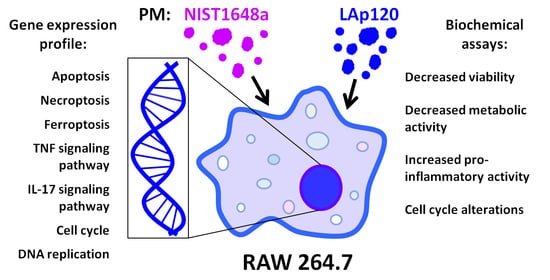
Gene Expression Changes Induced by Exposure of RAW 264.7 Macrophages to Particulate Matter of Air Pollution: The Role of Endotoxins
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Preparation of the PM Suspensions
2.3. Cell Culture and Treatment
2.4. Viability Assay
2.5. Metabolic Activity Assay
2.6. Assay for NO Release
2.7. Assessment of Cell Proliferation and the Cell Cycle
2.8. Assessment of the Endotoxin Content
2.9. Isolation of RNA
2.10. Microarray Hybridization
2.11. Microarray Data Analysis
2.12. Statistical Analysis
3. Results
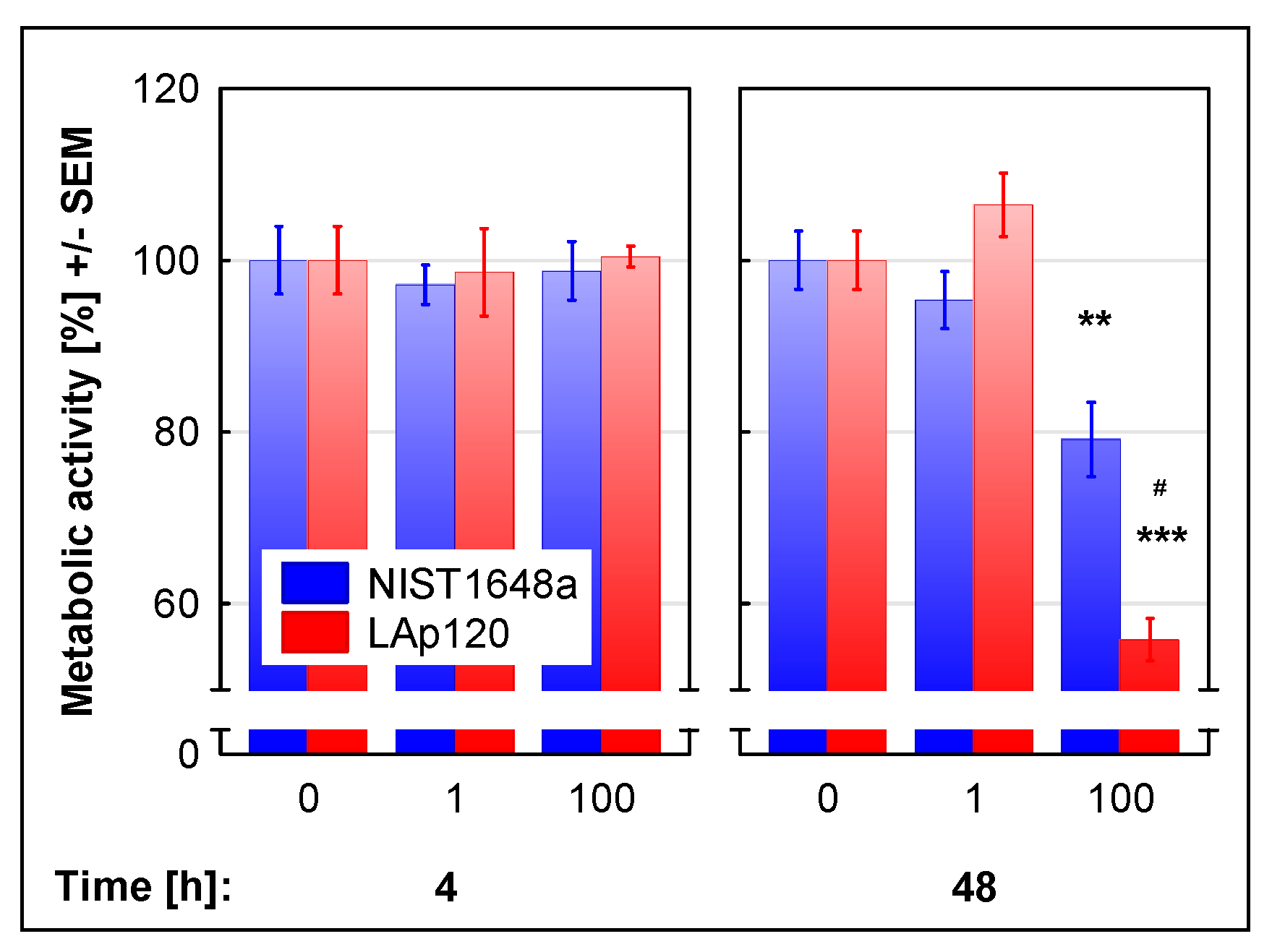
3.1. The Effect of PM on Cell Viability and Metabolic Activity
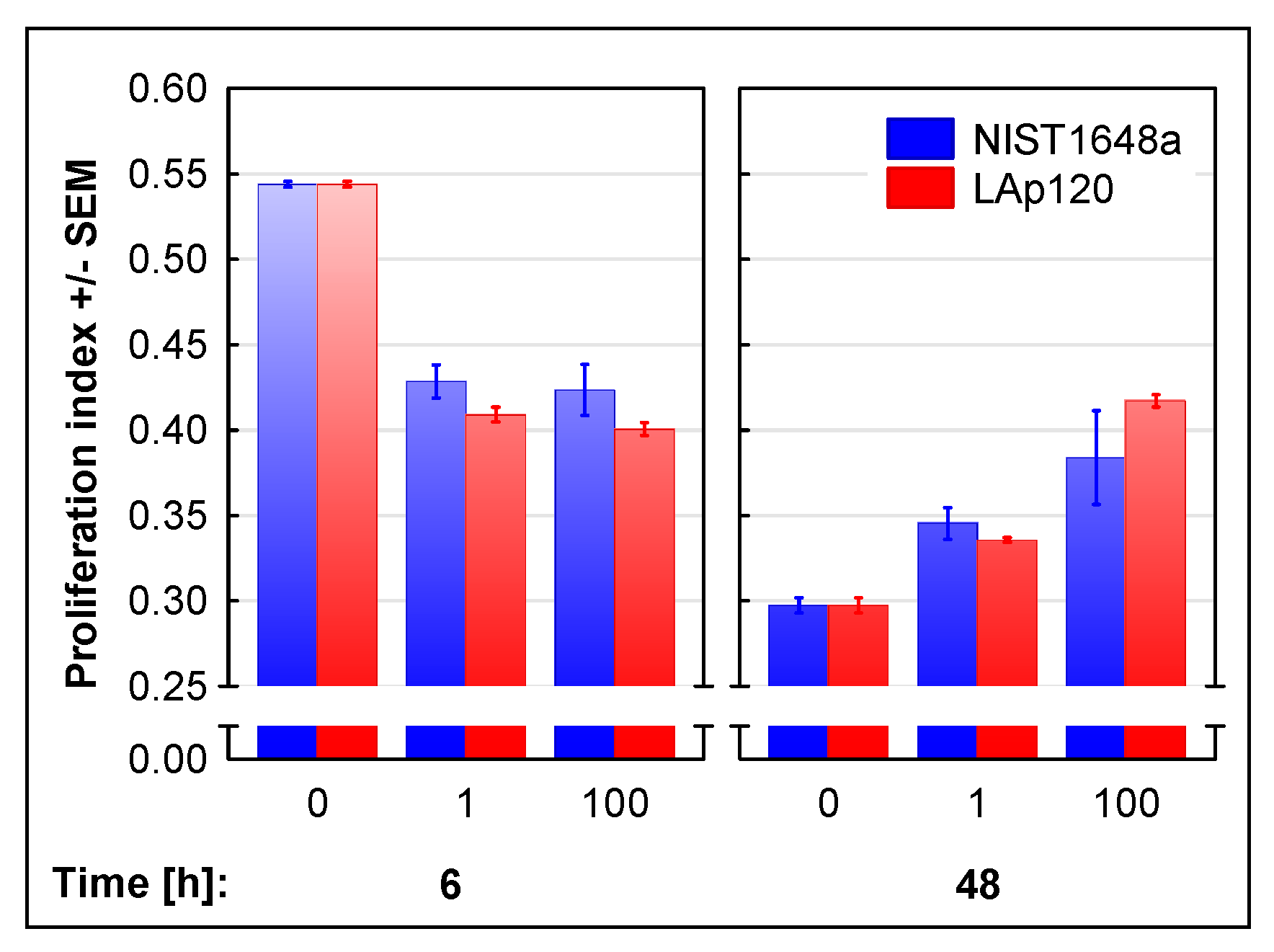
3.2. The Effect of PM on Cell Proliferation and Cell Cycle Distribution
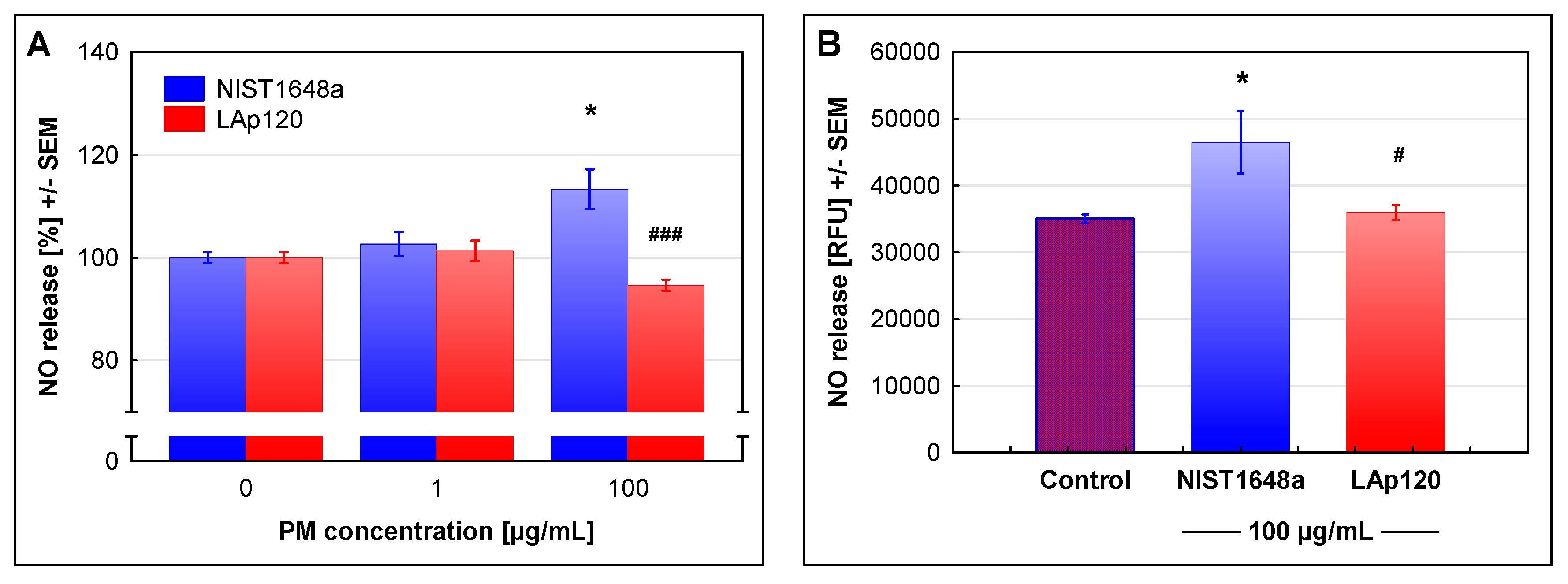
3.3. The Effect of PM on NO Release
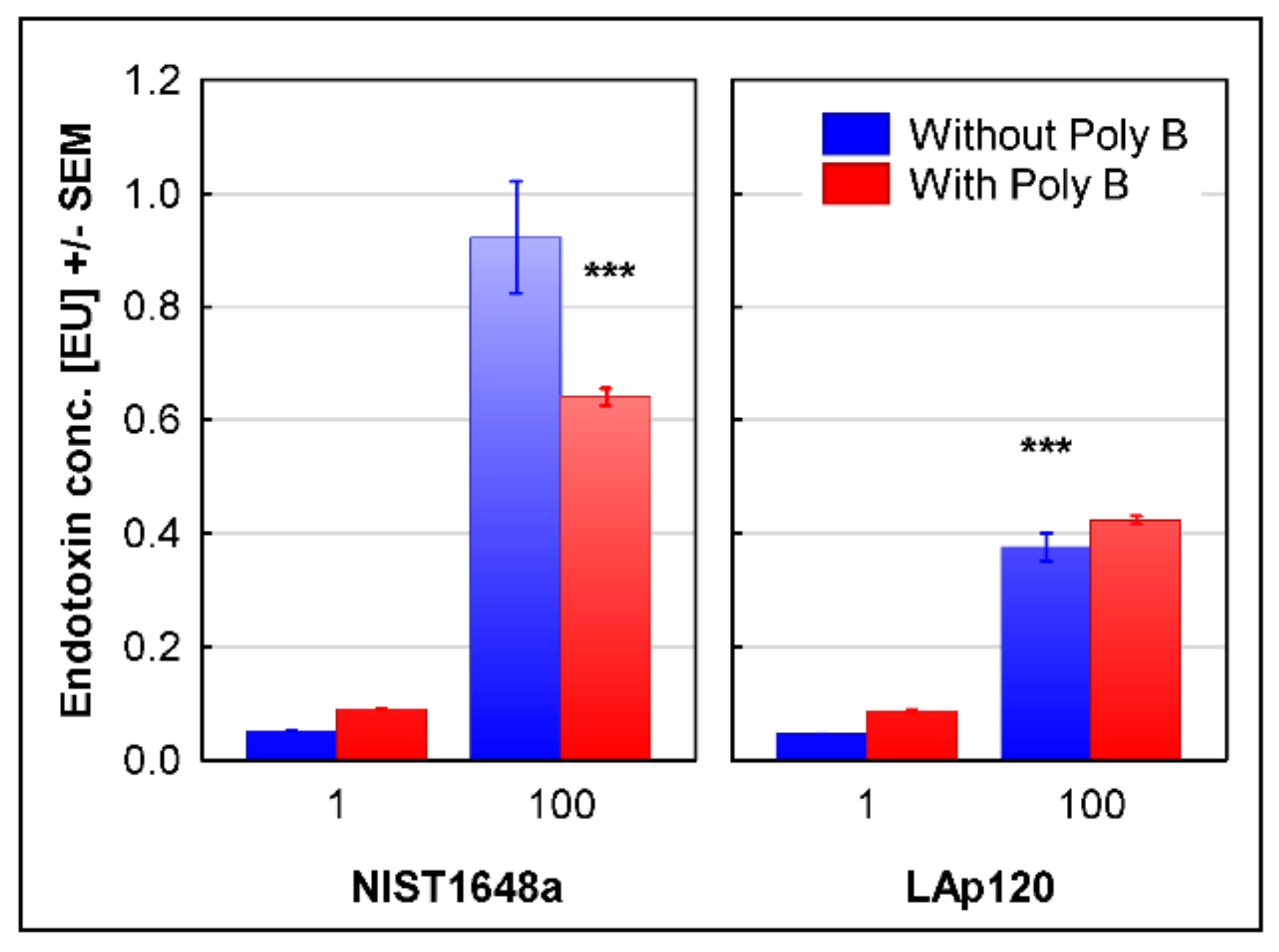
3.4. The Endotoxin Content in Both PM Forms
3.5. Changes in Gene Expression
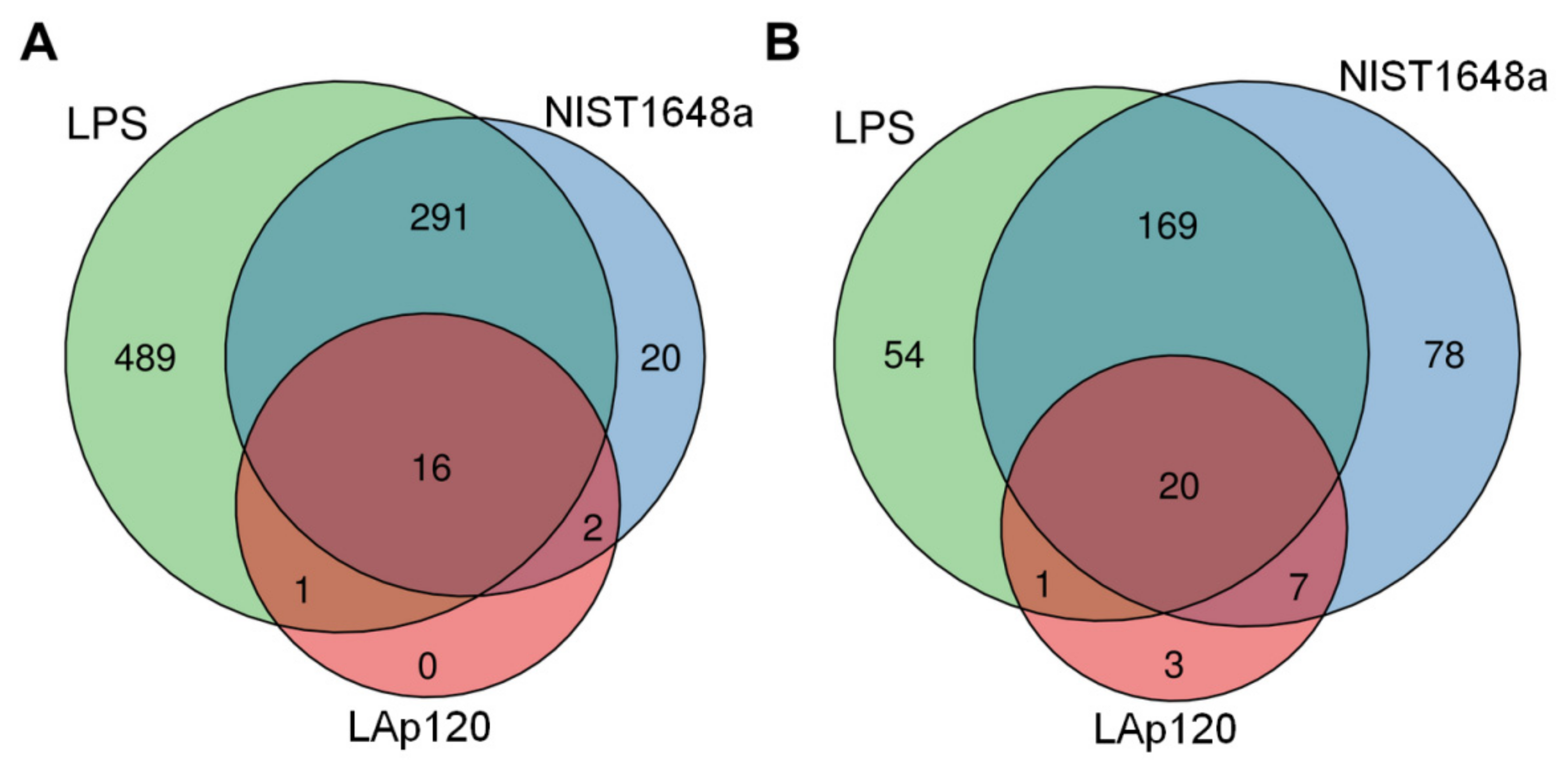
3.6. The Effect of PM or LPS on the Number of Regulated Genes
3.7. Association of the Changes Observed in Gene Expression and in Assessments of Cell Death
3.8. Association of the Changes Observed in Gene Expression and in Cell Cycle Distribution
3.9. Association of the Changes Observed in Gene Expression and Inflammatory Activation of the Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AHRs | aryl hydrocarbon receptors |
| ARG1 | arginase-1 |
| ARG2 | arginase-2 |
| BBB | blood–brain barrier |
| CDK | cyclin-dependent kinase |
| cDNA | complementary DNA |
| cRNA | complementary RNA |
| CSE | cigarette smoke extract |
| DAF-2 DA | 4,5-diaminofluorescein diacetate |
| DMEM | Dulbecco’s modified Eagle’s medium |
| DMSO | dimethyl sulfoxide |
| EU | endotoxin content |
| FBS | fetal bovine serum |
| HPA | hypothalamus–pituitary–adrenal axis |
| IL-1α | interleukin-1alpha |
| IL-1β | interleukin-1beta |
| IL-6 | interleukin 6 |
| IL-8 | interleukin 8 |
| IL-10 | interleukin 10 |
| IL-17 | interleukin 17 |
| iNOS | inducible nitric oxide synthase |
| LAL | limulus amebocyte lysate |
| LPS | lipopolysaccharide |
| MAPK | mitogen-activated protein kinase |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| NF-κB | nuclear factor-kappa B |
| NO | nitric oxide |
| PAH | polycyclic aromatic hydrocarbons |
| PBS | phosphate buffered saline |
| PI | propidium iodide |
| PM | particulate matter |
| Poly B | Polymyxin B |
| RFU | relative fluorescence units |
| ROS | reactive oxygen species |
| TLR4 | Toll-like receptor-4 |
| TNF-α | tumor necrosis factor-α |
| VOSO4 | vanadyl sulfate |
References
- Dominski, F.H.; Lorenzetti Branco, J.H.; Buonanno, G.; Stabile, L.; Gameiro da Silva, M.; Andrade, A. Effects of Air Pollution on Health: A Mapping Review of Systematic Reviews and Meta-Analyses. Environ. Res. 2021, 201, 111487. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Alvarez, A. Air Pollution and Life Expectancy in Europe: Does Investment in Renewable Energy Matter? Sci. Total Environ. 2021, 792, 148480. [Google Scholar] [CrossRef] [PubMed]
- Somboonsin, P.; Canudas-Romo, V. Mortality Attributable to Fine Particulate Matter in Asia, 2000-2015: A Cross-Sectional Cause-of-Death Analysis. BMJ Open 2021, 11, e043605. [Google Scholar] [CrossRef] [PubMed]
- Alemayehu, Y.A.; Asfaw, S.L.; Terfie, T.A. Exposure to Urban Particulate Matter and Its Association with Human Health Risks. Environ. Sci. Pollut. Res. Int. 2020, 27, 27491–27506. [Google Scholar] [CrossRef]
- Rönkkö, T.; Timonen, H. Overview of Sources and Characteristics of Nanoparticles in Urban Traffic-Influenced Areas. J. Alzheimers Dis. 2019, 72, 15–28. [Google Scholar] [CrossRef] [Green Version]
- Ali, M.U.; Liu, G.; Yousaf, B.; Ullah, H.; Abbas, Q.; Munir, M.A.M. A Systematic Review on Global Pollution Status of Particulate Matter-Associated Potential Toxic Elements and Health Perspectives in Urban Environment. Environ. Geochem. Health 2019, 41, 1131–1162. [Google Scholar] [CrossRef]
- Harrison, R.M. Airborne Particulate Matter. Philos. Trans. A Math. Phys. Eng. Sci. 2020, 378, 20190319. [Google Scholar] [CrossRef]
- Hu, W.; Wang, Y.; Wang, T.; Ji, Q.; Jia, Q.; Meng, T.; Ma, S.; Zhang, Z.; Li, Y.; Chen, R.; et al. Ambient Particulate Matter Compositions and Increased Oxidative Stress: Exposure-Response Analysis among High-Level Exposed Population. Environ. Int. 2021, 147, 106341. [Google Scholar] [CrossRef]
- Tao, S.; Xu, Y.; Chen, M.; Zhang, H.; Huang, X.; Li, Z.; Pan, B.; Peng, R.; Zhu, Y.; Kan, H.; et al. Exposure to Different Fractions of Diesel Exhaust PM2.5 Induces Different Levels of Pulmonary Inflammation and Acute Phase Response. Ecotoxicol. Environ. Saf. 2021, 210, 111871. [Google Scholar] [CrossRef]
- Xin, L.; Wang, J.; Sun, J.; Zhang, C.; Tong, X.; Wan, J.; Feng, J.; Tian, H.; Zhang, Z. Cellular Effects of PM2.5 from Suzhou, China: Relationship to Chemical Composition and Endotoxin Content. Environ. Sci. Pollut. Res. Int. 2021, 28, 287–299. [Google Scholar] [CrossRef]
- Lyu, Y.; Su, S.; Wang, B.; Zhu, X.; Wang, X.; Zeng, E.Y.; Xing, B.; Tao, S. Seasonal and Spatial Variations in the Chemical Components and the Cellular Effects of Particulate Matter Collected in Northern China. Sci. Total Environ. 2018, 627, 1627–1637. [Google Scholar] [CrossRef]
- Mazgaeen, L.; Gurung, P. Recent Advances in Lipopolysaccharide Recognition Systems. Int. J. Mol. Sci. 2020, 21, 379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maes, M.; Kubera, M.; Leunis, J.-C. The Gut-Brain Barrier in Major Depression: Intestinal Mucosal Dysfunction with an Increased Translocation of LPS from Gram Negative Enterobacteria (Leaky Gut) Plays a Role in the Inflammatory Pathophysiology of Depression. Neuro Endocrinol. Lett. 2008, 29, 117–124. [Google Scholar] [PubMed]
- Yue, Y.; Chen, H.; Setyan, A.; Elser, M.; Dietrich, M.; Li, J.; Zhang, T.; Zhang, X.; Zheng, Y.; Wang, J.; et al. Size-Resolved Endotoxin and Oxidative Potential of Ambient Particles in Beijing and Zürich. Environ. Sci. Technol. 2018, 52, 6816–6824. [Google Scholar] [CrossRef]
- Thomson, E.M.; Breznan, D.; Karthikeyan, S.; MacKinnon-Roy, C.; Vuong, N.Q.; Dabek-Zlotorzynska, E.; Celo, V.; Charland, J.-P.; Kumarathasan, P.; Brook, J.R.; et al. Contrasting Biological Potency of Particulate Matter Collected at Sites Impacted by Distinct Industrial Sources. Part. Fibre Toxicol. 2016, 13, 65. [Google Scholar] [CrossRef] [Green Version]
- Jalava, P.I.; Happo, M.S.; Huttunen, K.; Sillanpää, M.; Hillamo, R.; Salonen, R.O.; Hirvonen, M.-R. Chemical and Microbial Components of Urban Air PM Cause Seasonal Variation of Toxicological Activity. Environ. Toxicol. Pharmacol. 2015, 40, 375–387. [Google Scholar] [CrossRef]
- Chirino, Y.I.; Sánchez-Pérez, Y.; Osornio-Vargas, Á.R.; Rosas, I.; García-Cuellar, C.M. Sampling and Composition of Airborne Particulate Matter (PM10) from Two Locations of Mexico City. Data Brief 2015, 4, 353–356. [Google Scholar] [CrossRef] [Green Version]
- Nagappan, A.; Park, S.B.; Lee, S.-J.; Moon, Y. Mechanistic Implications of Biomass-Derived Particulate Matter for Immunity and Immune Disorders. Toxics 2021, 9, 18. [Google Scholar] [CrossRef]
- Glencross, D.A.; Ho, T.-R.; Camiña, N.; Hawrylowicz, C.M.; Pfeffer, P.E. Air Pollution and Its Effects on the Immune System. Free Radic. Biol. Med. 2020, 151, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A.; Chawla, A.; Pollard, J.W. Macrophage Biology in Development, Homeostasis and Disease. Nature 2013, 496, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Hiraiwa, K.; van Eeden, S.F. Contribution of Lung Macrophages to the Inflammatory Responses Induced by Exposure to Air Pollutants. Mediat. Inflamm. 2013, 2013, 619523. [Google Scholar] [CrossRef] [Green Version]
- Mills, C.D.; Ley, K. M1 and M2 Macrophages: The Chicken and the Egg of Immunity. J. Innate Immun. 2014, 6, 716–726. [Google Scholar] [CrossRef] [PubMed]
- Jankowska-Kieltyka, M.; Roman, A.; Mikrut, M.; Kowalska, M.; van Eldik, R.; Nalepa, I. Metabolic Response of RAW 264.7 Macrophages to Exposure to Crude Particulate Matter and a Reduced Content of Organic Matter. Toxics 2021, 9, 205. [Google Scholar] [CrossRef] [PubMed]
- Rath, M.; Müller, I.; Kropf, P.; Closs, E.I.; Munder, M. Metabolism via Arginase or Nitric Oxide Synthase: Two Competing Arginine Pathways in Macrophages. Front. Immunol. 2014, 5, 532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valacchi, G.; Magnani, N.; Woodby, B.; Ferreira, S.M.; Evelson, P. Particulate Matter Induces Tissue OxInflammation: From Mechanism to Damage. Antioxid. Redox Signal. 2020, 33, 308–326. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.-Y.; Wang, N.; Li, S.; Hong, M.; Wang, X.; Feng, Y. The Reactive Oxygen Species in Macrophage Polarization: Reflecting Its Dual Role in Progression and Treatment of Human Diseases. Oxid. Med. Cell Longev. 2016, 2016, 2795090. [Google Scholar] [CrossRef] [Green Version]
- Alexander, C.; Rietschel, E.T. Bacterial Lipopolysaccharides and Innate Immunity. J. Endotoxin Res. 2001, 7, 167–202. [Google Scholar] [CrossRef]
- Cho, C.-C.; Hsieh, W.-Y.; Tsai, C.-H.; Chen, C.-Y.; Chang, H.-F.; Lin, C.-S. In Vitro and In Vivo Experimental Studies of PM2.5 on Disease Progression. Int. J. Environ. Res. Public Health 2018, 15, 1380. [Google Scholar] [CrossRef] [Green Version]
- Chauhan, V.; Breznan, D.; Goegan, P.; Nadeau, D.; Karthikeyan, S.; Brook, J.R.; Vincent, R. Effects of Ambient Air Particles on Nitric Oxide Production in Macrophage Cell Lines. Cell Biol. Toxicol. 2004, 20, 221–239. [Google Scholar] [CrossRef]
- Zhong, Y.; Liao, J.; Hu, Y.; Wang, Y.; Sun, C.; Zhang, C.; Wang, G. PM2.5 Upregulates MicroRNA-146a-3p and Induces M1 Polarization in RAW264.7 Cells by Targeting Sirtuin1. Int. J. Med. Sci. 2019, 16, 384–393. [Google Scholar] [CrossRef] [Green Version]
- Happo, M.S.; Hirvonen, M.-R.; Uski, O.; Kasurinen, S.; Kelz, J.; Brunner, T.; Obernberger, I.; Jalava, P.I. Particulate Emissions from Modern and Old Technology Wood Combustion Induce Distinct Time-Dependent Patterns of Toxicological Responses in Vitro. Toxicol In Vitro 2017, 44, 164–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Institute of Standards and Technology. Certificate of Analysis, Standard Reference Material® 1648a, Urban Particulate Matter. 2015. Available online: https://www-s.nist.gov/srmors/view_cert.cfm?srm=1648A (accessed on 7 July 2022).
- Mikrut, M.; Regiel-Futyra, A.; Samek, L.; Macyk, W.; Stochel, G.; van Eldik, R. Generation of Hydroxyl Radicals and Singlet Oxygen by Particulate Matter and Its Inorganic Components. Environ. Pollut. 2018, 238, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Zamai, L.; Canonico, B.; Luchetti, F.; Ferri, P.; Melloni, E.; Guidotti, L.; Cappellini, A.; Cutroneo, G.; Vitale, M.; Papa, S. Supravital Exposure to Propidium Iodide Identifies Apoptosis on Adherent Cells. Cytometry 2001, 44, 57–64. [Google Scholar] [CrossRef]
- Roman, A.; Kuśmierczyk, J.; Kreiner, G.; Nalepa, I. Assessment of Leukocyte Activity in Mice Devoid of the Glucocorticoid Receptor in the Noradrenergic System (GRDBHCre). Immunobiology 2018, 223, 227–238. [Google Scholar] [CrossRef]
- Zhang, H.; Du, G.; Zhang, J. Assay of Mitochondrial Functions by Resazurin in Vitro. Acta Pharmacol. Sin. 2004, 25, 385–389. [Google Scholar]
- O’Brien, J.; Wilson, I.; Orton, T.; Pognan, F. Investigation of the Alamar Blue (Resazurin) Fluorescent Dye for the Assessment of Mammalian Cell Cytotoxicity. Eur. J. Biochem. 2000, 267, 5421–5426. [Google Scholar] [CrossRef]
- Marcinkiewicz, J.; Pater, M.; Grabowska, A. An Improved Experimental Model for the Study of in Vitro Release of Nitric Oxide by Murine Peritoneal Macrophages. Arch. Immunol. Ther. Exp. 1994, 42, 95–99. [Google Scholar]
- Itoh, Y.; Ma, F.H.; Hoshi, H.; Oka, M.; Noda, K.; Ukai, Y.; Kojima, H.; Nagano, T.; Toda, N. Determination and Bioimaging Method for Nitric Oxide in Biological Specimens by Diaminofluorescein Fluorometry. Anal. Biochem. 2000, 287, 203–209. [Google Scholar] [CrossRef]
- Kojima, H.; Sakurai, K.; Kikuchi, K.; Kawahara, S.; Kirino, Y.; Nagoshi, H.; Hirata, Y.; Nagano, T. Development of a Fluorescent Indicator for Nitric Oxide Based on the Fluorescein Chromophore. Chem. Pharm. Bull. 1998, 46, 373–375. [Google Scholar] [CrossRef] [Green Version]
- Nicoletti, I.; Migliorati, G.; Pagliacci, M.C.; Grignani, F.; Riccardi, C. A Rapid and Simple Method for Measuring Thymocyte Apoptosis by Propidium Iodide Staining and Flow Cytometry. J. Immunol. Methods 1991, 139, 271–279. [Google Scholar] [CrossRef]
- Wersto, R.P.; Chrest, F.J.; Leary, J.F.; Morris, C.; Stetler-Stevenson, M.A.; Gabrielson, E. Doublet Discrimination in DNA Cell-Cycle Analysis. Cytometry 2001, 46, 296–306. [Google Scholar] [CrossRef]
- Bertho, A.L.; Santiago, M.A.; Coutinho, S.G. Flow Cytometry in the Study of Cell Death. Mem. Inst. Oswaldo Cruz 2000, 95, 429–433. [Google Scholar] [CrossRef] [Green Version]
- Kudryavtseva, N.N.; Shurlygina, A.V.; Melnikova, E.V.; Tenditnik, M.V.; Bondar, N.P.; Panteleeva, N.G.; Smagin, D.A.; Kolesnikov, N.N.; Trufakin, V.A. Cell Cycle Arrest in the Thymus and Spleen in Male Mice under Conditions of Chronic Social Defeat Stress: Effects of Diazepam. Bull. Exp. Biol. Med. 2011, 151, 411–414. [Google Scholar] [CrossRef] [PubMed]
- Morrison, D.C.; Jacobs, D.M. Binding of Polymyxin B to the Lipid A Portion of Bacterial Lipopolysaccharides. Immunochemistry 1976, 13, 813–818. [Google Scholar] [CrossRef]
- Tsuji, K.; Martin, P.A.; Bussey, D.M. Automation of Chromogenic Substrate Limulus Amebocyte Lysate Assay Method for Endotoxin by Robotic System. Appl. Environ. Microbiol. 1984, 48, 550–555. [Google Scholar] [CrossRef] [Green Version]
- Kuleshov, M.V.; Jones, M.R.; Rouillard, A.D.; Fernandez, N.F.; Duan, Q.; Wang, Z.; Koplev, S.; Jenkins, S.L.; Jagodnik, K.M.; Lachmann, A.; et al. Enrichr: A Comprehensive Gene Set Enrichment Analysis Web Server 2016 Update. Nucleic Acids Res. 2016, 44, W90–W97. [Google Scholar] [CrossRef] [Green Version]
- Foto, M.; Plett, J.; Berghout, J.; Miller, J.D. Modification of the Limulus Amebocyte Lysate Assay for the Analysis of Glucan in Indoor Environments. Anal. Bioanal. Chem. 2004, 379, 156–162. [Google Scholar] [CrossRef]
- Zídek, Z.; Kmoníčková, E.; Kostecká, P.; Jansa, P. Microfiltration Method of Removal of Bacterial Contaminants and Their Monitoring by Nitric Oxide and Limulus Assays. Nitric Oxide 2013, 28, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Niu, F.; Yu, Y.; Li, Z.; Ren, Y.; Li, Z.; Ye, Q.; Liu, P.; Ji, C.; Qian, L.; Xiong, Y. Arginase: An Emerging and Promising Therapeutic Target for Cancer Treatment. Biomed. Pharmacother. 2022, 149, 112840. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, M.S.; de Oliveira Galvão, M.F.; Batistuzzo de Medeiros, S.R. Cell Death Pathways of Particulate Matter Toxicity. Chemosphere 2017, 188, 32–48. [Google Scholar] [CrossRef]
- De Stefano, D.; Carnuccio, R.; Maiuri, M.C. Nanomaterials Toxicity and Cell Death Modalities. J. Drug Deliv. 2012, 2012, 167896. [Google Scholar] [CrossRef] [Green Version]
- Darzynkiewicz, Z.; Bruno, S.; Del Bino, G.; Gorczyca, W.; Hotz, M.A.; Lassota, P.; Traganos, F. Features of Apoptotic Cells Measured by Flow Cytometry. Cytometry 1992, 13, 795–808. [Google Scholar] [CrossRef] [PubMed]
- Aleshin, V.A.; Artiukhov, A.V.; Oppermann, H.; Kazantsev, A.V.; Lukashev, N.V.; Bunik, V.I. Mitochondrial Impairment May Increase Cellular NAD(P)H: Resazurin Oxidoreductase Activity, Perturbing the NAD(P)H-Based Viability Assays. Cells 2015, 4, 427–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ścibior, A.; Szychowski, K.A.; Zwolak, I.; Dachowska, K.; Gmiński, J. In Vitro Effect of Vanadyl Sulfate on Cultured Primary Astrocytes: Cell Viability and Oxidative Stress Markers. J. Appl. Toxicol. 2020, 40, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Ardon-Dryer, K.; Mock, C.; Reyes, J.; Lahav, G. The Effect of Dust Storm Particles on Single Human Lung Cancer Cells. Environ. Res. 2020, 181, 108891. [Google Scholar] [CrossRef] [PubMed]
- Shan, X.; Liu, L.; Li, G.; Xu, K.; Liu, B.; Jiang, W. PM2.5 and the Typical Components Cause Organelle Damage, Apoptosis and Necrosis: Role of Reactive Oxygen Species. Sci. Total Environ. 2021, 782, 146785. [Google Scholar] [CrossRef]
- Wei, H.; Yuan, W.; Yu, H.; Geng, H. Cytotoxicity Induced by Fine Particulate Matter (PM2.5) via Mitochondria-Mediated Apoptosis Pathway in Rat Alveolar Macrophages. Environ. Sci. Pollut. Res. Int. 2021, 28, 25819–25829. [Google Scholar] [CrossRef]
- Xu, F.; Luo, M.; He, L.; Cao, Y.; Li, W.; Ying, S.; Chen, Z.; Shen, H. Necroptosis Contributes to Urban Particulate Matter-Induced Airway Epithelial Injury. Cell Physiol. Biochem. 2018, 46, 699–712. [Google Scholar] [CrossRef]
- Kiraz, Y.; Adan, A.; Kartal Yandim, M.; Baran, Y. Major Apoptotic Mechanisms and Genes Involved in Apoptosis. Tumour Biol. 2016, 37, 8471–8486. [Google Scholar] [CrossRef] [Green Version]
- Pentimalli, F.; Grelli, S.; Di Daniele, N.; Melino, G.; Amelio, I. Cell Death Pathologies: Targeting Death Pathways and the Immune System for Cancer Therapy. Genes Immun. 2019, 20, 539–554. [Google Scholar] [CrossRef]
- Park, M.Y.; Ha, S.E.; Vetrivel, P.; Kim, H.H.; Bhosale, P.B.; Abusaliya, A.; Kim, G.S. Differences of Key Proteins between Apoptosis and Necroptosis. Biomed. Res. Int. 2021, 2021, 3420168. [Google Scholar] [CrossRef] [PubMed]
- Belizário, J.; Vieira-Cordeiro, L.; Enns, S. Necroptotic Cell Death Signaling and Execution Pathway: Lessons from Knockout Mice. Mediat. Inflamm. 2015, 2015, 128076. [Google Scholar] [CrossRef] [Green Version]
- Mohammadinejad, R.; Moosavi, M.A.; Tavakol, S.; Vardar, D.Ö.; Hosseini, A.; Rahmati, M.; Dini, L.; Hussain, S.; Mandegary, A.; Klionsky, D.J. Necrotic, Apoptotic and Autophagic Cell Fates Triggered by Nanoparticles. Autophagy 2019, 15, 4–33. [Google Scholar] [CrossRef] [Green Version]
- Pu, F.; Chen, F.; Zhang, Z.; Shi, D.; Zhong, B.; Lv, X.; Tucker, A.B.; Fan, J.; Li, A.J.; Qin, K.; et al. Ferroptosis as a Novel Form of Regulated Cell Death: Implications in the Pathogenesis, Oncometabolism and Treatment of Human Cancer. Genes Dis. 2022, 9, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tang, M. PM2.5 Induces Ferroptosis in Human Endothelial Cells through Iron Overload and Redox Imbalance. Environ. Pollut. 2019, 254, 112937. [Google Scholar] [CrossRef]
- Liu, Z.; Xia, X.; Lv, X.; Song, E.; Song, Y. Iron-Bearing Nanoparticles Trigger Human Umbilical Vein Endothelial Cells Ferroptotic Responses by Promoting Intracellular Iron Level. Environ. Pollut. 2021, 287, 117345. [Google Scholar] [CrossRef] [PubMed]
- Sepand, M.R.; Maghsoudi, A.S.; Shadboorestan, A.; Mirnia, K.; Aghsami, M.; Raoufi, M. Cigarette Smoke-Induced Toxicity Consequences of Intracellular Iron Dysregulation and Ferroptosis. Life Sci. 2021, 281, 119799. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, J.; Chen, C.; Song, Y.; Pan, J. Transcriptomic Analysis of Key Genes and Pathways in Human Bronchial Epithelial Cells BEAS-2B Exposed to Urban Particulate Matter. Environ. Sci. Pollut. Res. Int. 2021, 28, 9598–9609. [Google Scholar] [CrossRef]
- Somborac-Bačura, A.; Rumora, L.; Novak, R.; Rašić, D.; Dumić, J.; Čepelak, I.; Žanić-Grubišić, T. Differential Expression of Heat Shock Proteins and Activation of Mitogen-Activated Protein Kinases in A549 Alveolar Epithelial Cells Exposed to Cigarette Smoke Extract. Exp. Physiol. 2018, 103, 1666–1678. [Google Scholar] [CrossRef]
- Marchetti, S.; Longhin, E.; Bengalli, R.; Avino, P.; Stabile, L.; Buonanno, G.; Colombo, A.; Camatini, M.; Mantecca, P. In Vitro Lung Toxicity of Indoor PM10 from a Stove Fueled with Different Biomasses. Sci. Total Environ. 2019, 649, 1422–1433. [Google Scholar] [CrossRef]
- Wang, B.Y.; Cai, Y.; Zheng, K.; Huang, H.Y.; Qin, X.Y.; Xu, X.Y. PM 2.5-Induced Alterations of Gene Expression in HBE Cells Revealed by Gene Chip Analysis. Biomed. Environ. Sci. 2020, 33, 213–216. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Tang, W.; Wang, L.; Xue, W.; Yao, W.; Zhong, Y.; Qiu, X.; Li, Y.; Chen, Y.; Wang, H.; et al. Transcriptomics Changes and the Candidate Pathway in Human Macrophages Induced by Different PM2.5 Extracts. Environ. Pollut. 2021, 289, 117890. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ghio, A.J.; Gao, M.; Wei, K.; Rosen, G.D.; Upadhyay, D. Ambient Particulate Matter Induces Alveolar Epithelial Cell Cycle Arrest: Role of G1 Cyclins. FEBS Lett. 2007, 581, 5315–5320. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Yang, D.; Yang, B.; Li, B.; Guo, J.; Xiao, C. PM2.5 Induces Cell Cycle Arrest through Regulating MTOR/P70S6K1 Signaling Pathway. Exp. Ther. Med. 2019, 17, 4371–4378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.; Shi, Y.; Asweto, C.O.; Feng, L.; Yang, X.; Zhang, Y.; Hu, H.; Duan, J.; Sun, Z. Fine Particle Matters Induce DNA Damage and G2/M Cell Cycle Arrest in Human Bronchial Epithelial BEAS-2B Cells. Environ. Sci. Pollut. Res. Int. 2017, 24, 25071–25081. [Google Scholar] [CrossRef]
- Rieder, C.L. Mitosis in Vertebrates: The G2/M and M/A Transitions and Their Associated Checkpoints. Chromosome Res. 2011, 19, 291–306. [Google Scholar] [CrossRef] [Green Version]
- Longhin, E.; Holme, J.A.; Gutzkow, K.B.; Arlt, V.M.; Kucab, J.E.; Camatini, M.; Gualtieri, M. Cell Cycle Alterations Induced by Urban PM2.5 in Bronchial Epithelial Cells: Characterization of the Process and Possible Mechanisms Involved. Part. Fibre Toxicol. 2013, 10, 63. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Liu, Q.; Liu, Y.; Qi, X.; Wang, X. Cell Cycle Arrest of Human Bronchial Epithelial Cells Modulated by Differences in Chemical Components of Particulate Matter. RSC Adv. 2021, 11, 10582–10591. [Google Scholar] [CrossRef] [PubMed]
- Arias-Pérez, R.D.; Taborda, N.A.; Gómez, D.M.; Narvaez, J.F.; Porras, J.; Hernandez, J.C. Inflammatory Effects of Particulate Matter Air Pollution. Environ. Sci. Pollut. Res. Int. 2020, 27, 42390–42404. [Google Scholar] [CrossRef]
- Øvrevik, J.; Refsnes, M.; Låg, M.; Brinchmann, B.C.; Schwarze, P.E.; Holme, J.A. Triggering Mechanisms and Inflammatory Effects of Combustion Exhaust Particles with Implication for Carcinogenesis. Basic Clin. Pharmacol. Toxicol. 2017, 121 (Suppl. 3), 55–62. [Google Scholar] [CrossRef] [Green Version]
- Guerra, E.; Oliveira, T.; Trancoso, I.A.; Lorençoni, M.F.; Souza Júnior, A.D.; Campagnaro, B.P.; Coco, L.Z.; Weitzel Dias Carneiro, M.T.; do Espírito Santo Lemos, M.; Endringer, D.C.; et al. Toxicological Effects of Air Settled Particles from the Vitoria Metropolitan Area Mediated by Oxidative Stress, pro-Inflammatory Mediators and NFΚB Pathway. Environ. Res. 2022, 204, 112015. [Google Scholar] [CrossRef]
- Jalava, P.I.; Salonen, R.O.; Pennanen, A.S.; Sillanpää, M.; Hälinen, A.I.; Happo, M.S.; Hillamo, R.; Brunekreef, B.; Katsouyanni, K.; Sunyer, J.; et al. Heterogeneities in Inflammatory and Cytotoxic Responses of RAW 264.7 Macrophage Cell Line to Urban Air Coarse, Fine, and Ultrafine Particles from Six European Sampling Campaigns. Inhal. Toxicol. 2007, 19, 213–225. [Google Scholar] [CrossRef]
- Salonen, R.O.; Hälinen, A.I.; Pennanen, A.S.; Hirvonen, M.-R.; Sillanpää, M.; Hillamo, R.; Shi, T.; Borm, P.; Sandell, E.; Koskentalo, T.; et al. Chemical and in Vitro Toxicologic Characterization of Wintertime and Springtime Urban-Air Particles with an Aerodynamic Diameter below 10 Microm in Helsinki. Scand. J. Work Environ. Health 2004, 30 (Suppl. 2), 80–90. [Google Scholar] [PubMed]
- Schins, R.P.F.; Lightbody, J.H.; Borm, P.J.A.; Shi, T.; Donaldson, K.; Stone, V. Inflammatory Effects of Coarse and Fine Particulate Matter in Relation to Chemical and Biological Constituents. Toxicol. Appl. Pharmacol. 2004, 195, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, H.; Sadeghi, S.; Taghipour, N.; Roshani, M.; Amani, D.; Ghazanfari, T.; Mosaffa, N. The Effects of Particulate Matter on C57BL/6 Peritoneal and Alveolar Macrophages. Iran. J. Allergy Asthma Immunol. 2020, 19, 647–659. [Google Scholar] [CrossRef]
- Gałuszka, A.; Stec, M.; Węglarczyk, K.; Kluczewska, A.; Siedlar, M.; Baran, J. Transition Metal Containing Particulate Matter Promotes Th1 and Th17 Inflammatory Response by Monocyte Activation in Organic and Inorganic Compounds Dependent Manner. Int. J. Environ. Res. Public Health 2020, 17, 1227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jankowska-Kieltyka, M.; Roman, A.; Nalepa, I. The Air We Breathe: Air Pollution as a Prevalent Proinflammatory Stimulus Contributing to Neurodegeneration. Front. Cell Neurosci. 2021, 15, 647643. [Google Scholar] [CrossRef]
- Mutlu, E.A.; Comba, I.Y.; Cho, T.; Engen, P.A.; Yazıcı, C.; Soberanes, S.; Hamanaka, R.B.; Niğdelioğlu, R.; Meliton, A.Y.; Ghio, A.J.; et al. Inhalational Exposure to Particulate Matter Air Pollution Alters the Composition of the Gut Microbiome. Environ. Pollut. 2018, 240, 817–830. [Google Scholar] [CrossRef]
- Mutlu, E.A.; Engen, P.A.; Soberanes, S.; Urich, D.; Forsyth, C.B.; Nigdelioglu, R.; Chiarella, S.E.; Radigan, K.A.; Gonzalez, A.; Jakate, S.; et al. Particulate Matter Air Pollution Causes Oxidant-Mediated Increase in Gut Permeability in Mice. Part. Fibre Toxicol. 2011, 8, 19. [Google Scholar] [CrossRef] [Green Version]
- Sherwin, E.; Dinan, T.G.; Cryan, J.F. Recent Developments in Understanding the Role of the Gut Microbiota in Brain Health and Disease. Ann. N. Y. Acad. Sci. 2018, 1420, 5–25. [Google Scholar] [CrossRef]
- Rudzki, L.; Maes, M. The Microbiota-Gut-Immune-Glia (MGIG) Axis in Major Depression. Mol. Neurobiol. 2020, 57, 4269–4295. [Google Scholar] [CrossRef] [PubMed]
- Eicher, T.P.; Mohajeri, M.H. Overlapping Mechanisms of Action of Brain-Active Bacteria and Bacterial Metabolites in the Pathogenesis of Common Brain Diseases. Nutrients 2022, 14, 2661. [Google Scholar] [CrossRef] [PubMed]
- Vogel, C.F.A.; Van Winkle, L.S.; Esser, C.; Haarmann-Stemmann, T. The Aryl Hydrocarbon Receptor as a Target of Environmental Stressors-Implications for Pollution Mediated Stress and Inflammatory Responses. Redox Biol. 2020, 34, 101530. [Google Scholar] [CrossRef]
- O’Driscoll, C.A.; Gallo, M.E.; Fechner, J.H.; Schauer, J.J.; Mezrich, J.D. Real-World PM Extracts Differentially Enhance Th17 Differentiation and Activate the Aryl Hydrocarbon Receptor (AHR). Toxicology 2019, 414, 14–26. [Google Scholar] [CrossRef]
- Jia, Y.-Y.; Wang, Q.; Liu, T. Toxicity Research of PM2.5 Compositions In Vitro. Int. J. Environ. Res. Public Health 2017, 14, 232. [Google Scholar] [CrossRef] [Green Version]
- Grytting, V.S.; Chand, P.; Låg, M.; Øvrevik, J.; Refsnes, M. The Pro-Inflammatory Effects of Combined Exposure to Diesel Exhaust Particles and Mineral Particles in Human Bronchial Epithelial Cells. Part. Fibre Toxicol. 2022, 19, 14. [Google Scholar] [CrossRef] [PubMed]




| Cluster and Term | Gene Name | NIST1648a | LAp120 | ||
|---|---|---|---|---|---|
| 4 h | 48 h | 4 h | 48 h | ||
| Cluster 1: Apoptosis | Birc3 | 0.0161 | 1 | 0.3763 | 1 |
| Daxx | 1.50 × 10−5 | 1 | 1 | 1 | |
| Fas | 9.20 × 10−5 | 0.0250 | 0.0225 | 0.8011 | |
| Gadd45b | 0.0162 | 1 | 1 | 1 | |
| Nfkb1 | 0.0023 | 1 | 0.2698 | 1 | |
| Nfkbia | 4.78 × 10−4 | 1 | 0.0220 | 1 | |
| Tnf | 1.50 × 10−5 | 9.20 × 10−5 | 0.0016 | 0.0409 | |
| Traf1 | 0.0010 | 1 | 0.1156 | 1 | |
| Cluster 1: Necroptosis | Birc3 | 0.0161 | 1 | 0.3763 | 1 |
| Fas | 9.20 × 10−5 | 0.0250 | 0.0225 | 0.8011 | |
| Jak2 | 0.0110 | 1 | 1 | 1 | |
| Nlrp3 | 0.0072 | 1 | 0.2144 | 1 | |
| Stat2 | 0.0251 | 1 | 1 | 1 | |
| Tnf | 1.50 × 10−5 | 9.60 × 10−5 | 0.0016 | 0.0409 | |
| Tnfaip3 | 1.10 × 10−4 | 1 | 1 | 1 | |
| Zbp1 | 5.46 × 10−4 | 1 | 1 | 1 | |
| Cluster 5: Ferroptosis | Gclm | 0.0016 | 0.0041 | 0.0135 | 0.0204 |
| Hmox1 | 0.0266 | 0.0139 | 0.0462 | 0.0169 | |
| Slc40a1 | 0.0028 | 0.3026 | 3.31 × 10−4 | 0.0159 | |
| Cluster and Term | Gene Name | NIST1648a | LAp120 | ||
|---|---|---|---|---|---|
| 4 h | 48 h | 4 h | 48 h | ||
| Cluster 6: Cell cycle | Bub1 | 0.1272 | 6.71 × 10−4 | 1 | 1 |
| Ccna2 | 0.0826 | 0.0023 | 0.1973 | 1 | |
| Ccne2 | 0.0036 | 4.30 × 10−5 | 0.2351 | 1 | |
| Cdc20 | 1 | 5.70 × 10−5 | 1 | 0.0011 | |
| Cdc6 | 0.0948 | 6.20 × 10−4 | 0.979 | 1 | |
| Cdkn2c | 7.72 × 10−4 | 0.0032 | 0.0536 | 0.7181 | |
| Chek2 | 0.0033 | 0.3525 | 1 | 1 | |
| Mcm6 | 2.70 × 10−5 | 1.8. × 10−4 | 0.2187 | 1 | |
| Mcm7 | 0.0309 | 0.0042 | 1 | 1 | |
| Plk1 | 0.0691 | 6.00 × 10−5 | 1 | 0.0711 | |
| Tfdp2 | 9.21 × 10−4 | 0.0062 | 0.2147 | 1 | |
| Ttk | 0.0044 | 3.90 × 10−5 | 1 | 0.0485 | |
| Wee1 | 0.0178 | 3.58 × 10−4 | 0.4424 | 1 | |
| Cluster 7: Cell cycle | Bub1b | 0.0247 | 4.50 × 10−5 | 1 | 0.0054 |
| Ccnb1 | 0.1038 | 0.0311 | 1 | 1 | |
| Ccnb2 | 1 | 0.0027 | 1 | 0.0352 | |
| Cdc25c | 0.0513 | 0.0150 | 1 | 1 | |
| Cdk1 | 1 | 0.0118 | 1 | 1 | |
| Espl1 | 1 | 0.0365 | 1 | 1 | |
| Mad2l1 | 0.0087 | 0.0040 | 0.1029 | 1 | |
| Mcm5 | 0.1021 | 0.0074 | 1 | 1 | |
| Cluster and Term | Gene Name | NIST1648a | LAp120 | ||
|---|---|---|---|---|---|
| 4 h | 48 h | 4 h | 48 h | ||
| Cluster 1 | Nos2 | 0.0471 | 0.1388 | 1 | 1 |
| Cluster 3 | Arg2 | 0.0492 | 0.0151 | 1 | 1 |
| Cluster 1: TNF signaling pathway | Birc3 | 0.0161 | 1 | 0.3763 | 1 |
| Ccl5 | 9.73 × 10−4 | 0.0128 | 1 | 1 | |
| Csf1 | 1.10 × 10−5 | 0.0152 | 0.0086 | 1 | |
| Csf2 | 3.80 × 10−5 | 1 | 1 | 1 | |
| Cxcl2 | 7.00 × 10−6 | 0.0248 | 9.20 × 10−4 | 1 | |
| Cxcl3 | 3.98 × 10−4 | 0.2441 | 1 | 0.2036 | |
| Edn1 | 0.0410 | 1 | 1 | 1 | |
| Fas | 9.20 × 10−5 | 0.0250 | 0.0225 | 0.8011 | |
| Icam1 | 2.93 × 10−4 | 1 | 0.2066 | 1 | |
| Il6 | 9.69 × 10−4 | 0.0942 | 1 | 1 | |
| Irf1 | 0.0555 | 1 | 1 | 1 | |
| Junb | 0.1259 | 0.0666 | 1 | 1 | |
| Lif | 0.0020 | 1 | 1 | 1 | |
| Lta | 0.0021 | 0.6914 | 0.9670 | 1 | |
| Nfkb1 | 0.0023 | 1 | 0.2698 | 1 | |
| Nfkbia | 4.78 × 10−4 | 1 | 0.02120 | 1 | |
| Ptgs2 | 4.90 × 10−5 | 3.09 × 10−4 | 0.0014 | 1 | |
| Tnf | 1.50 × 10−5 | 9.60 × 10−5 | 0.0016 | 0.0409 | |
| Tnfaip3 | 1.10 × 10−4 | 1 | 1 | 1 | |
| Traf1 | 0.0010 | 1 | 0.1156 | 1 | |
| Cluster 3: IL-17 signaling pathway | Ccl2 | 3.59 × 10−4 | 0.0034 | 1 | 1 |
| Ccl7 | 0.0149 | 6.07 × 10−4 | 1 | 1 | |
| Lcn2 | 2.64 × 10−4 | 0.0547 | 0.6715 | 1 | |
| Mmp9 | 0.0202 | 1 | 0.6668 | 1 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roman, A.; Korostyński, M.; Jankowska-Kieltyka, M.; Piechota, M.; Hajto, J.; Nalepa, I. Gene Expression Changes Induced by Exposure of RAW 264.7 Macrophages to Particulate Matter of Air Pollution: The Role of Endotoxins. Biomolecules 2022, 12, 1100. https://doi.org/10.3390/biom12081100
Roman A, Korostyński M, Jankowska-Kieltyka M, Piechota M, Hajto J, Nalepa I. Gene Expression Changes Induced by Exposure of RAW 264.7 Macrophages to Particulate Matter of Air Pollution: The Role of Endotoxins. Biomolecules. 2022; 12(8):1100. https://doi.org/10.3390/biom12081100
Chicago/Turabian StyleRoman, Adam, Michał Korostyński, Monika Jankowska-Kieltyka, Marcin Piechota, Jacek Hajto, and Irena Nalepa. 2022. "Gene Expression Changes Induced by Exposure of RAW 264.7 Macrophages to Particulate Matter of Air Pollution: The Role of Endotoxins" Biomolecules 12, no. 8: 1100. https://doi.org/10.3390/biom12081100
APA StyleRoman, A., Korostyński, M., Jankowska-Kieltyka, M., Piechota, M., Hajto, J., & Nalepa, I. (2022). Gene Expression Changes Induced by Exposure of RAW 264.7 Macrophages to Particulate Matter of Air Pollution: The Role of Endotoxins. Biomolecules, 12(8), 1100. https://doi.org/10.3390/biom12081100