Plasma Kallikrein-Activated TGF-β Is Prognostic for Poor Overall Survival in Patients with Pancreatic Ductal Adenocarcinoma and Associates with Increased Fibrogenesis
Abstract
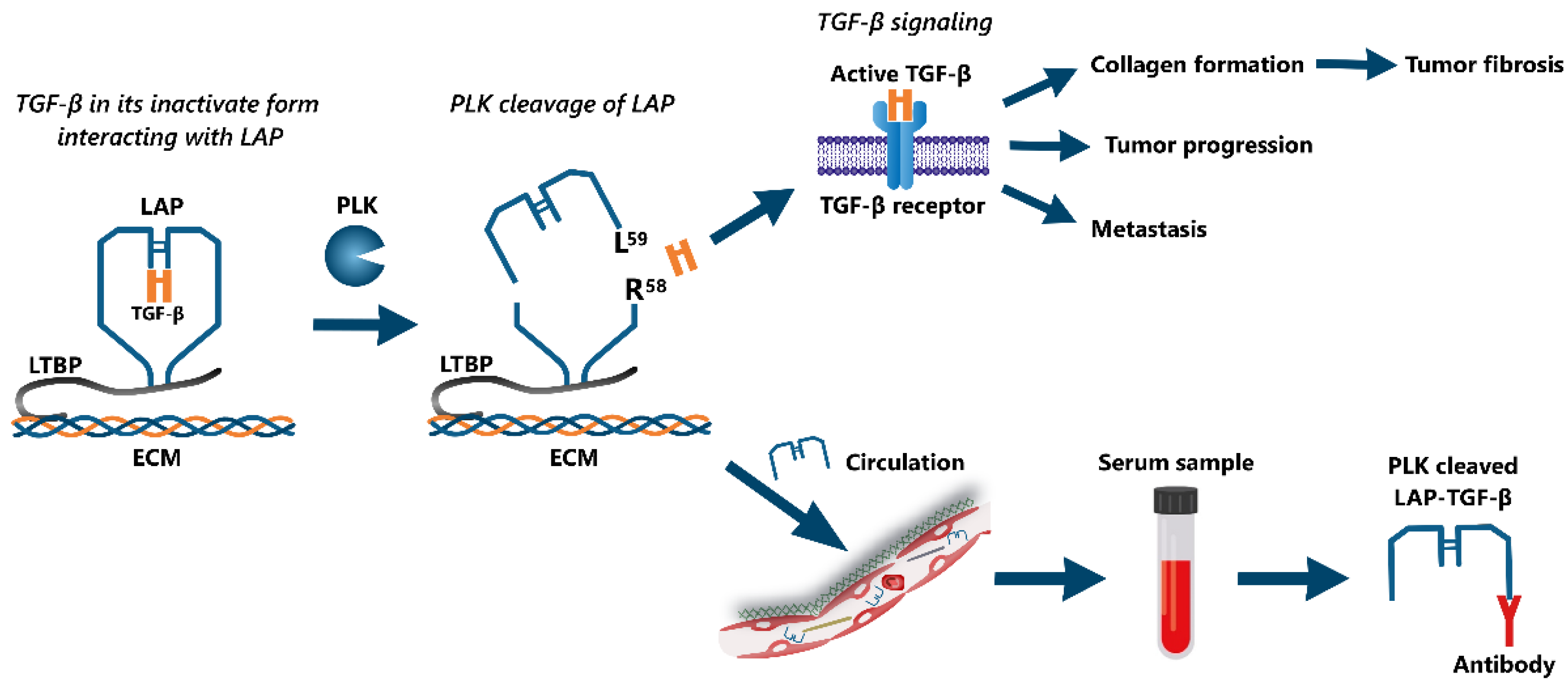
:1. Introduction
2. Materials and Methods
2.1. Target Identification and Antibody Specificity
2.2. Assay Development and Validation
2.3. PLK-Cleaved LAP-TGF-β Assay Protocol
2.4. Assessment of Type III and Type VI Collagen Formation
2.5. Subjects
2.6. Statistics
3. Results
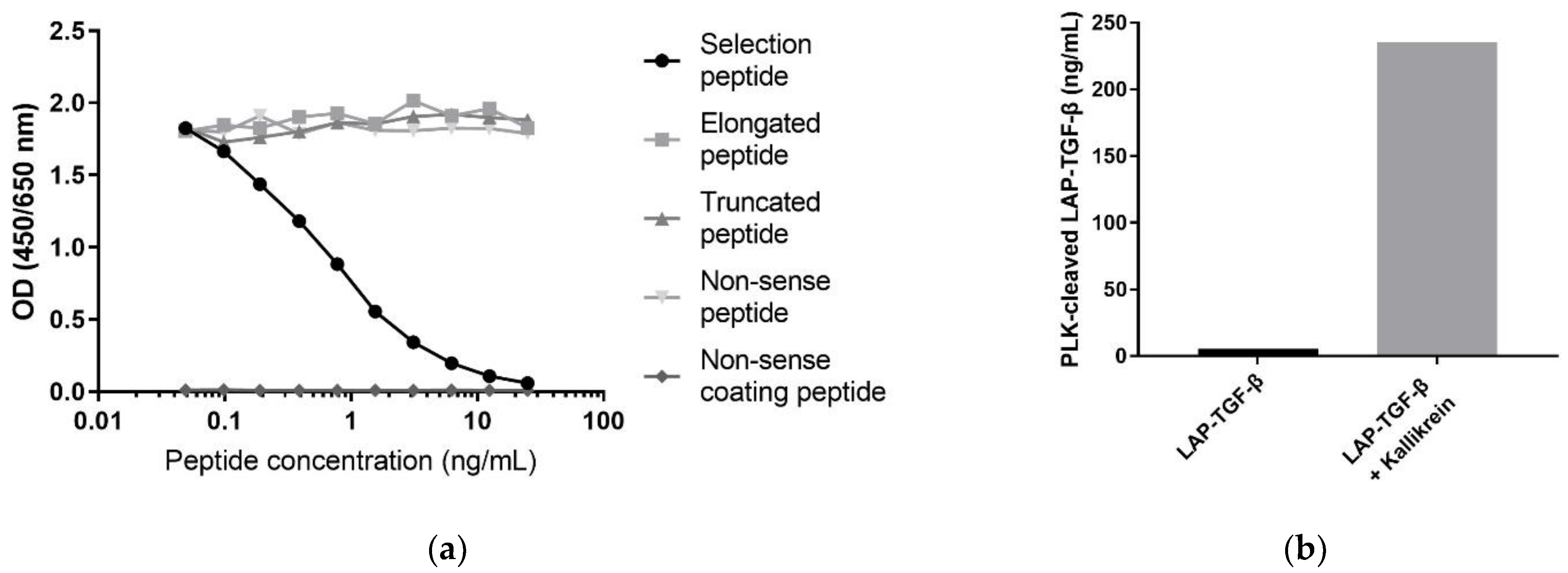
3.1. Technical Evaluation of the PLK-Cleaved LAP-TGF-β ELISA
3.2. Specificity of the PLK-Cleaved LAP-TGF-β ELISA
3.3. PLK-Cleaved LAP-TGF-β Levels Were Correlated in Matched Serum and Plasma Samples
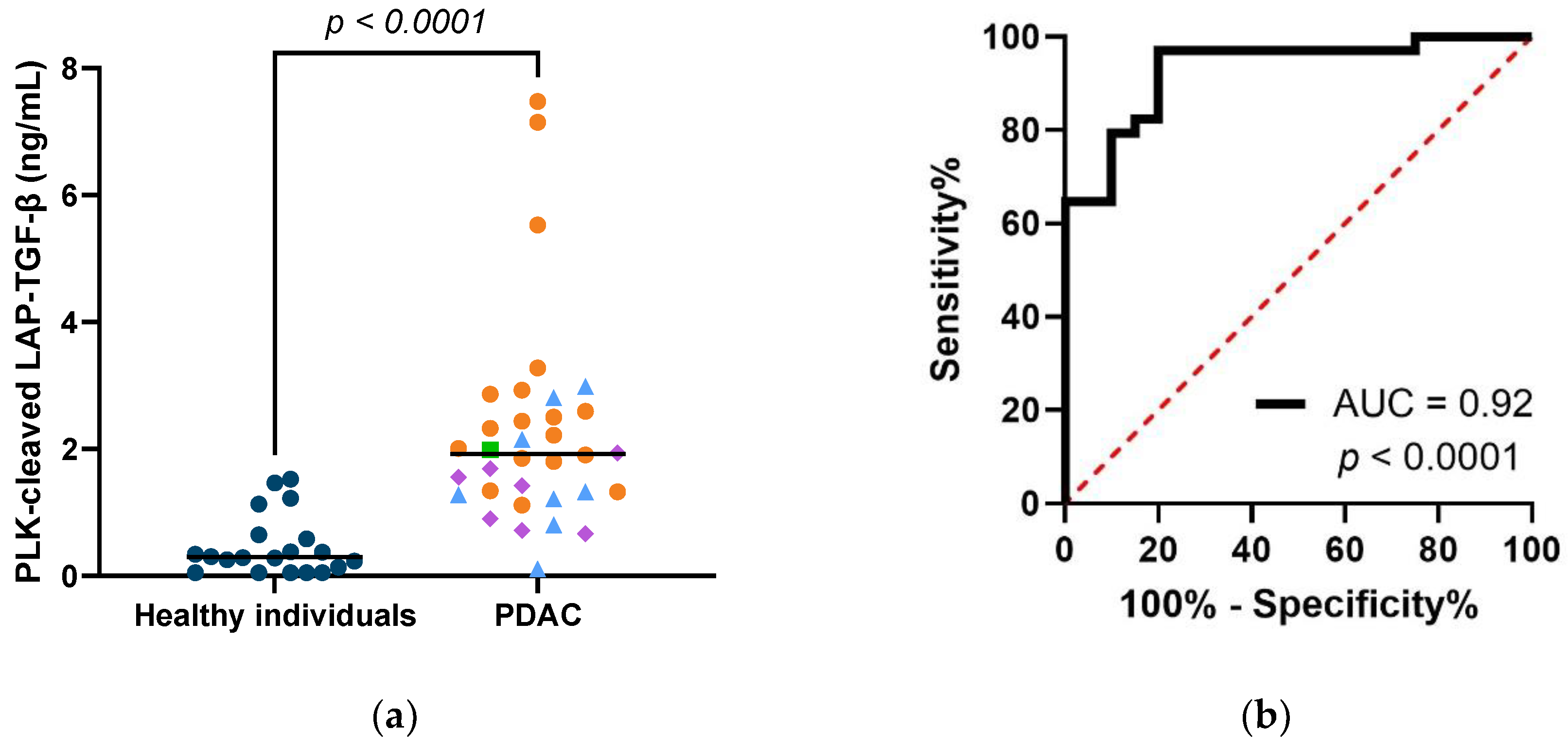
3.4. PLK-Cleaved LAP-TGF-β Is Elevated in Serum from Patients with PDAC, and Shows Diagnostic Potential
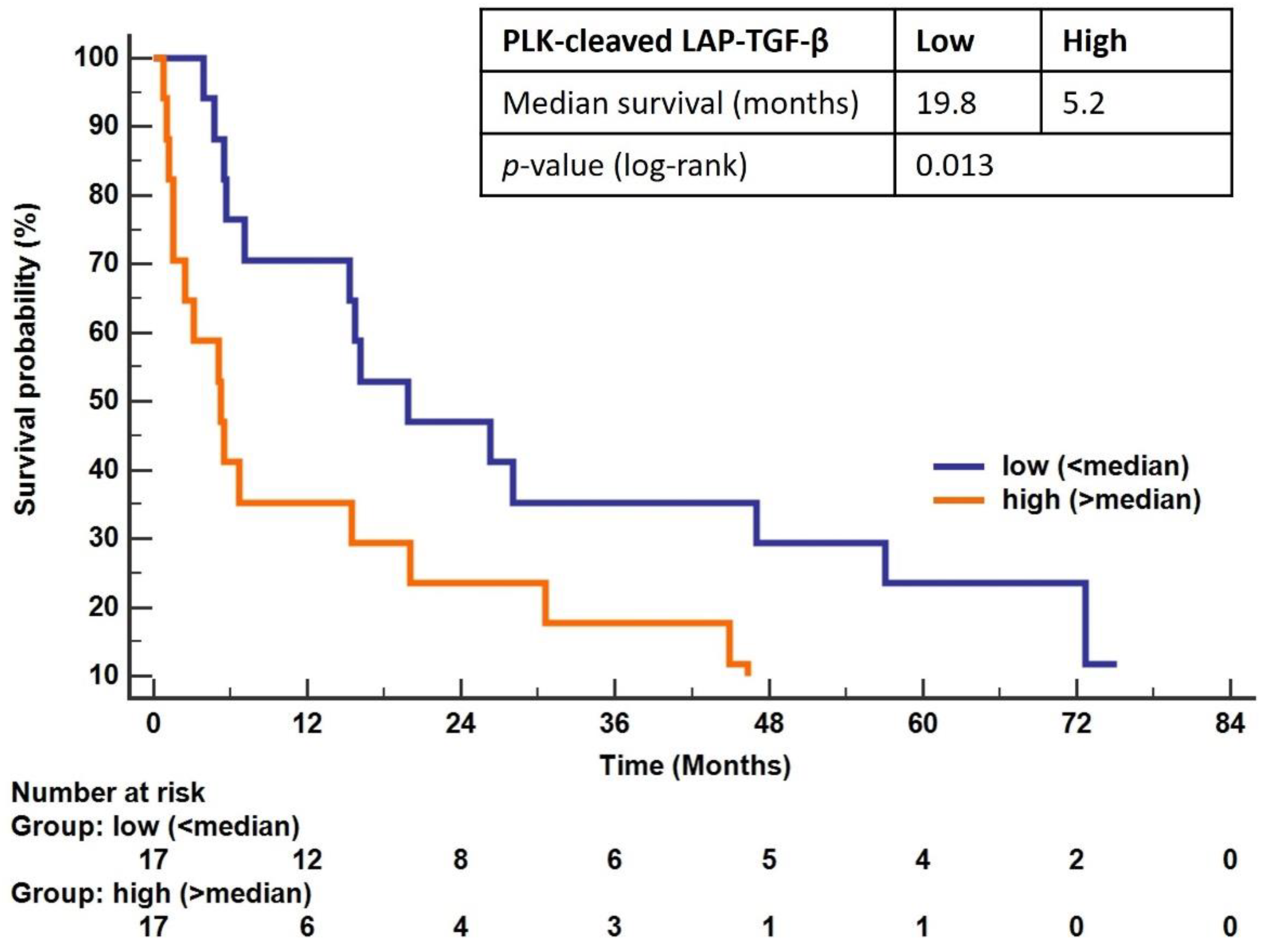
3.5. Overall Survival in Patients with PDAC Is Associated with PLK-Cleaved LAP-TGF-β
3.6. High PLK-Cleaved LAP-TGF-β Levels Associate with Both Collagen Type III and Type VI Formation in Patients with PDAC
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2022. CA. Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Mattiuzzi, C.; Lippi, G. Current Cancer Epidemiology. J. Epidemiol. Glob. Health 2019, 9, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Bulle, A.; Lim, K.H. Beyond Just a Tight Fortress: Contribution of Stroma to Epithelial-Mesenchymal Transition in Pancreatic Cancer. Signal Transduct. Target. Ther. 2020, 5, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Willumsen, N.; Jensen, C.; Green, G.; Nissen, N.I.; Neely, J.; Nelson, D.M.; Pedersen, R.S.; Frederiksen, P.; Chen, I.M.; Boisen, M.K.; et al. Fibrotic Activity Quantified in Serum by Measurements of Type III Collagen Pro-Peptides Can Be Used for Prognosis across Different Solid Tumor Types. Cell. Mol. Life Sci. 2022, 79, 204. [Google Scholar] [CrossRef] [PubMed]
- Feig, C.; Gopinathan, A.; Neesse, A.; Chan, D.S.; Cook, N.; Tuveson, D. a The Pancreas Cancer Microenvironment. Clin. Cancer Res. 2012, 18, 4266–4276. [Google Scholar] [CrossRef]
- Nissen, N.I.; Johansen, A.Z.; Chen, I.; Johansen, J.S.; Pedersen, R.S.; Hansen, C.P.; Karsdal, M.A.; Willumsen, N. Collagen Biomarkers Quantify Fibroblast Activity In Vitro and Predict Survival in Patients with Pancreatic Ductal Adenocarcinoma. Cancers 2022, 14, 819. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Song, E. Turning Foes to Friends: Targeting Cancer-Associated Fibroblasts. Nat. Rev. Drug Discov. 2019, 18, 99–115. [Google Scholar] [CrossRef]
- Meng, X.; Nikolic-Paterson, D.J.; Lan, H.Y. TGF-β: The Master Regulator of Fibrosis. Nat. Rev. Nephrol. 2016, 12, 325–338. [Google Scholar] [CrossRef]
- Rice, L.M.; Padilla, C.M.; McLaughlin, S.R.; Mathes, A.; Ziemek, J.; Goummih, S.; Nakerakanti, S.; York, M.; Farina, G.; Whitfield, M.L.; et al. Fresolimumab Treatment Decreases Biomarkers and Improves Clinical Symptoms in Systemic Sclerosis Patients. J. Clin. Investig. 2015, 125, 2795–2807. [Google Scholar] [CrossRef]
- Schwabe, R.F.; Tabas, I.; Pajvani, U.B. Mechanisms of Fibrosis Development in Nonalcoholic Steatohepatitis. Gastroenterology 2020, 158, 1913–1928. [Google Scholar] [CrossRef]
- Yamamoto, T.; Noble, N.A.; Miller, D.E.; Border, W.A. Sustained Expression of TGF-Β1 Underlies Development of Progressive Kidney Fibrosis. Kidney Int. 1994, 45, 916–927. [Google Scholar] [CrossRef] [PubMed]
- Principe, D.R.; Timbers, K.E.; Atia, L.G.; Koch, R.M.; Rana, A. TGFβ Signaling in the Pancreatic Tumor Microenvironment. Cancers 2021, 13, 5086. [Google Scholar] [CrossRef] [PubMed]
- Seoane, J.; Gomis, R.R. TGF-β Family Signaling in Tumor Suppression and Cancer Progression. Cold Spring Harb. Perspect. Biol. 2017, 9, a022277. [Google Scholar] [CrossRef]
- Ueshima, E.; Fujimori, M.; Kodama, H.; Felsen, D.; Chen, J.; Durack, J.C.; Solomon, S.B.; Coleman, J.A.; Srimathveeravalli, G. Macrophage-Secreted TGF-β 1 Contributes to Fibroblast Activation and Ureteral Stricture after Ablation Injury. Am. J. Physiol. Physiol. 2019, 317, F52–F64. [Google Scholar] [CrossRef] [PubMed]
- Acerbi, I.; Cassereau, L.; Dean, I.; Shi, Q.; Au, A.; Park, C.; Chen, Y.Y.; Liphardt, J.; Hwang, E.S.; Weaver, V.M. Human Breast Cancer Invasion and Aggression Correlates with ECM Stiffening and Immune Cell Infiltration. Integr. Biol. 2015, 7, 1120–1134. [Google Scholar] [CrossRef]
- Nagaraj, N.S.; Datta, P.K. Targeting the Transforming Growth Factor-β Signaling Pathway in Human Cancer. Expert Opin. Investig. Drugs 2010, 19, 77–91. [Google Scholar] [CrossRef]
- Assoian, R.K.; Komoriya, A.; Meyers, C.A.; Miller, D.M.; Sporn, M.B. Transforming Growth Factor-Beta in Human Platelets. Identification of a Major Storage Site, Purification, and Characterization. J. Biol. Chem. 1983, 258, 7155–7160. [Google Scholar] [CrossRef]
- Liu, M.; Fu, X.; Jiang, L.; Ma, J.; Zheng, X.; Wang, S.; Guo, H.; Tian, T.; Nan, K.; Wang, W. Colon Cancer Cells Secreted CXCL11 via RBP-Jκ to Facilitated Tumour-associated Macrophage-induced Cancer Metastasis. J. Cell. Mol. Med. 2021, 25, 10575–10590. [Google Scholar] [CrossRef]
- Hayashi, H.; Sakai, T. Biological Significance of Local TGF-β Activation in Liver Diseases. Front. Physiol. 2012, 3, 1–11. [Google Scholar] [CrossRef]
- Dallas, S.L.; Sivakumar, P.; Jones, C.J.P.; Chen, Q.; Peters, D.M.; Mosher, D.F.; Humphries, M.J.; Kielty, C.M. Fibronectin Regulates Latent Transforming Growth Factor-β (TGFβ) by Controlling Matrix Assembly of Latent TGFβ-Binding Protein-1. J. Biol. Chem. 2005, 280, 18871–18880. [Google Scholar] [CrossRef] [Green Version]
- Lyons, R.M.; Gentry, L.E.; Purchio, A.F.; Moses, H.L. Mechanism of Activation of Latent Recombinant Transforming Growth Factor Beta 1 by Plasmin. J. Cell Biol. 1990, 110, 1361–1367. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Stamenkovic, I. Cell Surface-Localized Matrix Metalloproteinase-9 Proteolytically Activates TGF-β and Promotes Tumor Invasion and Angiogenesis. Genes Dev. 2000, 14, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Bourd-Boittin, K.; Bonnier, D.; Leyme, A.; Mari, B.; Tuffery, P.; Samson, M.; Ezan, F.; Baffet, G.; Theret, N. Protease Profiling of Liver Fibrosis Reveals the ADAM Metallopeptidase with Thrombospondin Type 1 Motif, 1 as a Central Activator of Transforming Growth Factor Beta. Hepatology 2011, 54, 2173–2184. [Google Scholar] [CrossRef]
- Hara, M.; Kirita, A.; Kondo, W.; Matsuura, T.; Nagatsuma, K.; Dohmae, N.; Ogawa, S.; Imajoh-Ohmi, S.; Friedman, S.L.; Rifkin, D.B.; et al. LAP Degradation Product Reflects Plasma Kallikrein-Dependent TGF-β Activation in Patients with Hepatic Fibrosis. Springerplus 2014, 3, 221. [Google Scholar] [CrossRef] [PubMed]
- Hara, M.; Inoue, I.; Yamazaki, Y.; Kirita, A.; Matsuura, T.; Friedman, S.L.; Rifkin, D.B.; Kojima, S. L59 TGF-β LAP Degradation Products Serve as a Promising Blood Biomarker for Liver Fibrogenesis in Mice. Fibrogenesis Tissue Repair 2015, 8, 17. [Google Scholar] [CrossRef]
- O’Brien, P.J.; Ramanathan, R.; Yingling, J.M.; Baselga, J.; Rothenberg, M.L.; Carducci, M.; Daly, T.; Adcock, D.; Lahn, M. Analysis and Variability of TGFbeta Measurements in Cancer Patients with Skeletal Metastases. Biologics 2008, 2, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Mancini, D.; Monteagudo, J.; Suárez-Fariñas, M.; Bander, J.; Varshney, R.; Gonzalez, J.; Coller, B.S.; Ahamed, J. New Methodologies to Accurately Assess Circulating Active Transforming Growth Factor-Β1 Levels: Implications for Evaluating Heart Failure and the Impact of Left Ventricular Assist Devices. Transl. Res. 2018, 192, 15–29. [Google Scholar] [CrossRef]
- Yokoyama, H.; Masaki, T.; Inoue, I.; Nakamura, M.; Mezaki, Y.; Saeki, C.; Oikawa, T.; Saruta, M.; Takahashi, H.; Ikegami, M.; et al. Histological and Biochemical Evaluation of Transforming Growth Factor-β Activation and Its Clinical Significance in Patients with Chronic Liver Disease. Heliyon 2019, 5, e01231. [Google Scholar] [CrossRef]
- Kropf, J.; Schurek, J.O.; Wollner, A.; Gressner, A.M. Immunological Measurement of Transforming Growth Factor-Beta I (TGF- Β1) in Blood; Assay Development and Comparison. Clin. Chem. 1997, 43, 1965–1974. [Google Scholar] [CrossRef]
- Coffey, R.J.; Kost, L.J.; Lyons, R.M.; Moses, H.L.; LaRusso, N.F. Hepatic Processing of Transforming Growth Factor Beta in the Rat. Uptake, Metabolism, and Biliary Excretion. J. Clin. Investig. 1987, 80, 750–757. [Google Scholar] [CrossRef] [Green Version]
- Teraoka, R.; Hara, M.; Kikuta, K.; Hirooka, Y.; Furutani, Y.; Shimosegawa, T.; Masamune, A.; Kojima, S. Plasma Kallikrein-Dependent Transforming Growth Factor-β Activation in Patients With Chronic Pancreatitis and Pancreatic Cancer. Pancreas 2017, 46, e20–e22. [Google Scholar] [CrossRef] [PubMed]
- Combet, C.; Blanchet, C.; Geourjon, C.; Deléage, G. NPS@: Network Protein Sequence Analysis. Trends Biochem. Sci. 2000, 25, 147–150. [Google Scholar] [CrossRef]
- Nissen, N.I.; Kehlet, S.; Johansen, A.Z.; Chen, I.M.; Karsdal, M.; Johansen, J.S.; Diab, H.M.H.; Jørgensen, L.N.; Sun, S.; Manon-Jensen, T.; et al. Noninvasive Prognostic Biomarker Potential of Quantifying the Propeptides of Type XI Collagen Alpha-1 Chain (PRO-C11) in Patients with Pancreatic Ductal Adenocarcinoma. Int. J. Cancer 2021, 149, 228–238. [Google Scholar] [CrossRef] [PubMed]
- Gefter, M.L.; Margulies, D.H.; Scharff, M.D. A Simple Method for Polyethylene Glycol-Promoted Hybridization of Mouse Myeloma Cells. Somatic Cell Genet. 1977, 3, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.J.; Nedergaard, A.F.; Sun, S.; Veidal, S.S.; Larsen, L.; Zheng, Q.; Suetta, C.; Henriksen, K.; Christiansen, C.; Karsdal, M.A.; et al. The Neo-Epitope Specific PRO-C3 ELISA Measures True Formation of Type III Collagen Associated with Liver and Muscle Parameters. Am. J. Transl. Res. 2013, 5, 303–315. [Google Scholar] [PubMed]
- Sun, S.; Henriksen, K.; Karsdal, M.A.; Byrjalsen, I.; Rittweger, J.; Armbrecht, G.; Belavy, D.L.; Felsenberg, D.; Nedergaard, A.F. Collagen Type III and VI Turnover in Response to Long-Term Immobilization. PLoS ONE 2015, 10, e0144525. [Google Scholar] [CrossRef]
- Chen, I.M.; Willumsen, N.; Dehlendorff, C.; Johansen, A.Z.; Jensen, B.V.; Hansen, C.P.; Hasselby, J.P.; Bojesen, S.E.; Pfeiffer, P.; Nielsen, S.E.; et al. Clinical Value of Serum Hyaluronan and Propeptide of Type III Collagen in Patients with Pancreatic Cancer. Int. J. Cancer 2020, 146, 2913–2922. [Google Scholar] [CrossRef]
- Chen, I.; Jensen, B.V.; Bojesen, S.E.; Johansen, A.Z.; Schultz, N.A.; Hansen, C.P.; Hasselby, J.P.; Holl, N.H.; Nissen, M.H.B.; Bjerregaard, J.K.; et al. Identification of New Biomarkers in Patients with Pancreatic Cancer (BIOPAC): A Study Protocol of an Open Cohort Study. Cancer Sci. Ther. 2019, 11, 232–239. [Google Scholar]
- Meyer, A.; Wang, W.; Qu, J.; Croft, L.; Degen, J.L.; Coller, B.S.; Ahamed, J. Platelet TGF-Β1 Contributions to Plasma TGF-Β1, Cardiac Fibrosis, and Systolic Dysfunction in a Mouse Model of Pressure Overload. Blood 2012, 119, 1064–1074. [Google Scholar] [CrossRef]
- Grainger, D.J.; Mosedale, D.E.; Metcalfe, J.C.; Weissberg, P.L.; Kemp, P.R. Active and Acid-Activatable TGF-β in Human Sera, Platelets and Plasma. Clin. Chim. Acta 1995, 235, 11–31. [Google Scholar] [CrossRef]
- Willumsen, N.; Bager, C.; Karsdal, M.A. Matrix Metalloprotease Generated Fragments of Type VI Collagen Have Serum Biomarker Potential in Cancer–A Proof of Concept Study. Transl. Oncol. 2019, 12, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Kerbert, A.J.C.; Gupta, S.; Alabsawy, E.; Dobler, I.; Lønsmann, I.; Hall, A.; Nielsen, S.H.; Nielsen, M.J.; Gronbaek, H.; Amoros, À.; et al. Biomarkers of Extracellular Matrix Formation Are Associated with Acute-on-Chronic Liver Failure. JHEP Reports 2021, 3, 100355. [Google Scholar] [CrossRef] [PubMed]
- Rønnow, S.R.; Dabbagh, R.Q.; Genovese, F.; Nanthakumar, C.B.; Barrett, V.J.; Good, R.B.; Brockbank, S.; Cruwys, S.; Jessen, H.; Sorensen, G.L.; et al. Prolonged Scar-in-a-Jar: An in Vitro Screening Tool for Anti-Fibrotic Therapies Using Biomarkers of Extracellular Matrix Synthesis. Respir. Res. 2020, 21, 108. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Liang, Y.; Yin, Q.; Liu, S.; Wang, Q.; Tang, Y.; Cao, C. Clinical and Prognostic Significance of Serum Transforming Growth Factor-Beta1 Levels in Patients with Pancreatic Ductal Adenocarcinoma. Braz. J. Med. Biol. Res. 2016, 49. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Bang, J.; Nam, A.; Park, J.E.; Jin, M.H.; Bang, Y.; Oh, D. The Prognostic Role of Soluble TGF-beta and Its Dynamics in Unresectable Pancreatic Cancer Treated with Chemotherapy. Cancer Med. 2020, 9, 43–51. [Google Scholar] [CrossRef]
- Li, J.; Shen, C.; Wang, X.; Lai, Y.; Zhou, K.; Li, P.; Liu, L.; Che, G. Prognostic Value of TGF-β in Lung Cancer: Systematic Review and Meta-Analysis. BMC Cancer 2019, 19, 691. [Google Scholar] [CrossRef]
- Lin, T.-H.; Shao, Y.-Y.; Chan, S.-Y.; Huang, C.-Y.; Hsu, C.-H.; Cheng, A.-L. High Serum Transforming Growth Factor-Β1 Levels Predict Outcome in Hepatocellular Carcinoma Patients Treated with Sorafenib. Clin. Cancer Res. 2015, 21, 3678–3684. [Google Scholar] [CrossRef]
- Rouce, R.H.; Shaim, H.; Sekine, T.; Weber, G.; Ballard, B.; Ku, S.; Barese, C.; Murali, V.; Wu, M.-F.; Liu, H.; et al. The TGF-β/SMAD Pathway Is an Important Mechanism for NK Cell Immune Evasion in Childhood B-Acute Lymphoblastic Leukemia. Leukemia 2016, 30, 800–811. [Google Scholar] [CrossRef]
- Teixeira, A.F.; ten Dijke, P.; Zhu, H.-J. On-Target Anti-TGF-β Therapies Are Not Succeeding in Clinical Cancer Treatments: What Are Remaining Challenges? Front. Cell Dev. Biol. 2020, 8, 1–18. [Google Scholar] [CrossRef]
- Ciardiello, D.; Elez, E.; Tabernero, J.; Seoane, J. Clinical Development of Therapies Targeting TGFβ: Current Knowledge and Future Perspectives. Ann. Oncol. 2020, 31, 1336–1349. [Google Scholar] [CrossRef]
- Kim, B.-G.; Malek, E.; Choi, S.H.; Ignatz-Hoover, J.J.; Driscoll, J.J. Novel Therapies Emerging in Oncology to Target the TGF-β Pathway. J. Hematol. Oncol. 2021, 14, 55. [Google Scholar] [CrossRef] [PubMed]
- Huynh, L.; Hipolito, C.; ten Dijke, P. A Perspective on the Development of TGF-β Inhibitors for Cancer Treatment. Biomolecules 2019, 9, 743. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Clinical Variables | Patients with PDAC (n = 39) | Healthy Individuals (n = 20) |
|---|---|---|
| Age (years), Median (min–max) | 69 (52–79) | 58 (45–72) |
| Gender, n (%) Male Female | 17 (50%) 17 (50%) | 10 (50%) 10 (50%) |
| Number of metastatic sites, n (%) 0 site ≥1 site | 16 (47%) 18 (53%) | |
| Body mass index (BMI), Median (min-max) | 23 (16–31) | |
| Stage 1 2 3 4 | 1 (3%) 7 (21%) 8 (24%) 18 (53%) | |
| Performance status, n (%) 0 1 2 Unknown | 13 (38%) 13 (38%) 3 (9%) 5 (15%) |
| Test | Results |
|---|---|
| IC50 | 0.34 ng/mL |
| Measurement range (LLMR-ULMR) | 0.05–2.2 ng/mL |
| Detection range (LLOD-ULOD) | 0.02–10.8 ng/mL |
| Dilution recovery (1:2, 1:4, 1:8) | 115%, 114%, 101% |
| Spiking recovery (serum in serum) | 94% |
| Interference (Hemoglobin, low/high conc) | 101%/100% |
| Interference (biotin, low/high conc) | 102%/105% |
| Interference (lipids, low/high conc) | 98%/100% |
| Inter-assay variation | 11% |
| Intra-assay variation | 3% |
| Analyte stability (48 h 4 °C/24 h 20 °C) | 98%/88% |
| Freeze/thaw stability up to four cycles | 113% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pedersen, R.S.; Nissen, N.I.; Jensen, C.; Thorlacius-Ussing, J.; Manon-Jensen, T.; Olesen, M.L.; Langholm, L.L.; Diab, H.M.H.; Jorgensen, L.N.; Hansen, C.P.; et al. Plasma Kallikrein-Activated TGF-β Is Prognostic for Poor Overall Survival in Patients with Pancreatic Ductal Adenocarcinoma and Associates with Increased Fibrogenesis. Biomolecules 2022, 12, 1315. https://doi.org/10.3390/biom12091315
Pedersen RS, Nissen NI, Jensen C, Thorlacius-Ussing J, Manon-Jensen T, Olesen ML, Langholm LL, Diab HMH, Jorgensen LN, Hansen CP, et al. Plasma Kallikrein-Activated TGF-β Is Prognostic for Poor Overall Survival in Patients with Pancreatic Ductal Adenocarcinoma and Associates with Increased Fibrogenesis. Biomolecules. 2022; 12(9):1315. https://doi.org/10.3390/biom12091315
Chicago/Turabian StylePedersen, Rasmus S., Neel I. Nissen, Christina Jensen, Jeppe Thorlacius-Ussing, Tina Manon-Jensen, Majken L. Olesen, Lasse L. Langholm, Hadi M. H. Diab, Lars N. Jorgensen, Carsten P. Hansen, and et al. 2022. "Plasma Kallikrein-Activated TGF-β Is Prognostic for Poor Overall Survival in Patients with Pancreatic Ductal Adenocarcinoma and Associates with Increased Fibrogenesis" Biomolecules 12, no. 9: 1315. https://doi.org/10.3390/biom12091315






