Exploring Biomolecular Self-Assembly with Far-Infrared Radiation
Abstract
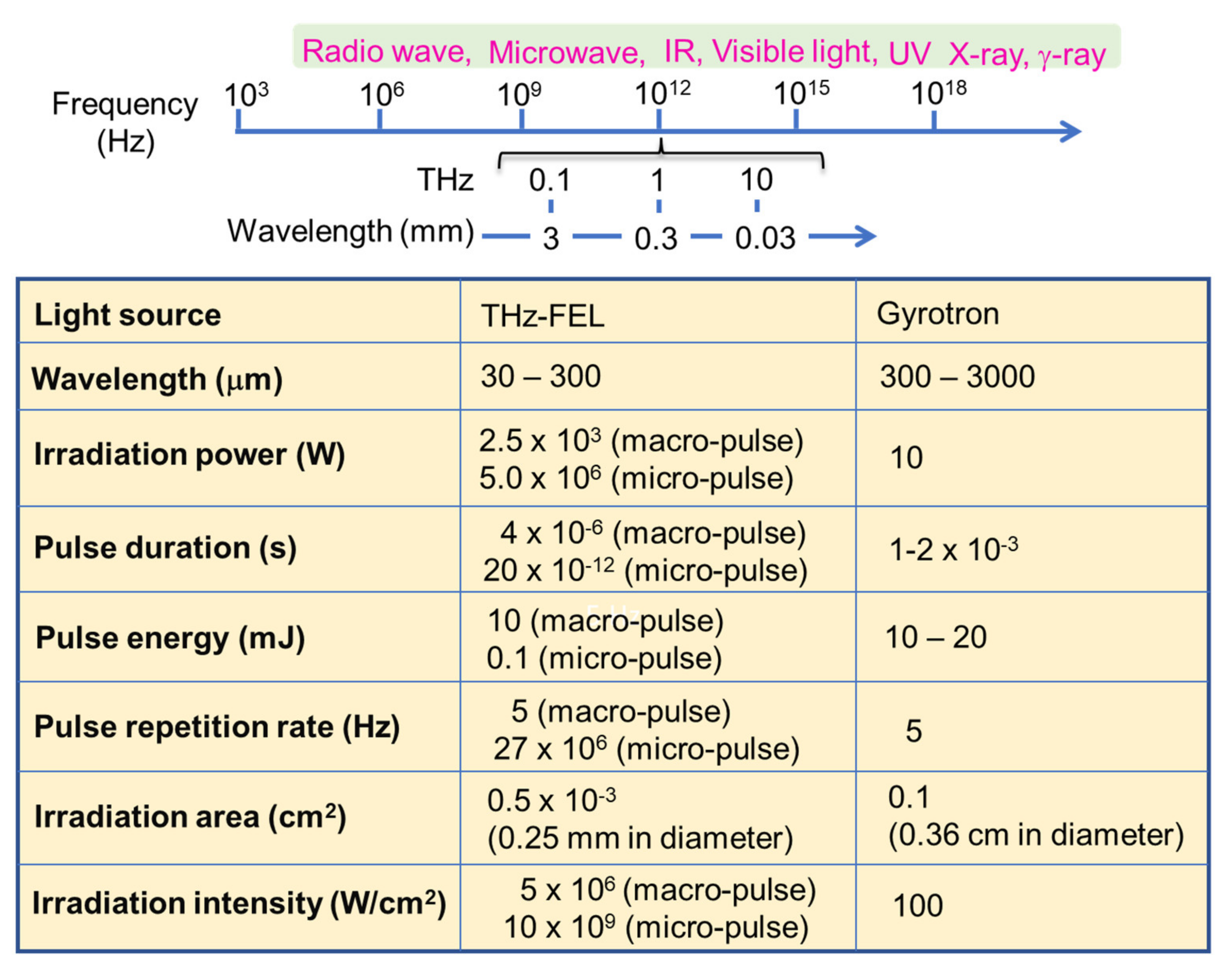
:1. Introduction
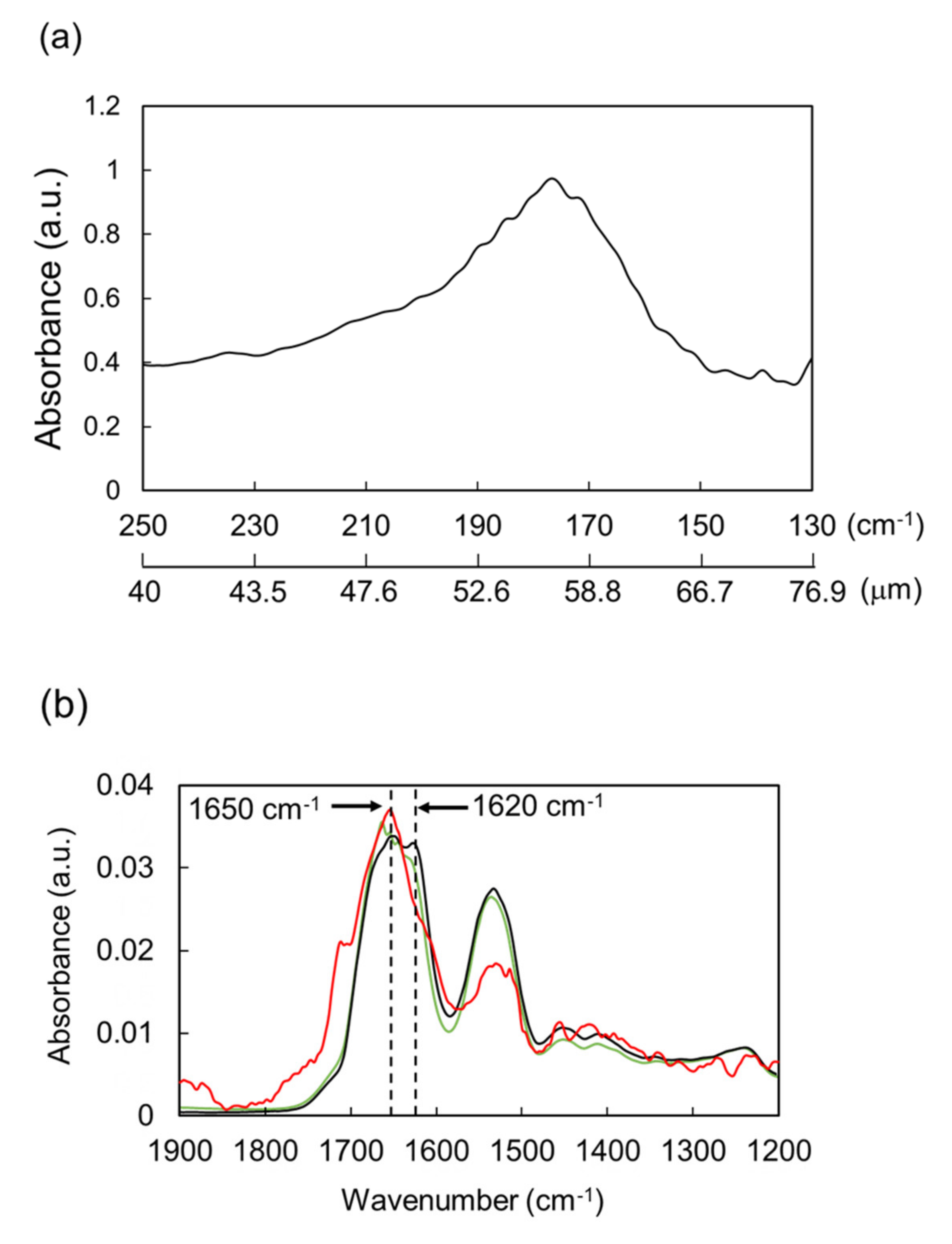
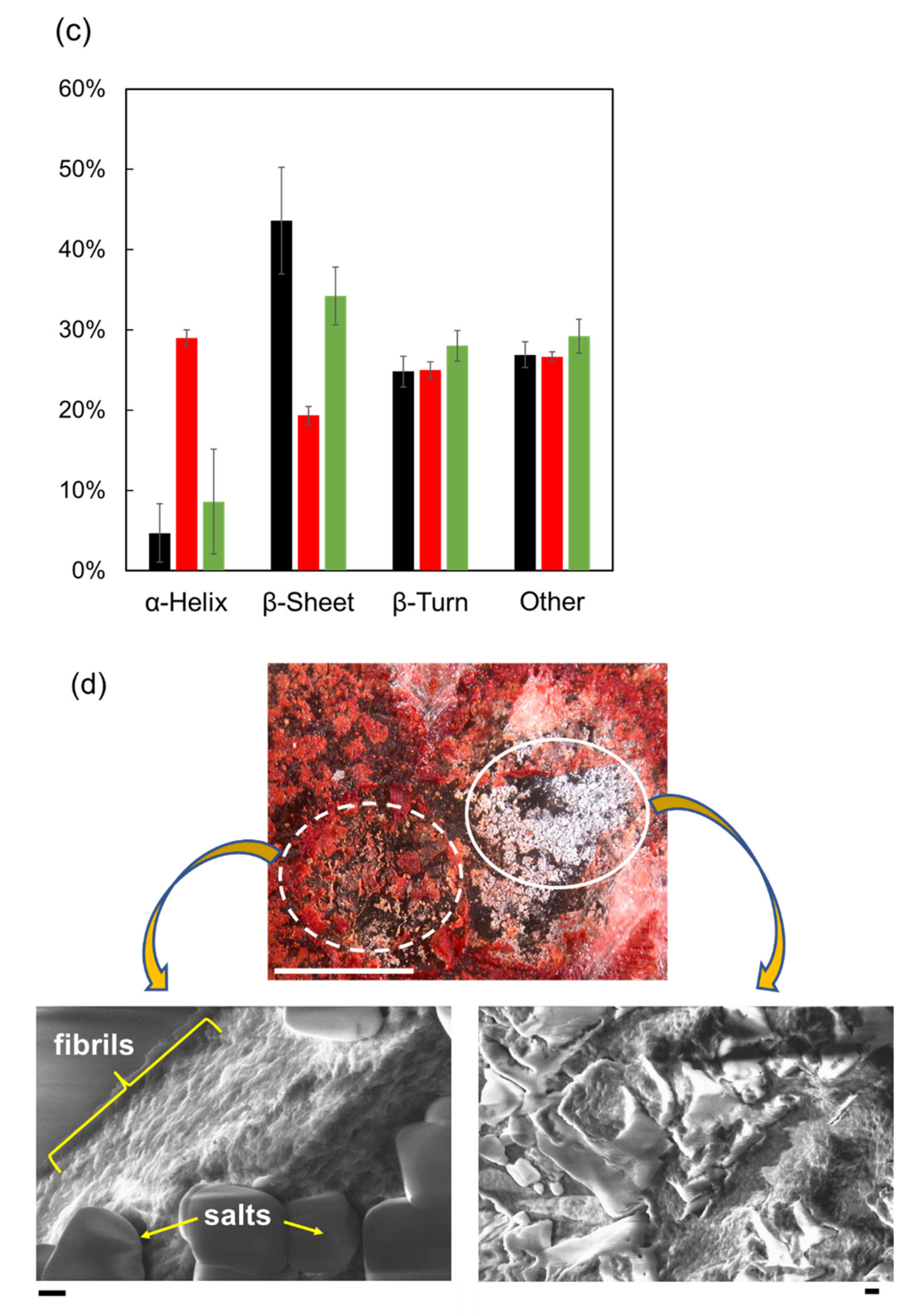
2. Dissociation of Amyloid Fibril by THz-FEL Irradiation
3. Promotion of Amyloid Fibrillation by the Submillimeter Wave from Gyrotron
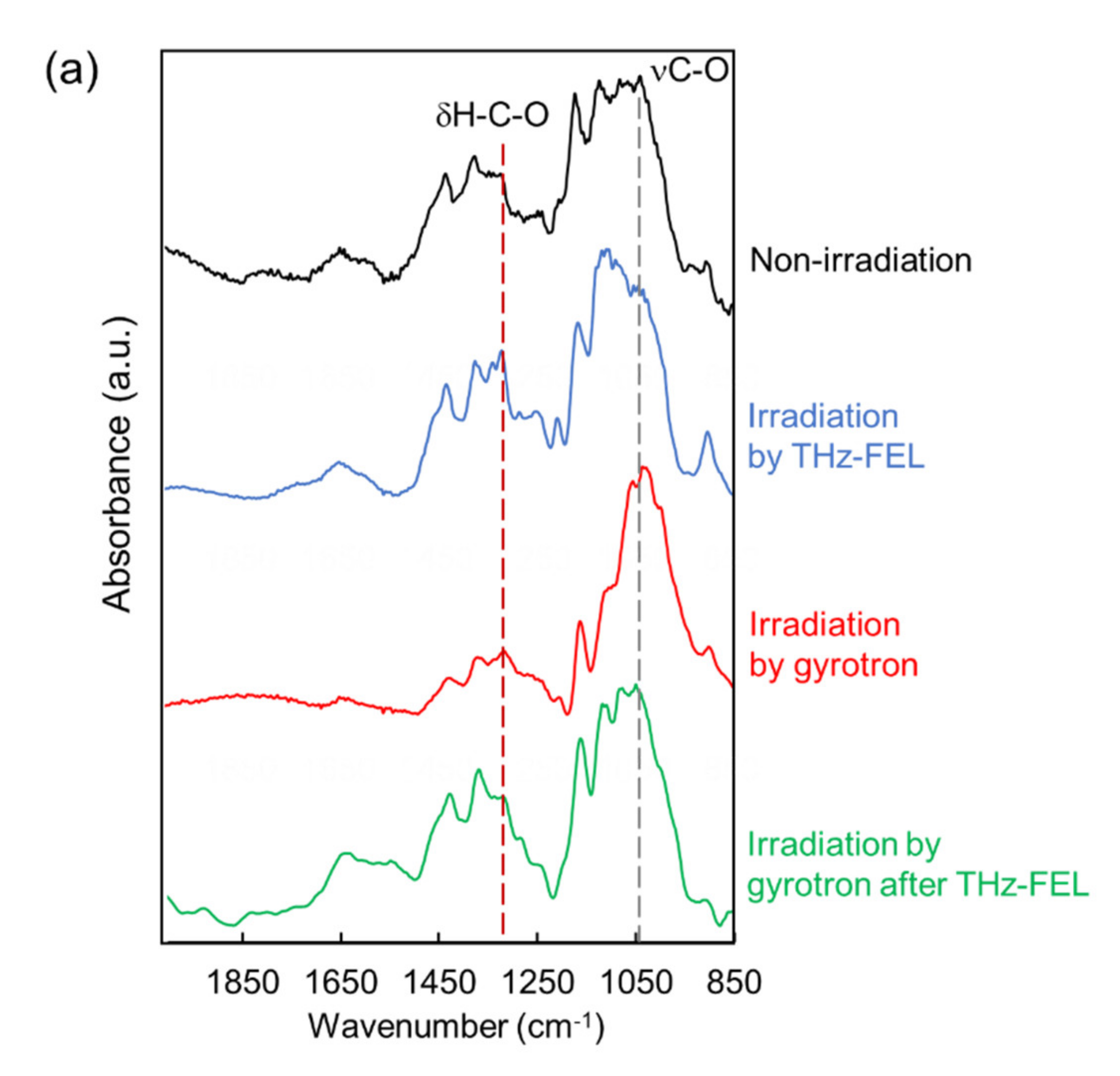
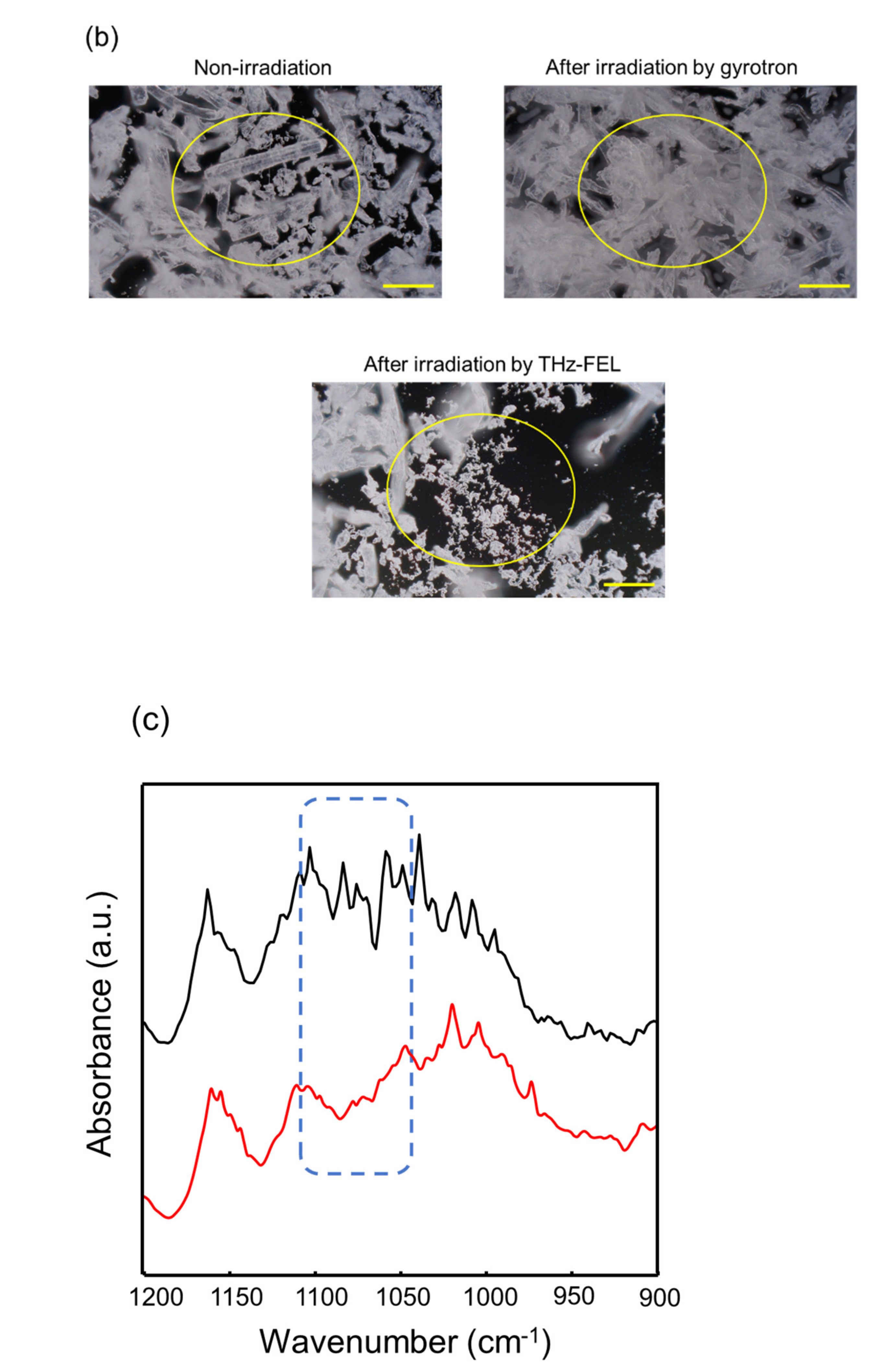
4. Dissociation and Re-Association of Cellulose Fiber with Far-Infrared Radiation
5. Future Aspect of the Use of Far-Infrared Radiation in Biological and Material Fields
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pedersen, S.L.; Tofteng, A.P.; Malik, L.; Jensen, K.J. Microwave heating in solid-phase peptide synthesis. Chem. Soc. Rev. 2011, 41, 1826–1844. [Google Scholar] [CrossRef] [PubMed]
- Horikoshi, S.; Serpone, N. Microwave Flow Chemistry as a Methodology in Organic Syntheses, Enzymatic Reactions, and Nanoparticle Syntheses. Chem. Rec. 2018, 19, 118–139. [Google Scholar] [CrossRef] [PubMed]
- Wilmink, G.J.; Rivest, B.D.; Roth, C.C.; Ibey, B.L.; Payne, J.A.; Cundin, L.X.; Grundt, J.E.; Peralta, X.; Mixon, D.G.; Roach, W.P. In vitro investigation of the biological effects associated with human dermal fibroblasts exposed to 2.52 THz radiation. Lasers Surg. Med. 2010, 43, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Vatansever, F.; Hamblin, M.R. Far infrared radiation (FIR): Its biological effects and medical applications. Photon- Lasers Med. 2012, 1, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Chiang, I.N.; Pu, Y.S.; Huang, C.Y.; Young, T.H. Far infrared radiation promotes rabbit renal proximal tubule cell proliferation and functional characteristics, and protects against cisplatin-induced nephrotoxicity. PLoS ONE 2017, 12, e0180872. [Google Scholar] [CrossRef]
- Tewari, P.; Garritano, J.; Bajwa, N.; Sung, S.; Huang, H.; Wang, D.; Grundfest, W.; Ennis, D.B.; Ruan, D.; Brown, E.; et al. Methods for registering and calibrating in vivo terahertz images of cutaneous burn wounds. Biomed. Opt. Express 2018, 10, 322–337. [Google Scholar] [CrossRef]
- Nibali, V.C.; Havenith, M. New Insights into the Role of Water in Biological Function: Studying Solvated Biomolecules Using Terahertz Absorption Spectroscopy in Conjunction with Molecular Dynamics Simulations. J. Am. Chem. Soc. 2014, 136, 12800–12807. [Google Scholar] [CrossRef]
- Wilmink, G.J.; Grundt, J.E. Invited Review Article: Current State of Research on Biological Effects of Terahertz Radiation. J. Infrared Millim. Terahertz Waves 2011, 32, 1074–1122. [Google Scholar] [CrossRef]
- Irizawa, A.; Fujimoto, M.; Kawase, K.; Kato, R.; Fujiwara, H.; Higashiya, A.; Macis, S.; Tomarchio, L.; Lupi, S.; Marcelli, A.; et al. Spatially Resolved Spectral Imaging by A THz-FEL. Condens. Matter 2020, 5, 38. [Google Scholar] [CrossRef]
- Kawasaki, T.; Tsukiyama, K.; Irizawa, A. Dissolution of a fibrous peptide by terahertz free electron laser. Sci. Rep. 2019, 9, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Kawasaki, T.; Yamaguchi, Y.; Ueda, T.; Ishikawa, Y.; Yaji, T.; Ohta, T.; Tsukiyama, K.; Idehara, T.; Saiki, M.; Tani, M. Irradi-ation effect of a submillimeter wave from 420 GHz gyrotron on amyloid peptides in vitro. Biomed. Opt. Express 2020, 11, 5341–5351. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, T.; Yamaguchi, Y.; Kitahara, H.; Irizawa, A.; Tani, M. Regulation of amyloid fibrils by high-power terahertz radiation. J. Jpn. Soc. Infrared Sci. Technol. 2022, 31, 52–59. [Google Scholar]
- Isoyama, G. Development of a Free-Electron Laser in the Terahertz Region. JAS4QoL 2020, 6, 1–10. [Google Scholar]
- Idehara, T.; Sabchevski, S.P.; Glyavin, M.; Mitsudo, S. The Gyrotrons as Promising Radiation Sources for THz Sensing and Imaging. Appl. Sci. 2020, 10, 980. [Google Scholar] [CrossRef]
- Ryan, H.; van Bentum, J.; Maly, T. A ferromagnetic shim insert for NMR magnets – Towards an integrated gyrotron for DNP-NMR spectroscopy. J. Magn. Reson. 2017, 277, 1–7. [Google Scholar] [CrossRef]
- Nanni, E.A.; Barnes, A.B.; Griffin, R.G.; Temkin, R.J. THz Dynamic Nuclear Polarization NMR. IEEE Trans. Terahertz Sci. Technol. 2011, 1, 145–163. [Google Scholar] [CrossRef]
- Maly, T.; Debelouchina, G.T.; Bajaj, V.S.; Hu, K.-N.; Joo, C.-G.; Mak–Jurkauskas, M.L.; Sirigiri, J.R.; van der Wel, P.C.A.; Herzfeld, J.; Temkin, R.J.; et al. Dynamic nuclear polarization at high magnetic fields. J. Chem. Phys. 2008, 128, 052211. [Google Scholar] [CrossRef]
- Mattsson, M.-O.; Simkó, M. Emerging medical applications based on non-ionizing electromagnetic fields from 0 Hz to 10 THz. Med. Devices-Evid. Res. 2019, 12, 347–368. [Google Scholar] [CrossRef]
- Kok, H.P.; Cressman, E.N.K.; Ceelen, W.; Brace, C.L.; Ivkov, R.; Grüll, H.; ter Haar, G.; Wust, P.; Crezee, J. Heating technology for malignant tumors: A review. Int. J. Hyperthermia. 2020, 37, 711–741. [Google Scholar] [CrossRef]
- Fändrich, M.; Nyström, S.; Nilsson, K.P.R.; Böckmann, A.; LeVine III, H.; Hammarström, P. Amyloid fibril polymorphism - a challenge for molecular imaging and therapy. J. Intern. Med. 2018, 283, 218–237. [Google Scholar] [CrossRef]
- Chuang, E.; Hori, A.M.; Hesketh, C.D.; Shorter, J. Amyloid assembly and disassembly. J. Cell Sci. 2018, 131, jcs189928. [Google Scholar] [CrossRef] [PubMed]
- Erskine, E.; MacPhee, C.E.; Stanley-Wall, N.R. Functional Amyloid and Other Protein Fibers in the Biofilm Matrix. J. Mol. Biol. 2018, 430, 3642–3656. [Google Scholar] [CrossRef] [PubMed]
- Fowler, D.M.; Koulov, A.V.; Balch, W.E.; Kelly, J.W. Functional amyloid – from bacteria to humans. Trends Biochem. Sci. 2007, 32, 5. [Google Scholar] [CrossRef] [PubMed]
- Fowler, D.M.; Koulov, A.V.; Alory-Jost, C.; Marks, M.; E Balch, W.; Kelly, J.W. Functional Amyloid Formation within Mammalian Tissue. PLoS Biol. 2005, 4, e6. [Google Scholar] [CrossRef]
- Siniukova, V.A.; Sopova, J.V.; Galkina, S.A.; Galkin, A.P. Search for functional amyloid structures in chicken and fruit fly female reproductive cells. Prion 2020, 14, 278–282. [Google Scholar] [CrossRef]
- Sun, H.; Marelli, B. Polypeptide templating for designer hierarchical materials. Nat. Commun. 2020, 11, 1–13. [Google Scholar] [CrossRef]
- True, H.L.; Lindquist, S.L. A yeast prion provides a mechanism for genetic variation and phenotypic diversity. Nature 2000, 407, 477–483. [Google Scholar] [CrossRef]
- Das, S.; Jacob, R.S.; Patel, K.; Singh, N.; Maji, S.K. Amyloid Fibrils: Versatile Biomaterials for Cell Adhesion and Tissue Engineering Applications. Biomacromolecules 2018, 19, 1826–1839. [Google Scholar] [CrossRef]
- Hilderbrand, A.M.; Ford, E.N.; Guo, C.; Sloppy, J.D.; Kloxin, A.M. Hierarchically structured hydrogels utilizing multifunctional assembling peptides for 3D cell culture. Biomater. Sci. 2020, 8, 1256–1269. [Google Scholar] [CrossRef]
- Kokotidou, C.; Jonnalagadda, S.V.R.; Orr, A.A.; Vrentzos, G.; Kretsovali, A.; Tamamis, P.; Mitraki, A. Designer Amyloid Cell-Penetrating Peptides for Potential Use as Gene Transfer Vehicles. Biomolecules 2019, 10, 7. [Google Scholar] [CrossRef]
- Barros, S.; Whitaker, S.K.; Sukthankar, P.; Avila, L.A.; Gudlur, S.; Warner, M.; Beltrão, E.I.; Tomich, J.M. A review of solute encapsulating nanoparticles used as delivery systems with emphasis on branched amphipathic peptide capsules. Arch. Biochem. Biophys. 2016, 596, 22–42. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Zhao, J.; Han, Q.; Hu, X.; Wang, D.; Zhang, X.; Yang, P. One-Step Assembly of a Biomimetic Biopolymer Coating for Particle Surface Engineering. Adv. Mater. 2018, 30, e1802851. [Google Scholar] [CrossRef] [PubMed]
- Krebs, M.R.; Wilkins, D.K.; Chung, E.W.; Pitkeathly, M.C.; Chamberlain, A.K.; Zurdo, J.; Robinson, C.; Dobson, C.M. Formation and seeding of amyloid fibrils from wild-type hen lysozyme and a peptide fragment from the β-domain. J. Mol. Biol. 2000, 300, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, T.; Izumi, Y.; Ohori, G.; Kitahara, H.; Furuya, T.; Yamamoto, K.; Matsuo, K.; Tani, M.; Tsukiyama, K. Study on Irradiation Effect of Mid-Infrared Free Electron Laser on Hen Egg-White Lysozyme by Using Terahertz-Time Domain Spec-troscopy and Synchrotron-Radiation Vacuum-Ultraviolet Circular-Dichroism Spectroscopy. J. Infrared Milli. Terahz. Waves 2019, 40, 998–1009. [Google Scholar]
- Usoltsev, D.; Sitnikova, V.; Kajava, A.; Uspenskaya, M. Systematic FTIR Spectroscopy Study of the Secondary Structure Changes in Human Serum Albumin under Various Denaturation Conditions. Biomolecules 2019, 9, 359. [Google Scholar] [CrossRef]
- Komatsu, H.; Liu, L.; Murray, I.V.J.; Axelsen, P.H. A mechanistic link between oxidative stress and membrane mediated am-yloidogenesis revealed by infrared spectroscopy. Biochim. Biophys. Acta 2007, 1768, 1913–1922. [Google Scholar] [CrossRef]
- Fawzi, M.M.; Gawdat, H.I.; Mahmoud, S.B.; El-Hawary, M.S.; Rashed, L.A.; Esmat, S.M. Fractional Carbon Dioxide Laser is Effective in Amelioration of Pruritus in Primary Cutaneous Amyloidosis: A Clinical and Biochemical Study. Lasers Surg. Med. 2020, 53, 482–487. [Google Scholar] [CrossRef]
- Giraldo, R. Amyloid Assemblies: Protein Legos at a Crossroads in Bottom-Up Synthetic Biology. ChemBioChem 2010, 11, 2347–2357. [Google Scholar] [CrossRef]
- Narayanan, T.; Konovalov, O. Synchrotron Scattering Methods for Nanomaterials and Soft Matter Research. Materials 2020, 13, 752. [Google Scholar] [CrossRef]
- Squires, A.M.; Devlin, G.L.; Gras, S.L.; Tickler, A.K.; MacPhee, C.E.; Dobson, C.M. X-ray Scattering Study of the Effect of Hydration on the Cross-β Structure of Amyloid Fibrils. J. Am. Chem. Soc. 2006, 128, 11738–11739. [Google Scholar] [CrossRef]
- Iwata, K.; Fujiwara, T.; Matsuki, Y.; Akutsu, H.; Takahashi, S.; Naiki, H.; Goto, Y. 3D structure of amyloid protofilaments of β2-microglobulin fragment probed by solid-state NMR. Proc. Natl. Acad. Sci. USA 2006, 103, 18119–18124. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, S.; Harata, M.; Idehara, T.; Konagaya, K.; Yokoyama, G.; Hoshina, H.; Ogawa, Y. Actin polymerization is activated by terahertz irradiation. Sci. Rep. 2018, 8, 9990. [Google Scholar] [CrossRef] [PubMed]
- Bianchet, R.T.; Cubas, A.L.V.; Machado, M.M.; Moecke, E.H.S. Applicability of bacterial cellulose in cosmetics – bibliometric review. Biotechnol. Rep. 2020, 27, e00502. [Google Scholar] [CrossRef] [PubMed]
- Chien, H.-W.; Tsai, M.-Y.; Kuo, C.-J.; Lin, C.-L. Well-Dispersed Silver Nanoparticles on Cellulose Filter Paper for Bacterial Removal. Nanomaterials 2021, 11, 595. [Google Scholar] [CrossRef]
- Qin, C.; Yao, M.; Liu, Y.; Yang, Y.; Zong, Y.; Zhao, H. MFC/NFC-Based Foam/Aerogel for Production of Porous Materials: Preparation, Properties and Applications. Materials 2020, 13, 5568. [Google Scholar] [CrossRef]
- Wang, X.; Yao, C.; Wang, F.; Li, Z. Cellulose-Based Nanomaterials for Energy Applications. Small 2017, 13, 42. [Google Scholar] [CrossRef]
- Zwawi, M. A Review on Natural Fiber Bio-Composites; Surface Modifications and Applications. Molecules 2021, 26, 404. [Google Scholar] [CrossRef]
- Mazurek, S.; Mucciolo, A.; Humbel, B.M.; Nawrath, C. Transmission Fourier transform infrared microspectroscopy allows simultaneous assessment of cutin and cell-wall polysaccharides of Arabidopsis petals. Plant J. 2013, 74, 880–891. [Google Scholar] [CrossRef]
- Kawasaki, T.; Sakai, T.; Zen, H.; Sumitomo, Y.; Nogami, K.; Hayakawa, K.; Yaji, T.; Ohta, T.; Tsukiyama, K.; Hayakawa, Y. Cellulose Degradation by Infrared Free Electron Laser. Energy Fuels 2020, 34, 9064–9068. [Google Scholar] [CrossRef]
- Rockwood, D.N.; Preda, R.C.; Yücel, T.; Wang, X.; Lovett, M.L.; Kaplan, D.L. Materials fabrication from Bombyx mori silk fibroin. Nat. Protoc. 2011, 6, 1612–1631. [Google Scholar] [CrossRef]
- Bossi, A.M.; Bucciarelli, A.; Maniglio, D. Molecularly Imprinted Silk Fibroin Nanoparticles. ACS Appl. Mater. Interfaces. 2021, 13, 31431–31439. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Cavaco-Paulo, A.; Xu, B.; Martins, M. Polymeric Hydrogel Coating for Modulating the Shape of Keratin Fiber. Front. Chem. 2019, 7, 749. [Google Scholar] [CrossRef] [PubMed]
- Gough, C.R.; Callaway, K.; Spencer, E.; Leisy, K.; Jiang, G.; Yang, S.; Hu, X. Biopolymer-Based Filtration Materials. ACS Omega 2021, 6, 11804–11812. [Google Scholar] [CrossRef]
- Nam, K.; Maruyama, C.L.; Wang, C.-S.; Trump, B.G.; Lei, P.; Andreadis, S.T.; Baker, O.J. Laminin-111-derived peptide conjugated fibrin hydrogel restores salivary gland function. PLoS ONE 2017, 12, e0187069. [Google Scholar] [CrossRef] [PubMed]
- Besser, R.R.; Bowles, A.C.; Alassaf, A.; Carbonero, D.; Claure, I.; Jones, E.; Reda, J.; Wubker, L.; Batchelor, W.; Ziebarth, N.; et al. Enzymatically crosslinked gelatin–laminin hydrogels for applications in neuromuscular tissue engineering. Biomater. Sci. 2019, 8, 591–606. [Google Scholar] [CrossRef] [PubMed]
- Schräder, C.U.; Heinz, A.; Majovsky, P.; Karaman Mayack, B.; Brinckmann, J.; Sippl, W.; Schmelzer, C.E.H. Elastin is heterogeneously cross-linked. J. Biol. Chem. 2018, 293, 15107–15119. [Google Scholar] [CrossRef]
- Roberts, S.; Dzuricky, M.; Chilkoti, A. Elastin-like polypeptides as models of intrinsically disordered proteins. FEBS Lett. 2015, 589, 2477–2486. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawasaki, T.; Yamaguchi, Y.; Kitahara, H.; Irizawa, A.; Tani, M. Exploring Biomolecular Self-Assembly with Far-Infrared Radiation. Biomolecules 2022, 12, 1326. https://doi.org/10.3390/biom12091326
Kawasaki T, Yamaguchi Y, Kitahara H, Irizawa A, Tani M. Exploring Biomolecular Self-Assembly with Far-Infrared Radiation. Biomolecules. 2022; 12(9):1326. https://doi.org/10.3390/biom12091326
Chicago/Turabian StyleKawasaki, Takayasu, Yuusuke Yamaguchi, Hideaki Kitahara, Akinori Irizawa, and Masahiko Tani. 2022. "Exploring Biomolecular Self-Assembly with Far-Infrared Radiation" Biomolecules 12, no. 9: 1326. https://doi.org/10.3390/biom12091326
APA StyleKawasaki, T., Yamaguchi, Y., Kitahara, H., Irizawa, A., & Tani, M. (2022). Exploring Biomolecular Self-Assembly with Far-Infrared Radiation. Biomolecules, 12(9), 1326. https://doi.org/10.3390/biom12091326








