar] [CrossRef]
- Verkerke, H.; Dias-Baruffi, M.; Cummings, R.D.; Arthur, C.M.; Stowell, S.R. Galectins: An Ancient Family of Carbohydrate Binding Proteins with Modern Functions. In Galectins; Methods in Molecular Biology; Humana Press: New York, NY, USA, 2022; Volume 2442, pp. 1–40. [Google Scholar] [CrossRef]
- Cengiz, T.; Turkboylari, S.; Gencler, O.S.; Anlar, O. The Roles of Galectin-3 and Galectin-4 in the Idiopatic Parkinson Disease and Its Progression. Clin. Neurol. Neurosurg. 2019, 184, 105373. [Google Scholar] [CrossRef]
- Stancic, M.; van Horssen, J.; Thijssen, V.L.; Gabius, H.-J.; van der Valk, P.; Hoekstra, D.; Baron, W. Increased Expression of Distinct Galectins in Multiple Sclerosis Lesions. Neuropathol. Appl. Neurobiol. 2011, 37, 654–671. [Google Scholar] [CrossRef]
- Zubiri, I.; Lombardi, V.; Bremang, M.; Mitra, V.; Nardo, G.; Adiutori, R.; Lu, C.-H.; Leoni, E.; Yip, P.; Yildiz, O.; et al. Tissue-Enhanced Plasma Proteomic Analysis for Disease Stratification in Amyotrophic Lateral Sclerosis. Mol. Neurodegener. 2018, 13, 60. [Google Scholar] [CrossRef]
- Boza-Serrano, A.; Ruiz, R.; Sanchez-Varo, R.; Garcia-Revilla, J.; Yang, Y.; Jimenez-Ferrer, I.; Paulus, A.; Wennstroem, M.; Vilalta, A.; Allendorf, D.; et al. Galectin-3, a Novel Endogenous TREM2 Ligand, Detrimentally Regulates Inflammatory Response in Alzheimer’s Disease. ACTA Neuropathol. 2019, 138, 251–273. [Google Scholar] [CrossRef] [Green Version]
- Hara, A.; Niwa, M.; Noguchi, K.; Kanayama, T.; Niwa, A.; Matsuo, M.; Hatano, Y.; Tomita, H. Galectin-3 as a Next-Generation Biomarker for Detecting Early Stage of Various Diseases. Biomolecules 2020, 10, 389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puigdellívol, M.; Allendorf, D.H.; Brown, G.C. Sialylation and Galectin-3 in Microglia-Mediated Neuroinflammation and Neurodegeneration. Front. Cell. Neurosci. 2020, 14, 162. [Google Scholar] [CrossRef] [PubMed]
- Siew, J.J.; Chen, H.-M.; Chen, H.-Y.; Chen, H.-L.; Chen, C.-M.; Soong, B.-W.; Wu, Y.-R.; Chang, C.-P.; Chan, Y.-C.; Lin, C.-H.; et al. Galectin-3 Is Required for the Microglia-Mediated Brain Inflammation in a Model of Huntington’s Disease. Nat. Commun. 2019, 10, 3473. [Google Scholar] [CrossRef] [Green Version]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hozo, S.P.; Djulbegovic, B.; Hozo, I. Estimating the Mean and Variance from the Median, Range, and the Size of a Sample. BMC Med. Res. Methodol. 2005, 5, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rohatgi, A. Web Plot Digitizer (Version No. 4.5). Windows. California. 2021. Available online: https://automeris.io/WebPlotDigitizer (accessed on 13 June 2022).
- DerSimonian, R.; Laird, N. Meta-Analysis in Clinical Trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Mantel, N.; Haenszel, W. Statistical Aspects of the Analysis of Data from Retrospective Studies of Disease. J. Natl. Cancer Inst. 1959, 22, 719–748. [Google Scholar]
- Duval, S.; Tweedie, R. A Nonparametric “Trim and Fill” Method of Accounting for Publication Bias in Meta-Analysis. J. Am. Stat. Assoc. 2000, 95, 89–98. [Google Scholar] [CrossRef]
- Kato, T.; Kurita, K.; Seino, T.; Kadoya, T.; Horie, H.; Wada, M.; Kawanami, T.; Daimon, M.; Hirano, A. Galectin-1 Is a Component of Neurofilamentous Lesions in Sporadic and Familial Amyotrophic Lateral Sclerosis. Biochem. Biophys. Res. Commun. 2001, 282, 166–172. [Google Scholar] [CrossRef]
- De Jong, C.G.H.M.; Stancic, M.; Pinxterhuis, T.H.; van Horssen, J.; van Dam, A.-M.; Gabius, H.-J.; Baron, W. Galectin-4, a Negative Regulator of Oligodendrocyte Differentiation, Is Persistently Present in Axons and Microglia/Macrophages in Multiple Sclerosis Lesions. J. Neuropathol. Exp. Neurol. 2018, 77, 1024–1038. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.-J.; Shi, Q.; Yang, X.-D.; Li, J.-L.; Ma, Y.; Xiao, K.; Chen, C.; Han, J.; Dong, X.-P. Increases of Galectin-1 and Its S-Nitrosylated Form in the Brain Tissues of Scrapie-Infected Rodent Models and Human Prion Diseases. Mol. Neurobiol. 2017, 54, 3707–3716. [Google Scholar] [CrossRef] [PubMed]
- Yazar, T.; Olgun Yazar, H.; Cihan, M. Evaluation of Serum Galectin-3 Levels at Alzheimer Patients by Stages: A Preliminary Report. Acta Neurol. Belg. 2021, 121, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Marques, T.M.; van Rumund, A.; Bruinsma, I.B.; Wessels, H.J.C.T.; Gloerich, J.; Esselink, R.A.J.; Bloem, B.R.; Kuiperij, H.B.; Verbeek, M.M. Cerebrospinal Fluid Galectin-1 Levels Discriminate Patients with Parkinsonism from Controls. Mol. Neurobiol. 2019, 56, 5067–5074. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Niu, Y.; Yue, C.-X.; Fu, S.; Wang, R.-T. Increased Ileal Bile Acid Binding Protein and Galectin-9 Are Associated with Mild Cognitive Impairment and Alzheimer’s Disease. J. Psychiatr. Res. 2019, 119, 102–106. [Google Scholar] [CrossRef]
- Yan, J.; Xu, Y.; Zhang, L.; Zhao, H.; Jin, L.; Liu, W.-G.; Weng, L.-H.; Li, Z.-H.; Chen, L. Increased Expressions of Plasma Galectin-3 in Patients with Amyotrophic Lateral Sclerosis. Chin. Med. J. 2016, 129, 2797–2803. [Google Scholar] [CrossRef]
- Zhou, J.-Y.; Afjehi-Sadat, L.; Asress, S.; Duong, D.M.; Cudkowicz, M.; Glass, J.D.; Peng, J. Galectin-3 Is a Candidate Biomarker for Amyotrophic Lateral Sclerosis: Discovery by a Proteomics Approach. J. Proteome Res. 2010, 9, 5133–5141. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Zhang, S.; Lin, F.; Chu, W.; Yue, S. Elevated Galectin-3 Levels in the Serum of Patients With Alzheimer’s Disease. Am. J. Alzheimers Dis. Other Dement. 2015, 30, 729–732. [Google Scholar] [CrossRef]
- Ashraf, G.M.; Baeesa, S.S. Investigation of Gal-3 Expression Pattern in Serum and Cerebrospinal Fluid of Patients Suffering From Neurodegenerative Disorders. Front. Neurosci. 2018, 12, 430. [Google Scholar] [CrossRef] [Green Version]
- Tian, P.-C.; Wang, H.-L.; Chen, G.-H.; Li, J.-H.; Zheng, C.; Wang, Y.; Tian, P.-C.; Wang, H.-L.; Chen, G.-H.; Li, J.-H.; et al. Elevated Interictal Serum Galectin-3 Levels in Intractable Epilepsy. Neurol. India 2016, 64, 233–236. [Google Scholar] [CrossRef]
- Burman, J.; Svenningsson, A. Cerebrospinal Fluid Concentration of Galectin-9 Is Increased in Secondary Progressive Multiple Sclerosis. J. Neuroimmunol. 2016, 292, 40–44. [Google Scholar] [CrossRef]
- Basso, M.; Giraudo, S.; Corpillo, D.; Bergamasco, B.; Lopiano, L.; Fasano, M. Proteome Analysis of Human Substantia Nigra in Parkinson’s Disease. Proteomics 2004, 4, 3943–3952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Werner, C.J.; Haussen, R.H.; Mall, G.; Wolf, S. Proteome Analysis of Human Substantia Nigra in Parkinson’s Disease. Proteome Sci. 2008, 6, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tao, C.-C.; Cheng, K.-M.; Ma, Y.-L.; Hsu, W.-L.; Chen, Y.-C.; Fuh, J.-L.; Lee, W.-J.; Chao, C.-C.; Lee, E.H.Y. Galectin-3 Promotes A Beta Oligomerization and A Beta Toxicity in a Mouse Model of Alzheimer’s Disease. Cell Death Differ. 2020, 27, 192–209. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.-C.; Chang, K.-H.; Chiang, M.-C.; Chen, C.-M. Alterations of Plasma Galectin-3 and C3 Levels in Patients with Parkinson’s Disease. Brain Sci. 2021, 11, 1515. [Google Scholar] [CrossRef]
- Wada, M.; Ono, S.; Kadoya, T.; Kawanami, T.; Kurita, K.; Kato, T. Decreased Galectin-1 Immunoreactivity of the Skin in Amyotrophic Lateral Sclerosis. J. Neurol. Sci. 2003, 208, 67–70. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Collecting Data. In Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: Chichester, UK, 2019. [Google Scholar]
- Burguillos, M.A.; Svensson, M.; Schulte, T.; Boza-Serrano, A.; Garcia-Quintanilla, A.; Kavanagh, E.; Santiago, M.; Viceconte, N.; Oliva-Martin, M.J.; Osman, A.M.; et al. Microglia-Secreted Galectin-3 Acts as a Toll-like Receptor 4 Ligand and Contributes to Microglial Activation. Cell Rep. 2015, 10, 1626–1638. [Google Scholar] [CrossRef] [Green Version]
- Itabashi, T.; Arima, Y.; Kamimura, D.; Higuchi, K.; Bando, Y.; Takahashi-Iwanaga, H.; Murakami, M.; Watanabe, M.; Iwanaga, T.; Nio-Kobayashi, J. Cell- and Stage-Specific Localization of Galectin-3, a β-Galactoside-Binding Lectin, in a Mouse Model of Experimental Autoimmune Encephalomyelitis. Neurochem. Int. 2018, 118, 176–184. [Google Scholar] [CrossRef]
- Kajitani, K.; Nomaru, H.; Ifuku, M.; Yutsudo, N.; Dan, Y.; Miura, T.; Tsuchimoto, D.; Sakumi, K.; Kadoya, T.; Horie, H.; et al. Galectin-1 Promotes Basal and Kainate-Induced Proliferation of Neural Progenitors in the Dentate Gyrus of Adult Mouse Hippocampus. Cell Death Differ. 2009, 16, 417–427. [Google Scholar] [CrossRef] [Green Version]
- Tai, X.Y.; Bernhardt, B.; Thom, M.; Thompson, P.; Baxendale, S.; Koepp, M.; Bernasconi, N. Review: Neurodegenerative Processes in Temporal Lobe Epilepsy with Hippocampal Sclerosis: Clinical, Pathological and Neuroimaging Evidence. Neuropathol. Appl. Neurobiol. 2018, 44, 70–90. [Google Scholar] [CrossRef]
- Wolinski, P.; Ksiazek-Winiarek, D.; Glabinski, A. Cytokines and Neurodegeneration in Epileptogenesis. Brain Sci. 2022, 12, 380. [Google Scholar] [CrossRef] [PubMed]
- Ramírez Hernández, E.; Sánchez-Maldonado, C.; Mayoral Chávez, M.A.; Hernández-Zimbrón, L.F.; Patricio Martínez, A.; Zenteno, E.; Limón Pérez de León, I.D. The therapeutic potential of galectin-1 and galectin-3 in the treatment of neurodegenerative diseases. Expert Rev. Neurother. 2022, 20, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.B.; Yoon, H.J.; Chang, C.Y.; Koh, H.S.; Jeon, S.H.; Park, E.J. Galectin-3 exerts cytokine-like regulatory actions through the JAK-STAT pathway. J. Immunol. 2010, 185, 7037–7046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoyos, H.C.; Marder, M.; Ulrich, R.; Gudi, V.; Stangel, M.; Rabinovich, G.A.; Pasquini, L.A.; Pasquini, J.M. The Role of Galectin-3: From Oligodendroglial Differentiation and Myelination to Demyelination and Remyelination Processes in a Cuprizone-Induced Demyelination Model. Adv. Exp. Med. Biol. 2016, 949, 311–332. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.-R.; Al Rasebi, Z.; Mensah-Brown, E.; Shahin, A.; Xu, D.; Goodyear, C.S.; Fukada, S.Y.; Liu, F.-T.; Liew, F.Y.; Lukic, M.L. Galectin-3 Deficiency Reduces the Severity of Experimental Autoimmune Encephalomyelitis. J. Immunol. 2009, 182, 1167–1173. [Google Scholar] [CrossRef]
- Li, Y.; Chen, N.; Wu, C.; Lu, Y.; Gao, G.; Duan, C.; Yang, H.; Lu, L. Galectin-1 Attenuates Neurodegeneration in Parkinson’s Disease Model by Modulating Microglial MAPK/IκB/NFκB Axis through Its Carbohydrate-Recognition Domain. Brain Behav. Immun. 2020, 83, 214–225. [Google Scholar] [CrossRef]
- Shen, Z.; Xu, H.; Song, W.; Hu, C.; Guo, M.; Li, J.; Li, J. Galectin-1 ameliorates perioperative neurocognitive disorders in aged mice. CNS Neurosci. Ther. 2021, 27, 842–856. [Google Scholar] [CrossRef]
- Starossom, S.C.; Mascanfroni, I.D.; Imitola, J.; Cao, L.; Raddassi, K.; Hernandez, S.F.; Bassil, R.; Croci, D.O.; Cerliani, J.P.; Delacour, D.; et al. Galectin-1 deactivates classically activated microglia and protects from inflammation-induced neurodegeneration. Immunity 2012, 37, 249–263. [Google Scholar] [CrossRef] [Green Version]
- Quintá, H.R.; Pasquini, J.M.; Rabinovich, G.A.; Pasquini, L.A. Glycan-dependent binding of galectin-1 to neuropilin-1 promotes axonal regeneration after spinal cord injury. Cell Death Differ. 2014, 21, 941–955. [Google Scholar] [CrossRef] [Green Version]
- Qu, W.S.; Wang, Y.H.; Wang, J.P.; Tang, Y.X.; Zhang, Q.; Tian, D.S.; Yu, Z.Y.; Xie, M.J.; Wang, W. Galectin-1 enhances astrocytic BDNF production and improves functional outcome in rats following ischemia. Neurochem. Res. 2010, 35, 1716–1724. [Google Scholar] [CrossRef]
- Falcon, B.; Noad, J.; McMahon, H.; Randow, F.; Goedert, M. Galectin-8-Mediated Selective Autophagy Protects against Seeded Tau Aggregation. J. Biol. Chem. 2018, 293, 2438–2451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pardo, E.; Barake, F.; Godoy, J.A.; Oyanadel, C.; Espinoza, S.; Metz, C.; Retamal, C.; Massardo, L.; Tapia-Rojas, C.; Inestrosa, N.C.; et al. GALECTIN-8 Is a Neuroprotective Factor in the Brain That Can Be Neutralized by Human Autoantibodies. Mol. Neurobiol. 2019, 56, 7774–7788. [Google Scholar] [CrossRef] [PubMed]
- Pardo, E.; Cárcamo, C.; Uribe-San Martín, R.; Ciampi, E.; Segovia-Miranda, F.; Curkovic-Peña, C.; Montecino, F.; Holmes, C.; Tichauer, J.E.; Acuña, E.; et al. Galectin-8 as an Immunosuppressor in Experimental Autoimmune Encephalomyelitis and a Target of Human Early Prognostic Antibodies in Multiple Sclerosis. PLoS ONE 2017, 12, e0177472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nio-Kobayashi, J.; Itabashi, T. Galectins and Their Ligand Glycoconjugates in the Central Nervous System Under Physiological and Pathological Conditions. Front. Neuroanat. 2021, 15, 767330. [Google Scholar] [CrossRef] [PubMed]
- Steelman, A.J.; Smith, R., 3rd; Welsh, C.J.; Li, J. Galectin-9 protein is up-regulated in astrocytes by tumor necrosis factor and promotes encephalitogenic T-cell apoptosis. J. Biol. Chem. 2013, 288, 23776–23787. [Google Scholar] [CrossRef] [Green Version]
- Steelman, A.J.; Li, J. Astrocyte galectin-9 potentiates microglial TNF secretion. J. Neuroinflamm. 2014, 11, 144. [Google Scholar] [CrossRef] [Green Version]
- Liang, T.; Ma, C.; Wang, T.; Deng, R.; Ding, J.; Wang, W.; Xu, Z.; Li, X.; Li, H.; Sun, Q.; et al. Galectin-9 Promotes Neuronal Restoration via Binding TLR-4 in a Rat Intracerebral Hemorrhage Model. Neuromol. Med. 2021, 23, 267–284. [Google Scholar] [CrossRef]
- Cioca, A.; Muntean, D.; Bungardean, C.; Raica, M.; Cimpean, A.M. Expression and Distribution of Galectin-3 in Chromophobe and Papillary Carcinomas. Anticancer Res. 2018, 38, 259–263. [Google Scholar] [CrossRef] [Green Version]
- Van den Brûle, F.A.; Waltregny, D.; Liu, F.T.; Castronovo, V. Alteration of the Cytoplasmic/Nuclear Expression Pattern of Galectin-3 Correlates with Prostate Carcinoma Progression. Int. J. Cancer 2000, 89, 361–367. [Google Scholar] [CrossRef]




| First Author and Year | Case Nationality | Region | Disease | Number of Patients | Controls | Female % Patients | Median or Mean Age Patients | Median or Mean Age Controls | Study Design | Detected Sample | Assay Method | Galectins Tested | Units |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Boza-Serrano, 2019 [11] | Dutch and Swedish | Europe | AD | 6 | 5 | 16.6 | 74.6 | 74.8 | CS | Brain | WB | Gal-3 | FC |
| Yazar, 2021 [24] | Turkish | Western Asia | AD | 57 | 61 | 61.4 | 79.1 | 77.7 | CS | Serum | ELISA | Gal-3 | pg/mL |
| Cengiz, 2019 [8] | Turkish | Western Asia | PD | 60 | 30 | 40 | 72.5 | 72 | CS | Serum | ELISA | Gal-3 and -4 | pg/mL |
| Marques, 2019 [25] | Dutch | Europe | PD | 37 | 44 | 35 | 57 | 58 | CS | CSF | ELISA | Gal-1 | ng/mg |
| Wang, 2019 [26] | Chinese | East Asia | AD | 115 | 115 | 64.3 | 75.7 | 75.9 | CS | Serum | ELISA | Gal-9 | pg/mL |
| Yan, 2016 [27] | Chinese | East Asia | ALS | 51 | 60 | 96 | 54.8 | 55.5 | CS | Plasma | ELISA | Gal-3 | ng/mL |
| Zhou, 2010 [28] | American | America | ALS | 27 | 14 | - | - | - | CS | CSF | ELISA | Gal-3 | ng/mL |
| Wang, 2015 [29] | Chinese | East Asia | AD | 41 | 46 | 46.3 | 71.2 | 69.8 | CS | Serum | ELISA | Gal-3 | ng/mL |
| Ashraf, 2018 [30] | Saudi | Western Asia | AD | 31 | 50 | 41.9 | 66.8 | 74.9 | CS | Serum | ELISA | Gal-3 | ng/mL |
| ALS | 19 | - | 42.1 | 64.1 | CS | Serum | ELISA | Gal-3 | ng/mL | ||||
| Tian, 2016 [31] | Chinese | East Asia | Epilepsy | 38 | 26 | 55 | 32.5 | - | CS | Serum | ELISA | Gal-3 | ng/mL |
| Burman, 2016 [32] | Swiss | Europe | MS | 94 | 25 | - | 46 | 48 | CSF | ELISA | Gal-9 | pg/mL | |
| Stancic, 2011 [9] | Dutch | Europe | MS | 17 | 8 | - | 61 | 76.6 | CS | Brain | WB | Gal-1, -3, -8, and -9 | FC |
| Basso, 2004 [33] | Dutch | Europe | PD | 4 | 4 | 25 | 75 | 70 | CS | Brain | 2-DE and MALDI-TOF-MS | Gal-1 | relative volume in 2-DE |
| Werner, 2008 [34] | German | Europe | PD | 5 | 5 | 40 | 84.2 | 77.4 | CS | Brain | 2-DE and MALDI-TOF-MS | Gal-1 | relative volume in 2-DE |
| Tao, 2020 [35] | - | - | AD | 101 | 52 | - | - | - | CS | Brain and serum | WB and ELISA | Gal-3 and -1 | FC and ng/mL |
| Wu, 2021 [36] | Taiwanese | East Asia | PD | 56 | 46 | 43 | 64.8 | 65.9 | CS | Plasma | ELISA | Gal-3 | ng/mL |
| Siew, 2019 [14] | Taiwanese | East Asia | HD | 31 | 21 | 72 | 51 | 54 | CS | Plasma and brain | ELISA and RTqPCR | Gal-3 | ng/mL |
| Articles included in the qualitative analysis | |||||||||||||
| Wada, 2003 [37] | ALS | 12 | 10 | 33 | 58.3 | 60.1 | CS | Skin | IHC | Gal-1 | pixels | ||
| Zubiri, 2018 [10] | British | Europe | ALS | 12 | 6 | 33 | PP | Plasma | LC-MS/MS | Gal-1 and -3 | FC | ||
| Kato, 2001 [21] | - | - | ALS | IHC | Gal-1 | ||||||||
| de Jong, 2018 [22] | - | - | SM | 25 | 11 | - | - | - | CS | Brain | IHC | Gal-4 | x |
| Guo, 2017 [23] | Chinese | East Asia | human prion diseases | 5 | 3 | - | - | - | CS | Brain | WB | Gal-1 | FC |
| Subgroup | Studies | Test for Association | Test for Heterogeneity | Analytical Model | |||
|---|---|---|---|---|---|---|---|
| SMD | 95% CI | p | I2 | p | |||
| Neurodegenerative disease and galectin type | |||||||
| AD and Gal-3 | 6 | 0.64 | [0.45, 0.83] | <0.00001 | 44% | p = 0.11 | FEM |
| PD and Gal-1 | 3 | 0.30 | [−1.16, 1.77] | 0.68 | 80% | 0.006 | REM |
| PD and Gal-3 | 2 | 0.58 | [0.28, 0.88] | 0.0001 | 0% | 0.45 | FEM |
| SM and Gal-9 | 2 | 1.03 | [0.62, 1.44] | <0.00001 | 0% | 0.61 | FEM |
| Region | |||||||
| Western Asia | 3 | 0.60 | [0.40, 0.79] | <0.00001 | 0% | 0.98 | FEM |
| East Asia | 6 | 0.70 | [−0.46, 1.86] | 0.24 | 98% | <0.00001 | REM |
| Europe | 6 | 0.76 | [0.10, 1.41] | 0.02 | 82% | <0.00001 | REM |
| American | 1 | 0.44 | [−0.21, 1.09] | 0.19 | NA | NA | NA |
| Detection method | |||||||
| ELISA | 13 | 0.50 | [−0.03, 1.03] | 0.06 | 95% | <0.00001 | REM |
| Western blot | 3 | 0.87 | [0.47, 1.27] | <0.0001 | 32% | 0.18 | FEM |
| 2-DE and MALDI-TOF-MS | 2 | 1.03 | [−0.01, 2.07] | 0.05 | 0% | 0.66 | FEM |
| sample analyzed | |||||||
| Serum and plasma | 10 | 0.58 | [−0.04, 1.19] | 0.07 | 96% | <0.00001 | REM |
| Brain | 6 | 0.93 | [0.57, 1.30] | <0.00001 | 9% | 0.36 | FEM |
| CSF | 3 | 0.20 | [−0.94, 1.35] | 0.73 | 93% | <0.00001 | REM |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos-Martínez, E.; Ramos-Martínez, I.; Sánchez-Betancourt, I.; Ramos-Martínez, J.C.; Peña-Corona, S.I.; Valencia, J.; Saucedo, R.; Almeida-Aguirre, E.K.P.; Cerbón, M. Association between Galectin Levels and Neurodegenerative Diseases: Systematic Review and Meta-Analysis. Biomolecules 2022, 12, 1062. https://doi.org/10.3390/biom12081062
Ramos-Martínez E, Ramos-Martínez I, Sánchez-Betancourt I, Ramos-Martínez JC, Peña-Corona SI, Valencia J, Saucedo R, Almeida-Aguirre EKP, Cerbón M. Association between Galectin Levels and Neurodegenerative Diseases: Systematic Review and Meta-Analysis. Biomolecules. 2022; 12(8):1062. https://doi.org/10.3390/biom12081062
Chicago/Turabian StyleRamos-Martínez, Edgar, Iván Ramos-Martínez, Iván Sánchez-Betancourt, Juan Carlos Ramos-Martínez, Sheila Irais Peña-Corona, Jorge Valencia, Renata Saucedo, Ericka Karol Pamela Almeida-Aguirre, and Marco Cerbón. 2022. "Association between Galectin Levels and Neurodegenerative Diseases: Systematic Review and Meta-Analysis" Biomolecules 12, no. 8: 1062. https://doi.org/10.3390/biom12081062
APA StyleRamos-Martínez, E., Ramos-Martínez, I., Sánchez-Betancourt, I., Ramos-Martínez, J. C., Peña-Corona, S. I., Valencia, J., Saucedo, R., Almeida-Aguirre, E. K. P., & Cerbón, M. (2022). Association between Galectin Levels and Neurodegenerative Diseases: Systematic Review and Meta-Analysis. Biomolecules, 12(8), 1062. https://doi.org/10.3390/biom12081062







