Biotransformation of d-Xylose-Rich Rice Husk Hydrolysate by a Rice Paddy Soil Bacterium, Priestia sp. Strain JY310, to Low Molecular Weight Poly(3-hydroxybutyrate)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Reducing SugarRHH
2.2. Isolation of PHA-Producing Bacteria
2.3. Identification of a PHB-Producing Bacterial Isolate
2.4. Effects of Culture Conditions on Bacterial Growth and PHB Production
2.5. Batch Fermentation of Strain JY310
2.6. Isolation and Purification of PHA
2.7. Analytical Methods
3. Results and Discussion
3.1. Phylogenetic Identification of a PHB-Accumulating Bacterial Isolate
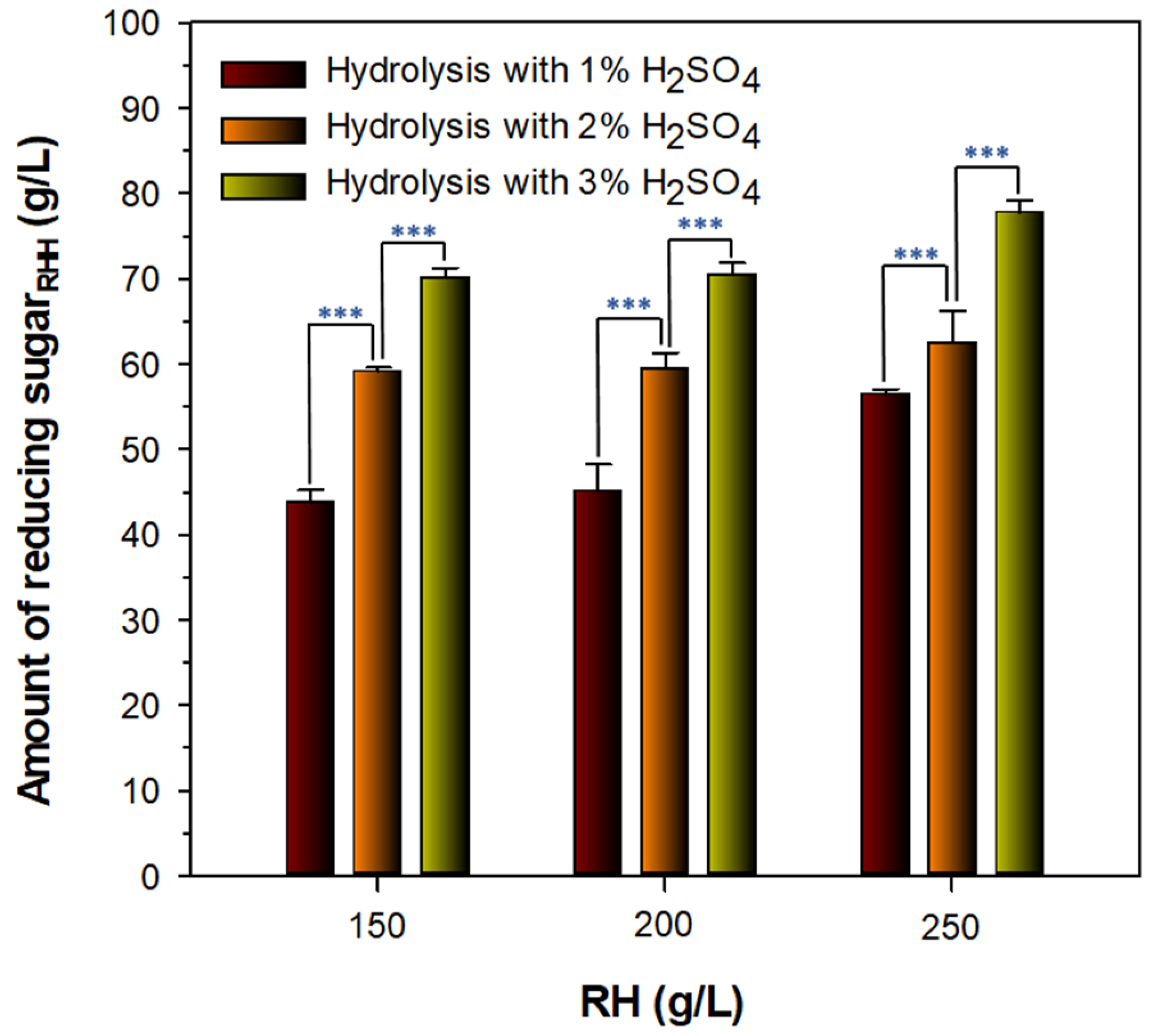
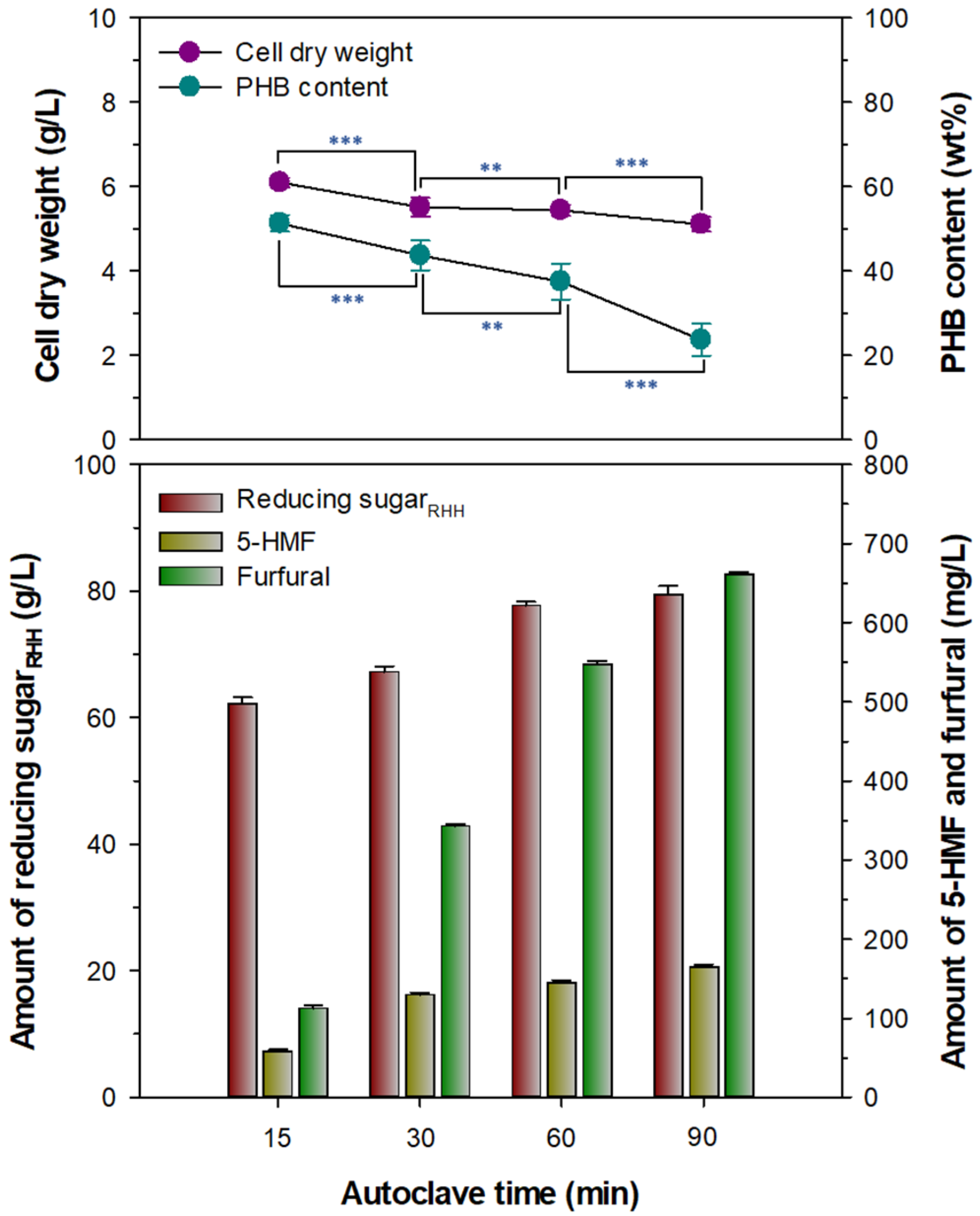
3.2. Preparation of Fermentable Reducing SugarRHH for PHB Production
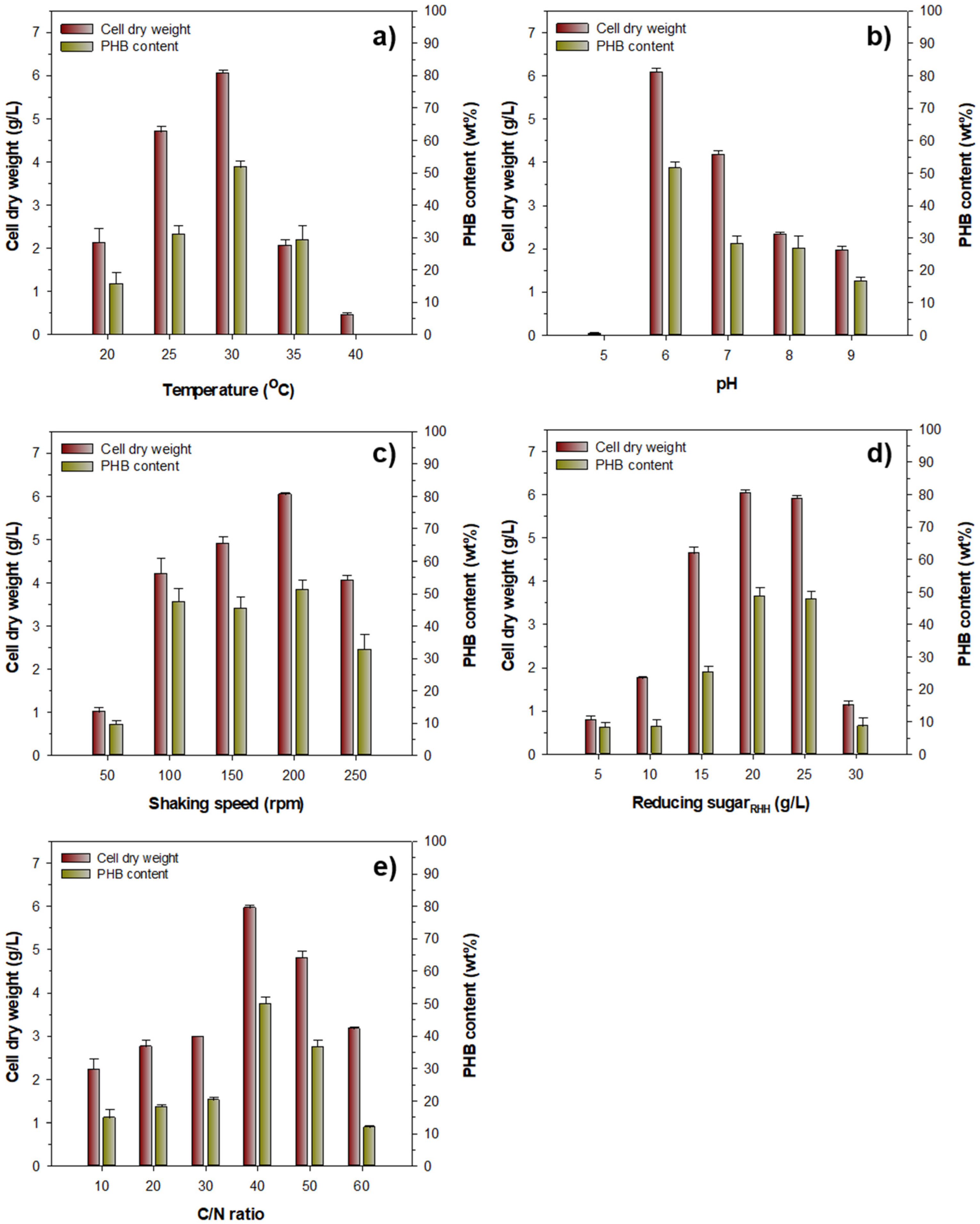
3.3. Optimization of Culture Conditions for Bacterial Growth and PHB Production
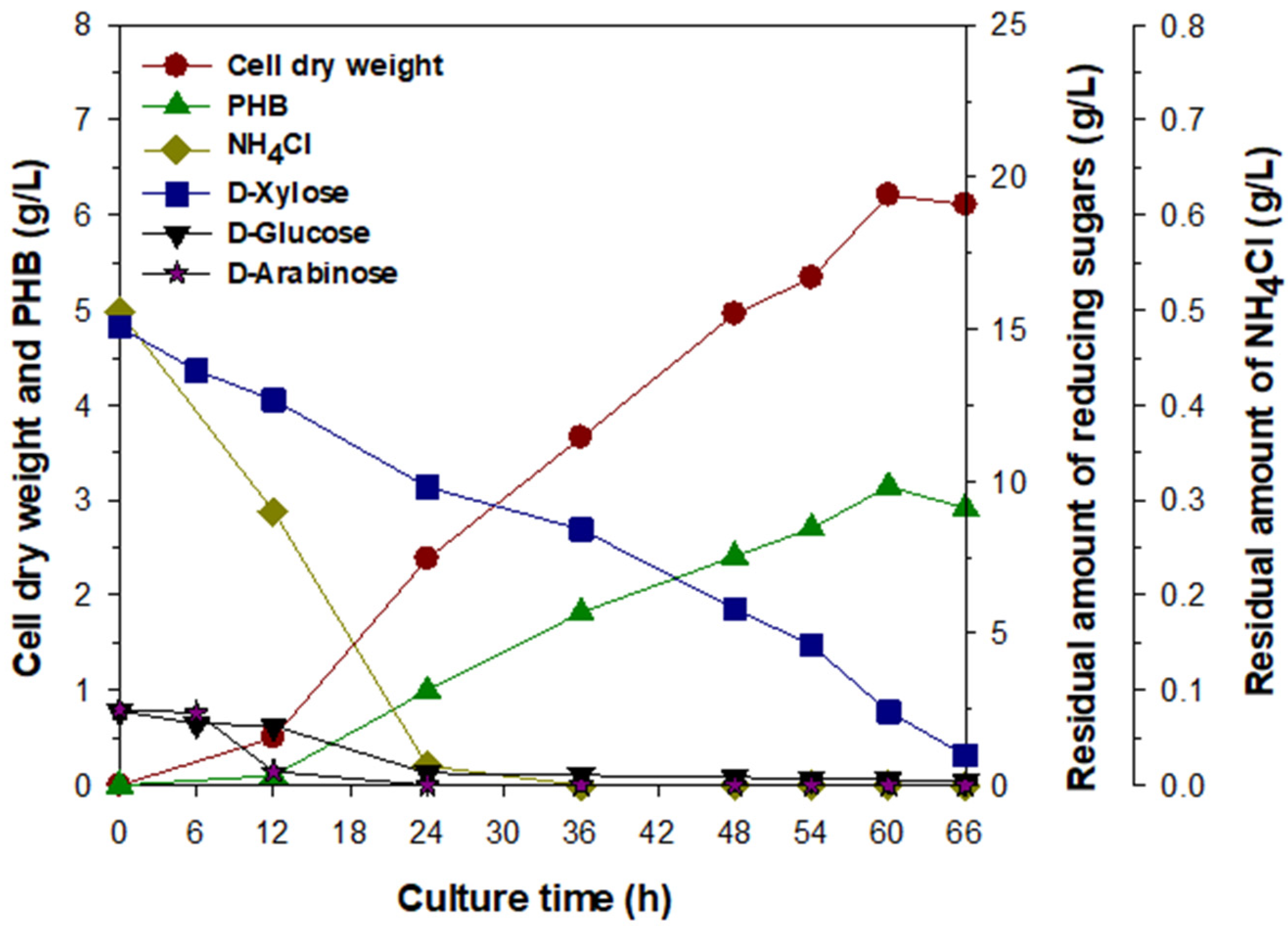
3.4. Bacterial Production of PHB by Batch Fermentation under Optimized Culture Conditions
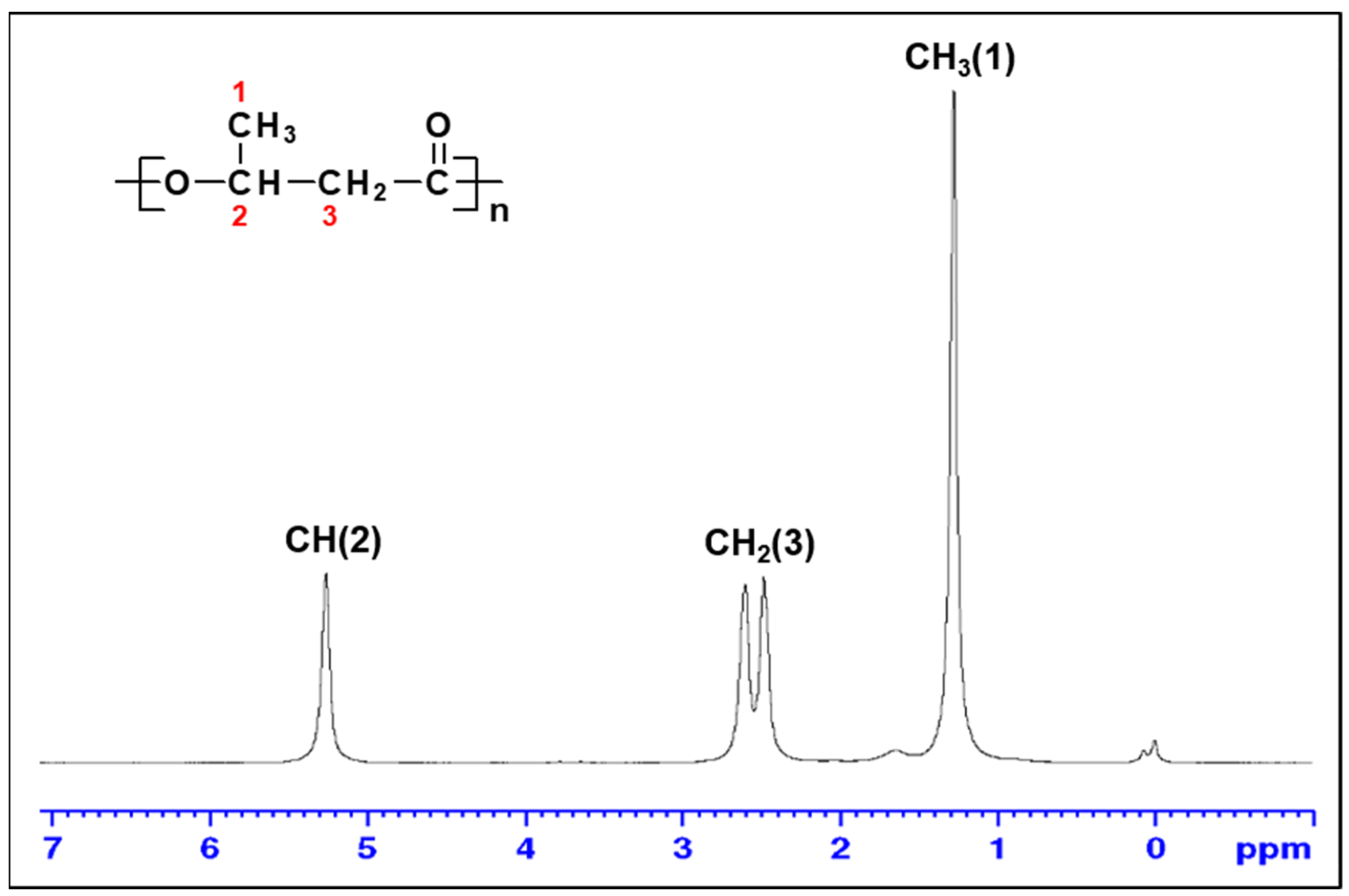
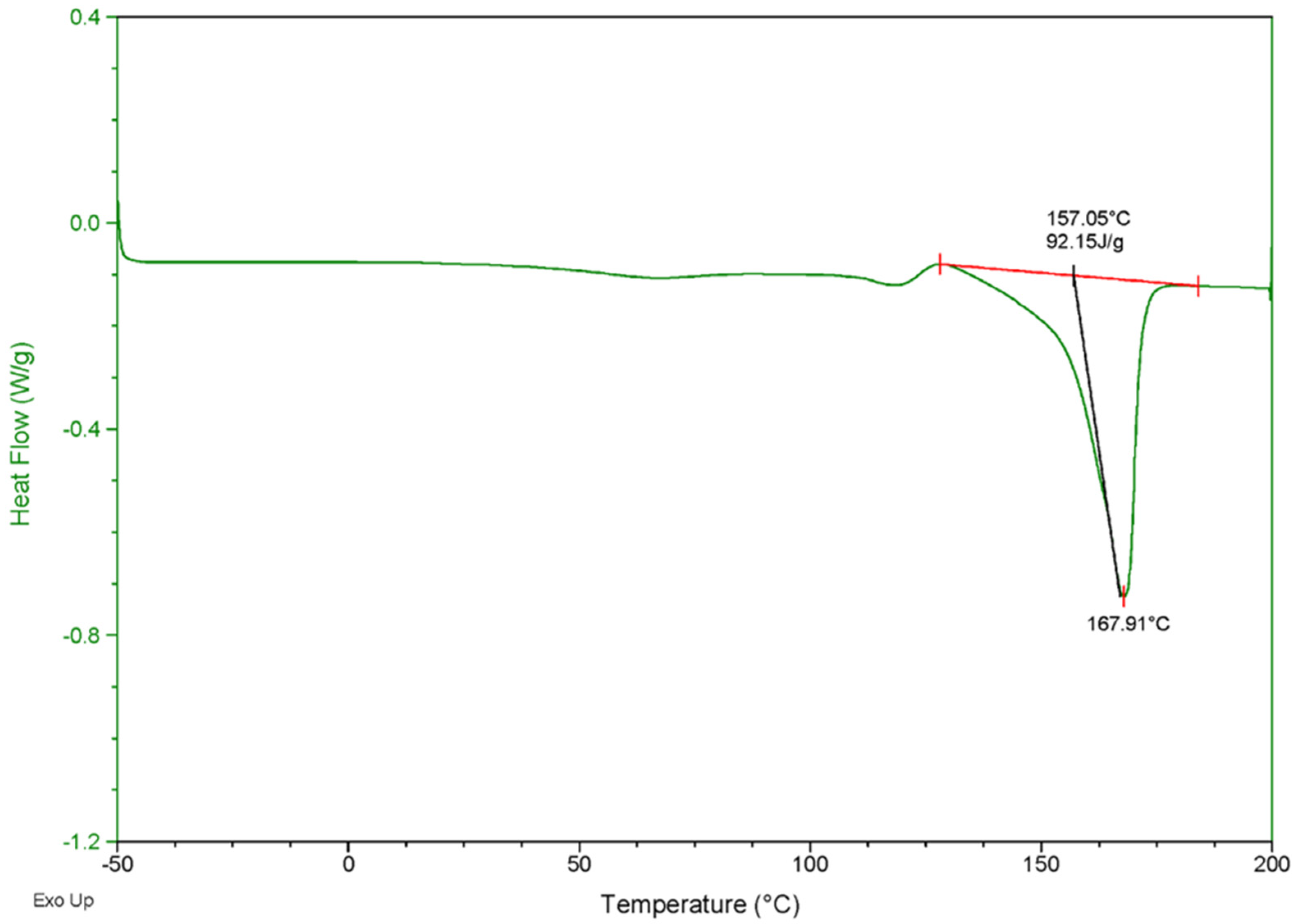
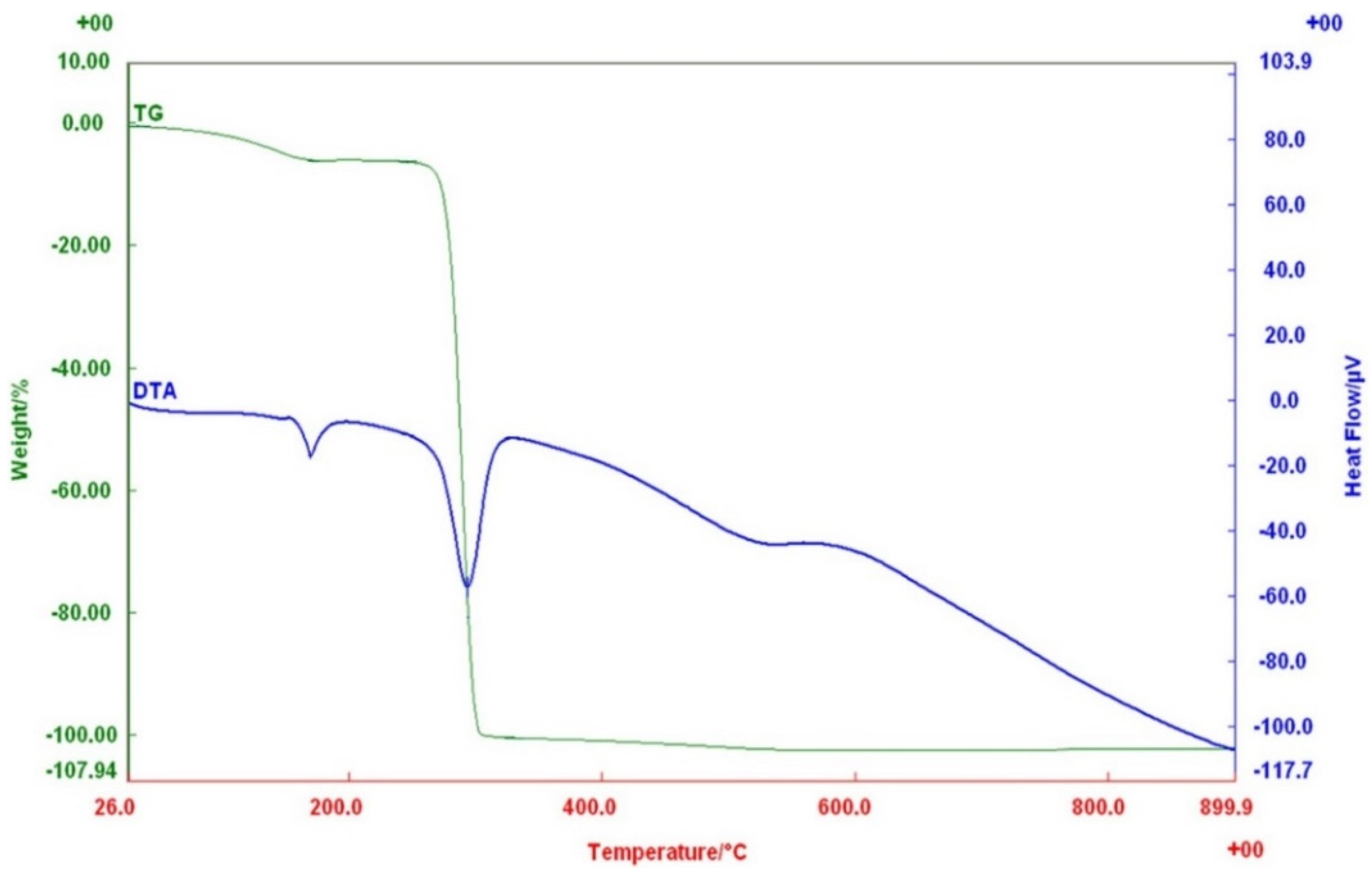
3.5. Characterization of PHB Biosynthesized by Priestia sp. Strain JY310
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jayanth, D.; Kumar, P.S.; Nayak, G.C.; Kumar, J.S.; Pal, S.K.; Rajasekar, R. A review on biodegradable polymeric materials striving towards the attainment of green environment. J. Polym. Environ. 2018, 26, 838–865. [Google Scholar] [CrossRef]
- Peng, Y.; Wu, P.; Schartup, A.T.; Zhang, Y. Plastic waste release caused by COVID-19 and its fate in the global ocean. Proc. Natl. Acad. Sci. USA 2021, 118, e2111530118. [Google Scholar] [CrossRef] [PubMed]
- Anjum, A.; Zuber, M.; Zia, K.M.; Noreen, A.; Anjum, M.N.; Tabasum, S. Microbial production of polyhydroxyalkanoates (PHAs) and its copolymers: A review of recent advancements. Int. J. Biol. Macromol. 2016, 89, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic waste inputs from land into the ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, K.; Dumont, M.-J.; Del Rio, L.F.; Orsat, V. Producing PHAs in the bioeconomy—Towards a sustainable bioplastic. Sustain. Prod. Consump. 2017, 9, 58–70. [Google Scholar] [CrossRef]
- Amaro, T.M.M.M.; Rosa, D.; Comi, G.; Iacumin, L. Prospects for the use of whey for polyhydroxyalkanoate (PHA) production. Front. Microbiol. 2019, 10, 992. [Google Scholar] [CrossRef]
- Shah, A.A.; Hasan, F.; Hameed, A.; Ahmed, S. Biological degradation of plastics: A comprehensive review. Biotechnol. Adv. 2008, 26, 246–265. [Google Scholar] [CrossRef]
- Sudesh, K.; Abe, H.; Doi, Y. Synthesis, structure and properties of polyhydroxyalkanoates: Biological polyesters. Prog. Polym. Sci. 2000, 25, 1503–1555. [Google Scholar] [CrossRef]
- Tokiwa, Y.; Calabia, B.P.; Ugwu, C.U.; Aiba, S. Biodegradability of plastics. Int. J. Mol. Sci. 2009, 10, 3722–3742. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.Y.; Kim, H.W.; Chung, M.G.; Rhee, Y.H. Biosynthesis, modification, and biodegradation of bacterial medium-chain-length polyhydroxyalkanoates. J. Microbiol. 2007, 45, 87–97. [Google Scholar]
- McAdam, B.; Fournet, M.B.; McDonald, P.; Mojicevic, M. Production of polyhydroxybutyrate (PHB) and factors impacting its chemical and mechanical characteristics. Polymers 2020, 12, 2908. [Google Scholar] [CrossRef] [PubMed]
- Hermann-Krauss, C.; Koller, M.; Muhr, A.; Fasl, H.; Stelzer, F.; Braunegg, G. Archaeal production of polyhydroxyalkanoate (PHA) co- and terpolyesters from biodiesel industry-derived by products. Archaea 2013, 2013, 129268. [Google Scholar] [CrossRef] [Green Version]
- Harding, K.G.; Dennis, J.S.; von Blottnitz, H.; Harrison, S.T.L. Environmental analysis of plastic production processes: Comparing petroleum-based polypropylene and polyethylene with biologically-based poly-β-hydroxybutyric acid using life cycle analysis. J. Biotechnol. 2007, 130, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Rhee, Y.H. Biodegradation of microbial and synthetic polyesters by fungi. Appl. Microbiol. Biotechnol. 2003, 61, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Haddadi, M.H.; Asadolahi, R.; Negahdari, B. The bioextraction of bioplastics with focus on polyhydroxybutyrate: A review. Int. J. Environ. Sci. Technol. 2019, 16, 3935–3948. [Google Scholar] [CrossRef]
- Kim, B.S. Production of poly(3-hydroxybutyrate) from inexpensive substrates. Enzyme Microb. Technol. 2000, 27, 774–777. [Google Scholar] [CrossRef]
- Colombo, B.; Calvo, M.V.; Sciarria, T.P.; Scaglia, B.; Kizito, S.S.; D’Imporzano, G.; Adani, F. Biohydrogen and polyhydroxyalkanoates (PHA) as products of a two-steps bioprocess from deproteinized dairy wastes. Waste Manag. 2019, 95, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Mannina, G.; Presti, D.; Montiel-Jarillo, G.; Carrera, J.; Suárez-Ojeda, M.E. Recovery of polyhydroxyalkanoates (PHAs) from wastewater: A review. Bioresour. Technol. 2020, 297, 122478. [Google Scholar] [CrossRef] [PubMed]
- Sandhya, M.; Aravind, J.; Kanmani, P. Production of polyhydroxyalkanoates from Ralstonia eutropha using paddy straw as cheap substrate. Int. J. Environ. Sci. Technol. 2013, 10, 47–54. [Google Scholar] [CrossRef]
- Gang, S.; Lee, W.; Kwon, K.; Kim, T.; Kim, J.-S.; Chung, C.-W. Production of polyhydroxybutyrate from Ralstonia eutropha H-16 using Makgeolli lees enzymatic hydrolysate. J. Polym. Envion. 2019, 27, 2182–2188. [Google Scholar] [CrossRef]
- Silva, L.F.; Taciro, M.K.; Michelin Ramos, M.E.; Carter, J.M.; Pradella, J.G.C.; Gomez, J.G.C. Poly-3-hydroxybutyrate (P3HB) produced by bacteria from xylose, glucose and sugarcane bagasse hydrolysate. J. Ind. Microbiol. Biotechnol. 2004, 31, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Sirohi, R.; Singh, A.; Tarafdar, A.; Shahi, N.C.; Verma, A.K.; Kushwaha, A. Cellulase production from pre-treated pea hulls using Trichoderma reesei under submerged fermentation. Waste Biomass Valori. 2019, 10, 2651–2659. [Google Scholar] [CrossRef]
- Ventorino, V.; Aliberti, A.; Faraco, V.; Robertiello, A.; Giacobbe, S.; Ercolini, D.; Amore, A.; Fagnano, M.; Pepe, O. Exploring the microbiota dynamics related to vegetable biomasses degradation and study of lignocellulose-degrading bacteria for industrial biotechnological application. Sci. Rep. 2015, 5, 8161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silambarasan, S.; Logeswari, P.; Sivaramakrishnan, R.; Pugazhendhi, A.; Kamaraj, B.; Ruiz, A.; Ramadoss, G.; Cornejo, P. Polyhydroxybutyrate production from ultrasound-aided alkaline pretreated finger millet straw using Bacillus megaterium strain CAM12. Bioresour. Technol. 2021, 325, 124632. [Google Scholar] [CrossRef]
- Bansal, V.; Ahmad, A.; Sastry, M. Fungus-mediated biotransformation of amorphous silica in rice husk to nanocrystalline silica. J. Am. Chem. Soc. 2006, 128, 14059–14066. [Google Scholar] [CrossRef]
- Hong, S.-G.; Hsu, H.-W.; Ye, M.-T. Thermal properties and applications of low molecular weight polyhydroxybutyrate. J. Therm. Anal. Calorim. 2013, 111, 1243–1250. [Google Scholar] [CrossRef]
- Xu, W.; Liang, L.; Zhu, M. Determination of sugars in molasses by HPLC following solid-phase extraction. Int. J. Food Prop. 2015, 18, 547–557. [Google Scholar] [CrossRef]
- Gao, X.; Wen, Y.; Qu, D.; An, L.; Luan, S.; Jiang, W.; Zong, W.; Liu, W.; Sun, Z. Interference effect of alcohol on Nessler’s reagent in photocatalytic nitrogen fixation. ACS Sustain. Chem. Eng. 2018, 6, 5342–5348. [Google Scholar] [CrossRef]
- Jönsson, L.; Alriksson, B.; Nilvebrant, N.-O. Bioconversion of lignocellulose: Inhibitors and detoxification. Biotechnol. Biofuels 2013, 6, 16. [Google Scholar] [CrossRef] [Green Version]
- Pan, W.; Perrotta, J.A.; Stipanovic, A.J.; Nomura, C.T.; Nakas, J.P. Production of polyhydroxyalkanoates by Burkholderia cepacia ATCC 17759 using a detoxified sugar maple hemicellulosic hydrolysate. J. Ind. Microbiol. Biotechnol. 2012, 39, 459–469. [Google Scholar] [CrossRef]
- Guarnieri, M.T.; Franden, M.A.; Johnson, C.W.; Beckham, G.T. Conversion and assimilation of furfural and 5-(hydroxymethyl)furfural by Pseudomonas putida KT2440. Metab. Eng. Commun. 2017, 4, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Lopes, M.S.G.; Gomez, J.G.C.; Taciro, M.K.; Mendonça, T.T.; Silva, L.F. J. Polyhydroxyalkanoate biosynthesis and simultaneous remotion of organic inhibitors from sugarcane bagasse hydrolysate by Burkholderia sp. Ind. Microbiol. Biotechnol. 2014, 41, 1353–1363. [Google Scholar] [CrossRef] [PubMed]
- Sindhu, R.; Silviya, N.; Binod, P.; Pandey, A. Pentose-rich hydrolysate from acid pretreated rice straw as a carbon source for the production of poly-3-hydroxybutyrate. Biochem. Eng. J. 2013, 78, 67–72. [Google Scholar] [CrossRef]
- Lee, S.M.; Lee, H.-J.; Kim, S.H.; Suh, M.J.; Cho, J.Y.; Ham, S.; Song, H.-S.; Bhatia, S.K.; Gurav, R.; Jeon, J.-M.; et al. Engineering of Shewanella marisflavi BBL25 for biomass-based polyhydroxybutyrate production and evaluation of its performance in electricity production. Int. J. Biol. Macromol. 2021, 183, 1669–1675. [Google Scholar] [CrossRef] [PubMed]
- Heng, K.-S.; Hatti-Kaul, R.; Adam, F.; Fukui, T.; Sudesh, K. Conversion of rice husks to polyhydroxyalkanoates (PHA) via a three-step process: Optimized alkaline pretreatment, enzymatic hydrolysis, and biosynthesis by Burkholderia cepacia USM (JCM 15050). J. Chem. Technol. Biotechnol. 2017, 92, 100–108. [Google Scholar] [CrossRef]
- Cesário, M.T.; Raposo, R.S.; de Almeida, M.C.M.D.; van Keulen, F.; Ferreira, B.S.; da Fonseca, M.M.R. Enhanced bioproduction of poly-3-hydroxybutyrate from wheat straw lignocellulosic hydrolysates. New Biotechnol. 2014, 31, 104–113. [Google Scholar] [CrossRef] [Green Version]
- Dietrich, K.; Dumont, M.-J.; Schwinghamer, T.; Orsat, V.; Del Rio, L.F. Model study to assess softwood hemicellulose hydrolysates as the carbon source for PHB production in Paraburkholderia sacchari IPT 101. Biomacromolecules 2018, 19, 188–200. [Google Scholar] [CrossRef]
- Dietrich, K.; Oliveira-Filho, E.R.; Dumont, M.-J.; Gomez, J.G.C.; Taciro, M.K.; da Silva, L.F.; Orsat, V.; Del Rio, L.F. Increasing PHB production with an industrially scalable hardwood hydrolysate as a carbon source. Ind. Crops Prod. 2020, 154, 112703. [Google Scholar] [CrossRef]
- Annamalai, N.; Sivakumar, N. Production of polyhydroxybutyrate from wheat bran hydrolysate using Ralstonia eutropha through microbial fermentation. J. Biotechnol. 2016, 237, 13–17. [Google Scholar] [CrossRef]
- Saratale, G.D.; Oh, M.-K. Characterization of poly-3-hydroxybutyrate (PHB) produced from Ralstonia eutropha using an alkali-pretreated biomass feedstock. Int. J. Biol. Macromol. 2015, 80, 627–635. [Google Scholar] [CrossRef]
- Saratale, R.G.; Saratale, G.D.; Cho, S.K.; Kim, D.S.; Ghodake, G.S.; Kadam, A.; Kumar, G.; Bharagava, R.N.; Banu, R.; Shin, H.S. Pretreatment of kenaf (Hibiscus cannabinus L.) biomass feedstock for polyhydroxybutyrate (PHB) production and characterization. Bioresour. Technol. 2019, 282, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Cho, D.-H.; Jung, H.J.; Kim, B.; Kim, S.H.; Bhatia, S.K.; Gurav, R.; Jeon, J.-M.; Yoon, J.-J.; Kim, W.; et al. Finding of novel polyhydroxybutyrate producer Loktanella sp. SM43 capable of balanced utilization of glucose and xylose from lignocellulosic biomass. Int. J. Biol. Macromol. 2022, 208, 809–818. [Google Scholar] [CrossRef] [PubMed]
- de Souza, L.; Manasa, Y.; Shivakumar, S. Bioconversion of lignocellulosic substrates for the production of polyhydroxyalkanoates. Biocatal. Agric. Biotechnol. 2020, 28, 101754. [Google Scholar] [CrossRef]
- Kucera, D.; Pernicová, I.; Kovalcik, A.; Koller, M.; Mullerova, L.; Sedlacek, P.; Mravec, F.; Nebesarova, J.; Kalina, M.; Marova, I.; et al. Characterization of the promising poly(3-hydroxybutyrate) producing halophilic bacterium Halomonas halophila. Bioresour. Technol. 2018, 256, 552–556. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L.; Li, T.; Shen, Y.; Luo, J. Acid soaking followed by steam flash-explosion pretreatment to enhance saccharification of rice husk for poly(3-hydroxybutyrate) production. Int. J. Biol. Macromol. 2020, 160, 446–455. [Google Scholar] [CrossRef]
- Oliveira-Filho, E.R.; Gomez, J.G.C.; Taciro, M.K.; Silva, L.F. Burkholderia sacchari (synonym Paraburkholderia sacchari): An industrial and versatile bacterial chassis for sustainable biosynthesis of polyhydroxyalkanoates and other bioproducts. Bioresour. Technol. 2021, 337, 125472. [Google Scholar] [CrossRef]
- Sindhu, R.; Ammu, B.; Binod, P.; Sreelatha, K.; Deepthi, K.; Ramachandran, B.; Soccol, C.R.; Pandey, A. Production and characterization of poly-3-hydroxybutyrate from crude glycerol by Bacillus sphaericus NII 0838 and improving its thermal properties by blending with other polymers. Braz. Arch. Biol. Technol. 2011, 54, 783–794. [Google Scholar] [CrossRef] [Green Version]
- Penkhrue, W.; Jendrossek, D.; Khanongnuch, C.; Pathom-aree, W.; Aizawa, T.; Behrens, R.L.; Lumyong, S. Response surface method for polyhydroxybutyrate (PHB) bioplastic accumulation in Bacillus drentensis BP17 using pineapple peel. PLoS ONE 2020, 15, e0230443. [Google Scholar] [CrossRef] [Green Version]
- Lim, H.N.; Lee, Y.; Hussein, R. Fundamental relationship between operon organization and gene expression. Proc. Natl. Acad. Sci. USA 2011, 108, 10626–10631. [Google Scholar] [CrossRef] [Green Version]
- Castillo, T.; Flores, C.; Segura, D.; Espín, G.; Sanguino, J.; Cabrera, E.; Barreto, J.; Díaz-Barrera, A.; Peña, C. Production of polyhydroxybutyrate (PHB) of high and ultra-high molecular weight by Azotobacter vinelandii in batch and fed-batch cultures. J. Chem. Technol. Biotechnol. 2017, 92, 1809–1816. [Google Scholar] [CrossRef]


| Strain | Carbon Source | Preparation Method | CDW (g/L) | PHB Content (wt%) | PHB (g/L) | Scale | Reference |
|---|---|---|---|---|---|---|---|
| Priestia sp. strain JY310 | Rice husk hydrolysate | Thermochemical hydrolysis | 6.2 | 50.4 | 3.1 | Batch bioreactor | This study |
| Burkholderia cepacia USM | Rice husk hydrolysate | Thermochemical and enzymatic hydrolysis | 7.8 | 50.0 | 3.9 | Batch Bioreactor | [35] |
| Burkholderia cepacia IPT 048 | Sugarcane bagasse hydrolysate | Thermochemical hydrolysis | 4.4 | 53.0 | 2.3 | Batch bioreactor | [21] |
| Burkholderia sacchari IPT 101 | Sugarcane bagasse hydrolysate | Thermochemical hydrolysis | 4.4 | 62.0 | 2.7 | Batch bioreactor | [21] |
| Burkholderia sacchari DSM 17165 | Wheat straw hydrolysate | AFEX process and enzymatic hydrolysis | 7.7 | 57.0 | 4.4 | Fed-batch bioreactor | [36] |
| Paraburkholderia sacchari IPT 101 | Softwood hemicellulose hydrolysates | Thermochemical hydrolysis | 7.1 | 80.5 | 5.7 | Shake Flask | [37] |
| Paraburkholderia sacchari IPT 101 | Sugarcane hydrolysate | Thermochemical hydrolysis | 40.3 | 55.0 | 22.0 | Fed-batch bioreactor | [38] |
| Paraburkholderia sacchari IPT 101 | Hardwood hydrolysate | Thermomechanical pulping and enzymatic hydrolysis | 59.5 | 58.0 | 34.5 | Fed-batch bioreactor | [38] |
| Shewanella marisflavi BBL25 | Barley straw hydrolysate | Thermochemical and enzymatic hydrolysis | 5.8 | 56.0 | 3.2 | Shake Flask | [34] |
| Ralstonia eutropha NCIMB 11599 | Wheat bran hydrolysate | Thermochemical and enzymatic hydrolysis | 24.5 | 62.5 | 15.3 | Shake Flask | [39] |
| Ralstonia eutropha ATCC 17699 | Rice paddy straw hydrolysate | Thermochemical and enzymatic hydrolysis | 15.5 | 63.7 | 9.8 | Shake Flask | [40] |
| Ralstonia eutropha ATCC 17699 | Kenaf hydrolysate | Thermochemical and enzymatic hydrolysis | 17.9 | 81.0 | 10.1 | Shake Flask | [41] |
| Loktanella sp. SM43 | Pine tree hydrolysate | Thermochemical and enzymatic hydrolysis | 4.68 | 78.0 | 3.6 | Shake Flask | [42] |
| Bacillus firmus NII 0830 | Rice straw hydrolysate | Thermochemical hydrolysis | 1.9 | 89.0 | 1.6 | Shake flask | [33] |
| Bacillus megaterium Ti3 | Corn husk hydrolysate | Biological hydrolysis | 1.7 | 59.0 | 1.0 | Shake flask | [43] |
| Halomonas halophila CCM 3662 | Spent coffee grounds hydrolysate | Thermochemical hydrolysis | 3.5 | 61.9 | 2.1 | Shake flask | [44] |
| Source | Carbon Substrate | Tg (°C) | Tm (°C) | Td (°C) | Mn (g/mol) | Mw (g/mol) | PDI (Mw/Mn) | Reference |
|---|---|---|---|---|---|---|---|---|
| Priestia sp. strain JY310 | Rice husk hydrolysate | NI a | 167.9 | 268.1 | 16,200 | 76,800 | 4.73 | This study |
| Cupriavidus necator | Rice husk hydrolysate | NI | 175.1 | 280.0 | 124,000 | 135,000 | 1.09 | [45] |
| Shewanella marisflavi BBL25 | Barley straw hydrolysate | NI | 176.7 | ND b | 1,548,000 | 1,403,000 | 1.10 | [34] |
| Ralstonia eutropha ATCC 17699 | Rice paddy straw hydrolysate | 10.0 | 172.2 | 283.5 | ND | ND | ND | [40] |
| Ralstonia eutropha ATCC 17699 | Kenaf hydrolysate | NI | 171.6 | 292.8 | ND | ND | ND | [41] |
| Loktanella sp. SM43 | Pine tree hydrolysate | NI | 176.7 | ND | 511,000 | 810,000 | 1.58 | [42] |
| Standard PHB | - | NI | 176.0 | 302.0 | 82,400 | 264,000 | 3.21 | [46,47] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.-Y.; Kim, M.-H.; Kim, J.-S.; Yun, B.-R.; Kim, D.Y.; Chung, C.-W. Biotransformation of d-Xylose-Rich Rice Husk Hydrolysate by a Rice Paddy Soil Bacterium, Priestia sp. Strain JY310, to Low Molecular Weight Poly(3-hydroxybutyrate). Biomolecules 2023, 13, 131. https://doi.org/10.3390/biom13010131
Lee J-Y, Kim M-H, Kim J-S, Yun B-R, Kim DY, Chung C-W. Biotransformation of d-Xylose-Rich Rice Husk Hydrolysate by a Rice Paddy Soil Bacterium, Priestia sp. Strain JY310, to Low Molecular Weight Poly(3-hydroxybutyrate). Biomolecules. 2023; 13(1):131. https://doi.org/10.3390/biom13010131
Chicago/Turabian StyleLee, Jae-Yeong, Min-Hwan Kim, Jong-Sik Kim, Bo-Ram Yun, Do Young Kim, and Chung-Wook Chung. 2023. "Biotransformation of d-Xylose-Rich Rice Husk Hydrolysate by a Rice Paddy Soil Bacterium, Priestia sp. Strain JY310, to Low Molecular Weight Poly(3-hydroxybutyrate)" Biomolecules 13, no. 1: 131. https://doi.org/10.3390/biom13010131






