Enzymatic Potential of Filamentous Fungi as a Biological Pretreatment for Acidogenic Fermentation of Coffee Waste
Abstract
:1. Introduction
2. Materials and Methods
2.1. Substrate
2.2. Microorganisms
2.3. Enzyme Production
2.4. SCG Pretreatments
2.4.1. Physicochemical
2.4.2. Biological
2.5. Acidogenic Fermentation
2.5.1. Inoculum
2.5.2. Experimental Set-Up
2.6. Analytical Methods
2.6.1. Determination of SCOAs and Monomeric Sugars
2.6.2. Chemical Oxygen Demand (COD)
2.7. Calculations
2.7.1. COD Conversions
2.7.2. Acidification Degree (AD)
2.7.3. Yields, Rates, and Productivities
2.7.4. Odd-to-Even Ratio of SCOAs
3. Results and Discussion
3.1. Pretreatment Efficiency
3.2. Acidogenic Fermentation
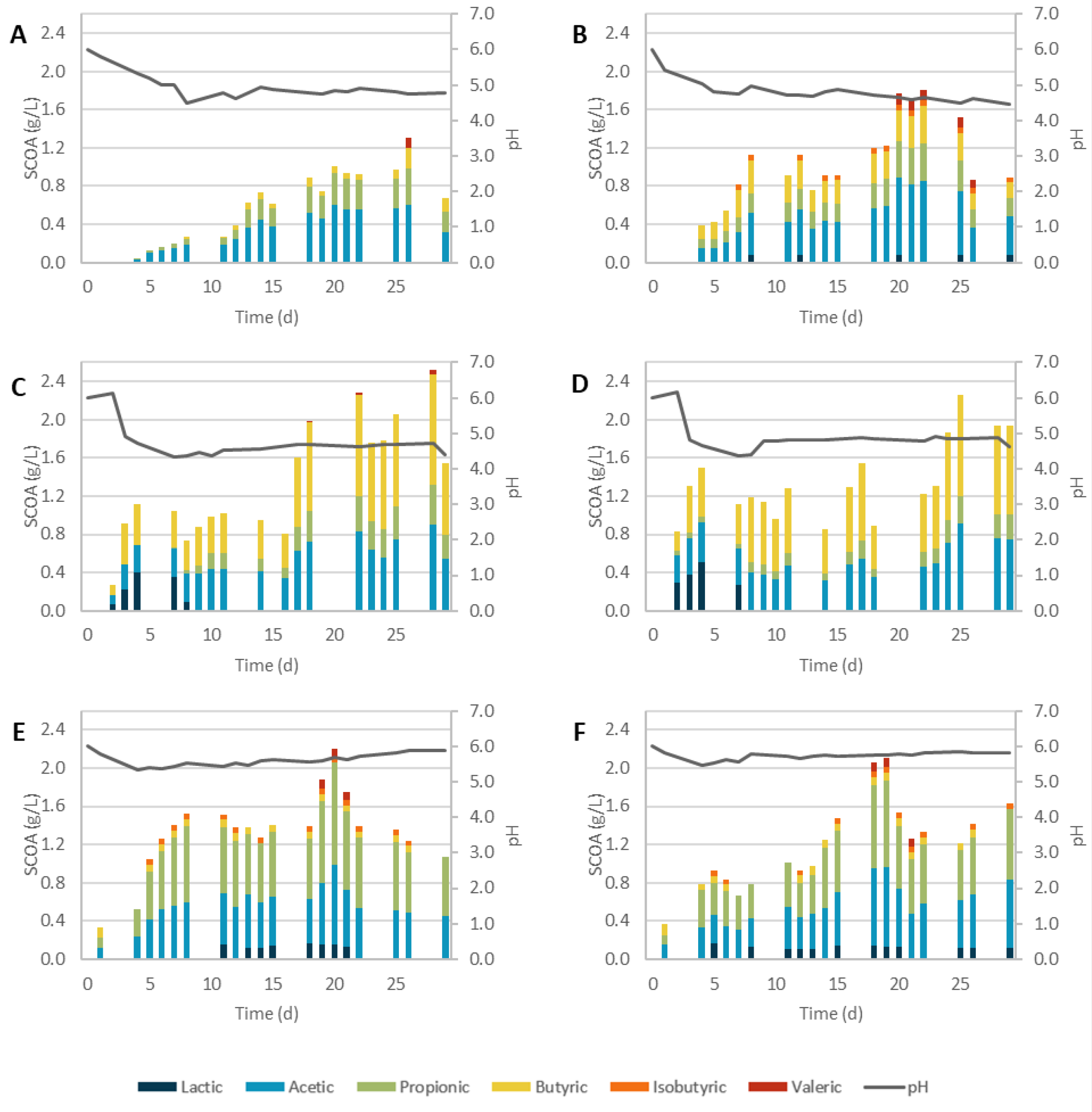
3.2.1. Physicochemical Pretreatments
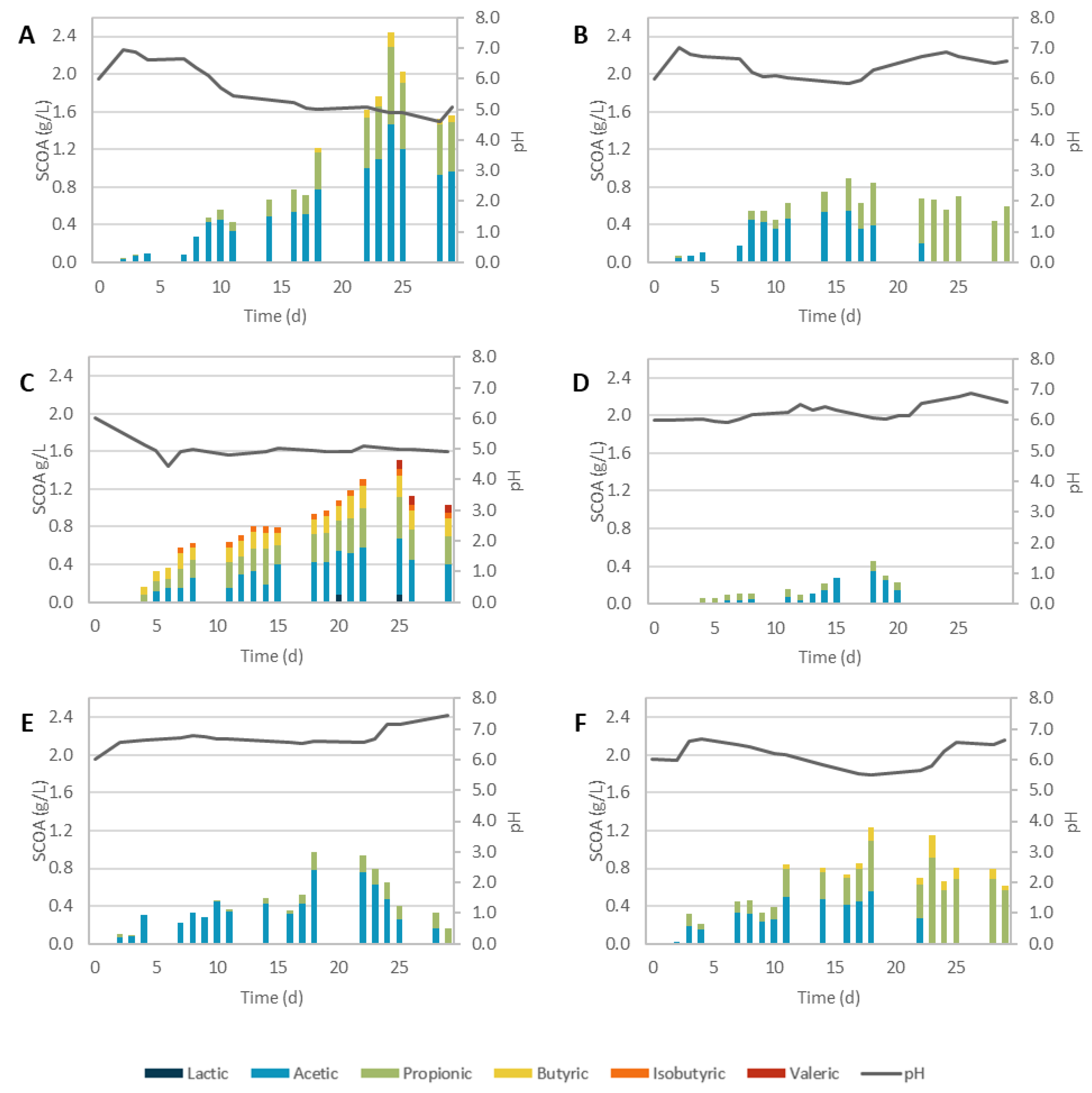
3.2.2. Biological Pretreatments
3.2.3. Overall Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- International Coffee Organization. The Value of Coffee-Sustainability, Inclusiveness, and Resilience; ICO: London, UK, 2020. [Google Scholar]
- International Coffee Organization. Coffee Market Report—September 2021; ICO: London, UK, 2021. [Google Scholar]
- Tsai, W.T.; Liu, S.C.; Hsieh, C.H. Preparation and fuel properties of biochars from the pyrolysis of exhausted coffee residue. J. Anal. Appl. Pyrolysis 2012, 93, 63–67. [Google Scholar] [CrossRef]
- McNutt, J.; He, Q. Spent coffee grounds: A review on current utilization. J. Ind. Eng. Chem. 2019, 71, 78–88. [Google Scholar] [CrossRef]
- Lee, W.S.; Chua, A.S.M.; Yeoh, H.K.; Ngoh, G.C. A review of the production and applications of waste-derived volatile fatty acids. Chem. Eng. J. 2014, 235, 83–99. [Google Scholar] [CrossRef]
- Sun, L.; Gong, M.; Lv, X.; Huang, Z.; Gu, Y.; Li, J.; Du, G.; Liu, L. Current advance in biological production of short-chain organic acid. Appl. Microbiol. Biotechnol. 2020, 104, 9109–9124. [Google Scholar] [CrossRef] [PubMed]
- Serafim, L.S.; Lemos, P.C.; Albuquerque, M.G.E.; Reis, M.A.M. Strategies for PHA production by mixed cultures and renewable waste materials. Appl. Microbiol. Biotechnol. 2008, 81, 615–628. [Google Scholar] [CrossRef]
- Koller, M.; Miranda, M.; Dias, D.S.; Braunegg, G. Producing microbial polyhydroxyalkanoate (PHA) biopolyesters in a sustainable manner. New Biotechnol. 2017, 37, 24–38. [Google Scholar] [CrossRef]
- Zhou, M.; Yan, B.; Wong, J.W.C.; Zhang, Y. Enhanced volatile fatty acids production from anaerobic fermentation of food waste: A mini-review focusing on acidogenic metabolic pathways. Bioresour. Technol. 2018, 248, 68–78. [Google Scholar] [CrossRef]
- Hashemi, B.; Sarker, S.; Lamb, J.J.; Lien, K.M. Yield improvements in anaerobic digestion of lignocellulosic feedstocks. J. Clean. Prod. 2021, 288, 125447. [Google Scholar] [CrossRef]
- Ramos-Suarez, M.; Zhang, Y.; Outram, V. Current Perspectives on Acidogenic Fermentation to Produce Volatile Fatty Acids from Waste; Springer: Cham, The Netherlands, 2021; Volume 20, ISBN 0123456789. [Google Scholar]
- Tsafrakidou, P.; Bekatorou, A.; Koutinas, A.A.; Kordulis, C.; Banat, I.M.; Petsi, T.; Sotiriou, M. Acidogenic Fermentation of Wheat Straw After Chemical and Microbial Pretreatment for Biofuel Applications. Energy Convers. Manag. 2018, 160, 509–517. [Google Scholar] [CrossRef]
- Bahreini, G.; Nazari, L.; Ho, D.; Flannery, C.C.; Elbeshbishy, E.; Santoro, D.; Nakhla, G. Enzymatic pre-treatment for enhancement of primary sludge fermentation. Bioresour. Technol. 2020, 305, 123071. [Google Scholar] [CrossRef]
- Islam, M.S.; Zhang, Z.; Qu, S.; Liu, C.L.; Guo, C.; Liu, C.Z. Coproduction of hydrogen and volatile fatty acids via integrated two-step fermentation of sweet sorghum stalks by alkaline and enzymatic treatment. Biomass Bioenergy 2021, 145, 105923. [Google Scholar] [CrossRef]
- Wösten, H.A.B. Filamentous fungi for the production of enzymes, chemicals and materials. Curr. Opin. Biotechnol. 2019, 59, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Soccol, C.R.; da Costa, E.S.F.; Letti, L.A.J.; Karp, S.G.; Woiciechowski, A.L.; de Souza Vandenberghe, L.P. Recent developments and innovations in solid state fermentation. Biotechnol. Res. Innov. 2017, 1, 52–71. [Google Scholar] [CrossRef]
- Téllez-Téllez, M.; Fernández, F.J.; Montiel-González, A.M.; Sánchez, C.; Díaz-Godínez, G. Growth and laccase production by Pleurotus ostreatus in submerged and solid-state fermentation. Appl. Microbiol. Biotechnol. 2008, 81, 675–679. [Google Scholar] [CrossRef]
- Jiménez, S.; Velásquez, C.; Mejía, F.; Arias, M.; Hormaza, A. Comparative studies of pure cultures and a consortium of white-rot fungi to degrade a binary mixture of dyes by solid-state fermentation and performance at different scales. Int. Biodeterior. Biodegrad. 2019, 145, 104772. [Google Scholar] [CrossRef]
- Córdoba, M.K.A.; Ríos, H.A. Biotechnological applications and potential uses of the mushroom Tramestes versicolor. Vitae 2012, 19, 70–76. [Google Scholar]
- Cardoso, W.S.; Soares, F.E.D.F.; Queiroz, P.V.; Tavares, G.P.; Santos, F.A.; Sufiate, B.L.; Kasuya, M.C.M.; de Queiroz, J.H. Minimum cocktail of cellulolytic multi-enzyme complexes obtained from white rot fungi via solid-state fermentation. 3 Biotech 2018, 8, 46. [Google Scholar] [CrossRef]
- Herrera Bravo de Laguna, I.; Toledo Marante, F.J.; Mioso, R. Enzymes and bioproducts produced by the ascomycete fungus Paecilomyces variotii. J. Appl. Microbiol. 2015, 119, 1455–1466. [Google Scholar] [CrossRef]
- Pereira, J.; De Melo, M.M.R.; Silva, C.M.; Lemos, P.C.; Serafim, L.S. Impact of a pretreatment step on the acidogenic fermentation of spent coffee grounds. Bioengineering 2022, 9, 362. [Google Scholar] [CrossRef]
- Roy, B.P.; Archibald, F. Effects of kraft pulp and lignin on Trametes versicolor carbon metabolism. Appl. Environ. Microbiol. 1993, 59, 1855–1863. [Google Scholar] [CrossRef]
- De Melo, M.M.R.; Barbosa, H.M.A.; Passos, C.P.; Silva, C.M. Supercritical fluid extraction of spent coffee grounds: Measurement of extraction curves, oil characterization and economic analysis. J. Supercrit. Fluids 2014, 86, 150–159. [Google Scholar] [CrossRef]
- Clesceri, L.; Greenberg, A.; Eaton, A. Standard Methods for the Examination of Water and Wastewater; American Water Works Association: Denver, CO, USA, 1999; ISBN 0-87553-47-8. [Google Scholar]
- Nguyen, Q.A.; Cho, E.J.; Lee, D.S.; Bae, H.J. Development of an advanced integrative process to create valuable biosugars including manno-oligosaccharides and mannose from spent coffee grounds. Bioresour. Technol. 2019, 272, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Getachew, A.T.; Chun, B.S. Influence of pretreatment and modifiers on subcritical water liquefaction of spent coffee grounds: A green waste valorization approach. J. Clean. Prod. 2017, 142, 3719–3727. [Google Scholar] [CrossRef]
- Kim, D. Physico-chemical conversion of lignocellulose: Inhibitor effects and detoxification strategies: A mini review. Molecules 2018, 23, 309. [Google Scholar] [CrossRef]
- López-Linares, J.C.; García-Cubero, M.T.; Coca, M.; Lucas, S. Efficient biobutanol production by acetone-butanol-ethanol fermentation from spent coffee grounds with microwave assisted dilute sulfuric acid pretreatment. Bioresour. Technol. 2021, 320, 124348. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Machado, E.M.S.; Martins, S.; Teixeira, J.A. Production, Composition, and Application of Coffee and Its Industrial Residues. Food Bioprocess Technol. 2011, 4, 661–672. [Google Scholar] [CrossRef]
- Juarez, G.F.Y.; Pabiloña, K.B.C.; Manlangit, K.B.L.; Go, A.W. Direct Dilute Acid Hydrolysis of Spent Coffee Grounds: A New Approach in Sugar and Lipid Recovery. Waste Biomass Valorization 2018, 9, 235–246. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Machado, E.M.S.; Carneiro, L.M.; Teixeira, J.A. Sugars metabolism and ethanol production by different yeast strains from coffee industry wastes hydrolysates. Appl. Energy 2012, 92, 763–768. [Google Scholar] [CrossRef]
- Kim, D.; Kim, J.; Lee, C. Effect of Mild-Temperature Thermo-Alkaline Pretreatment on the Solubilization and Anaerobic Digestion of Spent Coffee Grounds. Energies 2018, 11, 865. [Google Scholar] [CrossRef]
- Ballesteros, L.F.; Cerqueira, M.A.; Teixeira, J.A.; Mussatto, S.I. Characterization of polysaccharides extracted from spent coffee grounds by alkali pretreatment. Carbohydr. Polym. 2015, 127, 347–354. [Google Scholar] [CrossRef]
- Simões, J.; Madureira, P.; Nunes, F.M.; do Rosário Domingues, M.; Vilanova, M.; Coimbra, M.A. Immunostimulatory properties of coffee mannans. Mol. Nutr. Food Res. 2009, 53, 1036–1043. [Google Scholar] [CrossRef] [PubMed]
- Acevedo, F.; Rubilar, M.; Scheuermann, E.; Cancino, B.; Uquiche, E.; Garcés, M.; Inostroza, K.; Shene, C. Spent coffee grounds as a renewable source of bioactive compounds. J. Biobased Mater. Bioenergy 2013, 7, 420–428. [Google Scholar] [CrossRef]
- Kovalcik, A.; Kucera, D.; Matouskova, P.; Pernicova, I.; Obruca, S.; Kalina, M.; Enev, V.; Marova, I. Influence of removal of microbial inhibitors on PHA production from spent coffee grounds employing Halomonas halophila. J. Environ. Chem. Eng. 2018, 6, 3495–3501. [Google Scholar] [CrossRef]
- Wang, X.; Guo, Y.; Zhou, J.; Sun, G. Structural changes of poplar wood lignin after supercritical pretreatment using carbon dioxide and ethanol-water as co-solvents. RSC Adv. 2017, 7, 8314–8322. [Google Scholar] [CrossRef]
- Amaral, P.F.F.; Fernandes, D.L.A.; Tavares, A.P.M.; Xavier, A.B.M.R.; Cammarota, M.C.; Coutinho, J.A.P.; Coelho, M.A.Z. Decolorization of Dyes from textile wastewater by Trametes versicolor. Environ. Technol. 2004, 25, 1313–1320. [Google Scholar] [CrossRef] [PubMed]
- Angeloni, S.; Sagratini, G.; Nzekoue, F.K.; Torregiani, E.; Navarini, L.; Caprioli, G. An analytical method for the simultaneous quantification of 30 bioactive compounds in spent coffee ground by HPLC-MS/MS. J. Mass Spectrometry 2020, 55, e4519. [Google Scholar] [CrossRef] [PubMed]
- Janusz, G.; Pawlik, A.; Sulej, J.; Świderska-Burek, U.; Jarosz-Wilkolazka, A.; Paszczyński, A. Lignin degradation: Microorganisms, enzymes involved, genomes analysis and evolution. FEMS Microbiol. Rev. 2017, 41, 941–962. [Google Scholar] [CrossRef]
- Brijwani, K.; Rigdon, A.; Vadlani, P. V Fungal Laccases: Production, Function, and Applications in Food Processing. Enz. Res. 2010, 2010, 149748. [Google Scholar] [CrossRef]
- Dvořáčková, H.; Dvořáček, J.; González, P.H.; Vlček, V. Effect of different soil amendments on soil buffering capacity. PLoS ONE 2022, 17, e0263456. [Google Scholar] [CrossRef]
- Queirós, D.; Sousa, R.; Pereira, S.R.; Serafim, L.S. Valorization of a Pulp Industry By-Product through the Production of Short-Chain Organic Acids. Fermentation 2017, 3, 20. [Google Scholar] [CrossRef]
- Arroja, L.; Capela, I.; Nadais, H.; Serafim, L.S.; Silva, F. Acidogenic Valorisation of High Strength Waste Products from Food Industry. In Industrial Waste; InTech: Pittsburgh, PA, USA, 2012; pp. 227–252. ISBN 978-953-51-0253-3. [Google Scholar]
- Castilla-Archilla, J.; Papirio, S.; Lens, P.N.L. Two step process for volatile fatty acid production from brewery spent grain: Hydrolysis and direct acidogenic fermentation using anaerobic granular sludge. Process Biochem. 2021, 100, 272–283. [Google Scholar] [CrossRef]
- Kumar, A.N.; Mohan, S.V. Acidogenic valorization of vegetable waste for short chain carboxylic acids and biohydrogen production: Influence of pretreatment and pH. J. Clean. Prod. 2018, 203, 1055–1066. [Google Scholar] [CrossRef]
- Girotto, F.; Lavagnolo, M.C.; Pivato, A. Spent Coffee Grounds Alkaline Pre-treatment as Biorefinery Option to Enhance their Anaerobic Digestion Yield. Waste Biomass Valorization 2018, 9, 2565–2570. [Google Scholar] [CrossRef]
- Girotto, F.; Kusch, S.; Lavagnolo, M.C. Biological metabolites recovery from beverage production solid residues through acidogenic fermentation. Detritus 2019, 5, 19–28. [Google Scholar] [CrossRef]
- Kucharska, K.; Rybarczyk, P.; Hołowacz, I.; Łukajtis, R.; Glinka, M.; Kamiński, M. Pretreatment of lignocellulosic materials as substrates for fermentation processes. Molecules 2018, 23, 2937. [Google Scholar] [CrossRef]
- Xin, X.; She, Y.; Hong, J. Insights into microbial interaction profiles contributing to volatile fatty acids production via acidogenic fermentation of waste activated sludge assisted by calcium oxide pretreatment. Bioresour. Technol. 2021, 320, 124287. [Google Scholar] [CrossRef]
- Lin, L.; Li, R.H.; Li, X.Y. Recovery of organic resources from sewage sludge of Al-enhanced primary sedimentation by alkali pretreatment and acidogenic fermentation. J. Clean. Prod. 2018, 172, 3334–3341. [Google Scholar] [CrossRef]
- Szumacher-Strabel, M.; Zmora, P.; Roj, E.; Stochmal, A.; Pers-Kamczyc, E.; Urbańczyk, A.; Oleszek, W.; Lechniak, D.; Cieślak, A. The potential of the wild dog rose (Rosa canina) to mitigate in vitro rumen methane production. J. Anim. Feed Sci. 2011, 20, 285–299. [Google Scholar] [CrossRef]
- Hernández, D.; Solana, M.; Riaño, B.; García-González, M.C.; Bertucco, A. Biofuels from microalgae: Lipid extraction and methane production from the residual biomass in a biorefinery approach. Bioresour. Technol. 2014, 170, 370–378. [Google Scholar] [CrossRef]
- Monlau, F.; Sambusiti, C.; Barakat, A.; Quéméneur, M.; Trably, E.; Steyer, J.P.; Carrère, H. Do furanic and phenolic compounds of lignocellulosic and algae biomass hydrolyzate inhibit anaerobic mixed cultures? A comprehensive review. Biotechnol. Adv. 2014, 32, 934–951. [Google Scholar] [CrossRef]
- Wan, C.; Li, Y. Fungal pretreatment of lignocellulosic biomass. Biotechnol. Adv. 2012, 30, 1447–1457. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.R.; Ivanuša, S.; Evtuguin, D.V.; Serafim, L.S.; Xavier, A.M.R.B. Biological treatment of eucalypt spent sulphite liquors: A way to boost the production of second generation bioethanol. Bioresour. Technol. 2012, 103, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Lord, K.A.; Lacey, J.; Cayley, G.R.; Manlove, R. Fatty acids as substrates and inhibitors of fungi from propionic acid treated hay. Trans. Br. Mycol. Soc. 1981, 77, 41–45. [Google Scholar] [CrossRef]
- Ravindran, R.; Hassan, S.S.; Williams, G.A.; Jaiswal, A.K. A review on bioconversion of agro-industrial wastes to industrially important enzymes. Bioengineering 2018, 5, 93. [Google Scholar] [CrossRef]
- Tišma, M.; Planinić, M.; Bucić-Kojić, A.; Panjičko, M.; Zupančič, G.D.; Zelić, B. Corn silage fungal-based solid-state pretreatment for enhanced biogas production in anaerobic co-digestion with cow manure. Bioresour. Technol. 2018, 253, 220–226. [Google Scholar] [CrossRef]
- Kourmentza, C.; Economou, C.N.; Tsafrakidou, P.; Kornaros, M. Spent coffee grounds make much more than waste: Exploring recent advances and future exploitation strategies for the valorization of an emerging food waste stream. J. Clean. Prod. 2018, 172, 980–992. [Google Scholar] [CrossRef]
- Fang, W.; Zhang, P.; Zhang, X.; Zhu, X.; van Lier, J.B.; Spanjers, H. White rot fungi pretreatment to advance volatile fatty acid production from solid-state fermentation of solid digestate: Efficiency and mechanisms. Energy 2018, 162, 534–541. [Google Scholar] [CrossRef]
- Akyol, Ç.; Ince, O.; Bozan, M.; Ozbayram, E.G.; Ince, B. Biological pretreatment with Trametes versicolor to enhance methane production from lignocellulosic biomass: A metagenomic approach. Ind. Crops Prod. 2019, 140, 111659. [Google Scholar] [CrossRef]
- Fang, W.; Zhang, X.; Zhang, P.; Carol Morera, X.; van Lier, J.B.; Spanjers, H. Evaluation of white rot fungi pretreatment of mushroom residues for volatile fatty acid production by anaerobic fermentation: Feedstock applicability and fungal function. Bioresour. Technol. 2020, 297, 122447. [Google Scholar] [CrossRef]
- Mardetko, N.; Trontel, A.; Novak, M.; Pavlečić, M.; Ljubas, B.D.; Grubišić, M.; Tominac, V.P.; Ludwig, R.; Šantek, B. Screening of lignocellulolytic enzyme activities in fungal species and sequential solid-state and submerged cultivation for the production of enzyme cocktails. Polymers 2021, 13, 3736. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, T.; Lim, J.; Xie, J.; Zhang, B.; Yao, T.; Hamaker, B.R. Fabrication of a soluble crosslinked corn bran arabinoxylan matrix supports a shift to butyrogenic gut bacteria. Food Funct. 2019, 10, 4497–4504. [Google Scholar] [CrossRef] [PubMed]
- Rusli, N.D.; Azmi, M.A.; Mat, K.; Hasnita, C.H.; Wan-Zahari, M.; Azhar, K.; Zamri-Saad, M.; Hassim, H.A. The Effect of Physical and Biological Pre-treatments of Oil Palm Fronds on in vitro Ruminal Degradability. Pertanika. J. Trop. Agric. Sci. 2019, 42, 791–805. [Google Scholar]
- Yue, Z.Q.; Xu, Y.Z.; Wang, C.; Liu, Q.; Guo, G.; Huo, W.J.; Zhang, J.; Chen, L.; Pei, C.X.; Zhang, Y.L.; et al. Effects of dietary laccase supplementation on growth performance, nutrient digestion, rumen fermentation and microbiota in dairy bulls. Anim. Feed Sci. Technol. 2020, 269, 114645. [Google Scholar] [CrossRef]
- Chang, H.F.; Chang, W.C.; Tsai, C.Y. Synthesis of poly (3-hydroxybutyrate/3-hydroxyvalerate) from propionate-fed activated sludge under various carbon sources. Bioresour. Technol. 2012, 113, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.C.; Serafim, L.S.; Nadais, H.; Arroja, L.; Capela, I. Acidogenic fermentation towards valorisation of organic waste streams into volatile fatty acids. Chem. Biochem. Eng. Q. 2013, 27, 467–476. [Google Scholar]
- Calero, R.; Iglesias-Iglesias, R.; Kennes, C.; Veiga, M.C. Organic loading rate effect on the acidogenesis of cheese whey: A comparison between UASB and SBR reactors. Environ. Technol. 2018, 39, 3046–3054. [Google Scholar] [CrossRef] [PubMed]
| Pretreatment | Experimental Conditions | |
|---|---|---|
| Acid Hydrolysis | AH | 5% H2SO4 at 121 °C for 1 h in autoclave |
| Basic Hydrolysis | BH | 2% NaOH at 121 °C for 1 h in autoclave |
| Supercritical Extraction | SC | CO2 extraction at 300 bar, 50 °C, 12 gCO2/min for 2 h |
| Supercritical Extraction + Acid Hydrolysis | SC + AH | CO2 extraction at 300 bar, 50 °C, 12 gCO2/min for 2 h followed by 5% H2SO4 at 121 °C for 1 h in autoclave |
| Supercritical Extraction + Basic Hydrolysis | SC + BH | CO2 extraction at 300 bar, 50 °C, 12 gCO2/min for 2 h followed by 2% NaOH at 121 °C for 1 h in autoclave |
| Solid-State Fermentation | TvSSF | 28 °C for 3 months, 1:4 (w/v) MC with T. versicolor |
| PvSSF | 28 °C for 3 months, 1:4 (w/v) MC with P. variotii | |
| Submerged Fermentation | TvSmF | 28 °C, 180 rpm for 15 d with T. versicolor |
| PvSmF | 28 °C, 180 rpm for 15 d with P. variotii | |
| Enzymatic Hydrolysis | TvEH | Enzymatic extract obtained from T. versicolor at 40 °C, 100 rpm for 7 d |
| PvEH | Enzymatic extract obtained from P. variotii at 40 °C, 100 rpm for 7 d | |
| Pretreatment | Sugars (g/L) | %Yield (gSugar/gSCG) |
|---|---|---|
| AH | 2.23 | 5.95 |
| BH | 0.03 | 0.08 |
| SC + AH | 1.51 | 4.03 |
| SC + BH | 0.03 | 0.08 |
| Assay | Lactic | Acetic | Propionic | Isobutyric | Butyric | Valeric | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SCOAs Max † | r initial * | SCOAs Max † | r initial * | SCOAs Max † | r initial * | SCOAs Max † | r initial * | SCOAs Max † | r initial * | SCOAs Max † | r initial * | |
| SCG_C | - | - | 0.56 | 0.025 | 0.39 | 0.007 | - | - | 0.21 | 0.036 | 0.10 | 0.020 |
| SCG_AH | 0.40 | 0.097 | 0.90 | 0.081 | 0.43 | 0.024 | - | - | 1.15 | 0.132 | 0.05 | 0.003 |
| SCG_BH | 0.17 | 0.041 | 0.83 | 0.080 | 1.07 | 0.089 | 0.07 | 0.014 | 0.08 | 0.037 | 0.09 | 0.042 |
| SCG_SC | 0.08 | 0.005 | 0.85 | 0.058 | 0.40 | 0.028 | 0.06 | 0.030 | 0.39 | 0.048 | 0.11 | 0.051 |
| SCG_AH + SC | 0.50 | 0.124 | 0.92 | 0.108 | 0.28 | 0.022 | - | - | 1.06 | 0.155 | - | - |
| SCG_BH + SC | 0.15 | 0.031 | 0.84 | 0.078 | 0.90 | 0.097 | 0.06 | 0.011 | 0.18 | 0.042 | 0.10 | 0.032 |
| SCG_TvSSF | 0.08 | 0.026 | 0.60 | 0.054 | 0.44 | 0.030 | 0.07 | 0.027 | 0.24 | 0.025 | 0.10 | 0.032 |
| SCG_PvSSF | - | - | 0.34 | 0.022 | 0.11 | 0.016 | - | - | - | - | - | - |
| SCG_TvSmF | - | - | 1.46 | 0.025 | 0.54 | 0.059 | - | - | 0.15 | 0.018 | - | - |
| SCG_PvSmF | - | - | 0.55 | 0.047 | 0.87 | 0.020 | - | - | - | - | - | - |
| SCG_PvEH | - | - | 0.56 | 0.050 | 0.91 | 0.015 | - | - | 0.24 | 0.009 | - | - |
| SCG_TvEH | - | - | 0.78 | 0.066 | 0.19 | 0.015 | - | - | - | - | - | - |
| Assay | pH Max SCOA | SCOAs (gCOD/L) | Odd-to-Even Ratio | AD (%) | Prod. (gCOD/L.d) |
|---|---|---|---|---|---|
| SCG_C | 4.82 | 1.31 | 0.60 | 13% | 0.050 |
| SCG_AH | 4.71 | 2.52 | 0.23 | 23% | 0.090 |
| SCG_BH | 5.70 | 2.21 | 1.28 | 22% | 0.111 |
| SCG_SC | 4.65 | 1.8 | 0.38 | 31% | 0.082 |
| SCG_SE + AH | 4.86 | 2.26 | 0.14 | 12% | 0.090 |
| SCG_SE + BH | 5.75 | 2.1 | 1.01 | 14% | 0.117 |
| SCG_TvSSF | 5.00 | 1.51 | 0.60 | 15% | 0.060 |
| SCG_PvSSF | 6.03 | 0.45 | 0.31 | 5% | 0.024 |
| SCG_TvSmF | 4.90 | 2.44 | 0.51 | 48% | 0.102 |
| SCG_PvSmF | 5.85 | 0.89 | 0.63 | 12% | 0.056 |
| SCG_TvEH | 6.61 | 0.97 | 0.25 | 19% | 0.054 |
| SCG_PvEH | 5.52 | 1.23 | 0.77 | 26% | 0.068 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, J.; Cachinho, A.; de Melo, M.M.R.; Silva, C.M.; Lemos, P.C.; Xavier, A.M.R.B.; Serafim, L.S. Enzymatic Potential of Filamentous Fungi as a Biological Pretreatment for Acidogenic Fermentation of Coffee Waste. Biomolecules 2022, 12, 1284. https://doi.org/10.3390/biom12091284
Pereira J, Cachinho A, de Melo MMR, Silva CM, Lemos PC, Xavier AMRB, Serafim LS. Enzymatic Potential of Filamentous Fungi as a Biological Pretreatment for Acidogenic Fermentation of Coffee Waste. Biomolecules. 2022; 12(9):1284. https://doi.org/10.3390/biom12091284
Chicago/Turabian StylePereira, Joana, Ana Cachinho, Marcelo M. R. de Melo, Carlos M. Silva, Paulo C. Lemos, Ana M. R. B. Xavier, and Luísa S. Serafim. 2022. "Enzymatic Potential of Filamentous Fungi as a Biological Pretreatment for Acidogenic Fermentation of Coffee Waste" Biomolecules 12, no. 9: 1284. https://doi.org/10.3390/biom12091284










