Charge Transport across Proteins inside Proteins: Tunneling across Encapsulin Protein Cages and the Effect of Cargo Proteins
Abstract
:1. Introduction
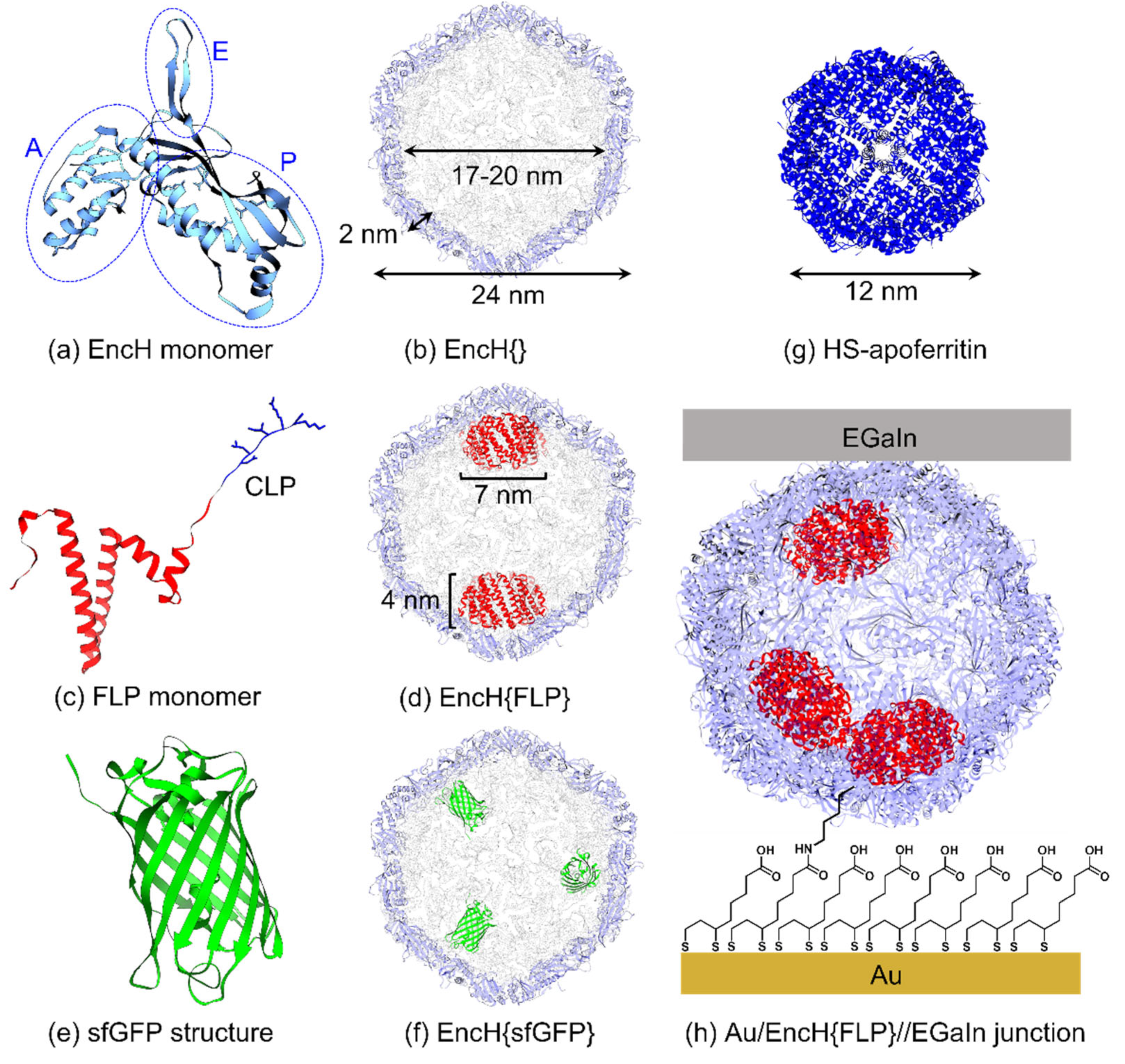
2. Materials and Methods
2.1. Protein Production and Purification
2.1.1. Cloning and Production
2.1.2. Purification
2.2. Sodium Dodecyl Sulfate PolyAcrylamide Gel Electrophoresis (SDS-PAGE)
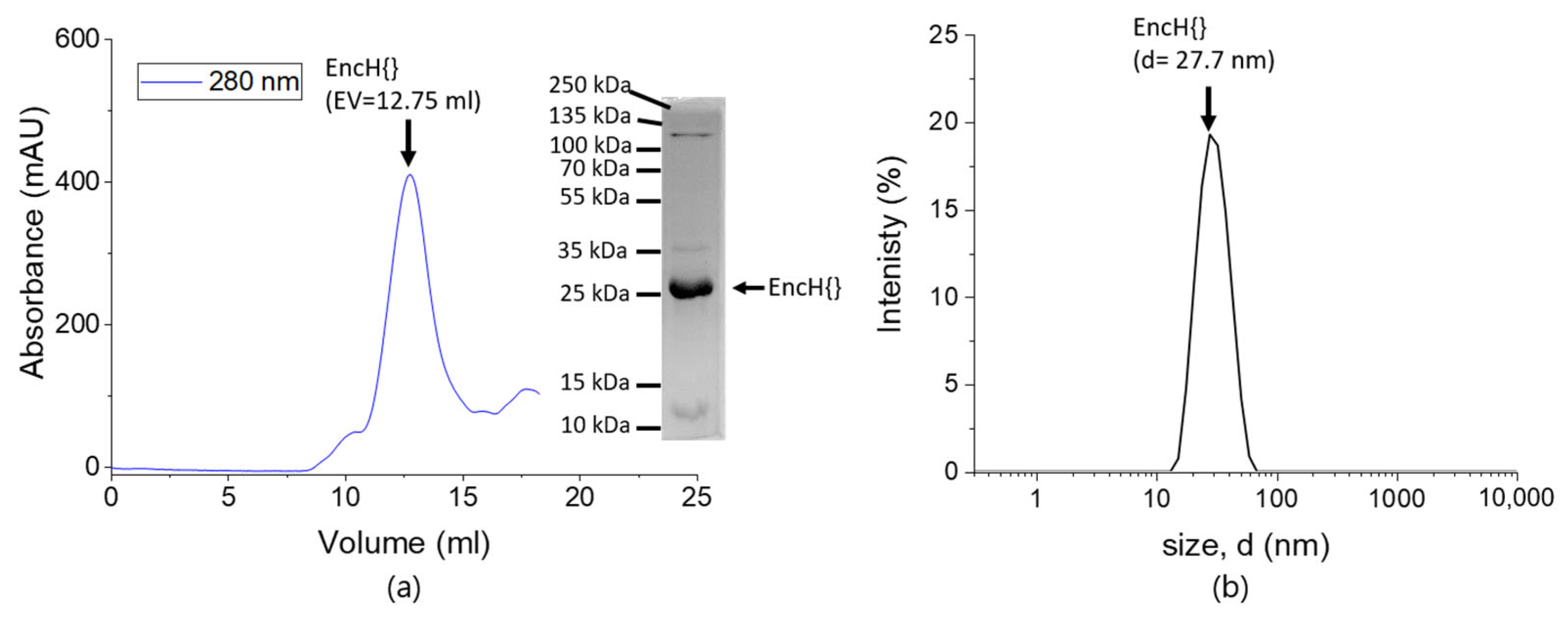
2.3. Dynamic Light Scattering (DLS)
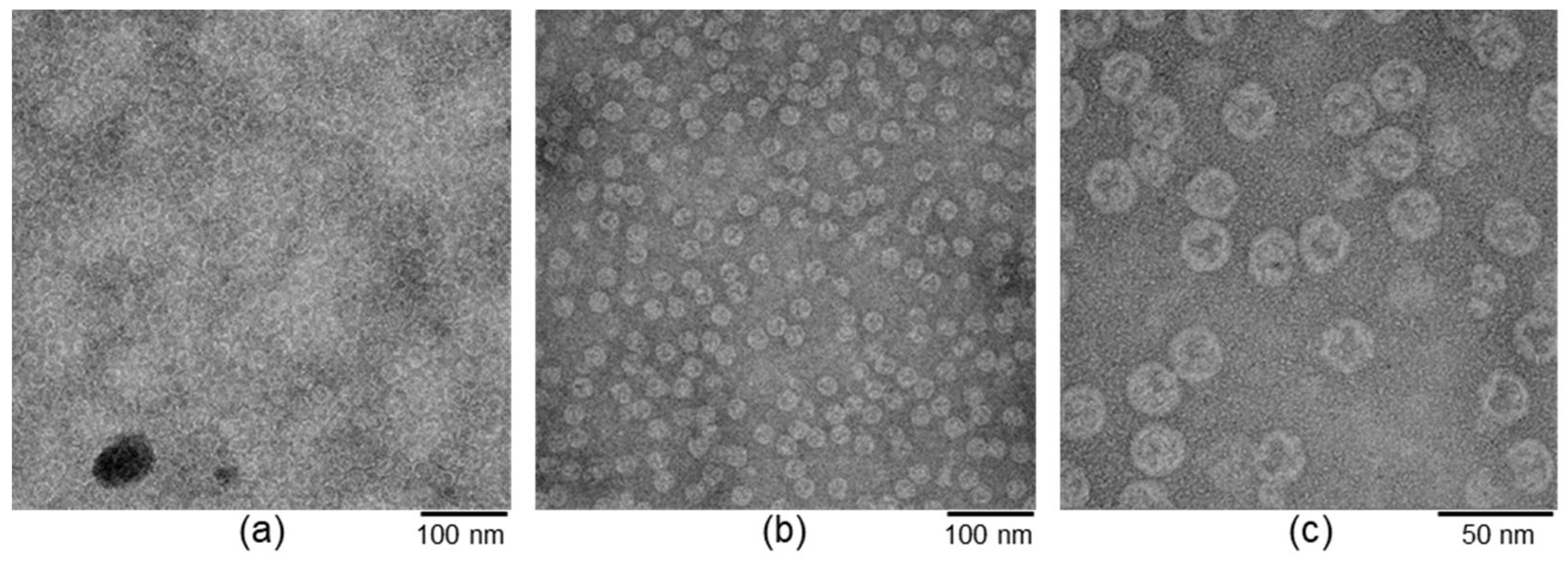
2.4. Transmission Electron Microscopy (TEM)
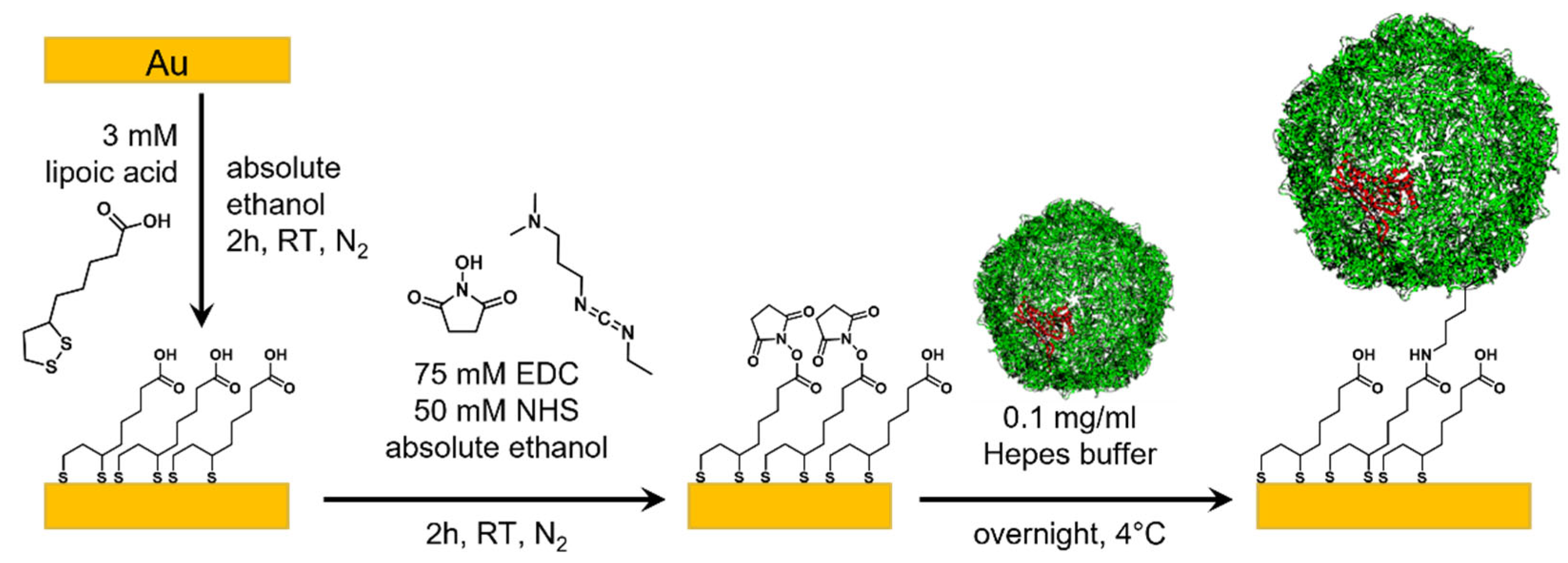
2.5. Template-Stripped Au
2.6. AFM Measurements
2.7. Current-Voltage Measurements
3. Results and Discussion
3.1. Experimental Design
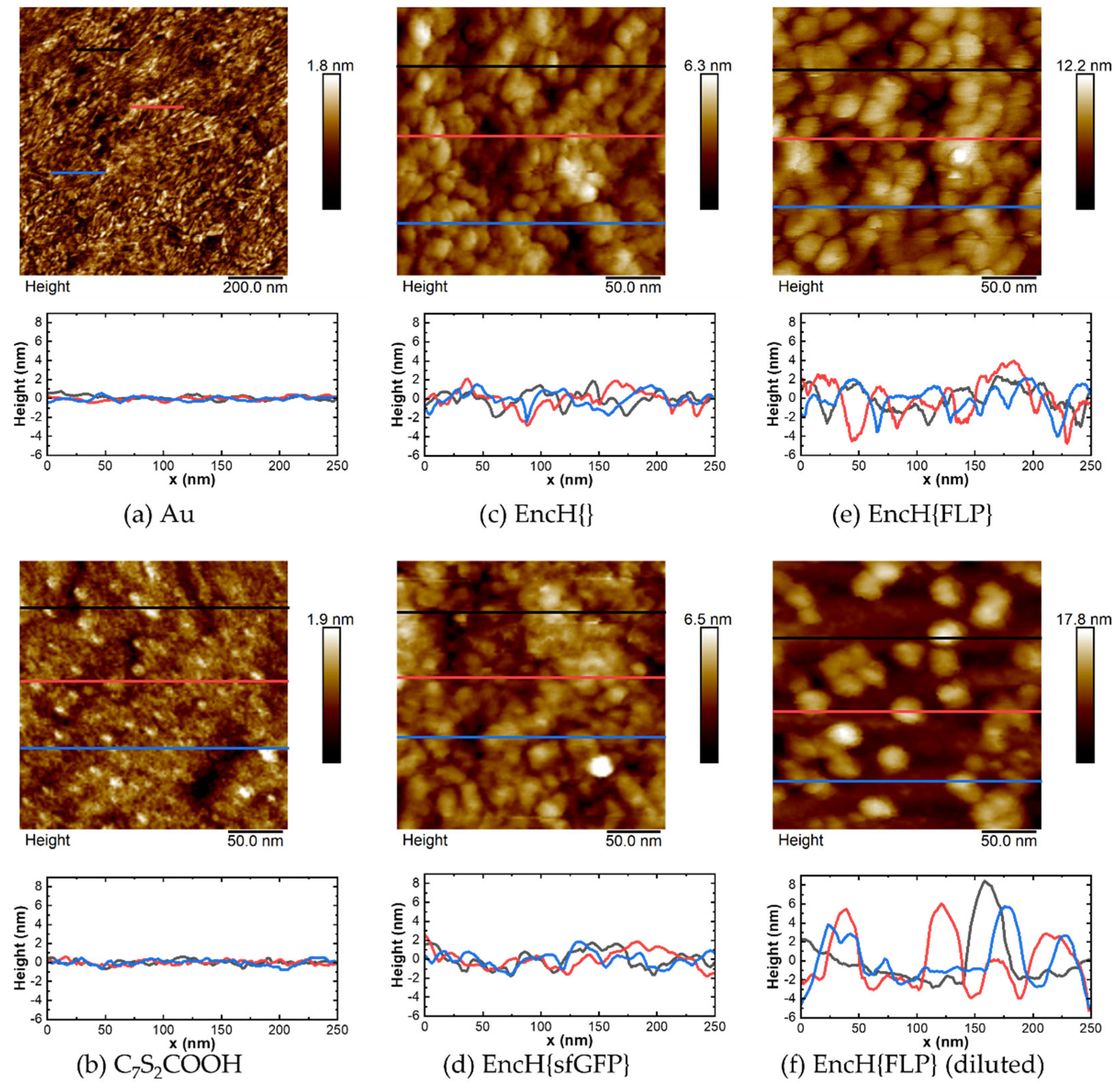
3.2. Surface Characterization of EncH Self-Assembled Monolayers
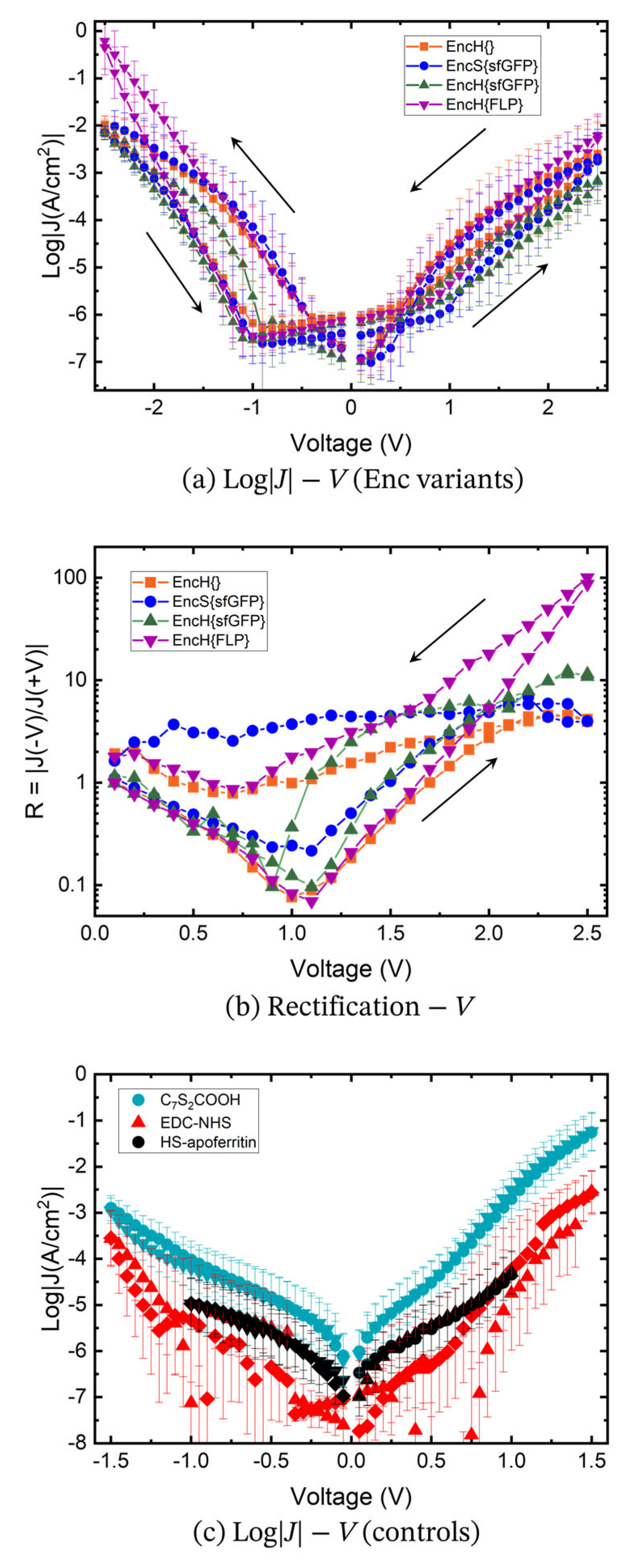
3.3. Charge Transport Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Simon, D.T.; Gabrielsson, E.O.; Tybrandt, K.; Berggren, M. Organic Bioelectronics: Bridging the Signaling Gap between Biology and Technology. Chem. Rev. 2016, 116, 13009–13041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Zhang, Y.; Liang, Z.; Cao, Y.; Han, Z.; Feng, X. Flexible Inorganic Bioelectronics. NPJ Flex. Electron. 2020, 4, 2. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Osgood, R.M.; Dinneen, S.R.; Koker, B.D.; Pang, R.; Deravi, L.F. Natural Light-Scattering Nanoparticles Enable Visible through Short-Wave Infrared Color Modulation. Adv. Opt. Mater. 2018, 6, 1701369. [Google Scholar] [CrossRef]
- Jutz, G.; van Rijn, P.; Santos Miranda, B.; Böker, A. Ferritin: A Versatile Building Block for Bionanotechnology. Chem. Rev. 2015, 115, 1653–1701. [Google Scholar] [CrossRef] [PubMed]
- Cahen, D.; Pecht, I.; Sheves, M. What Can We Learn from Protein-Based Electron Transport Junctions? J. Phys. Chem. Lett. 2021, 12, 11598–11603. [Google Scholar] [CrossRef]
- Zhuravel, R.; Huang, H.; Polycarpou, G.; Polydorides, S.; Motamarri, P.; Katrivas, L.; Rotem, D.; Sperling, J.; Zotti, L.A.; Kotlyar, A.B.; et al. Backbone Charge Transport in Double-Stranded DNA. Nat. Nanotechnol. 2020, 15, 836–840. [Google Scholar] [CrossRef]
- Li, Y.; Artés, J.M.; Demir, B.; Gokce, S.; Mohammad, H.M.; Alangari, M.; Anantram, M.P.; Oren, E.E.; Hihath, J. Detection and Identification of Genetic Material via Single-Molecule Conductance. Nat. Nanotechnol. 2018, 13, 1167–1173. [Google Scholar] [CrossRef]
- Ha, T.Q.; Planje, I.J.; White, J.R.G.; Aragonès, A.C.; Díez-Pérez, I. Charge Transport at the Protein–Electrode Interface in the Emerging Field of BioMolecular Electronics. Curr. Opin. Electrochem. 2021, 28, 100734. [Google Scholar] [CrossRef]
- Cox-Pridmore, D.M.; Castro, F.A.; Silva, S.R.P.; Camelliti, P.; Zhao, Y. Emerging Bioelectronic Strategies for Cardiovascular Tissue Engineering and Implantation. Small 2022, 18, 2105281. [Google Scholar] [CrossRef]
- Jonsson, A.; Inal, S.; Uguz, I.; Williamson, A.J.; Kergoat, L.; Rivnay, J.; Khodagholy, D.; Berggren, M.; Bernard, C.; Malliaras, G.G.; et al. Bioelectronic Neural Pixel: Chemical Stimulation and Electrical Sensing at the Same Site. Proc. Natl. Acad. Sci. USA 2016, 113, 9440–9445. [Google Scholar] [CrossRef]
- Giordano, G.; Carlotti, M.; Mazzolai, B. A Perspective on Cephalopods Mimicry and Bioinspired Technologies toward Proprioceptive Autonomous Soft Robots. Adv. Mater. Technol. 2021, 6, 2100437. [Google Scholar] [CrossRef]
- Bostick, C.D.; Mukhopadhyay, S.; Pecht, I.; Sheves, M.; Cahen, D.; Lederman, D. Protein Bioelectronics: A Review of What We Do and Do Not Know. Rep. Prog. Phys. 2018, 81, 026601. [Google Scholar] [CrossRef] [Green Version]
- Sepunaru, L.; Friedman, N.; Pecht, I.; Sheves, M.; Cahen, D. Temperature-Dependent Solid-State Electron Transport through Bacteriorhodopsin: Experimental Evidence for Multiple Transport Paths through Proteins. J. Am. Chem. Soc. 2012, 134, 4169–4176. [Google Scholar] [CrossRef]
- Alami, F.A.; Soni, S.; Borrini, A.; Nijhuis, C.A. Perspective—Temperature Dependencies and Charge Transport Mechanisms in Molecular Tunneling Junctions Induced by Redox-Reactions. ECS J. Solid State Sci. Technol. 2022, 11, 055005. [Google Scholar] [CrossRef]
- Gupta, N.K.; Karuppannan, S.K.; Pasula, R.R.; Vilan, A.; Martin, J.; Xu, W.; May, E.M.; Pike, A.R.; Astier, H.P.A.G.; Salim, T.; et al. Temperature-Dependent Coherent Tunneling across Graphene–Ferritin Biomolecular Junctions. ACS Appl. Mater. Interfaces 2022, 14, 44665–44675. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Dutta, S.; Pecht, I.; Sheves, M.; Cahen, D. Conjugated Cofactor Enables Efficient Temperature-Independent Electronic Transport Across ∼6 Nm Long Halorhodopsin. J. Am. Chem. Soc. 2015, 137, 11226–11229. [Google Scholar] [CrossRef]
- Liu, Y.; Qiu, X.; Soni, S.; Chiechi, R.C. Charge Transport through Molecular Ensembles: Recent Progress in Molecular Electronics. Chem. Phys. Rev. 2021, 2, 021303. [Google Scholar] [CrossRef]
- Amdursky, N.; Marchak, D.; Sepunaru, L.; Pecht, I.; Sheves, M.; Cahen, D. Electronic Transport via Proteins. Adv. Mater. 2014, 26, 7142–7161. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Karuppannan, S.K.; Guo, C.; Fereiro, J.A.; Bergren, A.; Mukundan, V.; Qiu, X.; Castañeda Ocampo, O.E.; Chen, X.; Chiechi, R.C.; et al. Solid-State Protein Junctions: Cross-Laboratory Study Shows Preservation of Mechanism at Varying Electronic Coupling. iScience 2020, 23, 101099. [Google Scholar] [CrossRef]
- Gupta, N.K.; Pasula, R.R.; Karuppannan, S.K.; Ziyu, Z.; Tadich, A.; Cowie, B.; Qi, D.-C.; Bencok, P.; Lim, S.; Nijhuis, C.A. Switching of the Mechanism of Charge Transport Induced by Phase Transitions in Tunnel Junctions with Large Biomolecular Cages. J. Mater. Chem. C Mater. 2021, 9, 10768–10776. [Google Scholar] [CrossRef]
- Beratan, D.N. Why Are DNA and Protein Electron Transfer So Different? Annu. Rev. Phys. Chem. 2019, 70, 71–97. [Google Scholar] [CrossRef] [PubMed]
- Fourmond, V.; Léger, C. Protein Electrochemistry: Questions and Answers. In Biophotoelectrochemistry: From Bioelectrochemistry to Biophotovoltaics; Springer: Cham, Switzerland, 2016; Volume 158, pp. 1–41. [Google Scholar]
- Paleček, E.; Tkáč, J.; Bartošík, M.; Bertók, T.; Ostatná, V.; Paleček, J. Electrochemistry of Nonconjugated Proteins and Glycoproteins. Toward Sensors for Biomedicine and Glycomics. Chem. Rev. 2015, 115, 2045–2108. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.K.; Wilkinson, E.A.; Karuppannan, S.K.; Bailey, L.; Vilan, A.; Zhang, Z.; Qi, D.C.; Tadich, A.; Tuite, E.M.; Pike, A.R.; et al. Role of Order in the Mechanism of Charge Transport across Single-Stranded and Double-Stranded DNA Monolayers in Tunnel Junctions. J. Am. Chem. Soc. 2021, 143, 20309–20319. [Google Scholar] [CrossRef] [PubMed]
- Ron, I.; Pecht, I.; Sheves, M.; Cahen, D. Proteins as Solid-State Electronic Conductors. Acc. Chem. Res. 2010, 43, 945–953. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.K.; Okamoto, N.; Karuppannan, S.K.; Pasula, R.R.; Ziyu, Z.; Qi, D.-C.; Lim, S.; Nakamura, M.; Nijhuis, C.A. The Role of Structural Order in the Mechanism of Charge Transport across Tunnel Junctions with Various Iron-Storing Proteins. Small 2022, 18, 2203338. [Google Scholar] [CrossRef]
- Kumar, K.S.; Pasula, R.R.; Lim, S.; Nijhuis, C.A. Long-Range Tunneling Processes across Ferritin-Based Junctions. Adv. Mater. 2016, 28, 1824–1830. [Google Scholar] [CrossRef]
- Jin, Y.; Friedman, N.; Sheves, M.; He, T.; Cahen, D. Bacteriorhodopsin (BR) as an Electronic Conduction Medium: Current Transport through BR-Containing Monolayers. Proc. Natl. Acad. Sci. USA 2006, 103, 8601–8606. [Google Scholar] [CrossRef] [Green Version]
- Castañeda Ocampo, O.E.; Gordiichuk, P.; Catarci, S.; Gautier, D.A.; Herrmann, A.; Chiechi, R.C. Mechanism of Orientation-Dependent Asymmetric Charge Transport in Tunneling Junctions Comprising Photosystem I. J. Am. Chem. Soc. 2015, 137, 8419–8427. [Google Scholar] [CrossRef]
- Qiu, X.; Castañeda Ocampo, O.; de Vries, H.W.; van Putten, M.; Loznik, M.; Herrmann, A.; Chiechi, R.C. Self-Regenerating Soft Biophotovoltaic Devices. ACS Appl. Mater. Interfaces 2018, 10, 37625–37633. [Google Scholar] [CrossRef] [Green Version]
- Futera, Z.; Ide, I.; Kayser, B.; Garg, K.; Jiang, X.; van Wonderen, J.H.; Butt, J.N.; Ishii, H.; Pecht, I.; Sheves, M.; et al. Coherent Electron Transport across a 3 Nm Bioelectronic Junction Made of Multi-Heme Proteins. J. Phys. Chem. Lett. 2020, 11, 9766–9774. [Google Scholar] [CrossRef]
- Garg, K.; Ghosh, M.; Eliash, T.; van Wonderen, J.H.; Butt, J.N.; Shi, L.; Jiang, X.; Zdenek, F.; Blumberger, J.; Pecht, I.; et al. Direct Evidence for Heme-Assisted Solid-State Electronic Conduction in Multi-Heme c -Type Cytochromes. Chem. Sci. 2018, 9, 7304–7310. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Artés, J.M.; Hihath, J. Long-Range Charge Transport in Adenine-Stacked RNA:DNA Hybrids. Small 2016, 12, 432–437. [Google Scholar] [CrossRef]
- Bixon, M.; Giese, B.; Wessely, S.; Langenbacher, T.; Michel-Beyerle, M.E.; Jortner, J. Long-Range Charge Hopping in DNA. Proc. Natl. Acad. Sci. USA 1999, 96, 11713–11716. [Google Scholar] [CrossRef] [Green Version]
- Bixon, M.; Jortner, J. Long-Range and Very Long-Range Charge Transport in DNA. Chem. Phys. 2002, 281, 393–408. [Google Scholar] [CrossRef]
- Chi, Q.; Zhang, J.; Andersen, J.E.T.; Ulstrup, J. Ordered Assembly and Controlled Electron Transfer of the Blue Copper Protein Azurin at Gold (111) Single-Crystal Substrates. J. Phys. Chem. B 2001, 105, 4669–4679. [Google Scholar] [CrossRef]
- Fereiro, J.A.; Yu, X.; Pecht, I.; Sheves, M.; Cuevas, J.C.; Cahen, D. Tunneling Explains Efficient Electron Transport via Protein Junctions. Proc. Natl. Acad. Sci. USA 2018, 115, E4577–E4583. [Google Scholar] [CrossRef] [Green Version]
- Qiu, X.; Chiechi, R.C. Printable Logic Circuits Comprising Self-Assembled Protein Complexes. Nat. Commun. 2022, 13, 2312. [Google Scholar] [CrossRef]
- Yan, H.; Bergren, A.J.; McCreery, R.; della Rocca, M.L.; Martin, P.; Lafarge, P.; Lacroix, J.C. Activationless Charge Transport across 4.5 to 22 Nm in Molecular Electronic Junctions. Proc. Natl. Acad. Sci. USA 2013, 110, 5326–5330. [Google Scholar] [CrossRef] [Green Version]
- Michel-Souzy, S.; Hamelmann, N.M.; Zarzuela-Pura, S.; Paulusse, J.M.J.; Cornelissen, J.J.L.M. Introduction of Surface Loops as a Tool for Encapsulin Functionalization. Biomacromolecules 2021, 22, 5234–5242. [Google Scholar] [CrossRef]
- LaFrance, B.J.; Cassidy-Amstutz, C.; Nichols, R.J.; Oltrogge, L.M.; Nogales, E.; Savage, D.F. The Encapsulin from Thermotoga Maritima Is a Flavoprotein with a Symmetry Matched Ferritin-like Cargo Protein. Sci. Rep. 2021, 11, 22810. [Google Scholar] [CrossRef]
- Sutter, M.; Boehringer, D.; Gutmann, S.; Günther, S.; Prangishvili, D.; Loessner, M.J.; Stetter, K.O.; Weber-Ban, E.; Ban, N. Structural Basis of Enzyme Encapsulation into a Bacterial Nanocompartment. Nat. Struct. Mol. Biol. 2008, 15, 939–947. [Google Scholar] [CrossRef] [PubMed]
- Putri, R.M.; Allende-Ballestero, C.; Luque, D.; Klem, R.; Rousou, K.A.; Liu, A.; Traulsen, C.H.H.; Rurup, W.F.; Koay, M.S.T.; Castón, J.R.; et al. Structural Characterization of Native and Modified Encapsulins as Nanoplatforms for in Vitro Catalysis and Cellular Uptake. ACS Nano 2017, 11, 12796–12804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, R.; White, D.; Demirel, Y.; Kelly, R.; Noll, K.; Blum, P. Uncoupling Fermentative Synthesis of Molecular Hydrogen from Biomass Formation in Thermotoga Maritima. Appl. Environ. Microbiol. 2018, 84, e00998-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nesbø, C.L.; Dlutek, M.; Doolittle, W.F. Recombination in Thermotoga: Implications for Species Concepts and Biogeography. Genetics 2006, 172, 759–769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelson, K.E.; Clayton, R.A.; Gill, S.R.; Gwinn, M.L.; Dodson, R.J.; Haft, D.H.; Hickey, E.K.; Peterson, J.D.; Nelson, W.C.; Ketchum, K.A.; et al. Evidence for Lateral Gene Transfer between Archaea and Bacteria from Genome Sequence of Thermotoga Maritima. Nature 1999, 399, 323–329. [Google Scholar] [CrossRef]
- Rurup, W.F.; Snijder, J.; Koay, M.S.T.; Heck, A.J.R.; Cornelissen, J.J.L.M. Self-Sorting of Foreign Proteins in a Bacterial Nanocompartment. J. Am. Chem. Soc. 2014, 136, 3828–3832. [Google Scholar] [CrossRef]
- Sangeeth, C.S.S.; Wan, A.; Nijhuis, C.A. Equivalent Circuits of a Self-Assembled Monolayer-Based Tunnel Junction Determined by Impedance Spectroscopy. J. Am. Chem. Soc. 2014, 136, 11134–11144. [Google Scholar] [CrossRef]
- Chiechi, R.C.; Weiss, E.A.; Dickey, M.D.; Whitesides, G.M. Eutectic Gallium–Indium (EGaIn): A Moldable Liquid Metal for Electrical Characterization of Self-Assembled Monolayers. Angew. Chem. Int. Ed. 2008, 47, 142–144. [Google Scholar] [CrossRef]
- Cademartiri, L.; Thuo, M.M.; Nijhuis, C.A.; Reus, W.F.; Tricard, S.; Barber, J.R.; Sodhi, R.N.S.; Brodersen, P.; Kim, C.; Chiechi, R.C.; et al. 0.7nm Thick: Electrical Resistance of Ag TS-S(CH 2) n -1CH 3//Ga 2O 3/EGaIn Tunneling Junctions. J. Phys. Chem. C 2012, 116, 10848–10860. [Google Scholar] [CrossRef] [Green Version]
- Amini, S.; Chen, X.; Chua, J.Q.I.; Tee, J.S.; Nijhuis, C.A.; Miserez, A. Interplay between Interfacial Energy, Contact Mechanics, and Capillary Forces in EGaIn Droplets. ACS Appl. Mater. Interfaces 2022, 14, 28074–28084. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [Green Version]
- Jeong, J.-Y.; Yim, H.-S.; Ryu, J.-Y.; Lee, H.S.; Lee, J.-H.; Seen, D.-S.; Kang, S.G. One-Step Sequence- and Ligation-Independent Cloning as a Rapid and Versatile Cloning Method for Functional Genomics Studies. Appl. Environ. Microbiol. 2012, 78, 5440–5443. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Hu, H.; Trasobares, J.; Nijhuis, C.A. Rectification Ratio and Tunneling Decay Coefficient Depend on the Contact Geometry Revealed by in Situ Imaging of the Formation of EGaIn Junctions. ACS Appl. Mater. Interfaces 2019, 11, 21018–21029. [Google Scholar] [CrossRef]
- Reus, W.F.; Thuo, M.M.; Shapiro, N.D.; Nijhuis, C.A.; Whitesides, G.M. The SAM, Not the Electrodes, Dominates Charge Transport in Metal-Monolayer//Ga 2O 3/Gallium-Indium Eutectic Junctions. ACS Nano 2012, 6, 4806–4822. [Google Scholar] [CrossRef] [Green Version]
- Chiechi, R.C. GitHub—Rchiechi/GaussFit: Scientific Python for Processing EGaIn and CP-AFM Data. Available online: https://github.com/rchiechi/gaussfit (accessed on 15 December 2022).
- Zhang, Y.; Soni, S.; Krijger, T.L.; Gordiichuk, P.; Qiu, X.; Ye, G.; Jonkman, H.T.; Herrmann, A.; Zojer, K.; Zojer, E.; et al. Tunneling Probability Increases with Distance in Junctions Comprising Self-Assembled Monolayers of Oligothiophenes. J. Am. Chem. Soc. 2018, 140, 15048–15055. [Google Scholar] [CrossRef] [Green Version]
- Grabarek, Z.; Gergely, J. Zero-Length Crosslinking Procedure with the Use of Active Esters. Anal. Biochem. 1990, 185, 131–135. [Google Scholar] [CrossRef]
- Han, Y.; Maglione, M.S.; Diez Cabanes, V.; Casado-Montenegro, J.; Yu, X.; Karuppannan, S.K.; Zhang, Z.; Crivillers, N.; Mas-Torrent, M.; Rovira, C.; et al. Reversal of the Direction of Rectification Induced by Fermi Level Pinning at Molecule–Electrode Interfaces in Redox-Active Tunneling Junctions. ACS Appl. Mater. Interfaces 2020, 12, 55044–55055. [Google Scholar] [CrossRef]
- Weiss, E.A.; Kaufman, G.K.; Kriebel, J.K.; Li, Z.; Schalek, R.; Whitesides, G.M. Si/SiO2-Templated Formation of Ultraflat Metal Surfaces on Glass, Polymer, and Solder Supports: Their Use as Substrates for Self-Assembled Monolayers. Langmuir 2007, 23, 9686–9694. [Google Scholar] [CrossRef]
- Qiu, X.; Ivasyshyn, V.; Qiu, L.; Enache, M.; Dong, J.; Rousseva, S.; Portale, G.; Stöhr, M.; Hummelen, J.C.; Chiechi, R.C. Thiol-Free Self-Assembled Oligoethylene Glycols Enable Robust Air-Stable Molecular Electronics. Nat. Mater. 2020, 19, 330–337. [Google Scholar] [CrossRef]
| Oligo | Sequence 5′-3′ | Characteristics |
|---|---|---|
| OSMS3 | AAGGAGATATACATATGGTGAACATGGAATTTCTG | PCR Tmenc forward for NdeI site in pDuet MCS2 |
| OKB23 | GCGTGGCCGGCCGATATCTCACTTTTCGAACTGCGGGTGGCTCCAACCACCACCACCACCGAACTTTAGAAGAATCAA | PCR Tmenc reverse for EcoRV site in pDuet MCS2 |
| OSMS36 | AGGAGATATACCATGGCAGATCAGTACCAC | PCR flp forward for NcoI site in pDuet MCS1 |
| OSMS37 | CCGCAAGCTTGTCGACTCAGAGCTTCCTTATG | PCR flp reverse for SalI site in pDuet MCS1 |
| Plasmid | Characteristics | Origin |
|---|---|---|
| pCDFDuet-Tmenc127H10 | SmR, PT7, Expression of Tm encapsulin with a 10 Histidine tag after residue 127 (EncH) | Michel-Souzy et al. [40] |
| pRSFDuet-TmencCstrep | KmR, PT7, Expression of Tm encapsulin with a Strep-tagII after the C terminus (EncS) | this study |
| pETDuet-sfGFPEflp | ApR, PT7, Expression of the sfGFP fuserazmakd with the CLP of Flp (cargo) | Michel-Souzy et al. [40] |
| pETDuet-Flp | ApR, PT7, Ferredoxin-like protein (physiological cargo of Tm encapsulin) | this study |
| SAM | AFM Roughness a | EGaIn Junction Parameters | ||||
|---|---|---|---|---|---|---|
| 250 × 250 nm | 1 × 1 um | Voltage Range (V) c | Non-Shorting Junctions c | Total No. of Scans c | Yield (%) c | |
| Bare Au | n.a. | 0.25 | n.a. | n.a. | n.a. | n.a. |
| C7S2COOH | 0.25 | 0.28 | 1.5 | 22 | 440 | 76 |
| EDC-NHS | n.a. | 0.75 | 1.5 | 16 | 257 | 73 |
| HP-apoferritin | n.a. | n.a. | 1 | 17 | 340 | 74 |
| EncH{} | 0.89 | 1.19 | 1.5 (2.5) | 15 (15) | 278 (300) | 79 (83) |
| EncH{sfGFP} | 0.92 | 1.18 | 1.5 (2.5) | 15 (15) | 290 (300) | 88 (100) |
| EncS{sfGFP} | n.a. | n.a. | 1.5 (2.5) | 19 (17) | 380 (340) | 73 (74) |
| EncH{FLP} | 1.80 [2.72] b | 2.02 [3.08] b | 1.5 (2.5) | 12 (18) | 240 (360) | 67 (90) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zinelli, R.; Soni, S.; Cornelissen, J.J.L.M.; Michel-Souzy, S.; Nijhuis, C.A. Charge Transport across Proteins inside Proteins: Tunneling across Encapsulin Protein Cages and the Effect of Cargo Proteins. Biomolecules 2023, 13, 174. https://doi.org/10.3390/biom13010174
Zinelli R, Soni S, Cornelissen JJLM, Michel-Souzy S, Nijhuis CA. Charge Transport across Proteins inside Proteins: Tunneling across Encapsulin Protein Cages and the Effect of Cargo Proteins. Biomolecules. 2023; 13(1):174. https://doi.org/10.3390/biom13010174
Chicago/Turabian StyleZinelli, Riccardo, Saurabh Soni, Jeroen J. L. M. Cornelissen, Sandra Michel-Souzy, and Christian A. Nijhuis. 2023. "Charge Transport across Proteins inside Proteins: Tunneling across Encapsulin Protein Cages and the Effect of Cargo Proteins" Biomolecules 13, no. 1: 174. https://doi.org/10.3390/biom13010174
APA StyleZinelli, R., Soni, S., Cornelissen, J. J. L. M., Michel-Souzy, S., & Nijhuis, C. A. (2023). Charge Transport across Proteins inside Proteins: Tunneling across Encapsulin Protein Cages and the Effect of Cargo Proteins. Biomolecules, 13(1), 174. https://doi.org/10.3390/biom13010174








