In Vitro Models of Ovarian Cancer: Bridging the Gap between Pathophysiology and Mechanistic Models
Abstract
1. Introduction
2. The Pathophysiology of Ovarian Cancers
2.1. The OC Environment
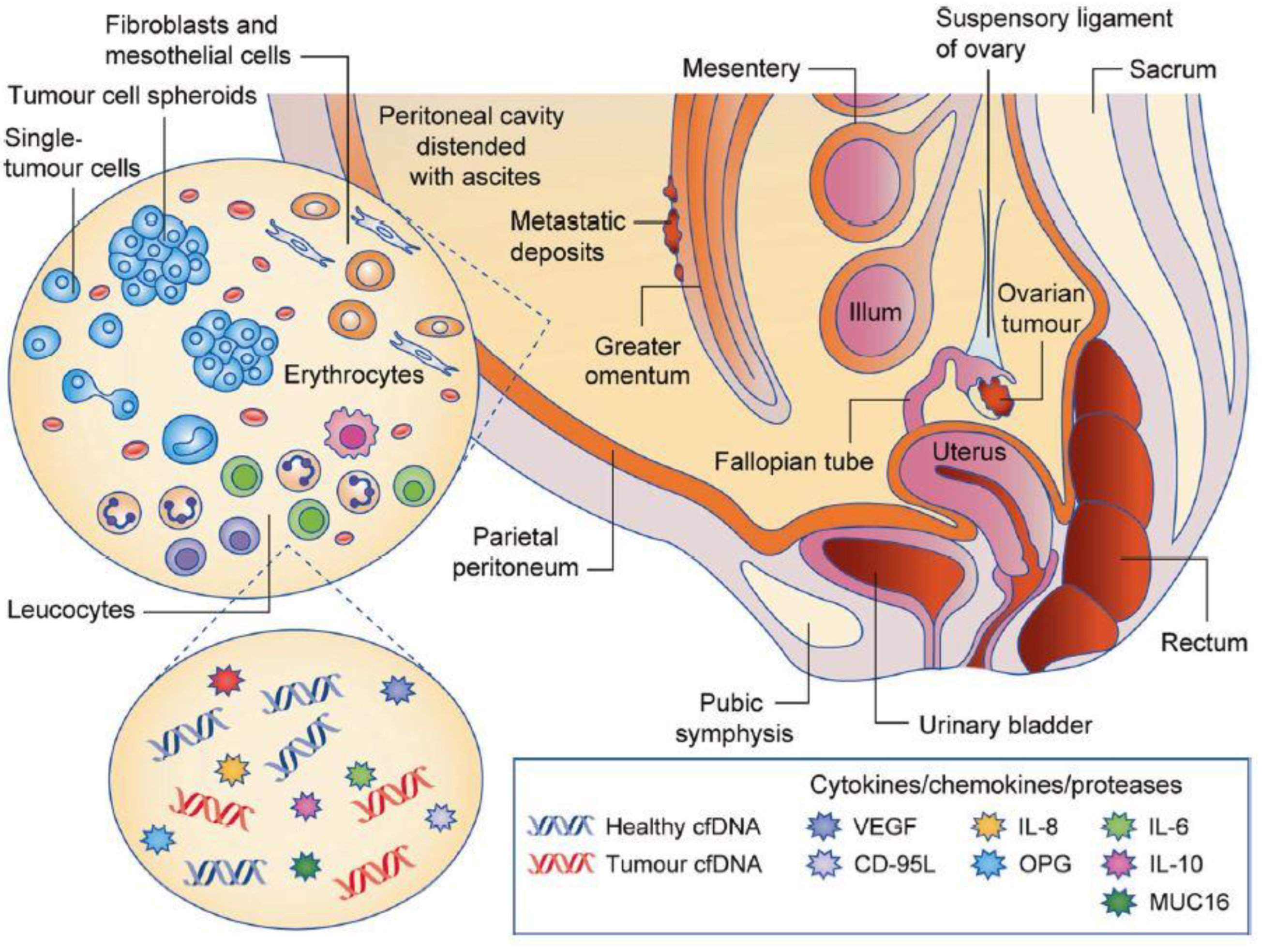
2.1.1. The Peritoneal Cavity and the Accumulation of Ascites
2.1.2. The Cellular Environment
2.2. Heterogeneities in OC Cells: From Genetics to Signaling Pathways
2.3. Phenotypic Heterogeneities and the Epithelial-to-Mesenchymal Transition
2.3.1. The Epithelial-to-Mesenchymal Transition
2.3.2. The Role of Cancer Stem Cells in EMT
2.3.3. Spheroids: Complexity, Invasive Dynamics, and Chemoresistance
2.4. The Mechanical Microenvironment of OC: From Scaffold to Circulating Environment
2.4.1. The Extracellular Matrix and Its Mechanosensing
2.4.2. The Importance of Fluids in OC: Soluble Factors and Shear Stress
3. Reconstructing OC in Its Multidimensional Environment
3.1. Co-Culture, Which Cells?
3.2. Spheroid Models
3.3. ECM and Scaffold
3.4. Contribution of Microfluidics: Consideration of Shear Forces in Mechanotransduction
3.4.1. Examples of Microfluidic Setups
3.4.2. Contribution of Microfluidics in Understanding the Progression of Ovarian Cancer
How Microfluidic Shear Stress Impact Ovarian Cancer
Shear Stress in Chemoresistance
3.4.3. Vascularized Microfluidic Models
4. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Torre, L.A.; Trabert, B.; DeSantis, C.E.; Miller, K.D.; Samimi, G.; Runowicz, C.D.; Gaudet, M.M.; Jemal, A.; Siegel, R.L. Ovarian Cancer Statistics, 2018. CA Cancer J. Clin. 2018, 68, 284–296. [Google Scholar] [CrossRef]
- Cortez, A.J.; Tudrej, P.; Kujawa, K.A.; Lisowska, K.M. Advances in Ovarian Cancer Therapy. Cancer Chemother. Pharmacol. 2018, 81, 17–38. [Google Scholar] [CrossRef] [PubMed]
- Kuroki, L.; Guntupalli, S.R. Treatment of Epithelial Ovarian Cancer. BMJ 2020, 371, m3773. [Google Scholar] [CrossRef]
- Karnezis, A.N.; Cho, K.R. Preclinical Models of Ovarian Cancer: Pathogenesis, Problems, and Implications for Prevention BACKGROUND-Ovarian Cancer Histotypes and Origins. Clin. Obstet. Gynecol. 2017, 60, 789–800. [Google Scholar] [CrossRef] [PubMed]
- Park, S.E.; Georgescu, A.; Huh, D. Organoids-on-a-Chip. Science 2019, 364, 960–965. [Google Scholar] [CrossRef]
- Ford, C.E.; Werner, B.; Hacker, N.F.; Warton, K. The Untapped Potential of Ascites in Ovarian Cancer Research and Treatment. Br. J. Cancer 2020, 123, 9–16. [Google Scholar] [CrossRef]
- Ahmed, N.; Kadife, E.; Raza, A.; Short, M.; Jubinsky, P.T.; Kannourakis, G. Ovarian Cancer, Cancer Stem Cells and Current Treatment Strategies: A Potential Role of Magmas in the Current Treatment Methods. Cells 2020, 9, 719. [Google Scholar] [CrossRef]
- Yang-Hartwich, Y.; Gurrea-Soteras, M.; Sumi, N.; Joo, W.D.; Holmberg, J.C.; Craveiro, V.; Alvero, A.B.; Mor, G. Ovulation and Extra-Ovarian Origin of Ovarian Cancer. Sci. Rep. 2014, 4, srep06116. [Google Scholar] [CrossRef]
- Coffman, L.G.; Burgos-Ojeda, D.; Wu, R.; Cho, K.; Bai, S.; Buckanovich, R.J. New Models of Hematogenous Ovarian Cancer Metastasis Demonstrate Preferential Spread to the Ovary and a Requirement for the Ovary for Abdominal Dissemination. Transl. Res. 2016, 175, 92–102.e2. [Google Scholar] [CrossRef]
- Kim, O.; Park, E.Y.; Klinkebie, D.L.; Pack, S.D.; Shin, Y.H.; Abdullaev, Z.; Emerson, R.E.; Coffey, D.M.; Kwon, S.Y.; Creighton, C.J.; et al. In Vivo Modeling of Metastatic Human Highgrade Serous Ovarian Cancer in Mice. PLoS Genet. 2020, 16, e1008808. [Google Scholar] [CrossRef]
- Jia, D.; Nagaoka, Y.; Katsumata, M.; Orsulic, S. Inflammation Is a Key Contributor to Ovarian Cancer Cell Seeding. Sci. Rep. 2018, 8, 12394. [Google Scholar] [CrossRef] [PubMed]
- Nagy, J.A.; Herzberg, K.T.; Dvorak, J.M.; Dvorak, H.F. Pathogenesis of Malignant Ascites Formation: Initiating Events That Lead to Fluid Accumulation. Cancer Res. 1993, 53, 2631–2643. [Google Scholar] [PubMed]
- Shen-Gunther, J.; Mannel, R.S. Ascites as a Predictor of Ovarian Malignancy. Gynecol. Oncol. 2002, 87, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Kipps, E.; Tan, D.S.P.; Kaye, S.B. Meeting the Challenge of Ascites in Ovarian Cancer: New Avenues for Therapy and Research Europe PMC Funders Group. Nat. Rev. Cancer 2013, 13, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Dean, D.C.; Hornicek, F.J.; Shi, H.; Duan, Z. Exosomes Promote Pre-Metastatic Niche Formation in Ovarian Cancer. Mol. Cancer 2019, 18, 124. [Google Scholar] [CrossRef]
- Butcher, D.T.; Alliston, T.; Weaver, V.M. A Tense Situation: Forcing Tumour Progression. Nat. Rev. Cancer 2009, 9, 108–122. [Google Scholar] [CrossRef]
- Kenny, H.A.; Krausz, T.; Yamada, S.D.; Lengyel, E. Use of a Novel 3D Culture Model to Elucidate the Role of Mesothelial Cells, Fibroblasts and Extra-Cellular Matrices on Adhesion and Invasion of Ovarian Cancer Cells to the Omentum. Int. J. Cancer 2007, 121, 1463–1472. [Google Scholar] [CrossRef]
- Erez, N.; Truitt, M.; Olson, P.; Hanahan, D. Cancer-Associated Fibroblasts Are Activated in Incipient Neoplasia to Orchestrate Tumor-Promoting Inflammation in an NF-ΚB-Dependent Manner. Cancer Cell 2010, 17, 135–147. [Google Scholar] [CrossRef]
- Song, M.; Yeku, O.O.; Rafiq, S.; Purdon, T.; Dong, X.; Zhu, L.; Zhang, T.; Wang, H.; Yu, Z.; Mai, J.; et al. Tumor Derived UBR5 Promotes Ovarian Cancer Growth and Metastasis through Inducing Immunosuppressive Macrophages. Nat. Commun. 2020, 11, 6298. [Google Scholar] [CrossRef]
- Hensler, M.; Kasikova, L.; Fiser, K.; Rakova, J.; Skapa, P.; Laco, J.; Lanickova, T.; Pecen, L.; Truxova, I.; Vosahlikova, S.; et al. M2-like Macrophages Dictate Clinically Relevant Immunosuppression in Metastatic Ovarian Cancer. J. Immunother. Cancer 2020, 8, e000979. [Google Scholar] [CrossRef]
- Raghavan, S.; Mehta, P.; Xie, Y.; Lei, Y.L.; Mehta, G. Ovarian Cancer Stem Cells and Macrophages Reciprocally Interact through the WNT Pathway to Promote Pro-Tumoral and Malignant Phenotypes in 3D Engineered Microenvironments. J. Immunother. Cancer 2019, 7, 190. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, V.; Tallapragada, S.; Schaar, B.; Kamat, K.; Chanana, A.M.; Zhang, Y.; Patel, S.; Parkash, V.; Rinker-Schaeffer, C.; Folkins, A.K.; et al. Omental Macrophages Secrete Chemokine Ligands That Promote Ovarian Cancer Colonization of the Omentum via CCR1. Commun. Biol. 2020, 3, 524. [Google Scholar] [CrossRef] [PubMed]
- Carroll, M.J.; Fogg, K.C.; Patel, H.A.; Krause, H.B.; Mancha, A.S.; Patankar, M.S.; Weisman, P.S.; Barroilhet, L.; Kreeger, P.K. Alternatively-Activated Macrophages Upregulate Mesothelial Expression of p-Selectin to Enhance Adhesion of Ovarian Cancer Cells. Cancer Res. 2018, 78, 3560–3573. [Google Scholar] [CrossRef]
- Nieman, K.M.; Kenny, H.A.; Penicka, C.v.; Ladanyi, A.; Buell-Gutbrod, R.; Zillhardt, M.R.; Romero, I.L.; Carey, M.S.; Mills, G.B.; Hotamisligil, G.S.; et al. Adipocytes Promote Ovarian Cancer Metastasis and Provide Energy for Rapid Tumor Growth. Nat. Med. 2011, 17, 1498–1503. [Google Scholar] [CrossRef] [PubMed]
- Nieman, K.M.; Romero, I.L.; van Houten, B.; Lengyel, E.; Biophys, B.; Author, A. Adipose Tissue and Adipocytes Supports Tumorigenesis and Metastasis # NIH Public Access Author Manuscript. Biochim. Biophys. Acta 2013, 1831, 1533–1541. [Google Scholar] [CrossRef] [PubMed]
- The Cancer Genome Atlas Research Network; Bell, D.; Berchuck, A.; Birrer, M.; Chien, J.; Cramer, D.W.; Dao, F.; Dhir, R.; Disaia, P.; Gabra, H.; et al. Integrated Genomic Analyses of Ovarian Carcinoma. Nature 2011, 474, 609–615. [Google Scholar] [CrossRef]
- Aunoble, B.; Anches Eliane Didier, R.S.; Bignon, Y. Major Oncogenes and Tumor Suppressor Genes Involved in Epithelial Ovarian Cancer (Review). Int. J. Oncol. 2000, 16, 567–576. [Google Scholar] [CrossRef]
- Izar, B.; Tirosh, I.; Stover, E.H.; Wakiro, I.; Cuoco, M.S.; Alter, I.; Rodman, C.; Leeson, R.; Su, M.J.; Shah, P.; et al. A Single-Cell Landscape of High-Grade Serous Ovarian Cancer. Nat. Med. 2020, 26, 1271–1279. [Google Scholar] [CrossRef]
- Bamford, S.; Dawson, E.; Forbes, S.; Clements, J.; Pettett, R.; Dogan, A.; Flanagan, A.; Teague, J.; Futreal, P.A.; Stratton, M.R.; et al. The COSMIC (Catalogue of Somatic Mutations in Cancer) Database and Website. Br. J. Cancer 2004, 91, 355–358. [Google Scholar] [CrossRef]
- Liu, X.; Gao, Y.; Lu, Y.; Zhang, J.; Li, L.; Yin, F. Oncogenes Associated with Drug Resistance in Ovarian Cancer. J. Cancer Res. Clin. Oncol. 2015, 141, 381–395. [Google Scholar] [CrossRef]
- Natanzon, Y.; Goode, E.L.; Cunningham, J.M. Epigenetics in Ovarian Cancer. Semin. Cancer Biol. 2018, 51, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wen, J.; Wang, L.; Wen, Q.; Wu, J.; Bie, M. Fluid Shear Stress-Induced IL-8/CXCR Signaling in Human Ovarian Cancer Cells. Transl. Cancer Res. 2019, 8, 1591–1601. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Zhang, L.; Liu, P. CXCR7 Signaling Induced Epithelial–Mesenchymal Transition by AKT and ERK Pathways in Epithelial Ovarian Carcinomas. Tumor. Biol. 2015, 36, 1679–1683. [Google Scholar] [CrossRef]
- Wakefield, L.M.; Hill, C.S. Beyond TGFβ: Roles of Other TGFβ Superfamily Members in Cancer. Nat. Rev. Cancer 2013, 13, 328–341. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Shonibare, Z.; Monavarian, M.; Arend, R.C.; Lee, N.Y.; Inman, G.J.; Mythreye, K. TGFβ Signaling Networks in Ovarian Cancer Progression and Plasticity. Clin. Exp. Metastasis 2021, 38, 139–161. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Song, C.; Zhang, T.; Zhou, Q.; Zhang, X.; Wang, J.; Xu, B.; Zhang, X.; Liu, X.; Ying, X. Wilms’ Tumor 1 (WT1) Promotes Ovarian Cancer Progression by Regulating E-Cadherin and ERK1/2 Signaling. Cell Cycle 2020, 19, 2662–2675. [Google Scholar] [CrossRef] [PubMed]
- Sui, X.; Jiao, Y.-N.; Yang, L.-H.; Liu, J. MiR-9 Accelerates Epithelial-Mesenchymal Transition of Ovarian Cancer Cells via Inhibiting e-Cadherin. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 209–216. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, T.; Qin, S.; Wang, R.; Li, Y.; Zhou, Z.; Chen, Y.; Wu, Q.; Su, F. Effects of UPF1 Expression on EMT Process by Targeting E-Cadherin, N-Cadherin, Vimentin and Twist in a Hepatocellular Carcinoma Cell Line. Mol. Med. Rep. 2019, 19, 2137–2143. [Google Scholar] [CrossRef]
- Klymenko, Y.; Johnson, J.; Bos, B.; Lombard, R.; Campbell, L.; Loughran, E.; Stack, M.S. Heterogeneous Cadherin Expression and Multicellular Aggregate Dynamics in Ovarian Cancer Dissemination. Neoplasia 2017, 19, 549–563. [Google Scholar] [CrossRef]
- Klymenko, Y.; Kim, O.; Loughran, E.; Yang, J.; Lombard, R.; Alber, M.; Stack, M.S. Cadherin Composition and Multicellular Aggregate Invasion in Organotypic Models of Epithelial Ovarian Cancer Intraperitoneal Metastasis. Oncogene 2017, 36, 5840–5851. [Google Scholar] [CrossRef]
- Moreno-Bueno, G.; Peinado, H.; Molina, P.; Olmeda, D.; Cubillo, E.; Santos, V.; Palacios, J.; Portillo, F.; Cano, A. The Morphological and Molecular Features of the Epithelial-to-Mesenchymal Transition. Nat. Protoc. 2009, 4, 1591–1613. [Google Scholar] [CrossRef]
- Xu, W.; Mezencev, R.; Kim, B.; Wang, L.; McDonald, J.; Sulchek, T. Cell Stiffness Is a Biomarker of the Metastatic Potential of Ovarian Cancer Cells. PLoS ONE 2012, 7, e46609. [Google Scholar] [CrossRef] [PubMed]
- Gorges, T.M.; Tinhofer, I.; Drosch, M.; Röse, L.; Zollner, T.M.; Krahn, T.; von Ahsen, O. Circulating Tumour Cells Escape from EpCAM-Based Detection Due to Epithelial-to-Mesenchymal Transition. BMC Cancer 2012, 12, 178. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-Y.; Lin, H.-H.; Tang, M.-J.; Wang, Y.-K. Vimentin Contributes to Epithelial-Mesenchymal Transition Cancer Cell Mechanics by Mediating Cytoskeletal Organization and Focal Adhesion Maturation. Oncotarget 2015, 6, 15966–15983. [Google Scholar] [CrossRef]
- Yang, J.; Antin, P.; Berx, G.; Blanpain, C.; Brabletz, T.; Bronner, M.; Campbell, K.; Cano, A.; Casanova, J.; Christofori, G.; et al. Guidelines and Definitions for Research on Epithelial–Mesenchymal Transition. Nat. Rev. 2020, 21, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Carduner, L.; Leroy-Dudal, J.; Picot, C.R.; Gallet, O.; Carreiras, F.; Kellouche, S. Ascites-Induced Shift along Epithelial-Mesenchymal Spectrum in Ovarian Cancer Cells: Enhancement of Their Invasive Behavior Partly Dependant on Av Integrins. Clin. Exp. Metastasis 2014, 31, 675–688. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.Z.; Miow, Q.H.; Miki, Y.; Noda, T.; Mori, S.; Huang, R.Y.; Thiery, J.P. Epithelial-mesenchymal Transition Spectrum Quantification and Its Efficacy in Deciphering Survival and Drug Responses of Cancer Patients. EMBO Mol. Med. 2014, 6, 1279–1293. [Google Scholar] [CrossRef] [PubMed]
- Kan, T.; Wang, W.; Ip, P.P.; Zhou, S.; Wong, A.S.; Wang, X.; Yang, M. Single-Cell EMT-Related Transcriptional Analysis Revealed Intra-Cluster Heterogeneity of Tumor Cell Clusters in Epithelial Ovarian Cancer Ascites. Oncogene 2020, 39, 4227–4240. [Google Scholar] [CrossRef] [PubMed]
- Loret, N.; Denys, H.; Tummers, P.; Berx, G. The Role of Epithelial-to-Mesenchymal Plasticity in Ovarian Cancer Progression and Therapy Resistance. Cancers 2019, 11, 838. [Google Scholar] [CrossRef]
- Cheng, J.C.; Auersperg, N.; Leung, P.C.K. TGF-Beta Induces Serous Borderline Ovarian Tumor Cell Invasion by Activating EMT but Triggers Apoptosis in Low-Grade Serous Ovarian Carcinoma Cells. PLoS ONE 2012, 7, e42436. [Google Scholar] [CrossRef][Green Version]
- Flesken-Nikitin, A.; Hwang, C.I.; Cheng, C.Y.; Michurina, T.v.; Enikolopov, G.; Nikitin, A.Y. Ovarian Surface Epithelium at the Junction Area Contains a Cancer-Prone Stem Cell Niche. Nature 2013, 495, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Marjanovic, N.D.; Weinberg, R.A.; Chaffer, C.L. Cell Plasticity and Heterogeneity in Cancer. Clin. Chem. 2013, 59, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Babahosseini, H.; Ketene, A.N.; Schmelz, E.M.; Roberts, P.C.; Agah, M. Biomechanical Profile of Cancer Stem-like/Tumor Initiating Cells Derived from a Progressive Ovarian Cancer Model. Nanomedicine 2014, 10, 1013–1019. [Google Scholar] [CrossRef]
- Jiang, H.; Lin, X.; Liu, Y.; Gong, W.; Ma, X.; Yu, Y.; Xie, Y.; Sun, X.; Feng, Y.; Janzen, V.; et al. Transformation of Epithelial Ovarian Cancer Stemlike Cells into Mesenchymal Lineage via EMT Results in Cellular Heterogeneity and Supports Tumor Engraftment. Mol. Med. 2012, 18, 1197–1208. [Google Scholar] [CrossRef]
- Akhter, M.Z.; Sharawat, S.K.; Kumar, V.; Kochat, V.; Equbal, Z.; Ramakrishnan, M.; Kumar, U.; Mathur, S.; Kumar, L.; Mukhopadhyay, A. Aggressive Serous Epithelial Ovarian Cancer Is Potentially Propagated by EpCAM + CD45 + Phenotype. Oncogene 2018, 37, 2089–2103. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.; Asad, M.; Heong, V.; Wong, M.K.; Tan, T.Z.; Ye, J.; Kuay, K.T.; Thiery, J.P.; Scott, C.; Huang, R.Y.J. The FZD7-TWIST1 Axis Is Responsible for Anoikis Resistance and Tumorigenesis in Ovarian Carcinoma. Mol. Oncol. 2019, 13, 757–780. [Google Scholar] [CrossRef]
- Chen, Y.C.; Humphries, B.; Brien, R.; Gibbons, A.E.; Chen, Y.T.; Qyli, T.; Haley, H.R.; Pirone, M.E.; Chiang, B.; Xiao, A.; et al. Functional Isolation of Tumor-Initiating Cells Using Microfluidic-Based Migration Identifies Phosphatidylserine Decarboxylase as a Key Regulator. Sci. Rep. 2018, 8, 244. [Google Scholar] [CrossRef]
- Gao, Q.; Yang, Z.; Xu, S.; Li, X.; Yang, X.; Jin, P.; Liu, Y.; Zhou, X.; Zhang, T.; Gong, C.; et al. Heterotypic CAF-Tumor Spheroids Promote Early Peritoneal Metastatis of Ovarian Cancer. J. Exp. Med. 2019, 216, 688–703. [Google Scholar] [CrossRef]
- Zhang, S.; Balch, C.; Chan, M.W.; Lai, H.C.; Matei, D.; Schilder, J.M.; Yan, P.S.; Huang, T.H.M.; Nephew, K.P. Identification and Characterization of Ovarian Cancer-Initiating Cells from Primary Human Tumors. Cancer Res. 2008, 68, 4311–4320. [Google Scholar] [CrossRef]
- Mansour, H.; Hassan, G.; Afify, S.M.; Yan, T.; Seno, A.; Seno, M. Metastasis Model of Cancer Stem Cell-Derived Tumors. Methods Protoc. 2020, 3, 60. [Google Scholar] [CrossRef]
- Li, S.S.; Ip, C.K.M.; Tang, M.Y.H.; Tang, M.K.S.; Tong, Y.; Zhang, J.; Hassan, A.A.; Mak, A.S.C.; Yung, S.; Chan, T.M.; et al. Sialyl Lewisx-P-Selectin Cascade Mediates Tumor–Mesothelial Adhesion in Ascitic Fluid Shear Flow. Nat. Commun. 2019, 10, 2406. [Google Scholar] [CrossRef]
- al Habyan, S.; Kalos, C.; Szymborski, J.; McCaffrey, L. Multicellular Detachment Generates Metastatic Spheroids during Intra-Abdominal Dissemination in Epithelial Ovarian Cancer. Oncogene 2018, 37, 5127–5135. [Google Scholar] [CrossRef] [PubMed]
- Bilandzic, M.; Rainczuk, A.; Green, E.; Fairweather, N.; Jobling, T.W.; Plebanski, M.; Stephens, A.N. Keratin-14 (KRT14) Positive Leader Cells Mediate Mesothelial Clearance and Invasion by Ovarian Cancer Cells. Cancers 2019, 11, 1228. [Google Scholar] [CrossRef] [PubMed]
- Chaffer, C.L.; San Juan, B.P.; Lim, E.; Weinberg, R.A. EMT, Cell Plasticity and Metastasis. Cancer Metastasis Rev. 2016, 35, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Carduner, L.; Picot, C.R.; Leroy-Dudal, J.; Blay, L.; Kellouche, S.; Carreiras, F. Cell Cycle Arrest or Survival Signaling through Av Integrins, Activation of PKC and ERK1/2 Lead to Anoikis Resistance of Ovarian Cancer Spheroids. Exp. Cell Res. 2014, 320, 329–342. [Google Scholar] [CrossRef]
- Matte, I.; Legault, C.M.; Garde-Granger, P.; Laplante, C.; Bessette, P.; Rancourt, C.; Piché, A. Mesothelial Cells Interact with Tumor Cells for the Formation of Ovarian Cancer Multicellular Spheroids in Peritoneal Effusions. Clin. Exp. Metastasis 2016, 33, 839–852. [Google Scholar] [CrossRef] [PubMed]
- Raghavan, S.; Mehta, P.; Ward, M.R.; Bregenzer, M.E.; Fleck, E.M.A.; Tan, L.; McLean, K.; Buckanovich, R.J.; Mehta, G. Personalized Medicine–Based Approach to Model Patterns of Chemoresistance and Tumor Recurrence Using Ovarian Cancer Stem Cell Spheroids. Clin. Cancer Res. 2017, 23, 6934–6945. [Google Scholar] [CrossRef]
- Casagrande, N.; Borghese, C.; Agostini, F.; Durante, C.; Mazzucato, M.; Colombatti, A.; Aldinucci, D. In Ovarian Cancer Multicellular Spheroids, Platelet Releasate Promotes Growth, Expansion of Aldh+ and Cd133+ Cancer Stem Cells, and Protection against the Cytotoxic Effects of Cisplatin, Carboplatin and Paclitaxel. Int. J. Mol. Sci. 2021, 22, 3019. [Google Scholar] [CrossRef]
- Liao, J.; Qian, F.; Tchabo, N.; Mhawech-Fauceglia, P.; Beck, A.; Qian, Z.; Wang, X.; Huss, W.J.; Lele, S.B.; Morrison, C.D.; et al. Ovarian Cancer Spheroid Cells with Stem Cell-like Properties Contribute to Tumor Generation, Metastasis and Chemotherapy Resistance through Hypoxia-Resistant Metabolism. PLoS ONE 2014, 9, e84941. [Google Scholar] [CrossRef]
- McKenzie, A.J.; Hicks, S.R.; Svec, K.v.; Naughton, H.; Edmunds, Z.L.; Howe, A.K. The Mechanical Microenvironment Regulates Ovarian Cancer Cell Morphology, Migration, and Spheroid Disaggregation. Sci. Rep. 2018, 8, 7228. [Google Scholar] [CrossRef]
- Ricciardelli, C.; Rodgers, R.J. Extracellular Matrix of Ovarian Tumors. Semin. Reprod. Med. 2006, 24, 270–282. [Google Scholar] [CrossRef] [PubMed]
- Stenbäck, F.; Wasenius, V.-M. Basement Membrane Structures in Tumors of the Ovary. Europ. J. Obstet. Gynec. Reprod. Biol 1985, 20, 357–371. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.-H.; Smith, E.R.; Cohen, C.; Wu, H.; Patriotis, C.; Godwin, A.K.; Hamilton, T.C.; Xu, X.-X. Molecular Events Associated with Dysplastic Morphologic Transformation and Initiation of Ovarian Tumorigenicity. Cancer 2002, 94, 2380–2392. [Google Scholar] [CrossRef] [PubMed]
- Roland, I.H.; Yang, W.-L.; Yang, D.-H.; Daly, M.B.; Ozols, R.F.; Hamilton, T.C.; Lynch, H.T.; Godwin, A.K.; Xu, X.-X.; Xu Robert Ozols, X.-X.F.; et al. Loss of Surface and Cyst Epithelial Basement Membranes and Preneoplastic Morphologic Changes in Prophylactic Oophorectomies. Cancer 2003, 98, 2607–2623. [Google Scholar] [CrossRef] [PubMed]
- Nadiarnykh, O.; Lacomb, R.B.; Brewer, M.A.; Campagnola, P.J. Alterations of the Extracellular Matrix in Ovarian Cancer Studied by Second Harmonic Generation Imaging Microscopy. BMC Cancer 2010, 10, 94. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, N.D.; Brewer, M.A.; Utzinger, U. Endogenous Optical Biomarkers of Ovarian Cancer Evaluated with Multiphoton Microscopy. Cancer Epidemiol. Biomarkers. Prev. 2007, 16, 2048–2057. [Google Scholar] [CrossRef]
- Carey, S.P.; D’Alfonso, T.M.; Shin, S.J.; Reinhart-King, C.A. Mechanobiology of Tumor Invasion: Engineering Meets Oncology. Crit. Rev. Oncol. Hematol. 2012, 83, 170–183. [Google Scholar] [CrossRef]
- Chaudhuri, O.; Cooper-white, J.; Janmey, P.A.; Mooney, D.J.; Shenoy, V.B. Effects of Extracellular Matrix Viscoelasticity on Cellular Behaviour. Nature 2020, 584, 535–546. [Google Scholar] [CrossRef]
- Zhou, N.; Ma, X.; Zhang, T.; Chen, X.; Guo, Z.; Ma, D.; Gu, N. Enrichment of Ovarian Cancer Stem Cells by PEG Cross-Linked PMVE-Co-MA Hydrogel with Controllable Elastic Modulus. J. Nanosci. Nanotechnol. 2016, 16, 12134–12144. [Google Scholar] [CrossRef]
- Dikovsky, D.; Bianco-Peled, H.; Seliktar, D. Defining the Role of Matrix Compliance and Proteolysis in Three-Dimensional Cell Spreading and Remodeling. Biophys. J. 2008, 94, 2914–2925. [Google Scholar] [CrossRef]
- McGrail, D.J.; Kieu, Q.M.N.; Dawson, M.R. The Malignancy of Metastatic Ovarian Cancer Cells Is Increased on Soft Matrices through a Mechanosensitive Rho-ROCK Pathway. J. Cell Sci. 2014, 127, 2621–2626. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Zhang, T.; Fang, K.; Dou, J.; Zhou, N.; Ma, X.; Gu, N. The Effects of Macroporosity and Stiffness of Poly[(Methyl Vinyl Ether)-: Alt -(Maleic Acid)] Cross-Linked Egg White Simulations of an Aged Extracellular Matrix on the Proliferation of Ovarian Cancer Cells. RSC Adv. 2016, 6, 43892–43900. [Google Scholar] [CrossRef]
- Pearce, O.M.T.; Delaine-Smith, R.M.; Maniati, E.; Nichols, S.; Wang, J.; Böhm, S.; Rajeeve, V.; Ullah, D.; Chakravarty, P.; Jones, R.R.; et al. Deconstruction of a Metastatic Tumor Microenvironment Reveals a Common Matrix Response in Human Cancers. Cancer Discov. 2018, 8, 304–319. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.C.; Jin, J.K.; Cheng, C.J.; Huang, C.F.; Song, J.H.; Huang, M.; Brown, W.S.; Zhang, S.; Yu-Lee, L.Y.; Yeh, E.T.; et al. Targeting Constitutively Activated Β1 Integrins Inhibits Prostate Cancer Metastasis. Mol. Cancer Res. 2013, 11, 405–417. [Google Scholar] [CrossRef] [PubMed]
- Carduner, L.; Agniel, R.; Kellouche, S.; Picot, C.R.; Blanc-Fournier, C.; Leroy-Dudal, J.; Carreiras, F. Ovarian Cancer Ascites-Derived Vitronectin and Fibronectin: Combined Purification, Molecular Features and Effects on Cell Response. Biochim. Biophys. Acta 2013, 1830, 4885–4897. [Google Scholar] [CrossRef] [PubMed]
- Mikuła-Pietrasik, J.; Uruski, P.; Szubert, S.; Szpurek, D.; Sajdak, S.; Tykarski, A.; Książek, K. Malignant Ascites Determine the Transmesothelial Invasion of Ovarian Cancer Cells. Int. J. Biochem. Cell Biol. 2017, 92, 6–13. [Google Scholar] [CrossRef]
- Matte, I.; Garde-Granger, P.; Bessette, P.; Piché, A. Ascites from Ovarian Cancer Patients Stimulates MUC16 Mucin Expression and Secretion in Human Peritoneal Mesothelial Cells through an Akt-Dependent Pathway. BMC Cancer 2019, 19, 406. [Google Scholar] [CrossRef]
- Heyman, L.; Leroy-Dudal, J.; Fernandes, J.; Seyer, D.; Dutoit, S.; Carreiras, F. Mesothelial Vitronectin Stimulates Migration of Ovarian Cancer Cells. Cell Biol. Int. 2010, 34, 493–502. [Google Scholar] [CrossRef]
- Estermann, M.; Huang, Y.L.; Septiadi, D.; Ritz, D.; Liang, C.Y.; Jacob, F.; Drasler, B.; Petri-Fink, A.; Heinzelmann-Schwarz, V.; Rothen-Rutishauser, B. Patient-Derived and Artificial Ascites Have Minor Effects on MeT-5A Mesothelial Cells and Do Not Facilitate Ovarian Cancer Cell Adhesion. PLoS ONE 2020, 15, e0241500. [Google Scholar] [CrossRef]
- Bascetin, R.; Laurent-Issartel, C.; Blanc-Fournier, C.; Vendrely, C.; Kellouche, S.; Carreiras, F.; Gallet, O.; Leroy-Dudal, J. A Biomimetic Model of 3D Fluid Extracellular Macromolecular Crowding Microenvironment Fine-Tunes Ovarian Cancer Cells Dissemination Phenotype. Biomaterials 2021, 269, 120610. [Google Scholar] [CrossRef]
- Gokturk, H.S.; Demir, M.; Ozturk, N.A.; Unler, G.K.; Kulaksizoglu, S.; Kozanoglu, I.; Serin, E.; Yilmaz, U. The Role of Ascitic Fluid Viscosity in the Differential Diagnosis of Ascites. Can. J. Gastroenterol. 2010, 24, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Klymenko, Y.; Wates, R.B.; Weiss-Bilka, H.; Lombard, R.; Liu, Y.; Campbell, L.; Kim, O.; Wagner, D.; Ravosa, M.J.; Stack, M.S. Modeling the Effect of Ascites-Induced Compression on Ovarian Cancer Multicellular Aggregates. Dis. Model. Mech. 2018, 11, dmm034199. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.F.; Chang, C.A.; Lee, D.Y.; Lee, P.L.; Yeh, Y.M.; Yeh, C.R.; Cheng, K.; Chien, S.; Chiu, J.J. Tumor Cell Cycle Arrest Induced by Shear Stress: Roles of Integrins and Smad. Proc. Natl. Acad. Sci. USA 2008, 105, 3927–3932. [Google Scholar] [CrossRef]
- Hyler, A.R.; Baudoin, N.C.; Brown, M.S.; Stremler, M.A.; Cimini, D.; Davalos, R.v.; Schmelz, E.M. Fluid Shear Stress Impacts Ovarian Cancer Cell Viability, Subcellular Organization, and Promotes Genomic Instability. PLoS ONE 2018, 13, e0194170. [Google Scholar] [CrossRef]
- Masiello, T.; Dhall, A.; Hemachandra, L.; Tokranova, N.; Melendez, J.; Castracane, J. A Dynamic Culture Method to Produce Ovarian Cancer Spheroids under Physiologically-Relevant Shear Stress. Cells 2018, 7, 277. [Google Scholar] [CrossRef]
- Ip, C.K.M.; Li, S.S.; Tang, M.Y.H.; Sy, S.K.H.; Ren, Y.; Shum, H.C.; Wong, A.S.T. Stemness and Chemoresistance in Epithelial Ovarian Carcinoma Cells under Shear Stress. Sci. Rep. 2016, 6, 26788. [Google Scholar] [CrossRef] [PubMed]
- Kenny, H.A.; Lal-Nag, M.; White, E.A.; Shen, M.; Chiang, C.Y.; Mitra, A.K.; Zhang, Y.; Curtis, M.; Schryver, E.M.; Bettis, S.; et al. Quantitative High Throughput Screening Using a Primary Human Three-Dimensional Organotypic Culture Predicts in Vivo Efficacy. Nat. Commun. 2015, 6, 6220. [Google Scholar] [CrossRef] [PubMed]
- Slack-Davis, J.K.; Atkins, K.A.; Harrer, C.; Daniel Hershey, E.; Conaway, M. Vascular Cell Adhesion Molecule-1 Is a Regulator of Ovarian Cancer Peritoneal Metastasis. Cancer Res. 2009, 69, 1469–1476. [Google Scholar] [CrossRef]
- Rieppi, M.; Vergani, V.; Gatto, C.; Zanetta, G.; Allavena, P.; Taraboletti, G.; Giavazzi, R. Mesothelial Cells Induce the Motility of Human Ovarian Carcinoma Cells. Int. J. Cancer 1999, 80, 303–307. [Google Scholar] [CrossRef]
- Burleson, K.M.; Casey, R.C.; Skubitz, K.M.; Pambuccian, S.E.; Oegema, T.R.; Skubitz, A.P.N. Ovarian Carcinoma Ascites Spheroids Adhere to Extracellular Matrix Components and Mesothelial Cell Monolayers. Gynecol. Oncol. 2004, 93, 170–181. [Google Scholar] [CrossRef]
- Dai, L.; Wang, C.; Song, K.; Wang, W.; Di, W. Activation of SphK1 by Adipocytes Mediates Epithelial Ovarian Cancer Cell Proliferation. J. Ovarian. Res. 2021, 14, 62. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Wang, R.; Xiao, H. Adipocytes Induce the Resistance of Ovarian Cancer to Carboplatin through ANGPTL4. Oncol. Rep. 2020, 44, 927–938. [Google Scholar] [CrossRef] [PubMed]
- Delaine-Smith, R.M.; Maniati, E.; Malacrida, B.; Nichols, S.; Roozitalab, R.; Jones, R.R.; Lecker, L.S.M.; Pearce, O.M.T.; Knight, M.M.; Balkwill, F.R. Modelling TGFβR and Hh Pathway Regulation of Prognostic Matrisome Molecules in Ovarian Cancer. iScience 2021, 24, 102674. [Google Scholar] [CrossRef] [PubMed]
- Malacrida, B.; Pearce, O.M.T.; Balkwill, F.R. Building in Vitro 3D Human Multicellular Models of High-Grade Serous Ovarian Cancer. STAR Protoc. 2022, 3, 101086. [Google Scholar] [CrossRef]
- Malacrida, B.; Nichols, S.; Maniati, E.; Jones, R.; Delanie-Smith, R.; Roozitalab, R.; Tyler, E.J.; Thomas, M.; Boot, G.; Mackerodt, J.; et al. A Human Multi-Cellular Model Shows How Platelets Drive Production of Diseased Extracellular Matrix and Tissue Invasion. iScience 2021, 24, 102676. [Google Scholar] [CrossRef]
- Wei, C.; Mei, J.; Tang, L.; Liu, Y.; Li, D.; Li, M.; Zhu, X. 1-Methyl-Tryptophan Attenuates Regulatory T Cells Differentiation Due to the Inhibition of Estrogen-IDO1-MRC2 Axis in Endometriosis. Cell Death Dis. 2016, 7, e2489. [Google Scholar] [CrossRef]
- Peters, P.N.; Schryver, E.M.; Lengyel, E.; Kenny, H. Modeling the Early Steps of Ovarian Cancer Dissemination in an Organotypic Culture of the Human Peritoneal Cavity. J. Vis. Exp. 2015, 2015, e53541. [Google Scholar] [CrossRef]
- Lal-Nag, M.; McGee, L.; Guha, R.; Lengyel, E.; Kenny, H.A.; Ferrer, M. A High-Throughput Screening Model of the Tumor Microenvironment for Ovarian Cancer Cell Growth. SLAS Discov. 2017, 22, 494–506. [Google Scholar] [CrossRef]
- Watters, K.M.; Bajwa, P.; Kenny, H.A. Organotypic 3D Models of the Ovarian Cancer Tumor Microenvironment. Cancers 2018, 10, 265. [Google Scholar] [CrossRef]
- Kutova, O.M.; Sencha, L.M.; Pospelov, A.D.; Dobrynina, O.E.; Brilkina, A.A.; Cherkasova, E.I.; Balalaeva, I.V. Comparative Analysis of Cell–Cell Contact Abundance in Ovarian Carcinoma Cells Cultured in Two- and Three-dimensional in Vitro Models. Biology 2020, 9, 446. [Google Scholar] [CrossRef]
- Patra, B.; Lateef, M.A.; Brodeur, M.N.; Fleury, H.; Carmona, E.; Péant, B.; Provencher, D.; Mes-Masson, A.M.; Gervais, T. Carboplatin Sensitivity in Epithelial Ovarian Cancer Cell Lines: The Impact of Model Systems. PLoS ONE 2020, 15, e0244549. [Google Scholar] [CrossRef] [PubMed]
- Weiswald, L.B.; Bellet, D.; Dangles-Marie, V. Spherical Cancer Models in Tumor Biology. Neoplasia 2015, 17, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Lee, K.S.; Bang, J.H.; Kim, Y.E.; Kim, M.C.; Oh, K.W.; Lee, S.H.; Kang, J.Y. 3-Dimensional Cell Culture for on-Chip Differentiation of Stem Cells in Embryoid Body. Lab Chip 2011, 11, 874–882. [Google Scholar] [CrossRef]
- Kim, C.; Bang, J.H.; Kim, Y.E.; Lee, S.H.; Kang, J.Y. On-Chip Anticancer Drug Test of Regular Tumor Spheroids Formed in Microwells by a Distributive Microchannel Network. Lab Chip 2012, 12, 4135–4142. [Google Scholar] [CrossRef]
- Chen, Y.C.; Lou, X.; Zhang, Z.; Ingram, P.; Yoon, E. High-Throughput Cancer Cell Sphere Formation for Characterizing the Efficacy of Photo Dynamic Therapy in 3D Cell Cultures. Sci. Rep. 2015, 5, 12175. [Google Scholar] [CrossRef] [PubMed]
- Suarez, J.S.; Main, H.G.; Muralidhar, G.G.; Elfituri, O.; Xu, H.L.; Kajdacsy-Balla, A.A.; Barbolina, M.V. CD44 Regulates Formation of Spheroids and Controls Organ-Specific Metastatic Colonization in Epithelial Ovarian Carcinoma. Mol. Cancer Res. 2019, 17, 1801–1814. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Huang, B.; Rofaani, E.; Hu, J.; Liu, Y.; Pitingolo, G.; Wang, L.; Shi, J.; Aimé, C.; Chen, Y. Fabrication of Micro-Cages and Caged Tumor Spheroids for Microfluidic Chip-Based Assays. Microelectron. Eng. 2020, 225, 111256. [Google Scholar] [CrossRef]
- Dadgar, N.; Gonzalez-Suarez, A.M.; Fattahi, P.; Hou, X.; Weroha, J.S.; Gaspar-Maia, A.; Stybayeva, G.; Revzin, A. A Microfluidic Platform for Cultivating Ovarian Cancer Spheroids and Testing Their Responses to Chemotherapies. Microsyst. Nanoeng. 2020, 6, 93. [Google Scholar] [CrossRef]
- Te Kuo, C.; Chiang, C.L.; Huang, R.Y.J.; Lee, H.; Wo, A.M. Configurable 2D and 3D Spheroid Tissue Cultures on Bioengineered Surfaces with Acquisition of Epithelial-Mesenchymal Transition Characteristics. NPG Asia Mater. 2012, 4, e27–e28. [Google Scholar] [CrossRef]
- Monteiro, C.F.; Santos, S.C.; Custódio, C.A.; Mano, J.F. Human Platelet Lysates-Based Hydrogels: A Novel Personalized 3D Platform for Spheroid Invasion Assessment. Adv. Sci. 2020, 7, 1902398. [Google Scholar] [CrossRef]
- Loessner, D.; Stok, K.S.; Lutolf, M.P.; Hutmacher, D.W.; Clements, J.A.; Rizzi, S.C. Bioengineered 3D Platform to Explore Cell-ECM Interactions and Drug Resistance of Epithelial Ovarian Cancer Cells. Biomaterials 2010, 31, 8494–8506. [Google Scholar] [CrossRef]
- Loessner, D.; Meinert, C.; Kaemmerer, E.; Martine, L.C.; Yue, K.; Levett, P.A.; Klein, T.J.; Melchels, F.P.W.; Khademhosseini, A.; Hutmacher, D.W. Functionalization, Preparation and Use of Cell-Laden Gelatin Methacryloyl-Based Hydrogels as Modular Tissue Culture Platforms. Nat. Protoc. 2016, 11, 727–746. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Cai, G.H.; Liang, J.; Ao, D.S.; Wang, H.; Yang, Z.H. Three-Dimensional Culture and Clinical Drug Responses of a Highly Metastatic Human Ovarian Cancer HO-8910PM Cells in Nanofibrous Microenvironments of Three Hydrogel Biomaterials. J. Nanobiotechnology 2020, 18, 90. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Ma, X.; Hu, W.; Ren, P.; Zhao, Y.; Zhang, T. Effect of RGD Content in Poly(Ethylene Glycol)-Crosslinked Poly(Methyl Vinyl Ether-Alt-Maleic Acid) Hydrogels on the Expansion of Ovarian Cancer Stem-like Cells. Mater. Sci. Eng. C 2021, 118, 111477. [Google Scholar] [CrossRef] [PubMed]
- Foty, R. A Simple Hanging Drop Cell Culture Protocol for Generation of 3D Spheroids. J. Vis. Exp. 2011, 51, e2720. [Google Scholar] [CrossRef]
- Das, T.; Meunier, L.; Barbe, L.; Provencher, D.; Guenat, O.; Gervais, T.; Mes-Masson, A.M. Empirical Chemosensitivity Testing in a Spheroid Model of Ovarian Cancer Using a Microfluidics-Based Multiplex Platform. Biomicrofluidics 2013, 7, 011805. [Google Scholar] [CrossRef]
- Castaneda, F.; Kinne, R.K.H. Short Exposure to Millimolar Concentrations of Ethanol Induces Apoptotic Cell Death in Multicellular HepG2 Spheroids. J. Cancer Res. Clin. Oncol. 2000, 126, 305–310. [Google Scholar] [CrossRef]
- Phelan, M.A.; Gianforcaro, A.L.; Gerstenhaber, J.A.; Lelkes, P.I. An Air Bubble-Isolating Rotating Wall Vessel Bioreactor for Improved Spheroid/Organoid Formation. Tissue Eng. Part C Methods 2019, 25, 479–488. [Google Scholar] [CrossRef]
- Kellouche, S.; Fernandes, J.; Leroy-Dudal, J.; Gallet, O.; Dutoit, S.; Poulain, L.; Carreiras, F. Initial Formation of IGROV1 Ovarian Cancer Multicellular Aggregates Involves Vitronectin. Tumor. Biol. 2010, 31, 129–139. [Google Scholar] [CrossRef]
- Wambecke, A.; Laurent-Issartel, C.; Leroy-Dudal, J.; Giffard, F.; Cosson, F.; Lubin-Germain, N.; Uziel, J.; Kellouche, S.; Carreirasid, F. Evaluation of the Potential of a New Ribavirin Analog Impairing the Dissemination of Ovarian Cancer Cells. PLoS ONE 2019, 14, e0225860. [Google Scholar] [CrossRef]
- Chen, C.; He, Y.; Lopez, E.; Carreiras, F.; Yamada, A.; Schanne-Klein, M.C.; Lambert, A.; Chen, Y.; Aimé, C. High-Throughput Tuning of Ovarian Cancer Spheroids for on-Chip Invasion Assays. Micro Nano Eng. 2022, 15, 100138. [Google Scholar] [CrossRef]
- Velletri, T.; Villa, C.E.; Cilli, D.; Barzaghi, B.; Lo Riso, P.; Lupia, M.; Luongo, R.; López-Tobón, A.; De Simone, M.; Bonnal, R.J.P.; et al. Single cell-derived spheroids capture the self-renewing subpopulations of metastatic ovarian cancer. Cell Death Differ. 2022, 29, 614–626. [Google Scholar] [CrossRef] [PubMed]
- Alessandri, K.; Sarangi, B.R.; Gurchenkov, V.V.; Sinha, B.; Kießling, T.R.; Fetler, L.; Rico, F.; Scheuring, S.; Lamaze, C.; Simon, A.; et al. Cellular Capsules as a Tool for Multicellular Spheroid Production and for Investigating the Mechanics of Tumor Progression in Vitro. Proc. Natl. Acad. Sci. USA 2013, 110, 14843–14848. [Google Scholar] [CrossRef]
- Liu, L.; ichiro Kamei, K.; Yoshioka, M.; Nakajima, M.; Li, J.; Fujimoto, N.; Terada, S.; Tokunaga, Y.; Koyama, Y.; Sato, H.; et al. Nano-on-Micro Fibrous Extracellular Matrices for Scalable Expansion of Human ES/IPS Cells. Biomaterials 2017, 124, 47–54. [Google Scholar] [CrossRef]
- Girard, Y.K.; Wang, C.; Ravi, S.; Howell, M.C.; Mallela, J.; Alibrahim, M.; Green, R.; Hellermann, G.; Mohapatra, S.S.; Mohapatra, S. A 3D Fibrous Scaffold Inducing Tumoroids: A Platform for Anticancer Drug Development. PLoS ONE 2013, 8, e0075345. [Google Scholar] [CrossRef] [PubMed]
- Dems, D.; Rodrigues da Silva, J.; Hélary, C.; Wien, F.; Marchand, M.; Debons, N.; Muller, L.; Chen, Y.; Schanne-Klein, M.-C.; Laberty-Robert, C.; et al. Native Collagen: Electrospinning of Pure, Cross-Linker-Free, Self-Supported Membrane. ACS Appl. Bio Mater. 2020, 3, 2948–2957. [Google Scholar] [CrossRef]
- Moghadas, H.; Saidi, M.S.; Kashaninejad, N.; Kiyoumarsioskouei, A.; Nguyen, N.T. Fabrication and Characterization of Low-Cost, Bead-Free, Durable and Hydrophobic Electrospun Membrane for 3D Cell Culture. Biomed. Microdevices 2017, 19, 74. [Google Scholar] [CrossRef] [PubMed]
- Eslami Amirabadi, H.; SahebAli, S.; Frimat, J.P.; Luttge, R.; den Toonder, J.M.J. A Novel Method to Understand Tumor Cell Invasion: Integrating Extracellular Matrix Mimicking Layers in Microfluidic Chips by “Selective Curing”. Biomed. Microdevices 2017, 19, 92. [Google Scholar] [CrossRef]
- Xu, F.; Celli, J.; Rizvi, I.; Moon, S.; Hasan, T.; Demirci, U. A Three-Dimensional in Vitro Ovarian Cancer Coculture Model Using a High-Throughput Cell Patterning Platform. Biotechnol. J. 2011, 6, 204–212. [Google Scholar] [CrossRef]
- Kuo, C.-Y.; Guo, T.; Cabrera-Luque, J.; Arumugasaamy, N.; Bracaglia, L.; Garcia-Vivas, A.; Santoro, M.; Baker, H.; Fisher, J.; Kim, P. Placental Basement Membrane Proteins Are Required for Effective Cytotrophoblast Invasion in a 3D Bioprinted Placenta Model. J. Biomed. Mater. Res. A 2018, 106, 1476–1487. [Google Scholar] [CrossRef]
- Stejskalová, A.; Fincke, V.; Nowak, M.; Schmidt, Y.; Borrmann, K.; von Wahlde, M.K.; Schäfer, S.D.; Kiesel, L.; Greve, B.; Götte, M. Collagen I Triggers Directional Migration, Invasion and Matrix Remodeling of Stroma Cells in a 3D Spheroid Model of Endometriosis. Sci. Rep. 2021, 11, 4115. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.; Buckley, M.; Scalise, C.B.; Katre, A.A.; Dholakia, J.J.; Crossman, D.; Birrer, M.J.; Berry, J.L.; Arend, R.C. Understanding the Effect of Mechanical Forces on Ovarian Cancer Progression. Gynecol. Oncol. 2021, 162, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Iwanicki, M.P.; Davidowitz, R.A.; Ng, M.R.; Besser, A.; Muranen, T.; Merritt, M.; Danuser, G.; Ince, T.; Brugge, J.S. Ovarian Cancer Spheroids Use Myosin-Generated Force to Clear the Mesothelium. Cancer. Discov. 2011, 1, 144–157. [Google Scholar] [CrossRef] [PubMed]
- Sodek, K.L.; Brown, T.J.; Ringuette, M.J. Collagen I but Not Matrigel Matrices Provide an MMP-Dependent Barrier to Ovarian Cancer Cell Penetration. BMC Cancer 2008, 8, 223. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Kurniawan, N.A.; Kumar, A.P.; Rajagopalan, R.; Lim, C.T. Effects of Migrating Cell-Induced Matrix Reorganization on 3D Cancer Cell Migration. Cell Mol. Bioeng. 2014, 7, 205–217. [Google Scholar] [CrossRef]
- Berger, A.J.; Linsmeier, K.; Kreeger, P.K.; Masters, K.S. Decoupling the Effects of Stiffness and Fiber Density on Cellular Behaviors via an Interpenetrating Network of Gelatin- Methacrylate and Collagen. Biomaterials 2017, 141, 125–135. [Google Scholar] [CrossRef]
- Pieuchot, L.; Marteau, J.; Guignandon, A.; dos Santos, T.; Brigaud, I.; Chauvy, P.-F.; Cloatre, T.; Ponche, A.; Petithory, T.; Rougerie, P.; et al. Curvotaxis Directs Cell Migration through Cell-Scale Curvature Landscapes. Nat. Commun. 2018, 9, 3995. [Google Scholar] [CrossRef]
- Chen, C.; Ibrahim, Z.; Marchand, M.F.; Piolot, T.; Kamboj, S.; Carreiras, F.; Yamada, A.; Schanne-Klein, M.C.; Chen, Y.; Lambert, A.; et al. Three-Dimensional Collagen Topology Shapes Cell Morphology, beyond Stiffness. ACS Biomater. Sci. Eng. 2022, 8, 5284–5294. [Google Scholar] [CrossRef]
- Guzman, A.; Ziperstein, M.J.; Kaufman, L.J. The Effect of Fibrillar Matrix Architecture on Tumor Cell Invasion of Physically Challenging Environments. Biomaterials 2014, 35, 6954–6963. [Google Scholar] [CrossRef]
- Ziółkowska, K.; Kwapiszewski, R.; Stelmachowska, A.; Chudy, M.; Dybko, A.; Brzózka, Z. Development of a Three-Dimensional Microfluidic System for Long-Term Tumor Spheroid Culture. Sens. Actuators B Chem. 2012, 173, 908–913. [Google Scholar] [CrossRef]
- Sleeboom, J.J.F.; Amirabadi, H.E.; Nair, P.; Sahlgren, C.M.; den Toonder, J.M.J. Metastasis in Context: Modeling the Tumor Microenvironment with Cancer-on-a-Chip Approaches. Dis. Model. Mech. 2018, 11, dmm033100. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Kong, B.; Li, D. A New Tool for Probing of Cell-Cell Communication: Human Embryonic Germ Cells Inducing Apoptosis of SKOV3 Ovarian Cancer Cells on a Microfluidic Chip. Biotechnol. Lett. 2008, 30, 1537–1543. [Google Scholar] [CrossRef] [PubMed]
- Li, S.S.; Ip, C.K.M.; Tang, M.Y.H.; Sy, S.K.H.; Yung, S.; Chan, T.M.; Yang, M.; Shum, H.C.; Wong, A.S.T. Modeling Ovarian Cancer Multicellular Spheroid Behavior in a Dynamic 3D Peritoneal Microdevice. J. Vis. Exp. 2017, 2017, e55337. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, I.; Gurkan, U.A.; Tasoglu, S.; Alagic, N.; Celli, J.P.; Mensah, L.B.; Mai, Z.; Demirci, U.; Hasan, T. Flow Induces Epithelial-Mesenchymal Transition, Cellular Heterogeneity and Biomarker Modulation in 3D Ovarian Cancer Nodules. Proc. Natl. Acad. Sci. USA 2013, 110, E1974–E1983. [Google Scholar] [CrossRef] [PubMed]
- Avraham-Chakim, L.; Elad, D.; Zaretsky, U.; Kloog, Y.; Jaffa, A.; Grisaru, D. Fluid-Flow Induced Wall Shear Stress and Epithelial Ovarian Cancer Peritoneal Spreading. PLoS ONE 2013, 8, e0060965. [Google Scholar] [CrossRef]
- van der Meer, A.D.; Poot, A.A.; Feijen, J.; Vermes, I. Analyzing Shear Stress-Induced Alignment of Actin Filaments in Endothelial Cells with a Microfluidic Assay. Biomicrofluidics 2010, 4, 011103. [Google Scholar] [CrossRef][Green Version]
- Hassan, A.A.; Artemenko, M.; Tang, M.K.; Shi, Z.; Chen, L.-Y.; Lai, H.-C.; Yang, Z.; Shum, H.-C.; Wong, A.S.T. Ascitic Fluid Shear Stress in Concert with Hepatocyte Growth Factor Drive Stemness and Chemoresistance of Ovarian Cancer Cells via the C-Met-PI3K/Akt-MiR-199a-3p Signaling Pathway. Cell Death Dis. 2022, 13, 537. [Google Scholar] [CrossRef]
- Saha, B.; Mathur, T.; Handley, K.F.; Hu, W.; Afshar-Kharghan, V.; Sood, A.K.; Jain, A. OvCa-Chip microsystem recreates vascular endothelium-mediated platelet extravasation in ovarian cancer. Blood Adv. 2020, 4, 3329–3342. [Google Scholar] [CrossRef]
- Saha, B.; Mathur, T.; Tronolone, J.J.; Chokshi, M.; Lokhande, G.K.; Selahi, A.; Gaharwar, A.K.; Afshar-Kharghan, V.; Sood, A.K.; Bao, G.; et al. Human tumor microenvironment chip evaluates the consequences of platelet extravasation and combinatorial antitumor-antiplatelet therapy in ovarian cancer. Sci. Adv. 2021, 7, eabg5283. [Google Scholar] [CrossRef]
- Ibrahim, L.I.; Hajal, C.; Offeddu, G.S.; Gillrie, M.R.; Kamm, R.D. Omentum-on-a-chip: A multicellular, vascularized microfluidic model of the human peritoneum for the study of ovarian cancer metastases. Biomaterials 2022, 288, 121728. [Google Scholar] [CrossRef]













Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopez, E.; Kamboj, S.; Chen, C.; Wang, Z.; Kellouche, S.; Leroy-Dudal, J.; Carreiras, F.; Lambert, A.; Aimé, C. In Vitro Models of Ovarian Cancer: Bridging the Gap between Pathophysiology and Mechanistic Models. Biomolecules 2023, 13, 103. https://doi.org/10.3390/biom13010103
Lopez E, Kamboj S, Chen C, Wang Z, Kellouche S, Leroy-Dudal J, Carreiras F, Lambert A, Aimé C. In Vitro Models of Ovarian Cancer: Bridging the Gap between Pathophysiology and Mechanistic Models. Biomolecules. 2023; 13(1):103. https://doi.org/10.3390/biom13010103
Chicago/Turabian StyleLopez, Elliot, Sahil Kamboj, Changchong Chen, Zixu Wang, Sabrina Kellouche, Johanne Leroy-Dudal, Franck Carreiras, Ambroise Lambert, and Carole Aimé. 2023. "In Vitro Models of Ovarian Cancer: Bridging the Gap between Pathophysiology and Mechanistic Models" Biomolecules 13, no. 1: 103. https://doi.org/10.3390/biom13010103
APA StyleLopez, E., Kamboj, S., Chen, C., Wang, Z., Kellouche, S., Leroy-Dudal, J., Carreiras, F., Lambert, A., & Aimé, C. (2023). In Vitro Models of Ovarian Cancer: Bridging the Gap between Pathophysiology and Mechanistic Models. Biomolecules, 13(1), 103. https://doi.org/10.3390/biom13010103






