Approaches and Vectors for Efficient Cochlear Gene Transfer in Adult Mouse Models
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. AAV Vector Construction
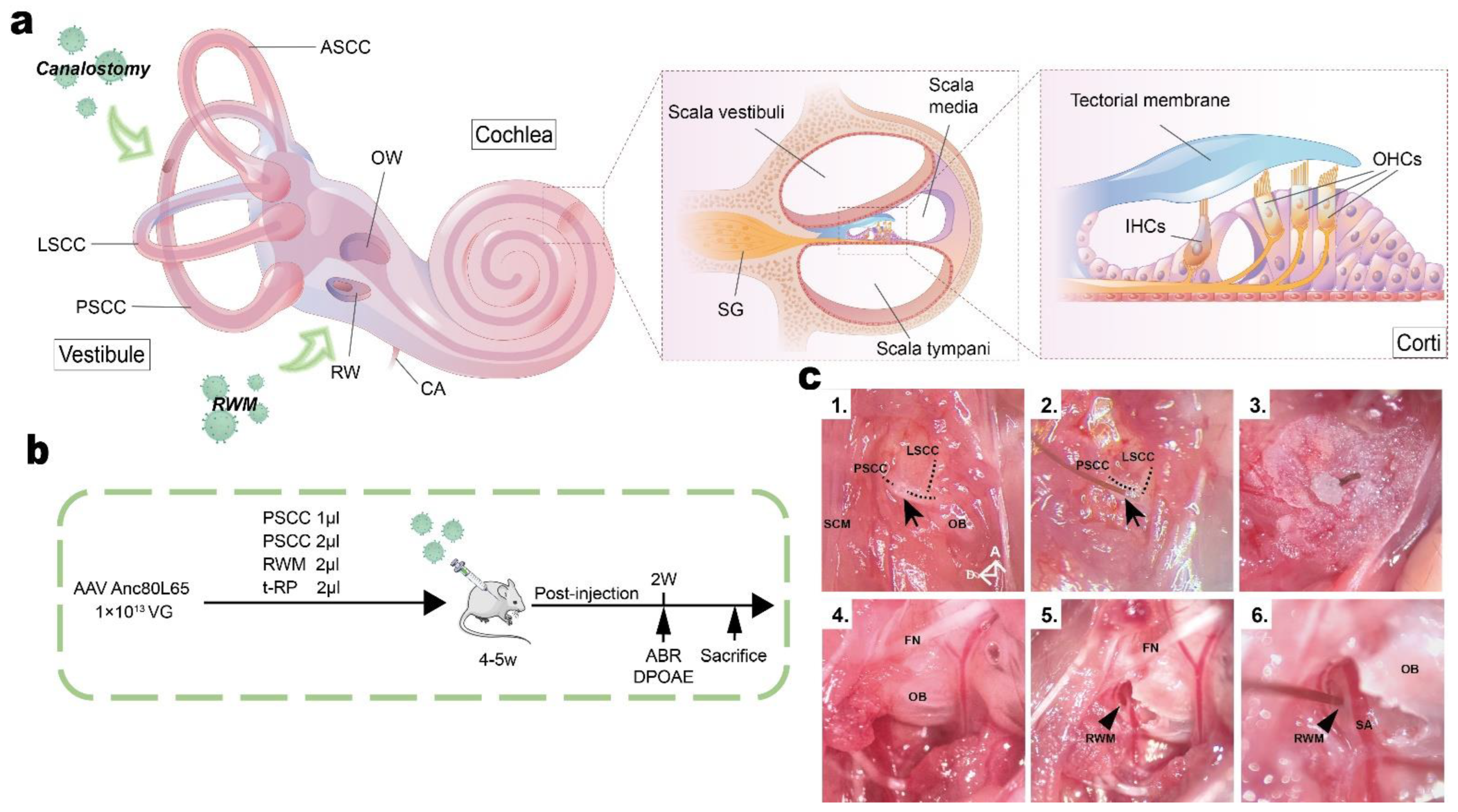
2.3. Animal Surgery: PSCC Canalostomy
2.4. Animal Surgery: RWM Injection
2.5. Animal Surgery: Tubing-RWM+PSCC Injection (t-RP)
2.6. Hearing Tests
2.7. Fixation and Preparation of the Samples
2.8. Immunohistochemistry and Confocal Microscopy
2.9. Hematoxylin and Eosin (H&E) Staining
2.10. Cell Counting
2.11. Statistical Analyses
3. Results
3.1. In Vivo Delivery of AAV-Anc80L65 into Adult Cochleae via Different Injection Routes
3.2. Canalostomy Resulted in Robust Transduction of HCs throughout the Cochlea
3.3. Preservation of Hearing Function via PSCC Canalostomy in Adult Mice
3.4. Local Injection in the Inner Ear Allows AAV Vectors to Spread to the Brain
3.5. AAV-Anc80L65 Showed More Efficient Transduction in Cochlear HCs and SGNs
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deafness and hearing loss. Available online: https://www.who.int/news-room/fact-sheets/detail/deafness-and-hearing-loss (accessed on 15 September 2022).
- Lesica, N.A. Why Do Hearing Aids Fail to Restore Normal Auditory Perception? Trends Neurosci. 2018, 41, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Gyorgy, B.; Nist-Lund, C.; Pan, B.; Asai, Y.; Karavitaki, K.D.; Kleinstiver, B.P.; Garcia, S.P.; Zaborowski, M.P.; Solanes, P.; Spataro, S.; et al. Allele-specific gene editing prevents deafness in a model of dominant progressive hearing loss. Nat. Med. 2019, 25, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
- Akil, O.; Dyka, F.; Calvet, C.; Emptoz, A.; Lahlou, G.; Nouaille, S.; Boutet de Monvel, J.; Hardelin, J.P.; Hauswirth, W.W.; Avan, P.; et al. Dual AAV-mediated gene therapy restores hearing in a DFNB9 mouse model. Proc. Natl. Acad. Sci. USA 2019, 116, 4496–4501. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Tao, Y.; Lamas, V.; Huang, M.; Yeh, W.H.; Pan, B.; Hu, Y.J.; Hu, J.H.; Thompson, D.B.; Shu, Y.; et al. Treatment of autosomal dominant hearing loss by in vivo delivery of genome editing agents. Nature 2018, 553, 217–221. [Google Scholar] [CrossRef]
- Guo, Y.; Han, L.; Han, S.; Tang, H.; Wang, S.; Cui, C.; Chen, B.; Li, H.; Shu, Y. Specific knockdown of Htra2 by CRISPR-CasRx prevents acquired sensorineural hearing loss in mice. Mol. Ther. Nucleic Acids 2022, 28, 643–655. [Google Scholar] [CrossRef]
- Gu, X.; Wang, D.; Xu, Z.; Wang, J.; Guo, L.; Chai, R.; Li, G.; Shu, Y.; Li, H. Prevention of acquired sensorineural hearing loss in mice by in vivo Htra2 gene editing. Genome Biol. 2021, 22, 86. [Google Scholar] [CrossRef]
- Xue, Y.; Hu, X.; Wang, D.; Li, D.; Li, Y.; Wang, F.; Huang, M.; Gu, X.; Xu, Z.; Zhou, J.; et al. Gene editing in a Myo6 semi-dominant mouse model rescues auditory function. Mol. Ther. 2022, 30, 105–118. [Google Scholar] [CrossRef]
- Gu, X.; Hu, X.; Wang, D.; Xu, Z.; Wang, F.; Li, D.; Li, G.L.; Yang, H.; Li, H.; Zuo, E.; et al. Treatment of autosomal recessive hearing loss via in vivo CRISPR/Cas9-mediated optimized homology-directed repair in mice. Cell Res. 2022, 32, 699–702. [Google Scholar] [CrossRef]
- Cui, C.; Wang, D.; Huang, B.; Wang, F.; Chen, Y.; Lv, J.; Zhang, L.; Han, L.; Liu, D.; Chen, Z.Y.; et al. Precise detection of CRISPR-Cas9 editing in hair cells in the treatment of autosomal dominant hearing loss. Mol. Ther. Nucleic Acids 2022, 29, 400–412. [Google Scholar] [CrossRef]
- Xiao, Q.; Xu, Z.; Xue, Y.; Xu, C.; Han, L.; Liu, Y.; Wang, F.; Zhang, R.; Han, S.; Wang, X.; et al. Rescue of autosomal dominant hearing loss by in vivo delivery of mini dCas13X-derived RNA base editor. Sci. Transl. Med. 2022, 14, eabn0449. [Google Scholar] [CrossRef]
- Zheng, Z.; Li, G.; Cui, C.; Wang, F.; Wang, X.; Xu, Z.; Guo, H.; Chen, Y.; Tang, H.; Wang, D.; et al. Preventing autosomal-dominant hearing loss in Bth mice with CRISPR/CasRx-based RNA editing. Signal Transduct. Target. Ther. 2022, 7, 79. [Google Scholar] [CrossRef] [PubMed]
- Zuris, J.A.; Thompson, D.B.; Shu, Y.; Guilinger, J.P.; Bessen, J.L.; Hu, J.H.; Maeder, M.L.; Joung, J.K.; Chen, Z.Y.; Liu, D.R. Cationic lipid-mediated delivery of proteins enables efficient protein-based genome editing in vitro and in vivo. Nat. Biotechnol. 2015, 33, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Walters, B.J.; Zuo, J. Postnatal development, maturation and aging in the mouse cochlea and their effects on hair cell regeneration. Hear. Res. 2013, 297, 68–83. [Google Scholar] [CrossRef] [PubMed]
- Drummond, M.C.; Belyantseva, I.A.; Friderici, K.H.; Friedman, T.B. Actin in hair cells and hearing loss. Hear. Res. 2012, 288, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.K.; Chang, K.W. GJB2-associated hearing loss: Systematic review of worldwide prevalence, genotype, and auditory phenotype. Laryngoscope 2014, 124, E34–E53. [Google Scholar] [CrossRef]
- Song, M.H.; Jung, J.; Rim, J.H.; Choi, H.J.; Lee, H.J.; Noh, B.; Lee, J.S.; Gee, H.Y.; Choi, J.Y. Genetic Inheritance of Late-Onset, Down-Sloping Hearing Loss and Its Implications for Auditory Rehabilitation. Ear Hear. 2020, 41, 114–124. [Google Scholar] [CrossRef]
- Fortunato, G.; Marciano, E.; Zarrilli, F.; Mazzaccara, C.; Intrieri, M.; Calcagno, G.; Vitale, D.F.; La Manna, P.; Saulino, C.; Marcelli, V.; et al. Paraoxonase and superoxide dismutase gene polymorphisms and noise-induced hearing loss. Clin. Chem. 2004, 50, 2012–2018. [Google Scholar] [CrossRef][Green Version]
- Unal, M.; Tamer, L.; Dogruer, Z.N.; Yildirim, H.; Vayisoglu, Y.; Camdeviren, H. N-acetyltransferase 2 gene polymorphism and presbycusis. Laryngoscope 2005, 115, 2238–2241. [Google Scholar] [CrossRef]
- Konings, A.; Van Laer, L.; Pawelczyk, M.; Carlsson, P.I.; Bondeson, M.L.; Rajkowska, E.; Dudarewicz, A.; Vandevelde, A.; Fransen, E.; Huyghe, J.; et al. Association between variations in CAT and noise-induced hearing loss in two independent noise-exposed populations. Hum. Mol. Genet. 2007, 16, 1872–1883. [Google Scholar] [CrossRef]
- Shu, Y.; Tao, Y.; Wang, Z.; Tang, Y.; Li, H.; Dai, P.; Gao, G.; Chen, Z.-Y. Identification of Adeno-Associated Viral Vectors That Target Neonatal and Adult Mammalian Inner Ear Cell Subtypes. Hum. Gene Ther. 2016, 27, 687–699. [Google Scholar] [CrossRef]
- Chien, W.W.; McDougald, D.S.; Roy, S.; Fitzgerald, T.S.; Cunningham, L.L. Cochlear gene transfer mediated by adeno-associated virus: Comparison of two surgical approaches. Laryngoscope 2015, 125, 2557–2564. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Okada, T.; Sheykholeslami, K.; Shimazaki, K.; Nomoto, T.; Muramatsu, S.; Kanazawa, T.; Takeuchi, K.; Ajalli, R.; Mizukami, H.; et al. Specific and efficient transduction of Cochlear inner hair cells with recombinant adeno-associated virus type 3 vector. Mol. Ther. 2005, 12, 725–733. [Google Scholar] [CrossRef]
- Yoshimura, H.; Shibata, S.B.; Ranum, P.T.; Smith, R.J.H. Enhanced viral-mediated cochlear gene delivery in adult mice by combining canal fenestration with round window membrane inoculation. Sci. Rep. 2018, 8, 2980. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.Z.; Saleh, J.; Isgrig, K.T.; Cunningham, L.L.; Chien, W.W. Hearing Loss after Round Window Surgery in Mice Is due to Middle Ear Effusion. Audiol. Neurotol. 2016, 21, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.-Y.; He, L.; Qu, T.-F.; Liu, Y.-Y.; Liu, K.; Wang, G.-P.; Gong, S.-S. Canalostomy As a Surgical Approach to Local Drug Delivery into the Inner Ears of Adult and Neonatal Mice. J. Vis. Exp. 2018, 135, 57351. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Choi, J.W.; Ishibashi, Y.; Isgrig, K.; Grati, M.; Bennett, J.; Chien, W. Refining surgical techniques for efficient posterior semicircular canal gene delivery in the adult mammalian inner ear with minimal hearing loss. Sci. Rep. 2021, 11, 18856. [Google Scholar] [CrossRef]
- Tao, Y.; Huang, M.; Shu, Y.; Ruprecht, A.; Wang, H.; Tang, Y.; Vandenberghe, L.H.; Wang, Q.; Gao, G.; Kong, W.J.; et al. Delivery of Adeno-Associated Virus Vectors in Adult Mammalian Inner-Ear Cell Subtypes Without Auditory Dysfunction. Hum. Gene Ther. 2018, 29, 492–506. [Google Scholar] [CrossRef]
- Suzuki, J.; Hashimoto, K.; Xiao, R.; Vandenberghe, L.H.; Liberman, M.C. Cochlear gene therapy with ancestral AAV in adult mice: Complete transduction of inner hair cells without cochlear dysfunction. Sci. Rep. 2017, 7, 45524. [Google Scholar] [CrossRef]
- Omichi, R.; Yoshimura, H.; Shibata, S.B.; Vandenberghe, L.H.; Smith, R.J.H. Hair Cell Transduction Efficiency of Single- and Dual-AAV Serotypes in Adult Murine Cochleae. Mol. Ther. Methods Clin. Dev. 2020, 17, 1167–1177. [Google Scholar] [CrossRef]
- Hu, X.; Wang, J.; Yao, X.; Xiao, Q.; Xue, Y.; Wang, S.; Shi, L.; Shu, Y.; Li, H.; Yang, H. Screened AAV variants permit efficient transduction access to supporting cells and hair cells. Cell Discov. 2019, 5, 49. [Google Scholar] [CrossRef]
- Tan, F.; Chu, C.; Qi, J.; Li, W.; You, D.; Li, K.; Chen, X.; Zhao, W.; Cheng, C.; Liu, X.; et al. AAV-ie enables safe and efficient gene transfer to inner ear cells. Nat. Commun. 2019, 10, 3733. [Google Scholar] [CrossRef] [PubMed]
- Landegger, L.D.; Pan, B.; Askew, C.; Wassmer, S.J.; Gluck, S.D.; Galvin, A.; Taylor, R.; Forge, A.; Stankovic, K.M.; Holt, J.R.; et al. A synthetic AAV vector enables safe and efficient gene transfer to the mammalian inner ear. Nat. Biotechnol. 2017, 35, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, S.; Pattison, L.A.; Doleschall, B.; Rickman, R.H.; Blake, H.; Callejo, G.; Heppenstall, P.A.; Smith, E.S.J. Intraarticular Adeno-Associated Virus Serotype AAV-PHP.S-Mediated Chemogenetic Targeting of Knee-Innervating Dorsal Root Ganglion Neurons Alleviates Inflammatory Pain in Mice. Arthritis Rheumatol. 2020, 72, 1749–1758. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.Y.; Jang, M.J.; Yoo, B.B.; Greenbaum, A.; Ravi, N.; Wu, W.L.; Sanchez-Guardado, L.; Lois, C.; Mazmanian, S.K.; Deverman, B.E.; et al. Engineered AAVs for efficient noninvasive gene delivery to the central and peripheral nervous systems. Nat. Neurosci. 2017, 20, 1172–1179. [Google Scholar] [CrossRef]
- Akil, O.; Lustig, L. AAV-Mediated Gene Delivery to the Inner Ear. Methods Mol. Biol. 2019, 1950, 271–282. [Google Scholar] [CrossRef]
- Yoshimura, H.; Shibata, S.B.; Ranum, P.T.; Moteki, H.; Smith, R.J.H. Targeted Allele Suppression Prevents Progressive Hearing Loss in the Mature Murine Model of Human TMC1 Deafness. Mol. Ther. 2019, 27, 681–690. [Google Scholar] [CrossRef]
- Kilpatrick, L.A.; Li, Q.; Yang, J.; Goddard, J.C.; Fekete, D.M.; Lang, H. Adeno-associated virus-mediated gene delivery into the scala media of the normal and deafened adult mouse ear. Gene Ther. 2011, 18, 569–578. [Google Scholar] [CrossRef]
- Kang, W.; Zhao, X.; Sun, Z.; Dong, T.; Jin, C.; Tong, L.; Zhu, W.; Tao, Y.; Wu, H. Adeno-associated virus vector enables safe and efficient Cas9 activation in neonatal and adult Cas9 knockin murine cochleae. Gene Ther. 2020, 27, 392–405. [Google Scholar] [CrossRef]
- Loeb, J.E.; Cordier, W.S.; Harris, M.E.; Weitzman, M.D.; Hope, T.J. Enhanced expression of transgenes from adeno-associated virus vectors with the woodchuck hepatitis virus posttranscriptional regulatory element: Implications for gene therapy. Hum. Gene Ther. 1999, 10, 2295–2305. [Google Scholar] [CrossRef]
- Nass, S.A.; Mattingly, M.A.; Woodcock, D.A.; Burnham, B.L.; Ardinger, J.A.; Osmond, S.E.; Frederick, A.M.; Scaria, A.; Cheng, S.H.; O’Riordan, C.R. Universal Method for the Purification of Recombinant AAV Vectors of Differing Serotypes. Mol. Ther. Methods Clin. Dev. 2018, 9, 33–46. [Google Scholar] [CrossRef]
- Talaei, S.; Schnee, M.E.; Aaron, K.A.; Ricci, A.J. Dye Tracking Following Posterior Semicircular Canal or Round Window Membrane Injections Suggests a Role for the Cochlea Aqueduct in Modulating Distribution. Front. Cell Neurosci. 2019, 13, 471. [Google Scholar] [CrossRef] [PubMed]
- Blanc, F.; Bemelmans, A.P.; Affortit, C.; Josephine, C.; Puel, J.L.; Mondain, M.; Wang, J. A Single Cisterna Magna Injection of AAV Leads to Binaural Transduction in Mice. Front. Cell Dev. Biol. 2021, 9, 783504. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Zhang, L.; Wang, D.; Chen, B.; Shu, Y. Approaches and Vectors for Efficient Cochlear Gene Transfer in Adult Mouse Models. Biomolecules 2023, 13, 38. https://doi.org/10.3390/biom13010038
Zhao Y, Zhang L, Wang D, Chen B, Shu Y. Approaches and Vectors for Efficient Cochlear Gene Transfer in Adult Mouse Models. Biomolecules. 2023; 13(1):38. https://doi.org/10.3390/biom13010038
Chicago/Turabian StyleZhao, Yu, Longlong Zhang, Daqi Wang, Bing Chen, and Yilai Shu. 2023. "Approaches and Vectors for Efficient Cochlear Gene Transfer in Adult Mouse Models" Biomolecules 13, no. 1: 38. https://doi.org/10.3390/biom13010038
APA StyleZhao, Y., Zhang, L., Wang, D., Chen, B., & Shu, Y. (2023). Approaches and Vectors for Efficient Cochlear Gene Transfer in Adult Mouse Models. Biomolecules, 13(1), 38. https://doi.org/10.3390/biom13010038







