Thiophosphate Analogs of Coenzyme A and Its Precursors—Synthesis, Stability, and Biomimetic Potential
Abstract
:1. Introduction
2. Materials and Methods
2.1. Protein Expression and Purification
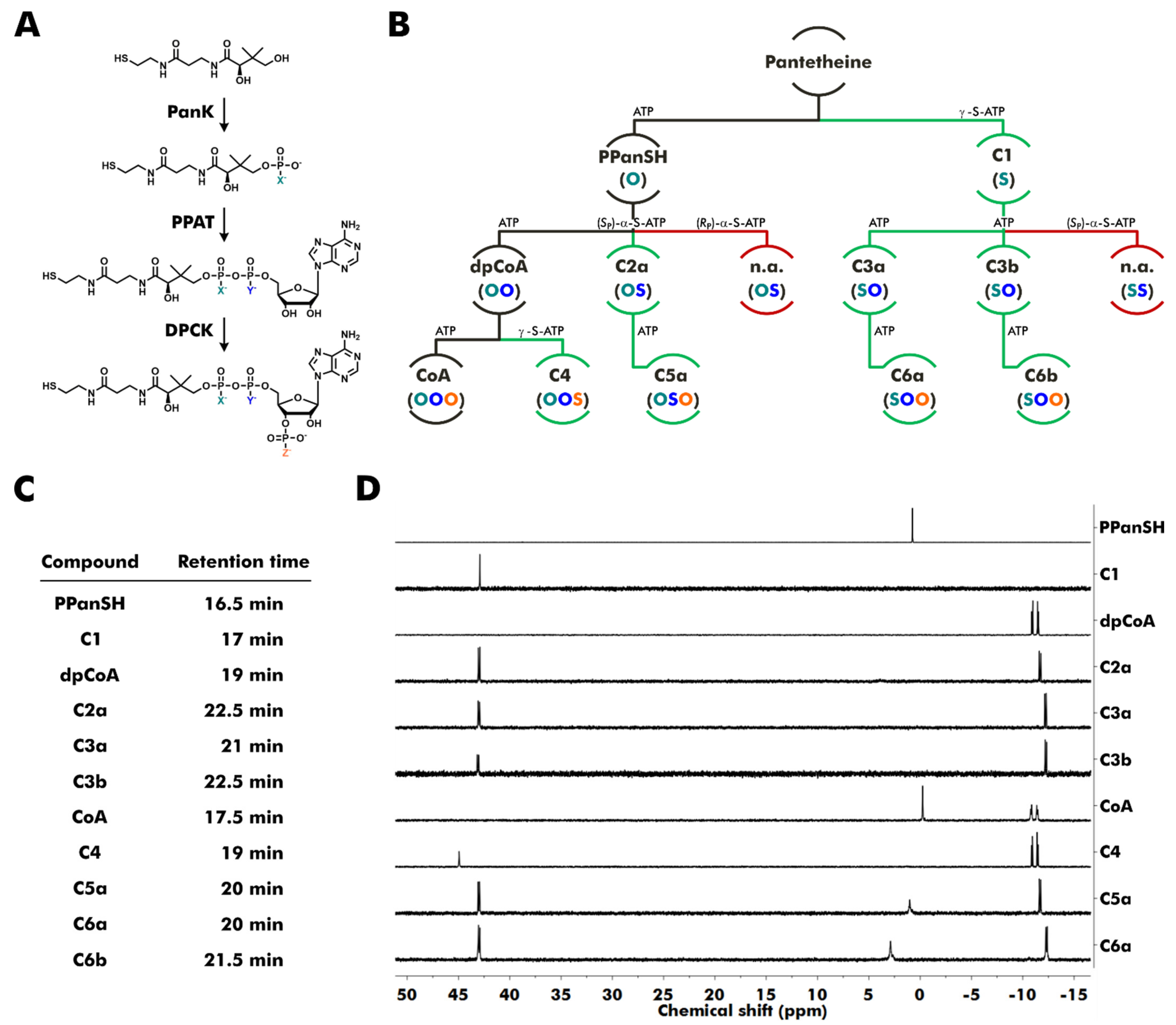
2.2. Enzymatic Synthesis of Thiophosphate Analogs of PPanSH, dpCoA, and CoA
2.3. Synthesis of Calcium Hopantenate (HoPan)
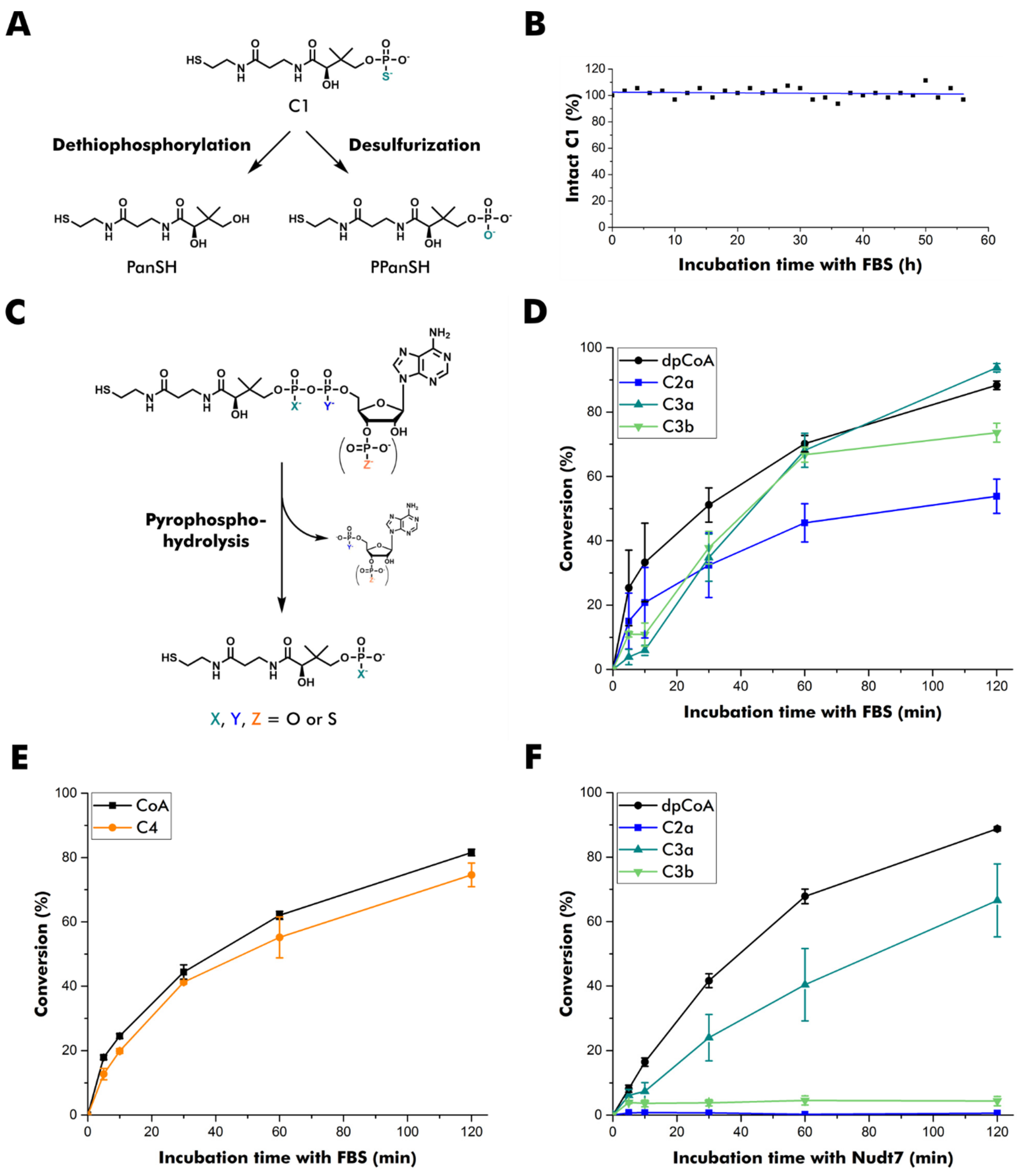
2.4. Serum Stability of C1
2.5. Pyrophosphate Hydrolysis in Fetal Bovine Serum (FBS)
2.6. Pyrophosphate Hydrolysis by Human Nudt7
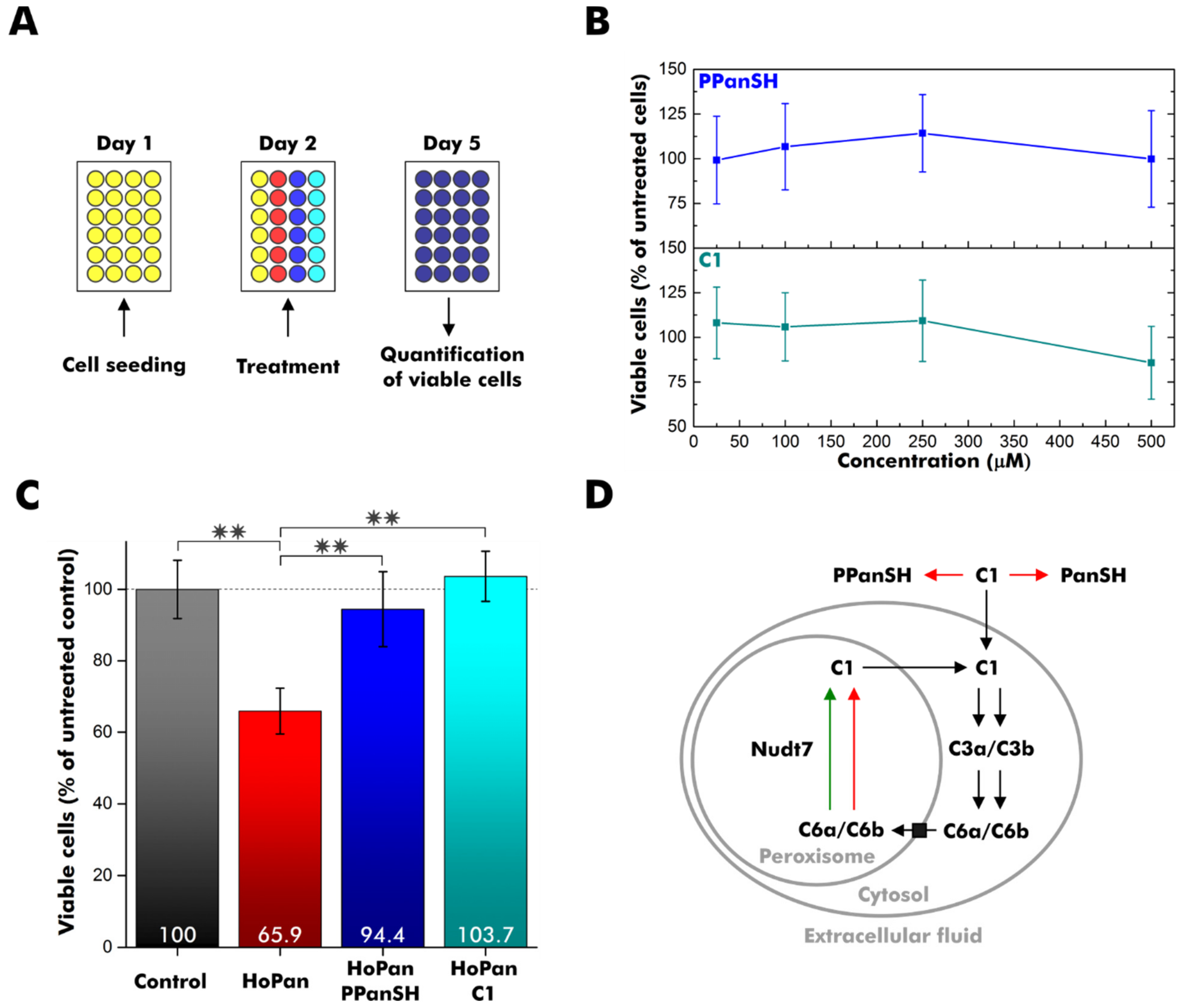
2.7. HEK 293T Cell Experiments
3. Results
3.1. Enzymatic Synthesis of Thiophosphate Analogs
3.2. Stability of Thiophosphate Analogs
3.3. C1 Treatment Rescues CoA-Deficient Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pietrocola, F.; Galluzzi, L.; Bravo-San Pedro, J.M.; Madeo, F.; Kroemer, G. Acetyl Coenzyme A: A Central Metabolite and Second Messenger. Cell Metab. 2015, 21, 805–821. [Google Scholar] [CrossRef] [PubMed]
- Davaapil, H.; Tsuchiya, Y.; Gout, I. Signalling functions of coenzyme A and its derivatives in mammalian cells. Biochem. Soc. Trans. 2014, 42, 1056–1062. [Google Scholar] [CrossRef]
- Gout, I. Coenzyme A, protein CoAlation and redox regulation in mammalian cells. Biochem. Soc. Trans. 2018, 46, 721–728. [Google Scholar] [CrossRef]
- Trefely, S.; Lovell, C.D.; Snyder, N.W.; Wellen, K.E. Compartmentalised acyl-CoA metabolism and roles in chromatin regulation. Mol. Metab. 2020, 38, 100941. [Google Scholar] [CrossRef]
- Srinivasan, B.; Baratashvili, M.; van der Zwaag, M.; Kanon, B.; Colombelli, C.; Lambrechts, R.A.; Schaap, O.; Nollen, E.A.; Podgoršek, A.; Kosec, G.; et al. Extracellular 4′-phosphopantetheine is a source for intracellular coenzyme A synthesis. Nat. Chem. Biol. 2015, 11, 784–792. [Google Scholar] [CrossRef]
- Martinez David, L.; Tsuchiya, Y.; Gout, I. Coenzyme A biosynthetic machinery in mammalian cells. Biochem. Soc. Trans. 2014, 42, 1112–1117. [Google Scholar] [CrossRef]
- Zhou, B.; Westaway, S.K.; Levinson, B.; Johnson, M.A.; Gitschier, J.; Hayflick, S.J. A novel pantothenate kinase gene (PANK2) is defective in Hallervorden-Spatz syndrome. Nat. Genet. 2001, 28, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Thakur, N.; Klopstock, T.; Jackowski, S.; Kuscer, E.; Tricta, F.; Videnovic, A.; Jinnah, H.A. Rational Design of Novel Therapies for Pantothenate Kinase–Associated Neurodegeneration. Mov. Disord. 2021, 36, 2005–2016. [Google Scholar] [CrossRef] [PubMed]
- Iankova, V.; Karin, I.; Klopstock, T.; Schneider, S.A. Emerging Disease-Modifying Therapies in Neurodegeneration with Brain Iron Accumulation (NBIA) Disorders. Front. Neurol. 2021, 12, 629414. [Google Scholar] [CrossRef]
- Jeong, S.Y.; Hogarth, P.; Placzek, A.; Gregory, A.M.; Fox, R.; Zhen, D.; Hamada, J.; van der Zwaag, M.; Lambrechts, R.; Jin, H.; et al. 4′-Phosphopantetheine corrects CoA, iron, and dopamine metabolic defects in mammalian models of PKAN. EMBO Mol. Med. 2019, 11, e10489. [Google Scholar] [CrossRef]
- Gasmi, L.; McLennan, A.G. The mouse Nudt7 gene encodes a peroxisomal nudix hydrolase specific for coenzyme A and its derivatives. Biochem. J. 2001, 357, 33–38. [Google Scholar] [CrossRef]
- Ofman, R.; Speijer, D.; Leen, R.; Wanders, R.J.A. Proteomic analysis of mouse kidney peroxisomes: Identification of RP2p as a peroxisomal nudix hydrolase with acyl-CoA diphosphatase activity. Biochem. J. 2006, 393, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Kerr, E.W.; Shumar, S.A.; Leonardi, R. Nudt8 is a novel CoA diphosphohydrolase that resides in the mitochondria. FEBS Lett. 2019, 593, 1133–1143. [Google Scholar] [CrossRef] [PubMed]
- Zhyvoloup, A.; Nemazanyy, I.; Panasyuk, G.; Valovka, T.; Fenton, T.; Rebholz, H.; Wang, M.-L.; Foxon, R.; Lyzogubov, V.; Usenko, V.; et al. Subcellular Localization and Regulation of Coenzyme A Synthase. J. Biol. Chem. 2003, 278, 50316–50321. [Google Scholar] [CrossRef]
- Mlynarska-Cieslak, A.; Depaix, A.; Grudzien-Nogalska, E.; Sikorski, P.J.; Warminski, M.; Kiledjian, M.; Jemielity, J.; Kowalska, J. Nicotinamide-Containing Di- and Trinucleotides as Chemical Tools for Studies of NAD-Capped RNAs. Org. Lett. 2018, 20, 7650–7655. [Google Scholar] [CrossRef]
- Grudzien-Nogalska, E.; Jemielity, J.; Kowalska, J.; Darzynkiewicz, E.; Rhoads, R.E. Phosphorothioate cap analogs stabilize mRNA and increase translational efficiency in mammalian cells. RNA 2007, 13, 1745–1755. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, N.; Butler, D.C.D.; Svrzikapa, N.; Mohapatra, S.; Zlatev, I.; Sah, D.W.Y.; Meena; Standley, S.M.; Lu, G.; Apponi, L.H.; et al. Control of phosphorothioate stereochemistry substantially increases the efficacy of antisense oligonucleotides. Nat. Biotechnol. 2017, 35, 845–851. [Google Scholar] [CrossRef]
- Zhang, Y.-M.; Chohnan, S.; Virga, K.G.; Stevens, R.D.; Ilkayeva, O.R.; Wenner, B.R.; Bain, J.R.; Newgard, C.B.; Lee, R.E.; Rock, C.O.; et al. Chemical Knockout of Pantothenate Kinase Reveals the Metabolic and Genetic Program Responsible for Hepatic Coenzyme A Homeostasis. Chem. Biol. 2007, 14, 291–302. [Google Scholar] [CrossRef]
- Bibart, R.T.; Vogel, K.W.; Drueckhammer, D.G. Development of a Second Generation Coenzyme A Analogue Synthon. J. Org. Chem. 1999, 64, 2903–2909. [Google Scholar] [CrossRef]
- Nazi, I.; Koteva, K.P.; Wright, G.D. One-pot chemoenzymatic preparation of coenzyme A analogues. Anal. Biochem. 2004, 324, 100–105. [Google Scholar] [CrossRef]
- Meier, J.L.; Mercer, A.C.; Rivera, H.; Burkart, M.D. Synthesis and Evaluation of Bioorthogonal Pantetheine Analogues for in Vivo Protein Modification. J. Am. Chem. Soc. 2006, 128, 12174–12184. [Google Scholar] [CrossRef] [PubMed]
- van Wyk, M.; Strauss, E. One-pot preparation of coenzyme A analogues via an improved chemo-enzymatic synthesis of pre-CoA thioester synthons. Chem. Commun. 2007, 398–400. [Google Scholar] [CrossRef] [PubMed]
- Strauss, E.; de Villiers, M.; Rootman, I. Biocatalytic Production of Coenzyme A Analogues. Chemcatchem 2010, 2, 929–937. [Google Scholar] [CrossRef]
- Rothmann, M.; Kang, M.; Villa, R.; Ntai, I.; La Clair, J.J.; Kelleher, N.L.; Chapman, E.; Burkart, M.D. Metabolic Perturbation of an Essential Pathway: Evaluation of a Glycine Precursor of Coenzyme A. J. Am. Chem. Soc. 2013, 135, 5962–5965. [Google Scholar] [CrossRef]
- Mouterde, L.M.M.; Stewart, J.D. An Efficient Chemoenzymatic Synthesis of Coenzyme A and Its Disulfide. Org. Process Res. Dev. 2016, 20, 954–959. [Google Scholar] [CrossRef]
- Sapkota, K.; Huang, F. Efficient one-pot enzymatic synthesis of dephospho coenzyme A. Bioorg. Chem. 2018, 76, 23–27. [Google Scholar] [CrossRef]
- Guan, J.; Barnard, L.; Cresson, J.; Hoegl, A.; Chang, J.H.; Strauss, E.; Auclair, K. Probing the ligand preferences of the three types of bacterial pantothenate kinase. Bioorg. Med. Chem. 2018, 26, 5896–5902. [Google Scholar] [CrossRef]
- Duncan, D.; Auclair, K. The coenzyme A biosynthetic pathway: A new tool for prodrug bioactivation. Arch. Biochem. Biophys. 2019, 672, 108069. [Google Scholar] [CrossRef]
- Izard, T. The crystal structures of phosphopantetheine adenylyltransferase with bound substrates reveal the enzyme’s catalytic mechanism11Edited by K. Nagai. J. Mol. Biol. 2002, 315, 487–495. [Google Scholar] [CrossRef]
- Shumar, S.A.; Kerr, E.W.; Geldenhuys, W.J.; Montgomery, G.E.; Fagone, P.; Thirawatananond, P.; Saavedra, H.; Gabelli, S.B.; Leonardi, R. Nudt19 is a renal CoA diphosphohydrolase with biochemical and regulatory properties that are distinct from the hepatic Nudt7 isoform. J. Biol. Chem. 2018, 293, 4134–4148. [Google Scholar] [CrossRef]
- Naquet, P.; Kerr, E.W.; Vickers, S.D.; Leonardi, R. Regulation of coenzyme A levels by degradation: The ‘Ins and Outs’. Prog. Lipid Res. 2020, 78, 101028. [Google Scholar] [CrossRef] [PubMed]
- Di Meo, I.; Colombelli, C.; Srinivasan, B.; de Villiers, M.; Hamada, J.; Jeong, S.Y.; Fox, R.; Woltjer, R.L.; Tepper, P.G.; Lahaye, L.L.; et al. Acetyl-4′-phosphopantetheine is stable in serum and prevents phenotypes induced by pantothenate kinase deficiency. Sci. Rep. 2017, 7, 11260. [Google Scholar] [CrossRef] [PubMed]
- Goosen, R.; Strauss, E. Simultaneous quantification of coenzyme A and its salvage pathway intermediates in in vitro and whole cell-sourced samples. Rsc. Adv. 2017, 7, 19717–19724. [Google Scholar] [CrossRef]
- Dunigan, D.D.; Waters, S.B.; Owen, T.C. Aqueous Soluble Tetrazolium/Formazan Mts as an Indicator of Nadh-Dependent and Nadph-Dependent Dehydrogenase-Activity. Biotechniques 1995, 19, 640–649. [Google Scholar] [PubMed]
- Mostert, K.J.; Sharma, N.; van der Zwaag, M.; Staats, R.; Koekemoer, L.; Anand, R.; Sibon, O.C.M.; Strauss, E. The Coenzyme A Level Modulator Hopantenate (HoPan) Inhibits Phosphopantotenoylcysteine Synthetase Activity. ACS Chem. Biol. 2021, 16, 2401–2414. [Google Scholar] [CrossRef]
- Reilly, S.-J.; Tillander, V.; Ofman, R.; Alexson, S.E.H.; Hunt, M.C. The Nudix Hydrolase 7 is an Acyl-CoA Diphosphatase Involved in Regulating Peroxisomal Coenzyme A Homeostasis. J. Biochem. 2008, 144, 655–663. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Löcherer, C.; Tosun, E.; Backes, H.; Jäschke, A. Thiophosphate Analogs of Coenzyme A and Its Precursors—Synthesis, Stability, and Biomimetic Potential. Biomolecules 2022, 12, 1065. https://doi.org/10.3390/biom12081065
Löcherer C, Tosun E, Backes H, Jäschke A. Thiophosphate Analogs of Coenzyme A and Its Precursors—Synthesis, Stability, and Biomimetic Potential. Biomolecules. 2022; 12(8):1065. https://doi.org/10.3390/biom12081065
Chicago/Turabian StyleLöcherer, Christian, Elif Tosun, Hannah Backes, and Andres Jäschke. 2022. "Thiophosphate Analogs of Coenzyme A and Its Precursors—Synthesis, Stability, and Biomimetic Potential" Biomolecules 12, no. 8: 1065. https://doi.org/10.3390/biom12081065





