Edaravone Attenuated Angiotensin II-Induced Atherosclerosis and Abdominal Aortic Aneurysms in Apolipoprotein E-Deficient Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice and Study Protocol
2.2. Blood Pressure Measurement
2.3. Total Cholesterol Concentration Measurements
2.4. Quantification of Aneurysms and Atherosclerosis
2.5. Histological Analysis
2.6. Gelatin Zymography
2.7. Isolation of Peritoneal Macrophages
2.8. Peritoneal Macrophage Treatment
2.9. Real-time Polymerase Chain Reaction
2.10. Statistics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moll, F.L.; Powell, J.T.; Fraedrich, G.; Verzini, F.; Haulon, S.; Waltham, M.; van Herwaarden, J.A.; Holt, P.J.; van Keulen, J.W.; Rantner, B.; et al. Management of abdominal aortic aneurysms clinical practice guidelines of the European society for vascular surgery. Eur. J. Vasc. Endovasc. Surg. 2011, 41 (Suppl. S1), S1–S58. [Google Scholar] [CrossRef]
- Bown, M.J.; Sutton, A.J.; Bell, P.R.; Sayers, R.D. A meta-analysis of 50 years of ruptured abdominal aortic aneurysm repair. Br. J. Surg. 2002, 89, 714–730. [Google Scholar] [CrossRef]
- Hoornweg, L.L.; Storm-Versloot, M.N.; Ubbink, D.T.; Koelemay, M.J.; Legemate, D.A.; Balm, R. Meta analysis on mortality of ruptured abdominal aortic aneurysms. Eur. J. Vasc. Endovasc. Surg. 2008, 35, 558–570. [Google Scholar] [CrossRef] [PubMed]
- LeFevre, M.L. Screening for abdominal aortic aneurysm: U.S. Preventive Services Task Force recommendation statement. Ann. Intern. Med. 2014, 161, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Umebayashi, R.; Uchida, H.A.; Wada, J. Abdominal aortic aneurysm in aged population. Aging 2018, 10, 3650–3651. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, H.; Okuyama, M.; Uchida, H.A.; Kakio, Y.; Umebayashi, R.; Okuyama, Y.; Fujii, Y.; Ozawa, S.; Yoshida, M.; Oshima, Y.; et al. Chronic Kidney Disease Is Positively and Diabetes Mellitus Is Negatively Associated with Abdominal Aortic Aneurysm. PLoS ONE 2016, 11, e0164015. [Google Scholar] [CrossRef] [PubMed]
- Erbel, R.; Aboyans, V.; Boileau, C.; Bossone, E.; Bartolomeo, R.D.; Eggebrecht, H.; Evangelista, A.; Falk, V.; Frank, H.; Gaemperli, O.; et al. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur. Heart J. 2014, 35, 2873–2926. [Google Scholar] [CrossRef]
- Hirsch, A.T.; Haskal, Z.J.; Hertzer, N.R.; Bakal, C.W.; Creager, M.A.; Halperin, J.L.; Hiratzka, L.F.; Murphy, W.R.; Olin, J.W.; Puschett, J.B.; et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): A collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients with Peripheral Arterial Disease): Endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation 2006, 113, e463–e654. [Google Scholar] [CrossRef]
- Rooke, T.W.; Hirsch, A.T.; Misra, S.; Sidawy, A.N.; Beckman, J.A.; Findeiss, L.K.; Golzarian, J.; Gornik, H.L.; Halperin, J.L.; Jaff, M.R.; et al. 2011 ACCF/AHA Focused Update of the Guideline for the Management of Patients With Peripheral Artery Disease (updating the 2005 guideline): A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2011, 58, 2020–2045. [Google Scholar] [CrossRef]
- Schanzer, A.; Oderich, G.S. Management of Abdominal Aortic Aneurysms. N. Engl. J. Med. 2021, 385, 1690–1698. [Google Scholar] [CrossRef] [PubMed]
- Marx, J.L. Oxygen free radicals linked to many diseases. Science 1987, 235, 529–531. [Google Scholar] [CrossRef] [PubMed]
- Griendling, K.K.; Sorescu, D.; Ushio-Fukai, M. NAD(P)H oxidase: Role in cardiovascular biology and disease. Circ. Res. 2000, 86, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Murugesan, P.; Huang, K.; Cai, H. NADPH oxidases and oxidase crosstalk in cardiovascular diseases: Novel therapeutic targets. Nat. Rev. Cardiol. 2020, 17, 170–194. [Google Scholar] [CrossRef] [PubMed]
- Daugherty, A.; Manning, M.W.; Cassis, L.A. Angiotensin II promotes atherosclerotic lesions and aneurysms in apolipoprotein E-deficient mice. J. Clin. Investig. 2000, 105, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Uchida, H.A.; Poduri, A.; Subramanian, V.; Cassis, L.A.; Daugherty, A. Urokinase-type plasminogen activator deficiency in bone marrow-derived cells augments rupture of angiotensin II-induced abdominal aortic aneurysms. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 2845–2852. [Google Scholar] [CrossRef] [PubMed]
- Patelis, N.; Moris, D.; Schizas, D.; Damaskos, C.; Perrea, D.; Bakoyiannis, C.; Liakakos, T.; Georgopoulos, S. Animal models in the research of abdominal aortic aneurysms development. Physiol. Res. 2017, 66, 899–915. [Google Scholar] [CrossRef] [PubMed]
- Longo, G.M.; Xiong, W.; Greiner, T.C.; Zhao, Y.; Fiotti, N.; Baxter, B.T. Matrix metalloproteinases 2 and 9 work in concert to produce aortic aneurysms. J. Clin. Investig. 2002, 110, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Pyo, R.; Lee, J.K.; Shipley, J.M.; Curci, J.A.; Mao, D.; Ziporin, S.J.; Ennis, T.L.; Shapiro, S.D.; Senior, R.M.; Thompson, R.W. Targeted gene disruption of matrix metalloproteinase-9 (gelatinase B) suppresses development of experimental abdominal aortic aneurysms. J. Clin. Investig. 2000, 105, 1641–1649. [Google Scholar] [CrossRef] [PubMed]
- Davis, F.M.; Daugherty, A.; Lu, H.S. Updates of Recent Aortic Aneurysm Research. Arterioscler. Thromb. Vasc. Biol. 2019, 39, e83–e90. [Google Scholar] [CrossRef] [PubMed]
- Muniappan, L.; Okuyama, M.; Javidan, A.; Thiagarajan, D.; Jiang, W.; Moorleghen, J.J.; Yang, L.; Balakrishnan, A.; Howatt, D.A.; Uchida, H.A.; et al. Inducible Depletion of Calpain-2 Mitigates Abdominal Aortic Aneurysm in Mice. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 1694–1709. [Google Scholar] [CrossRef] [PubMed]
- Otaka, N.; Uchida, H.A.; Okuyama, M.; Hada, Y.; Onishi, Y.; Kakio, Y.; Takeuchi, H.; Umebayashi, R.; Tanabe, K.; Subramanian, V.; et al. Vasohibin-2 Aggravates Development of Ascending Aortic Aneurysms but not Abdominal Aortic Aneurysms nor Atherosclerosis in ApoE-Deficient Mice. Am. J. Hypertens. 2021, 34, 467–475. [Google Scholar] [CrossRef]
- Saraff, K.; Babamusta, F.; Cassis, L.A.; Daugherty, A. Aortic dissection precedes formation of aneurysms and atherosclerosis in angiotensin II-infused, apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1621–1626. [Google Scholar] [CrossRef]
- Shimizu, K.; Mitchell, R.N.; Libby, P. Inflammation and cellular immune responses in abdominal aortic aneurysms. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 987–994. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Kuwahara, T.; Watanabe, K.; Watanabe, K. Antioxidant activity of 3-methyl-1-phenyl-2-pyrazolin-5-one. Redox Rep. 1996, 2, 333–338. [Google Scholar] [CrossRef]
- Tsujita, K.; Shimomura, H.; Kawano, H.; Hokamaki, J.; Fukuda, M.; Yamashita, T.; Hida, S.; Nakamura, Y.; Nagayoshi, Y.; Sakamoto, T.; et al. Effects of edaravone on reperfusion injury in patients with acute myocardial infarction. Am. J. Cardiol. 2004, 94, 481–484. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, K.; Tancharoen, S.; Takeshige, N.; Yoshitomi, M.; Morioka, M.; Murai, Y.; Tanaka, E. The efficacy of edaravone (radicut), a free radical scavenger, for cardiovascular disease. Int. J. Mol. Sci. 2013, 14, 13909–13930. [Google Scholar] [CrossRef] [PubMed]
- Xi, H.; Akishita, M.; Nagai, K.; Yu, W.; Hasegawa, H.; Eto, M.; Kozaki, K.; Toba, K. Potent free radical scavenger, edaravone, suppresses oxidative stress-induced endothelial damage and early atherosclerosis. Atherosclerosis 2007, 191, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Aoki, T.; Nishimura, M.; Kataoka, H.; Ishibashi, R.; Nozaki, K.; Hashimoto, N. Reactive oxygen species modulate growth of cerebral aneurysms: A study using the free radical scavenger edaravone and p47phox(-/-) mice. Lab. Investig. 2009, 89, 730–741. [Google Scholar] [CrossRef]
- Radhakrishnan, G.; Suzuki, R.; Maeda, H.; Yamamoto, M.; Hirose, N.; Gopalrao, R.K.; Lee, G.H.; Hayashi, Y.; Rao, P.; Sasaguri, S. Inhibition of neointimal hyperplasia development by MCI-186 is correlated with downregulation of nuclear factor-kappaB pathway. Circ. J. 2008, 72, 800–806. [Google Scholar] [CrossRef]
- Yamashita, T.; Kamiya, T.; Deguchi, K.; Inaba, T.; Zhang, H.; Shang, J.; Miyazaki, K.; Ohtsuka, A.; Katayama, Y.; Abe, K. Dissociation and protection of the neurovascular unit after thrombolysis and reperfusion in ischemic rat brain. J. Cereb. Blood Flow Metab. 2009, 29, 715–725. [Google Scholar] [CrossRef]
- Umebayashi, R.; Uchida, H.A.; Kakio, Y.; Subramanian, V.; Daugherty, A.; Wada, J. Cilostazol Attenuates Angiotensin II-Induced Abdominal Aortic Aneurysms but Not Atherosclerosis in Apolipoprotein E-Deficient Mice. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 903–912. [Google Scholar] [CrossRef] [PubMed]
- Okuyama, M.; Uchida, H.A.; Hada, Y.; Kakio, Y.; Otaka, N.; Umebayashi, R.; Tanabe, K.; Fujii, Y.; Kasahara, S.; Subramanian, V.; et al. Exogenous Vasohibin-2 Exacerbates Angiotensin II-Induced Ascending Aortic Dilation in Mice. Circ. Rep. 2019, 1, 155–161. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Uchida, H.A.; Sugiyama, H.; Takiue, K.; Kikumoto, Y.; Inoue, T.; Makino, H. Development of angiotensin II-induced abdominal aortic aneurysms is independent of catalase in mice. J. Cardiovasc. Pharmacol. 2011, 58, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, V.; Moorleghen, J.J.; Balakrishnan, A.; Howatt, D.A.; Chishti, A.H.; Uchida, H.A. Calpain-2 compensation promotes angiotensin II-induced ascending and abdominal aortic aneurysms in calpain-1 deficient mice. PLoS ONE 2013, 8, e72214. [Google Scholar] [CrossRef]
- Uchida, H.A.; Kristo, F.; Rateri, D.L.; Lu, H.; Charnigo, R.; Cassis, L.A.; Daugherty, A. Total lymphocyte deficiency attenuates AngII-induced atherosclerosis in males but not abdominal aortic aneurysms in apoE deficient mice. Atherosclerosis 2010, 211, 399–403. [Google Scholar] [CrossRef]
- Howatt, D.A.; Balakrishnan, A.; Moorleghen, J.J.; Muniappan, L.; Rateri, D.L.; Uchida, H.A.; Takano, J.; Saido, T.C.; Chishti, A.H.; Baud, L.; et al. Leukocyte Calpain Deficiency Reduces Angiotensin II-Induced Inflammation and Atherosclerosis But Not Abdominal Aortic Aneurysms in Mice. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 835–845. [Google Scholar] [CrossRef]
- Tsang, K.M.; Knutsen, R.H.; Billington, C.J., Jr.; Lindberg, E.; Steenbock, H.; Fu, Y.P.; Wardlaw-Pickett, A.; Liu, D.; Malide, D.; Yu, Z.X.; et al. Copper-Binding Domain Variation in a Novel Murine Lysyl Oxidase Model Produces Structurally Inferior Aortic Elastic Fibers Whose Failure Is Modified by Age, Sex, and Blood Pressure. Int. J. Mol. Sci. 2022, 23, 6749. [Google Scholar] [CrossRef]
- Foote, K.; Reinhold, J.; Yu, E.P.K.; Figg, N.L.; Finigan, A.; Murphy, M.P.; Bennett, M.R. Restoring mitochondrial DNA copy number preserves mitochondrial function and delays vascular aging in mice. Aging Cell 2018, 17, e12773. [Google Scholar] [CrossRef]
- Subramanian, V.; Uchida, H.A.; Ijaz, T.; Moorleghen, J.J.; Howatt, D.A.; Balakrishnan, A. Calpain inhibition attenuates angiotensin II-induced abdominal aortic aneurysms and atherosclerosis in low-density lipoprotein receptor-deficient mice. J. Cardiovasc. Pharmacol. 2012, 59, 66–76. [Google Scholar] [CrossRef]
- Sánchez-Infantes, D.; Nus, M.; Navas-Madroñal, M.; Fité, J.; Pérez, B.; Barros-Membrilla, A.J.; Soto, B.; Martínez-González, J.; Camacho, M.; Rodriguez, C.; et al. Oxidative Stress and Inflammatory Markers in Abdominal Aortic Aneurysm. Antioxidants 2021, 10, 602. [Google Scholar] [CrossRef]
- Sawada, H.; Hao, H.; Naito, Y.; Oboshi, M.; Hirotani, S.; Mitsuno, M.; Miyamoto, Y.; Hirota, S.; Masuyama, T. Aortic iron overload with oxidative stress and inflammation in human and murine abdominal aortic aneurysm. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1507–1514. [Google Scholar] [CrossRef] [PubMed]
- Wiernicki, I.; Parafiniuk, M.; Kolasa-Wołosiuk, A.; Gutowska, I.; Kazimierczak, A.; Clark, J.; Baranowska-Bosiacka, I.; Szumilowicz, P.; Gutowski, P. Relationship between aortic wall oxidative stress/proteolytic enzyme expression and intraluminal thrombus thickness indicates a novel pathomechanism in the progression of human abdominal aortic aneurysm. Faseb. J. 2019, 33, 885–895. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, A.; Müglich, M.; Wolk, S.; Khorzom, Y.; Sabarstinski, P.; Kopaliani, I.; Egorov, D.; Horn, F.; Brunssen, C.; Giebe, S.; et al. Induction of Heme Oxygenase-1 Is Linked to the Severity of Disease in Human Abdominal Aortic Aneurysm. J. Am. Heart Assoc. 2021, 10, e022747. [Google Scholar] [CrossRef] [PubMed]
- Emeto, T.I.; Moxon, J.V.; Au, M.; Golledge, J. Oxidative stress and abdominal aortic aneurysm: Potential treatment targets. Clin. Sci. 2016, 130, 301–315. [Google Scholar] [CrossRef] [PubMed]
- Chai, H.; Tao, Z.; Qi, Y.; Qi, H.; Chen, W.; Xu, Y.; Zhang, L.; Chen, H.; Chen, X. IKK Epsilon Deficiency Attenuates Angiotensin II-Induced Abdominal Aortic Aneurysm Formation in Mice by Inhibiting Inflammation, Oxidative Stress, and Apoptosis. Oxidative Med. Cell. Longev. 2020, 2020, 3602824. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Lee, E.; Song, W.; Ricciotti, E.; Rader, D.J.; Lawson, J.A.; Puré, E.; FitzGerald, G.A. Microsomal prostaglandin E synthase-1 deletion suppresses oxidative stress and angiotensin II-induced abdominal aortic aneurysm formation. Circulation 2008, 117, 1302–1309. [Google Scholar] [CrossRef] [PubMed]
- Satoh, K.; Nigro, P.; Matoba, T.; O’Dell, M.R.; Cui, Z.; Shi, X.; Mohan, A.; Yan, C.; Abe, J.; Illig, K.A.; et al. Cyclophilin A enhances vascular oxidative stress and the development of angiotensin II-induced aortic aneurysms. Nat. Med. 2009, 15, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.; Gavrila, D.; McCormick, M.L.; Miller, F.J., Jr.; Daugherty, A.; Cassis, L.A.; Dellsperger, K.C.; Weintraub, N.L. Deletion of p47phox attenuates angiotensin II-induced abdominal aortic aneurysm formation in apolipoprotein E-deficient mice. Circulation 2006, 114, 404–413. [Google Scholar] [CrossRef]
- Gavrila, D.; Li, W.G.; McCormick, M.L.; Thomas, M.; Daugherty, A.; Cassis, L.A.; Miller, F.J., Jr.; Oberley, L.W.; Dellsperger, K.C.; Weintraub, N.L. Vitamin E inhibits abdominal aortic aneurysm formation in angiotensin II-infused apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1671–1677. [Google Scholar] [CrossRef]
- Lu, W.W.; Jia, L.X.; Ni, X.Q.; Zhao, L.; Chang, J.R.; Zhang, J.S.; Hou, Y.L.; Zhu, Y.; Guan, Y.F.; Yu, Y.R.; et al. Intermedin1-53 Attenuates Abdominal Aortic Aneurysm by Inhibiting Oxidative Stress. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 2176–2190. [Google Scholar] [CrossRef]
- Morimoto, K.; Hasegawa, T.; Tanaka, A.; Wulan, B.; Yu, J.; Morimoto, N.; Okita, Y.; Okada, K. Free-radical scavenger edaravone inhibits both formation and development of abdominal aortic aneurysm in rats. J. Vasc. Surg. 2012, 55, 1749–1758. [Google Scholar] [CrossRef] [PubMed]
- Ross, R. Atherosclerosis--an inflammatory disease. N. Engl. J. Med. 1999, 340, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. Inflammation in atherosclerosis. Nature 2002, 420, 868–874. [Google Scholar] [CrossRef] [PubMed]
- Gallino, A.; Aboyans, V.; Diehm, C.; Cosentino, F.; Stricker, H.; Falk, E.; Schouten, O.; Lekakis, J.; Amann-Vesti, B.; Siclari, F.; et al. Non-coronary atherosclerosis. Eur. Heart J. 2014, 35, 1112–1119. [Google Scholar] [CrossRef]
- Rader, D.J.; Daugherty, A. Translating molecular discoveries into new therapies for atherosclerosis. Nature 2008, 451, 904–913. [Google Scholar] [CrossRef]
- Weiss, D.; Kools, J.J.; Taylor, W.R. Angiotensin II-induced hypertension accelerates the development of atherosclerosis in apoE-deficient mice. Circulation 2001, 103, 448–454. [Google Scholar] [CrossRef]
- Lu, H.; Cassis, L.A.; Daugherty, A. Atherosclerosis and arterial blood pressure in mice. Curr. Drug Targets 2007, 8, 1181–1189. [Google Scholar] [CrossRef]
- Wang, Q.; Ren, J.; Morgan, S.; Liu, Z.; Dou, C.; Liu, B. Monocyte chemoattractant protein-1 (MCP-1) regulates macrophage cytotoxicity in abdominal aortic aneurysm. PLoS ONE 2014, 9, e92053. [Google Scholar] [CrossRef]
- Moehle, C.W.; Bhamidipati, C.M.; Alexander, M.R.; Mehta, G.S.; Irvine, J.N.; Salmon, M.; Upchurch, G.R., Jr.; Kron, I.L.; Owens, G.K.; Ailawadi, G. Bone marrow-derived MCP1 required for experimental aortic aneurysm formation and smooth muscle phenotypic modulation. J. Thorac. Cardiovasc. Surg. 2011, 142, 1567–1574. [Google Scholar] [CrossRef]
- Xiao, J.; Wei, Z.; Chen, X.; Chen, W.; Zhang, H.; Yang, C.; Shang, Y.; Liu, J. Experimental abdominal aortic aneurysm growth is inhibited by blocking the JAK2/STAT3 pathway. Int. J. Cardiol. 2020, 312, 100–106. [Google Scholar] [CrossRef]
- Qin, Z.; Bagley, J.; Sukhova, G.; Baur, W.E.; Park, H.J.; Beasley, D.; Libby, P.; Zhang, Y.; Galper, J.B. Angiotensin II-induced TLR4 mediated abdominal aortic aneurysm in apolipoprotein E knockout mice is dependent on STAT3. J. Mol. Cell. Cardiol. 2015, 87, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.Y.; Cheng, Z.; Zhou, Y.Z.; Zhao, Y.; Li, J.M.; Zhou, X.M.; Peng, H.L.; Zhang, G.S.; Liao, X.B.; Fu, X.M. A novel STAT3 inhibitor attenuates angiotensin II-induced abdominal aortic aneurysm progression in mice through modulating vascular inflammation and autophagy. Cell Death Dis. 2020, 11, 131. [Google Scholar] [CrossRef]
- Chen, H.; Chen, Y.; Wang, X.; Yang, J.; Huang, C. Edaravone attenuates myocyte apoptosis through the JAK2/STAT3 pathway in acute myocardial infarction. Free Radic. Res. 2020, 54, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Tanaka, M.; Yuki, S.; Hirai, M.; Yamamoto, Y. How is edaravone effective against acute ischemic stroke and amyotrophic lateral sclerosis? J. Clin. Biochem. Nutr. 2018, 62, 20–38. [Google Scholar] [CrossRef] [PubMed]
- Bailly, C. Potential use of edaravone to reduce specific side effects of chemo-, radio- and immuno-therapy of cancers. Int. Immunopharmacol. 2019, 77, 105967. [Google Scholar] [CrossRef] [PubMed]
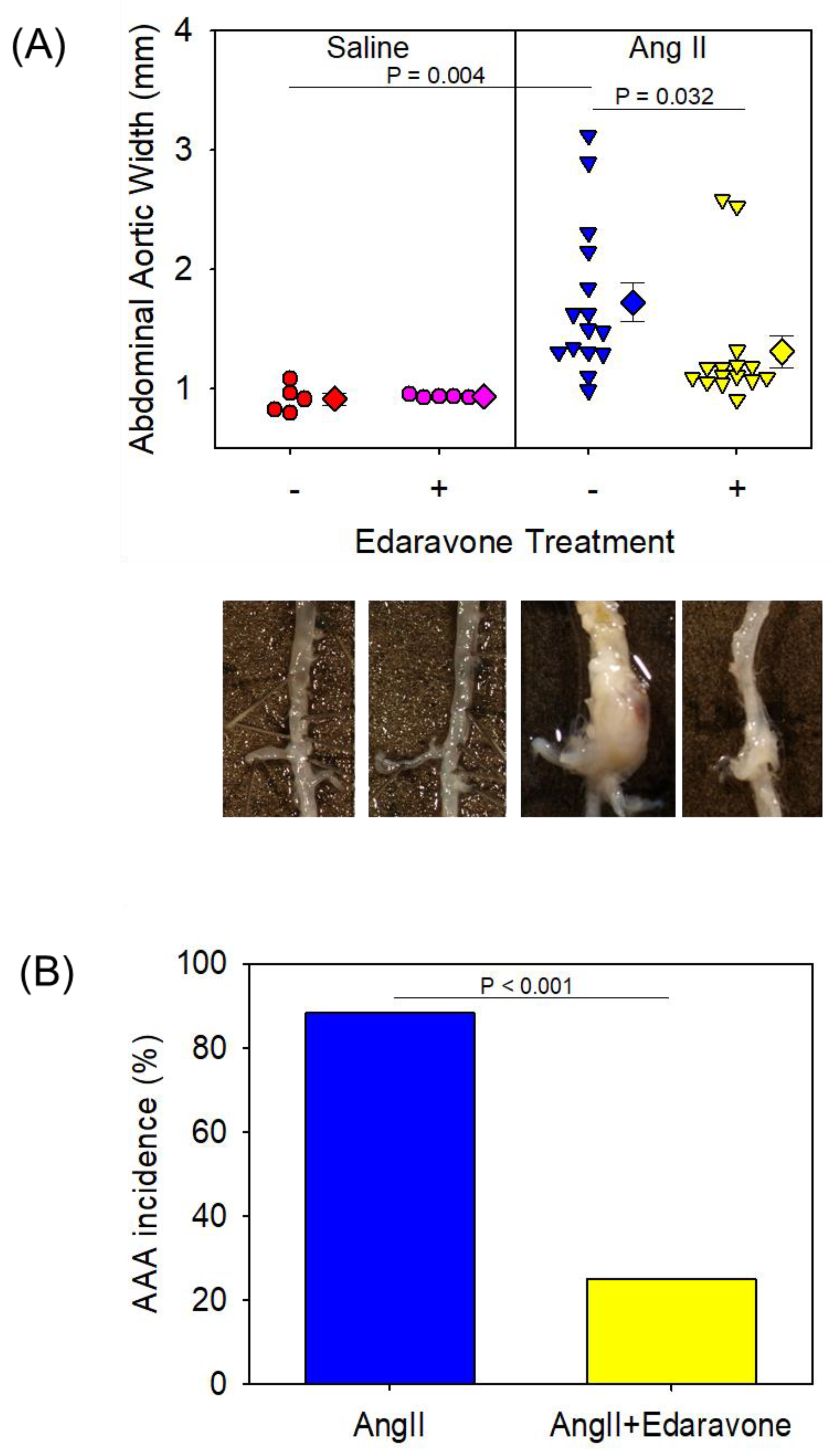


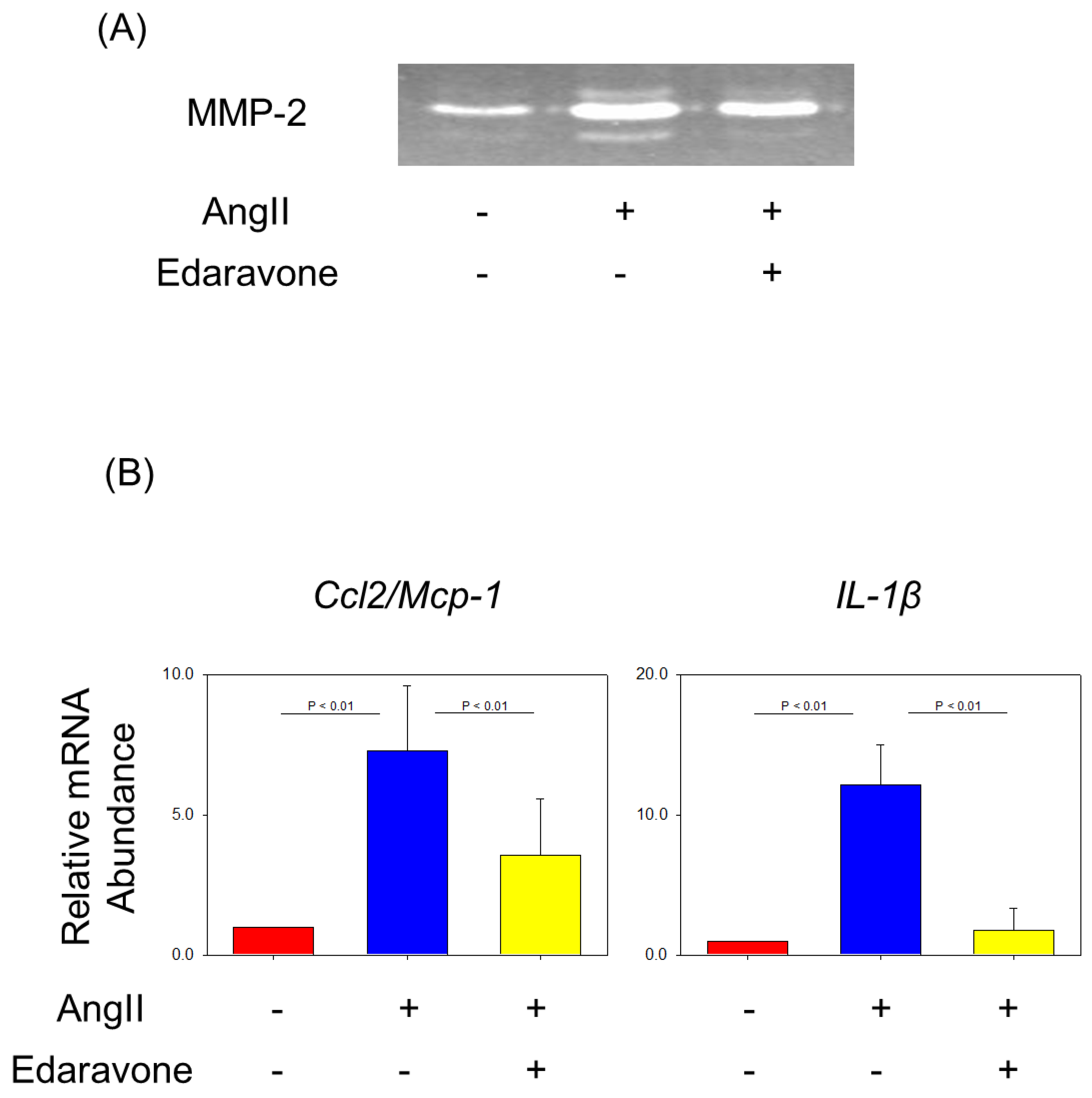

| Saline | AngII | |||
|---|---|---|---|---|
| Saline | Edaravone | Saline | Edaravone | |
| N | 5 | 5 | 17 | 16 |
| Body Weight (g) | 28 ± 1 | 28 ± 0 | 28 ± 1 | 27 ± 1 |
| SBP (mmHg) | 97 ± 4 | 96 ± 4 | 122 ± 3 * | 128 ± 3 * |
| T.Cho (mg/dl) | 444 ± 38 | 476 ± 58 | 466 ± 27 | 464 ± 16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uchida, H.A.; Takatsuka, T.; Hada, Y.; Umebayashi, R.; Takeuchi, H.; Shikata, K.; Subramanian, V.; Daugherty, A.; Wada, J. Edaravone Attenuated Angiotensin II-Induced Atherosclerosis and Abdominal Aortic Aneurysms in Apolipoprotein E-Deficient Mice. Biomolecules 2022, 12, 1117. https://doi.org/10.3390/biom12081117
Uchida HA, Takatsuka T, Hada Y, Umebayashi R, Takeuchi H, Shikata K, Subramanian V, Daugherty A, Wada J. Edaravone Attenuated Angiotensin II-Induced Atherosclerosis and Abdominal Aortic Aneurysms in Apolipoprotein E-Deficient Mice. Biomolecules. 2022; 12(8):1117. https://doi.org/10.3390/biom12081117
Chicago/Turabian StyleUchida, Haruhito A., Tetsuharu Takatsuka, Yoshiko Hada, Ryoko Umebayashi, Hidemi Takeuchi, Kenichi Shikata, Venkateswaran Subramanian, Alan Daugherty, and Jun Wada. 2022. "Edaravone Attenuated Angiotensin II-Induced Atherosclerosis and Abdominal Aortic Aneurysms in Apolipoprotein E-Deficient Mice" Biomolecules 12, no. 8: 1117. https://doi.org/10.3390/biom12081117
APA StyleUchida, H. A., Takatsuka, T., Hada, Y., Umebayashi, R., Takeuchi, H., Shikata, K., Subramanian, V., Daugherty, A., & Wada, J. (2022). Edaravone Attenuated Angiotensin II-Induced Atherosclerosis and Abdominal Aortic Aneurysms in Apolipoprotein E-Deficient Mice. Biomolecules, 12(8), 1117. https://doi.org/10.3390/biom12081117







