Cadmium Activates EGFR/STAT5 Signaling to Overcome Calcium Chelation and Promote Epithelial to Mesenchymal Transition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Cadmium Treatments
2.3. Real-Time Quantitative PCR
2.4. Immunofluorescence Assay
2.5. Western Blot
2.6. Statistical Analysis
3. Results
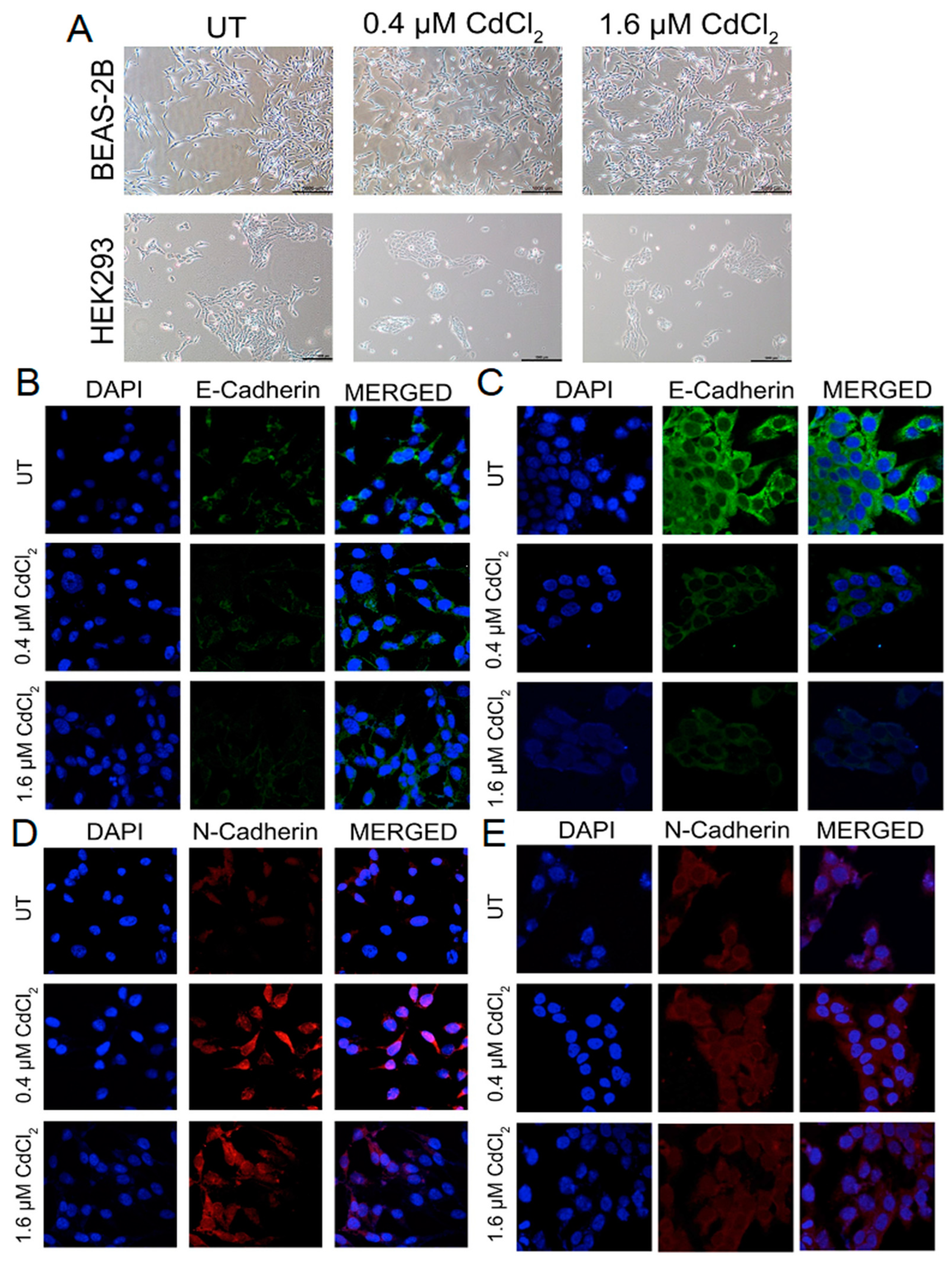
3.1. Epithelial Cells Overcome Cadmium-Mediated Loss of E-Cadherin by Increasing N-Cadherin
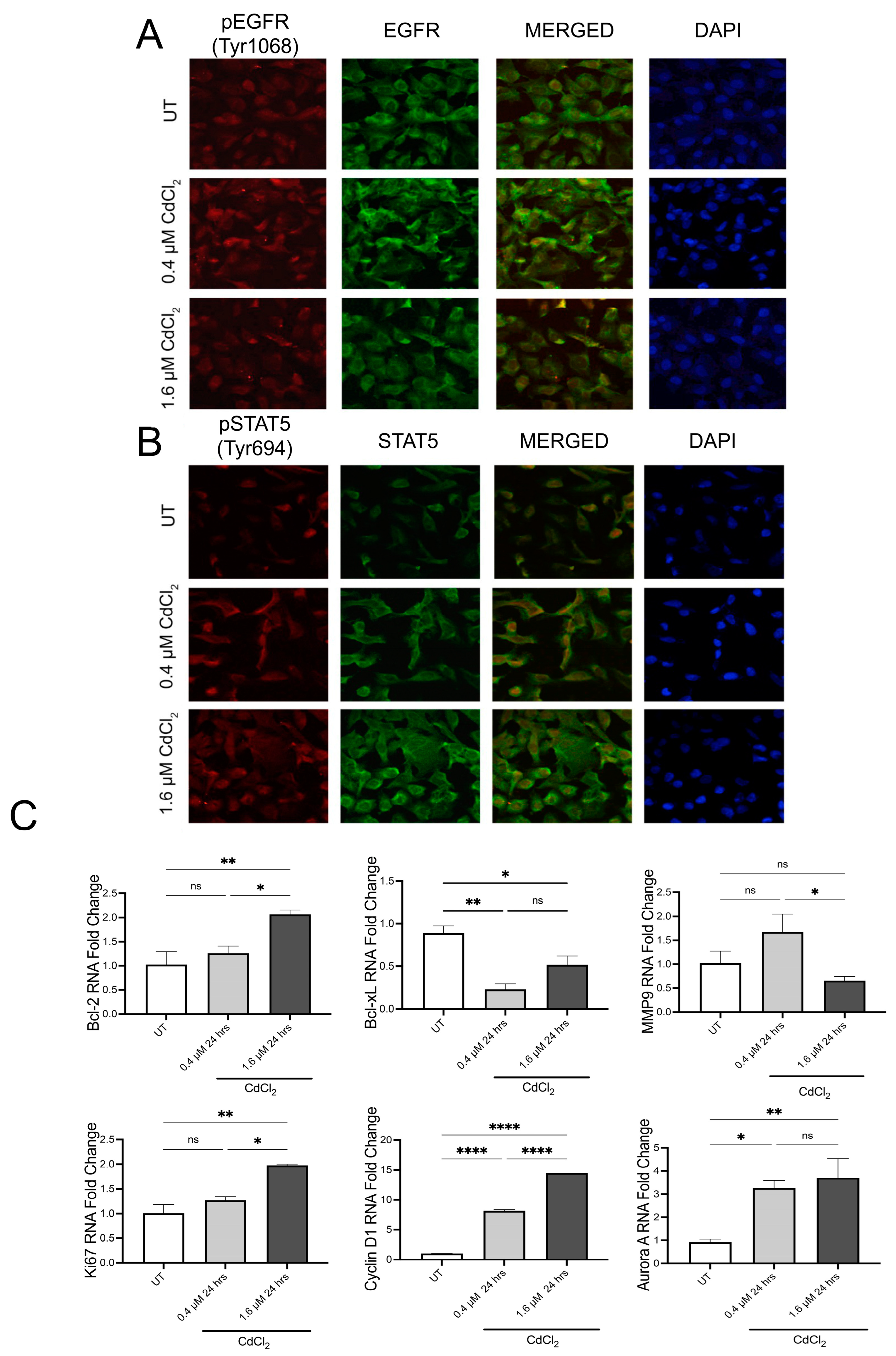
3.2. Low Doses of Cadmium Increased Phosphorylation of EGFR and STAT5 after 24 h
3.3. Cells Undergoing Chronic Exposure to Low Doses of Cadmium Maintain Cadherin Switch
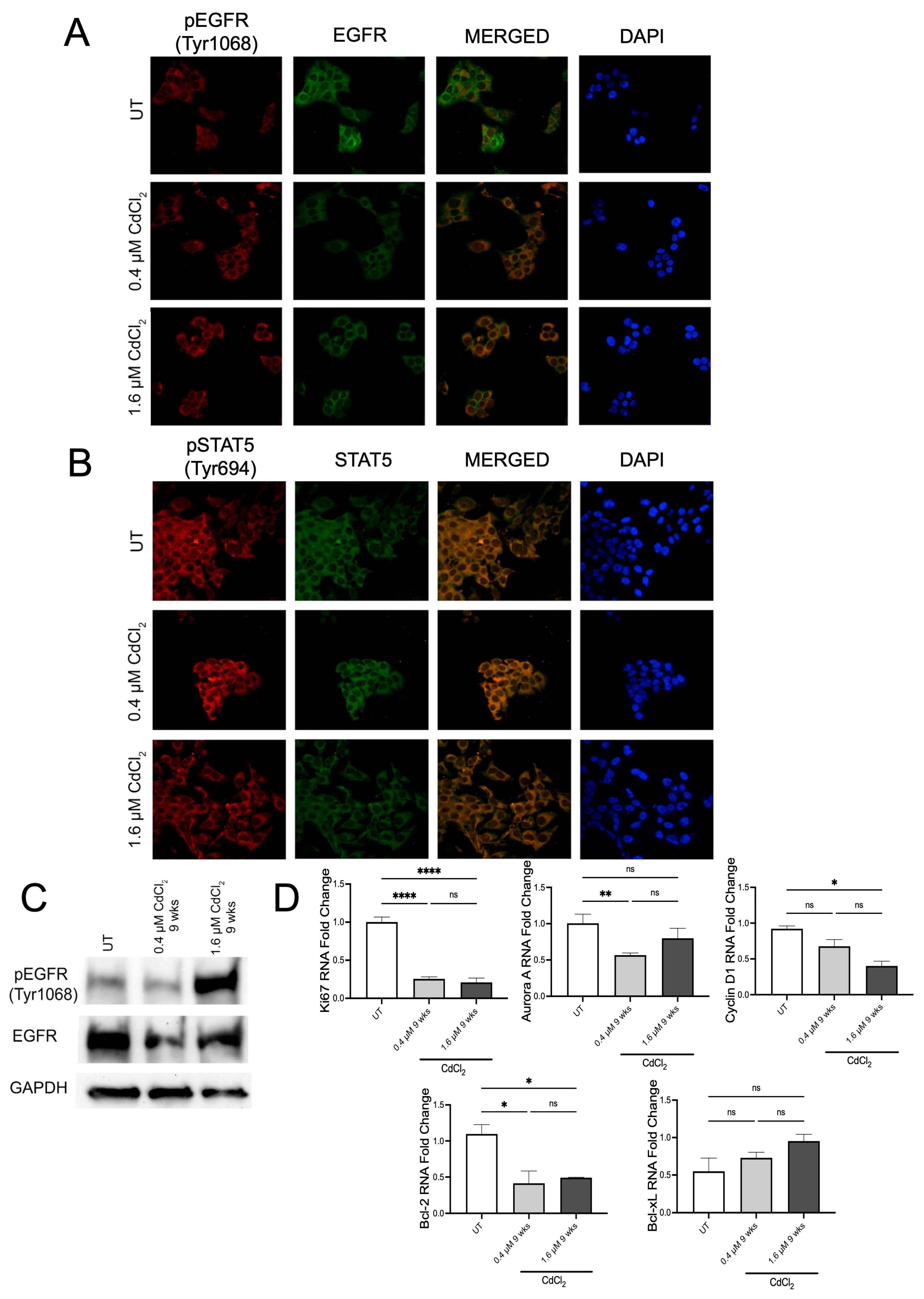
3.4. Chronic Exposure to Cadmium Sustained EGFR/STAT5 Signaling
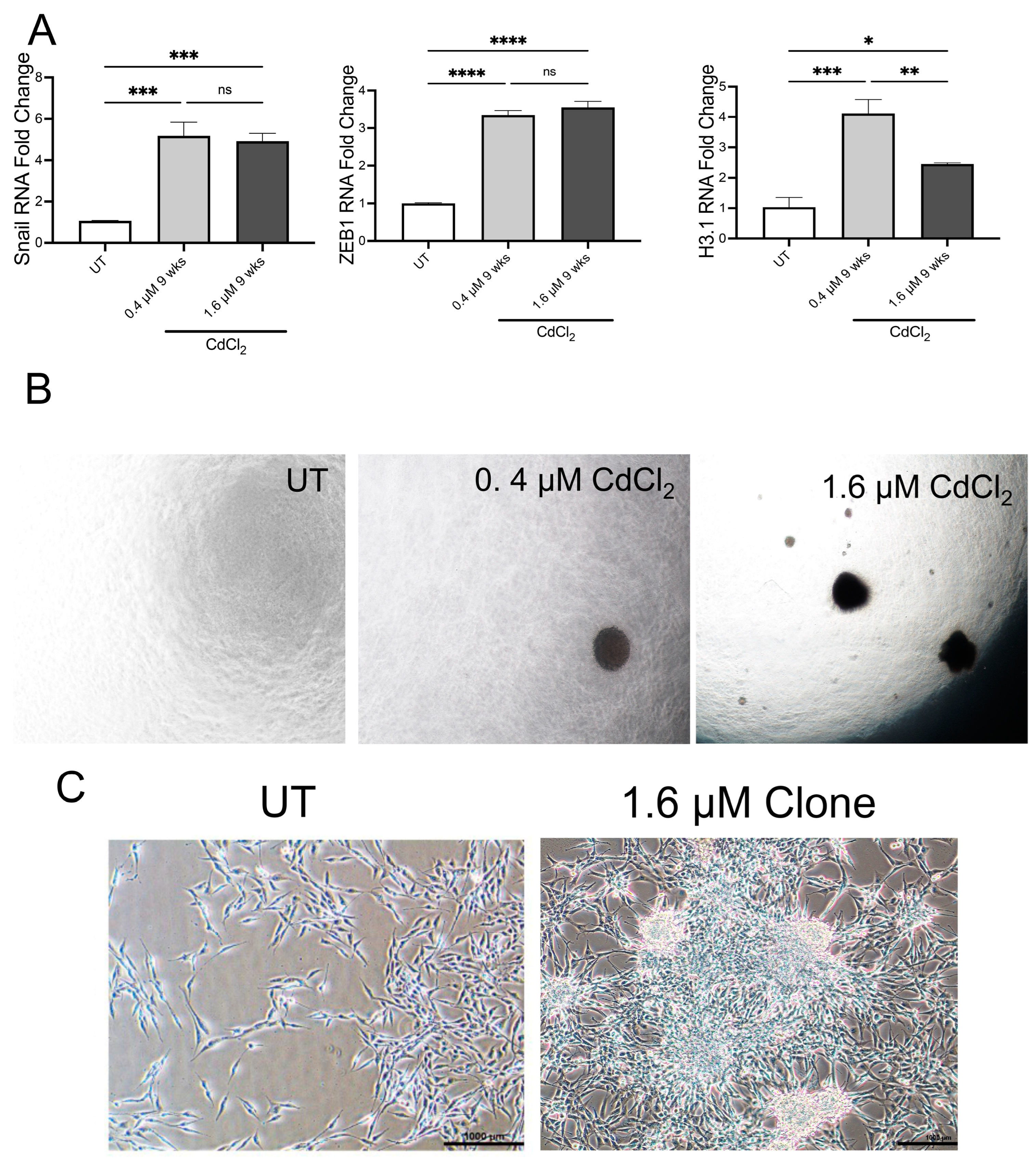
3.5. Chronic Exposure to Low Doses of Cadmium Induces EMT
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nordberg, G.F. Lung cancer and exposure to environmental cadmium. Lancet Oncol. 2006, 7, 99–101. [Google Scholar] [CrossRef] [PubMed]
- Nordberg, G.F.; Åkesson, A.; Nogawa, K.; Nordberg, M. Chapter 7—Cadmium. In Handbook on the Toxicology of Metals, 5th ed.; Nordberg, G.F., Costa, M., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 141–196. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. Cadmium and Cadmium Compounds IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer Monographs: Lyon, France, 1993; Volume 58, pp. 119–237. [Google Scholar]
- Centers for Disease Control and Prevention. Cadmium Toxicity: Who Is at Risk of Cadmium Exposure? Centers for Disease Control and Prevention: Atlanta, GA, USA, 2013. Available online: https://www.atsdr.cdc.gov/csem/cadmium/Who-Is-at-Risk.html (accessed on 6 December 2022).
- Itoh, H.; Iwasaki, M.; Sawada, N.; Takachi, R.; Kasuga, Y.; Yokoyama, S.; Onuma, H.; Nishimura, H.; Kusama, R.; Yokoyama, K.; et al. Dietary cadmium intake and breast cancer risk in Japanese women: A case–control study. Int. J. Hyg. Environ. Health 2014, 217, 70–77. [Google Scholar] [CrossRef]
- Zhang, P.; Sun, Y.; Ma, L. ZEB1: At the crossroads of epithelial-mesenchymal transition, metastasis and therapy resistance. Cell Cycle 2015, 14, 481–487. [Google Scholar] [CrossRef] [Green Version]
- Qian, X.; Karpova, T.; Sheppard, A.M.; McNally, J.; Lowy, D.R. E-cadherin-mediated adhesion inhibits ligand-dependent activation of diverse receptor tyrosine kinases. EMBO J. 2004, 23, 1739–1748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramirez Moreno, M.; Bulgakova, N.A. The Cross-Talk between EGFR and E-Cadherin. Front. Cell Dev. Biol. 2021, 9, 828673. [Google Scholar] [CrossRef]
- Bashir, N.A.; Ragab, E.S.; Khabour, O.F.; Khassawneh, B.Y.; Alfaqih, M.A.; Momani, J.A. The Association between Epidermal Growth Factor Receptor (EGFR) Gene Polymorphisms and Lung Cancer Risk. Biomolecules 2018, 8, 53. [Google Scholar] [CrossRef] [Green Version]
- Brand, T.M.; Iida, M.; Li, C.; Wheeler, D.L. The nuclear epidermal growth factor receptor signaling network and its role in cancer. Discov. Med. 2011, 12, 419–432. [Google Scholar] [PubMed]
- Hong, S.Y.; Kao, Y.R.; Lee, T.C.; Wu, C.W. Upregulation of E3 Ubiquitin Ligase CBLC Enhances EGFR Dysregulation and Signaling in Lung Adenocarcinoma. Cancer Res. 2018, 78, 4984–4996. [Google Scholar] [CrossRef] [Green Version]
- Kamiza, A.B.; Wang, W.C.; You, J.F.; Tang, R.; Wang, Y.T.; Chien, H.T.; Lai, C.H.; Chiu, L.L.; Lo, T.P.; Hung, K.Y.; et al. EGFR, SMAD7, and TGFBR2 Polymorphisms Are Associated with Colorectal Cancer in Patients with Lynch Syndrome. Anticancer Res. 2018, 38, 5983–5990. [Google Scholar] [CrossRef] [PubMed]
- Majumder, A.; Ray, S.; Banerji, A. Epidermal growth factor receptor-mediated regulation of matrix metalloproteinase-2 and matrix metalloproteinase-9 in MCF-7 breast cancer cells. Mol. Cell Biochem. 2019, 452, 111–121. [Google Scholar] [CrossRef]
- Makinoshima, H.; Takita, M.; Matsumoto, S.; Yagishita, A.; Owada, S.; Esumi, H.; Tsuchihara, K. Epidermal growth factor receptor (EGFR) signaling regulates global metabolic pathways in EGFR-mutated lung adenocarcinoma. J. Biol. Chem. 2014, 289, 20813–20823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roos, A.; Dhruv, H.D.; Peng, S.; Inge, L.J.; Tuncali, S.; Pineda, M.; Millard, N.; Mayo, Z.; Eschbacher, J.M.; Loftus, J.C.; et al. EGFRvIII-Stat5 Signaling Enhances Glioblastoma Cell Migration and Survival. Mol. Cancer Res. 2018, 16, 1185–1195. [Google Scholar] [CrossRef] [Green Version]
- Hung, L.Y.; Tseng, J.T.; Lee, Y.C.; Xia, W.; Wang, Y.N.; Wu, M.L.; Chuang, Y.H.; Lai, C.H.; Chang, W.C. Nuclear epidermal growth factor receptor (EGFR) interacts with signal transducer and activator of transcription 5 (STAT5) in activating Aurora-A gene expression. Nucleic Acids Res. 2008, 36, 4337–4351. [Google Scholar] [CrossRef] [PubMed]
- Quesnelle, K.M.; Boehm, A.L.; Grandis, J.R. STAT-mediated EGFR signaling in cancer. J. Cell Biochem. 2007, 102, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Tao, Y.; Jiang, Y.; Xu, Y.; Yan, B.; Chen, X.; Xiao, L.; Cao, Y. Nuclear epidermal growth factor receptor interacts with transcriptional intermediary factor 2 to activate cyclin D1 gene expression triggered by the oncoprotein latent membrane protein 1. Carcinogenesis 2012, 33, 1468–1478. [Google Scholar] [CrossRef]
- Wang, S.C.; Hung, M.C. Nuclear translocation of the epidermal growth factor receptor family membrane tyrosine kinase receptors. Clin. Cancer Res. 2009, 15, 6484–6489. [Google Scholar] [CrossRef] [Green Version]
- Basham, B.; Sathe, M.; Grein, J.; McClanahan, T.; D’Andrea, A.; Lees, E.; Rascle, A. In vivo identification of novel STAT5 target genes. Nucleic Acids Res. 2008, 36, 3802–3818. [Google Scholar] [CrossRef] [Green Version]
- Igelmann, S.; Neubauer, H.A.; Ferbeyre, G. STAT3 and STAT5 Activation in Solid Cancers. Cancers 2019, 11, 1428. [Google Scholar] [CrossRef] [Green Version]
- Leong, P.L.; Xi, S.; Drenning, S.D.; Dyer, K.F.; Wentzel, A.L.; Lerner, E.C.; Smithgall, T.E.; Grandis, J.R. 652 Differential function of STAT5 isoforms in head and neck cancer growth control. Oncogene 2002, 21, 2846–2853. [Google Scholar] [CrossRef]
- Kim, K.; Melough, M.M.; Vance, T.M.; Noh, H.; Koo, S.I.; Chun, O.K. Dietary Cadmium Intake and Sources in the US. Nutrients 2019, 11, 2. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6356330/ (accessed on 7 December 2022). [CrossRef] [Green Version]
- Fitzpatrick, M. Measuring Cell Fluorescence Using Imagej; The Open Lab Book v1.0. 2014. Available online: https://theolb.readthedocs.io/en/latest/imaging/measuring-cell-fluorescence-using-imagej.html (accessed on 7 December 2022).
- Talati, P.G.; Gu, L.; Ellsworth, E.M.; Girondo, M.A.; Trerotola, M.; Hoang, D.T.; Leiby, B.; Dagvadorj, A.; McCue, P.A.; Lallas, C.D.; et al. Jak2-stat5a/B signaling induces epithelial-to-mesenchymal transition and stem-like cell properties in prostate cancer. Am. J. Pathol. 2015, 185, 2505–2522. [Google Scholar] [CrossRef] [Green Version]
- Liao, Z.; Nevalainen, M.T. Targeting transcription factor Stat5a/b as a therapeutic strategy for prostate cancer. Am. J. Transl. Res. 2011, 3, 133–138. [Google Scholar] [PubMed]
- Cartularo, L.; Kluz, T.; Cohen, L.; Shen, S.S.; Costa, M. Molecular Mechanisms of Malignant Transformation by Low Dose Cadmium in Normal Human Bronchial Epithelial Cells. PLoS ONE 2016, 11, e0155002. [Google Scholar] [CrossRef] [Green Version]
- Xiao, C.; Liu, Y.; Xie, C.; Tu, W.; Xia, Y.; Costa, M.; Zhou, X. Cadmium induces histone H3 lysine methylation by inhibiting histone demethylase activity. Toxicol. Sci. 2015, 145, 80–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, I.; Damdimopoulou, P.; Stenius, U.; Halldin, K. Cadmium at nanomolar concentrations activates Raf-MEK-ERK1/2 MAPKs signaling via EGFR in human cancer cell lines. Chem. Biol. Interact. 2015, 231, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Tillo, E.; Lazaro, A.; Torrent, R.; Cuatrecasas, M.; Vaquero, E.C.; Castells, A.; Engel, P.; Postigo, A. ZEB1 represses E-cadherin and induces an EMT by recruiting the SWI/SNF chromatin-remodeling protein BRG1. Oncogene 2010, 29, 3490–3500. [Google Scholar] [CrossRef] [Green Version]
- Aban, C.E.; Lombardi, A.; Neiman, G.; Biani, M.C.; La Greca, A.; Waisman, A.; Moro, L.N.; Sevlever, G.; Miriuka, S.; Luzzani, C. Downregulation of E-cadherin in pluripotent stem cells triggers partial EMT. Sci. Rep. 2021, 11, 2048. [Google Scholar] [CrossRef]
- Takeda, T.; Tsubaki, M.; Matsuda, T.; Kimura, A.; Jinushi, M.; Obana, T.; Takegami, M.; Nishida, S. EGFR inhibition reverses epithelialmesenchymal transition, and decreases tamoxifen resistance via Snail and Twist downregulation in breast cancer cells. Oncol. Rep. 2022, 47, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hu, J.; Wang, Y.; Ye, W.; Zhang, X.; Ju, H.; Xu, D.; Liu, L.; Ye, D.; Zhang, L.; et al. EGFR activation induced Snail-dependent EMT and myc-dependent PD-L1 in human salivary adenoid cystic carcinoma cells. Cell Cycle 2018, 17, 1457–1470. [Google Scholar] [CrossRef] [Green Version]
- Yochum, Z.A.; Socinski, M.A.; Burns, T.F. Paradoxical functions of ZEB1 in EGFR-mutant lung cancer: Tumor suppressor and driver of therapeutic resistance. J. Thorac. Dis. 2016, 8, E1528–E1531. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.; Chen, Q.Y.; Wang, Z.; Zhu, Y.; Kluz, T.; Tan, W.; Li, J.; Wu, F.; Fang, L.; Zhang, X.; et al. Polyadenylation of Histone H3.1 mRNA Promotes Cell Transformation by Displacing H3.3 from Gene Regulatory Elements. Iscience 2020, 23, 101518. [Google Scholar] [CrossRef] [PubMed]



| Gene | Forward Prime (5′ → 3′) | Reverse Primer (5′ → 3′) |
|---|---|---|
| Bcl-2 | ATCGCCCTGTGGATGACTGAG | CGCCAGGAGAAATCAAACAGAGG |
| Bcl-xL | CTGAATCGGAGATGGAGACC | TGGGATGTCAGGTCACTGAA |
| MMP9 | ATCCAGTTTGGTGTCGCGGAGC | GAAGGGGAAGACGCACAGCT |
| AURKA | GGAGAGCTTAAAATTGCAGATTTT | GCTCCAGAGATCCTTCTCAT |
| Cyclin-D1 | CTTCCTCTCCAAAATGCCAG | AGAGATGGAAGGGGGAAAGA |
| Ki67 | GAGGTGTGCAGAAAATCCAAA | CTGTCCCTATGACTTCTGGTTGT |
| ZEB1 | AGCAGTGAAAGAGAAGGGAATGC | GGTCCTCTTCAGGTGCCTCAG |
| SNAIL | ACTGCAACAAGGAATACCTCAG | GCACTGGTACTTCTTGACATCTG |
| H3.1 | ACGCCAAGCGGGTGACTAT | TCTCGCCGCGGATACG |
| GAPDH | AGGGCTGCTTTTAACTCTGGT | CCCCACTTGATTTTGGAGGGA |
| Primary Antibody | Host Species Species | Dilution |
|---|---|---|
| EGFR (Santa Cruz Biotechnology, Dallas, TX) | Mouse | 1:100 |
| p-EGFR (Tyr1068) (Cell Signaling, Danvers, MA) | Rabbit | 1:100 |
| STAT5 (Santa Cruz Biotechnology, Dallas, TX) | Mouse | 1:100 |
| p-STAT5 (Tyr694) (Cell Signaling, Danvers, MA) | Rabbit | 1:100 |
| N-Cadherin (Cell Signaling, Danvers, MA) | Rabbit | 1:100 |
| E-Cadherin (Cell Signaling, Danvers, MA) | Mouse | 1:100 |
| Primary Antibody | Host Species Species | Dilution | Secondary Antibody | Dilution |
|---|---|---|---|---|
| N-Cadherin (Cell signaling, Danvers, MA) | Rabbit | 1:1000 | Goat anti-Rabbit IgG HRP-linked | 1:2000 |
| E-Cadherin (Cell signaling, Danvers, MA) | Mouse | 1:1000 | Horse anti-Mouse IgG HRP-linked | 1:1000 |
| EGFR (Santa Cruz Biotechnology, Dallas, TX) | Mouse | 1:1000 | Goat anti-Rabbit IgG HRP-linked | 1:2000 |
| p-EGFR (Tyr1068) (Cell Signaling, Danvers, MA) | Rabbit | 1:1000 | Goat anti-Rabbit IgG HRP-linked | 1:1000 |
| GAPDH (Proteintech, Rosemont, IL) | Mouse | 1:10,000 | Horse anti-Mouse IgG HRP-linked | 1:2000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stavrou, A.; Ortiz, A.; Costa, M. Cadmium Activates EGFR/STAT5 Signaling to Overcome Calcium Chelation and Promote Epithelial to Mesenchymal Transition. Biomolecules 2023, 13, 116. https://doi.org/10.3390/biom13010116
Stavrou A, Ortiz A, Costa M. Cadmium Activates EGFR/STAT5 Signaling to Overcome Calcium Chelation and Promote Epithelial to Mesenchymal Transition. Biomolecules. 2023; 13(1):116. https://doi.org/10.3390/biom13010116
Chicago/Turabian StyleStavrou, Aikaterini, Angelica Ortiz, and Max Costa. 2023. "Cadmium Activates EGFR/STAT5 Signaling to Overcome Calcium Chelation and Promote Epithelial to Mesenchymal Transition" Biomolecules 13, no. 1: 116. https://doi.org/10.3390/biom13010116





