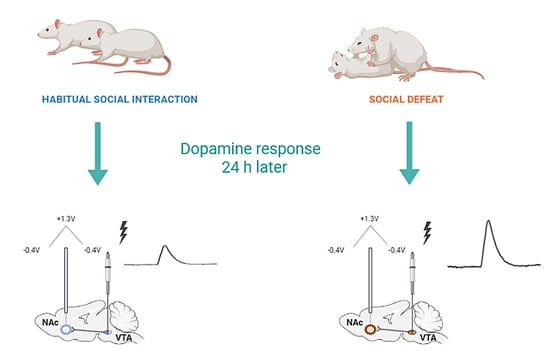
Short-Term Consequences of Single Social Defeat on Accumbal Dopamine and Behaviors in Rats
Abstract
1. Introduction
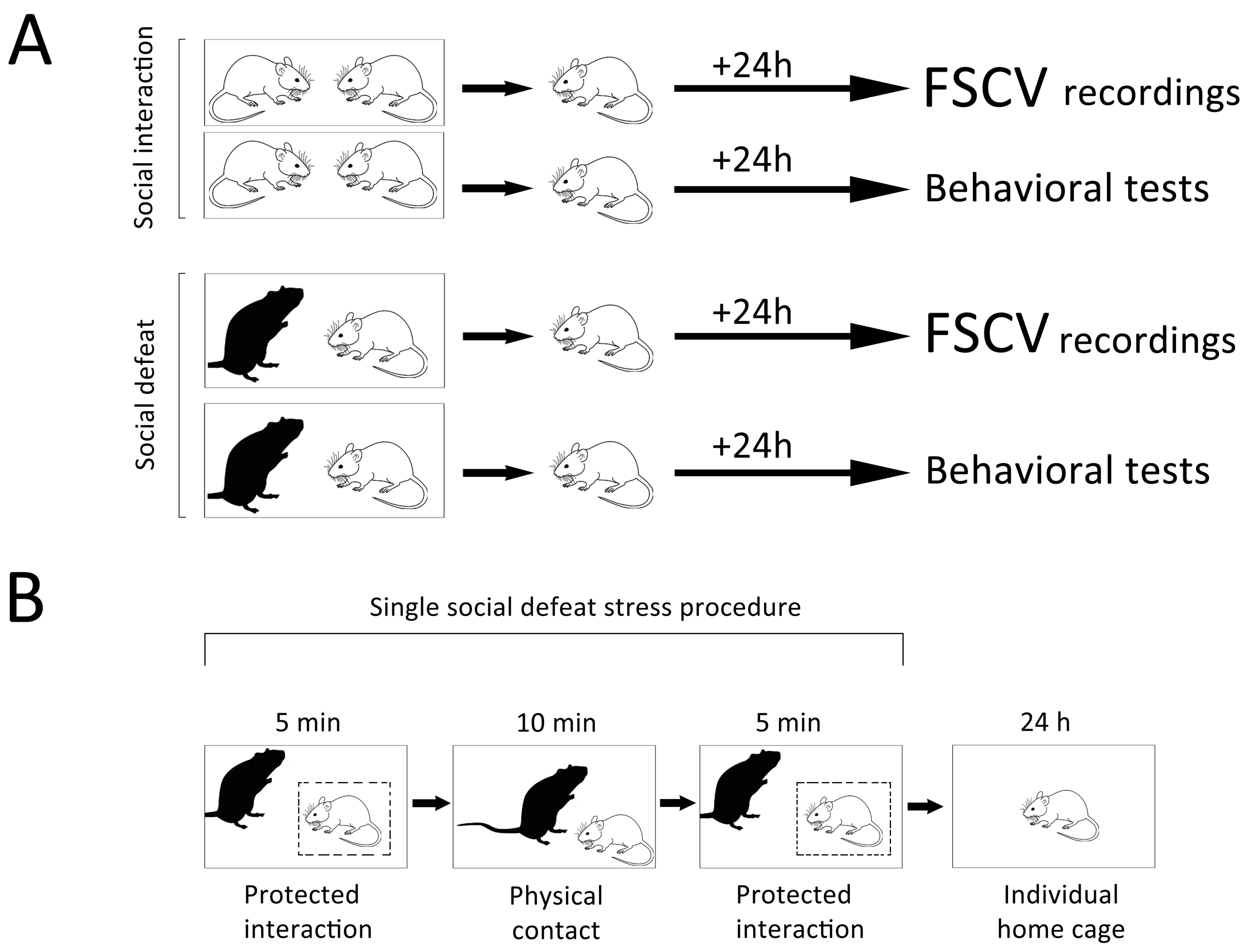
2. Materials and Methods
2.1. Animals and Behavioral Tests
2.2. FSCV Recordings of Dopamine Release in Rat Nucleus Accumbens
2.3. Histology
2.4. Statistical Analysis
3. Results
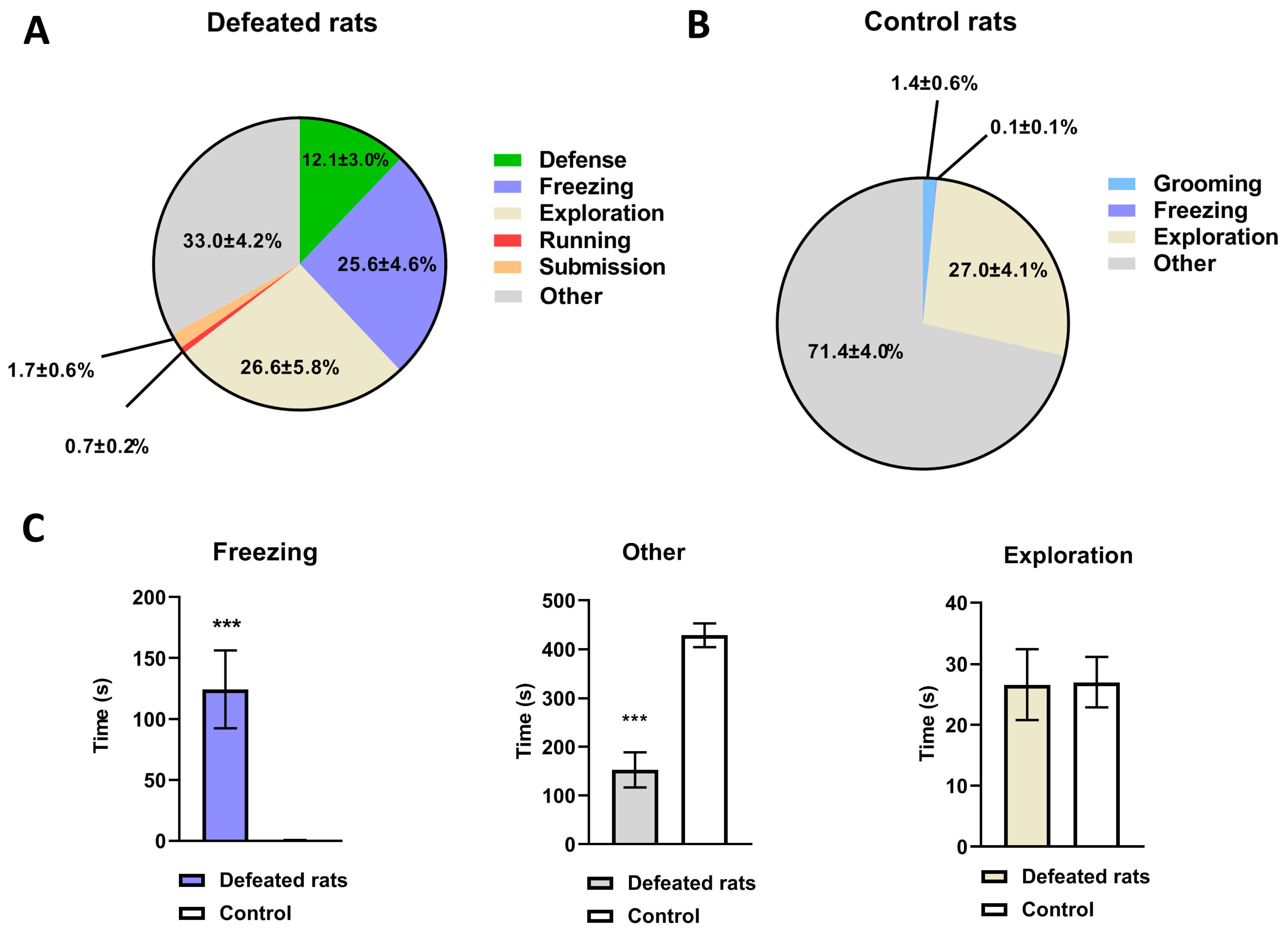
3.1. Behavioral Changes Observed during a Single SD Session
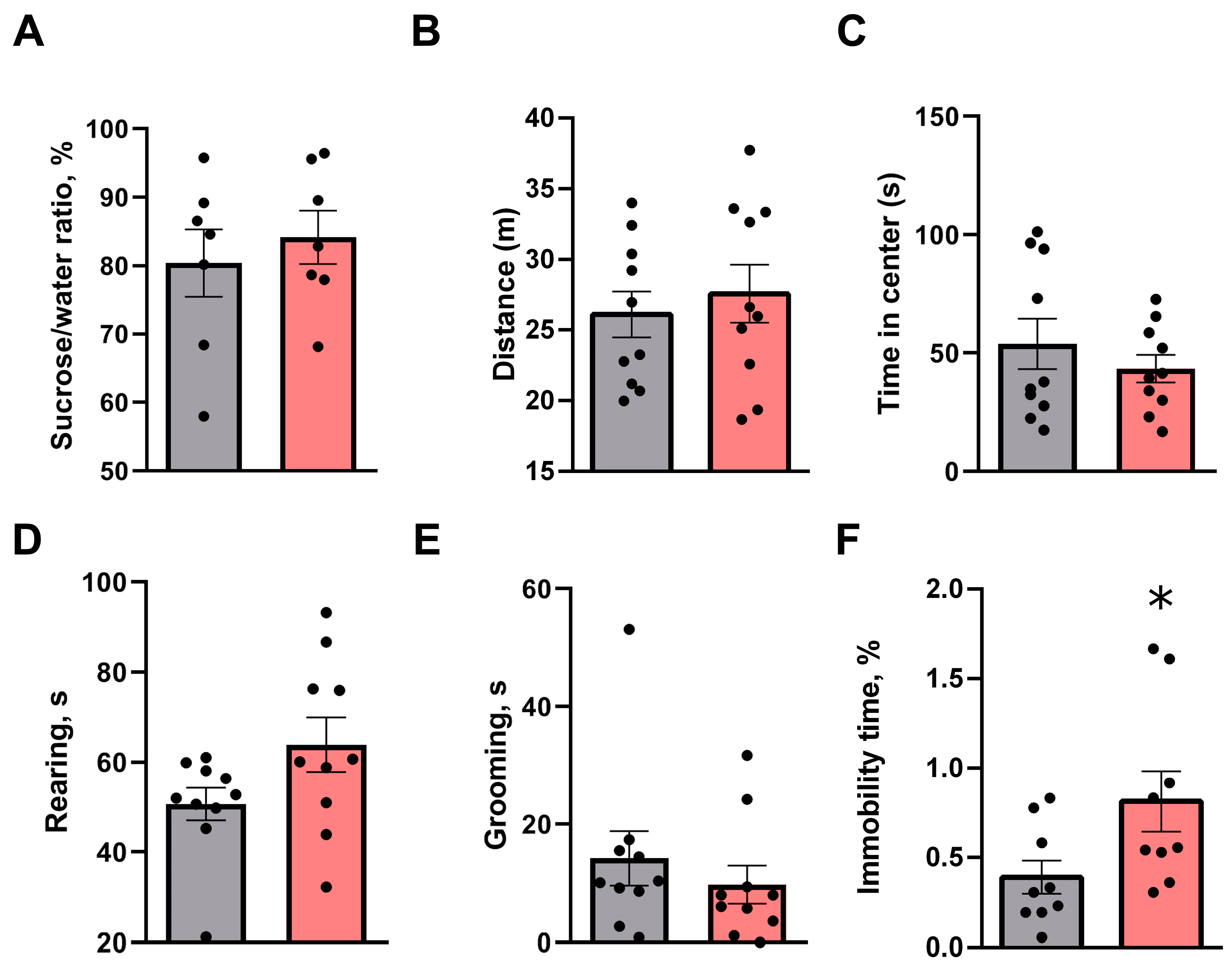
3.2. Short-Term Behavioral Consequences Observed 24 h Following a Single SD Session
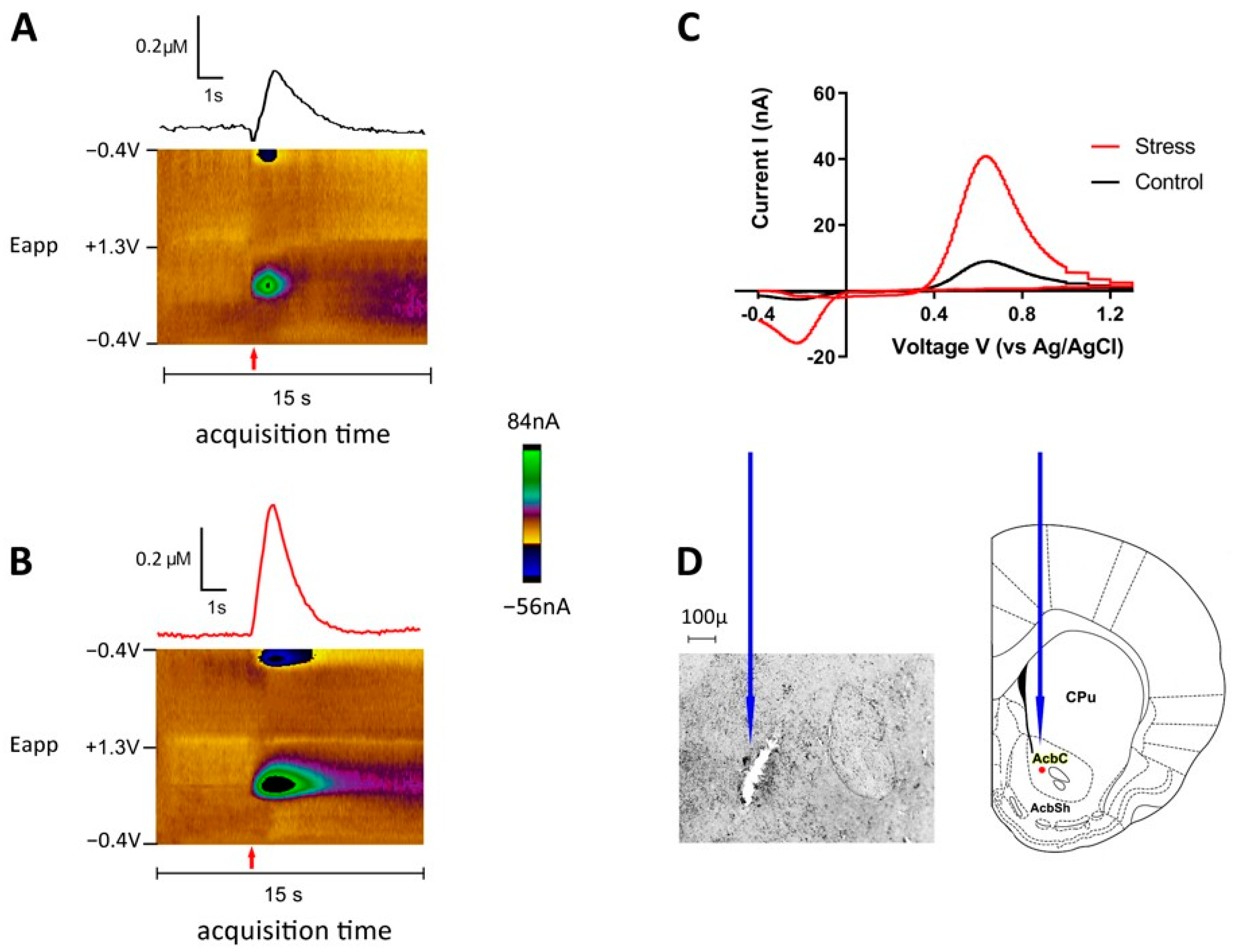
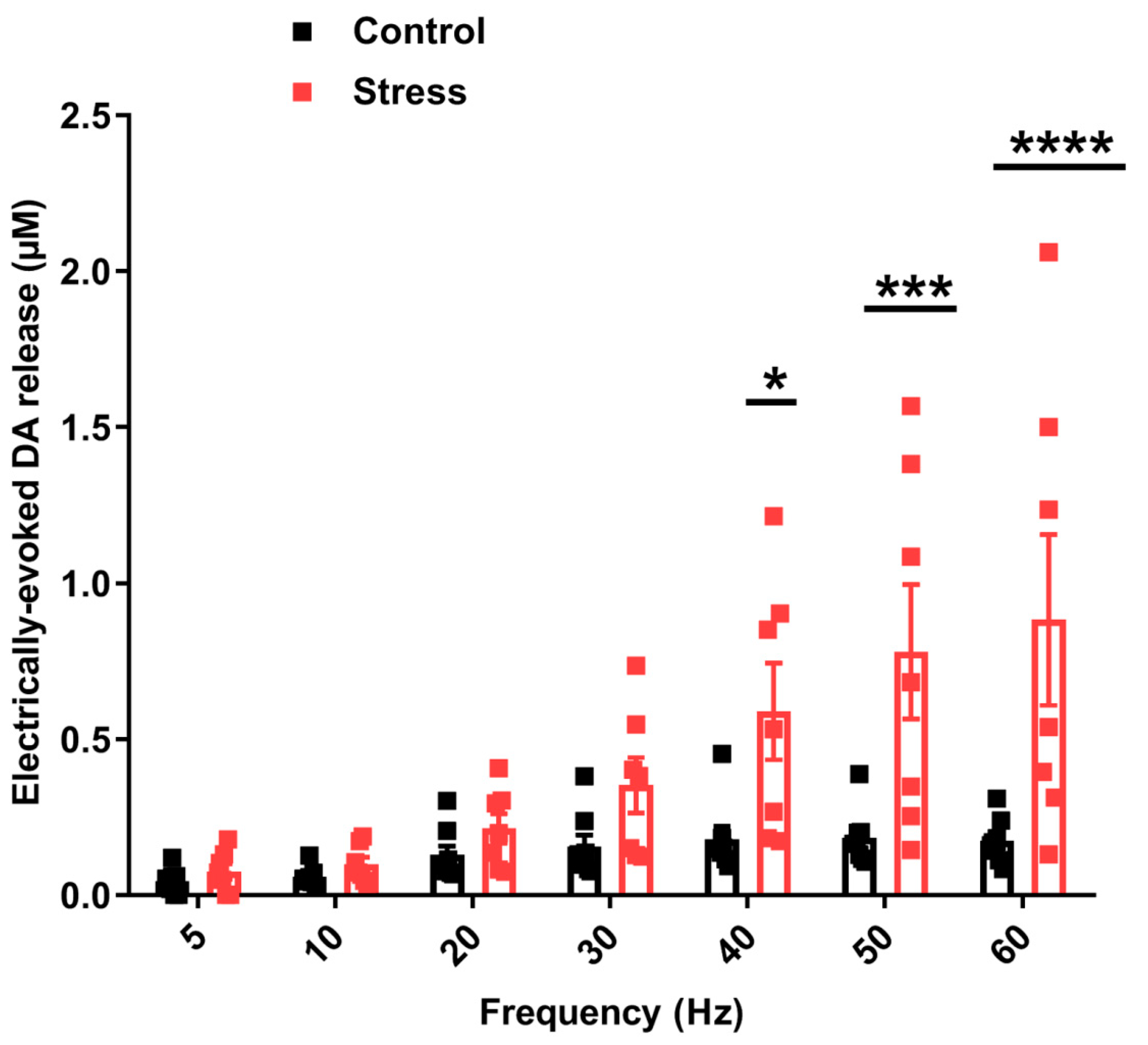
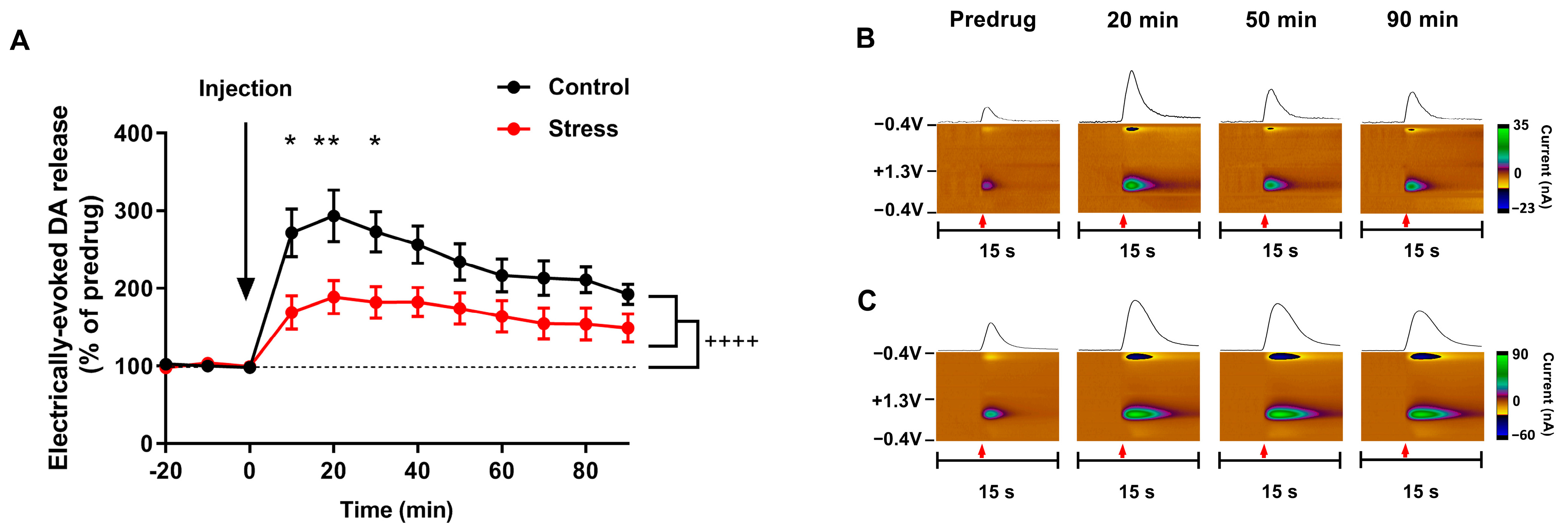
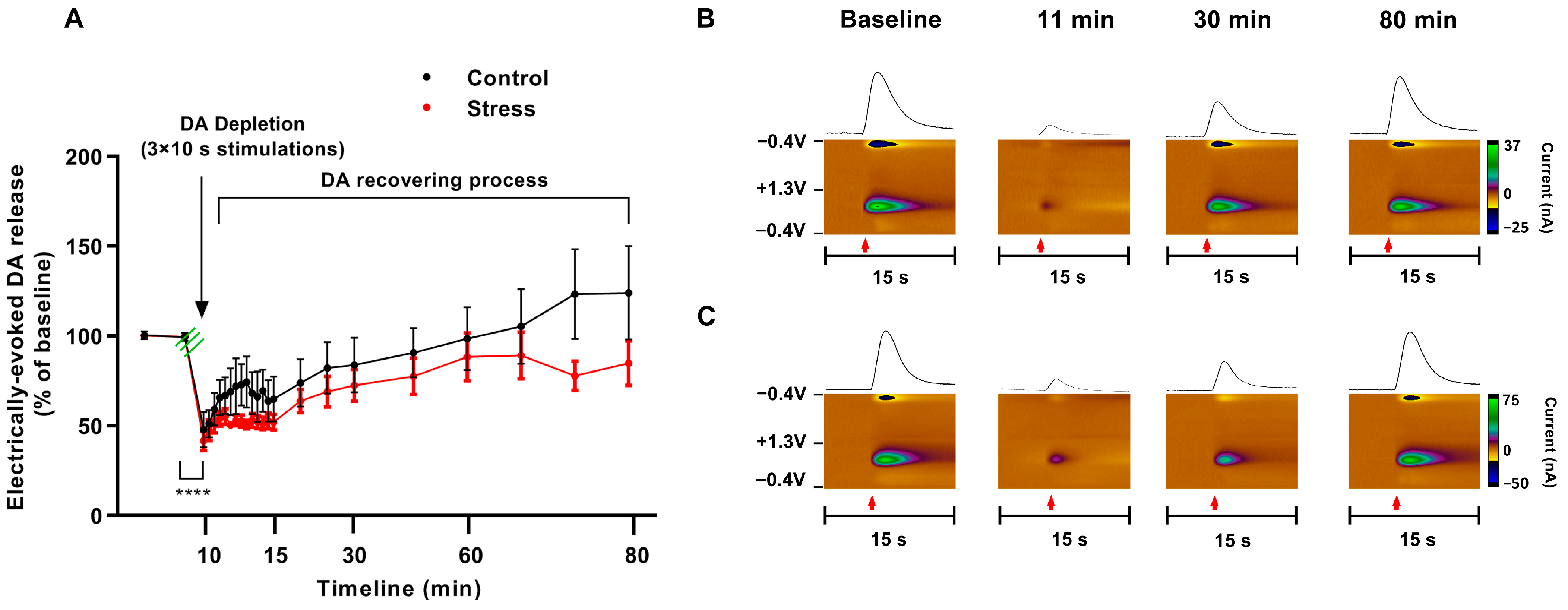
3.3. Consequences of Single SD Session for Dopamine Dynamics in Rat Nucleus Accumbens
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Biltz, R.G.; Sawicki, C.M.; Sheridan, J.F.; Godbout, J.P. The Neuroimmunology of Social-Stress-Induced Sensitization. Nat. Immunol. 2022, 23, 1527–1535. [Google Scholar] [CrossRef] [PubMed]
- Gorlova, A.; Ortega, G.; Waider, J.; Bazhenova, N.; Veniaminova, E.; Proshin, A.; Kalueff, A.V.; Anthony, D.C.; Lesch, K.P.; Strekalova, T. Stress-Induced Aggression in Heterozygous TPH2 Mutant Mice Is Associated with Alterations in Serotonin Turnover and Expression of 5-HT6 and AMPA Subunit 2A Receptors. J. Affect. Disord. 2020, 272, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Holly, E.N.; Miczek, K.A. Ventral Tegmental Area Dopamine Revisited: Effects of Acute and Repeated Stress. Psychopharmacology 2016, 233, 163–186. [Google Scholar] [CrossRef] [PubMed]
- Strekalova, T.; Svirin, E.; Waider, J.; Gorlova, A.; Cespuglio, R.; Kalueff, A.; Pomytkin, I.; Schmitt-Boehrer, A.G.; Lesch, K.P.; Anthony, D.C. Altered Behaviour, Dopamine and Norepinephrine Regulation in Stressed Mice Heterozygous in TPH2 Gene. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 108, 110155. [Google Scholar] [CrossRef]
- Deal, A.L.; Park, J.; Weiner, J.L.; Budygin, E.A. Stress Alters the Effect of Alcohol on Catecholamine Dynamics in the Basolateral Amygdala. Front. Behav. Neurosci. 2021, 15, 1–10. [Google Scholar] [CrossRef]
- Miczek, K.A.; Yap, J.J.; Covington, H.E. Social Stress, Therapeutics and Drug Abuse: Preclinical Models of Escalated and Depressed Intake. Pharmacol. Ther. 2008, 120, 102–128. [Google Scholar] [CrossRef]
- Miczek, K.A.; Thompson, M.L.; Tornatzky, W. Short and Long Term Physiological and Neurochemical Adaptations to Social Conflict. In Psychobiology of Stress; Springer: Dordrecht, The Netherlands, 1990. [Google Scholar]
- Silberman, Y.; Bajo, M.; Chappell, A.M.; Christian, D.T.; Cruz, M.; Diaz, M.R.; Kash, T.; Lack, A.K.; Messing, R.O.; Siggins, G.R.; et al. Neurobiological Mechanisms Contributing to Alcohol-Stress-Anxiety Interactions. Alcohol 2009, 43, 509. [Google Scholar] [CrossRef]
- Saal, D.; Dong, Y.; Bonci, A.; Malenka, R.C. Drugs of Abuse and Stress Trigger a Common Synaptic Adaptation in Dopamine Neurons. Neuron 2003, 37, 577–582. [Google Scholar] [CrossRef]
- Steger, J.S.; Land, B.B.; Lemos, J.C.; Chavkin, C.; Phillips, P.E.M. Insidious Transmission of a Stress-Related Neuroadaptation. Front. Behav. Neurosci. 2020, 14, 564054. [Google Scholar] [CrossRef]
- Charney, D.S.; Manji, H.K. Life Stress, Genes, and Depression: Multiple Pathways Lead to Increased Risk and New Opportunities for Intervention. Sci. STKE 2004, 2004, re5. [Google Scholar] [CrossRef]
- Huhman, K.L. Social Conflict Models: Can They Inform Us about Human Psychopathology? Horm. Behav. 2006, 50, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Grinevich, V.P.; Krupitsky, E.M.; Gainetdinov, R.R.; Budygin, E.A. Linking Ethanol-Addictive Behaviors With Brain Catecholamines: Release Pattern Matters. Front. Behav. Neurosci. 2021, 15, 795030. [Google Scholar] [CrossRef] [PubMed]
- Reguilón, M.D.; Montagud-Romero, S.; Ferrer-Pérez, C.; Roger-Sánchez, C.; Aguilar, M.A.; Miñarro, J.; Rodríguez-Arias, M. Dopamine D2 Receptors Mediate the Increase in Reinstatement of the Conditioned Rewarding Effects of Cocaine Induced by Acute Social Defeat. Eur. J. Pharmacol. 2017, 799, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Grinevich, V.P.; Zakirov, A.N.; Berseneva, U.V.; Gerasimova, E.V.; Gainetdinov, R.R.; Budygin, E.A. Applying a Fast-Scan Cyclic Voltammetry to Explore Dopamine Dynamics in Animal Models of Neuropsychiatric Disorders. Cells 2022, 11, 1533. [Google Scholar] [CrossRef] [PubMed]
- Belujon, P.; Grace, A.A. Dopamine System Dysregulation in Major Depressive Disorders. Int. J. Neuropsychopharmacol. 2017, 20, 1036. [Google Scholar] [CrossRef] [PubMed]
- Drury, S.S.; Theall, K.P.; Keats, B.J.B. The Role of the Dopamine Transporter (DAT) in the Development of PTSD in Preschool Children. J. Trauma. Stress 2009, 22, 534. [Google Scholar] [CrossRef]
- Seidemann, R.; Duek, O.; Jia, R.; Levy, I.; Harpaz-Rotem, I. The Reward System and Post-Traumatic Stress Disorder: Does Trauma Affect the Way We Interact With Positive Stimuli? Chronic Stress 2021, 5, 2470547021996006. [Google Scholar] [CrossRef]
- Bloomfield, M.A.; McCutcheon, R.A.; Kempton, M.; Freeman, T.P.; Howes, O. The Effects of Psychosocial Stress on Dopaminergic Function and the Acute Stress Response. Elife 2019, 8, e46797. [Google Scholar] [CrossRef]
- Malikowska-Racia, N.; Sałat, K.; Nowaczyk, A.; Fijałkowski, Ł.; Popik, P. Dopamine D2/D3 Receptor Agonists Attenuate PTSD-like Symptoms in Mice Exposed to Single Prolonged Stress. Neuropharmacology 2019, 155, 1–9. [Google Scholar] [CrossRef]
- Miczek, K.A.; Nikulina, E.M.; Shimamoto, A.; Covington, H.E. Escalated or Suppressed Cocaine Reward, Tegmental BDNF, and Accumbal Dopamine Caused by Episodic versus Continuous Social Stress in Rats. J. Neurosci. 2011, 31, 9848–9857. [Google Scholar] [CrossRef]
- Prabhu, V.V.; Nguyen, T.B.; Cui, Y.; Oh, Y.E.; Lee, K.H.; Bagalkot, T.R.; Chung, Y.C. Effects of Social Defeat Stress on Dopamine D2 Receptor Isoforms and Proteins Involved in Intracellular Trafficking. Behav. Brain Funct. 2018, 14, 16. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M.; Calvo-Torrent, A.; Pico-Alfonso, M.A. Social Defeat and Subordination as Models of Social Stress in Laboratory Rodents: A Review. Aggress. Behav. 1998, 24, 241–256. [Google Scholar] [CrossRef]
- Avgustinovich, D.F.; Kovalenko, I.L.; Kudryavtseva, N.N. A Model of Anxious Depression: Persistence of Behavioral Pathology. Neurosci. Behav. Physiol. 2005, 35, 917–924. [Google Scholar] [CrossRef] [PubMed]
- Hammels, C.; Pishva, E.; De Vry, J.; van den Hove, D.L.A.; Prickaerts, J.; van Winkel, R.; Selten, J.P.; Lesch, K.P.; Daskalakis, N.P.; Steinbusch, H.W.M.; et al. Defeat Stress in Rodents: From Behavior to Molecules. Neurosci. Biobehav. Rev. 2015, 59, 111–140. [Google Scholar] [CrossRef] [PubMed]
- Hollis, F.; Kabbaj, M. Social Defeat as an Animal Model for Depression. ILAR J. 2014, 55, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Whitaker, A.M.; Gilpin, N.W.; Edwards, S. Animal Models of Post-Traumatic Stress Disorder and Recent Neurobiological Insights. Behav. Pharmacol. 2014, 25, 398. [Google Scholar] [CrossRef]
- Shimamoto, A.; DeBold, J.F.; Holly, E.N.; Miczek, K.A. Blunted Accumbal Dopamine Response to Cocaine Following Chronic Social Stress in Female Rats: Exploring a Link between Depression and Drug Abuse. Psychopharmacology 2011, 218, 271. [Google Scholar] [CrossRef]
- Meerlo, P.; Overkamp, G.J.F.; Koolhaas, J.M. Behavioural and Physiological Consequences of a Single Social Defeat in Roman High- and Low-Avoidance Rats. Psychoneuroendocrinology 1997, 22, 155–168. [Google Scholar] [CrossRef]
- Bohus, B.; Benus, R.F.; Fokkema, D.S.; Koolhaas, J.M.; Nyakas, C.; van Oortmerssen, G.A.; Prins, A.J.A.; de Ruiter, A.J.H.; Scheurink, A.J.W.; Steffens, A.B. Neuroendocrine States and Behavioral and Physiological Stress Responses. Prog. Brain Res. 1987, 72, 57–70. [Google Scholar] [CrossRef]
- Anstrom, K.K.; Miczek, K.A.; Budygin, E.A. Increased Phasic Dopamine Signaling in the Mesolimbic Pathway during Social Defeat in Rats. Neuroscience 2009, 161, 3–12. [Google Scholar] [CrossRef]
- Holly, E.N.; Debold, J.F.; Miczek, K.A. Increased Mesocorticolimbic Dopamine during Acute and Repeated Social Defeat Stress: Modulation by Corticotropin Releasing Factor Receptors in the Ventral Tegmental Area. Psychopharmacology 2015, 232, 4469–4479. [Google Scholar] [CrossRef] [PubMed]
- Verbitsky, A.; Dopfel, D.; Zhang, N. Rodent Models of Post-Traumatic Stress Disorder: Behavioral Assessment. Transl. Psychiatry 2020, 10, 132. [Google Scholar] [CrossRef] [PubMed]
- Pulliam, J.; Dawaghreh, J.; Alema-Mensah, E.; Plotsky, E. Social Defeat Stress Produces Prolonged Alterations in Acoustic Startle and Body Weight Gain in Male Long Evans Rats. J. Psychiatr. Res. 2010, 44, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Vestring, S.; Serchov, T.; Normann, C. Animal Models of Depression-Chronic Despair Model (Cdm). J. Vis. Exp. 2021, 2021, 62579. [Google Scholar] [CrossRef] [PubMed]
- Koolhaas, J.M.; Meerlo, P.; Boer, S.F.D.E.; Strubbe, J.H.; Bohus, B. The Temporal Dynamics of the Stress Response. Neurosci. Biobehav. Rev. 1997, 21, 775–782. [Google Scholar] [CrossRef]
- Graziane, N.M.; Polter, A.M.; Briand, L.A.; Pierce, R.C.; Kauer, J.A. Kappa Opioid Receptors Regulate Stress-Induced Cocaine Seeking and Synaptic Plasticity. Neuron 2013, 77, 942–954. [Google Scholar] [CrossRef]
- Tan, K.R.; Yvon, C.; Turiault, M.; Mirzabekov, J.J.; Doehner, J.; Labouèbe, G.; Deisseroth, K.; Tye, K.M.; Lüscher, C. GABA Neurons of the VTA Drive Conditioned Place Aversion. Neuron 2012, 73, 1173–1183. [Google Scholar] [CrossRef] [PubMed]
- Van Zessen, R.; Phillips, J.L.; Budygin, E.A.; Stuber, G.D. Activation of VTA GABA Neurons Disrupts Reward Consumption. Neuron 2012, 73, 1184–1194. [Google Scholar] [CrossRef]
- Anstrom, K.K.; Woodward, D.J. Restraint Increases Dopaminergic Burst Firing in Awake Rats. Neuropsychopharmacology 2005, 30, 1832–1840. [Google Scholar] [CrossRef]
- Henry, J.P.; Liu, Y.Y.; Nadra, W.E.; Qian, C.G.; Mormede, P.; Lemaire, V.; Ely, D.; Hendley, E.D. Psychosocial Stress Can Induce Chronic Hypertension in Normotensive Strains of Rats. Hypertens. 1993, 21, 714–723. [Google Scholar] [CrossRef]
- Snyder, B.; Duong, P.; Tenkorang, M.; Wilson, E.N.; Cunningham, R.L. Rat Strain and Housing Conditions Alter Oxidative Stress and Hormone Responses to Chronic Intermittent Hypoxia. Front. Physiol. 2018, 9, 1554. [Google Scholar] [CrossRef] [PubMed]
- Acero-Castillo, M.C.; Ardila-Figueroa, M.C.; Botelho de Oliveira, S. Anhedonic Type Behavior and Anxiety Profile of Wistar-UIS Rats Subjected to Chronic Social Isolation. Front. Behav. Neurosci. 2021, 15, 103. [Google Scholar] [CrossRef] [PubMed]
- He, L.W.; Zeng, L.; Tian, N.; Li, Y.; He, T.; Tan, D.M.; Zhang, Q.; Tan, Y. Optimization of Food Deprivation and Sucrose Preference Test in SD Rat Model Undergoing Chronic Unpredictable Mild Stress. Anim. Model. Exp. Med. 2020, 3, 69. [Google Scholar] [CrossRef] [PubMed]
- Strekalova, T.; Spanagel, R.; Bartsch, D.; Henn, F.; Gass, P. Stress-Induced Anhedonia in Mice Is Associated with Deficits in Forced Swimming and Exploration. Neuropsychopharmacology 2004, 29, 2007–2017. [Google Scholar] [CrossRef] [PubMed]
- Porsolt, R.D.; Le Pichon, M.; Jalfre, M. Depression: A New Animal Model Sensitive to Antidepressant Treatments. Nature 1977, 266, 730–732. [Google Scholar] [CrossRef]
- Park, J.; Bucher, E.S.; Budygin, E.A.; Mark Wightman, R. Norepinephrine and Dopamine Transmission in 2 Limbic Regions Differentially Respond to Acute Noxious Stimulation. Pain 2015, 156, 318–327. [Google Scholar] [CrossRef]
- Oleson, E.B.; Salek, J.; Bonin, K.D.; Jones, S.R.; Budygin, E.A. Real-Time Voltammetric Detection of Cocaine-Induced Dopamine Changes in the Striatum of Freely Moving Mice. Neurosci. Lett. 2009, 467, 144–146. [Google Scholar] [CrossRef]
- Phillips, P.E.M.; Johns, J.M.; Lubin, D.A.; Budygin, E.A.; Gainetdinov, R.R.; Lieberman, J.A.; Wightman, R.M. Presynaptic Dopaminergic Function Is Largely Unaltered in Mesolimbic and Mesostriatal Terminals of Adult Rats That Were Prenatally Exposed to Cocaine. Brain Res. 2003, 961, 63. [Google Scholar] [CrossRef]
- Park, J.; Aragona, B.J.; Kile, B.M.; Carelli, R.M.; Wightman, R.M. In Vivo Voltammetric Monitoring of Catecholamine Release in Subterritories of the Nucleus Accumbens Shell. Neuroscience 2010, 169, 132–142. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 6th ed.; Academic Press: Cambridge, MA, USA, 2006. [Google Scholar]
- Keeney, A.; Jessop, D.S.; Harbuz, M.S.; Marsden, C.A.; Hogg, S.; Blackburn-Munro, R.E. Differential Effects of Acute and Chronic Social Defeat Stress on Hypothalamic-Pituitary-Adrenal Axis Function and Hippocampal Serotonin Release in Mice. J. Neuroendocrinol. 2006, 18, 330–338. [Google Scholar] [CrossRef]
- Montagud-Romero, S.; Reguilón, M.D.; Rodríguez-Arias, M. Two Interconnected Worlds How Exposure to Social Stress Makes Us More Vulnerable to Drug Use. Metode 2022, 2022, 63–69. [Google Scholar] [CrossRef]
- Meerlo, P.; Overkamp, G.J.F.; Benning, M.A.; Koolhaas, J.M.; Van Den Hoofdakker, R.H. Long-Term Changes in Open Field Behaviour Following a Single Social Defeat in Rats Can Be Reversed by Sleep Deprivation. Physiol. Behav. 1996, 60, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Meerlo, P.; Sgoifo, A.; De Boer, S.F.; Koolhaas, J.M. Long-Lasting Consequences of a Social Conflict in Rats: Behavior during the Interaction Predicts Subsequent Changes in Daily Rhythms of Heart Rate, Temperature, and Activity. Behav. Neurosci. 1999, 113, 1283–1290. [Google Scholar] [CrossRef]
- Bass, C.E.; Grinevich, V.P.; Gioia, D.; Day-Brown, J.D.; Bonin, K.D.; Stuber, G.D.; Weiner, J.L.; Budygin, E.A. Optogenetic Stimulation of VTA Dopamine Neurons Reveals That Tonic but Not Phasic Patterns of Dopamine Transmission Reduce Ethanol Self-Administration. Front. Behav. Neurosci. 2013, 7, 173. [Google Scholar] [CrossRef] [PubMed]
- Budygin, E.A.; Bass, C.E.; Grinevich, V.P.; Deal, A.L.; Bonin, K.D.; Weiner, J.L. Opposite Consequences of Tonic and Phasic Increases in Accumbal Dopamine on Alcohol-Seeking Behavior. iScience 2020, 23, 100877. [Google Scholar] [CrossRef] [PubMed]
- Gonon, F.G. Nonlinear Relationship between Impulse Flow and Dopamine Released by Rat Midbrain Dopaminergic Neurons as Studied by in Vivo Electrochemistry. Neuroscience 1988, 24, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Niehaus, J.L.; Murali, M.; Kauer, J.A. Drugs of Abuse and Stress Impair LTP at Inhibitory Synapses in the Ventral Tegmental Area. Eur. J. Neurosci. 2010, 32, 108–117. [Google Scholar] [CrossRef]
- Daftary, S.S.; Panksepp, J.; Dong, Y.; Saal, D.B. Stress-Induced, Glucocorticoid-Dependent Strengthening of Glutamatergic Synapse Transmission in Midbrain Dopamine Neurons. Neurosci. Lett. 2009, 452, 273. [Google Scholar] [CrossRef]
- Deal, A.L.; Konstantopoulos, J.K.; Weiner, J.L.; Budygin, E.A. Exploring the Consequences of Social Defeat Stress and Intermittent Ethanol Drinking on Dopamine Dynamics in the Rat Nucleus Accumbens. Sci. Rep. 2018, 8, 332. [Google Scholar] [CrossRef]
- Donahue, R.J.; Landino, S.M.; Golden, S.A.; Carroll, F.I.; Russo, S.J.; Carlezon, W.A. Effects of Acute and Chronic Social Defeat Stress Are Differentially Mediated by the Dynorphin/Kappa-Opioid Receptor System. Behav. Pharmacol. 2015, 26, 654. [Google Scholar] [CrossRef]
- Ebner, S.R.; Roitman, M.F.; Potter, D.N.; Rachlin, A.B.; Chartoff, E.H. Depressive-like Effects of the Kappa Opioid Receptor Agonist Salvinorin A Are Associated with Decreased Phasic Dopamine Release in the Nucleus Accumbens. Psychopharmacology 2010, 210, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Karkhanis, A.N.; Rose, J.H.; Weiner, J.L.; Jones, S.R. Early-Life Social Isolation Stress Increases Kappa Opioid Receptor Responsiveness and Downregulates the Dopamine System. Neuropsychopharmacology 2016, 41, 2263–2274. [Google Scholar] [CrossRef] [PubMed]
- Holly, E.N.; Boyson, C.O.; Montagud-Romero, S.; Stein, D.J.; Gobrogge, K.L.; DeBold, J.F.; Miczek, K.A. Episodic Social Stress-Escalated Cocaine Self-Administration: Role of Phasic and Tonic Corticotropin Releasing Factor in the Anterior and Posterior Ventral Tegmental Area. J. Neurosci. 2016, 36, 4093–4105. [Google Scholar] [CrossRef] [PubMed]
- Hostetler, C.M.; Ryabinin, A.E. The CRF System and Social Behavior: A Review. Front. Neurosci. 2013, 7, 92. [Google Scholar] [CrossRef] [PubMed]
- Henckens, M.J.A.G.; Deussing, J.M.; Chen, A. Region-Specific Roles of the Corticotropin-Releasing Factor-Urocortin System in Stress. Nat. Rev. Neurosci. 2016, 17, 636–651. [Google Scholar] [CrossRef] [PubMed]
- Lemos, J.C.; Wanat, M.J.; Smith, J.S.; Reyes, B.A.S.; Hollon, N.G.; Van Bockstaele, E.J.; Chavkin, C.; Phillips, P.E.M. Severe Stress Switches CRF Action in the Nucleus Accumbens from Appetitive to Aversive. Nature 2012, 490, 402–406. [Google Scholar] [CrossRef]
- Lemos, J.C.; Shin, J.H.; Alvarez, V.A. Striatal Cholinergic Interneurons Are a Novel Target of Corticotropin Releasing Factor. J. Neurosci. 2019, 39, 5647–5661. [Google Scholar] [CrossRef]
- Michael, A.C.; Ikeda, M.; Justice, J.B. Dynamics of the Recovery of Releasable Dopamine Following Electrical Stimulation of the Medial Forebrain Bundle. Neurosci. Lett. 1987, 76, 81–86. [Google Scholar] [CrossRef]
- Michael, A.C.; Ikeda, M.; Justice, J.B. Mechanisms Contributing to the Recovery of Striatal Releasable Dopamine Following MFB Stimulation. Brain Res. 1987, 421, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Yavich, L. Two Simultaneously Working Storage Pools of Dopamine in Mouse Caudate and Nucleus Accumbens. Br. J. Pharmacol. 1996, 119, 869–876. [Google Scholar] [CrossRef]
- Suzuki, H.; Lucas, L.R. Neurochemical Correlates of Accumbal Dopamine D2 and Amygdaloid 5-HT 1B Receptor Densities on Observational Learning of Aggression. Cogn. Affect. Behav. Neurosci. 2015, 15, 460–474. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nemets, V.V.; Deal, A.L.; Sobolev, V.E.; Grinevich, V.P.; Gainetdinov, R.R.; Budygin, E.A. Short-Term Consequences of Single Social Defeat on Accumbal Dopamine and Behaviors in Rats. Biomolecules 2023, 13, 35. https://doi.org/10.3390/biom13010035
Nemets VV, Deal AL, Sobolev VE, Grinevich VP, Gainetdinov RR, Budygin EA. Short-Term Consequences of Single Social Defeat on Accumbal Dopamine and Behaviors in Rats. Biomolecules. 2023; 13(1):35. https://doi.org/10.3390/biom13010035
Chicago/Turabian StyleNemets, Vsevolod V., Alex L. Deal, Vladislav E. Sobolev, Vladimir P. Grinevich, Raul R. Gainetdinov, and Evgeny A. Budygin. 2023. "Short-Term Consequences of Single Social Defeat on Accumbal Dopamine and Behaviors in Rats" Biomolecules 13, no. 1: 35. https://doi.org/10.3390/biom13010035
APA StyleNemets, V. V., Deal, A. L., Sobolev, V. E., Grinevich, V. P., Gainetdinov, R. R., & Budygin, E. A. (2023). Short-Term Consequences of Single Social Defeat on Accumbal Dopamine and Behaviors in Rats. Biomolecules, 13(1), 35. https://doi.org/10.3390/biom13010035