Role of Adiponectin Peptide I (APNp1) in Age-Related Macular Degeneration
Abstract
1. Introduction
1.1. Age-Related Macular Degeneration
1.2. Adiponectin Peptide 1 (APNp1)
1.3. Topical Therapeutic Administration
1.4. Adeno-Associated Virus Therapeutic Delivery
2. Materials and Methods
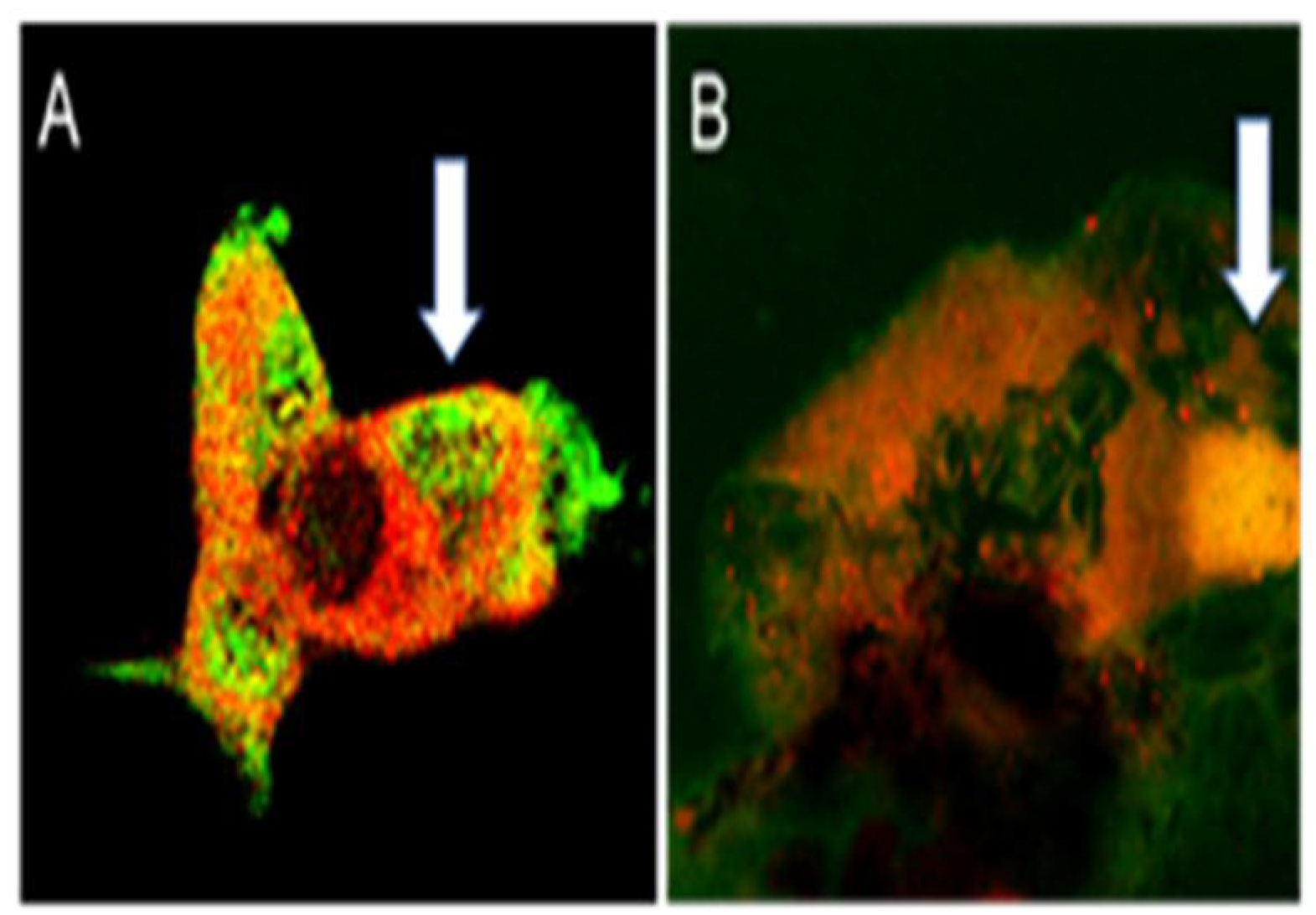
2.1. Colocalization of APNp1 and AdipoR1
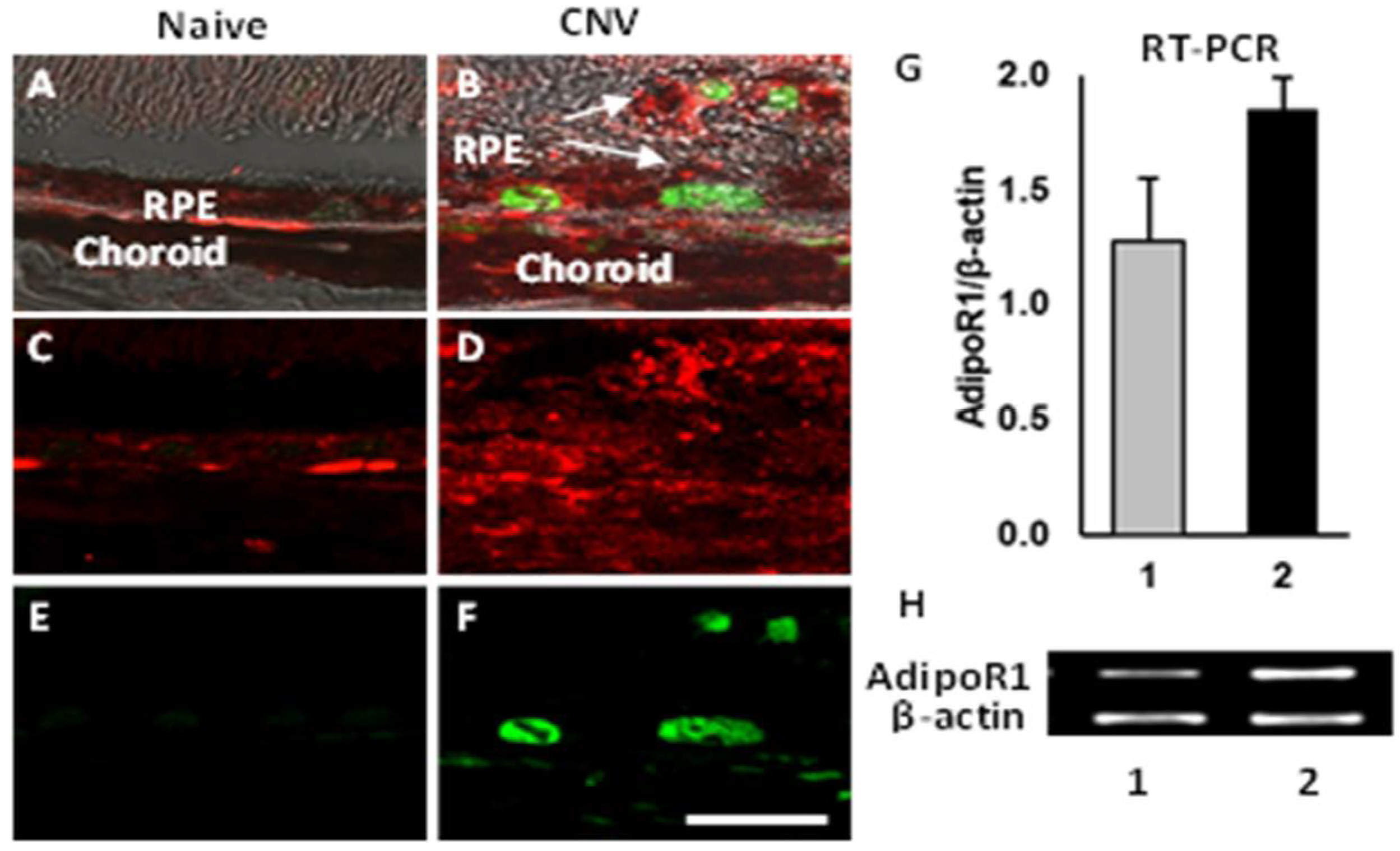
2.2. Expression of AdipoR1 and PCNA
2.3. Choroidal Endothelial Cells (CECs) Culture
2.4. Real-Time Quantitative RT-PCR (RT-qPCR)
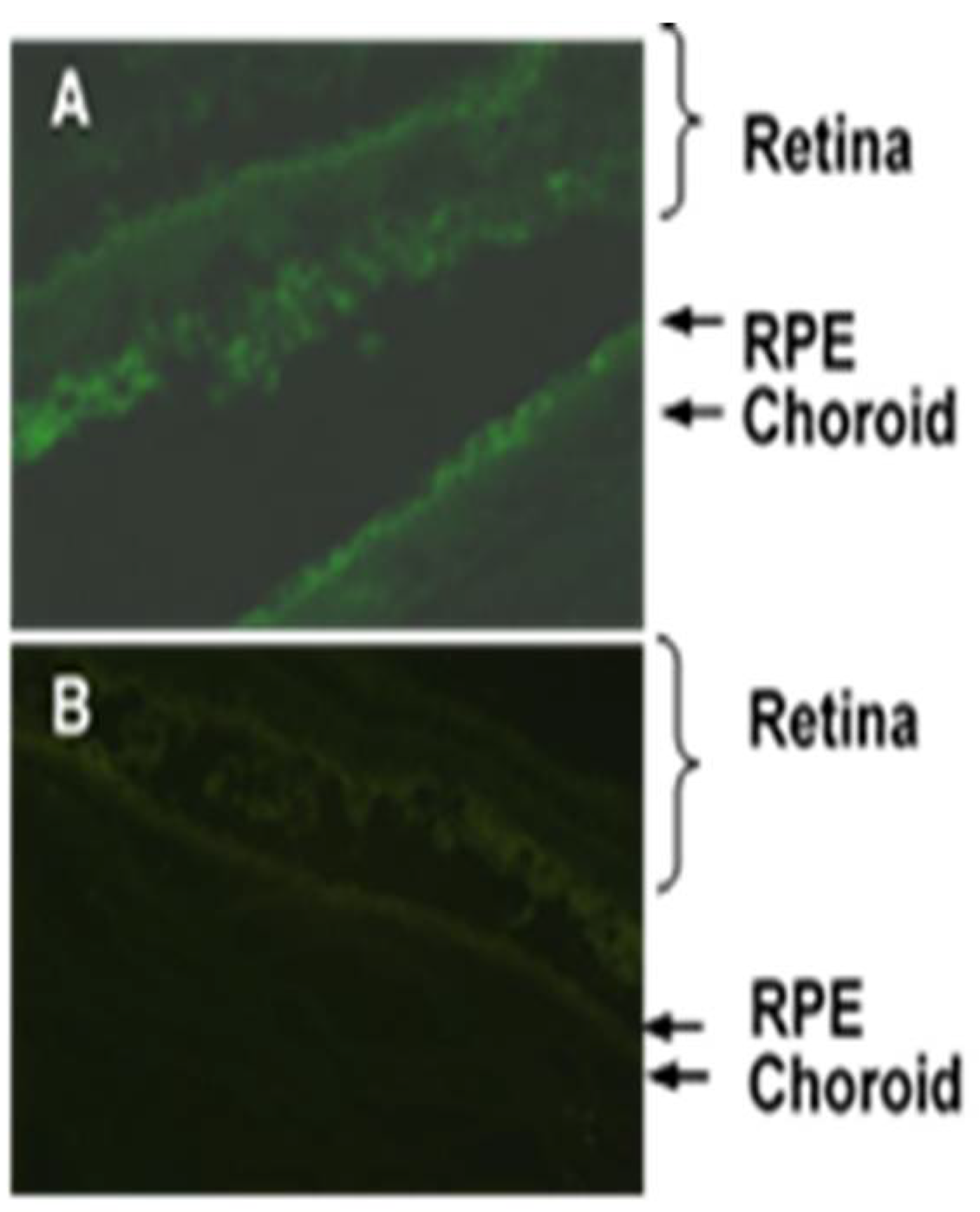
2.5. Topical Administration of APNp1
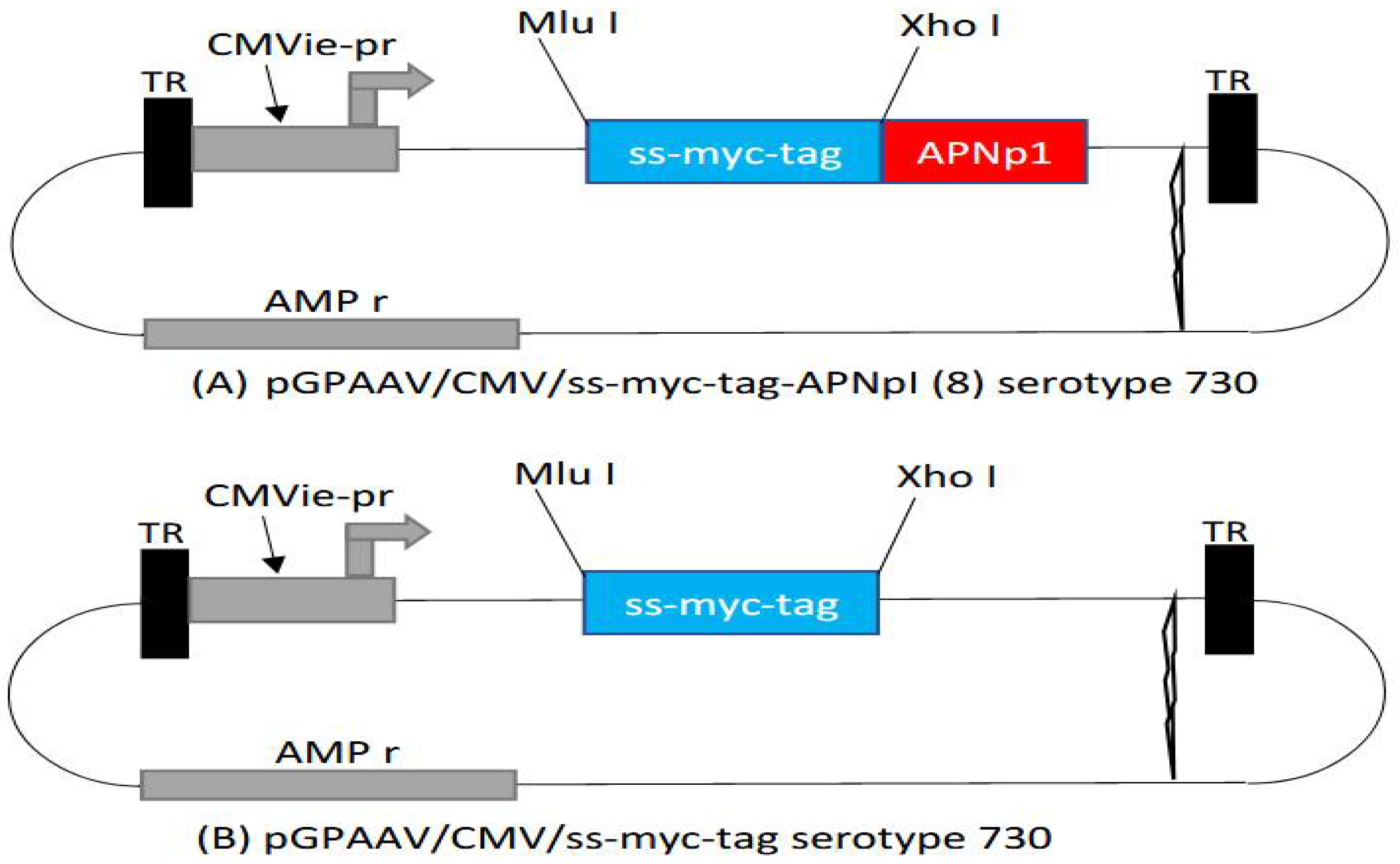
2.6. Adeno-Associated Virus (AAV) Delivery
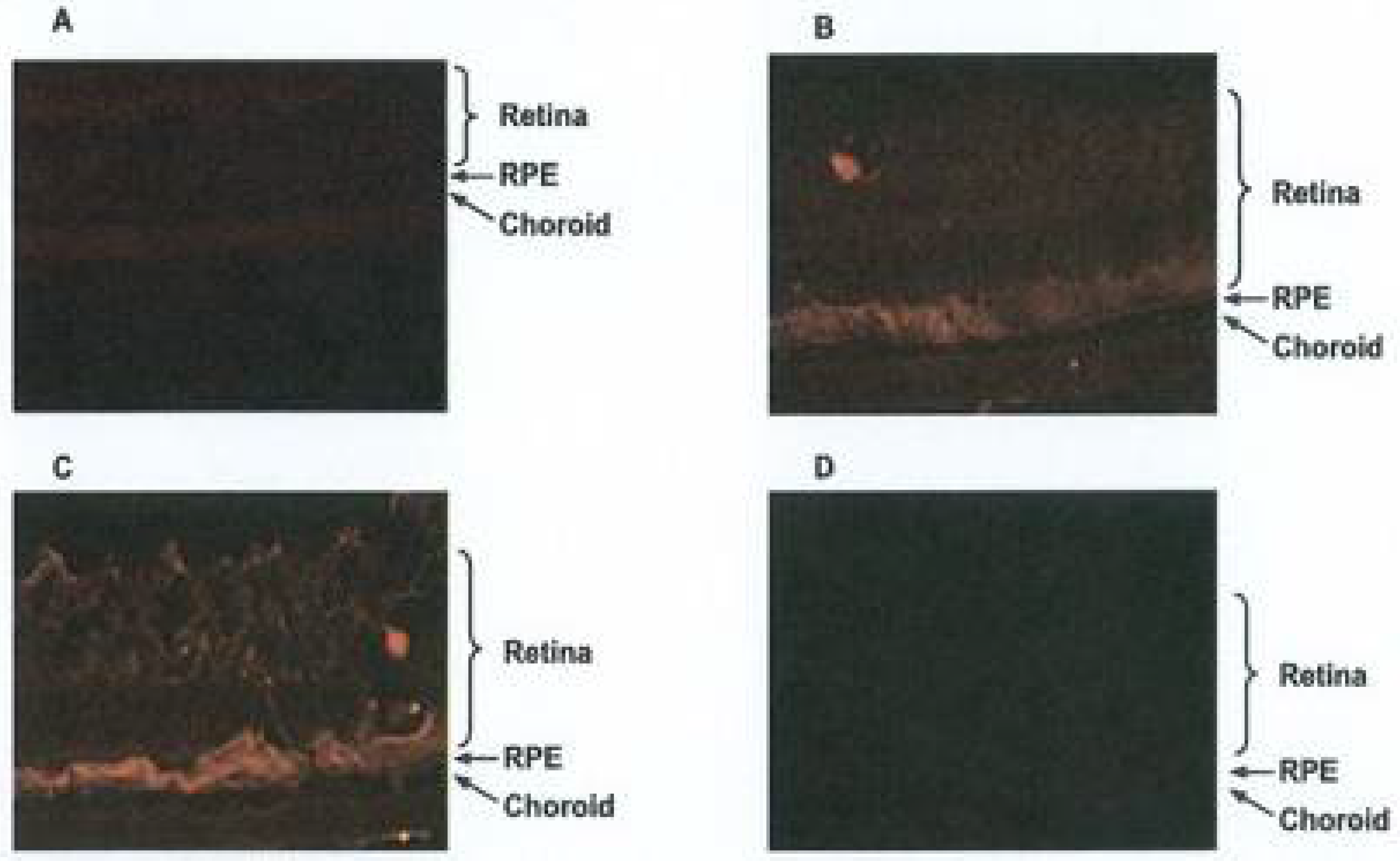
3. Results
3.1. Mechanism of Action of APNp1 on CECs and RPE
3.2. Topical Administration of APNp1
3.3. Adeno-Associated Virus (AAV) Delivery of APNp1
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Friedman, D.S.; O’Colmain, B.J.; Muñoz, B.; Tomany, S.C.; McCarty, C.; De Jong, P.T.V.M.; Nemesure, B.; Mitchell, P.; Kempen, J.; Congdon, N. Prevalence of age-related macular degeneration in the United States. Arch. Ophthalmol. 2004, 122, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Coleman, H.R.; Chan, C.C.; Ferris, F.L., 3rd; Chew, E.Y. Age-related macular degeneration. Lancet 2008, 372, 1835–1845. [Google Scholar] [CrossRef]
- Gehrs, K.M.; Anderson, D.H.; Johnson, L.V.; Hageman, G.S. Age-related macular degeneration--emerging pathogenetic and therapeutic concepts. Ann. Med. 2006, 38, 450–471. [Google Scholar] [CrossRef]
- Christoforidis, J.B.; Tecce, N.; Dell’Omo, R.; Mastropasqua, R.; Verolino, M.; Costagliola, C. Age related macular degeneration and visual disability. Curr. Drug Targets 2011, 12, 221–233. [Google Scholar] [CrossRef]
- Lim, L.S.; Mitchell, P.; Seddon, J.M.; Holz, F.G.; Wong, T.Y. Age-related macular degeneration. Lancet 2012, 379, 1728–1738. [Google Scholar] [CrossRef]
- Rein, D.B.; Wittenborn, J.S.; Zhang, X.; Honeycutt, A.A.; Lesesne, S.B.; Saaddine, J. Forecasting age-related macular degeneration through the year 2050: The potential impact of new treatments. Arch. Ophthalmol. 2009, 127, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Hageman, G.S.; Gehrs, K.; Johnson, L.V.; Anderson, D. Age-Related Macular Degeneration (AMD). In Webvision: The Organization of the Retina and Visual System; Kolb, H., Fernandez, E., Nelson, R., Eds.; John A. Moran Eye Center: Salt Lake City, UT, USA, 2008. [Google Scholar]
- De Jong, P.T. Age-related macular degeneration. N. Engl. J. Med. 2006, 355, 1474–1485. [Google Scholar] [CrossRef]
- Seddon, J.M.; Rosner, B. Validated prediction models for macular degeneration progression and predictors of visual acuity loss identify high-risk individuals. Am. J. Ophthalmol. 2019, 198, 223–261. [Google Scholar] [CrossRef]
- Modenese, A.; Gobba, F. Macular degeneration and occupational risk factors: A systematic review. Int. Arch. Occup. Environ. Health 2019, 92, 1–11. [Google Scholar] [CrossRef]
- Ho, A.C.; Albini, T.A.; Brown, D.M.; Boyer, D.S.; Regillo, C.D.; Heier, J.S. The potential importance of detection of neovascular age-related macular degeneration when visual acuity is relatively good. JAMA Opthalmol. 2017, 135, 268–273. [Google Scholar] [CrossRef]
- Bakri, S.J.; Thorne, J.E.; Ho, A.C.; Ehlers, J.P.; Schoenberger, S.D.; Yeh, S.; Kim, S.J. Safety and efficacy of anti-vascular endothelial growth factor therapies for neovascular age-related macular degeneration: A report by the American Academy of Ophthalmology. Ophthalmology 2019, 126, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Holekamp, N. Review of Neovascular Age-Related Macular Degeneration Treatment Options. Am. J. Manag. Care 2019, 25, 172–181. [Google Scholar]
- Husain, D.; Ambati, B.; Adamis, A.P.; Miller, J.W. Mechanisms of age-related macular degeneration. Ophthalmol. Clin. N. Am. 2002, 15, 87–91. [Google Scholar] [CrossRef]
- Zeiss, C.J. Animals as models of age-related macular degeneration: An imperfect measure of the truth. Vet. Pathol. 2010, 47, 396–413. [Google Scholar] [CrossRef] [PubMed]
- Lyzogubov, V.V.; Tytarenko, R.G.; Bora, N.S.; Bora, P.S. Inhibitory role of adiponectin peptide I on rat choroidal neovascularization. Biochim. Biophys. Acta 2012, 1823, 1264–1272. [Google Scholar] [CrossRef] [PubMed]
- Lyzogubov, V.V.; Tytarenko, R.G.; Liu, J.; Bora, N.S.; Bora, P.S. Polyethylene glycol (PEG)-induced mouse model of choroidal neovascularization. J. Biol. Chem. 2011, 286, 16229–16237. [Google Scholar] [CrossRef] [PubMed]
- Bora, P.S.; Sohn, J.-H.; Cruz, J.M.C.; Jha, P.; Nishihori, H.; Wang, Y.; Kaliappan, S.; Kaplan, H.J.; Bora, N.S. Role of complement and complement membrane attack complex in laser-induced choroidal neovascularization. J. Immunol. 2005, 174, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Bora, N.S.; Kaliappan, S.; Jha, P.; Xu, Q.; Sohn, J.-H.; Dhaulakhandi, D.B.; Kaplan, H.J.; Bora, P.S. Complement activation via alternative pathway is critical in the development of laser-induced choroidal neovascularization: Role of factor B and factor H. J. Immunol. 2006, 177, 1872–1878. [Google Scholar] [CrossRef]
- Bora, P.S.; Kaliappan, S.; Lyzogubov, V.V.; Tytarenko, R.G.; Thotakura, S.; Viswanathan, T.; Bora, N.S. Expression of adiponectin in choroidal tissue and inhibition of laser induced choroidal neovascularization by adiponectin. FEBS Lett. 2007, 581, 1977–1982. [Google Scholar] [CrossRef]
- Zubilewicz, A.; Hecquet, C.; Jeanny, J.; Soubrane, G.; Courtois, Y.; Mascarelli, F. Proliferation of CECs requires dual signaling through both MAPK/ERK and PI 3-K/Akt pathways. Invest. Ophthalmol. Vis. Sci. 2001, 42, 488–496. [Google Scholar]
- Kadowaki, T.; Yamauchi, T.; Waki, H.; Iwabu, M.; Okada-Iwabu, M.; Nakamura, M. Adiponectin, adiponectin receptors, and epigenetic regulation of adipogenesis. Cold Spring Harb. Symp. Quant. Biol. 2011, 76, 257–265. [Google Scholar] [CrossRef]
- Kaarniranta, K.; Paananen, J.; Nevalainen, T.; Sorri, I.; Seitsonen, S.; Immonen, I.; Salminen, A.; Pulkkinen, L.; Uusitupa, M. Adiponectin receptor 1 gene (ADIPOR1) variant is associated with advanced age-related macular degeneration in Finnish population. Neurosci. Lett. 2012, 513, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Turer, A.T.; Scherer, P.E. Adiponectin: Mechanistic insights and clinical implications. Diabetologia 2012, 55, 2319–2326. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, S.; Miyoshi, Y.; Ishihara, H.; Noguchi, S. Growth-inhibitory effect of adiponectin via adiponectin receptor 1 on human breast cancer cells through inhibition of S-phase entry without inducing apoptosis. Breast Cancer Res. Treat. 2008, 112, 405–410. [Google Scholar] [CrossRef]
- Ogunwobi, O.O.; Beales, I.L. Globular adiponectin, acting via adiponectin receptor-1, inhibits leptin-stimulated oesophageal adenocarcinoma cell proliferation. Mol. Cell. Endocrinol. 2008, 285, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.L.; Qin, X.Q.; Xiang, Y.; Tan, Y.R.; Qu, X.P.; Liu, H.J. Adipokine adiponectin is a potential protector to human bronchial epithelial cell for regulating proliferation, wound repair and apoptosis: Comparison with leptin and resistin. Peptides 2013, 40, 34–41. [Google Scholar] [CrossRef]
- Tang, Y.T.; Hu, T.; Arterburn, M.; Boyle, B.; Bright, J.M.; Emtage, P.C.; Funk, W.D. PAQR proteins: A novel membrane receptor family defined by an ancient 7-transmembrane pass motif. J. Mol. Evol. 2005, 61, 372–380. [Google Scholar] [CrossRef]
- Patel, S.A.; Hoehn, K.; Lawrence, R.T.; Sawbridge, L.; Talbot, N.A.; Tomsig, J.L.; Turner, N.; Cooney, G.J.; Whitehead, J.; Kraegen, E.W.; et al. Overexpression of the adiponectin receptor AdipoR1 in rat skeletal muscle amplifies local insulin sensitivity. Endocrinology 2012, 153, 5231–5246. [Google Scholar] [CrossRef]
- Heiker, J.T.; Kosel, D.; Beck-Sickinger, A.G. Molecular mechanisms of signal transduction via adiponectin and adiponectin receptors. Biol. Chem. 2010, 391, 1005–1018. [Google Scholar] [CrossRef]
- Ishikawa, M.; Kitayama, J.; Yamauchi, T.; Kadowaki, T.; Maki, T.; Miyato, H.; Yamashita, H.; Nagawa, H. Adiponectin inhibits the growth and peritoneal metastasis of gastric cancer through its specific membrane receptors AdipoR1 and AdipoR2. Cancer Sci. 2007, 98, 1120–1127. [Google Scholar] [CrossRef]
- Shibata, M.A.; Jorcyk, C.L.; Liu, M.L.; Yoshidome, K.; Gold, L.G.; Green, J.E. The C3(1)/SV40 T antigen transgenic mouse model of prostate and mammary cancer. Toxicol. Pathol. 1998, 26, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Funahashi, T.; Nakamura, T.; Shimomura, I.; Maeda, K.; Kuriyama, H.; Takahashi, M.; Arita, Y.; Kihara, S.; Matsuzawa, Y. Role of adipocytokines on the pathogenesis of atherosclerosis in visceral obesity. Intern. Med. 1999, 38, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, T.; Kamon, J.; Ito, Y.; Tsuchida, A.; Yokomizo, T.; Kita, S.; Sugiyama, T.; Miyagishi, M.; Hara, K.; Tsunoda, M.; et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature 2003, 423, 762–769. [Google Scholar] [CrossRef] [PubMed]
- Tsukada, T.; Fushida, S.; Harada, S.; Terai, S.; Yagi, Y.; Kinoshita, J.; Oyama, K.; Tajima, H.; Fujita, H.; Ninomiya, I.; et al. Adiponectin receptor-1 expression is associated with good prognosis in gastric cancer. J. Exp. Clin. Cancer Res. 2011, 30, 107. [Google Scholar] [CrossRef]
- Cai, X.; Feng, M.; Chen, L.; Li, L.; Rong, Y.; Hu, X.; Wang, R.; Zhang, M.; Zhang, Y.; Zhang, M. Expression and role of adiponectin receptor 1 in lipopolysaccharide-induced proliferation of cultured rat adventitial fibroblasts. Cell Biol. Int. 2010, 34, 163–169. [Google Scholar] [CrossRef]
- Pierre, P.; Froment, P.; Negre, D.; Ramé, C.; Barateau, V.; Chabrolle, C.; LeComte, P.; Dupont, J. Role of adiponectin receptors, AdipoR1 and AdipoR2, in the steroidogenesis of the human granulosa tumor cell line, KGN. Hum. Reprod. 2009, 24, 2890–2901. [Google Scholar] [CrossRef]
- Luo, X.H.; Zhao, L.L.; Yuan, L.Q.; Wang, M.; Xie, H.; Liao, E.Y. Development of arterial calcification in adiponectin-deficient mice: Adiponectin regulates arterial calcification. J. Bone Min. Res. 2009, 24, 1461–1468. [Google Scholar] [CrossRef]
- Lee, M.H.; Klein, R.L.; El-Shewy, H.M.; Luttrell, D.K.; Luttrell, L.M. The adiponectin receptors AdipoR1 and AdipoR2 activate ERK1/2 through a Src/Ras-dependent pathway and stimulate cell growth. Biochemistry 2008, 47, 11682–11692. [Google Scholar] [CrossRef]
- Iwabu, M.; Yamauchi, T.; Okada-Iwabu, M.; Sato, K.; Nakagawa, T.; Funata, M.; Yamaguchi, M.; Namiki, S.; Nakayama, R.; Tabata, M.; et al. Adiponectin and AdipoR1 regulate PGC-1alpha and mitochondria by Ca2+ and AMPK/SIRT1. Nature 2010, 464, 1313–1319. [Google Scholar] [CrossRef]
- Yamauchi, T.; Nio, Y.; Maki, T.; Kobayashi, M.; Takazawa, T.; Iwabu, M.; Okada-Iwabu, M.; Kawamoto, S.; Kubota, N.; Kubota, T.; et al. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat. Med. 2007, 13, 332–339. [Google Scholar] [CrossRef]
- Kanazawa, I.; Yamaguchi, T.; Yano, S.; Yamauchi, M.; Yamamoto, M.; Sugimoto, T. Adiponectin and AMP kinase activator stimulate proliferation, differentiation, and mineralization of osteoblastic MC3T3-E1 cells. BMC Cell Biol. 2007, 8, 51. [Google Scholar] [CrossRef] [PubMed]
- Gasperini, J.L.; Fawzi, A.; Khondkaryan, A.; Lam, L.; Chong, L.P.; Eliott, D.; Walsh, A.C.; Hwang, J.; Sadda, S.R. Bevacizumab and ranibizumab tachyphylaxis in the treatment of choroidal neovascularisation. Br. J. Ophthalmol. 2012, 96, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Forooghian, F.; Cukras, C.; Meyerle, C.B.; Chew, E.Y.; Wong, W.T. Tachyphylaxis after intravitreal bevacizumab for exudative age-related macular degeneration. Retina 2009, 29, 723–731. [Google Scholar] [CrossRef]
- Forooghian, F.; Kertes, P.J.; Eng, K.T.; Agron, E.; Chew, E.Y. Alterations in the intraocular cytokine milieu after intravitreal bevacizumab. Investig. Ophthalmol. Vis. Sci. 2010, 51, 2388–2392. [Google Scholar] [CrossRef]
- Lai, C.M.; Shen, W.Y.; Brankov, M.; Lai, K.Y.; Barnett, N.L.; Lee, S.Y.; Yeo, Y.S.; Mathur, R.; Ho, J.E.S.; Pineda, P.; et al. Lont term evaluation of AAV mediated sFlt-1 gene therapy for ocular neovascularization in mice and monkeys. Mol. Ther. 2005, 12, 659–668. [Google Scholar] [CrossRef]
- Kong, F.; Li, W.; Li, X.; Zheng, Q.; Dai, X.; Zhou, X.; Boye, S.L.; Hauswirth, W.W.; Qu, J.; Pang, J.-J. Self- complementary AAV5 vector facilitates quicker transgene expression in photoreceptor and retinal epithelial cells of normal mouse. Exp. Eye Res. 2010, 90, 546–554. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lebherz, C.; Maguire, A.; Tang, W.; Bennett, J.; Wilson, J.M. Novel AAV serotypes for improved ocular gene transfer. J. Gene Med. 2008, 10, 375–382. [Google Scholar] [CrossRef]
- Bora, P.S.; Hu, Z.; Tezel, T.H.; Sohn, J.H.; Kang, S.G.; Cruz, J.M.; Bora, N.S.; Garen, A.; Kaplan, H.J. Immunotherapy for choroidal neovascularization in a laser-induced mouse model simulating exudative (wet) macular degeneration. Proc. Natl. Acad. Sci. USA 2003, 100, 2679–2684. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Logan, C.; Lyzogubov, V.; Bora, N.; Bora, P. Role of Adiponectin Peptide I (APNp1) in Age-Related Macular Degeneration. Biomolecules 2022, 12, 1232. https://doi.org/10.3390/biom12091232
Logan C, Lyzogubov V, Bora N, Bora P. Role of Adiponectin Peptide I (APNp1) in Age-Related Macular Degeneration. Biomolecules. 2022; 12(9):1232. https://doi.org/10.3390/biom12091232
Chicago/Turabian StyleLogan, Connor, Valeriy Lyzogubov, Nalini Bora, and Puran Bora. 2022. "Role of Adiponectin Peptide I (APNp1) in Age-Related Macular Degeneration" Biomolecules 12, no. 9: 1232. https://doi.org/10.3390/biom12091232
APA StyleLogan, C., Lyzogubov, V., Bora, N., & Bora, P. (2022). Role of Adiponectin Peptide I (APNp1) in Age-Related Macular Degeneration. Biomolecules, 12(9), 1232. https://doi.org/10.3390/biom12091232






