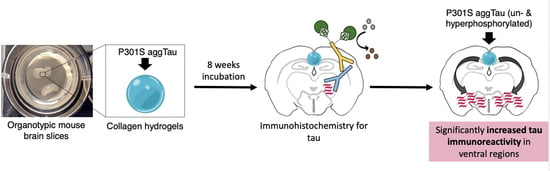
Spreading of P301S Aggregated Tau Investigated in Organotypic Mouse Brain Slice Cultures
Abstract
:1. Introduction
1.1. Tau Protein and AD
1.2. Tau and Propagation
1.3. Spreading in Organotypic Brain Slices
2. Materials and Methods
2.1. Animals
2.2. Tau Proteins
2.3. Organotypic Brain Slice Cultures
2.4. Collagen Hydrogels and Loading
2.5. Hyperphosphorylation and Aggregation of Tau
2.6. Western Blot
2.7. Release Experiments and Tau Detection
2.8. Immunohistochemistry
2.9. Lactate Dehydrogenase (LDH) Assay
2.10. Data Analysis and Statistics
3. Results
3.1. Culturing of Organotypic Brain Slices with Collagen Hydrogels
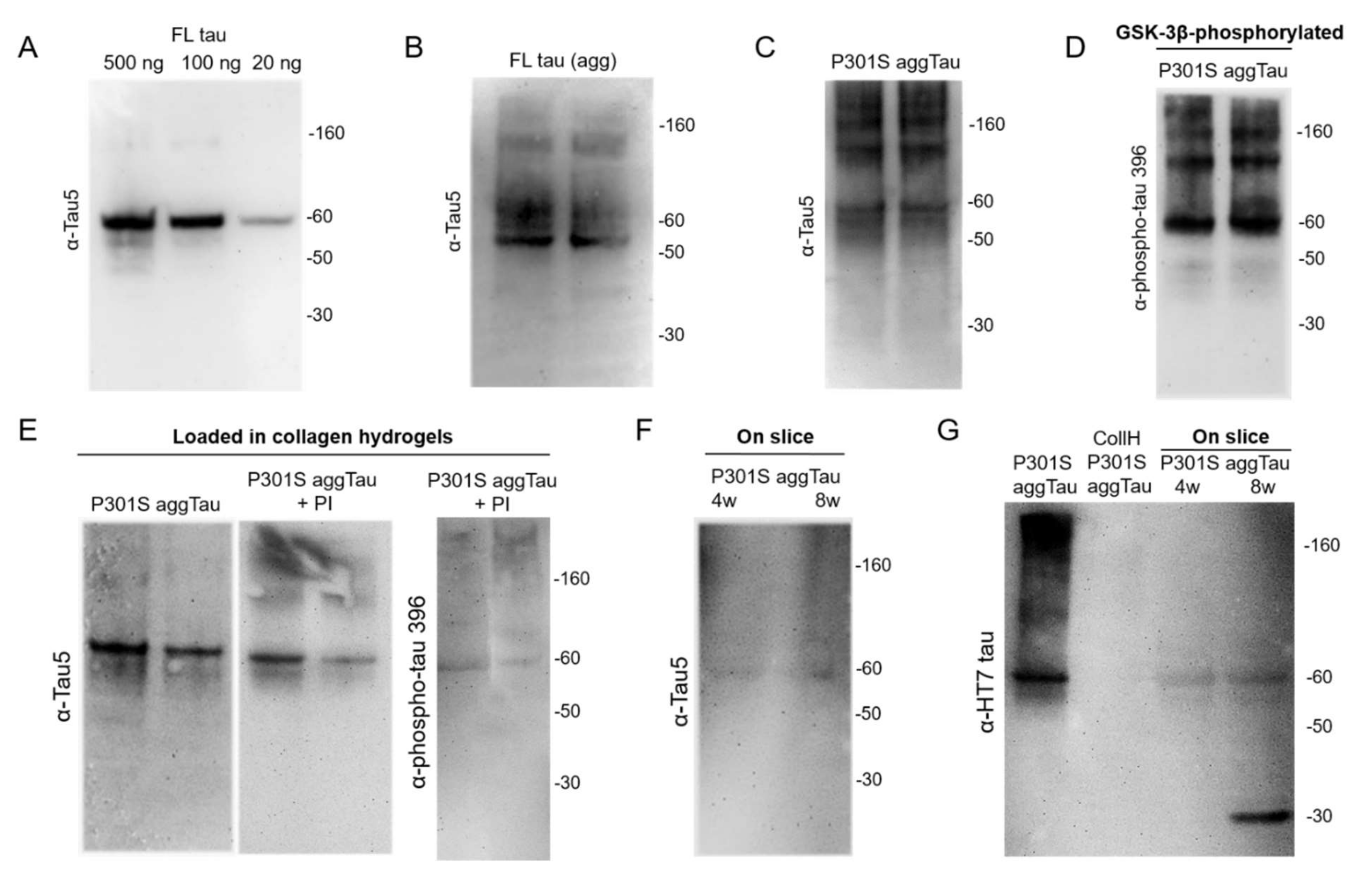
3.2. Characterization of the Tau Proteins
3.3. Loading of Tau Proteins into Collagen Hydrogels
3.4. Release of Tau from Collagen Hydrogels
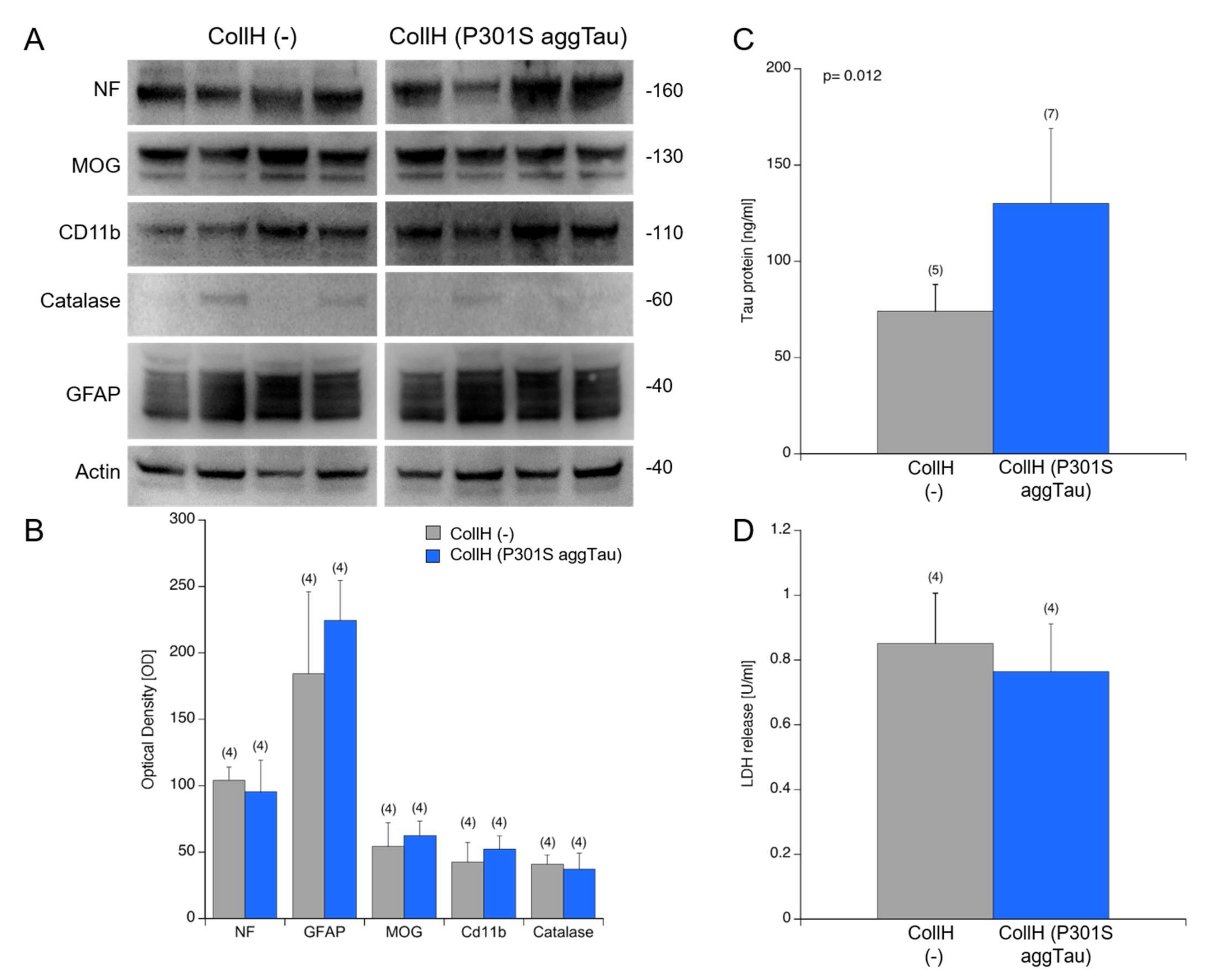
3.5. Viability of Organotypic Brain Slices
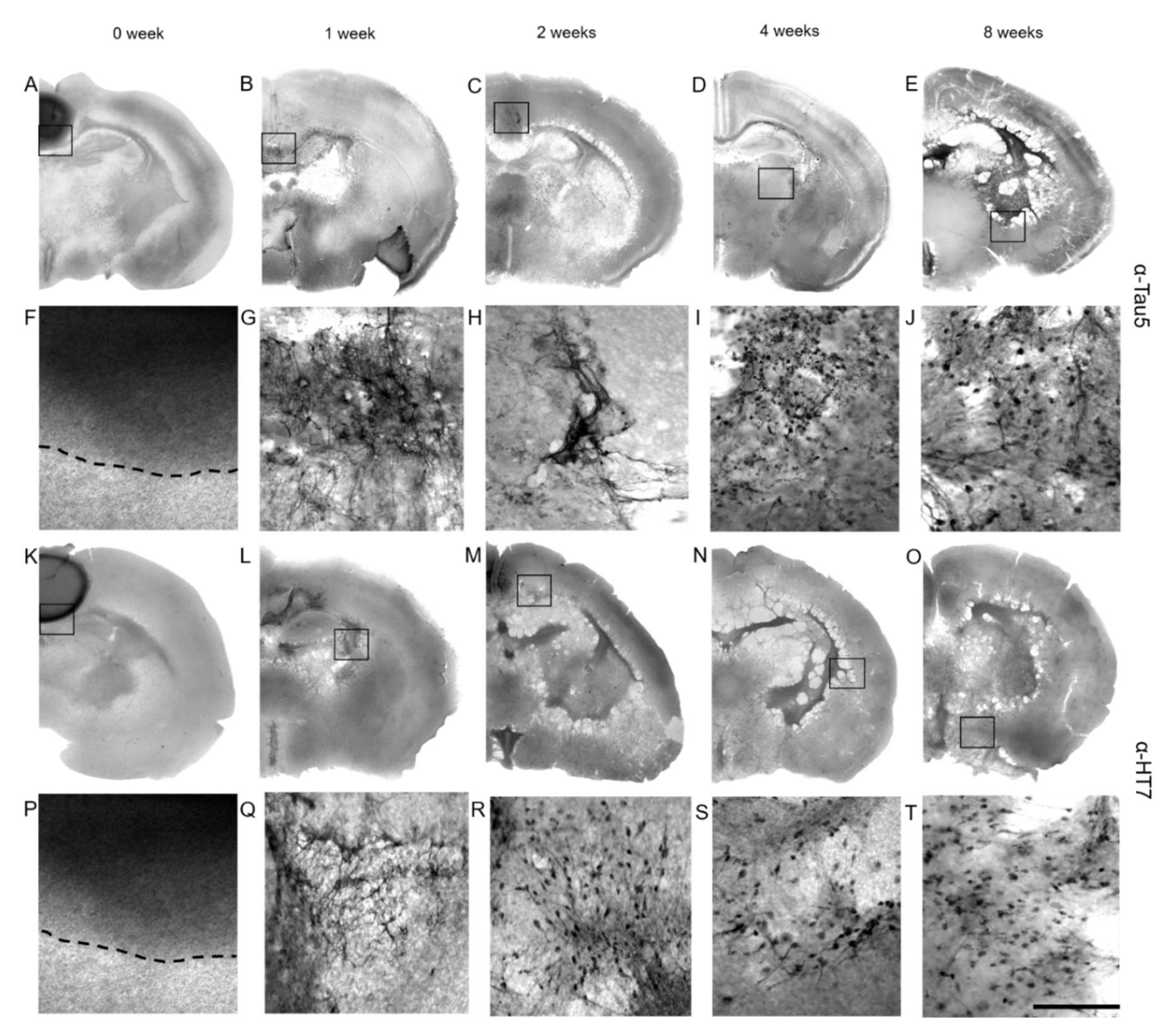
3.6. Immunostainings for Tau
3.7. Spread of P301S aggTau along Neuroanatomically Connected Pathways
3.8. Localization of P301S aggTau
4. Discussion
4.1. Viability of Organotypic Brain Slices to Study Tau Spreading
4.2. Collagen Hydrogels—Effectiveness as a Protein Delivery System
4.3. Tau Proteins Used in This Study
4.4. Spreading of P301S aggTau to Ventral Areas
4.5. Mechanism of Tau Spreading
4.6. Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| ALS | Amyotrophic lateral sclerosis |
| Aβ | Amyloid-β |
| BSA | Bovine serum albumin |
| CAA | Cerebral amyloid angiopathy |
| CSF | Cerebrospinal fluid |
| DAB | 3,3′-diaminobenzidine |
| ECM | Extracellular matrix |
| FL tau | Full-length tau |
| GFAP | Glial fibrillary acidic protein |
| GSK-3β | Glycogensynthase-kinase-3β |
| LDH | Lactate dehydrogenase |
| LDL | Low-density lipoprotein |
| LRP1 | LDL receptor-related protein 1 |
| MOG | Myelin-oligodendrocyte protein |
| NFT | Neurofibrillary tangle |
| P301S aggTau | P301S aggregated tau (active) |
| PBS | Phosphate-buffered saline |
| PD | Parkinson’s disease |
| PFA | Paraformaldehyde |
| PHF | Paired-helical filament |
| PIC | Protease inhibitor cocktail |
| PSP | Progressive supranuclear palsy |
| SD | Standard deviation |
| Δ306-311 Tau | Δ306-311 mutant tau |
| α-Syn | α-Synuclein |
References
- Prince, M.; Guerchet, M.; Prina, M. The Global Impact of Dementia 2013–2050; Alzheimer’s Disease International: London, UK, 2013. [Google Scholar]
- Guthier, S.; Rosa-Neto, P.; Morais, J.; Webster, C. World Alzheimer Report 2021: Journey through the Diagnosis of Dementia; Alzheimer’s Disease International: London, UK, 2021. [Google Scholar]
- Breijyeh, Z.; Karaman, R. Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Molecules 2020, 25, 5789. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Pozo, A.; Frosch, M.; Masliah, E.; Hyman, B. Neuropathological Alterations in Alzheimer Disease. Cold Spring Harb. Perspect. Med. 2011, 1, a006189. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, S.; Bacskai, B.; Hernandez-Guillamon, M.; Pruzin, J.; Sperling, R.; van Veluw, S. Cerebral amyloid angiopathy and Alzheimer disease—One peptide, two pathways. Nat. Rev. Neurol. 2019, 16, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Brunello, C.; Merezhko, M.; Uronen, R.; Huttunen, H. Mechanisms of secretion and spreading of pathological tau protein. Cell. Mol. Life Sci. 2020, 77, 1721–1744. [Google Scholar] [CrossRef] [Green Version]
- Sabbagh, J.; Dickey, C. The Metamorphic Nature of the Tau Protein: Dynamic Flexibility Comes at a Cost. Front. Neurosci. 2016, 10, 3. [Google Scholar] [CrossRef] [Green Version]
- Kolarova, M.; García-Sierra, F.; Bartos, A.; Ricny, J.; Ripova, D. Structure and Pathology of Tau Protein in Alzheimer Disease. Int. J. Alzheimers Dis. 2012, 2012, 731526. [Google Scholar] [CrossRef] [Green Version]
- Qiang, L.; Sun, X.; Austin, T.O.; Muralidharan, H.; Jean, D.C.; Liu, M.; Yu, W.; Baas, P.W. Tau Does Not Stabilize Axonal Microtubules but Rather Enables Them to Have Long Labile Domains. Curr. Biol. 2018, 28, 2181–2189. [Google Scholar] [CrossRef] [Green Version]
- Guo, T.; Noble, W.; Hanger, D. Roles of tau protein in health and disease. Acta Neuropathol. 2017, 133, 665–704. [Google Scholar] [CrossRef] [Green Version]
- Braak, H.; Braak, E. Staging of alzheimer’s disease-related neurofibrillary changes. Neurobiol. Aging 1995, 16, 271–278. [Google Scholar] [CrossRef]
- Mudher, A.; Colin, M.; Dujardin, S.; Medina, M.; Dewachter, I.; Alavi Naini, S.M.; Mandelkow, E.; Mandelkow, E.; Buée, L.; Goedert, M.; et al. What is the evidence that tau pathology spreads through prion-like propagation? Acta Neuropathol. Commun. 2017, 5, 99. [Google Scholar] [CrossRef]
- Kim, E.; Hwang, J.L.; Gaus, S.E.; Nana, A.L.; Deng, J.; Brown, J.A.; Spina, S.; Lee, M.J.; Ramos, E.M.; Grinberg, L.T.; et al. Evidence of corticofugal tau spreading in patients with frontotemporal dementia. Acta Neuropathol. 2020, 139, 27–43. [Google Scholar] [CrossRef] [PubMed]
- Kane, M.; Lipinski, W.; Callahan, M.; Bian, F.; Durham, R.; Schwarz, R.; Roher, A.E.; Walker, L.C. Evidence for Seeding of β-Amyloid by Intracerebral Infusion of Alzheimer Brain Extracts in β-Amyloid Precursor Protein-Transgenic Mice. J. Neurosci. 2000, 20, 3606–3611. [Google Scholar] [CrossRef] [Green Version]
- Stöhr, J.; Watts, J.; Mensinger, Z.; Oehler, A.; Grillo, S.; DeArmond, S.; Prusiner, S.B.; Giles, K. Purified and synthetic Alzheimer’s amyloid beta (Aβ) prions. Proc. Natl. Acad. Sci. USA 2012, 109, 11025–11030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luk, K.; Kehm, V.; Carroll, J.; Zhang, B.; O’Brien, P.; Trojanowski, J.; Lee, V.M.Y. Pathological α-Synuclein Transmission Initiates Parkinson-like Neurodegeneration in Nontransgenic Mice. Science 2012, 338, 949–953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uçar, B.; Stefanova, N.; Humpel, C. Spreading of Aggregated α-Synuclein in Sagittal Organotypic Mouse Brain Slices. Biomolecules 2022, 12, 163. [Google Scholar] [CrossRef]
- Clavaguera, F.; Bolmont, T.; Crowther, R.; Abramowski, D.; Frank, S.; Probst, A.; Fraser, G.; Stalder, A.K.; Beibel, M.; Staufenbiel, M.; et al. Transmission and spreading of tauopathy in transgenic mouse brain. Nat. Cell Biol. 2009, 11, 909–913. [Google Scholar] [CrossRef]
- Clavaguera, F.; Akatsu, H.; Fraser, G.; Crowther, R.A.; Frank, S.; Hench, J.; Probst, A.; Winkler, D.T.; Reichwald, J.; Staufenbiel, M.; et al. Brain homogenates from human tauopathies induce tau inclusions in mouse brain. Proc. Natl. Acad. Sci. USA 2013, 110, 9535–9540. [Google Scholar] [CrossRef] [Green Version]
- Lasagna-Reeves, C.; Castillo-Carranza, D.; Sengupta, U.; Guerrero-Munoz, M.; Kiritoshi, T.; Neugebauer, V.; Jackson, G.R.; Kayed, R. Alzheimer brain-derived tau oligomers propagate pathology from endogenous tau. Sci. Rep. 2012, 2, 700. [Google Scholar] [CrossRef]
- Guo, J.; Narasimhan, S.; Changolkar, L.; He, Z.; Stieber, A.; Zhang, B.; Gathagan, R.J.; Iba, M.; McBride, J.D.; Trojanowski, J.Q.; et al. Unique pathological tau conformers from Alzheimer’s brains transmit tau pathology in nontransgenic mice. J. Exp. Med. 2016, 213, 2635–2654. [Google Scholar] [CrossRef]
- Ahmed, Z.; Cooper, J.; Murray, T.; Garn, K.; McNaughton, E.; Clarke, H.; Parhizkar, S.; Ward, M.A.; Cavallini, A.; Jackson, S.; et al. A novel in vivo model of tau propagation with rapid and progressive neurofibrillary tangle pathology: The pattern of spread is determined by connectivity, not proximity. Acta Neuropathol. 2014, 127, 667–683. [Google Scholar] [CrossRef] [Green Version]
- Boluda, S.; Iba, M.; Zhang, B.; Raible, K.M.; Lee, V.M.Y.; Trojanowski, J.Q. Differential induction and spread of tau pathology in young PS19 tau transgenic mice following intracerebral injections of pathological tau from Alzheimer’s disease or corticobasal degeneration brains. Acta Neuropathol. 2015, 129, 221–237. [Google Scholar] [CrossRef] [Green Version]
- Iba, M.; Guo, J.; McBride, J.; Zhang, B.; Trojanowski, J.; Lee, V.M.Y. Synthetic Tau Fibrils Mediate Transmission of Neurofibrillary Tangles in a Transgenic Mouse Model of Alzheimer’s-Like Tauopathy. J. Neurosci. 2013, 33, 1024–1037. [Google Scholar] [CrossRef] [PubMed]
- Peeraer, E.; Bottelbergs, A.; Van Kolen, K.; Stancu, I.; Vasconcelos, B.; Mahieu, M.; Duytschaever, H.; Donck, L.V.; Torremans, A.; Sluydts, E.; et al. Intracerebral injection of preformed synthetic tau fibrils initiates widespread tauopathy and neuronal loss in the brains of tau transgenic mice. Neurobiol. Dis. 2015, 73, 83–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stancu, I.; Vasconcelos, B.; Ris, L.; Wang, P.; Villers, A.; Peeraer, E.; Buist, A.; Terwel, D.; Baatsen, P.; Oyelami, T.; et al. Templated misfolding of Tau by prion-like seeding along neuronal connections impairs neuronal network function and associated behavioral outcomes in Tau transgenic mice. Acta Neuropathol. 2015, 129, 875–894. [Google Scholar] [CrossRef] [Green Version]
- Kaufman, S.K.; Sander, D.W.; Thomas, T.L.; Ruchinskas, A.; Vaquer-Alicea, J.; Sharma, A.M.; Miller, T.M.; Diamond, M.I. Tau Prion Strains Dictate Patterns of Cell Pathology, Progression Rate, and Regional Vulnerability In Vivo. Neuron 2016, 92, 796–812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calafate, S.; Buist, A.; Miskiewicz, K.; Vijayan, V.; Daneels, G.; de Strooper, B.; de Wit, J.; Verstreken, P.; Moechars, D. Synaptic Contacts Enhance Cell-to-Cell Tau Pathology Propagation. Cell Rep. 2015, 11, 1176–1183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.; Hussaini, S.; Bastille, I.; Rodriguez, G.A.; Mrejeru, A.; Rilett, K.; Sanders, D.W.; Cook, C.; Fu, H.; Boonen, R.A.C.M.; et al. Neuronal activity enhances tau propagation and tau pathology in vivo. Nat. Neurosci. 2016, 19, 1085–1092. [Google Scholar] [CrossRef]
- Pooler, A.; Phillips, E.; Lau, D.; Noble, W.; Hanger, D. Physiological release of endogenous tau is stimulated by neuronal activity. EMBO Rep. 2013, 14, 389–394. [Google Scholar] [CrossRef]
- Holmes, B.; DeVos, S.; Kfoury, N.; Li, M.; Jacks, R.; Yanamandra, K.; Ouidja, M.O.; Brodsky, F.M.; Marasa, J.; Bagchi, D.P.; et al. Heparan sulfate proteoglycans mediate internalization and propagation of specific proteopathic seeds. Proc. Natl. Acad. Sci. USA 2013, 110, E3138–E3147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rauch, J.; Luna, G.; Guzman, E.; Audouard, M.; Challis, C.; Sibih, Y.E.; Leshuk, C.; Hernandez, I.; Wegmann, S.; Hyman, B.T.; et al. LRP1 is a master regulator of tau uptake and spread. Nature 2020, 580, 381–385. [Google Scholar] [CrossRef]
- Frost, B.; Jacks, R.; Diamond, M. Propagation of Tau Misfolding from the Outside to the Inside of a Cell. J. Biol. Chem. 2009, 284, 12845–12852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, J.; Lee, V. Seeding of Normal Tau by Pathological Tau Conformers Drives Pathogenesis of Alzheimer-like Tangles. J. Biol. Chem. 2011, 286, 15317–15331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Humpel, C. Organotypic brain slice cultures: A review. Neuroscience 2015, 305, 86–98. [Google Scholar] [CrossRef] [Green Version]
- Weis, C.; Marksteiner, J.; Humpel, C. Nerve growth factor and glial cell line-derived neurotrophic factor restore the cholinergic neuronal phenotype in organotypic brain slices of the basal nucleus of Meynert. Neuroscience 2001, 102, 129–138. [Google Scholar] [CrossRef]
- Duff, K.; Noble, W.; Gaynor, K.; Matsuoka, Y. Organotypic Slice Cultures from Transgenic Mice as Disease Model Systems. J. Mol. Neurosci. 2002, 19, 317–320. [Google Scholar] [CrossRef]
- Mewes, A.; Franke, H.; Singer, D. Organotypic Brain Slice Cultures of Adult Transgenic P301S Mice—A Model for Tauopathy Studies. PLoS ONE 2012, 7, e45017. [Google Scholar] [CrossRef] [PubMed]
- Harwell, C.; Coleman, M. Synaptophysin depletion and intraneuronal Aβ in organotypic hippocampal slice cultures from huAPP transgenic mice. Mol. Neurodegener. 2016, 11, 44. [Google Scholar] [CrossRef] [Green Version]
- Humpel, C. Organotypic vibrosections from whole brain adult Alzheimer mice (overexpressing amyloid-precursor-protein with the Swedish-Dutch-Iowa mutations) as a model to study clearance of beta-amyloid plaques. Front. Aging Neurosci. 2015, 7, 47. [Google Scholar] [CrossRef] [Green Version]
- Croft, C.; Noble, W. Preparation of organotypic brain slice cultures for the study of Alzheimer’s disease. F1000Res 2018, 7, 592. [Google Scholar] [CrossRef]
- McCarthy, J.; Virdee, J.; Brown, J.; Ursu, D.; Ahmed, Z.; Cavallini, A.; Nuthall, H.N. Development of P301S tau seeded organotypic hippocampal slice cultures to study potential therapeutics. Sci. Rep. 2021, 11, 10309. [Google Scholar] [CrossRef]
- Miller, L.; Mukadam, A.; Durrant, C.; Vaysburd, M.J.; Katsinelos, T.; Tuck, B.J.; Sanford, S.; Sheppard, O.; Knox, C.; Cheng, S.; et al. Tau assemblies do not behave like independently acting prion-like particles in mouse neural tissue. Acta Neuropathol. Commun. 2021, 9, 41. [Google Scholar] [CrossRef] [PubMed]
- Pineau, H.; Sim, V. POSCAbilities: The Application of the Prion Organotypic Slice Culture Assay to Neurodegenerative Disease Research. Biomolecules 2020, 10, 1079. [Google Scholar] [CrossRef] [PubMed]
- Moelgg, K.; Jummun, F.; Humpel, C. Spreading of Beta-Amyloid in Organotypic Mouse Brain Slices and Microglial Elimination and Effects on Cholinergic Neurons. Biomolecules 2021, 11, 434. [Google Scholar] [CrossRef] [PubMed]
- Humpel, C. Organotypic Brain Slice Cultures. Curr. Protoc. Immunol. 2018, 123, e59. [Google Scholar] [CrossRef] [PubMed]
- Ucar, B.; Humpel, C. Therapeutic efficacy of glial cell-derived neurotrophic factor loaded collagen scaffolds in ex vivo organotypic brain slice Parkinson’s disease models. Brain Res. Bull. 2019, 149, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Foidl, B.; Ucar, B.; Schwarz, A.; Rebelo, A.; Pandit, A.; Humpel, C. Nerve growth factor released from collagen scaffolds protects axotomized cholinergic neurons of the basal nucleus of Meynert in organotypic brain slices. J. Neurosci. Methods 2018, 295, 77–86. [Google Scholar] [CrossRef]
- Foidl, B.; Humpel, C. Differential Hyperphosphorylation of Tau-S199, -T231 and -S396 in Organotypic Brain Slices of Alzheimer Mice. A Model to Study Early Tau Hyperphosphorylation Using Okadaic Acid. Front. Aging Neurosci. 2018, 10, 113. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Balaji, V.; Kaniyappan, S.; Krüger, L.; Irsen, S.; Tepper, K.; Chandupatla, R.; Maetzler, W.; Schneider, A.; Mandelkow, E.; et al. The release and trans-synaptic transmission of Tau via exosomes. Mol. Neurodegener. 2017, 12, 5. [Google Scholar] [CrossRef] [Green Version]
- Sorushanova, A.; Delgado, L.; Wu, Z.; Shologu, N.; Kshirsagar, A.; Raghunath, R.; Mullen, A.M.; Bayon, Y.; Pandit, A.; Raghunath, M.; et al. The Collagen Suprafamily: From Biosynthesis to Advanced Biomaterial Development. Adv. Mater. 2019, 31, e1801651. [Google Scholar] [CrossRef] [Green Version]
- Ucar, B. Natural biomaterials in brain repair: A focus on collagen. Neurochem. Int. 2021, 146, 105033. [Google Scholar] [CrossRef]
- Buée, L.; Bussière, T.; Buée-Scherrer, V.; Delacourte, A.; Hof, P.R. Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain Res. Rev. 2000, 33, 95–130. [Google Scholar] [CrossRef]
- Pérez, M.; Valpuesta, J.; Medina, M.; Montejo de Garcini, E.; Avila, J. Polymerization of τ into Filaments in the Presence of Heparin: The Minimal Sequence Required for Tau- Tau Interaction. J. Neurochem. 1996, 67, 1183–1190. [Google Scholar] [CrossRef] [PubMed]
- Von Bergen, M.; Friedhoff, P.; Biernat, J.; Heberle, J.; Mandelkow, E.M.; Mandelkow, E. Assembly of tau protein into Alzheimer paired helical filaments depends on a local sequence motif ((306)VQIVYK(311)) forming beta structure. Proc. Natl. Acad. Sci. USA 2000, 97, 5129–5134. [Google Scholar] [CrossRef] [Green Version]
- Bugiani, O.; Murrell, J.; Giaccone, G.; Hasegawa, M.; Ghigo, G.; Tabaton, M.; Morbin, M.; Primavera, A.; Carella, F.; Solaro, C.; et al. Frontotemporal Dementia and Corticobasal Degeneration in a Family with a P301S Mutation in Tau. J. Neuropathol. Exp. Neurol. 1999, 58, 667–677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goedert, M.; Jakes, R. Mutations causing neurodegenerative tauopathies. Biochim. Biophys. Acta 2005, 1739, 240–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ling, H.; Kovacs, G.; Vonsattel, J.; Davey, K.; Mok, K.; Hardy, J.; Morris, H.R.; Warner, T.T.; Holton, J.L.; Revesz, T. Astrogliopathy predominates the earliest stage of corticobasal degeneration pathology. Brain 2016, 139, 3237–3252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amro, Z.; Yool, A.; Collins-Praino, L. The potential role of glial cells in driving the prion-like transcellular propagation of tau in tauopathies. Brain Behav. Immun. Health 2021, 14, 100242. [Google Scholar] [CrossRef]
- Asai, H.; Ikezu, S.; Tsunoda, S.; Medalla, M.; Luebke, J.; Haydar, T.; Wolozin, B.; Butovsky, O.; Kügler, S.; Ikezu, T. Depletion of microglia and inhibition of exosome synthesis halt tau propagation. Nat. Neurosci. 2015, 18, 1584–1593. [Google Scholar] [CrossRef]
- Arai, H.; Terajima, M.; Miura, M.; Higuchi, S.; Muramatsu, T.; Machida, N.; Seiki, H.; Takase, S.; Clark, C.M.; Lee, V.M.Y.; et al. Tau in cerebrospinal fluid: A potential diagnostic marker in Alzheimer’s disease. Ann. Neurol. 1995, 38, 649–652. [Google Scholar] [CrossRef]
- Hampel, H.; Blennow, K.; Shaw, L.; Hoessler, Y.; Zetterberg, H.; Trojanowski, J. Total and phosphorylated tau protein as biological markers of Alzheimer’s disease. Exp. Gerontol. 2010, 45, 30–40. [Google Scholar] [CrossRef] [Green Version]
- Katsinelos, T.; Zeitler, M.; Dimou, E.; Karakatsani, A.; Müller, H.; Nachman, E.; Steringer, J.P.; de Almodovar, C.R.; Nickel, W.; Jahn, T.R. Unconventional Secretion Mediates the Trans-cellular Spreading of Tau. Cell Rep. 2018, 23, 2039–2055. [Google Scholar] [CrossRef] [PubMed]
- Alavi Naini, S.; Soussi-Yanicostas, N. Heparan Sulfate as a Therapeutic Target in Tauopathies: Insights From Zebrafish. Front. Cell Dev. Biol. 2018, 6, 163. [Google Scholar] [CrossRef] [PubMed]
- Dujardin, S.; Bégard, S.; Caillierez, R.; Lachaud, C.; Delattre, L.; Carrier, S.; Loyens, A.; Galas, M.; Bousset, L.; Melki, R.; et al. Ectosomes: A New Mechanism for Non-Exosomal Secretion of Tau Protein. PLoS ONE 2014, 9, e100760. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Son, S.; Baik, S.; Yang, J.; Mook-Jung, I. Autophagy-Mediated Secretory Pathway is Responsible for Both Normal and Pathological Tau in Neurons. J. Alzheimers Dis. 2019, 70, 667–680. [Google Scholar] [CrossRef]
- Abounit, S.; Wu, J.; Duff, K.; Victoria, G.; Zurzolo, C. Tunneling nanotubes: A possible highway in the spreading of tau and other prion-like proteins in neurodegenerative diseases. Prion 2016, 10, 344–351. [Google Scholar] [CrossRef] [Green Version]
- Tardivel, M.; Bégard, S.; Bousset, L.; Dujardin, S.; Coens, A.; Melki, R.; Buée, L.; Colin, M. Tunneling nanotube (TNT)-mediated neuron-to neuron transfer of pathological Tau protein assemblies. Acta Neuropathol. Commun. 2016, 4, 117. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Kim, E.; Park, M.; Lee, E.; Namkoong, K. Organotypic hippocampal slice culture from the adult mouse brain: A versatile tool for translational neuropsychopharmacology. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 41, 36–43. [Google Scholar] [CrossRef]
- Jang, S.; Kim, H.; Kim, H.; Lee, S.; Kim, E.; Namkoong, K.; Kim, E. Long-Term Culture of Organotypic Hippocampal Slice from Old 3xTg-AD Mouse: An ex vivo Model of Alzheimer’s Disease. Psychiatry Investig. 2018, 15, 205–213. [Google Scholar] [CrossRef] [Green Version]
- Masters, C.; Bateman, R.; Blennow, K.; Rowe, C.; Sperling, R.; Cummings, J. Alzheimer’s disease. Nat. Rev. Dis. Primers 2015, 1, 15056. [Google Scholar] [CrossRef]


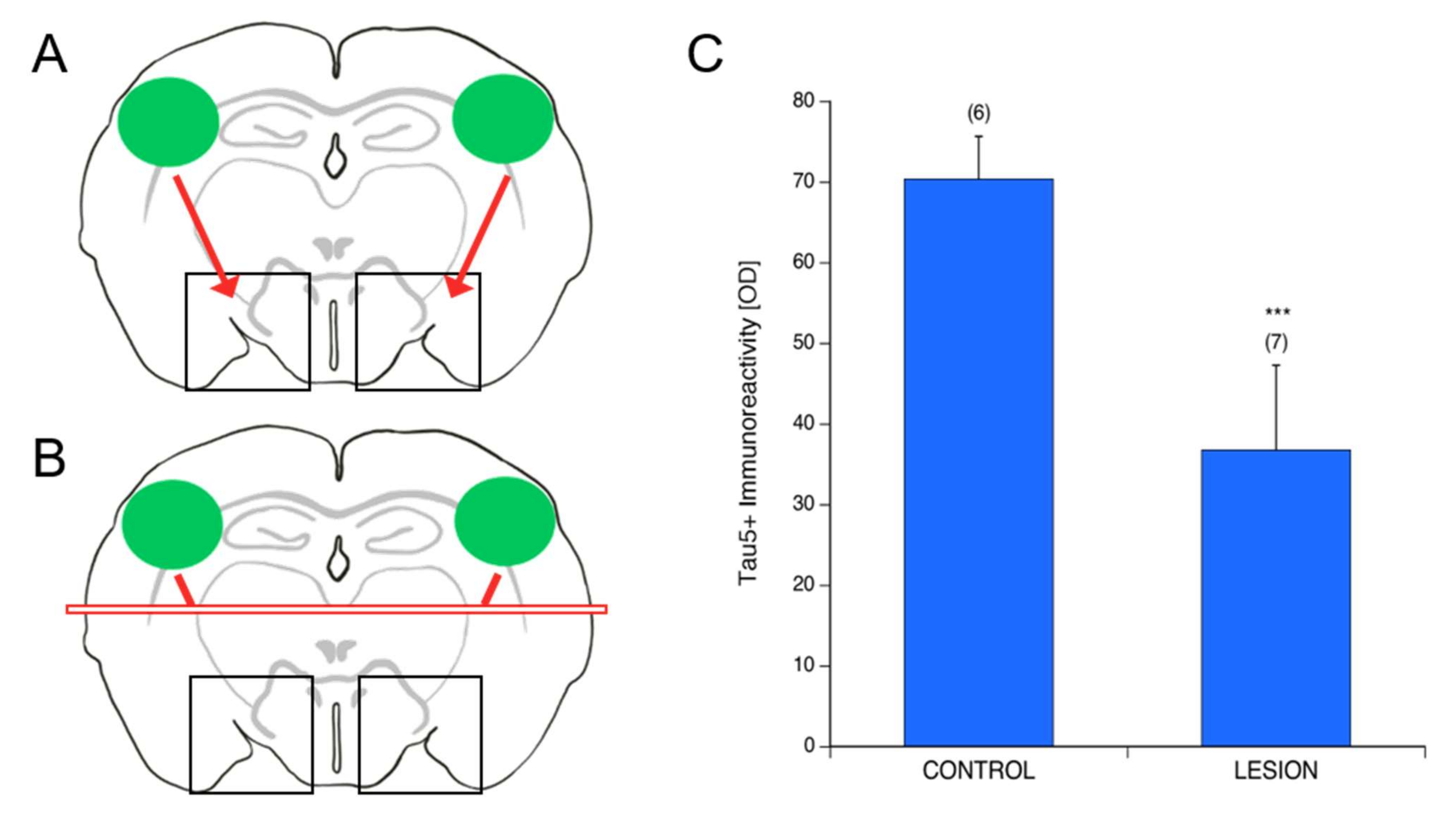
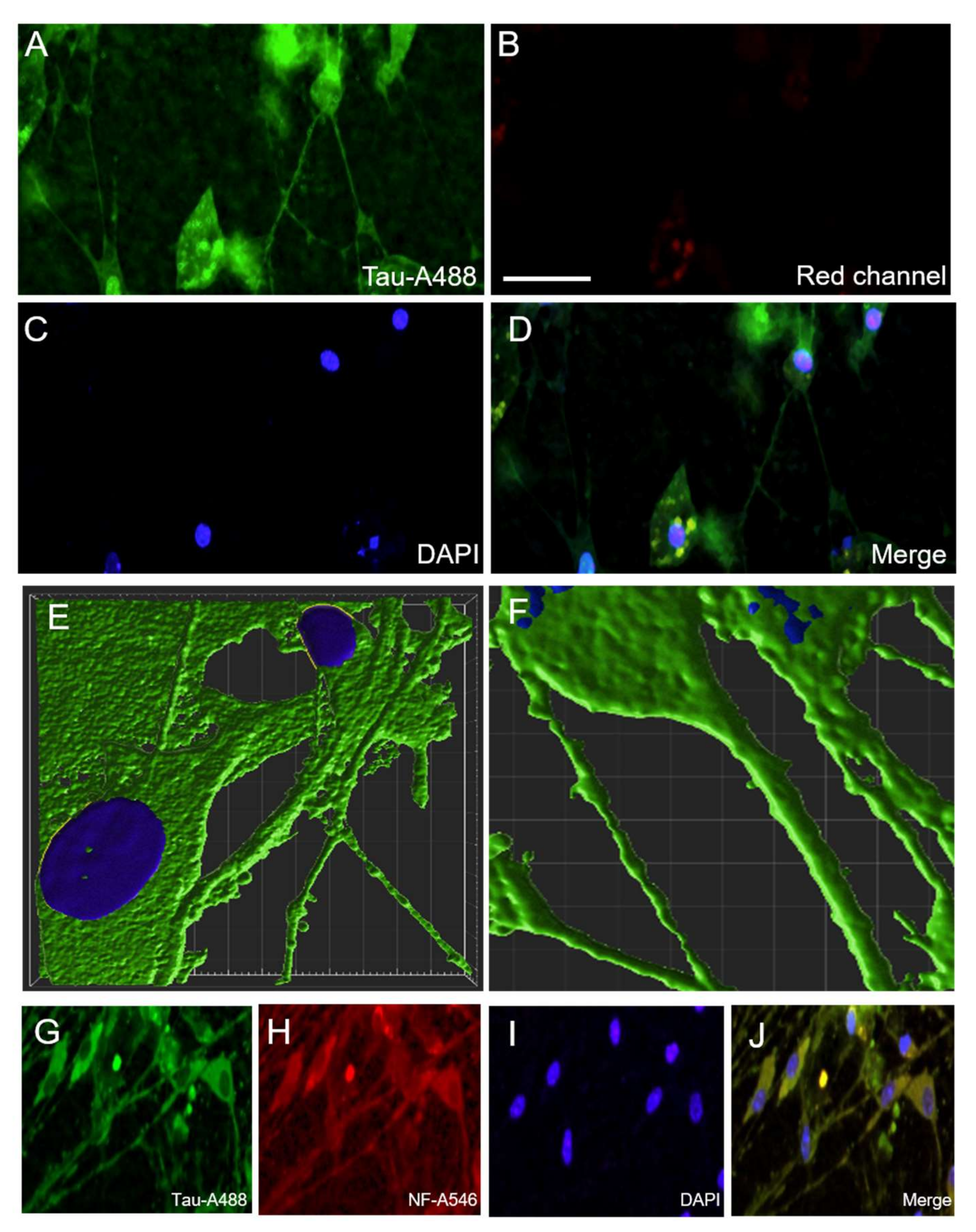
| Area 1 | Area 2 | Area 3 | |
|---|---|---|---|
| w/o (−) | 36 ± 8 (4) | 23 ± 10 (4) | 23 ± 8 (4) |
| PBS | 26 ± 7 (4) | 24 ± 7 (4) | 21 ± 6 (4) |
| CollH (−) | 28 ± 5 (12) vs. | 24 ± 7 (12) vs. | 29 ± 9 (12) vs. |
| CollH (FL tau) | 43 ± 16 (5) | 32 ± 17 (5) | 33 ± 14 (5) |
| CollH (Δ306-311 Tau) | 37 ± 20 (5) | 29 ± 12 (5) | 34 ± 18 (5) |
| CollH (P301S aggTau) | 30 ± 21 (14) | 30 ± 11 (14) | 69 ± 19 (14) *** |
| CollH (P301S aggTau-P*) | 25 ± 9 (3) | 24 ± 10 (3) | 72 ± 7 (9) *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korde, D.S.; Humpel, C. Spreading of P301S Aggregated Tau Investigated in Organotypic Mouse Brain Slice Cultures. Biomolecules 2022, 12, 1164. https://doi.org/10.3390/biom12091164
Korde DS, Humpel C. Spreading of P301S Aggregated Tau Investigated in Organotypic Mouse Brain Slice Cultures. Biomolecules. 2022; 12(9):1164. https://doi.org/10.3390/biom12091164
Chicago/Turabian StyleKorde, Dhwani S., and Christian Humpel. 2022. "Spreading of P301S Aggregated Tau Investigated in Organotypic Mouse Brain Slice Cultures" Biomolecules 12, no. 9: 1164. https://doi.org/10.3390/biom12091164
APA StyleKorde, D. S., & Humpel, C. (2022). Spreading of P301S Aggregated Tau Investigated in Organotypic Mouse Brain Slice Cultures. Biomolecules, 12(9), 1164. https://doi.org/10.3390/biom12091164