Race for the Cure: From the Oldest to the Newest Monoclonal Antibodies for Multiple Myeloma Treatment
Abstract
:1. Introduction
1.1. Targets of Antibody Compounds
- -
- CD38: it is a transmembrane glycoprotein belonging to the family of cyclic ADP ribose hydrolases and is widely expressed on the surface of hematopoietic and non-hematopoietic cell lines, with greater intensity on both the non-clonal and the clonal PCs. CD38 plays multiple functions: as a receptor, it interacts with CD31 on the surface of T cells to produce a great variety of inflammatory cytokines; as an enzyme, it is involved in the regulation of cellular calcium trafficking [3]. Its central role in the oncogenesis of MM has been shown by different in vitro assays: the increase of levels of nicotinamide adenine dinucleotide (NAD+) due to its enzymatic activity seems to be associated with resistance to apoptosis [4] and the subsequent production of cyclic ADP ribose (cADPR) seems to ease the tumor escape from the immune system [5]. High expression of CD38 has also been associated with the formation of nanotubes transferring mitochondria from the stromal cells to myeloma cells to increase their proliferation and survival [6].
- -
- SLAMF7: also referred to as CS1, CD2 subset-1, CRACC, and CD319, it is a surface protein belonging to the signaling lymphocyte activation molecule (SLAM) family. SLAMF7 is highly expressed by non-clonal and clonal PCs but can also be found on the surface of CD8+ T cells, NK cells, and mature dendritic cells. Even if its function is not fully understood yet, SLAMF7 seems to play a key role in the interaction between the myeloma cells and the microenvironment: in particular, the stimulation of this SLAM protein might recruit the immune cells of the bone marrow niche to support the survival of PCs [7].
- -
- BCMA: also known as tumor necrosis factor receptor superfamily member 17 (TNFRSF17), it actually belongs to the TNF-receptors superfamily. This protein is consistently expressed on the surface of normal and malignant B-cells and by mature B cells and has a crucial role in the survival of long-lived plasma cells in the bone marrow. In particular, the binding of the ligand B-cell activating factor (BAFF) and a proliferation-inducing ligand (APRIL) to BCMA, expressed on the surface of clonal plasma cells, results in the activation of survival and proliferation pathways and in multidrug resistance [8]. BCMA can also be solubilized by y-secretase and interfere with circulating B-cell activating factor (BAFF), which is required for normal B-cell development. Therefore, serum BCMA is elevated in myeloma patients and directly correlates with tumor burden and outcome [9,10].
- -
- Surface molecules: GPRC5D, FcRH5, CD138, CD40, CD74;
- -
- Signalling molecules: IL-6, RANKL, BAFF, VEGF, DKK1;
- -
- Immune checkpoint inhibitors: PD1, PDL1, CTLA4, KIR.
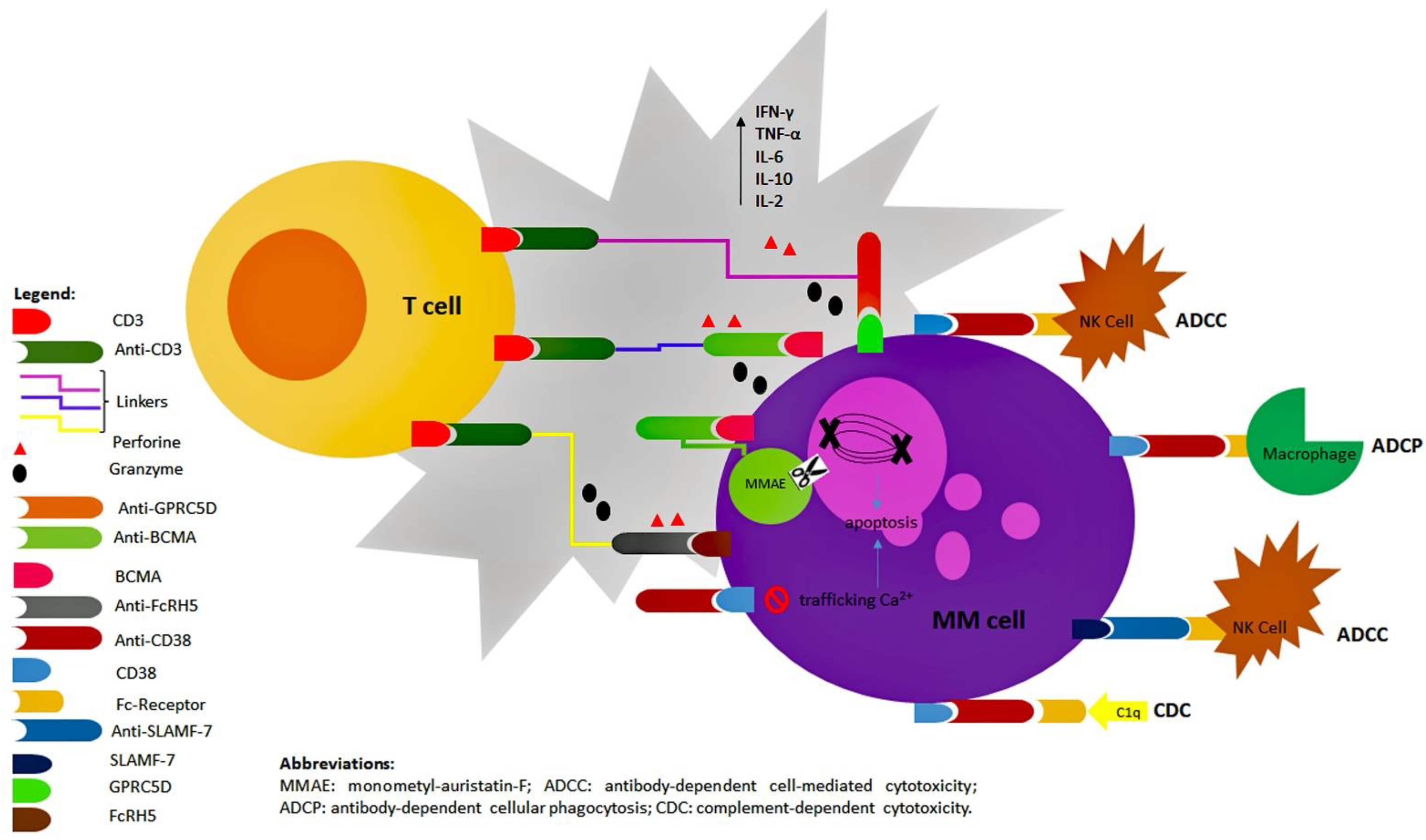
1.2. Mechanisms of Action of Antibody Compounds: Overview
2. Starting from the Oldest: The “Naked” Monoclonal Antibodies
2.1. Anti-CD38: Daratumumab and Isatuximab
2.1.1. Daratumumab: Mechanism of Action
2.1.2. Daratumumab in Relapsed/Refractory Multiple Myeloma
2.1.3. Daratumumab in Newly Diagnosed Multiple Myeloma
2.1.4. Daratumumab: Side Effects
2.1.5. Daratumumab: Recurrent Interferences with Laboratory Tests
2.1.6. Isatuximab: Mechanism of Action
2.1.7. Isatuximab in Relapsed/Refractory Multiple Myeloma
2.1.8. Isatuximab: Side Effects
2.1.9. Felzartamab
2.2. Anti-Slamf7: Elotuzumab
2.2.1. Elotuzumab: Mechanism of Action
2.2.2. Elotuzumab in Relapsed/Refractory Multiple Myeloma
2.2.3. Elotuzumab in Newly Diagnosed Multiple Myeloma
2.2.4. Elotuzumab: Side Effects
3. Arriving to the Newest: The “Combined” Monoclonal Antibodies
3.1. Antibody-Drug Conjugates: Belantamab-Mafodotin, AMG224, MEDI2228, HDP-101
3.1.1. Belantamab-Mafodotin: Mechanism of Action
3.1.2. Belantamab-Mafodotin for Relapsed/Refractory Multiple Myeloma
3.1.3. Belantamab-Mafodotin: Side Effects
3.1.4. Other Antibody-Drug Conjugates Targeting BCMA
- -
- AMG224 is a fully humanized IgG1 antibody directed against BCMA and conjugated to a maytansinoid, a derivative of a cytotoxic agent isolated from plants of the genus Maytenus. Its activity against MM has been evaluated in a phase I trial, which enrolled 42 patients with a median number of 7 prior lines of therapy: 29 received the drug in the dose-escalation phase and 11 in the dose-expansion [66]. In both phases, manageable hematologic side effects and grade 1-2 infusion-related reactions were reported. In addition, in this case, the treatment was associated with the onset of keratopathy requiring dose delays or dose modifications, with no need for discontinuation. The ORR was 23%, with a median duration of response of 14.7 months reported in the second cohort.
- -
- MEDI2228 is another anti-BCMA conjugated to a pyrrolobenzodiazepine with tumor activity due to its ability to link DNA and induce fatal damage. Based on preclinical studies showing efficacy against MM-cells, especially if combined with PIs [67], a phase I trial has investigated its use in 82 patients with RRMM [68]. The ORR was 61% with acceptable hematologic toxicities and no reported cases of keratopathy, even if 58.5% of patients experienced photophobia; in 41.5% of patients, this was grade 1 or 2, while it was grade 3 or 4 in 17.1%. In addition, considering the high rate of patients with prior exposure to daratumumab enrolled in this trial, MEDI2228 might be a worthy alternative for those relapsing after therapy with the “naked” moAbs, and further studies to confirm this observation are warranted.
- -
- HDP-101 differs from the other ADCs directed against BCMA since the payload is represented by α-amantin, a derivative of mushrooms genus Amanita, interfering with protein biosynthesis through inhibition of RNA polymerase II subunit A. Pre-clinical studies showed that this drug induces immunogenic cell death in myeloma cell lines, with a particular affinity towards those harbouring del17p, suggesting the possibility of using this high-risk cytogenetic aberration as a potential target in future [69].
3.2. Bispecific Antibodies
3.2.1. BiTEs Targeting BCMA
- -
- Teclistamab (JNJ-64007957): it is a humanized IgG4 binding simultaneously BCMA on the surface of MM cells and CD3 on the surface of T cells. In vitro assays showed that this drug induces cytotoxicity of BCMA+ cells through multiple mechanisms, including dose-dependent lysis, T-cell activation, and cytokine release, especially if in the presence of a γ-secretase inhibitor [71]. These observations have been confirmed in murine models and in ex vivo assays performed on bone marrow samples from MM patients. Based on these encouraging results, the safety and efficacy of teclistamab are being evaluated in the ongoing trial MajesTEC-1 (NCT03145181, Dimopoulos MA, National and Kapodistrian University of Athens School of Medicine, Greece). MejesTEC-1 is an open-label, single-arm, phase I trial in which teclistamab is administered intravenously or subcutaneously in different cohorts of RRMM patients with step-up dosing. To the last cut-off analysis, overall, 157 patients, with a median of six previous therapy lines, received at least one dose of the novel drug [72]. Forty of them were administered the recommended phase 2 dose (once per week 1500 μg/kg by subcutaneous route). In this last group, after a median follow-up of 6.1 months, the ORR was 65% and confirmed across all the subgroups, including 33 triple-class refractory patients. These responses were durable and deepened over time, with six evaluable MRD patients achieving MRD-negative complete response, evaluated by immunoglobulin gene rearrangement sequencing.
- -
- AMG420, formerly known as BI836909, is another BiTE targeting BCMA and inducing MM cell death through the same mechanism as teclistamab. Actually, studies performed in vitro on BCMA+ cell lines and in vivo on murine models and ex vivo assays on bone marrow samples from MM patients showed that this drug selectively mediates apoptosis of BCMA+ cells by recruiting T-cells [73]. NCT03836053 is the first-in-human, phase 1, a dose-escalation trial evaluating this novel drug [74]. It enrolled 42 patients with RRMM and a median of 5 prior therapy lines, including daratumumab. Extramedullary disease and prior allogenic transplant were exclusion criteria, representing a limit in the evaluation of the efficacy of AMG420 in the setting of patients with very aggressive diseases. After a median follow-up of 8 months, the ORR was 31% but at the maximum tolerated dose of 400 μg/die, the response rate increased to 70%. In the group exposed to this dose, MRD negativity was attained in 71% of cases even if it is evaluated per protocol by flow-cytometry.
- -
- AMG701 is a half-life extended anti-BCMA BiTE, formerly tested in cynomolgus monkeys in which it depleted aberrant plasma cells [75]. In further studies performed in MM cell lines and in autologous cells from RRMM patients, it showed to induce T-cell-dependent cellular cytotoxicity, especially if in combination with lenalidomide and pomalidomide [76]. The synergic effect of AMG701 with IMiDs might overcome the pro-tumor effect due to the microenvironment. Based on these results, the ongoing trial NCT03287908 is investigating in heavily pre-treated patients AMG701 monotherapy to assess the recommended phase 2 dose, to be also used in combination with pomalidomide. The initial results of this study showed in a population of 71 patients an ORR of 36%, increasing to 83% in those who underwent early escalation [77].
- -
- REGN5458 is a fully human BiTE with proven efficacy in vitro and in animal models, especially if combined with checkpoint inhibitors [78]. In these in vivo models, it showed an anti-tumor activity with faster kinetics than that of anti-BCMA CAR-T constructs injected in similar animals, suggesting the potential use of this novel drug to debulk very aggressive disease. NCT03761108 is the first-in-human trial aimed at establishing the safety and tolerability of REGN5458 in patients with RRMM. As of data cut-off, 68 patients, with the majority being penta-refractory, were treated in the dose-escalation cohort with full doses ranging from 3 to 400 mg [79]. The highest response rate was 73.7% and was observed among patients treated at 96 and 200 mg dose levels, with acceptable safety and tolerability profile.
- -
- CC-93269 is a BiTE characterized by a bivalent binding to BCMA and is under investigation in the trial NCT03486067 for patients with RRMM with no prior exposure to anti-BCMA therapy. The interim results on 30 patients showed an ORR of 43.3% with a complete response/stringent complete response of 16.7% and MRD-negativity achieved in the majority of responders [80].
- -
- Elranatamab, also known as PF-06863135 or PF-3135, is a humanized IgG2 anti-BCMA BiTE. Based on promising results of MagnetisMM-1, a phase 1 trial investigating efficacy and safety of elranatamab alone and in combination with IMiDs in RRMM, the phase-2 trial MagnetisMM-3 will evaluate this novel drug as a single agent in patients who should be at least triple refractory, also including those with prior exposure to other anti-BCMA [81].
- -
- TNB-383B is a unique anti-BCMA with major activity on effector T-cells more than on regulatory T cells if compared to similar drugs. The first-in-human, phase 1, dose-escalation/expansion study in RRMM with prior exposure to ≥3 lines showed an ORR of 79%, also confirmed in triple-refractory patients, again showing the efficacy of this class of drugs, especially in heavily pre-treated patients [82].
3.2.2. BiTEes Targeting BCMA: Side Effects
3.2.3. BiTEs Non-Targeting BCMA
3.2.4. BiTEs Non-Targeting BCMA: Anti-GPRC5D
3.2.5. BiTEs Non-Targeting BCMA: Anti-FCRH5
3.3. Other Antibodies: Overview
- -
- Indatuximab-ravtansine: also mentioned to a BT062, it is an anti-CD138 ADC. A phase I/II A study showed promising results in 35 heavily pre-treated patients, with ORR > 75% and mild-to-moderate gastro-enteric toxicity, but further investigations are needed to confirm them [94].
- -
- Siltuximab: it is an antibody targeting IL-6 that is involved in the growth of MM [95]. While its efficacy has been confirmed in other hematologic malignancies (i.e., Castelman disease), its use in MM is controversial. Even if it seems that it could delay progression from smoldering multiple myeloma to symptomatic forms [96], more evidence is required.
- -
- Denosumab: it is an inhibitor of RANK-L, which plays a leading role in the survival of osteoclasts. It might be of interest in the treatment of MM since it seems to slow progression through suppression of tumor escape in patients already on supportive treatment with this drug [97]. To date, in fact, this drug is approved in the setting of MM patients only to prevent disease-related bone fractures.
4. Overcoming the Newest: From the Microscopic to the Nanoscopic Therapeutic Approach
4.1. The Nanobodies: Overview
4.2. The Nanobodies: Experimental Combinations with Radionuclides and Nanoparticles
4.3. The Aptamers
5. Discussion: At the Finish Line or Still in the Race?
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Costa, L.J.; Gonsalves, W.I.; Kumar, S.K. Early mortality in multiple myeloma. Leukemia 2015, 29, 1616–1618. [Google Scholar] [CrossRef] [PubMed]
- Usmani, S.Z.; Hoering, A.; Cavo, M.; Miguel, J.S.; Goldschimdt, H.; Hajek, R.; Turesson, I.; Lahuerta, J.J.; Attal, M.; Barlogie, B.; et al. Clinical predictors of long-term survival in newly diagnosed transplant eligible multiple myeloma—An IMWG Research Project. Blood Cancer J. 2018, 8, 123. [Google Scholar] [CrossRef] [PubMed]
- Morandi, F.; Horenstein, A.L.; Costa, F.; Giuliani, N.; Pistoia, V.; Malavasi, F. CD38: A target for immunotherapeutic approaches in multiple myeloma. Front. Immunol. 2018, 9, 2722. [Google Scholar] [CrossRef] [PubMed]
- Cagnetta, A.; Cea, M.; Calimeri, T.; Acharya, C.; Fulciniti, M.; Tai, Y.T.; Hideshima, T.; Chauhan, D.; Zhong, M.Y.; Patrone, F.; et al. Intracellular NAD+ depletion enhances bortezomib-induced anti-myeloma activity. Blood 2013, 122, 1243–1255. [Google Scholar] [CrossRef]
- Chillemi, A.; Quarona, V.; Antonioli, L.; Ferrari, D.; Horenstein, A.L.; Malavasi, F. Roles and Modalities of Ectonucleotidases in Remodeling the Multiple Myeloma Niche. Front. Immunol. 2017, 8, 305. [Google Scholar] [CrossRef]
- Marlein, C.R.; Piddock, R.E.; Mistry, J.J.; Zaitseva, L.; Hellmich, C.; Horton, R.H.; Zhou, Z.; Auger, M.J.; Bowles, K.M.; Rushworth, S.A. CD38-Driven Mitochondrial Trafficking Promotes Bioenergetic Plasticity in Multiple Myeloma. Cancer Res. 2019, 79, 2285–2297. [Google Scholar] [CrossRef]
- Tai, Y.T.; Dillon, M.; Song, W.; Leiba, M.; Li, X.F.; Burger, P.; Lee, A.I.; Podar, K.; Hideshima, T.; Rice, A.G.; et al. Anti-CS1 humanized monoclonal antibody HuLuc63 inhibits myeloma cell adhesion and induces antibody-dependent cellular cytotoxicity in the bone marrow milieu. Blood 2008, 112, 1329–1337. [Google Scholar] [CrossRef]
- Tai, Y.T.; Acharya, C.; An, G.; Moschetta, M.; Zhong, M.Y.; Feng, X.; Cea, M.; Cagnetta, A.; Wen, K.; van Eenennaam, H.; et al. APRIL and BCMA promote human multiple myeloma growth and immunosuppression in the bone marrow microenvironment. Blood 2016, 127, 3225–3236. [Google Scholar] [CrossRef]
- Sanchez, E.; Li, M.; Kitto, A.; Li, J.; Wang, C.S.; Kirk, D.T.; Yellin, O.; Nichols, C.M.; Dreyer, M.P.; Ahles, C.P.; et al. Serum B-cell maturation antigen is elevated in multiple myeloma and correlates with disease status and survival. Br. J. Haematol. 2012, 158, 727–738. [Google Scholar] [CrossRef]
- Ghermezi, M.; Li, M.; Vardanyan, S.; Harutyunyan, N.M.; Gottlieb, J.; Berenson, A.; Spektor, T.M.; Andreu-Vieyra, C.; Petraki, S.; Sanchez, E.; et al. Serum B-cell maturation antigen: A novel biomarker to predict outcomes for multiple myeloma patients. Haematologica 2017, 102, 785–795. [Google Scholar] [CrossRef]
- van de Donk, N.W.C.J.; Usmani, S.Z. CD38 Antibodies in Multiple Myeloma: Mechanisms of Action and Modes of Resistance. Front. Immunol. 2018, 9, 2134. [Google Scholar] [CrossRef] [PubMed]
- Veillette, A.; Guo, H. CS1, a SLAM family receptor involved in immune regulation, is a therapeutic target in multiple myeloma. Crit. Rev. Oncol. Hematol. 2013, 88, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Coats, S.; Williams, M.; Kebble, B.; Dixit, R.; Tseng, L.; Yao, N.S.; Tice, D.A.; Soria, J.C. Antibody-drug conjugates: Future directions in clinical and translational strategies to improve the therapeutic index. Clin. Cancer Res. 2019, 25, 5441–5448. [Google Scholar] [CrossRef] [PubMed]
- Suurs, F.V.; Lub-de Hooge, M.N.; de Vries, E.; de Groot, D. A review of bispecific antibodies and antibody constructs in oncology and clinical challenges. Pharmacol. Ther. 2019, 201, 103–119. [Google Scholar] [CrossRef] [PubMed]
- Krejcik, J.; Casneuf, T.; Nijhof, I.S.; Verbist, B.; Bald, J.; Plesner, T.; Syed, K.; Liu, K.; van de Donk, N.W.; Weiss, B.M.; et al. Daratumumab depletes CD38+ immune regulatory cells, promotes T-cell expansion, and skews T-cell repertoire in multiple myeloma. Blood 2016, 128, 384–394. [Google Scholar] [CrossRef]
- Lokhorst, H.M.; Plesner, T.; Laubach, J.P.; Nahi, H.; Gimsing, P.; Hansson, M.; Minnema, M.C.; Lassen, U.; Krejcik, J.; Palumbo, A.; et al. Targeting CD38 with daratumumab monotherapy in multiple myeloma. N. Engl. J. Med. 2015, 373, 1207–1219. [Google Scholar] [CrossRef]
- Lonial, S.; Weiss, B.M.; Usmani, S.Z.; Singhal, S.; Chari, A.; Bahlis, N.J.; Belch, A.; Krishnan, A.; Vescio, R.A.; Mateos, M.V.; et al. Daratumumab monotherapy in patients with treatment-refractory multiple myeloma (SIRIUS): An open-label, randomised, phase 2 trial. Lancet 2016, 387, 1551–1560. [Google Scholar] [CrossRef]
- Usmani, S.Z.; Weiss, B.M.; Plesner, T.; Bahlis, N.J.; Belch, A.; Lonial, S.; Lokhorst, H.M.; Voorhees, P.M.; Richardson, P.G.; Chari, A.; et al. Clinical efficacy of daratumumab monotherapy in patients with heavily pretreated relapsed or refractory multiple myeloma. Blood 2016, 128, 37–44. [Google Scholar] [CrossRef]
- van der Veer, M.S.; de Weers, M.; van Kessel, B.; Bakker, J.M.; Wittebol, S.; Parren, P.W.; Lokhorst, H.M.; Mutis, T. The therapeutic human CD38 antibody daratumumab improves the anti-myeloma effect of newly emerging multi-drug therapies. Blood Cancer J. 2011, 1, e41. [Google Scholar] [CrossRef]
- Plesner, T.; Arkenau, H.T.; Gimsing, P.; Krejcik, J.; Lemech, C.; Minnema, M.C.; Lassen, U.; Laubach, J.P.; Palumbo, A.; Lisby, S.; et al. Phase ½ study of daratumumab, lenalidomide, and dexamethasone for relapsed multiple myeloma. Blood 2016, 128, 1821–1828. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Oriol, A.; Nahi, H.; San-Miguel, J.; Bahlis, N.J.; Usmani, S.Z.; Rabin, N.; Orlowski, R.Z.; Komarnicki, M.; Suzuki, K.; et al. Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N. Engl. J. Med. 2016, 375, 1319–1331. [Google Scholar] [CrossRef] [PubMed]
- Mateos, M.V.; Moreau, P.; Comenzo, R.; Bladé, J.; Benboubker, L.; de la Rubia, J.; Facon, T.; Fay, J.; Qin, X.; Masterson, T.; et al. An open-label, multicenter, phase 1b study of daratumumab in combination with pomalidomide-dexamethasone and with backbone regimens in patients with multiple myeloma. In Proceedings of the 20th Congress of the European Hematology Association, Vienna, Austria, 11–14 June 2015. [Google Scholar]
- Palumbo, A.; Chanan-Khan, A.; Weisel, K.; Nooka, A.K.; Masszi, T.; Beksac, M.; Spicka, I.; Hungria, V.; Munder, M.; Mateos, M.V.; et al. Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N. Engl. J. Med. 2016, 375, 754–766. [Google Scholar] [CrossRef] [PubMed]
- Chari, A.; Suvannasankha, A.; Fay, J.W.; Arnulf, B.; Kaufman, J.L.; Ifthikharuddin, J.J.; Weiss, B.M.; Krishnan, A.; Lentzsch, S.; Comenzo, R.; et al. Daratumumab plus pomalidomide and dexamethasone in relapsed and/or refractory multiple myeloma. Blood 2017, 130, 974–981. [Google Scholar] [CrossRef] [PubMed]
- Chari, A.; Martinez-Lopez, J.; Mateos, M.V.; Bladé, J.; Benboubker, L.; Oriol, A.; Arnulf, B.; Rodriguez-Otero, P.; Pineiro, L.; Jakubowiak, A.; et al. Daratumumab plus carfilzomib and dexamethasone in patients with relapsed or refractory multiple myeloma. Blood 2019, 134, 421–431. [Google Scholar] [CrossRef]
- Mateos, M.V.; Dimopoulos, M.A.; Cavo, M.; Suzuki, K.; Jakubowiak, A.; Knop, S.; Doyen, C.; Lucio, P.; Nagy, Z.; Kaplan, P.; et al. Daratumumab plus bortezomib, melphalan, and prednisone for untreated myeloma. N. Engl. J. Med. 2018, 378, 518–528. [Google Scholar] [CrossRef]
- Facon, T.; Kumar, S.; Plesner, T.; Orlowski, R.Z.; Moreau, P.; Bahlis, N.; Basu, S.; Nahi, H.; Hulin, C.; Quach, H.; et al. Daratumumab plus lenalidomide and dexamethasone for untreated myeloma. N. Engl. J. Med. 2019, 380, 2014–2115. [Google Scholar] [CrossRef] [PubMed]
- Moreau, P.; Attal, M.; Hulin, C.; Arnulf, B.; Belhadj, K.; Benboubker, L.; Béné, M.C.; Broijl, A.; Caillon, H.; Caillot, D.; et al. Bortezomib, thalidomide, and dexamethasone with or without daratumumab before and after autologous stem-cell transplantation for newly diagnosed multiple myeloma (CASSIOPEIA): A randomised, open-label, phase 3 study. Lancet 2019, 394, 29–38. [Google Scholar] [CrossRef]
- Nahi, H.; Chrobok, M.; Gran, C.; Lund, J.; Gruber, A.; Gahrton, G.; Ljungman, P.; Wagner, A.K.; Alici, E. Infectious complications and NK cell depletion following daratumumab treatment of Multiple Myeloma. PLoS ONE 2019, 14, e0211927. [Google Scholar] [CrossRef]
- Drgona, L.; Gudiol, C.; Lanini, S.; Salzberger, B.; Ippolito, G.; Mikulska, M. ESCMID Study Group for Infections in Compromised Hosts (ESGICH) Consensus Document on the safety of targeted and biological therapies: An infectious diseases perspective (Agents targeting lymphoid Multiple Myeloma 16 or myeloid cells surface antigens [II]: CD22, CD30, CD33, CD38, CD40, SLAMF-7 and CCR4). Clin. Microbiol. Infect. 2018, 24 (Suppl. 2), S83–S94. [Google Scholar]
- Al Hadidi, S.; Miller-Chism, C.N.; Kamble, R.; Mims, M. Safety Analysis of Five Randomized Controlled Studies of Daratumumab in Patients With Multiple Myeloma. Clin. Lymphoma Myeloma Leuk. 2020, 20, e579–e589. [Google Scholar] [CrossRef]
- Girmenia, C.; Cavo, M.; Corso, A.; Di Raimondo, F.; Musto, P.; Offidani, M.; Petrucci, M.T.; Rosato, A.; Barosi, G. Management of infectious risk of daratumumab therapy in multiple myeloma: A consensus-based position paper from an ad hoc Italian expert panel. Crit. Rev. Oncol. Hematol. 2022, 172, 103623. [Google Scholar] [CrossRef] [PubMed]
- Tzogani, K.; Penninga, E.; Schougaard Christiansen, M.L.; Hovgaard, D.; Sarac, S.B.; Camarero Jimenez, J.; Garcia, I.; Lafuente, M.; Sancho-López, A.; Salmonson, T.; et al. EMA Review of Daratumumab for the Treatment of Adult Patients with Multiple Myeloma. Oncologist 2018, 23, 594–602. [Google Scholar] [CrossRef]
- McCudden, C.R.; Voorhees, P.M.; Hainsworth, S.A.; Whinna, H.C.; Chapman, J.F.; Hammett-Stabler, C.A.; Willis, M.S. Interference of monoclonal antibody therapies with serum protein electrophoresis tests. Clin. Chem. 2010, 56, 1897–1899. [Google Scholar] [CrossRef] [PubMed]
- McCudden, C.; Axel, A.E.; Slaets, D.; Dejoie, T.; Clemens, P.L.; Frans, S.; Bald, J.; Plesner, T.; Jacobs, J.F.; van de Donk, N.W.; et al. Monitoring multiple myeloma patients treated with daratumumab: Teasing out monoclonal antibody interference. Clin. Chem. Lab. Med. 2016, 54, 1095–1104. [Google Scholar] [CrossRef] [PubMed]
- Santockyte, R.; Puig, O.; Zheng, N.; Ouyang, Z.; Titsch, C.; Zhang, Y.J.; Pillutla, R.; Zeng, J. High-Throughput Therapeutic Antibody Interference-Free High-Resolution Mass Spectrometry Assay for Monitoring M-Proteins in Multiple Myeloma. Anal. Chem. 2021, 93, 834–842. [Google Scholar] [CrossRef] [PubMed]
- Noori, S.; Verkleij, C.; Zajec, M.; Langerhorst, P.; Bosman, P.; de Rijke, Y.B.; Zweegman, S.; VanDuijn, M.; Luider, T.; van de Donk, N.; et al. Monitoring the M-protein of multiple myeloma patients treated with a combination of monoclonal antibodies: The laboratory solution to eliminate interference. Clin. Chem. Lab. Med. 2021, 59, 1963–1971. [Google Scholar] [CrossRef]
- Plesner, T.; van de Donk, N.; Richardson, P.G. Controversy in the Use of CD38 Antibody for Treatment of Myeloma: Is High CD38 Expression Good or Bad? Cells 2020, 9, 378. [Google Scholar] [CrossRef] [PubMed]
- Moreno, L.; Perez, C.; Zabaleta, A.; Manrique, I.; Alignani, D.; Ajona, D.; Blanco, L.; Lasa, M.; Maiso, P.; Rodriguez, I.; et al. The mechanism of action of the anti-CD38 monoclonal antibody isatuximab in multiple myeloma. Clin. Cancer Res. 2019, 25, 3176–3187. [Google Scholar] [CrossRef]
- Atanackovic, D.; Yousef, S.; Shorter, C.; Tantravahi, S.K.; Steinbach, M.; Iglesias, F.; Sborov, D.; Radhakrishnan, S.V.; Chiron, M.; Miles, R.; et al. In vivo vaccination effect in multiple myeloma patients treated with the monoclonal antibody isatuximab. Leukemia 2020, 34, 317–321. [Google Scholar] [CrossRef]
- Sanof-Aventis. SARCLISA (Isatuximab): EU Summary of Product Characteristics. 2021. Available online: https://www.ema.europa.eu (accessed on 22 April 2021).
- Attal, M.; Richardson, P.G.; Rajkumar, S.V.; San-Miguel, J.; Beksac, M.; Spicka, I.; Leleu, X.; Schjesvold, F.; Moreau, P.; Dimopoulos, M.A.; et al. Isatuximab plus pomalidomide and low-dose dexamethasone versus pomalidomide and low-dose dexamethasone in patients with relapsed and refractory multiple myeloma (ICARIA-MM): A randomised, multicentre, open-label, phase 3 study. Lancet 2019, 394, 2096–2107. [Google Scholar] [CrossRef]
- Richardson, P.G.; Perrot, A.; San-Miguel, J.; Beksac, M.; Spicka, I.; Leleu, X.; Schjesvold, F.; Moreau, P.; Dimopoulos, M.A.; Huang, J.S.; et al. Isatuximab plus pomalidomide and low-dose dexamethasone versus pomalidomide and low-dose dexamethasone in patients with relapsed and refractory multiple myeloma (ICARIA-MM): Follow-up analysis of a randomised, phase 3 study. Lancet Oncol. 2022, 23, 416–427. [Google Scholar] [CrossRef]
- Harrison, S.J.; Perrot, A.; Alegre, A.; Simpson, D.; Wang, M.C.; Spencer, A.; Delimpasi, S.; Hulin, C.; Sunami, K.; Facon, T.; et al. Subgroup analysis of ICARIA-MM study in relapsed/refractory multiple myeloma patients with high-risk cytogenetics. Br. J. Haematol. 2021, 194, 120–131. [Google Scholar] [CrossRef]
- Moreau, P.; Dimopoulos, M.A.; Mikhael, J.; Yong, K.; Capra, M.; Facon, T.; Hajek, R.; Špička, I.; Baker, R.; Kim, K.; et al. Isatuximab, carflzomib, and dexamethasone in relapsed multiple myeloma (IKEMA): A multicentre, open-label, randomised phase 3 trial. Lancet 2021, 397, 2361–2371. [Google Scholar] [CrossRef]
- Dimopoulos, M.; Bringhen, S.; Anttila, P.; Capra, M.; Cavo, M.; Cole, C.; Gasparetto, C.; Hungria, V.; Jenner, M.; Vorobyev, V.; et al. Isatuximab as monotherapy and combined with dexamethasone in patients with relapsed/refractory multiple myeloma. Blood 2021, 137, 1154–1165. [Google Scholar] [CrossRef] [PubMed]
- Boxhammer, R.; Weirather, J.; Steidl, S.; Endell, J. MOR202, a human anti-CD38 monoclonal antibody, mediates potent tumoricidal activity in vivo and shows synergistic efficacy in combination with different antineoplastic compounds. Blood 2015, 126, 3015. [Google Scholar] [CrossRef]
- Raab, M.S.; Engelhardt, M.; Blank, A.; Goldschmidt, H.; Agis, H.; Blau, I.W.; Einsele, H.; Ferstl, B.; Schub, N.; Röllig, C.; et al. MOR202, a novel anti-CD38 monoclonal antibody, in patients with relapsed or refractory multiple myeloma: A first-in-human, multicentre, phase 1-2a trial. Lancet Haematol. 2020, 7, e381–e394. [Google Scholar] [CrossRef]
- van Rhee, F.; Szmania, S.M.; Dillon, M.; van Abbema, A.M.; Li, X.; Stone, M.K.; Garg, T.K.; Shi, J.; Moreno-Bost, A.M.; Yun, R.; et al. Combinatorial efficacy of anti-CS1 monoclonal antibody elotuzumab (HuLuc63) and bortezomib against multiple myeloma. Mol. Cancer Ther. 2009, 8, 2616–2624. [Google Scholar] [CrossRef]
- Richardson, P.G.; Jagannath, S.; Moreau, P.; Jakubowiak, A.J.; Raab, M.S.; Facon, T.; Vij, R.; White, D.; Reece, D.E.; Benboubker, L.; et al. Elotuzumab in combination with lenalidomide and dexamethasone in patients with relapsed multiple myeloma: Final phase 2 results from the randomised, open-label, phase 1b-2 dose-escalation study. Lancet Haematol. 2015, 2, e516–e527. [Google Scholar] [CrossRef]
- Mateos, M.V.; Granell, M.; Oriol, A.; Martinez-Lopez, J.; Blade, J.; Hernandez, M.T.; Martín, J.; Gironella, M.; Lynch, M.; Bleickardt, E.; et al. Elotuzumab in combination with thalidomide and low-dose dexamethasone: A phase 2 single-arm safety study in patients with relapsed/refractory multiple myeloma. Br. J. Haematol. 2016, 175, 448–456. [Google Scholar] [CrossRef]
- Lonial, S.; Dimopoulos, M.; Palumbo, A.; White, D.; Grosicki, S.; Spicka, I.; Walter-Croneck, A.; Moreau, P.; Mateos, M.V.; Magen, H.; et al. ELOQUENT-2 Investigators. Elotuzumab therapy for relapsed or refractory multiple myeloma. N. Engl. J. Med. 2015, 373, 621–631. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Lonial, S.; White, D.; Moreau, P.; Weisel, K.; San-Miguel, J.; Shpilberg, O.; Grosicki, S.; Špička, I.; Walter-Croneck, A.; et al. Elotuzumab, lenalidomide, and dexamethasone in RRMM: Final overall survival results from the phase 3 randomized ELOQUENT-2 study. Blood Cancer J. 2020, 10, 91. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, M.A.; Dytfeld, D.; Grosicki, S.; Moreau, P.; Takezako, N.; Hori, M.; Leleu, X.; LeBlanc, R.; Suzuki, K.; Raab, M.S.; et al. Elotuzumab plus Pomalidomide and Dexamethasone for Multiple Myeloma. N. Engl. J. Med. 2018, 379, 1811–1822. [Google Scholar] [CrossRef]
- Kubo, K.; Hori, M.; Ohta, K.; Handa, H.; Hatake, K.; Matsumoto, M.; Hagiwara, S.; Ohashi, K.; Nakaseko, C.; Suzuki, K.; et al. Elotuzumab plus lenalidomide and dexamethasone for newly diagnosed multiple myeloma: A randomized, open-label, phase 2 study in Japan. Int. J. Hematol. 2020, 111, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, M.A.; Richardson, P.G.; Bahlis, N.J.; Grosicki, S.; Cavo, M.; Beksaç, M.; Legieć, W.; Liberati, A.M.; Goldschmidt, H.; Belch, A.; et al. Addition of elotuzumab to lenalidomide and dexamethasone for patients with newly diagnosed, transplantation ineligible multiple myeloma (ELOQUENT-1): An open-label, multicentre, randomised, phase 3 trial. Lancet Haematol. 2022, 9, e403–e414. [Google Scholar] [CrossRef]
- Tai, Y.T.; Mayes, P.A.; Acharya, C.; Zhong, M.Y.; Cea, M.; Cagnetta, A.; Craigen, J.; Yates, J.; Gliddon, L.; Fieles, W.; et al. Novel anti-B-cell maturation antigen antibody-drug conjugate (GSK2857916) selectively induces killing of multiple myeloma. Blood 2014, 123, 3128–3138. [Google Scholar] [CrossRef]
- Montes de Oca, R.; Alavi, A.S.; Vitali, N.; Bhattacharya, S.; Blackwell, C.; Patel, K.; Seestaller-Wehr, L.; Kaczynski, H.; Shi, H.; Dobrzynski, E.; et al. Belantamab Mafodotin (GSK2857916) Drives Immunogenic Cell Death and Immune-mediated Antitumor Responses In Vivo. Mol. Cancer Ther. 2021, 20, 1941–1955. [Google Scholar] [CrossRef] [PubMed]
- Trudel, S.; Lendvai, N.; Popat, R.; Voorhees, P.M.; Reeves, B.; Libby, E.N.; Richardson, P.G.; Hoos, A.; Gupta, I.; Bragulat, V.; et al. Antibody-drug conjugate, GSK2857916, in relapsed/refractory multiple myeloma: An update on safety and efficacy from dose expansion phase I study. Blood Cancer J. 2019, 9, 37. [Google Scholar] [CrossRef]
- Lonial, S.; Lee, H.C.; Badros, A.; Trudel, S.; Nooka, A.K.; Chari, A.; Abdallah, A.O.; Callander, N.; Lendvai, N.; Sborov, D.; et al. Belantamab mafodotin for relapsed or refractory multiple myeloma (DREAMM-2): A two-arm, randomised, open-label, phase 2 study. Lancet Oncol. 2020, 21, 207–221. [Google Scholar] [CrossRef]
- Lonial, S.; Lee, H.C.; Badros, A.; Trudel, S.; Nooka, A.K.; Chari, A.; Abdallah, A.O.; Callander, N.; Sborov, D.; Suvannasankha, A.; et al. Longer term outcomes with single-agent belantamab mafodotin in patients with relapsed or refractory multiple myeloma: 13-month follow-up from the pivotal DREAMM-2 study. Cancer 2021, 127, 4198–4212. [Google Scholar] [CrossRef]
- Nooka, A.K.; Stockerl-Goldstein, K.; Quch, H.; Forbes, A.; Mateos, M.V.; Khot, A.; Tan, A.; Abonour, R.; Chopra, B.; Rogers, R.; et al. DREAMM-6: Safety and tolerability of belantamab mafodotin in combination with bortezomib/dexamethasone in relapsed/refractory multiple myeloma (RRMM). J. Clin. Oncol. 2020, 38 (Suppl. 15), 8502. [Google Scholar] [CrossRef]
- Nooka, A.K.; Weisel, K.; van de Donk, N.W.; Routledge, D.; Otero, P.R.; Song, K.; Quach, H.; Callander, N.; Minnema, M.C.; Trudel, S.; et al. Belantamab mafodotin in combination with novel agents in relapsed/refractory multiple myeloma: DREAMM-5 study design. Future Oncol. 2021, 17, 1987–2003. [Google Scholar] [CrossRef] [PubMed]
- Farooq, A.V.; Degli Esposti, S.; Popat, R.; Thulasi, P.; Lonial, S.; Nooka, A.K.; Jakubowiak, A.; Sborov, D.; Zaugg, B.E.; Badros, A.Z.; et al. Corneal Epithelial Findings in Patients with Multiple Myeloma Treated with Antibody-Drug Conjugate Belantamab Mafodotin in the Pivotal, Randomized, DREAMM-2 Study. Ophthalmol. Ther. 2020, 9, 889–911. [Google Scholar] [CrossRef] [PubMed]
- Lonial, S.; Nooka, A.K.; Thulasi, P.; Badros, A.Z.; Jeng, B.H.; Callander, N.S.; Potter, H.A.; Sborov, D.; Zaugg, B.E.; Popat, R.; et al. Management of belantamab mafodotin-associated corneal events in patients with relapsed or refractory multiple myeloma (RRMM). Blood Cancer J. 2021, 11, 103. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.C.; Raje, N.S.; Landgren, O.; Upreti, V.V.; Wang, J.; Avilion, A.A.; Hu, X.; Rasmussen, E.; Ngarmchamnanrith, G.; Fujii, H.; et al. Phase 1 study of the anti-BCMA antibody-drug conjugate AMG 224 in patients with relapsed/refractory multiple myeloma. Leukemia 2021, 35, 255–258. [Google Scholar] [CrossRef]
- Xing, L.; Lin, L.; Yu, T.; Li, Y.; Cho, S.F.; Liu, J.; Wen, K.; Hsieh, P.A.; Kinneer, K.; Munshi, N. MEDI2228, a novel BCMA pyrrolobenzodiazepine antibody drug conjugate, overcomes drug resistance and synergizes with bortezomib and DNA damage response inhibitors in multiple myeloma. Clin. Lymphoma Myeloma 2019, 19, E154–E155. [Google Scholar]
- Kumar, S.K.; Migkou, M.; Bhutani, M.; Spencer, A.; Ailawadhi, S.; Kalff, A.; Walcott, F.; Pore, N.; Gibson, D.; Wang, F.; et al. Phase 1, first-in-human study of MEDI2228, a BCMA-targeted ADC in patients with relapsed/refractory multiple myeloma. Blood 2020, 136 (Suppl. 1), 26–27. [Google Scholar] [CrossRef]
- Singh, R.K.; Jones, R.J.; Hong, S.; Shirazi, F.; Wang, H.; Kuiatse, I.; Pahl, A.; Orlowski, R.Z. HDP101, a novel B-Cell Maturation Antigen (BCMA)-targeted antibody conjugated to α-Amanitin, is active against myeloma with preferential efficacy against pre-clinical models of deletion 17p. Blood 2018, 132 (Suppl. 1), 593. [Google Scholar] [CrossRef]
- Shah, N.; Chari, A.; Scott, E.; Mezzi, K.; Usmani, S.Z. B-cell maturation antigen (BCMA) in multiple myeloma: Rationale for targeting and current therapeutic approaches. Leukemia 2020, 34, 985–1005. [Google Scholar] [CrossRef]
- Pillarisetti, K.; Powers, G.; Luistro, L.; Babich, A.; Baldwin, E.; Li, Y.; Zhang, X.; Mendonça, M.; Majewski, N.; Nanjunda, R.; et al. Teclistamab is an active T cell-redirecting bispecific antibody against B-cell maturation antigen for multiple myeloma. Blood Adv. 2020, 4, 4538–4549. [Google Scholar] [CrossRef]
- Usmani, S.Z.; Garfall, A.L.; van de Donk, N.; Nahi, H.; San-Miguel, J.F.; Oriol, A.; Rosinol, L.; Chari, A.; Bhutani, M.; Karlin, L.; et al. Teclistamab, a B-cell maturation antigen × CD3 bispecific antibody, in patients with relapsed or refractory multiple myeloma (MajesTEC-1): A multicentre, open-label, single-arm, phase 1 study. Lancet 2021, 398, 665–674. [Google Scholar] [CrossRef]
- Hipp, S.; Tai, Y.T.; Blanset, D.; Deegen, P.; Wahl, J.; Thomas, O.; Rattel, B.; Adam, P.J.; Anderson, K.C.; Friedrich, M. A novel BCMA/CD3 bispecific T-cell engager for the treatment of multiple myeloma induces selective lysis in vitro and in vivo. Leukemia 2017, 31, 1743–1751. [Google Scholar] [CrossRef] [PubMed]
- Topp, M.S.; Duell, J.; Zugmaier, G.; Attal, M.; Moreau, P.; Langer, C.; Krönke, J.; Facon, T.; Salnikov, A.V.; Lesley, R.; et al. Anti-B-Cell Maturation Antigen BiTE Molecule AMG 420 Induces Responses in Multiple Myeloma. J. Clin. Oncol. 2020, 38, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, R.L.; Goyos, A.; Li, C.M.; Deegen, P.; Bogner, P.; Sternjak, A.; Thomas, O.; Klinger, M.; Wahl, J.; Friedrich, M.; et al. AMG 701 induces cytotoxicity of multiple myeloma cells and depletes plasma cells in cynomolgus monkeys. Blood Adv. 2020, 4, 4180–4194. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.F.; Lin, L.; Xing, L.; Li, Y.; Wen, K.; Yu, T.; Hsieh, P.A.; Munshi, N.; Wahl, J.; Matthes, K.; et al. The immunomodulatory drugs lenalidomide and pomalidomide enhance the potency of AMG 701 in multiple myeloma preclinical models. Blood Adv. 2020, 4, 4195–4207. [Google Scholar] [CrossRef]
- Harrison, S.J.; Minnema, M.C.; Lee, H.C.; Spencer, A.; Kapoor, P.; Madduri, D.; Larsen, J.; Ailawadhi, S.; Kaufman, J.L.; Raab, M.S.; et al. A phase 1 First in Human (FIH) study of AMG 701, an anti-B-Cell Maturation Antigen (BCMA) Half-Life Extended (HLE) BiTE® (bispecific T-cell engager) molecule, in Relapsed/Refractory (RR) Multiple Myeloma (MM). Blood 2020, 136, 28–29. [Google Scholar] [CrossRef]
- DiLillo, D.J.; Olson, K.; Mohrs, K.; Meagher, T.C.; Bray, K.; Sineshchekova, O.; Startz, T.; Kuhnert, J.; Retter, M.W.; Godin, S.; et al. A BCMAxCD3 bispecific T cell-engaging antibody demonstrates robust antitumor efficacy similar to that of anti-BCMA CAR T cells. Blood Adv. 2021, 5, 1291–1304. [Google Scholar] [CrossRef] [PubMed]
- Zonder, J.A.; Richter, J.; Bumma, N.; Brayer, J.; Hoffman, J.E.; Bensinger, W.I.; Wu, K.L.; Xu, L.; Chokshi, D.; Boyapati, A.; et al. Early, deep, and durable responses, and low rates of cytokine release syndrome with REGN5458, a BCMAxCD3 bispecific monoclonal antibody, in a phase 1/2 first-in-human study in patients with relapsed/refractory multiple myeloma (RRMM). Blood 2021, 138, 160. [Google Scholar] [CrossRef]
- Costa, L.; Wong, S.; Bermudez, A.; de la Rubia, J.; Mateos, M.V.; Ocio, E.M.; RodriguezOtero, P.; San Miguel, J.; Li, S.; Sarmiento, R.; et al. Interim results from the first phase 1 clinical study of the B-cell maturation antigen (BCMA) 2+1 T-cell engager (TCE) CC-93269 in patients (pts) with relapsed/refractory multiple myeloma (RRMM). EHA Libr. 2020, 295025, S205. [Google Scholar]
- Lesokhin, A.; Iida, S.; Stevens, D.; Gabayan, A.E.; Ma, W.D.; Sullivan, S.; Raab, M.S. MagnetisMM-3: An open-label, multicenter, non-randomized phase 2 study of elranatamab (PF-06863135) in patients with relapsed or refractory multiple myeloma. Blood 2021, 138, 1674. [Google Scholar] [CrossRef]
- Kumar, S.K.; D’Souza, A.; Shah, N.; Rodriguez, C.; Voorhees, P.M.; Bueno, O.F.; Buelow, B.; Freise, K.J.; Yue, S.; Pothacamury, R.K.; et al. A phase 1 first-in-human study of Tnb-383B, a BCMA x CD3 Bispecific T-cell redirecting antibody, in patients with relapsed/refractory multiple myeloma. Blood 2021, 138, 900. [Google Scholar] [CrossRef]
- Lee, D.W.; Gardner, R.; Porter, D.L.; Louis, C.U.; Ahmed, N.; Jensen, M.; Grupp, S.A.; Mackall, C.L. Current concepts in the diagnosis and management of cytokine release syndrome. Blood 2014, 124, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Munshi, N.C.; Anderson, L.D., Jr.; Shah, N.; Madduri, D.; Berdeja, J.; Lonial, S.; Raje, N.; Lin, Y.; Siegel, D.; Oriol, A.; et al. Idecabtagene Vicleucel in Relapsed and Refractory Multiple Myeloma. N. Engl. J. Med. 2021, 384, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Berdeja, J.G.; Madduri, D.; Usmani, S.Z.; Jakubowiak, A.; Agha, M.; Cohen, A.D.; Stewart, A.K.; Hari, P.; Htut, M.; Lesokhin, A. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): A phase 1b/2 open-label study. Lancet 2021, 398, 314–324. [Google Scholar] [CrossRef]
- van de Donk, N.W.C.J.; Usmani, S.Z.; Yong, K. CAR T-cell therapy for multiple myeloma: State of the art and prospects. Lancet Haematol. 2021, 8, e446–e461. [Google Scholar] [CrossRef]
- Da Vià, M.C.; Dietrich, O.; Truger, M.; Arampatzi, P.; Duell, J.; Heidemeier, A.; Zhou, X.; Danhof, S.; Kraus, S.; Chatterjee, M.; et al. Homozygous BCMA gene deletion in response to anti-BCMA CAR T cells in a patient with multiple myeloma. Nat. Med. 2021, 27, 616–619. [Google Scholar] [CrossRef]
- Truger, M.S.; Duell, J.; Zhou, X.; Heimeshoff, L.; Ruckdeschel, A.; John, M.; Riedel, A.; Hüper, S.; Peter, J.; Walter, W.; et al. Single- and double-hit events in genes encoding immune targets before and after T cell-engaging antibody therapy in MM. Blood Adv. 2021, 5, 3794–3798. [Google Scholar] [CrossRef]
- Smith, E.L.; Harrington, K.; Staehr, M.; Masakayan, R.; Jones, J.; Long, T.J.; Ng, K.Y.; Ghoddusi, M.; Purdon, T.J.; Wang, X.; et al. GPRC5D is a target for the immunotherapy of multiple myeloma with rationally designed CAR T cells. Sci. Transl. Med. 2019, 11, eaau7746. [Google Scholar] [CrossRef]
- Verkleij, C.; Broekmans, M.; van Duin, M.; Frerichs, K.A.; Kuiper, R.; de Jonge, A.V.; Kaiser, M.; Morgan, G.; Axel, A.; Boominathan, R.; et al. Preclinical activity and determinants of response of the GPRC5DxCD3 bispecific antibody talquetamab in multiple myeloma. Blood Adv. 2021, 5, 2196–2215. [Google Scholar] [CrossRef]
- Krishnan, A.Y.; Minnema, M.C.; Berdeja, J.G. Updated phase 1 results from MonumenTAL-1: First-in-human study of talquetamab, a G protein-coupled receptor family C group 5 member D x CD3 bispecific antibody, in patients with relapsed/refractory multiple myeloma. Blood 2021, 138, 158. [Google Scholar] [CrossRef]
- Polson, A.G.; Zheng, B.; Elkins, K.; Chang, W.; Du, C.; Dowd, P.; Yen, L.; Tan, C.; Hongo, J.A.; Koeppen, H.; et al. Expression pattern of the human FcRH/IRTA receptors in normal tissue and in B-chronic lymphocytic leukemia. Int. Immunol. 2006, 18, 1363–1373. [Google Scholar] [CrossRef]
- Trudel, S.; Cohen, A.D.; Krishnan, A.Y. Cevostamab monotherapy continues to show clinically meaningful activity and manageable safety in patients with heavily pre-treated relapsed/refractory multiple myeloma (RRMM): Updated results from an ongoing phase I study. Blood 2021, 138, 157. [Google Scholar] [CrossRef]
- Jagannath, S.; Heffner, L.T., Jr.; Ailawadhi, S.; Munshi, N.C.; Zimmerman, T.M.; Rosenblatt, J.; Lonial, S.; Chanan-Khan, A.; Ruehle, M.; Rharbaoui, F.; et al. Indatuximab Ravtansine (BT062) Monotherapy in Patients With Relapsed and/or Refractory Multiple Myeloma. Clin. Lymphoma Myeloma Leuk. 2019, 19, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Du, Z.; Wang, X.; Wang, G.; Li, W. Association of IL-6 Promoter and Receptor Polymorphisms with Multiple Myeloma Risk: A Systematic Review and Meta-Analysis. Genet. Test. Mol. Biomark. 2016, 20, 587–596. [Google Scholar] [CrossRef]
- Brighton, T.A.; Khot, A.; Harrison, S.J.; Ghez, D.; Weiss, B.M.; Kirsch, A.; Magen, H.; Gironella, M.; Oriol, A.; Streetly, M.; et al. Randomized, Double-Blind, Placebo-Controlled, Multicenter Study of Siltuximab in High-Risk Smoldering Multiple Myeloma. Clin. Cancer Res. 2019, 25, 3772–3775. [Google Scholar] [CrossRef]
- Desantis, V.; Savino, F.D.; Scaringella, A.; Potenza, M.A.; Nacci, C.; Frassanito, M.A.; Vacca, A.; Montagnani, M. The Leading Role of the Immune Microenvironment in Multiple Myeloma: A New Target with a Great Prognostic and Clinical Value. J. Clin. Med. 2022, 11, 2513. [Google Scholar] [CrossRef] [PubMed]
- Waldmann, H. Human Monoclonal Antibodies: The Benefits of Humanization. Methods Mol Biol. 2019, 1904, 1–10. [Google Scholar]
- Cruz, E.; Kayser, V. Monoclonal antibody therapy of solid tumors: Clinical limitations and novel strategies to enhance treatment efficacy. Biologics 2019, 13, 33–51. [Google Scholar] [CrossRef]
- Jovčevska, I.; Muyldermans, S. The Therapeutic Potential of Nanobodies. BioDrugs 2020, 34, 11–26. [Google Scholar] [CrossRef]
- Ackaert, C.; Smiejkowska, N.; Xavier, C.; Sterckx, Y.; Denies, S.; Stijlemans, B.; Elkrim, Y.; Devoogdt, N.; Caveliers, V.; Lahoutte, T.; et al. Immunogenicity Risk Profile of Nanobodies. Front. Immunol. 2021, 12, 632687. [Google Scholar] [CrossRef]
- Li, B.; Qin, X.; Mi, L.Z. Nanobodies: From structure to applications in non-injectable and bispecific biotherapeutic development. Nanoscale 2022, 14, 7110–7122. [Google Scholar] [CrossRef]
- de Marco, A. Cytoplasmic Production of Nanobodies and Nanobody-Based Reagents by Co-Expression of Sulfhydryl Oxidase and DsbC Isomerase. Methods Mol. Biol. 2022, 2446, 145–157. [Google Scholar] [PubMed]
- Puttemans, J.; Stijlemans, B.; Keyaerts, M.; Vander Meeren, S.; Renmans, W.; Fostier, K.; Debie, P.; Hanssens, H.; Rodak, M.; Pruszynski, M.; et al. The Road to Personalized Myeloma Medicine: Patient-specific Single-domain Antibodies for Anti-idiotypic Radionuclide Therapy. Mol. Cancer Ther. 2022, 21, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Minnix, M.; Adhikarla, V.; Caserta, E.; Poku, E.; Rockne, R.; Shively, J.E.; Pichiorri, F. Comparison of CD38-Targeted α- Versus β-Radionuclide Therapy of Disseminated Multiple Myeloma in an Animal Model. J. Nucl. Med. 2021, 62, 795–801. [Google Scholar] [CrossRef] [PubMed]
- De Veirman, K.; Puttemans, J.; Krasniqi, A.; Ertveldt, T.; Hanssens, H.; Romao, E.; Hose, D.; Goyvaert, C.; Vlummens, P.; Muyldermans, S.; et al. CS1-specific single-domain antibodies labeled with Actinium-225 prolong survival and increase CD8+ T cells and PD-L1 expression in Multiple Myeloma. Oncoimmunology 2021, 10, 2000699. [Google Scholar] [CrossRef]
- Giles, H.V.; Cook, M.A.; Drayson, M.T.; Cook, G.; Wright, N.J.; North, S.J.; Harding, S.; Cairns, D.A.; Hockaday, A.; Kaiser, M.F.; et al. Redefining nonmeasurable multiple myeloma using mass spectrometry. Blood 2022, 139, 946–950. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.; Parayath, N.; Ma, W.; Amiji, M.; Munshi, N.; Anderson, K.C. BCMA peptide-engineered nanoparticles enhance induction and function of antigen-specific CD8+ cytotoxic T lymphocytes against multiple myeloma: Clinical applications. Leukemia 2020, 34, 210–223. [Google Scholar] [CrossRef]
- Nigro, A.; Frattaruolo, L.; Fava, M.; De Napoli, I.; Greco, M.; Comandè, A.; De Santo, M.; Pellegrino, M.; Ricci, E.; Giordano, F.; et al. Bortezomib-Loaded Mesoporous Silica Nanoparticles Selectively Alter Metabolism and Induce Death in Multiple Myeloma Cells. Cancers 2020, 12, 2709. [Google Scholar] [CrossRef]
- Federico, C.; Alhallak, K.; Sun, J.; Duncan, K.; Azab, F.; Sudlow, G.P.; de la Puente, P.; Muz, B.; Kapoor, V.; Zhang, L.; et al. Tumor microenvironment-targeted nanoparticles loaded with bortezomib and ROCK inhibitor improve efficacy in multiple myeloma. Nat. Commun. 2020, 11, 6037. [Google Scholar] [CrossRef]
- Mølgaard, K.; Harwood, S.L.; Compte, M.; Merino, N.; Bonet, J.; Alvarez-Cienfuegos, A.; Mikkelsen, K.; Nuñez-Prado, N.; Alvarez-Mendez, A.; Sanz, L.; et al. Bispecific light T-cell engagers for gene-based immunotherapy of epidermal growth factor receptor (EGFR)-positive malignancies. Cancer Immunol. Immunother. 2018, 67, 1251–1260. [Google Scholar] [CrossRef]
- Tapia-Alveal, C.; Olsen, T.R.; Worgall, T.S. Personalized immunoglobulin aptamers for detection of multiple myeloma minimal residual disease in serum. Commun. Biol. 2020, 3, 781. [Google Scholar] [CrossRef]
- Dai, H.; Ye, M.; Peng, M.; Zhou, W.; Bai, H.; Xiao, X.; Ma, B.; Zhou, J.; Tang, S.; Yao, S.; et al. Aptamer TY04 inhibits the growth of multiple myeloma cells via cell cycle arrest. Tumour. Biol. 2014, 35, 7561–7568. [Google Scholar] [CrossRef]
- Zhou, W.; Zhang, Y.; Zeng, Y.; Peng, M.; Li, H.; Sun, S.; Ma, B.; Wang, Y.; Ye, M.; Liu, J. Screening and characterization of an Annexin A2 binding aptamer that inhibits the proliferation of myeloma cells. Biochimie 2018, 151, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gao, H.; Zhou, W.; Sun, S.; Zeng, Y.; Zhang, H.; Liang, L.; Xiao, X.; Song, J.; Ye, M.; et al. Targeting c-met receptor tyrosine kinase by the DNA aptamer SL1 as a potential novel therapeutic option for myeloma. J. Cell Mol. Med. 2018, 22, 5978–5990. [Google Scholar] [CrossRef] [PubMed]
- Catuogno, S.; Di Martino, M.T.; Nuzzo, S.; Esposito, C.L.; Tassone, P.; de Franciscis, V. An Anti-BCMA RNA Aptamer for miRNA Intracellular Delivery. Mol. Ther. Nucleic Acids. 2019, 18, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Schavgoulidze, A.; Cazaubiel, T.; Perrot, A.; Avet-Loiseau, H.; Corre, J. Multiple Myeloma: Heterogeneous in Every Way. Cancers 2021, 13, 1285. [Google Scholar] [CrossRef] [PubMed]
- Dahlhoff, J.; Manz, H.; Steinfatt, T.; Delgado-Tascon, J.; Seebacher, E.; Schneider, T.; Wilnit, A.; Mokhtari, Z.; Tabares, P.; Böckle, D.; et al. Transient regulatory T-cell targeting triggers immune control of multiple myeloma and prevents disease progression. Leukemia 2022, 36, 790–800. [Google Scholar] [CrossRef] [PubMed]
- Cooke, R.E.; Quinn, K.M.; Quach, H.; Harrison, S.; Prince, H.M.; Koldej, R.; Ritchie, D. Conventional Treatment for Multiple Myeloma Drives Premature Aging Phenotypes and Metabolic Dysfunction in T Cells. Front. Immunol. 2020, 11, 2153. [Google Scholar] [CrossRef]

| MECHANISM of ACTION | DRUG | SETTING |
|---|---|---|
| ANTI-CD38 | DARATUMUMAB | NDMM: (a) transplant-ineligible pts: - Dara-VMP (ALCYONE) - Dara-RD (MAIA). (b) transplant-eligible pts: - Dara-VTD (CASSIOPEIA) - Dara-VRD (PERSEUS; GRIFFIN) RRMM - Dara-RD (POLLUX) - Dara-VD (CASTOR) - Dara-PD (APOLLO) - Dara-KD (CANDOR) |
| ISATUXIMAB | RRMM - Isa-KD (IKEMA) - Isa-PD (ICARIA) - Isa SA - Isa-D | |
| FELZARTAMAB | RRMM - Fel SA - Fel-PD - Fel-RD | |
| ANTI-SLAMF-7 | ELOTUZUMAB | NDMM - Elo-RD (ELOQUENT-1) RRMM - Elo-RD (ELOQUENT-2) - Elo-PD (ELOQUENT-3) |
| ANTIBODY-DRUG CONJUGATES ANTI-BCMA | BELANTAMAB-MAFODOTIN | NDMM (a) Transplant-Eligible Pts: - Belamaf-VRD (NCT04802356) (b) Transplant-Ineligible Pts: - Belamaf-RD (NCT04808037) HRRMM - Belamaf SA (DREAMM-2) - Belamaf-VD/RD (DREAMM-6) - Belamaf-CPIs/ySIs (DREAMM-5) |
|---|---|---|
| AMG224 | highly pretreated RRMM | |
| MEDI2228 | highly pretreated RRMM | |
| HDP-101 | highly pretreated RRMM | |
| BISPECIFIC T-CELL ENGAGERS CD3xBCMA | TECLISTAMAB | highly pretreated RRMM - SA (MAJESTEC-1) |
| AMG420 | highly pretreated RRMM - SA (NCT03836053) | |
| AMG701 | highly pretreated RRMM - SA (NCT03287908) | |
| REGN5458 | highly pretreated RRMM - SA (NCT03761108) | |
| CC-93269 | highly pretreated RRMM - SA (NCT03486067) | |
| ELRANATAMAB | highly pretreated RRMM - SA (MAGNETISMM-3) | |
| BISPECIFIC T-CELL ENGAGERS CD3xOTHER TARGETS | TALQUETAMAB (CD3xGPRC5D) | highly pretreated RRMM - SA (MONUMETAL-1) |
| CEVOSTAMAB (CD3xFcRH5) | highly pretreated RRMM - SA (NCT03275103) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lapietra, G.; Fazio, F.; Petrucci, M.T. Race for the Cure: From the Oldest to the Newest Monoclonal Antibodies for Multiple Myeloma Treatment. Biomolecules 2022, 12, 1146. https://doi.org/10.3390/biom12081146
Lapietra G, Fazio F, Petrucci MT. Race for the Cure: From the Oldest to the Newest Monoclonal Antibodies for Multiple Myeloma Treatment. Biomolecules. 2022; 12(8):1146. https://doi.org/10.3390/biom12081146
Chicago/Turabian StyleLapietra, Gianfranco, Francesca Fazio, and Maria Teresa Petrucci. 2022. "Race for the Cure: From the Oldest to the Newest Monoclonal Antibodies for Multiple Myeloma Treatment" Biomolecules 12, no. 8: 1146. https://doi.org/10.3390/biom12081146
APA StyleLapietra, G., Fazio, F., & Petrucci, M. T. (2022). Race for the Cure: From the Oldest to the Newest Monoclonal Antibodies for Multiple Myeloma Treatment. Biomolecules, 12(8), 1146. https://doi.org/10.3390/biom12081146






