Generation and Functional Characterization of PLAP CAR-T Cells against Cervical Cancer Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. Generation of Retroviral Constructs and Retroviral Vectors
2.3. Generation of CAR T Cells
2.4. Flow Cytometry Analysis
2.5. Cytotoxicity Assay
2.6. Activation Assessment and Cytokine Measurement
2.7. Co-Culture Experiments
2.8. Statistical Analyses
3. Results
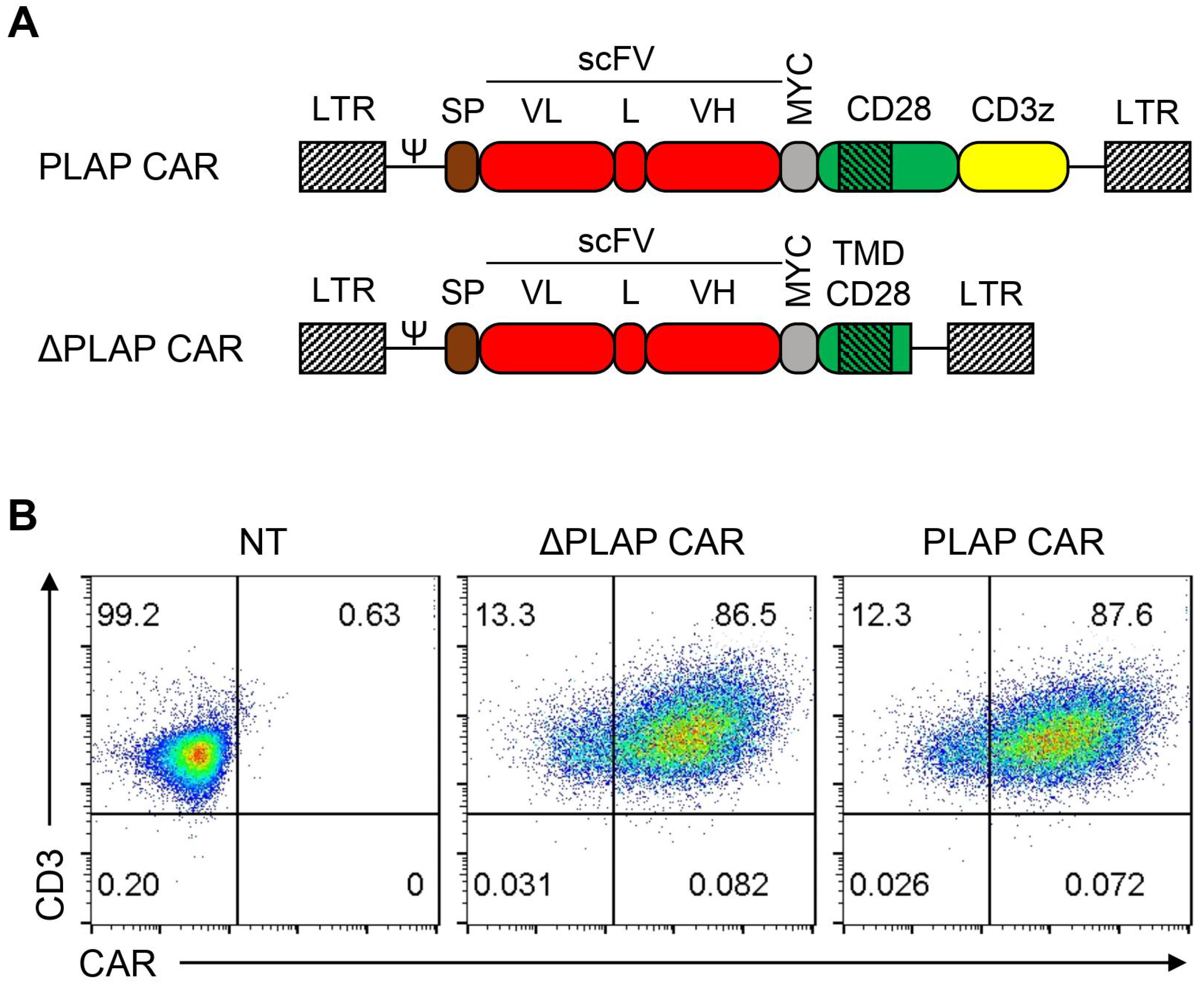
3.1. CAR Construct and its Expression on T Cells
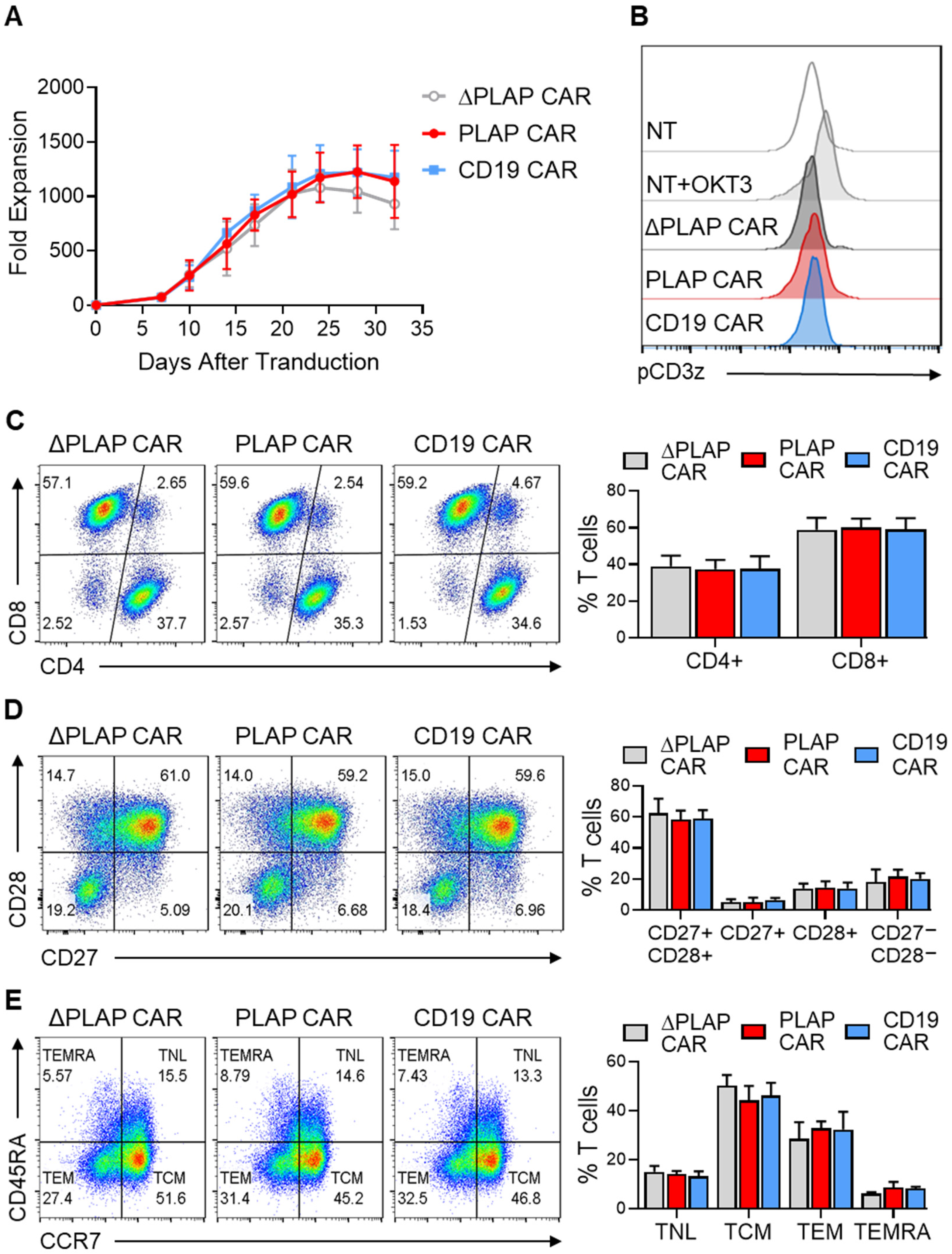
3.2. Tonic Signaling and Immunophenotype of CAR T Cells
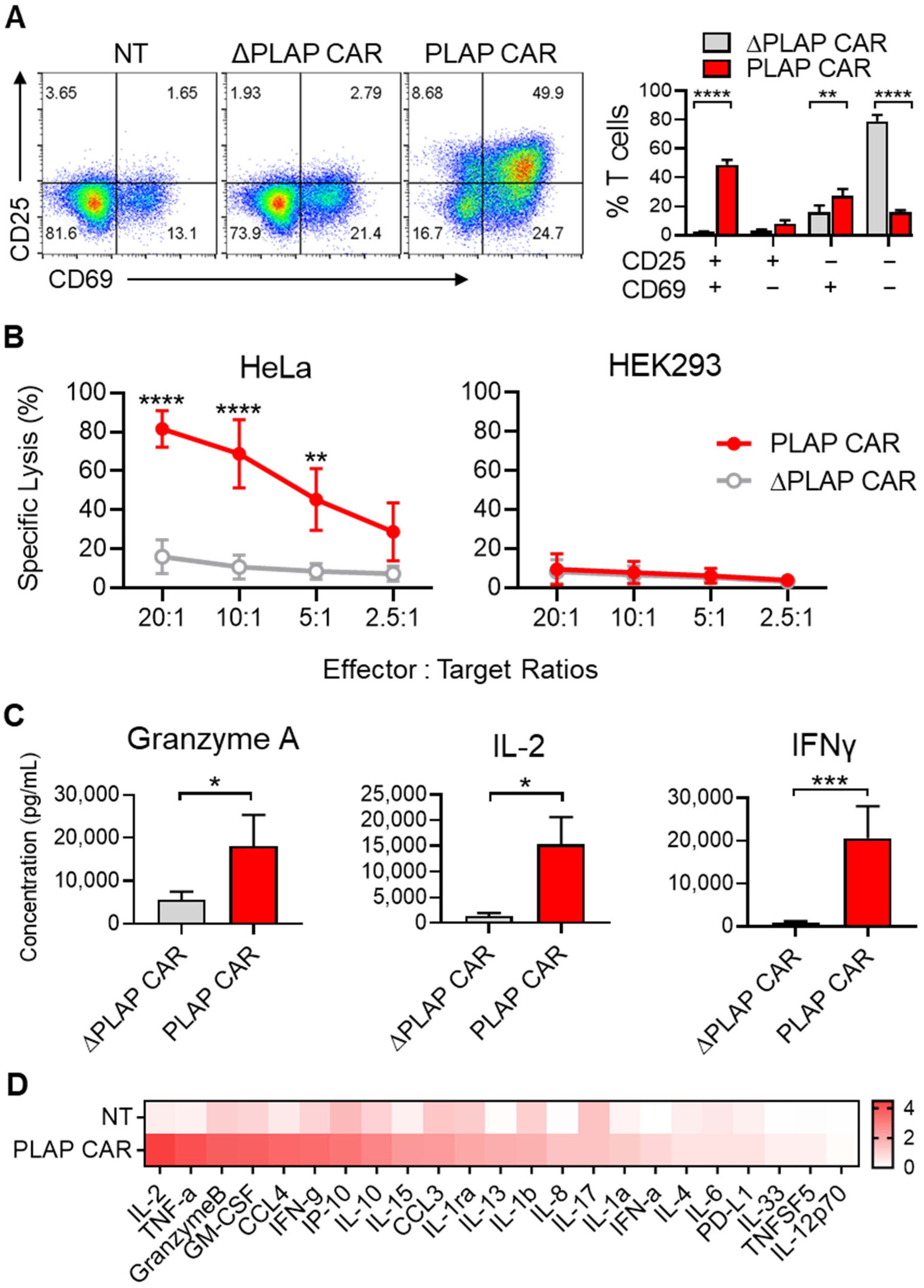
3.3. Activation and Functionality of CAR T Cells
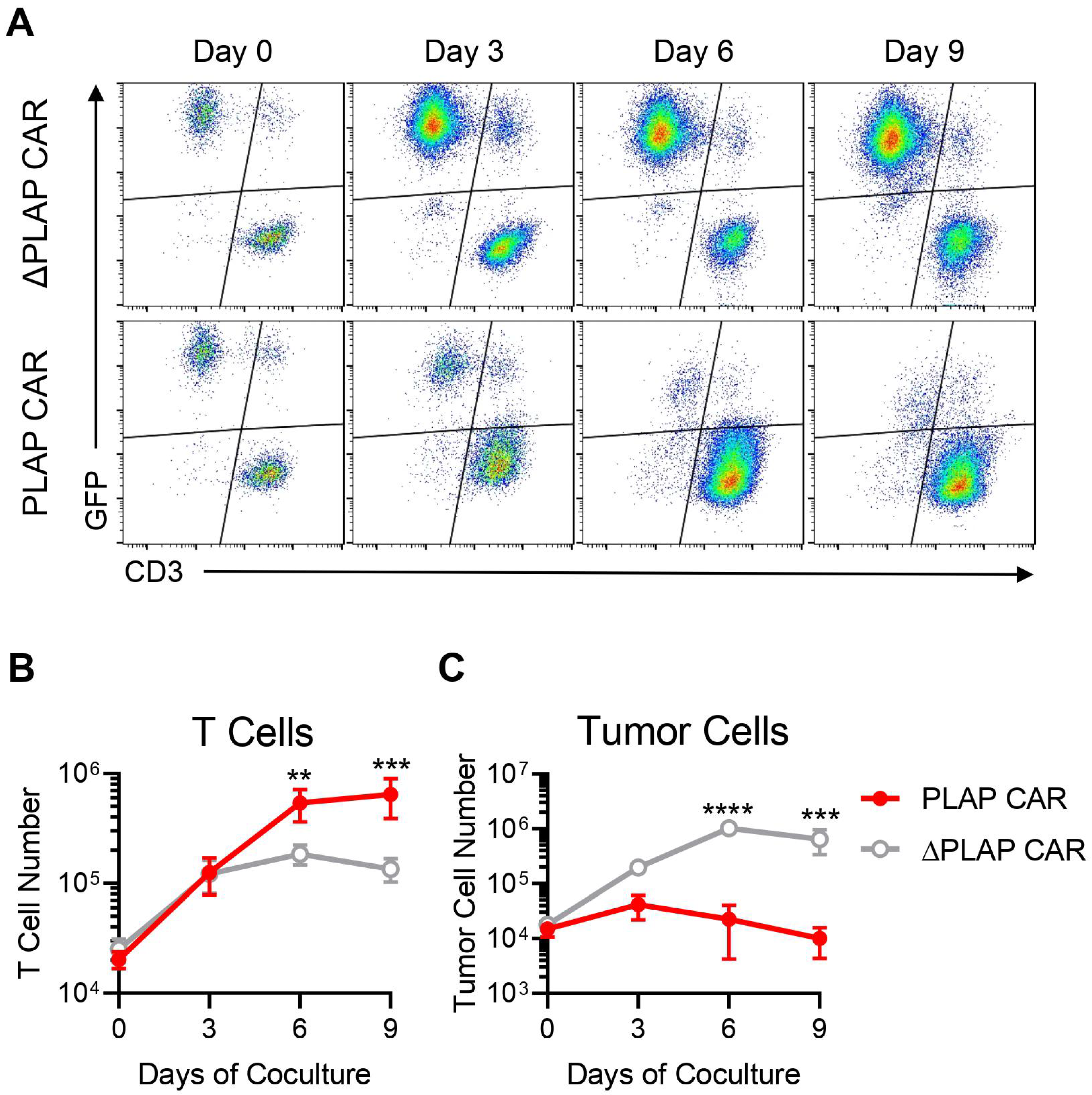
3.4. Anti-Tumor Activity of PLAP CAR T Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ochsenbein, A.F. Principles of tumor immunosurveillance and implications for immunotherapy. Cancer Gene Ther. 2002, 9, 1043–1055. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.; Clubb, J.D.; Chen, Y.Y. Engineering CAR-T cells for next-generation cancer therapy. Cancer Cell 2020, 38, 473–488. [Google Scholar] [CrossRef] [PubMed]
- Sadelain, M.; Rivière, I.; Brentjens, R. Targeting tumours with genetically enhanced T lymphocytes. Nat. Rev. Cancer 2003, 3, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Kalos, M.; Levine, B.L.; Porter, D.L.; Katz, S.; Grupp, S.A.; Bagg, A.; June, C.H. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci. Transl. Med. 2011, 3, 95ra73. [Google Scholar] [CrossRef] [PubMed]
- Fraietta, J.A.; Lacey, S.F.; Orlando, E.J.; Pruteanu-Malinici, I.; Gohil, M.; Lundh, S.; Boesteanu, A.C.; Wang, Y.; O’Connor, R.S.; Hwang, W.-T. Determinants of response and resistance to CD19 chimeric antigen receptor (CAR) T cell therapy of chronic lymphocytic leukemia. Nat. Med. 2018, 24, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Melenhorst, J.J.; Chen, G.M.; Wang, M.; Porter, D.L.; Chen, C.; Collins, M.A.; Gao, P.; Bandyopadhyay, S.; Sun, H.; Zhao, Z. Decade-long leukaemia remissions with persistence of CD4+ CAR T cells. Nature 2022, 602, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Gross, G.; Eshhar, Z. Therapeutic Potential of T Cell Chimeric Antigen Receptors (CARs) in Cancer Treatment: Counteracting Off-Tumor Toxicities for Safe CAR T Cell Therapy. Annu. Rev. Pharm. Toxicol. 2016, 56, 59–83. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Hao, H.; Yang, G.; Zhang, Y.; Fu, Y. Immunotherapy with CAR-modified T cells: Toxicities and overcoming strategies. J. Immunol. Res. 2018, 2018, 2386187. [Google Scholar] [CrossRef]
- Fishman, W.H. Clinical and biological significance of an isozyme tumor marker—PLAP. Clin. Biochem. 1987, 20, 387–392. [Google Scholar] [CrossRef]
- Rao, S.; Snaith, A.; Marino, D.; Cheng, X.; Lwin, S.; Orriss, I.; Hamdy, F.; Edwards, C. Tumour-derived alkaline phosphatase regulates tumour growth, epithelial plasticity and disease-free survival in metastatic prostate cancer. Br. J. Cancer 2017, 116, 227–236. [Google Scholar] [CrossRef]
- Koshida, K.; Yokoyama, K.; Imao, T.; Konaka, H.; Hirano, K.; Uchibayashi, T.; Namiki, M. Immunolocalization of anti-placental alkaline phosphatase monoclonal antibody in mice with testicular tumors and lymph node metastasis. Urol. Res. 1998, 26, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Angellis, D.; Fishman, W.H. Presence of the rare D-variant heat-stable, placental-type alkaline phosphatase in normal human testis. Cancer Res. 1980, 40, 1506–1510. [Google Scholar] [PubMed]
- Millán, J.; Eriksson, A.; Stigbrand, T. A possible new locus of alkaline phosphatase expressed in human testis. Hum. Genet. 1982, 62, 293–295. [Google Scholar] [CrossRef] [PubMed]
- Hamilton-Dutoit, S.J.; Lou, H.; Pallesen, G. The expression of placental alkaline phosphatase (PLAP) and PLAP-like enzymes in normal and neoplastic human tissues: An immunohistological survey using monoclonal antibodies. Apmis 1990, 98, 797–811. [Google Scholar] [CrossRef] [PubMed]
- Lonsdale, J.; Thomas, J.; Salvatore, M.; Phillips, R.; Lo, E.; Shad, S.; Hasz, R.; Walters, G.; Garcia, F.; Young, N. The genotype-tissue expression (GTEx) project. Nat. Genet. 2013, 45, 580–585. [Google Scholar] [CrossRef]
- Basiri, M.; Pahlavanneshan, S. Evaluation of Placental Alkaline Phosphatase Expression as A Potential Target of Solid Tumors Immunotherapy by Using Gene and Protein Expression Repositories. Cell J. 2021, 23, 717–721. [Google Scholar] [CrossRef]
- Oh, D.-Y.; Kim, S.; Choi, Y.-L.; Cho, Y.J.; Oh, E.; Choi, J.-J.; Jung, K.; Song, J.-Y.; Ahn, S.E.; Kim, B.-G. HER2 as a novel therapeutic target for cervical cancer. Oncotarget 2015, 6, 36219. [Google Scholar] [CrossRef]
- O’Hara, M.; Stashwick, C.; Haas, A.R.; Tanyi, J.L. Mesothelin as a target for chimeric antigen receptor-modified T cells as anticancer therapy. Immunotherapy 2016, 8, 449–460. [Google Scholar] [CrossRef]
- He, Y.; Li, X.-M.; Yin, C.-H.; Wu, Y.-M. Killing cervical cancer cells by specific chimeric antigen receptor-modified T cells. J. Reprod. Immunol. 2020, 139, 103115. [Google Scholar] [CrossRef]
- Goldstein, D.J.; Blasco, L.; Harris, H. Placental alkaline phosphatase in nonmalignant human cervix. Proc. Natl. Acad. Sci. USA 1980, 77, 4226–4228. [Google Scholar] [CrossRef] [Green Version]
- Reiswich, V.; Gorbokon, N.; Luebke, A.M.; Burandt, E.; Menz, A.; Kluth, M.; Hube-Magg, C.; Wittmer, C.; Weidemann, S.; Fraune, C. Pattern of placental alkaline phosphatase (PLAP) expression in human tumors: A tissue microarray study on 12,381 tumors. J. Pathol. Clin. Res. 2021, 7, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Bassi, G.; Favalli, N.; Pellegrino, C.; Onda, Y.; Scheuermann, J.; Cazzamalli, S.; Manz, M.G.; Neri, D. Specific Inhibitor of Placental Alkaline Phosphatase Isolated from a DNA-Encoded Chemical Library Targets Tumor of the Female Reproductive Tract. J. Med. Chem. 2021, 64, 15799–15809. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Berahovich, R.; Zhou, H.; Liu, X.; Li, F.; Xu, S.; Wei, Y.; Ouaret, D.; Bodmer, W.; Wu, L. PLAP-CAR T cells mediate high specific cytotoxicity against colon cancer cells. Front. Biosci. (Landmark Ed.) 2020, 25, 1765–1786. [Google Scholar] [PubMed]
- Waggoner, S.E. Cervical cancer. Lancet 2003, 361, 2217–2225. [Google Scholar] [CrossRef]
- Cohen, P.A.; Jhingran, A.; Oaknin, A.; Denny, L. Cervical cancer. Lancet 2019, 393, 169–182. [Google Scholar] [CrossRef]
- Ravenni, N.; Weber, M.; Neri, D. A human monoclonal antibody specific to placental alkaline phosphatase, a marker of ovarian cancer. MAbs 2014, 6, 86–94. [Google Scholar] [CrossRef]
- Gattinoni, L.; Speiser, D.E.; Lichterfeld, M.; Bonini, C. T memory stem cells in health and disease. Nat. Med. 2017, 23, 18–27. [Google Scholar] [CrossRef]
- Khan, I.; Zakaria, M.K.; Kumar, M.; Mani, P.; Chattopadhyay, P.; Sarkar, D.P.; Sinha, S. A novel placental like alkaline phosphatase promoter driven transcriptional silencing combined with single chain variable fragment antibody based virosomal delivery for neoplastic cell targeting [corrected]. J. Transl. Med. 2015, 13, 254. [Google Scholar] [CrossRef]
- Ajina, A.; Maher, J. Strategies to address chimeric antigen receptor tonic signaling. Mol. Cancer Ther. 2018, 17, 1795–1815. [Google Scholar] [CrossRef]
- Calderon, H.; Mamonkin, M.; Guedan, S. Analysis of CAR-mediated tonic signaling. In Chimeric Antigen Receptor T Cells; Springer: Berlin/Heidelberg, Germany, 2020; pp. 223–236. [Google Scholar]
- Long, A.H.; Haso, W.M.; Shern, J.F.; Wanhainen, K.M.; Murgai, M.; Ingaramo, M.; Smith, J.P.; Walker, A.J.; Kohler, M.E.; Venkateshwara, V.R. 4-1BB costimulation ameliorates T cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nat. Med. 2015, 21, 581–590. [Google Scholar] [CrossRef] [Green Version]
- Gomes-Silva, D.; Mukherjee, M.; Srinivasan, M.; Krenciute, G.; Dakhova, O.; Zheng, Y.; Cabral, J.M.; Rooney, C.M.; Orange, J.S.; Brenner, M.K. Tonic 4-1BB costimulation in chimeric antigen receptors impedes T cell survival and is vector-dependent. Cell Rep. 2017, 21, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, K.; Masutani, M.; Tachibana, M.; Okada, N. Impact of scFv structure in chimeric antigen receptor on receptor expression efficiency and antigen recognition properties. Biochem. Biophys. Res. Commun. 2020, 527, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Anagnostou, T.; Riaz, I.B.; Hashmi, S.K.; Murad, M.H.; Kenderian, S.S. Anti-CD19 chimeric antigen receptor T-cell therapy in acute lymphocytic leukaemia: A systematic review and meta-analysis. Lancet Haematol. 2020, 7, e816. [Google Scholar] [CrossRef]
- Hu, L.; Charwudzi, A.; Li, Q.; Zhu, W.; Tao, Q.; Xiong, S.; Zhai, Z. Anti-CD19 CAR-T cell therapy bridge to HSCT decreases the relapse rate and improves the long-term survival of R/R B-ALL patients: A systematic review and meta-analysis. Ann. Hematol. 2021, 100, 1003–1012. [Google Scholar] [CrossRef] [PubMed]
- Wagner, D.L.; Fritsche, E.; Pulsipher, M.A.; Ahmed, N.; Hamieh, M.; Hegde, M.; Ruella, M.; Savoldo, B.; Shah, N.N.; Turtle, C.J. Immunogenicity of CAR T cells in cancer therapy. Nat. Rev. Clin. Oncol. 2021, 18, 379–393. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, H.R.; Jamali, A.; Jafarzadeh, L.; Masoumi, E.; Alishah, K.; Fallah Mehrjardi, K.; Emami, S.A.H.; Noorbakhsh, F.; Till, B.G.; Hadjati, J. Construction and functional characterization of a fully human anti-CD19 chimeric antigen receptor (huCAR)-expressing primary human T cells. J. Cell. Physiol. 2019, 234, 9207–9215. [Google Scholar] [CrossRef]
- Johnson, L.A.; Scholler, J.; Ohkuri, T.; Kosaka, A.; Patel, P.R.; McGettigan, S.E.; Nace, A.K.; Dentchev, T.; Thekkat, P.; Loew, A. Rational development and characterization of humanized anti–EGFR variant III chimeric antigen receptor T cells for glioblastoma. Sci. Transl. Med. 2015, 7, 275ra222. [Google Scholar] [CrossRef]
- Brudno, J.N.; Lam, N.; Vanasse, D.; Shen, Y.-w.; Rose, J.J.; Rossi, J.; Xue, A.; Bot, A.; Scholler, N.; Mikkilineni, L. Safety and feasibility of anti-CD19 CAR T cells with fully human binding domains in patients with B-cell lymphoma. Nat. Med. 2020, 26, 270–280. [Google Scholar] [CrossRef]
- Adusumilli, P.S.; Cherkassky, L.; Villena-Vargas, J.; Colovos, C.; Servais, E.; Plotkin, J.; Jones, D.R.; Sadelain, M. Regional delivery of mesothelin-targeted CAR T cell therapy generates potent and long-lasting CD4-dependent tumor immunity. Sci. Transl. Med. 2014, 6, 261ra151. [Google Scholar] [CrossRef]
- Lanitis, E.; Poussin, M.; Hagemann, I.S.; Coukos, G.; Sandaltzopoulos, R.; Scholler, N.; Powell, D.J., Jr. Redirected antitumor activity of primary human lymphocytes transduced with a fully human anti-mesothelin chimeric receptor. Mol. Ther. 2012, 20, 633–643. [Google Scholar] [CrossRef] [Green Version]
- Turtle, C.J.; Hanafi, L.-A.; Berger, C.; Gooley, T.A.; Cherian, S.; Hudecek, M.; Sommermeyer, D.; Melville, K.; Pender, B.; Budiarto, T.M. CD19 CAR–T cells of defined CD4+: CD8+ composition in adult B cell ALL patients. J. Clin. Investig. 2016, 126, 2123–2138. [Google Scholar] [CrossRef] [PubMed]
- Klebanoff, C.A.; Gattinoni, L.; Torabi-Parizi, P.; Kerstann, K.; Cardones, A.R.; Finkelstein, S.E.; Palmer, D.C.; Antony, P.A.; Hwang, S.T.; Rosenberg, S.A. Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells. Proc. Natl. Acad. Sci. USA 2005, 102, 9571–9576. [Google Scholar] [CrossRef] [PubMed]
- Gattinoni, L.; Klebanoff, C.A.; Restifo, N.P. Paths to stemness: Building the ultimate antitumour T cell. Nat. Rev. Cancer 2012, 12, 671–684. [Google Scholar] [CrossRef] [PubMed]
- Gattinoni, L.; Lugli, E.; Ji, Y.; Pos, Z.; Paulos, C.M.; Quigley, M.F.; Almeida, J.R.; Gostick, E.; Yu, Z.; Carpenito, C. A human memory T cell subset with stem cell–like properties. Nat. Med. 2011, 17, 1290–1297. [Google Scholar] [CrossRef] [PubMed]
- Maus, M.V.; June, C.H. Making better chimeric antigen receptors for adoptive T-cell therapy. Clin. Cancer Res. 2016, 22, 1875–1884. [Google Scholar] [CrossRef]
- Kim, D.W.; Cho, J.-Y. Recent advances in allogeneic CAR-T cells. Biomolecules 2020, 10, 263. [Google Scholar] [CrossRef]
- Torres Chavez, A.; McKenna, M.K.; Canestrari, E.; Dann, C.T.; Ramos, C.A.; Lulla, P.; Leen, A.M.; Vera, J.F.; Watanabe, N. Expanding CAR T cells in human platelet lysate renders T cells with in vivo longevity. J. Immunother. Cancer 2019, 7, 330. [Google Scholar] [CrossRef]
- Berger, C.; Jensen, M.C.; Lansdorp, P.M.; Gough, M.; Elliott, C.; Riddell, S.R. Adoptive transfer of effector CD8+ T cells derived from central memory cells establishes persistent T cell memory in primates. J. Clin. Investig. 2008, 118, 294–305. [Google Scholar] [CrossRef]
- Graef, P.; Buchholz, V.R.; Stemberger, C.; Flossdorf, M.; Henkel, L.; Schiemann, M.; Drexler, I.; Höfer, T.; Riddell, S.R.; Busch, D.H. Serial transfer of single-cell-derived immunocompetence reveals stemness of CD8+ central memory T cells. Immunity 2014, 41, 116–126. [Google Scholar] [CrossRef]
- Bullock, T.N. Stimulating CD27 to quantitatively and qualitatively shape adaptive immunity to cancer. Curr. Opin. Immunol. 2017, 45, 82–88. [Google Scholar] [CrossRef] [Green Version]
- O’Neill, R.E.; Cao, X. Co-stimulatory and co-inhibitory pathways in cancer immunotherapy. Adv. Cancer Res. 2019, 143, 145–194. [Google Scholar] [PubMed]
- Cullen, S.; Martin, S. Mechanisms of granule-dependent killing. Cell Death Differ. 2008, 15, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Benmebarek, M.-R.; Karches, C.H.; Cadilha, B.L.; Lesch, S.; Endres, S.; Kobold, S. Killing mechanisms of chimeric antigen receptor (CAR) T cells. Int. J. Mol. Sci. 2019, 20, 1283. [Google Scholar] [CrossRef] [PubMed]
- Wabnitz, G.H.; Köcher, T.; Lohneis, P.; Stober, C.; Konstandin, M.H.; Funk, B.; Sester, U.; Wilm, M.; Klemke, M.; Samstag, Y. Costimulation induced phosphorylation of L-plastin facilitates surface transport of the T cell activation molecules CD69 and CD25. Eur. J. Immunol. 2007, 37, 649–662. [Google Scholar] [CrossRef] [PubMed]
| Target Protein | Clone | Fluorophore | Company | Cat. No. |
|---|---|---|---|---|
| cMYC | 9E10 | FITC | Sigma-Aldrich | F2047 |
| CD3 | UCHT1 | APC-Alexa Fluor 750 | Beckman Coulter (Brea, CA, USA) | A66329 |
| CD4 | 13B82 | Krome Orange | Beckman Coulter | A96417 |
| CD8 | SK1 | Pacific Blue | BioLegend (Fell, Germany) | 344718 |
| CD45RA | 5H9 | APC | BD Biosciences | 561210 |
| CCR7 | 150503 | Alexa Flour 700 | BD Biosciences | 561143 |
| CD27 | O323 | PE Cy7 | BD Biosciences | 567290 |
| CD28 | CD28.2 | PE Cy5 | BD Biosciences | 561791 |
| CD247(pY142) | K25-407.69 | Alexa Fluor 647 | BD Biosciences | 558489 |
| CD69 | TP1.55.3 | ECD | Beckman Coulter | 6607110 |
| CD25 | M-A251 | PE Cy5 | BD Biosciences | 555433 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yekehfallah, V.; Pahlavanneshan, S.; Sayadmanesh, A.; Momtahan, Z.; Ma, B.; Basiri, M. Generation and Functional Characterization of PLAP CAR-T Cells against Cervical Cancer Cells. Biomolecules 2022, 12, 1296. https://doi.org/10.3390/biom12091296
Yekehfallah V, Pahlavanneshan S, Sayadmanesh A, Momtahan Z, Ma B, Basiri M. Generation and Functional Characterization of PLAP CAR-T Cells against Cervical Cancer Cells. Biomolecules. 2022; 12(9):1296. https://doi.org/10.3390/biom12091296
Chicago/Turabian StyleYekehfallah, Vahid, Saghar Pahlavanneshan, Ali Sayadmanesh, Zahra Momtahan, Bin Ma, and Mohsen Basiri. 2022. "Generation and Functional Characterization of PLAP CAR-T Cells against Cervical Cancer Cells" Biomolecules 12, no. 9: 1296. https://doi.org/10.3390/biom12091296
APA StyleYekehfallah, V., Pahlavanneshan, S., Sayadmanesh, A., Momtahan, Z., Ma, B., & Basiri, M. (2022). Generation and Functional Characterization of PLAP CAR-T Cells against Cervical Cancer Cells. Biomolecules, 12(9), 1296. https://doi.org/10.3390/biom12091296








