Human Alpha-1 Antitrypsin Attenuates ENaC and MARCKS and Lowers Blood Pressure in Hypertensive Diabetic db/db Mice
Abstract
1. Introduction
2. Materials and Methods
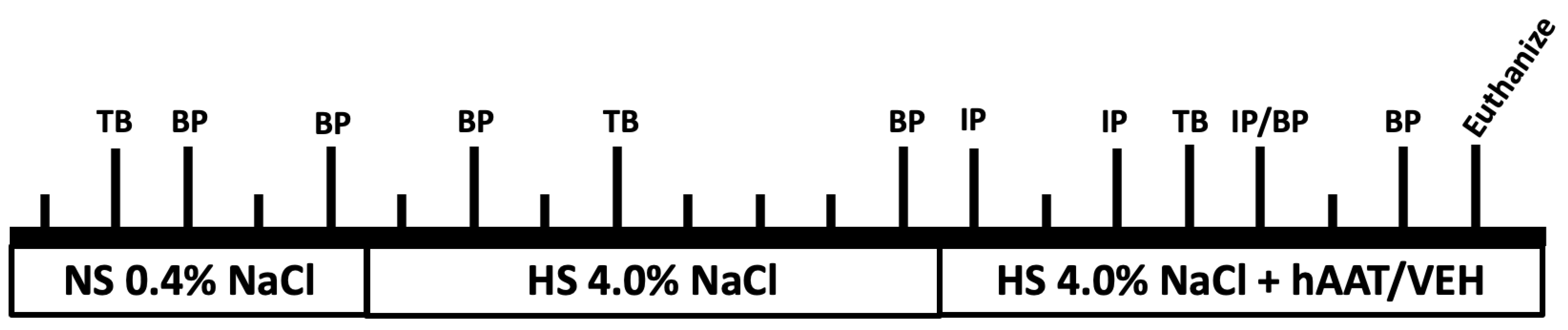
2.1. Experimental Design
2.2. Animals
2.3. Blood Pressure Measurements
2.4. Human AAT Injections and Detection
2.5. Tissue Processing
2.6. SDS-PAGE and Western Blotting
2.7. Cathepsin B and S Activity Assays
2.8. Immunohistochemistry
2.9. Measurement of Urine Osmolality
2.10. Measurements of Urine Electrolytes
2.11. Blood Glucose Measurements
2.12. Urinary Extracellular Vesicle Isolation and Nanoparticle Tracking Analysis
2.13. Transmission Electron Microscope Analysis of Freshly Isolated EVs
2.14. Lipidomics
2.15. Statistical Analysis
3. Results
3.1. Blood Glucose Levels in Salt Sensitive Hypertensive db/db Mice after hAAT Treatment
3.2. Circulating hAAT Levels in Hypertensive Diabetic db/db Mice after hAAT Administration
3.3. Presence of hAAT in Kidney Cortex Lysates from hAAT Treated Mice Compared to Control Mice
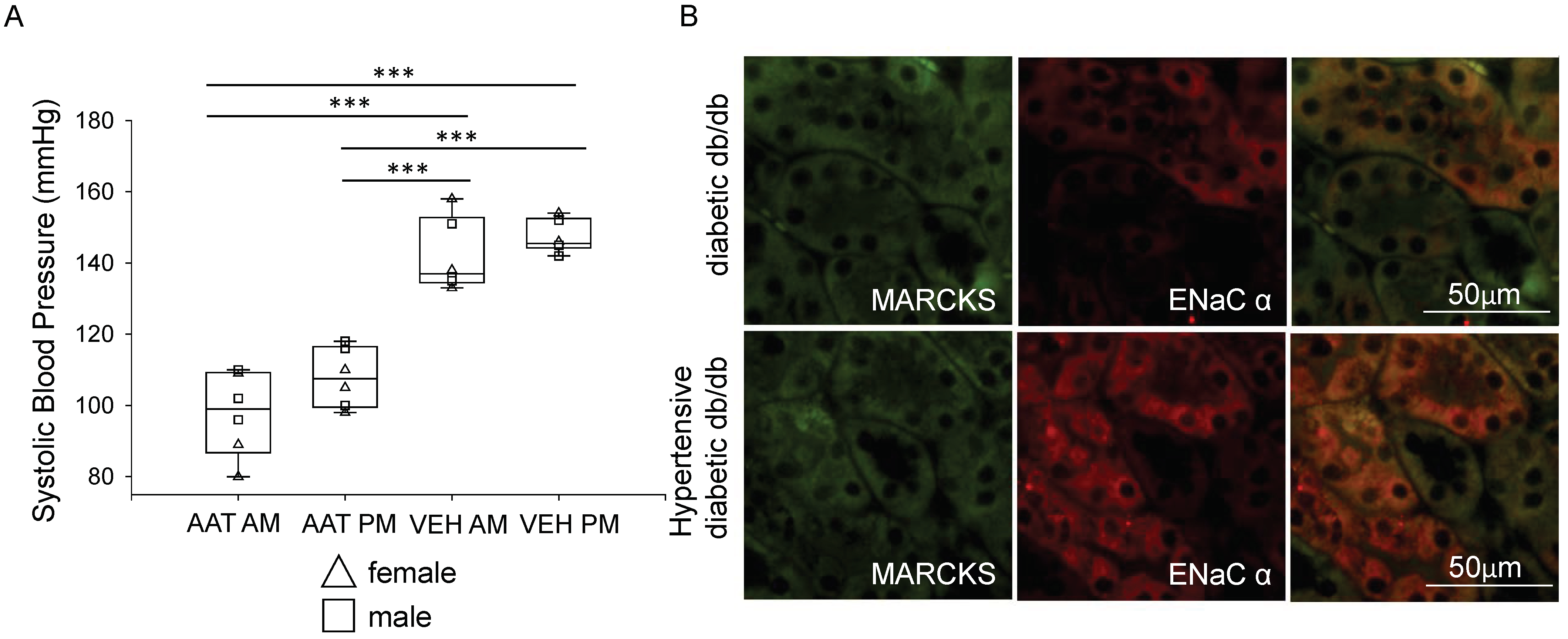
3.4. Administration of hAAT Decreases Systolic Blood Pressure in Hypertensive Diabetic db/db Mice
3.5. Urinary Osmolality and Electrolytes in Diabetic db/db Mice Treated with hAAT or Vehicle
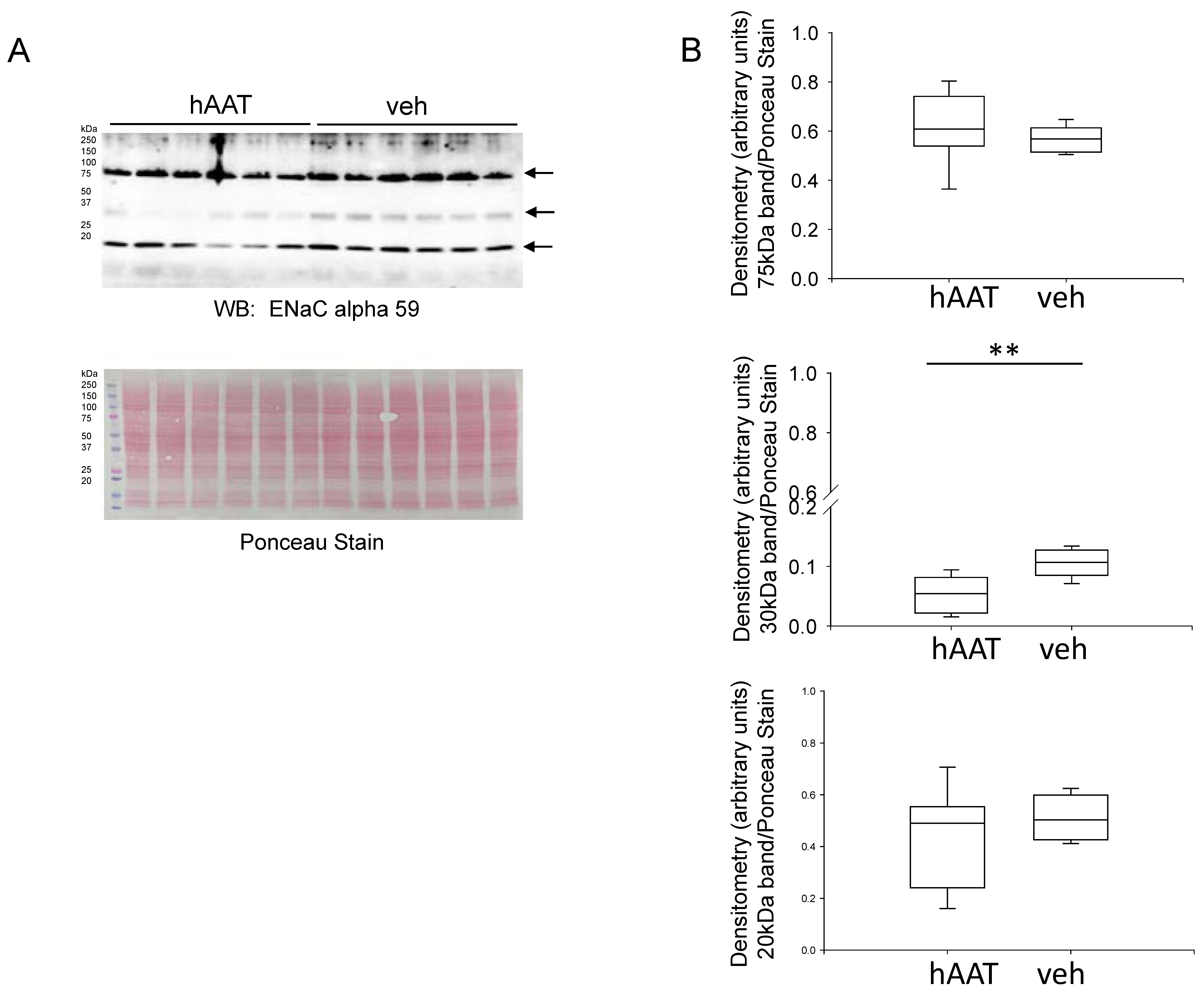
3.6. Effect of hAAT Administration on ENaC Alpha Subunit Protein Expression
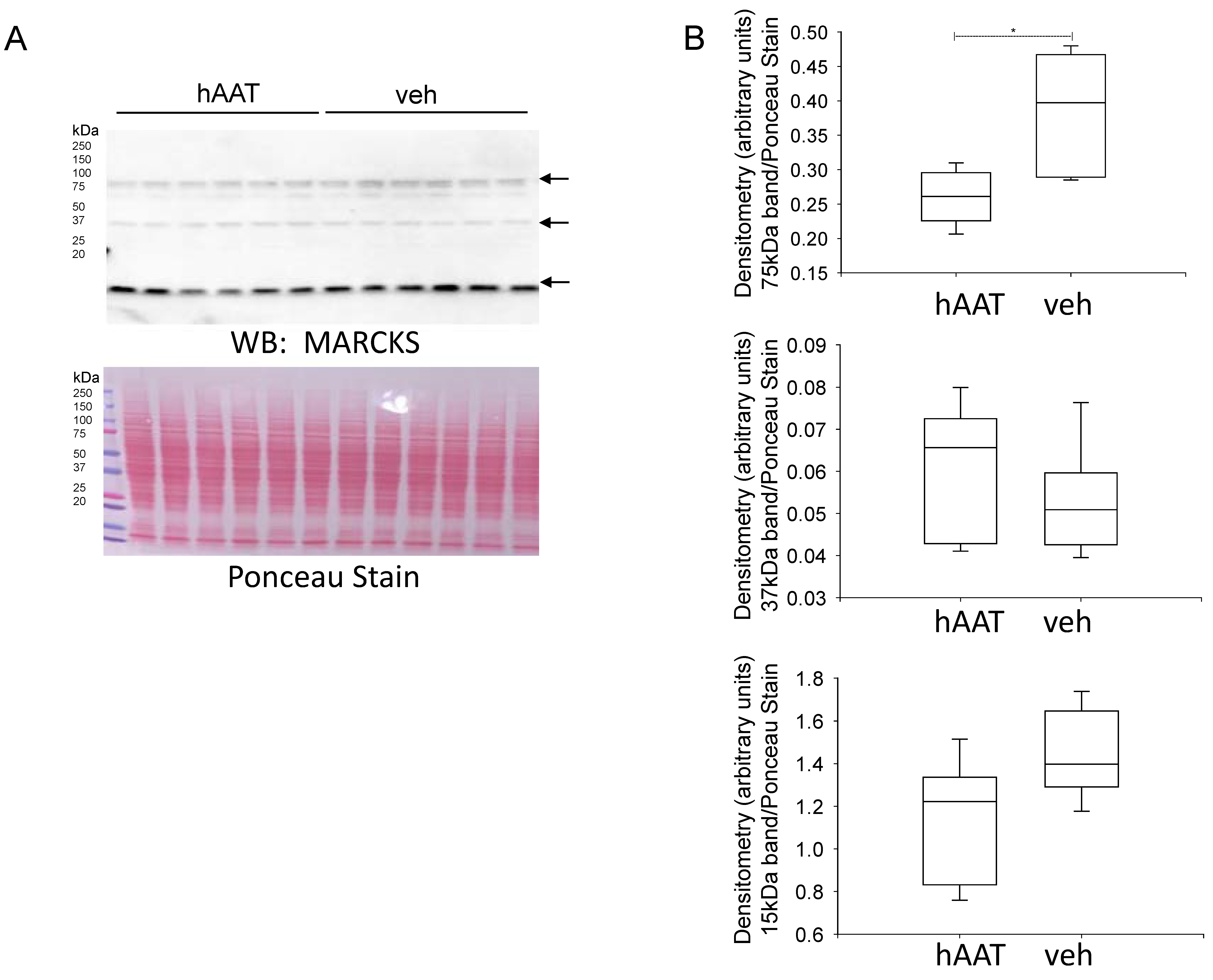
3.7. MARCKS Protein Expression Is Reduced in the Diabetic db/db Kidney after Treatment with hAAT
3.8. hAAT Treatment Attenuates Colocalization of MARCKS and ENaC Alpha Protein in the Diabetic db/db Kidney
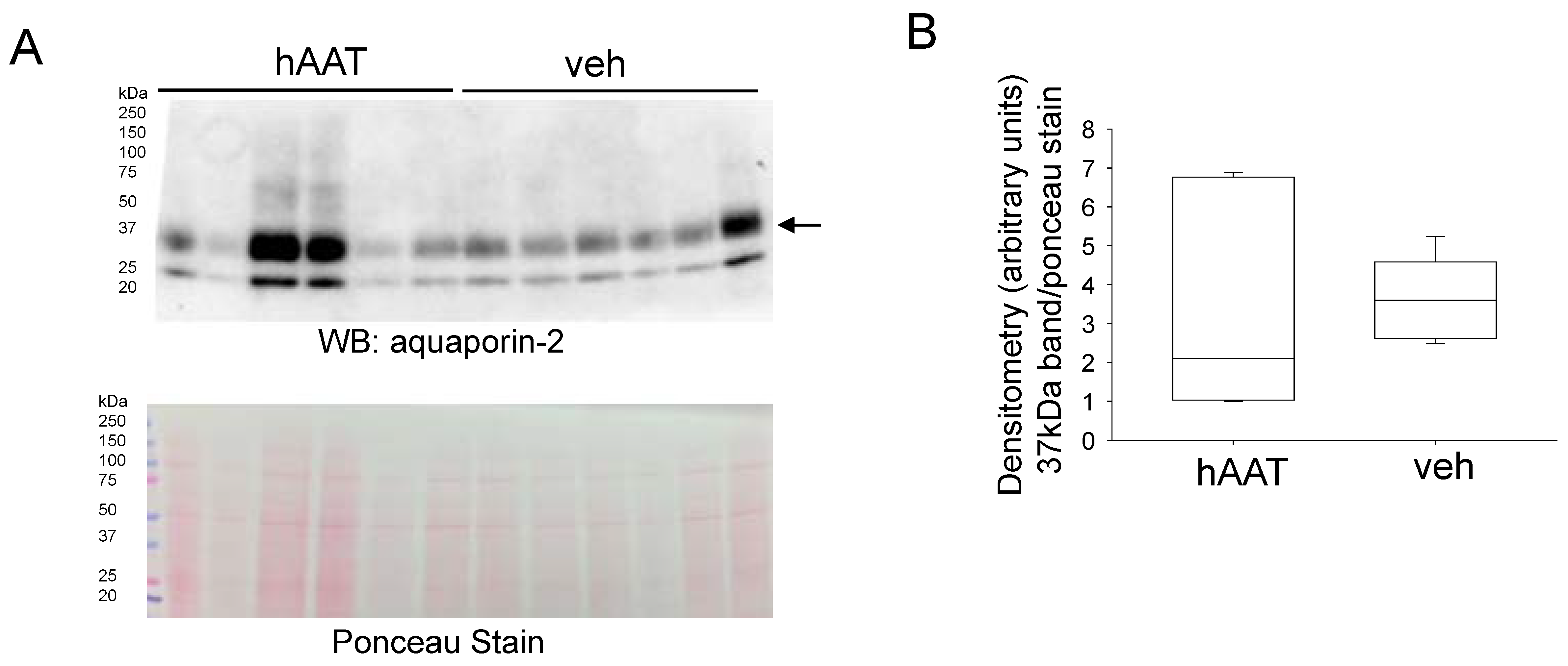
3.9. hAAT Treatment Does Not Alter Aquaporin-2 Protein Expression in the Diabetic Kidney
3.10. hAAT Treatment Does Not Alter NPRC Protein Expression in the Kidney of db/db Mice
3.11. hAAT Treatment Does Not Alter Cathepsin B or Kidney Kallikrein Protein Expression or Activity in db/db Mice
3.12. Characterization of Urinary EVs from db/db Mice Treated with hAAT or Vehicle
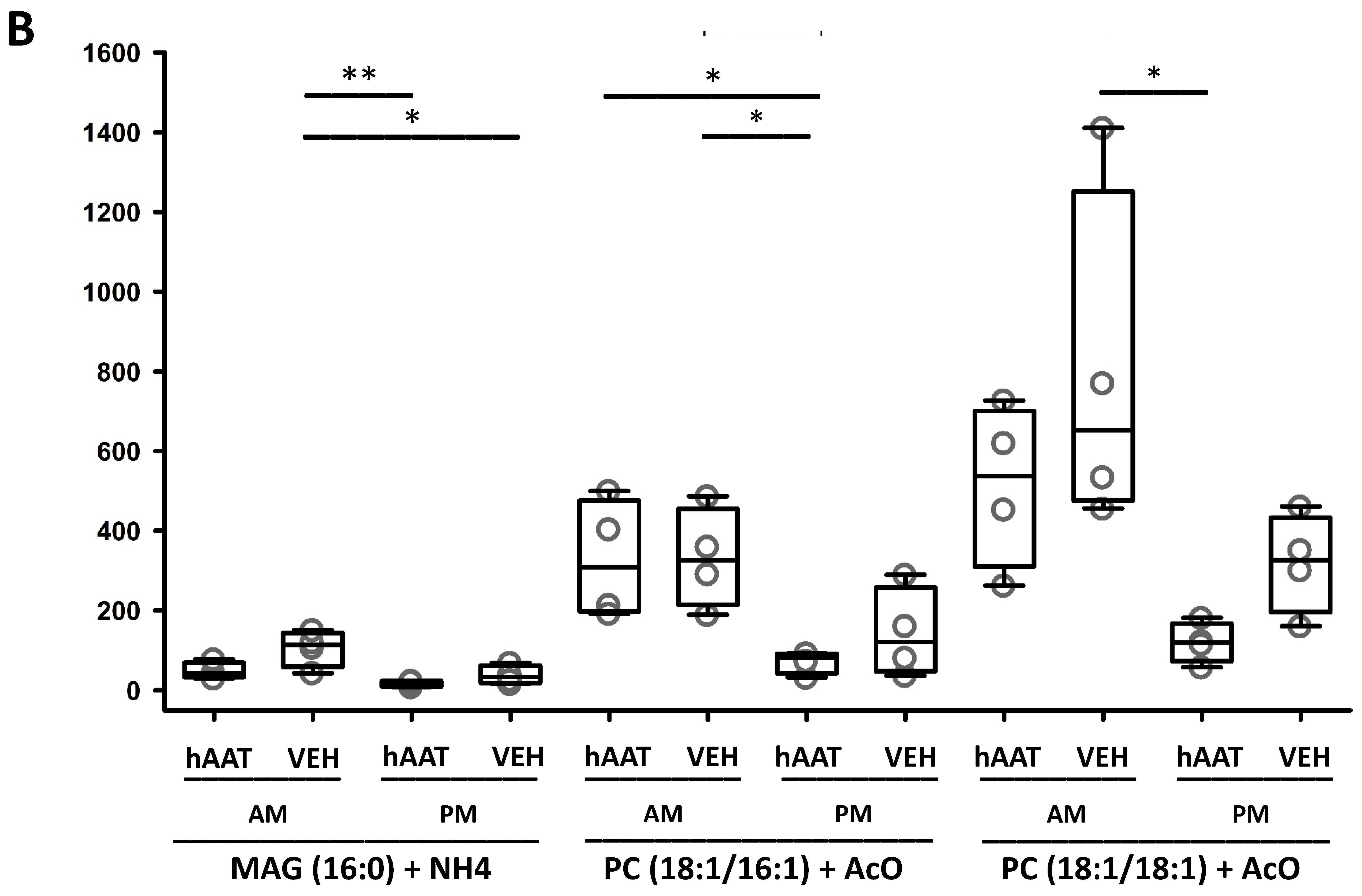
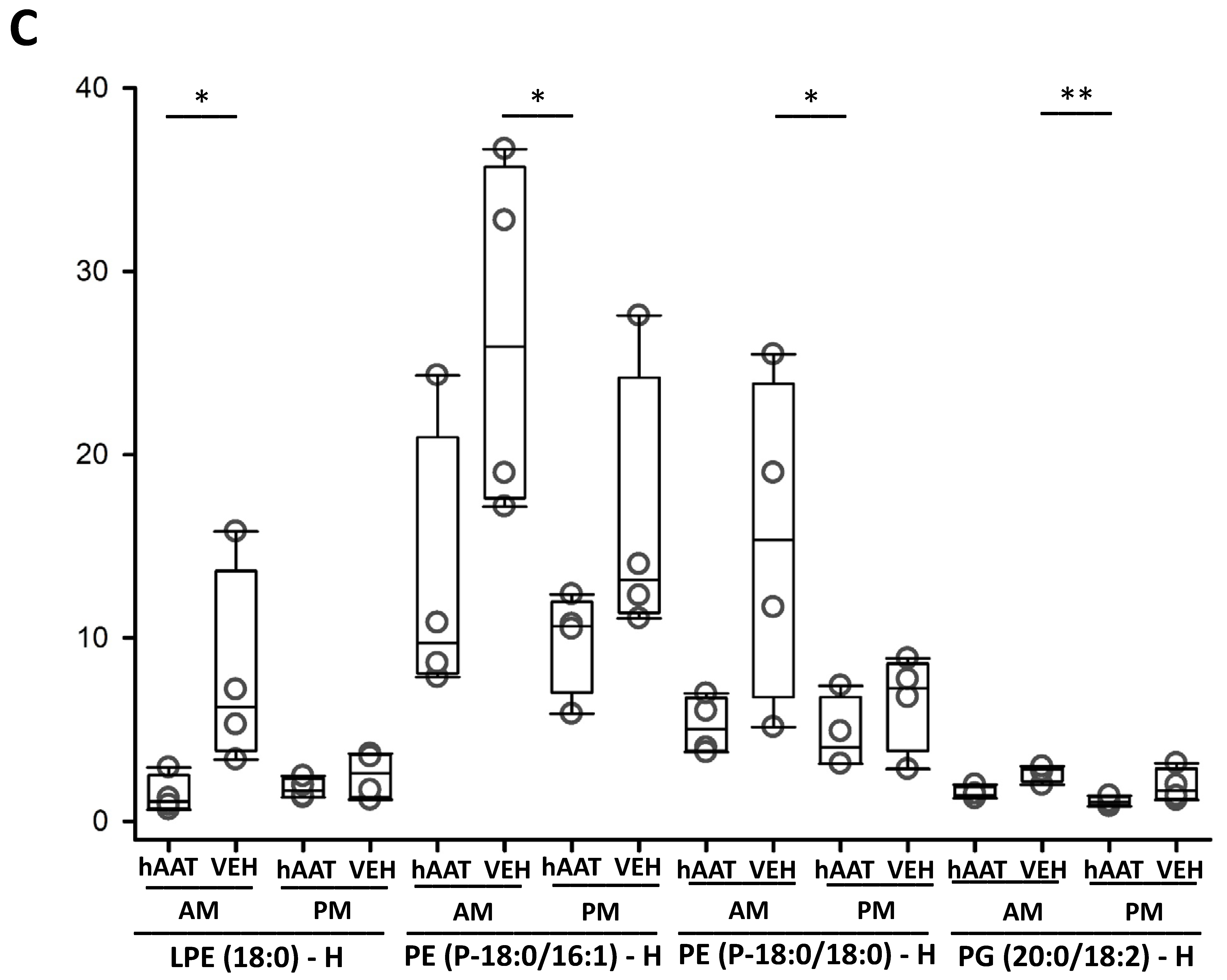
3.13. hAAT Treatment Increases Hexosylceramides in uEVs from Diabetic db/db Mice
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguat, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef]
- Unger, T.; Borghi, C.; Charchar, F.; Khan, N.A.; Poulter, N.R.; Prabhakaran, D.; Ramirez, A.; Schlaich, M.; Stergiou, G.S.; Tomaszewski, M.; et al. 2020 International Society of Hypertension Global Hypertension Practice Guidelines. Hypertension 2020, 75, 1334–1357. [Google Scholar] [CrossRef]
- Paneni, F.; Beckman, J.A.; Creager, M.A.; Cosentino, F. Diabetes and vascular disease: Pathophysiology, clinical consequences, and medical therapy: Part, I. Eur. Heart J. 2013, 34, 2436–2443. [Google Scholar] [CrossRef] [PubMed]
- Parving, H.H.; Andersen, A.R.; Smidt, U.M.; Hommel, E.; Mathiesen, E.R.; Svendsen, P.A. Effect of antihypertensive treatment on kidney function in diabetic nephropathy. Br. Med. J. (Clin. Res. Ed). 1987, 294, 1443–1447. [Google Scholar] [CrossRef] [PubMed]
- Frindt, G.; Palmer, L.G. Na channels in the rat connecting tubule. Am. J. Physiol. Renal. Physiol. 2004, 286, F669–F674. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Gao, Z.X.; Zhang, D.D.; Duan, X.P.; Terker, A.S.; Lin, D.H.; Wang, W.H. Effect of Angiotensin II on ENaC in the Distal Convoluted Tubule and in the Cortical Collecting Duct of Mineralocorticoid Receptor Deficient Mice. J. Am. Heart Assoc. 2020, 9, e014996. [Google Scholar] [CrossRef] [PubMed]
- Pacha, J.; Frindt, G.; Antonian, L.; Silver, R.B.; Palmer, L.G. Regulation of Na channels of the rat cortical collecting tubule by aldosterone. J. Gen. Physiol. 1993, 102, 25–42. [Google Scholar] [CrossRef] [PubMed]
- Pratt, J.H. Central role for ENaC in development of hypertension. J. Am. Soc. Nephrol. 2005, 16, 3154–3159. [Google Scholar] [CrossRef]
- He, J.; Yang, B. Aquaporins in Renal Diseases. Int. J. Mol. Sci. 2019, 20, 366. [Google Scholar] [CrossRef]
- Potter, L.R.; Yoder, A.R.; Flora, D.R.; Antos, L.K.; Dickey, D.M. Natriuretic peptides: Their structures, receptors, physiologic functions and therapeutic applications. Handb. Exp. Pharmacol. 2009, 191, 341–366. [Google Scholar] [CrossRef]
- McKie, P.M.; Cataliotti, A.; Huntley, B.K.; Martin, F.L.; Olson, T.M.; Burnett, J.C., Jr. A human atrial natriuretic peptide gene mutation reveals a novel peptide with enhanced blood pressure-lowering, renal-enhancing, and aldosterone-suppressing actions. J. Am. Coll. Cardiol. 2009, 54, 1024–1032. [Google Scholar] [CrossRef] [PubMed]
- Drexler, H.; Hirth, C.; Stasch, H.P.; Lu, W.; Neuser, D.; Just, H. Vasodilatory action of endogenous atrial natriuretic factor in a rat model of chronic heart failure as determined by monoclonal ANF antibody. Circ. Res. 1990, 66, 1371–1380. [Google Scholar] [CrossRef] [PubMed]
- Gardner, D.G.; Chen, S.; Glenn, D.J.; Grigsby, C.L. Molecular biology of the natriuretic peptide system: Implications for physiology and hypertension. Hypertension 2007, 49, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.J.; Alli, A.A.; Eaton, D.C.; Bao, H.F. ENaC is regulated by natriuretic peptide receptor-dependent cGMP signaling. Am. J. Physiol. Renal. Physiol. 2013, 304, F930–F937. [Google Scholar] [CrossRef][Green Version]
- Song, C.; Yue, Q.; Moseley, A.; Al-Khalili, O.; Wynne, B.M.; Ma, H.; Wang, L.; Eaton, D.C. Myristoylated alanine-rich C kinase substrate-like protein-1 regulates epithelial sodium channel activity in renal distal convoluted tubule cells. Am. J. Physiol. Cell Physiol. 2020, 319, C589–C604. [Google Scholar] [CrossRef]
- Alli, A.A.; Bao, H.F.; Alli, A.A.; Aldrugh, Y.; Song, J.Z.; Ma, H.P.; Yu, L.; Al-Khalili, O.; Douglas, C. Eaton Phosphatidylinositol phosphate-dependent regulation of Xenopus ENaC by MARCKS protein. Am. J. Physiol. Renal. Physiol. 2012, 303, F800–F811. [Google Scholar] [CrossRef]
- Pochynyuk, O.; Tong, Q.; Staruschenko, A.; Ma, H.P.; Stockand, J.D. Regulation of the epithelial Na+ channel (ENaC) by phosphatidylinositides. Am. J. Physiol. Renal. Physiol. 2006, 290, F949–F957. [Google Scholar] [CrossRef]
- Nesterov, V.; Dahlmann, A.; Bertog, M.; Korbmacher, C. Trypsin can activate the epithelial sodium channel (ENaC) in microdissected mouse distal nephron. Am. J. Physiol. Renal. Physiol. 2008, 295, F1052–F1062. [Google Scholar] [CrossRef] [PubMed]
- Bruns, J.B.; Carattino, M.D.; Sheng, S.; Maarouf, A.B.; Weisz, O.A.; Pilewski, J.M.; Hughey, R.P.; Kleyman, T.R. Epithelial Na+ channels are fully activated by furin- and prostasin-dependent release of an inhibitory peptide from the gamma-subunit. J. Biol. Chem. 2007, 282, 6153–6160. [Google Scholar] [CrossRef]
- Harris, M.; Garcia-Caballero, A.; Stutts, M.J.; Firsov, D.; Rossier, B.C. Preferential assembly of epithelial sodium channel (ENaC) subunits in Xenopus oocytes: Role of furin-mediated endogenous proteolysis. J. Biol. Chem. 2008, 283, 7455–7463. [Google Scholar] [CrossRef]
- Alli, A.A.; Song, J.Z.; Al-Khalili, O.; Bao, H.F.; Ma, H.P.; Alli, A.A.; Eaton, D.C. Cathepsin B is secreted apically from Xenopus 2F3 cells and cleaves the epithelial sodium channel (ENaC) to increase its activity. J. Biol. Chem. 2012, 287, 30073–30083. [Google Scholar] [CrossRef] [PubMed]
- Larionov, A.; Dahlke, E.; Kunke, M.; Zanon Rodriguez, L.; Schiessl, I.M.; Magnin, J.L.; Kern, U.; Alli, A.A.; Mollet, G.; Schilling, O.; et al. Cathepsin B increases ENaC activity leading to hypertension early in nephrotic syndrome. J. Cell. Mol. Med. 2019, 23, 6543–6553. [Google Scholar] [CrossRef]
- Haerteis, S.; Krappitz, M.; Bertog, M.; Krappitz, A.; Baraznenok, V.; Henderson, I.; Lindström, E.; Murphy, J.E.; Bunnett, N.W. Proteolytic activation of the epithelial sodium channel (ENaC) by the cysteine protease cathepsin-S. Pflugers Arch. 2012, 464, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Adachi, M.; Kitamura, K.; Miyoshi, T.; Narikiyo, T.; Iwashita, K.; Shiraishi, N.; Nonoguchi, H.; Tomita, K. Activation of epithelial sodium channels by prostasin in Xenopus oocytes. J. Am. Soc. Nephrol. 2001, 12, 1114–1121. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.B.; Chao, J.; Palmer, L.G. Tissue kallikrein activation of the epithelial Na channel. Am. J. Physiol. Renal. Physiol. 2012, 303, F540–F550. [Google Scholar] [CrossRef] [PubMed]
- Alli, A.; Yu, L.; Holzworth, M.; Richards, J.; Cheng, K.Y.; Lynch, I.J.; Wingo, C.S.; Gumz, M.L. Direct and indirect inhibition of the circadian clock protein Per1: Effects on ENaC and blood pressure. Am. J. Physiol. Renal. Physiol. 2019, 316, F807–F813. [Google Scholar] [CrossRef]
- Richards, J.; Ko, B.; All, S.; Cheng, K.Y.; Hoover, R.S.; Gumz, M.L. A role for the circadian clock protein Per1 in the regulation of the NaCl co-transporter (NCC) and the with-no-lysine kinase (WNK) cascade in mouse distal convoluted tubule cells. J. Biol. Chem. 2014, 289, 11791–11806. [Google Scholar] [CrossRef]
- Gumz, M.L.; Stow, L.R.; Lynch, I.J.; Greenlee, M.M.; Rudin, A.; Cain, B.D.; Weaver, D.R.; Wingo, C.S. The circadian clock protein Period 1 regulates expression of the renal epithelial sodium channel in mice. J. Clin. Investig. 2009, 119, 2423–2434. [Google Scholar] [CrossRef]
- Nikolaeva, S.; Pradervand, S.; Centeno, G.; Zavadova, V.; Tokonami, N.; Maillard, M.; Bonny, O.; Firsov, D. The circadian clock modulates renal sodium handling. J. Am. Soc. Nephrol. 2012, 23, 1019–1026. [Google Scholar] [CrossRef]
- Strnad, P.; McElvaney, N.G.; Lomas, D.A. Alpha1-Antitrypsin Deficiency. N. Engl. J. Med. 2020, 382, 1443–1455. [Google Scholar] [CrossRef]
- Scharpe, S.; Eid, M.; Cooreman, W.; Lauwers, A. Alpha-1-Anti-trypsin, an inhibitor of renin. Biochem. J. 1976, 153, 505–507. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Calero, L.; Martin-Lorenzo, M.; de la Cuesta, F.; Maroto, A.S.; Baldan-Martin, M.; Ruiz-Hurtado, G.; Pulido-Olmo, H.; Segura, J.; Barderas, M.G.; Ruilope, L.M.; et al. Urinary alpha-1 antitrypsin and CD59 glycoprotein predict albuminuria development in hypertensive patients under chronic renin-angiotensin system suppression. Cardiovasc. Diabetol. 2016, 15, 8. [Google Scholar] [CrossRef] [PubMed]
- de Loyola, M.B.; Dos Reis, T.T.A.; de Oliveira, G.; da Fonseca Palmeira, J.; Arganaraz, G.A.; Arganaraz, E.R. Alpha-1-antitrypsin: A possible host protective factor against COVID-19. Rev. Med. Virol. 2021, 31, e2157. [Google Scholar] [CrossRef] [PubMed]
- Grimstein, C.; Choi, Y.K.; Satoh, M.; Lu, Y.; Wang, X.; Campbell-Thompson, M.; Song, S. Combination of alpha-1 antitrypsin and doxycycline suppresses collagen-induced arthritis. J. Gene Med. 2010, 12, 35–44. [Google Scholar] [CrossRef]
- Akbar, M.A.; Cao, J.J.; Lu, Y.; Nardo, D.; Chen, M.J.; Elshikha, A.S.; Ahamed, R.; Brantly, M.; Holliday, L.S.; Song, S. Alpha-1 Antitrypsin Gene Therapy Ameliorates Bone Loss in Ovariectomy-Induced Osteoporosis Mouse Model. Hum. Gene Ther. 2016, 27, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Elshikha, A.S.; Yuan, Y.; Lu, Y.; Chen, M.J.; Abboud, G.; Akbar, M.A.; Plate, H.; Wolney, H.; Hoffmann, T.; Tagari, E.; et al. Alpha 1 Antitrypsin Gene Therapy Extends the Lifespan of Lupus-Prone Mice. Mol. Ther. Methods Clin. Dev. 2018, 11, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Koulmanda, M.; Bhasin, M.; Hoffman, L.; Fan, Z.; Qipo, A.; Shi, H.; Bonner-Weir, S.; Putheti, P.; Degauque, N.; Libermann, T.A.; et al. Curative and beta cell regenerative effects of alpha1-antitrypsin treatment in autoimmune diabetic NOD mice. Proc. Natl. Acad. Sci. USA 2008, 105, 16242–16247. [Google Scholar] [CrossRef]
- Song, S.; Goudy, K.; Campbell-Thompson, M.; Wasserfall, C.; Scott-Jorgensen, M.; Wang, J.; Tang, Q.; Crawford, J.; Ellis, T.; Atkinson, M. Recombinant adeno-associated virus-mediated alpha-1 antitrypsin gene therapy prevents type I diabetes in NOD mice. Gene Ther. 2004, 11, 181–186. [Google Scholar] [CrossRef]
- Zhang, B.; Lu, Y.; Campbell-Thompson, M.; Spencer, T.; Wasserfall, C.; Atkinson, M.; Song, S. Alpha1-antitrypsin protects beta-cells from apoptosis. Diabetes 2007, 56, 1316–1323. [Google Scholar] [CrossRef]
- Liu, L.P.; Gholam, M.F.; Elshikha, A.S.; Kawakibi, T.; Elmoujahid, N.; Moussa, H.H.; Song, S.; Alli, A.A. Transgenic Mice Overexpressing Human Alpha-1 Antitrypsin Exhibit Low Blood Pressure and Altered Epithelial Transport Mechanisms in the Inactive and Active Cycles. Front. Physiol. 2021, 12, 710313. [Google Scholar] [CrossRef]
- Elshikha, A.S.; Abboud, G.; van der Meijden-Erkelens, L.; Lu, Y.; Chen, M.-J.; Yuan, Y.; Ponjee, G.; Zeumer, L.; Satoh, M.; Morel, L.; et al. Alpha-1-Antitrypsin Ameliorates Pristane Induced Diffuse Alveolar Hemorrhage in Mice. J. Clin. Med. 2019, 8, 1341. [Google Scholar] [CrossRef] [PubMed]
- Alli, A.A.; Gower, W.R., Jr. Molecular approaches to examine the phosphorylation state of the C type natriuretic peptide receptor. J. Cell Biochem. 2010, 110, 985–994. [Google Scholar] [CrossRef]
- Chacko, K.M.; Nouri, M.Z.; Schramm, W.C.; Malik, Z.; Liu, L.P.; Denslow, N.D.; Alli, A.A. Tempol Alters Urinary Extracellular Vesicle Lipid Content and Release While Reducing Blood Pressure during the Development of Salt-Sensitive Hypertension. Biomolecules 2021, 11, 1804. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Glover, S.C.; Nouri, M.Z.; Tuna, K.M.; Mendoza Alvarez, L.B.; Ryan, L.K.; Shirley, J.F.; Tang, Y.; Denslow, N.D.; Alli, A.A. Lipidomic analysis of urinary exosomes from hereditary alpha-tryptasemia patients and healthy volunteers. FASEB Bioadv. 2019, 1, 624–638. [Google Scholar] [CrossRef]
- Posse de Chaves, E.; Sipione, S. Sphingolipids and gangliosides of the nervous system in membrane function and dysfunction. FEBS Lett. 2010, 584, 1748–1759. [Google Scholar] [CrossRef] [PubMed]
- Sheng, S.; Carattino, M.D.; Bruns, J.B.; Hughey, R.P.; Kleyman, T.R. Furin cleavage activates the epithelial Na+ channel by relieving Na+ self-inhibition. Am. J. Physiol. Renal. Physiol. 2006, 290, F1488–F1496. [Google Scholar] [CrossRef]
- Carattino, M.D.; Mueller, G.M.; Palmer, L.G.; Frindt, G.; Rued, A.C.; Hughey, R.P.; Kleyman, T.R. Prostasin interacts with the epithelial Na+ channel and facilitates cleavage of the gamma-subunit by a second protease. Am. J. Physiol. Renal. Physiol. 2014, 307, F1080–F1087. [Google Scholar] [CrossRef]
- Giunti, S.; Barit, D.; Cooper, M.E. Mechanisms of diabetic nephropathy: Role of hypertension. Hypertension 2006, 48, 519–526. [Google Scholar] [CrossRef]
- Hostetter, T.H.; Rennke, H.G.; Brenner, B.M. The case for intrarenal hypertension in the initiation and progression of diabetic and other glomerulopathies. Am. J. Med. 1982, 72, 375–380. [Google Scholar] [CrossRef]
- Zatz, R.; Meyer, T.W.; Rennke, H.G.; Brenner, B.M. Predominance of hemodynamic rather than metabolic factors in the pathogenesis of diabetic glomerulopathy. Proc. Natl. Acad. Sci. USA 1985, 82, 5963–5967. [Google Scholar] [CrossRef] [PubMed]
- Jella, K.K.; Yu, L.; Yue, Q.; Friedman, D.; Duke, B.J.; Alli, A.A. Exosomal GAPDH from Proximal Tubule Cells Regulate ENaC Activity. PLoS ONE 2016, 11, e0165763. [Google Scholar] [CrossRef] [PubMed]
- Sofi, M.H.; Heinrichs, J.; Dany, M.; Nguyen, H.; Dai, M.; Bastian, D.; Schutt, S.; Wu, Y.; Daenthanasanmak, A.; Gencer, S.; et al. Ceramide synthesis regulates T cell activity and GVHD development. JCI Insight. 2017, 2, e91701. [Google Scholar] [CrossRef] [PubMed]
- Dupre, T.V.; Doll, M.A.; Shah, P.P.; Sharp, C.N.; Siow, D.; Megyesi, J.; Shayman, J.; Bielawska, A.; Bielawski, J.; Beverly, L.J.; et al. Inhibiting glucosylceramide synthase exacerbates cisplatin-induced acute kidney injury. J. Lipid Res. 2017, 58, 1439–1452. [Google Scholar] [CrossRef] [PubMed]
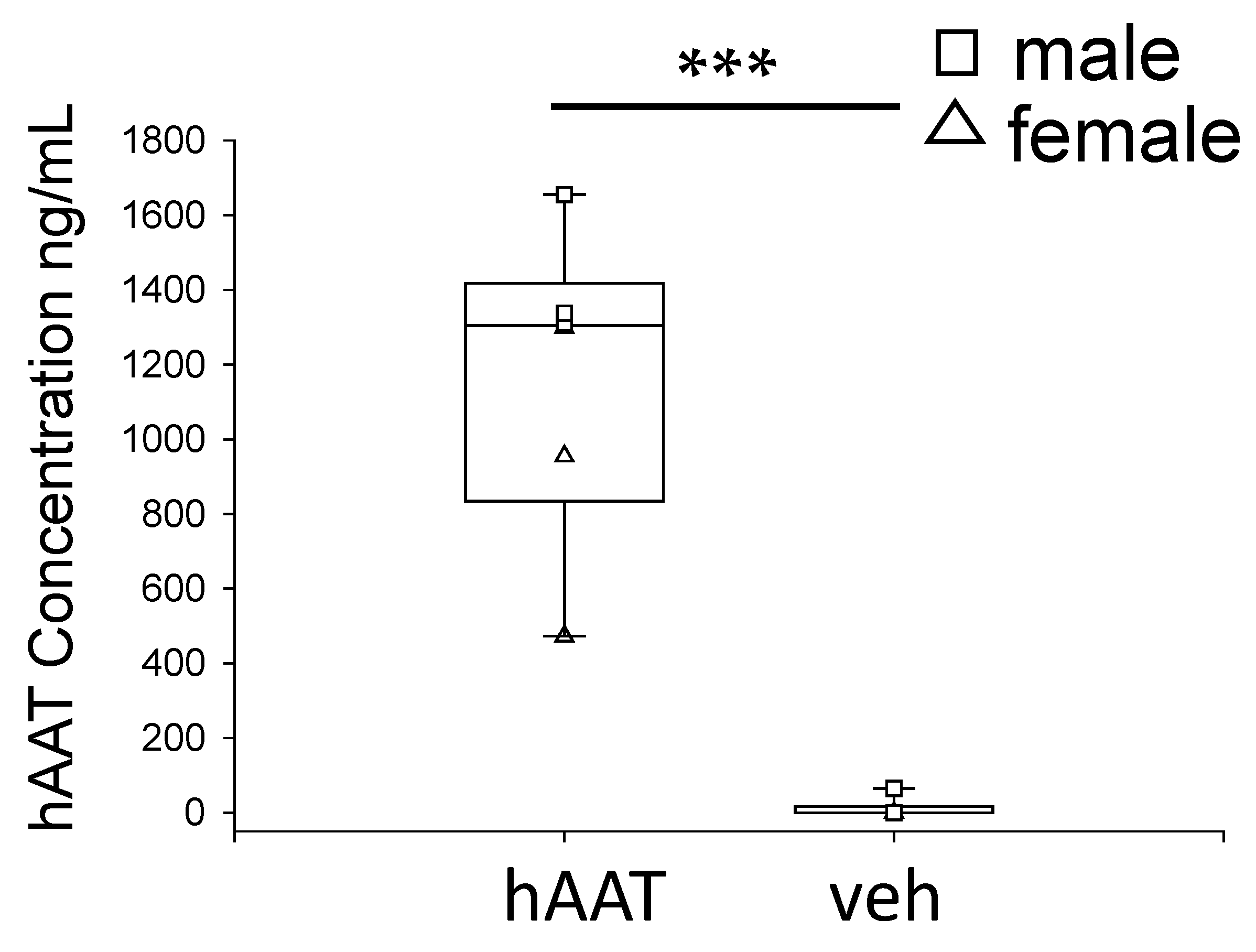





| GROUP | MEAN + SEM | p-VALUE | ||
|---|---|---|---|---|
| Na+ (mmol/L) | Active | AAT | 156.167 ± 40.402 | p = 0.793 |
| VEH | 126.5 ± 29.567 | |||
| Inactive | AAT | 130.333 ± 19.736 | ||
| VEH | 158.667 ± 19.857 | |||
| K+ (MMOL/L) | Active | AAT | 21.483 ± 4.572 | p = 0.861 |
| VEH | 19.533 ± 4.636 | |||
| Inactive | AAT | 23.133 ± 5.223 | ||
| VEH | 24.983 ± 4.107999 | |||
| CL+ (MMOL/L) | Active | AAT | 177.667 ± 35.819 | p = 0.953 |
| VEH | 154.333 ± 30.81 | |||
| Inactive | AAT | 169.167 ± 33.893 | ||
| VEH | 176.167 ± 27.368 | |||
| URINE OSMOLALITY (MOSM) | Active | AAT | 1268.16 ± 66.45 | p = 0.947 |
| VEH | 1295.5 ± 74.09 | |||
| Inactive | AAT | 1314.5 ± 90.74 | ||
| VEH | 1365.16 ± 193.31 |
| PROTEASE | GROUPS | MEAN ± SEM RELATIVE FLUORESCENCE UNITS (RFU) | STUDENT’S T-TEST COMPARISON | p-VALUE |
|---|---|---|---|---|
| CATHEPSIN S | AAT VEH | 27,099.000 ± 4915.036 22,441.500 ± 3486.513 | AAT vs. VEH | p = 0.457 |
| CATHEPSIN B | AAT VEH | 1972.333 ± 237.104 1829.833 ± 135.648 | AAT vs. VEH | p = 0.613 |
| CALPAIN | AAT VEH | 38,126.667 ± 1198.951 38,614.167 ± 1524.387 | AAT vs. VEH | p = 0.807 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lugo, C.I.; Liu, L.P.; Bala, N.; Morales, A.G.; Gholam, M.F.; Abchee, J.C.; Elmoujahid, N.; Elshikha, A.S.; Avdiaj, R.; Searcy, L.A.; et al. Human Alpha-1 Antitrypsin Attenuates ENaC and MARCKS and Lowers Blood Pressure in Hypertensive Diabetic db/db Mice. Biomolecules 2023, 13, 66. https://doi.org/10.3390/biom13010066
Lugo CI, Liu LP, Bala N, Morales AG, Gholam MF, Abchee JC, Elmoujahid N, Elshikha AS, Avdiaj R, Searcy LA, et al. Human Alpha-1 Antitrypsin Attenuates ENaC and MARCKS and Lowers Blood Pressure in Hypertensive Diabetic db/db Mice. Biomolecules. 2023; 13(1):66. https://doi.org/10.3390/biom13010066
Chicago/Turabian StyleLugo, Carlos I., Lauren P. Liu, Niharika Bala, Angelica G. Morales, Mohammed F. Gholam, Julia C. Abchee, Nasseem Elmoujahid, Ahmed Samir Elshikha, Rigena Avdiaj, Louis A. Searcy, and et al. 2023. "Human Alpha-1 Antitrypsin Attenuates ENaC and MARCKS and Lowers Blood Pressure in Hypertensive Diabetic db/db Mice" Biomolecules 13, no. 1: 66. https://doi.org/10.3390/biom13010066
APA StyleLugo, C. I., Liu, L. P., Bala, N., Morales, A. G., Gholam, M. F., Abchee, J. C., Elmoujahid, N., Elshikha, A. S., Avdiaj, R., Searcy, L. A., Denslow, N. D., Song, S., & Alli, A. A. (2023). Human Alpha-1 Antitrypsin Attenuates ENaC and MARCKS and Lowers Blood Pressure in Hypertensive Diabetic db/db Mice. Biomolecules, 13(1), 66. https://doi.org/10.3390/biom13010066






