Anti-Aging Activity and Modes of Action of Compounds from Natural Food Sources
Abstract
1. Introduction
2. Anti-Aging Effects of Compounds from Food Sources
2.1. Polysaccharides
2.1.1. Plant Polysaccharides
2.1.2. Algal Polysaccharides
2.1.3. Fungal Polysaccharides
2.2. Polyphenols
2.2.1. Phenolic Acids
2.2.2. Flavonoids
2.2.3. Stilbenes
2.2.4. Lignans
2.2.5. Tannins
2.3. Carotenoids
2.3.1. Carotenes
2.3.2. Xanthophylls
2.4. Sterols
2.4.1. Phytosterols
2.4.2. Animal Sterols
2.4.3. Fungal Sterols
2.5. Terpenoids
2.5.1. Hemiterpenoids
2.5.2. Monoterpenoids
2.5.3. Sesquiterpenoids
2.5.4. Diterpenoids
2.5.5. Triterpenes
2.6. Vitamins
2.6.1. Water-Soluble Vitamins
2.6.2. Fat-Soluble Vitamins
3. Anti-Aging Mechanisms of Compounds from Food Sources
3.1. Suppression of Oxidative Stress
3.2. Regulation of Age-Related Genes and Pathways
3.3. Immune Modulation
3.4. Regulation of Apoptosis
3.5. Intestinal Flora Regulation
3.6. Autophagy Regulation
3.7. Suppression of Cellular Senescence
3.8. Other Anti-Aging Mechanisms
4. Conclusions and Perspectives
- Most of the compounds are mixtures and therefore need further purification in order to determine the exact molecule that exhibits the bioactive activity.
- A compound often has multiple modes of action. Therefore, for specific compounds, their modes of action require in-depth research.
- Lifespan extension is a direct and key indicator for evaluating the anti-aging efficiency of bioactive compounds. It is relatively easy to detect the lifespan of animals with short life cycles such as nematodes and fruit flies, but it is difficult to do so for human beings as they have much longer lifespans. Therefore, we need to search for and identify suitable and intuitive anti-aging indicators to examine the life-extension effects of the compounds on humans.
- Previous anti-aging experiments are often limited to research on the short-term effects of the compounds. Therefore, long-term and large-scale clinical trials are urgently needed to investigate the anti-aging effects of compounds.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- World Population-Population Stat 2023. Available online: https://populationstat.com/ (accessed on 7 July 2023).
- World Population Prospects 2022. Available online: https://www.un.org/development/desa/pd/sites/www.un.org.development.desa.pd/files/wpp2022_summary_of_results.pdf (accessed on 5 June 2023).
- Schäfer, D. Aging, longevity, and diet: Historical remarks on calorie intake reduction. Gerontology 2005, 51, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Q.; Niu, Y.; Wei, K.; Wang, X.; Niu, B.; Zhang, L. Research progress on aging mechanism and drugs and the role of stem cells in anti-aging process. Exp. Gerontol. 2023, 179, 112248. [Google Scholar] [CrossRef] [PubMed]
- Klass, M.R. A method for the isolation of longevity mutants in the nematode Caenorhabditis elegans and initial results. Mech. Ageing. Dev. 1983, 22, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Otin, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef]
- Schmeer, C.; Kretz, A.; Wengerodt, D.; Stojiljkovic, M.; Witte, O.W. Dissecting aging and senescence-current concepts and open lessons. Cells 2019, 8, 1446. [Google Scholar] [CrossRef] [PubMed]
- Van Voorhies, W.A. Metabolism and aging in the nematode Caenorhabditis elegans. Free Radic. Biol. Med. 2002, 33, 587–596. [Google Scholar]
- Harman, D. The free radical theory of aging: Effect of age on serum copper levels. J. Gerontol. 1965, 20, 151–153. [Google Scholar] [CrossRef]
- Harman, D. The aging process. Proc. Natl. Acad. Sci. USA 1981, 78, 7124–7128. [Google Scholar] [CrossRef]
- Hayflick, L.; Moorhead, P.S. The serial cultivation of human diploid cell strains. Exp. Cell Res. 1961, 25, 585–621. [Google Scholar] [CrossRef]
- Olovnikov, A.M. Telomeres, telomerase, and aging: Origin of the theory. Exp. Gerontol. 1996, 31, 443–448. [Google Scholar] [CrossRef]
- Gensler, H.L.; Bernstein, H. DNA damage as the primary cause of aging. Q. Rev. Biol. 1981, 56, 279–303. [Google Scholar] [CrossRef] [PubMed]
- Orgel, L.E. The maintenance of the accuracy of protein synthesis and its relevance to ageing. Proc. Natl. Acad. Sci. USA 1963, 49, 517–521. [Google Scholar] [CrossRef]
- Weismann, A. Essays upon Heredity and Kindred Biological Problems; Poulton, E.B., Selmar, S., Arthur, S.E., Eds.; Clarendon Press: Oxford, UK, 1889. [Google Scholar]
- Davalli, P.; Mitic, T.; Caporali, A.; Lauriola, A.; D’Arca, D. ROS, cell senescence, and novel molecular mechanisms in aging and age-related diseases. Oxid. Med. Cell. Longev. 2016, 2016, 3565127. [Google Scholar] [CrossRef] [PubMed]
- Fulop, T.; Larbi, A.; Pawelec, G.; Khalil, A.; Cohen, A.A.; Hirokawa, K.; Witkowski, J.M.; Franceschi, C. Immunology of aging: The birth of inflammaging. Clin. Rev. Allergy. Immunol. 2023, 64, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Argüelles, S.; Guerrero-Castilla, A.; Cano, M.; Muñoz, M.F.; Ayala, A. Advantages and disadvantages of apoptosis in the aging process. Ann. N. Y. Acad. Sci. 2019, 1443, 20–33. [Google Scholar] [CrossRef]
- Lopez-Lluch, G.; Hernandez-Camacho, J.D.; Fernandez-Ayala, D.J.M.; Navas, P. Mitochondrial dysfunction in metabolism and ageing: Shared mechanisms and outcomes? Biogerontology 2018, 19, 461–480. [Google Scholar] [CrossRef]
- Mechchate, H.; El Allam, A.; El Omari, N.; El Hachlafi, N.; Shariati, M.A.; Wilairatana, P.; Mubarak, M.S.; Bouyahya, A. Vegetables and their bioactive compounds as anti-aging drugs. Molecules 2022, 27, 2316. [Google Scholar] [CrossRef]
- Hernandez, D.F.; Cervantes, E.L.; Luna-Vital, D.A.; Mojica, L. Food-derived bioactive compounds with anti-aging potential for nutricosmetic and cosmeceutical products. Crit. Rev. Food. Sci. Nutr. 2021, 61, 3740–3755. [Google Scholar] [CrossRef]
- Di Micco, R.; Krizhanovsky, V.; Baker, D.; d’Adda di Fagagna, F. Cellular senescence in ageing: From mechanisms to therapeutic opportunities. Nat. Rev. Mol. Cell Biol. 2021, 22, 75–95. [Google Scholar] [CrossRef]
- Herranz, N.; Gil, J. Mechanisms and functions of cellular senescence. J. Clin. Investig. 2018, 128, 1238–1246. [Google Scholar] [CrossRef]
- Lee, S.; Schmitt, C.A. The dynamic nature of senescence in cancer. Nat. Cell Biol. 2019, 21, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Chuang, S.Y.; Lin, C.H.; Fang, J.Y. Natural compounds and aging: Between autophagy and inflammasome. Biomed. Res. Int. 2014, 2014, e297293. [Google Scholar] [CrossRef] [PubMed]
- Tundis, R.; Loizzo, M.R.; Bonesi, M.; Menichini, F. Potential role of natural compounds against skin aging. Curr. Med. Chem. 2015, 22, 1515–1538. [Google Scholar] [CrossRef]
- Taoerdahong, H.; Kadeer, G.; Chang, J.; Kang, J.; Ma, X.; Yang, F. A review concerning the polysaccharides found in edible and medicinal plants in Xinjiang. Molecules 2023, 28, 2054. [Google Scholar] [CrossRef]
- Vetvicka, V.; Vetvickova, J. Immune enhancing effects of WB365, a novel combination of Ashwagandha (Withania somnifera) and Maitake (Grifola frondosa) extracts. N. Am. J. Med. Sci. 2011, 3, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Rossi, P.; Difrancia, R.; Quagliariello, V.; Savino, E.; Tralongo, P.; Randazzo, C.L.; Berretta, M. B-glucans from Grifola Frondosa and Ganoderma Lucidum in breast cancer: An example of complementary and integrative medicine. Oncotarget 2018, 9, 24837–24856. [Google Scholar] [CrossRef]
- Yu, Y.; Shen, M.; Song, Q.; Xie, J. Biological activities and pharmaceutical applications of polysaccharide from natural resources: A review. Carbohydr. Polym. 2018, 183, 91–101. [Google Scholar] [CrossRef]
- Tang, R.; Chen, X.; Dang, T.; Deng, Y.; Zou, Z.; Liu, Q.; Gong, G.; Song, S.; Ma, F.; Huang, L.; et al. Lycium barbarum polysaccharides extend the mean lifespan of Drosophila melanogaster. Food Funct. 2019, 10, 4231–4241. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, Q.; Fang, J.; Wang, C.; Wang, D.; Li, M. The anti-aging activity of Lycium barbarum polysaccharide extracted by yeast fermentation: In vivo and in vitro studies. Int. J. Biol. Macromol. 2022, 209, 2032–2041. [Google Scholar] [CrossRef]
- Zheng, X.; Wang, J.; Bi, F.; Li, Y.; Xiao, J.; Chai, Z.; Li, Y.; Miao, Z.; Wang, Y. Protective effects of Lycium barbarum polysaccharide on ovariectomy-induced cognition reduction in aging mice. Int. J. Mol. Med. 2021, 48, 121. [Google Scholar] [CrossRef]
- Li, H.; Li, Z.; Peng, L.; Jiang, N.; Liu, Q.; Zhang, E.; Liang, B.; Li, R.; Zhu, H. Lycium barbarum polysaccharide protects human keratinocytes against UVB-induced photo-damage. Free. Radic. Res. 2017, 51, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Li, X.; Dang, B.; Wu, F.; Wang, C.; Lin, C. Lycium barbarum polysaccharide protects HaCaT cells from PM2.5-induced apoptosis via inhibiting oxidative stress, ER stress and autophagy. Redox. Rep. 2022, 27, 32–44. [Google Scholar] [CrossRef]
- Xia, G.; Xin, N.; Liu, W.; Yao, H.; Hou, Y.; Qi, J. Inhibitory effect of Lycium barbarum polysaccharides on cell apoptosis and senescence is potentially mediated by the p53 signaling pathway. Mol. Med. Rep. 2014, 9, 1237–1241. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Feng, H.; Yu, Y.; Sun, M.; Liu, Y.; Li, T.; Sun, X.; Liu, S.; Sun, M. Antioxidant activities of the polysaccharides of Chuanminshen violaceum. Carbohyd. Polym. 2017, 157, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhong, X.; Sun, D.; Xu, L.; Shi, L.; Sui, J.; Liu, Y. Anti-aging effects of polysaccharides from ginseng extract residues in Caenorhabditis elegans. Int. J. Biol. Macromol. 2023, 225, 1072–1084. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Li, N.; Lu, X.; Shan, S.; Gao, X.; Cao, Y.; Lu, W. Sweet tea (Rubus Suavissmus S. Lee) polysaccharides promote the longevity of Caenorhabditis elegans through autophagy-dependent insulin and mitochondrial pathways. Int. J. Biol. Macromol. 2022, 207, 883–892. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Sun, J.; Gu, D.; Li, P.; Yao, L.; Shi, D.; Guo, S.; Liu, C. Antioxidant activities of sulfated Codonopsis polysaccharides in acute oxidative stress. J. Food. Biochem. 2021, 45, e13974. [Google Scholar] [CrossRef]
- Doan, V.M.; Chen, C.; Lin, X.; Nguyen, V.P.; Nong, Z.; Li, W.; Chen, Q.; Ming, J.; Xie, Q.; Huang, R. Yulangsan polysaccharide improves redox homeostasis and immune impairment in D-galactose-induced mimetic aging. Food Funct. 2015, 6, 1712–1718. [Google Scholar] [CrossRef]
- Zhang, M.; Cui, S.; Mao, B.; Zhang, Q.; Zhao, J.; Tang, X.; Chen, W. Urolithin A produced by novel microbial fermentation possesses anti-aging effects by improving mitophagy and reducing reactive oxygen species in Caenorhabditis elegans. J. Agric. Food. Chem. 2023, 71, 6348–6357. [Google Scholar] [CrossRef]
- Kim, J.E.; Jang, S.G.; Lee, C.H.; Lee, J.Y.; Park, H.; Kim, J.H.; Lee, S.; Kim, H.S.; Park, E.-Y.; Lee, K.W.; et al. Beneficial effects on skin health using polysaccharides from red ginseng by-product. J. Food Biochem. 2019, 43, e12961. [Google Scholar] [CrossRef]
- Pu, X.; Yu, S.; Fan, W.; Liu, L.; Ma, X.; Ren, J. Guiqi polysaccharide protects the normal human fetal lung fibroblast WI-38 cells from H2O2-induced premature senescence. Int. J. Clin. Exp. Pathol. 2015, 8, 4398–4407. [Google Scholar]
- Li, N.; Li, Q.; He, X.; Gao, X.; Wu, L.; Xiao, M.; Cai, W.; Liu, B.; Zeng, F. Antioxidant and anti-aging activities of Laminaria japonica polysaccharide in Caenorhabditis elegans based on metabonomic analysis. Int. J. Biol. Macromol. 2022, 221, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, X.; Pan, Y.; Wang, G.; Mao, G. The degraded polysaccharide from Pyropia haitanensis represses amyloid beta peptide-induced neurotoxicity and memory in vivo. Int. J. Biol. Macromol. 2019, 146, 725–729. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Z.; Zhou, H.; Sun, X.; Chen, X.; Xu, N. The anti-aging effects of Gracilaria lemaneiformis polysaccharide in Caenorhabditis elegans. Int. J. Biol. Macromol. 2019, 140, 600–604. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Zhang, Y.; Xu, M.; Chen, H.; Zou, H.; Zhang, X.; Tong, H.; You, C.; Wu, M. Proteomic landscape of liver tissue in old male mice that are long-term treated with polysaccharides from Sargassum fusiforme. Food. Funct. 2020, 11, 3632–3644. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; He, D.; Zhang, Y.; Yang, S.; Chen, L.; Wang, S.; Zou, H.; Liao, Z.; Zhang, X.; Wu, M. Sargassum fusiforme polysaccharides activate antioxidant defense by promoting Nrf2-dependent cytoprotection and ameliorate stress insult during aging. Food Funct. 2016, 7, 4576–4588. [Google Scholar] [CrossRef]
- Chen, P.; Yang, S.; Hu, C.; Zhao, Z.; Liu, J.; Cheng, Y.; Wang, S.; Chen, Q.; Yu, P.; Zhang, X.; et al. Sargassum fusiforme polysaccharide rejuvenates the small intestine in mice through altering its physiology and gut microbiota composition. Curr. Mol. Med. 2017, 17, 350–358. [Google Scholar] [PubMed]
- Zhang, X.; Liu, L.; Luo, J.; Peng, X. Anti-aging potency correlates with metabolites from in vitro fermentation of edible fungal polysaccharides using human fecal intestinal microflora. Food. Funct. 2022, 13, 11592–11603. [Google Scholar] [CrossRef]
- Tripodi, F.; Falletta, E.; Leri, M.; Angeloni, C.; Beghelli, D.; Giusti, L.; Milanesi, R.; Sampaio-Marques, B.; Ludovico, P.; Goppa, L.; et al. Anti-aging and neuroprotective properties of Grifola frondosa and Hericium erinaceus extracts. Nutrients 2022, 14, 4368. [Google Scholar] [CrossRef]
- Zhang, C.; Gao, Z.; Hu, C.; Zhang, J.; Sun, X.; Rong, C.; Jia, L. Antioxidant, antibacterial and anti-aging activities of intracellular zinc polysaccharides from Grifola frondosa SH-05. Int. J. Biol. Macromol. 2017, 95, 778–787. [Google Scholar] [CrossRef]
- Song, F.; Gao, Z.; Liu, W.; Li, H.; Zhang, Y.; Feng, Y.; Song, X.; Wang, W.; Zhang, J.; Huang, C.; et al. Characterization, antioxidant, anti-aging and organ protective effects of sulfated polysaccharides from Flammulina velutipes. Molecules 2019, 24, 3517. [Google Scholar]
- Duan, H.; Li, J.; Fan, L. Agaricus bisporus polysaccharides ameliorates behavioural deficits in D-galactose-induced aging mice: Mediated by gut microbiota. Foods 2023, 12, 424. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, M.; Zhang, C.; Tian, C.; Wang, X.; Song, X.; Jing, H.; Gao, Z.; Ren, Z.; Liu, W.; et al. Purification, in vitro antioxidant and in vivo anti-aging activities of soluble polysaccharides by enzyme-assisted extraction from Agaricus bisporus. Int. J. Biol. Macromol. 2018, 109, 457–466. [Google Scholar] [CrossRef]
- Li, S.; Liu, H.; Wang, W.; Wang, X.; Zhang, C.; Zhang, J.; Jing, H.; Ren, Z.; Gao, Z.; Song, X.; et al. Antioxidant and anti-aging effects of acidic-extractable polysaccharides by Agaricus bisporus. Int. J. Biol. Macromol. 2018, 106, 1297–1306. [Google Scholar] [CrossRef]
- Song, L.; Zhou, Y.; Ni, S.; Wang, X.; Yuan, J.; Zhang, Y.; Zhang, S. Dietary intake of β-glucans can prolong lifespan and exert an antioxidant action on aged fish Nothobranchius guentheri. Rejuvenation. Res. 2020, 23, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Yuan, J.; Ni, S.; Zhou, Y.; Wang, X.; Chen, Y.; Zhang, S. Enhancement of adaptive immune responses of aged mice by dietary intake of β-glucans, with special emphasis on anti-aging activity. Mol. Immunol. 2020, 117, 160–167. [Google Scholar] [CrossRef]
- Shen, T.; Duan, C.; Chen, B.; Li, M.; Ruan, Y.; Xu, D.; Shi, D.; Yu, D.; Li, J.; Wang, C. Tremella fuciformis polysaccharide suppresses hydrogen peroxide-triggered injury of human skin fibroblasts via upregulation of SIRT1. Mol. Med. Rep. 2017, 16, 1340–1346. [Google Scholar] [CrossRef]
- Yue, Y.; Li, Z.; Li, P.; Song, N.; Li, B.; Lin, W.; Liu, S. Antiviral activity of a polysaccharide from Laminaria japonica against enterovirus 71. Biomed. Pharmacother. 2017, 96, 256–262. [Google Scholar] [CrossRef]
- Ding, Q.; Yang, D.; Zhang, W.; Lu, Y.; Zhang, M.; Wang, L.; Li, X.; Zhou, L.; Wu, Q.; Pan, W.; et al. Antioxidant and anti-aging activities of the polysaccharide TLH-3 from Tricholoma lobayense. Int. J. Biol. Macromol. 2016, 85, 133–140. [Google Scholar] [CrossRef]
- Wu, Y.J.; Wei, Z.X.; Zhang, F.M.; Linhardt, R.J.; Sun, P.L.; Zhang, A.Q. Structure, bioactivities and applications of the polysaccharides from Tremella fuciformis mushroom: A review. Int. J. Biol. Macromol. 2019, 121, 1005–1010. [Google Scholar] [CrossRef]
- Singla, R.K.; Dubey, A.K.; Garg, A.; Sharma, R.K.; Fiorino, M.; Ameen, S.M.; Haddad, M.A.; Al-Hiary, M. Natural polyphenols: Chemical classification, definition of classes, subcategories, and structures. J. AOAC Int. 2019, 102, 1397–1400. [Google Scholar] [CrossRef] [PubMed]
- Sahiner, M.; Sahiner, N.; Sagbas, S.; Fullerton, M.L.; Blake, D.A. Fabrication of biodegradable poly (naringin) particles with antioxidant activity and low toxicity. ACS. Omega 2018, 3, 17359–17367. [Google Scholar] [CrossRef]
- Xu, D.P.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.J.; Li, H.B. Natural antioxidants in foods and medicinal plants: Extraction, assessment and resources. Int. J. Mol. Sci. 2017, 18, 96. [Google Scholar] [CrossRef]
- Uddin, N.; Muhammad, N.; Nisar, M.; Aisha; Ali, N.; Ullah, R.; Ali, E.A.; Khan, A.A.; Rahman, I.U.; Khan, A.; et al. Distribution of polyphenolic compounds, antioxidant potential, and free amino acids in Ziziphus fruits extract; a study for determining the influence of wider geography. Food. Sci. Nutr. 2022, 10, 1414–1430. [Google Scholar] [CrossRef] [PubMed]
- Besednova, N.N.; Andryukov, B.G.; Zaporozhets, T.S.; Kryzhanovsky, S.P.; Kuznetsova, T.A.; Fedyanina, L.N.; Makarenkova, I.D.; Zvyagintseva, T.N. Algae polyphenolic compounds and modern antibacterial strategies: Current achievements and immediate prospects. Biomedicines 2020, 8, 342. [Google Scholar] [CrossRef]
- Sun, C.; Zhao, C.; Guven, E.C.; Paoli, P.; Simal-Gandara, J.; Ramkumar, K.M.; Wang, S.; Buleu, F.; Pah, A.; Turi, V.; et al. Dietary polyphenols as antidiabetic agents: Advances and opportunities. Food. Front. 2020, 1, 18–44. [Google Scholar] [CrossRef]
- Niedzwiecki, A.; Roomi, M.; Kalinovsky, T.; Rath, M. Anticancer efficacy of polyphenols and their combinations. Nutrients 2016, 8, 552. [Google Scholar] [CrossRef]
- Meng, T.; Xiao, D.; Muhammed, A.; Deng, J.; Chen, L.; He, J. Anti-inflammatory action and mechanisms of resveratrol. Molecules 2021, 26, 229. [Google Scholar] [CrossRef]
- Zhou, D.D.; Luo, M.; Huang, S.Y.; Saimaiti, A.; Shang, A.; Gan, R.Y.; Li, H.B. Effects and mechanisms of resveratrol on aging and age-related diseases. Oxid. Med. Cell. Longev. 2021, 2021, 9932218. [Google Scholar] [CrossRef]
- Mateș, L.; Rusu, M.E.; Popa, D.S. Phytochemicals and biological activities of walnut septum: A systematic review. Antioxidants 2023, 12, 604. [Google Scholar] [CrossRef]
- Zheng, S.Q.; Huang, X.B.; Xing, T.K.; Ding, A.J.; Wu, G.S.; Luo, H.R. Chlorogenic acid extends the lifespan of Caenorhabditis elegans via insulin/IGF-1 signaling pathway. J. Gerontol. A. Biol. Sci. Med. Sci. 2017, 72, 464–472. [Google Scholar]
- Yue, Y.; Shen, P.; Xu, Y.; Park, Y. p-Coumaric acid improves oxidative and osmosis stress responses in Caenorhabditis elegans. J. Sci. Food. Agr. 2019, 99, 1190–1197. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Zetina, S.M.; González-Manzano, S.; Ayuda-Durán, B.; Santos-Buelga, C.; González-Paramás, A.M. Caffeic and dihydrocaffeic acids promote longevity and increase stress resistance in Caenorhabditis elegans by modulating expression of stress-related genes. Molecules 2021, 26, 1517. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, T.; Sakamoto, K. Quercetin enhances motility in aged and heat-stressed Caenorhabditis elegans nematodes by modulating both HSF-1 activity, and insulin-like and p38-MAPK signaling. PLoS. ONE 2020, 15, e0238528. [Google Scholar] [CrossRef]
- Kim, S.G.; Sung, J.Y.; Kim, J.R.; Choi, H.C. Quercetin-induced apoptosis ameliorates vascular smooth muscle cell senescence through AMP-activated protein kinase signaling pathway. Korean. J. Physiol. Pharmacol. 2020, 24, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Bermejo-Bescós, P.; Jiménez-Aliaga, K.L.; Benedí, J.; Martín-Aragón, S. A diet containing rutin ameliorates brain intracellular redox homeostasis in a mouse model of Alzheimer’s disease. Int. J. Mol. Sci. 2023, 24, 4863. [Google Scholar] [CrossRef]
- Chen, W.; Muller, D.; Richling, E.; Wink, M. Anthocyanin-rich purple wheat prolongs the life span of Caenorhabditis elegans probably by activating the DAF-16/FOXO transcription factor. J. Agric. Food. Chem. 2013, 61, 3047–3053. [Google Scholar] [CrossRef]
- Golubev, D.; Zemskaya, N.; Shevchenko, O.; Shaposhnikov, M.; Kukuman, D.; Patov, S.; Punegov, V.; Moskalev, A. Honeysuckle extract (Lonicera pallasii L.) exerts antioxidant properties and extends the lifespan and healthspan of Drosophila melanogaster. Biogerontology 2022, 23, 215–235. [Google Scholar] [CrossRef]
- Jayarathne, S.; Ramalingam, L.; Edwards, H.; Vanapalli, S.A.; Moustaid-Moussa, N. Tart cherry increases lifespan in Caenorhabditis elegans by altering metabolic signaling pathways. Nutrients 2020, 12, 1482. [Google Scholar] [CrossRef]
- Chandrashekara, K.T.; Shakarad, M.N. Aloe vera or resveratrol supplementation in larval diet delays adult aging in the fruit fly, Drosophila melanogaster. J. Gerontol. A-Biol. 2011, 66, 965–971. [Google Scholar] [CrossRef]
- Yu, X.; Li, G.R. Effects of resveratrol on longevity, cognitive ability and aging-related histological markers in the annual fish Nothobranchius guentheri. Exp. Gerontol. 2012, 47, 940–949. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Li, X.; Qiao, M.; Sun, X.; Li, G. Resveratrol alleviates inflammation and ER stress through SIRT1/NRF2 to delay ovarian aging in a short-lived fish. J. Gerontol. A. Biol. Sci. Med. Sci. 2023, 78, 596–602. [Google Scholar] [CrossRef]
- Gerhardt, E.; Graber, S.; Szego, E.M.; Moisoi, N.; Martins, L.M.; Outeiro, T.F.; Kermer, P. Idebenone and resveratrol extend lifespan and improve motor function of HtrA2 knockout mice. PLoS. ONE 2011, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.X.; Chai, Z.T.; Wang, B.; Zhou, G.X.; Cang, J.; Xue, Z.G.; Ge, S.J. Resveratrol alleviates learning and memory impairment in aged rats after general anesthesia with sevoflurane and nitrous oxide via SIRT1-p53 signaling pathway. Int. J. Clin. Exp. Med. 2016, 9, 21118–21130. [Google Scholar]
- Liu, S.; Zheng, Z.; Ji, S.; Liu, T.; Hou, Y.; Li, S.; Li, G. Resveratrol reduces senescence-associated secretory phenotype by SIRT1/NF-κB pathway in gut of the annual fish Nothobranchius guentheri. Fish Shellfish Immunol. 2018, 80, 473–479. [Google Scholar] [CrossRef]
- Yuan, J.; Lu, L.; Zhang, Z.; Zhang, S. Dietary intake of resveratrol enhances the adaptive immunity of aged rats. Rejuvenation Res. 2012, 15, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Ginés, C.; Cuesta, S.; Kireev, R.; García, C.; Rancan, L.; Paredes, S.D.; Vara, E.; Tresguerres, J.A.F. Protective effect of resveratrol against inflammation, oxidative stress and apoptosis in pancreas of aged SAMP8 mice. Exp. Gerontol. 2017, 90, 61–70. [Google Scholar] [CrossRef]
- Keowkase, R.; Shoomarom, N.; Bunargin, W.; Sitthithaworn, W.; Weerapreeyakul, N. Sesamin and sesamolin reduce amyloid-beta toxicity in a transgenic Caenorhabditis elegans. Biomed. Pharmacother. 2018, 107, 656–664. [Google Scholar] [CrossRef]
- Yaguchi, Y.; Komura, T.; Kashima, N.; Tamura, M.; Kage-Nakadai, E.; Saeki, S.; Terao, K.; Nishikawa, Y. Influence of oral supplementation with sesamin on longevity of Caenorhabditis elegans and the host defense. Eur. J. Nutr. 2014, 53, 1659–1668. [Google Scholar] [CrossRef]
- Saul, N.; Pietsch, K.; Menzel, R.; Sturzenbaum, S.R.; Steinberg, C.E. The longevity effect of tannic acid in Caenorhabditis elegans: Disposable soma meets hormesis. J. Gerontol. A. Biol. Sci. Med. Sci. 2010, 65, 626–635. [Google Scholar] [CrossRef]
- Saul, N.; Pietsch, K.; Sturzenbaum, S.R.; Menzel, R.; Steinberg, C.E. Diversity of polyphenol action in Caenorhabditis elegans: Between toxicity and longevity. J. Nat. Prod. 2011, 74, 1713–1720. [Google Scholar] [CrossRef]
- Chen, Y.; Onken, B.; Chen, H.; Zhang, X.; Driscoll, M.; Cao, Y.; Huang, Q. Healthy lifespan extension mediated by oenothein B isolated from Eucalyptus grandis ⅹ Eucalyptus urophylla GL9 in Caenorhabditis elegans. Food. Funct. 2020, 11, 2439–2450. [Google Scholar] [CrossRef]
- Chen, Y.; Onken, B.; Chen, H.; Xiao, S.; Liu, X.; Driscoll, M.; Cao, Y.; Huang, Q. Mechanism of longevity extension of Caenorhabditis elegans induced by pentagalloyl glucose isolated from eucalyptus leaves. J. Agric. Food. Chem. 2014, 62, 3422–3431. [Google Scholar] [CrossRef] [PubMed]
- Majidinia, M.; Karimian, A.; Alemi, F.; Yousefi, B.; Safa, A. Targeting miRNAs by polyphenols: Novel therapeutic strategy for aging. Biochem. Pharmacol. 2020, 173, 113688. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Chen, F.; Guo, Z.; Lei, J.; Zhou, B. Recent advancement in bioeffect, metabolism, stability, and delivery systems of apigenin, a natural flavonoid compound: Challenges and perspectives. Front. Nutr. 2023, 10, 1221227. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, M.F.; Ahmad, N.; Ahmed, Z.; Siddique, R.; Zeng, X.A.; Rahaman, A.; Muhammad Aadil, R.; Wahab, A. Novel extraction techniques and pharmaceutical activities of luteolin and its derivatives. J. Food. Biochem. 2019, 43, e12974. [Google Scholar] [CrossRef]
- El Menyiy, N.; Aboulaghras, S.; Bakrim, S.; Moubachir, R.; Taha, D.; Khalid, A.; Abdalla, A.N.; Algarni, A.S.; Hermansyah, A.; Ming, L.C.; et al. Genkwanin: An emerging natural compound with multifaceted pharmacological effects. Biomed. Pharmacother. 2023, 165, 115159. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Zhou, H.Y.; Cho, S.Y.; Kim, Y.S.; Lee, Y.S.; Jeong, C.S. Anti-inflammatory mechanisms of apigenin: Inhibition of cyclooxygenase-2 expression, adhesion of monocytes to human umbilical vein endothelial cells, and expression of cellular adhesion molecules. Arch. Pharm. Res. 2007, 30, 1318–1327. [Google Scholar] [CrossRef]
- Patel, M.; Singh, S. Apigenin attenuates functional and structural alterations via targeting NF-kB/Nrf2 signaling pathway in LPS-induced Parkinsonism in experimental rats: Apigenin attenuates LPS-induced Parkinsonism in experimental rats. Neurotox. Res. 2022, 40, 941–960. [Google Scholar] [CrossRef]
- Yan, F.J.; Chen, Y.S.; Azat, R.; Zheng, X.D. Mulberry anthocyanin extract ameliorates oxidative damage in HepG2 cells and prolongs the lifespan of Caenorhabditis elegans through MAPK and Nrf2 pathways. Oxid. Med. Cell. Longev. 2017, 2017, 7956158. [Google Scholar] [CrossRef]
- Malaguarnera, L. Influence of resveratrol on the immune response. Nutrients 2019, 11, 946. [Google Scholar] [CrossRef]
- Salehi, B.; Mishra, A.P.; Nigam, M.; Sener, B.; Kilic, M.; Sharifi-Rad, M.; Valere Tsouh Fokou, P.; Cruz-Martins, N.; Sharifi-Rad, J. Resveratrol: A double-edged sword in health benefits. Biomedicines 2018, 6, 91. [Google Scholar] [CrossRef]
- Zhang, J. Resveratrol inhibits insulin responses in a SirT1-independent pathway. Biochem. J. 2006, 397, 519–527. [Google Scholar] [CrossRef]
- Desjardins, D.; Cacho-Valadez, B.; Liu, J.L.; Wang, Y.; Yee, C.; Bernard, K.; Khaki, A.; Breton, L.; Hekimi, S. Antioxidants reveal an inverted U-shaped dose-response relationship between reactive oxygen species levels and the rate of aging in Caenorhabditis elegans. Aging Cell 2017, 16, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Legarreta, I.; López-Hernández, E.; Armenta-López, R.E.; García Barrientos, R. Pigmentos. In Química de Alimentos; Badui-Dergal, S., Ed.; Pearson: Mexico City, Mexico, 2013; p. 379. [Google Scholar]
- Schawartz, S.J.; Cooperstone, J.L.; Cichon, M.J.; von Elbe, J.H.; Giusti, M.M. Colorants. In Fennema’s Food Chemistry; Damodaran, S., Parkin, K.L., Eds.; CRC Press Taylor and Francis: Boca Raton, FL, USA, 2017; p. 681. [Google Scholar]
- Saini, R.K.; Nile, S.H.; Park, S.W. Carotenoids from fruits and vegetables: Chemistry, analysis, occurrence, bioavailability and biological activities. Food. Res. Int. 2015, 76, 735–750. [Google Scholar] [CrossRef] [PubMed]
- Torres-Rodríguez, A.; Salinas-Moreno, Y.; Valle-Guadarrama, S.; Alia-Tejacal, I. Soluble phenols and antioxidant activity in mamey sapote (Pouteria sapota) fruits in postharvest. Food. Res. Int. 2011, 44, 1956–1961. [Google Scholar] [CrossRef]
- Zheng, W.V.; Xu, W.; Li, Y.; Qin, J.; Zhou, T.; Li, D.; Xu, Y.; Cheng, X.; Xiong, Y.; Chen, Z. Anti-aging effect of β-carotene through regulating the KAT7-P15 signaling axis, inflammation and oxidative stress process. Cell. Mol. Biol. Lett. 2022, 27, 86. [Google Scholar] [CrossRef]
- Hu, W.; Dai, D.; Li, W. Anti-aging effect of Blakeslea trispora powder on adult mice. Biotechnol. Lett. 2013, 35, 1309–1315. [Google Scholar] [CrossRef]
- Liu, X.; Lin, X.; Zhang, S.; Guo, C.; Li, J.; Mi, Y.; Zhang, C. Lycopene ameliorates oxidative stress in the aging chicken ovary via activation of Nrf2/HO-1 pathway. Aging 2018, 10, 2016–2036. [Google Scholar] [CrossRef]
- Colmán-Martínez, M.; Martínez-Huélamo, M.; Valderas-Martínez, P.; Arranz-Martínez, S.; Almanza-Aguilera, E.; Corella, D.; Estruch, R.; Lamuela-Raventós, R.M. trans-Lycopene from tomato juice attenuates inflammatory biomarkers in human plasma samples: An intervention trial. Mol. Nutr. Food. Res. 2017, 61, 1600993. [Google Scholar] [CrossRef]
- Liu, C.B.; Wang, R.; Yi, Y.F.; Gao, Z.; Chen, Y.Z. Lycopene mitigates β-amyloid induced inflammatory response and inhibits NF-κB signaling at the choroid plexus in early stages of Alzheimer’s disease rats. J. Nutr. Biochem. 2018, 53, 66–71. [Google Scholar] [CrossRef]
- Krishnaswamy, V.K.D.; Alugoju, P.; Periyasamy, L. Effect of short-term oral supplementation of crocin on age-related oxidative stress, cholinergic, and mitochondrial dysfunction in rat cerebral cortex. Life Sci. 2020, 263, 118545. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Xiao, J.; Liu, H.; Yao, K.; Hou, H.; Cao, Y.; Liu, X. Astaxanthin attenuates oxidative stress and immune impairment in d-galactose-induced aging in rats by activating the Nrf2/Keap1 pathway and suppressing the NF-κB pathway. Food. Funct. 2020, 11, 8099–8111. [Google Scholar] [CrossRef] [PubMed]
- Al-Amin, M.M.; Akhter, S.; Hasan, A.T.; Alam, T.; Nageeb Hasan, S.M.; Saifullah, A.R.; Shohel, M. The antioxidant effect of astaxanthin is higher in young mice than aged: A region specific study on brain. Metab. Brain. Dis. 2015, 30, 1237–1246. [Google Scholar] [CrossRef]
- Sj, S.; Veerabhadrappa, B.; Subramaniyan, S.; Dyavaiah, M. Astaxanthin enhances the longevity of Saccharomyces cerevisiae by decreasing oxidative stress and apoptosis. FEMS Yeast Res. 2019, 19, foy113. [Google Scholar] [CrossRef]
- Wen, X.; Huang, A.; Hu, J.; Zhong, Z.; Liu, Y.; Li, Z.; Pan, X.; Liu, Z. Neuroprotective effect of astaxanthin against glutamate-induced cytotoxicity in HT22 cells: Involvement of the Akt/GSK-3β pathway. Neuroscience 2015, 303, 558–568. [Google Scholar] [CrossRef]
- Song, L.; Leng, K.; Xiao, K.; Zhang, S. Administration of krill oil extends lifespan of fish Nothobranchius guentheri via enhancement of antioxidant system and suppression of NF-κB pathway. Fish. Physiol. Biochem. 2022, 48, 1057–1073. [Google Scholar] [CrossRef]
- Li, J.; Xian, L.; Zheng, R.; Wang, Y.; Wan, X.; Liu, Y. Canthaxanthin shows anti-liver aging and anti-liver fibrosis effects by down-regulating inflammation and oxidative stress in vivo and in vitro. Int. Immunopharmacol. 2022, 110, 108942. [Google Scholar] [CrossRef]
- Philips, N.; Keller, T.; Hendrix, C.; Hamilton, S.; Arena, R.; Tuason, M.; Gonzalez, S. Regulation of the extracellular matrix remodeling by lutein in dermal fibroblasts, melanoma cells, and ultraviolet radiation exposed fibroblasts. Arch. Dermatol. Res. 2007, 299, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Chae, S.; Park, S.; Park, G. Lutein protects human retinal pigment epithelial cells from oxidative stress-induced cellular senescence. Mol. Med. Rep. 2018, 18, 5182–5190. [Google Scholar] [CrossRef]
- Hadad, N.; Levy, R. The synergistic anti-inflammatory effects of lycopene, lutein, β-carotene, and carnosic acid combinations via redox-based inhibition of NF-κB signaling. Free Rad. Biol. Med. 2012, 53, 1381–1391. [Google Scholar] [CrossRef]
- Morris, M.C.; Wang, Y.; Barnes, L.L.; Bennett, D.A.; Dawson-Hughes, B.; Booth, S.L. Nutrients and bioactives in green leafy vegetables and cognitive decline: Prospective study. Neurology 2018, 90, e214–e222. [Google Scholar] [CrossRef] [PubMed]
- Baba, S.A.; Malik, A.H.; Wani, Z.A.; Mohiuddin, T.; Shah, Z.; Abbas, N.; Ashraf, N. Phytochemical analysis and antioxidant activity of different tissue types of Crocus sativus and oxidative stress alleviating potential of saffron extract in plants, bacteria, and yeast. S. Afr. J. Bot. 2015, 99, 80–87. [Google Scholar] [CrossRef]
- Seabra, L.M.A.J.; Pedrosa, L.F.C. Astaxanthin: Structural and functional aspects. Rev. Nutrio. 2010, 23, 1041–1050. [Google Scholar] [CrossRef]
- Liu, X.; Liu, H.; Chen, Z.; Xiao, J.; Cao, Y. DAF-16 acts as the “hub” of astaxanthin’s anti-aging mechanism to improve aging-related physiological functions in Caenorhabditis elegans. Food. Funct. 2021, 12, 9098–9110. [Google Scholar] [CrossRef]
- Ding, F.; Zhao, Y. Astaxanthin induces transcriptomic responses associated with lifespan extension in Caenorhabditis elegans. Antioxidants 2022, 11, 2115. [Google Scholar] [CrossRef]
- Sudharshan, S.J.; Dyavaiah, M. Astaxanthin protects oxidative stress mediated DNA damage and enhances longevity in Saccharomyces cerevisiae. Biogerontology 2021, 22, 81–100. [Google Scholar] [CrossRef]
- Hartmann, M.A. Plant sterols and the membrane environment. Trends. Plant. Sci. 1998, 3, 170–175. [Google Scholar] [CrossRef]
- Piironen, V.; Lindsay, D.G.; Miettinen, T.A.; Toivo, J.; Lampi, A.M. Plant sterols: Biosynthesis, biological function and their importance to human nutrition. J. Sci. Food. Agric. 2000, 80, 939–966. [Google Scholar] [CrossRef]
- Chen, S.; Yong, T.; Zhang, Y.; Su, J.; Jiao, C.; Xie, Y. Anti-tumor and anti-angiogenic ergosterols from Ganoderma lucidum. Front. Chem. 2017, 5, 85. [Google Scholar] [CrossRef]
- Hu, Z.; He, B.; Ma, L.; Sun, Y.; Niu, Y.; Zeng, B. Recent advances in ergosterol biosynthesis and regulation mechanisms in Saccharomyces cerevisiae. Indian. J. Microbiol. 2017, 57, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.R.M.; Mohamed, G.A.; Al Haidari, R.A.; Soliman, A.A.; Asfour, H.Z.; Zayed, M.F. Fusaristerol A: A new cytotoxic and antifungal ergosterol fatty acid ester from the endophytic fungus Fusarium sp. associated with Mentha longifolia roots. Phcog. Mag. 2018, 14, 308–311. [Google Scholar] [CrossRef]
- Song, L.; Li, C.; Wu, F.; Zhang, S. Dietary intake of diosgenin delays aging of male fish Nothobranchius guentheri through modulation of multiple pathways that play prominent roles in ROS production. Biogerontology 2022, 23, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Haiyuan, Y.U.; Shen, X.; Liu, D.; Hong, M.; Lu, Y. The protective effects of β-sitosterol and vermicularin from Thamnolia vermicularis (Sw.) Ach. against skin aging in vitro. An. Acad. Bras. Cienc. 2019, 91, e20181088. [Google Scholar] [CrossRef]
- Ji, Z.H.; Xu, Z.Q.; Zhao, H.; Yu, X.Y. Neuroprotective effect and mechanism of daucosterol palmitate in ameliorating learning and memory impairment in a rat model of Alzheimer’s disease. Steroids 2017, 119, 31–35. [Google Scholar] [CrossRef]
- Hah, Y.S.; Lee, W.K.; Lee, S.; Kim, E.J.; Lee, J.H.; Lee, S.J.; Ji, Y.H.; Kim, S.G.; Lee, H.H.; Hong, S.Y.; et al. β-Sitosterol attenuates dexamethasone-induced muscle atrophy via regulating FoxO1-dependent signaling in C2C12 cell and mice model. Nutrients 2022, 14, 2894. [Google Scholar] [CrossRef]
- Shi, C.; Luo, X.; Wang, J.; Long, D. Incorporation of β-sitosterol into the membrane prevents tumor necrosis factor-α-induced nuclear factor-κB activation and gonadotropin-releasing hormone decline. Steroids 2015, 96, 1–6. [Google Scholar] [CrossRef]
- Jeremy, M.; Gurusubramanian, G.; Roy, V.K. Vitamin D3 regulates apoptosis and proliferation in the testis of D-galactose-induced aged rat model. Sci. Rep. 2019, 9, 14103. [Google Scholar] [CrossRef]
- Halicka, H.D.; Zhao, H.; Li, J.; Traganos, F.; Studzinski, G.P.; Darzynkiewicz, Z. Attenuation of constitutive DNA damage signaling by 1,25-dihydroxyvitamin D3. Aging 2012, 4, 270–278. [Google Scholar] [CrossRef]
- Sun, Y.; Lin, Y.; Cao, X.; Xiang, L.; Qi, J. Sterols from Mytilidae show anti-aging and neuroprotective effects via anti-oxidative activity. Int. J. Mol. Sci. 2014, 15, 21660–21673. [Google Scholar] [CrossRef]
- Weng, Y.; Xiang, L.; Matsuura, A.; Zhang, Y.; Huang, Q.; Qi, J. Ganodermasides A and B, two novel anti-aging ergosterols from spores of a medicinal mushroom Ganoderma lucidum on yeast via UTH1 gene. Bioorg. Med. Chem. 2010, 18, 999–1002. [Google Scholar] [CrossRef]
- Long, H.; Qiu, X.; Cao, L.; Han, R. Discovery of the signal pathways and major bioactive compounds responsible for the anti-hypoxia effect of Chinese cordyceps. J. Ethnopharmacol. 2021, 277, 114215. [Google Scholar] [CrossRef] [PubMed]
- Racette, S.B.; Lin, X.; Ma, L.; Ostlund, R.E., Jr. Natural dietary phytosterols. J. AOAC Int. 2015, 98, 679–684. [Google Scholar] [CrossRef]
- Nes, W.D. Biosynthesis of cholesterol and other sterols. Chem. Rev. 2011, 111, 6423–6451. [Google Scholar] [CrossRef]
- Moreau, R.A.; Whitaker, B.D.; Hicks, K.B. Phytosterols, phytostanols, and their conjugates in foods: Structural diversity, quantitative analysis, and health-promoting uses. Prog. Lipid. Res. 2002, 41, 457–500. [Google Scholar] [CrossRef] [PubMed]
- Cohn, J.S.; Kamili, A.; Wat, E.; Chung, R.W.; Tandy, S. Reduction in intestinal cholesterol absorption by various food components: Mechanisms and implications. Atheroscler. Suppl. 2010, 11, 45–48. [Google Scholar] [CrossRef]
- Gupta, A.K.; Savopoulos, C.G.; Ahuja, J.; Hatzitolios, A.I. Role of phytosterols in lipid-lowering: Current perspectives. QJM 2011, 104, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Ogbe, R.J.; Ochalefu, D.O.; Mafulul, S.G. A review on dietary phytosterols: Their occurrence, metabolism and health benefits. Asian. J. Plant. Sci. Res. 2015, 5, 10–21. [Google Scholar]
- Tieu, E.W.; Li, W.; Chen, J.; Baldisseri, D.M.; Slominski, A.T.; Tuckey, R.C. Metabolism of cholesterol, vitamin D3 and 20-hydroxyvitamin D3 incorporated into phospholipid vesicles by human CYP27A1. J. Steroid. Biochem. Mol. Biol. 2012, 129, 163–171. [Google Scholar] [CrossRef]
- Benedik, E. Sources of vitamin D for humans. Int. J. Vitam. Nutr. Res. 2022, 92, 118–125. [Google Scholar] [CrossRef]
- Blomberg Jensen, M.; Nielsen, J.E.; Jørgensen, A.; Rajpert-De Meyts, E.; Kristensen, D.M.; Jørgensen, N.; Skakkebaek, N.E.; Juul, A.; Leffers, H. Vitamin D receptor and vitamin D metabolizing enzymes are expressed in the human male reproductive tract. Hum. Reprod. 2010, 25, 1303–1311. [Google Scholar] [CrossRef] [PubMed]
- Shahrokhi, S.Z.; Ghaffari, F.; Kazerouni, F. Role of vitamin D in female reproduction. Clin. Chim. Acta. 2016, 455, 33–38. [Google Scholar] [CrossRef]
- Kwiecinski, G.G.; Petrie, G.I.; DeLuca, H.F. Vitamin D is necessary for reproductive functions of the male rat. J. Nutr. 1989, 119, 741–744. [Google Scholar] [CrossRef]
- Zaki, A.H.; Zahid, M.T.; Haiying, B. Bioactive compounds of the culinary-medicinal mushroom Leucocalocybe mongolica (Agaricomycetes): Pharmacological and therapeutic applications-A review. Int. J. Med. Mushrooms 2022, 24, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Maser, E.; Lanišnik Rižner, T. Steroids and microorganisms. J. Steroid. Biochem. Mol. Biol. 2012, 129, 1–3. [Google Scholar] [CrossRef]
- El-Baba, C.; Baassiri, A.; Kiriako, G.; Dia, B.; Fadlallah, S.; Moodad, S.; Darwiche, N. Terpenoids’ anti-cancer effects: Focus on autophagy. Apoptosis 2021, 26, 491–511. [Google Scholar] [CrossRef]
- Huang, M.; Lu, J.J.; Huang, M.Q.; Bao, J.L.; Chen, X.P.; Wang, Y.T. Terpenoids: Natural products for cancer therapy. Expert. Opin. Investig. Drugs 2012, 21, 1801–1818. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Vong, C.T.; Chen, H.; Gao, Y.; Lyu, P.; Qiu, L.; Zhao, M.; Liu, Q.; Zehua, C.; Zou, Y.; et al. Naturally occurring anticancer compounds: Shining from Chinese herbal medicine. Chin. Med. 2019, 14, 48. [Google Scholar] [CrossRef]
- Ju, S.; Seo, J.Y.; Lee, S.K.; Oh, J.; Kim, J.S. Oral administration of hydrolyzed red ginseng extract improves learning and memory capability of scopolamine-treated C57BL/6J mice via upregulation of Nrf2-mediated antioxidant mechanism. J. Ginseng. Res. 2021, 45, 108–118. [Google Scholar] [CrossRef]
- Tu, T.H.T.; Sharma, N.; Shin, E.J.; Tran, H.Q.; Lee, Y.J.; Jeong, J.H.; Jeong, J.H.; Nah, S.Y.; Tran, H.Y.P.; Byun, J.K.; et al. Ginsenoside Re protects trimethyltin-induced neurotoxicity via activation of IL-6-mediated phosphoinositol 3-kinase/Akt signaling in mice. Neurochem. Res. 2017, 42, 3125–3139. [Google Scholar] [CrossRef]
- Jiang, J.L.; Liang, J.; Yang, Y.Y.; Liu, S.K. Research progress on pharmacological and toxicological effects of Siraitia grosvenori. Mod. Prev. Med. 2020, 47, 2246–2248+2262. [Google Scholar]
- Schmucker, D.L. Age-related changes in liver structure and function: Implications for disease? Exp. Gerontol. 2005, 40, 650–659. [Google Scholar] [CrossRef]
- Shen, X.; Dong, X.; Han, Y.; Li, Y.; Ding, S.; Zhang, H.; Sun, Z.; Yin, Y.; Li, W.; Li, W. Ginsenoside Rg1 ameliorates glomerular fibrosis during kidney aging by inhibiting NOX4 and NLRP3 inflammasome activation in SAMP8 mice. Int. Immunopharmacol. 2020, 82, 106339. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ding, S.; Zhang, H.; Sun, Z.; Shen, X.; Sun, L.; Yin, Y.; Qun, S.; Li, W. Protective effects of ginsenoside Rg1 on neuronal senescence due to inhibition of NOX2 and NLRP1 inflammasome activation in SAMP8 mice. J. Funct. Foods 2020, 65, 103713. [Google Scholar] [CrossRef]
- Phulara, S.C.; Pandey, S.; Jha, A.; Chauhan, P.S.; Gupta, P.; Shukla, V. Hemiterpene compound, 3,3-dimethylallyl alcohol promotes longevity and neuroprotection in Caenorhabditis elegans. GeroScience 2021, 43, 791–807. [Google Scholar] [CrossRef]
- Omari, Z.; Kazunori, S.; Sabti, M.; Bejaoui, M.; Hafidi, A.; Gadhi, C.; Isoda, H. Dietary administration of cumin-derived cuminaldehyde induce neuroprotective and learning and memory enhancement effects to aging mice. Aging 2021, 13, 1671–1685. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhang, Q.; Amrouche, A.T.; Huang, W.; Lu, B. Asperuloside, the bioactive compound in the edible Eucommia ulmoides male flower, delays muscle aging by daf-16 mediated improvement in mitochondrial dysfunction. Food. Funct. 2023, 14, 5562–5575. [Google Scholar] [CrossRef]
- Kumar, K.J.S.; Vani, M.G.; Wang, S.Y. Limonene protects human skin keratinocytes against UVB-induced photodamage and photoaging by activating the Nrf2-dependent antioxidant defense system. Environ. Toxicol. 2022, 37, 2897–2909. [Google Scholar] [CrossRef]
- El-Far, A.H.; Mohamed, H.H.; Elsabagh, D.A.; Mohamed, S.A.; Noreldin, A.E.; Al Jaouni, S.K.; Alsenosy, A.A. Eugenol and carvacrol attenuate brain D-galactose-induced aging-related oxidative alterations in rats. Environ. Sci. Pollut. R. 2022, 29, 47436–47447. [Google Scholar] [CrossRef]
- Mu, H.Y.; Gao, Y.H.; Cao, G.C.; Jiang, J.Y.; Wang, H.B.; Zhao, W.M. Dihydro-β-agarofuran-type sesquiterpenoids from the seeds of Celastrus virens with lifespan-extending effect on the nematode Caenorhabditis elegans. Fitoterapia 2022, 158, 105165. [Google Scholar] [CrossRef]
- Mohankumar, A.; Kalaiselvi, D.; Thiruppathi, G.; Muthusaravanan, S.; Nivitha, S.; Levenson, C.; Tawata, S.; Sundararaj, P. α- and β-Santalols delay aging in Caenorhabditis elegans via preventing oxidative stress and protein aggregation. ACS. Omega 2020, 5, 32641–32654. [Google Scholar] [CrossRef]
- Chen, M.; Wen, H.; Zhou, S.; Yan, X.; Li, H. Patchouli alcohol inhibits D-gal induced oxidative stress and ameliorates the quality of aging cartilage via activating the Nrf2/HO-1 pathway in mice. Oxid. Med. Cell. Longev. 2022, 2022, 6821170. [Google Scholar] [CrossRef]
- Tsai, Y.C.; Lin, Y.C.; Huang, C.C.; Villaflores, O.B.; Wu, T.Y.; Huang, S.M.; Chin, T.Y. Hericium erinaceus mycelium and its isolated compound, erinacine A, ameliorate high-fat high-sucrose diet-induced metabolic dysfunction and spatial learning deficits in aging mice. J. Med. Food. 2019, 22, 469–478. [Google Scholar] [CrossRef]
- Lin, C.; Zhang, X.; Su, Z.; Xiao, J.; Lv, M.; Cao, Y.; Chen, Y. Carnosol improved lifespan and healthspan by promoting antioxidant capacity in Caenorhabditis elegans. Oxid. Med. Cell. Longev. 2019, 2019, 5958043. [Google Scholar] [CrossRef] [PubMed]
- Oliva, C.A.; Rivera, D.S.; Torres, A.K.; Lindsay, C.B.; Tapia-Rojas, C.; Bozinovic, F.; Inestrosa, N.C. Age-dependent behavioral and synaptic dysfunction impairment are improved with long-term andrographolide administration in long-lived female degus (Octodon degus). Int. J. Mol. Sci. 2023, 24, 1105. [Google Scholar] [CrossRef]
- Yang, Q.; Lin, J.; Zhang, H.; Liu, Y.; Kan, M.; Xiu, Z.; Chen, X.; Lan, X.; Li, X.; Shi, X.; et al. Ginsenoside compound K regulates amyloid β via the Nrf2/Keap1 signaling pathway in mice with scopolamine hydrobromide-induced memory impairments. J. Mol. Neurosci. 2019, 67, 62–71. [Google Scholar] [CrossRef]
- Zong, W.; Zeng, X.; Chen, S.; Chen, L.; Zhou, L.; Wang, X.; Gao, Q.; Zeng, G.; Hu, K.; Ou-Yang, D.S. Ginsenoside compound K attenuates cognitive deficits in vascular dementia rats by reducing the Aβ deposition. J. Pharmacol. Sci. 2019, 139, 223–230. [Google Scholar] [CrossRef]
- Kim, E.; Kim, N.; Yoo, S.; Hong, Y.H.; Han, S.Y.; Jeong, S.; Jeong, D.; Kim, J.H.; Cho, J.; Park, J. The skin protective effects of compound K, a metabolite of ginsenoside Rb1 from Panax ginseng. J. Ginseng Res. 2017, 42, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, Y.P.; He, Y.H.; Ding, J.C. Ginsenoside Rg1 performs anti-aging functions by suppressing mitochondrial pathway-mediated apoptosis and activating sirtuin 3 (SIRT3)/superoxide dismutase 2 (SOD2) pathway in Sca-1⁺ HSC/HPC cells of an aging rat model. Med. Sci. Monit. 2020, 26, e920666. [Google Scholar] [CrossRef]
- Yu, S.; Xia, H.; Guo, Y.; Qian, X.; Zou, X.; Yang, H.; Yin, M.; Liu, H. Ginsenoside Rb1 retards aging process by regulating cell cycle, apoptotic pathway and metabolism of aging mice. J. Ethnopharmacol. 2020, 255, 112746. [Google Scholar] [CrossRef] [PubMed]
- Lim, T.G.; Jeon, A.J.; Yoon, J.H.; Song, D.; Kim, J.E.; Kwon, J.Y.; Kim, J.R.; Kang, N.J.; Park, J.S.; Yeom, M.H.; et al. 20-O-β-D-glucopyranosyl-20(S)-protopanaxadiol, a metabolite of ginsenoside Rb1, enhances the production of hyaluronic acid through the activation of ERK and Akt mediated by Src tyrosin kinase in human keratinocytes. Int. J. Mol. Med. 2015, 35, 1388–1394. [Google Scholar] [CrossRef]
- Xu, Y.; Yuan, H.; Luo, Y.; Zhao, Y.J.; Xiao, J.H. Ganoderic acid D protects human amniotic mesenchymal stem cells against oxidative stress-induced senescence through the PERK/NRF2 signaling pathway. Oxid. Med. Cell. Longev. 2020, 2020, 8291413. [Google Scholar] [CrossRef]
- Yuan, H.; Xu, Y.; Luo, Y.; Zhang, J.R.; Zhu, X.X.; Xiao, J.H. Ganoderic acid D prevents oxidative stress-induced senescence by targeting 14-3-3ε to activate CaM/CaMKII/NRF2 signaling pathway in mesenchymal stem cells. Aging Cell 2022, 21, e13686. [Google Scholar] [CrossRef]
- Montilla, M.P.; Agil, A.; Navarro, M.C.; Jiménez, M.I.; García-Granados, A.; Parra, A.; Cabo, M.M. Antioxidant activity of maslinic acid, a triterpene derivative obtained from Olea europaea. Planta. Medica 2003, 69, 472–474. [Google Scholar]
- Bakhtiari, N.; Hosseinkhani, S.; Soleimani, M.; Hemmati, R.; Noori-Zadeh, A.; Javan, M.; Tashakor, A. Short-term ursolic acid promotes skeletal muscle rejuvenation through enhancing of SIRT1 expression and satellite cells proliferation. Biomed. Pharmacother. 2016, 78, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Gharibi, S.; Bakhtiari, N.; Elham-Moslemee-Jalalvand; Bakhtiari, F. Ursolic acid mediates hepatic protection through enhancing of anti-aging biomarkers. Curr Aging Sci. 2018, 11, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Ni, Y.; Jiang, B.; Yan, S.; Xu, B.; Fan, B.; Huang, H.; Chen, G. Anti-aging derivatives of cycloastragenol produced by biotransformation. Nat. Prod. Res. 2021, 35, 2685–2690. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, H.F.; Fuller, T.; Edwards, J.; Finger, D.; Molgora, B. Cycloastragenol extends T cell proliferation by increasing telomerase activity. J. Immunol. 2009, 18, 9030. [Google Scholar] [CrossRef]
- Choi, Y.H. Activation of the Nrf2/HO-1 signaling pathway contributes to the protective effects of platycodin D against oxidative stress-induced DNA damage and apoptosis in C2C12 myoblasts. Gen. Physiol. Biophys. 2020, 39, 519–530. [Google Scholar] [CrossRef]
- Nguyen, B.T.; Shin, E.J.; Jeong, J.H.; Sharma, N.; Nah, S.Y.; Ko, S.K.; Byun, J.K.; Lee, Y.; Lei, X.G.; Kim, D.J.; et al. Ginsenoside Re attenuates memory impairments in aged Klotho deficient mice via interactive modulations of angiotensin II AT1 receptor, Nrf2 and GPx-1 gene. Free. Radic. Biol. Med. 2022, 189, 2–19. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, D.; Li, L.; Han, Y.; Dong, X.; Yang, L.; Li, X.; Li, W.; Li, W. Ginsenoside Rg1 ameliorates aging-induced liver fibrosis by inhibiting the NOX4/NLRP3 inflammasome in SAMP8 mice. Mol. Med. Rep. 2021, 24, 801. [Google Scholar] [CrossRef]
- Zhong, S.J.; Wang, L.; Gu, R.Z.; Zhang, W.H.; Lan, R.; Qin, X.Y. Ginsenoside Rg1 ameliorates the cognitive deficits in D-galactose and AlCl3-induced aging mice by restoring FGF2-Akt and BDNF-TrkB signaling axis to inhibit apoptosis. Int. J. Med. Sci. 2020, 17, 1048–1055. [Google Scholar] [CrossRef]
- Lasekan, O. Identification of the aroma compounds in Vitex doniana sweet: Free and bound odorants. Chem. Cent. J. 2017, 11, 19. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Varoni, E.M.; Iriti, M.; Martorell, M.; Setzer, W.N.; Del Mar Contreras, M.; Salehi, B.; Soltani-Nejad, A.; Rajabi, S.; Tajbakhsh, M.; et al. Carvacrol and human health: A comprehensive review. Phytother. Res. 2018, 32, 1675–1687. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Zhao, W. Polyesterified sesquiterpenoids from the seeds of Celastrus paniculatus as lifespan-extending agents for the nematode Caenorhabditis elegans. J. Nat. Prod. 2020, 83, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Mu, X.; Zeng, J.; Xu, C.; Liu, J.; Zhang, M.; Li, C.; Chen, J.; Li, T.; Wang, Y. Ginsenoside Rg1 prevents cognitive impairment and hippocampus senescence in a rat model of D-galactose-induced aging. PLoS ONE 2014, 9, e101291. [Google Scholar] [CrossRef]
- Bakhtiari, N.; Hosseinkhani, S.; Tashakor, A.; Hemmati, R. Ursolic acid ameliorates aging-metabolic phenotype through promoting of skeletal muscle rejuvenation. Med. Hypotheses 2015, 85, 1–6. [Google Scholar] [CrossRef]
- Bjørklund, G.; Shanaida, M.; Lysiuk, R.; Butnariu, M.; Peana, M.; Sarac, I.; Strus, O.; Smetanina, K.; Chirumbolo, S. Natural compounds and products from an anti-aging perspective. Molecules 2022, 27, 7084. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A.; Iordache, F.; Stanca, L.; Geicu, O.I.; Bilteanu, L.; Serban, A.I. Antioxidant, anti-inflammatory and immunomodulatory roles of vitamins in COVID-19 therapy. Eur. J. Med. Chem. 2022, 232, 114175. [Google Scholar] [CrossRef]
- Ashor, A.W.; Brown, R.; Keenan, P.D.; Willis, N.D.; Siervo, M.; Mathers, J.C. Limited evidence for a beneficial effect of vitamin C supplementation on biomarkers of cardiovascular diseases: An umbrella review of systematic reviews and meta-analyses. Nutr. Res. 2019, 61, 1–12. [Google Scholar] [CrossRef]
- Zou, Y.X.; Ruan, M.H.; Luan, J.; Feng, X.; Chen, S.; Chu, Z.Y. Anti-aging effect of riboflavin via endogenous antioxidant in fruit fly Drosophila Melanogaster. J. Nutr. Health. Aging 2017, 21, 314–319. [Google Scholar] [CrossRef]
- Kim, H.M.; Byun, K.A.; Oh, S.; Yang, J.Y.; Park, H.J.; Chung, M.S.; Son, K.H.; Byun, K. A mixture of topical forms of polydeoxyribonucleotide, vitamin C, and niacinamide attenuated skin pigmentation and increased skin elasticity by modulating nuclear factor erythroid 2-like 2. Molecules 2022, 27, 1276. [Google Scholar] [CrossRef] [PubMed]
- Martín-Martínez, A.; Sánchez-Marzo, N.; Martínez-Casanova, D.; Abarquero-Cerezo, M.; Herranz-López, M.; Barrajón-Catalán, E.; Matabuena-Yzaguirre, M. High global antioxidant protection and stimulation of the collagen synthesis of new anti-aging product containing an optimized active mix. J. Cosmet. Dermatol. 2022, 21, 3993–4000. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Gu, Y.; Pang, Q.; Han, Q.; Li, A.; Wu, W.; Zhang, X.; Shi, Q.; Zhu, L.; Yu, H.; et al. Vitamin C inhibits lipid deposition through GSK-3β/mTOR signaling in the liver of zebrafish. Fish. Physiol. Biochem. 2020, 46, 383–394. [Google Scholar] [CrossRef]
- La Fata, G.; van Vliet, N.; Barnhoorn, S.; Brandt, R.M.C.; Etheve, S.; Chenal, E.; Grunenwald, C.; Seifert, N.; Weber, P.; Hoeijmakers, J.H.J.; et al. Vitamin E supplementation reduces cellular loss in the brain of a premature aging mouse model. J. Prev. Alzheimers. Dis. 2017, 4, 226–235. [Google Scholar] [PubMed]
- Liu, X.; Yang, Q.; Li, H.; Lan, X.; Kan, M.; Lin, J.; Wang, J.; Zhang, Z.; Ming, S.; Li, Z.; et al. The anti-aging effect of velvet antler polypeptide is dependent on modulation of the gut microbiota and regulation of the PPARα/APOE4 pathway. J. Integr. Neurosci. 2021, 20, 573–583. [Google Scholar]
- Amevor, F.K.; Cui, Z.; Du, X.; Ning, Z.; Deng, X.; Xu, D.; Shu, G.; Wu, Y.; Cao, X.; Shuo, W.; et al. Supplementation of dietary quercetin and vitamin E promotes the intestinal structure and immune barrier integrity in aged breeder hens. Front. Immunol. 2022, 13, 860889. [Google Scholar] [CrossRef] [PubMed]
- Ambrożewicz, E.; Muszyńska, M.; Tokajuk, G.; Grynkiewicz, G.; Žarković, N.; Skrzydlewska, E. Beneficial effects of vitamins K and D3 on redox balance of human osteoblasts cultured with hydroxyapatite-based biomaterials. Cells 2019, 8, 325. [Google Scholar] [CrossRef] [PubMed]
- Qu, Z.; Zhang, L.; Huang, W.; Zheng, S. Vitamin K2 enhances fat degradation to improve the survival of C. elegans. Front. Nutr. 2022, 9, 858481. [Google Scholar] [CrossRef]
- Pham, V.T.; Dold, S.; Rehman, A.; Bird, J.K.; Steinert, R.E. Vitamins, the gut microbiome and gastrointestinal health in humans. Nutr. Res. 2021, 95, 35–53. [Google Scholar] [CrossRef]
- Dym, O.; Eisenberg, D. Sequence-structure analysis of FAD-containing proteins. Protein Sci. 2001, 10, 1712–1728. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, D.R.; Libardi, S.H.; Skibsted, L.H. Riboflavin as a photosensitizer. Effects on human health and food quality. Food Funct. 2012, 3, 487–502. [Google Scholar] [CrossRef] [PubMed]
- Buehler, B.A. Vitamin B2: Riboflavin. J. Evid. Based Complement. Altern. Med. 2011, 16, 88–90. [Google Scholar] [CrossRef]
- Naveed, S.; Sajid, S. Degradation in pharmaceutical creams: Ascorbic acid demonstrating phenomenon. J. Bioequivalence. Bioavailab. 2016, 8, 80–83. [Google Scholar] [CrossRef]
- Mumtaz, S.; Ali, S.; Tahir, H.M.; Kazmi, S.A.R.; Shakir, H.A.; Mughal, T.A.; Mumtaz, S.; Summer, M.; Farooq, M.A. Aging and its treatment with vitamin C: A comprehensive mechanistic review. Mol. Biol. Rep. 2021, 48, 8141–8153. [Google Scholar] [CrossRef]
- Guida, J.L.; Agurs-Collins, T.; Ahles, T.A.; Campisi, J.; William Dale, W.; Demark-Wahnefried, W.; Dietrich, J.; Fuldner, R.; Gallicchio, L.; Green, P.A.; et al. Strategies to prevent or remediate cancer and treatment-related aging. J. Nat. Cancer. Inst. 2021, 113, 112–122. [Google Scholar]
- Markiewicz-Tomczyk, A.; Budzisz, E.; Erkiert-Polguj, A. Clinical evaluation of anti-aging effects of combined therapy-Azelaic acid, phytic acid, and vitamin C applied layer by layer in females with Fitzpatrick skin types II and III. J. Cosmet. Dermatol. 2022, 21, 6830–6839. [Google Scholar] [CrossRef]
- Goldberg, D.J.; Robinson, D.M.; Granger, C. Clinical evidence of the efficacy and safety of a new 3-in-1 anti-aging topical night serum-in-oil containing melatonin, bakuchiol, and ascorbyl tetraisopalmitate: 103 Females treated from 28 to 84 days. J. Cosmet. Dermatol. 2019, 18, 806–814. [Google Scholar] [CrossRef]
- Akulinina, I.; Stefanaki, I.; Pavlíčková, E.; Maiolino, M.; Hajduk, S.; Sápy, M.; Mertin, B.; Rijo, H.; Tekeli, Ö.; Valois, A.; et al. Topical formulation containing peptides and vitamin C in ampoules improves skin aging signs: Results of a large, international, observational study. J. Cosmet. Dermatol. 2022, 21, 3910–3916. [Google Scholar] [CrossRef]
- Rattanawiwatpong, P.; Wanitphakdeedecha, R.; Bumrungpert, A.; Maiprasert, M. Anti-aging and brightening effects of a topical treatment containing vitamin C, vitamin E, and raspberry leaf cell culture extract: A split-face, randomized controlled trial. J. Cosmet. Dermatol. 2020, 19, 671–676. [Google Scholar] [CrossRef]
- Handler, M.; Adams-Woodford, A.; Ayres, P.; Giancola, G.; Diaz, I. Facial aging improvement case study using a novel combination of retinol, niacinamide, and Terminalia Chebula. J. Drugs. Dermatol. 2022, 21, 784–788. [Google Scholar] [PubMed]
- Jang, S.I.; Jung, Y.C.; Suk, J.; Lee, S.; Han, J.; Suh, B.F.; Kim, E. A long term study of the difference in efficacy and effect rate of various concentrations of retinol (1500–6600 IU) in middle aged women. Arch. Dermatol. Res. 2023, 315, 1323–1332. [Google Scholar] [CrossRef]
- Abed, K.; Foucher, A.; Bernard, D.; Tancrède-Bohin, E.; Cavusoglu, N. One-year longitudinal study of the stratum corneum proteome of retinol and all-trans-retinoic acid treated human skin: An orchestrated molecular event. Sci. Rep. 2023, 13, 11196. [Google Scholar] [CrossRef] [PubMed]
- Kamal-Eldin, A.; Appelqvist, L.A. The chemistry and antioxidant properties of tocopherols and tocotrienols. Lipids 1996, 31, 671–701. [Google Scholar] [PubMed]
- Rigotti, A. Absorption, transport, and tissue delivery of vitamin E. Mol. Aspects. Med. 2007, 28, 423–436. [Google Scholar] [PubMed]
- Brigelius-Flohe, R.; Traber, M.G. Vitamin E: Function and metabolism. FASEB J. 1999, 13, 1145–1155. [Google Scholar] [CrossRef]
- Mocchegiani, E.; Costarelli, L.; Giacconi, R.; Malavolta, M.; Basso, A.; Piacenza, F.; Ostan, R.; Cevenini, E.; Gonos, E.S.; Franceschi, C.; et al. Vitamin E-gene interactions in aging and inflammatory age-related diseases: Implications for treatment. A systematic review. Ageing Res. Rev. 2014, 14, 81–101. [Google Scholar]
- Takahashi, T.; Nakaso, K.; Horikoshi, Y.; Hanaki, T.; Yamakawa, M.; Nakasone, M.; Kitagawa, Y.; Koike, T.; Matsura, T. Rice bran dietary supplementation improves neurological symptoms and loss of purkinje cells in vitamin E-deficient mice. Yonago. Acta. Med. 2016, 59, 188–195. [Google Scholar]
- Wang, L.; Chen, Q.; Zhuang, S.; Wen, Y.; Cheng, W.; Zeng, Z.; Jiang, T.; Tang, C. Effect of Anoectochilus roxburghii flavonoids extract on H2O2—Induced oxidative stress in LO2 cells and D-gal induced aging mice model. J. Ethnopharmacol. 2020, 254, 112670. [Google Scholar]
- Na Takuathung, M.; Klinjan, P.; Sakuludomkan, W.; Dukaew, N.; Inpan, R.; Kongta, R.; Chaiyana, W.; Teekachunhatean, S.; Koonrungsesomboon, N. Efficacy and safety of the genistein nutraceutical product containing vitamin E, vitamin B3, and ceramide on skin health in postmenopausal women: A randomized, double-blind, placebo-controlled clinical trial. J. Clin. Med. 2023, 12, 1326. [Google Scholar]
- Farris, P.; Yatskayer, M.; Chen, N.; Krol, Y.; Oresajo, C. Evaluation of efficacy and tolerance of a nighttime topical antioxidant containing resveratrol, baicalin, and vitamin e for treatment of mild to moderately photodamaged skin. J. Drugs. Dermatol. 2014, 13, 1467–1472. [Google Scholar] [PubMed]
- Fujitaka, Y.; Hamada, H.; Uesugi, D.; Kuboki, A.; Shimoda, K.; Iwaki, T.; Kiriake, Y.; Saikawa, T. Synthesis of daidzein glycosides, α-tocopherol glycosides, hesperetin glycosides by bioconversion and their potential for anti-allergic functional-foods and cosmetics. Molecules 2019, 24, 2975. [Google Scholar] [CrossRef] [PubMed]
- Popa, D.S.; Bigman, G.; Rusu, M.E. The role of vitamin K in humans: Implication in aging and age-associated diseases. Antioxidants 2021, 10, 566. [Google Scholar] [CrossRef] [PubMed]
- Simes, D.C.; Viegas, C.S.B.; Araújo, N.; Marreiros, C. Vitamin K as a powerful micronutrient in aging and age-related diseases: Pros and cons from clinical studies. Int. J. Mol. Sci. 2019, 20, 4150. [Google Scholar] [CrossRef]
- Simes, D.C.; Viegas, C.S.B.; Araújo, N.; Marreiros, C. Vitamin K as a diet supplement with impact in human health: Current evidence in age-related diseases. Nutrients 2020, 12, 138. [Google Scholar] [CrossRef]
- Booth, S.L.; Shea, M.K.; Barger, K.; Leurgans, S.E.; James, B.D.; Holland, T.M.; Agarwal, P.; Fu, X.; Wang, J.; Matuszek, G.; et al. Association of vitamin K with cognitive decline and neuropathology in community-dwelling older persons. Alzheimers Dement. 2022, 8, e12255. [Google Scholar] [CrossRef]
- van Ballegooijen, A.J.; van Putten, S.R.; Visser, M.; Beulens, J.W.; Hoogendijk, E.O. Vitamin K status and physical decline in older adults-the longitudinal aging study amsterdam. Maturitas 2018, 113, 73–79. [Google Scholar] [CrossRef]
- Shea, M.K.; Kritchevsky, S.B.; Loeser, R.F.; Booth, S.L. Vitamin K status and mobility limitation and disability in older adults: The health, aging, and body composition study. J. Gerontol. A. Biol. Sci. Med. Sci. 2020, 75, 792–797. [Google Scholar]
- Doisy, E.A.; Binkley, S.B.; Thayer, S.A.; Mc Kee, R.W. Vitamin K. Science 1940, 91, 58–62. [Google Scholar] [CrossRef]
- Nelsestuen, G.L.; Zytkovicz, T.H.; Howard, J.B. The mode of action of vitamin K. Identification of gamma-carboxyglutamic acid as a component of prothrombin. J. Biol. Chem. 1974, 249, 6347–6350. [Google Scholar] [CrossRef]
- Azuma, K.; Inoue, S. Multiple modes of vitamin K actions in aging-related musculoskeletal disorders. Int. J. Mol. Sci. 2019, 20, 2844. [Google Scholar] [CrossRef]
- Sim, M.; Lewis, J.R.; Prince, R.L.; Levinger, I.; Brennan-Speranza, T.C.; Palmer, C.; Bondonno, C.P.; Bondonno, N.P.; Devine, A.; Ward, N.C.; et al. The effects of vitamin K-rich green leafy vegetables on bone metabolism: A 4-week randomised controlled trial in middle-aged and older individuals. Bone Rep. 2020, 12, 100274. [Google Scholar] [CrossRef]
- Camacho-Barcia, L.; García-Gavilán, J.; Martínez-González, M.Á.; Fernández-Aranda, F.; Galié, S.; Corella, D.; Cuenca-Royo, A.; Romaguera, D.; Vioque, J.; Alonso-Gómez, A.M.; et al. Vitamin K dietary intake is associated with cognitive function in an older adult Mediterranean population. Age Ageing 2022, 51, afab246. [Google Scholar] [CrossRef]
- Shea, M.K.; Wang, J.; Barger, K.; Weiner, D.E.; Booth, S.L.; Seliger, S.L.; Anderson, A.H.; Deo, R.; Feldman, H.I.; Go, A.S.; et al. Cric study investigators. Vitamin K status and cognitive function in adults with chronic kidney disease: The chronic renal insufficiency cohort. Curr. Dev. Nutr. 2022, 6, nzac111. [Google Scholar] [CrossRef]
- Giordano, S.; Darley-Usmar, V.; Zhang, J.H. Autophagy as an essential cellular antioxidant pathway in neurodegenerative disease. Redox. Biol. 2013, 2, 82–90. [Google Scholar] [CrossRef]
- Sykiotis, G.P.; Bohmann, D. Keap1/Nrf2 signaling regulates oxidative stress tolerance and lifespan in Drosophila. Dev. Cell. 2008, 14, 76–85. [Google Scholar] [CrossRef]
- Magesh, S.; Chen, Y.; Hu, L. Small molecule modulators of Keap1-Nrf2-ARE pathway as potential preventive and therapeutic agents. Med. Res. Rev. 2012, 32, 687–726. [Google Scholar] [CrossRef] [PubMed]
- Mu, S.; Yang, W.; Huang, G. Antioxidant activities and mechanisms of polysaccharides. Chem. Biol. Drug Des. 2021, 97, 628–632. [Google Scholar] [CrossRef]
- Hsieh, T.C.; Lu, X.; Wang, Z.; Wu, J.M. Induction of quinone reductase NQO1 by resveratrol in human K562 cells involves the antioxidant response element ARE and is accompanied by nuclear translocation of transcription factor Nrf2. Med. Chem. 2006, 2, 275–285. [Google Scholar] [PubMed]
- Szewczyk, K.; Pietrzak, W.; Klimek, K.; Miazga-Karska, M.; Firlej, A.; Flisiński, M.; Grzywa-Celińska, A. Flavonoid and phenolic acids content and in vitro study of the potential anti-aging properties of Eutrema japonicum (Miq.) koidz cultivated in wasabi farm Poland. Int. J. Mol. Sci. 2021, 22, 6219. [Google Scholar] [CrossRef] [PubMed]
- Mu, X.; Zhang, Y.; Li, J.; Xia, J.; Chen, X.; Jing, P.; Song, X.; Wang, L.; Wang, Y. Angelica Sinensis polysaccharide prevents hematopoietic stem cells senescence in D-galactose-induced aging mouse model. Stem. Cells. Int. 2017, 2017, 3508907. [Google Scholar] [CrossRef]
- Rodier, F.; Campisi, J. Four faces of cellular senescence. J. Cell Biol. 2011, 192, 547–556. [Google Scholar] [CrossRef]
- Ruan, Y.; Dong, C.; Patel, J.; Duan, C.; Wang, X.; Wu, X.; Cao, Y.; Pu, L.; Lu, D.; Shen, T.; et al. SIRT1 suppresses doxorubicin-induced cardiotoxicity by regulating the oxidative stress and p38MAPK pathways. Cell Physiol. Biochem. 2015, 35, 1116–1124. [Google Scholar] [CrossRef]
- Ou, H.L.; Schumacher, B. DNA damage responses and p53 in the aging process. Blood 2018, 131, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Dutto, I.; Tillhon, M.; Cazzalini, O.; Stivala, L.A.; Prosperi, E. Biology of the cell cycle inhibitor p21 (CDKN1A): Molecular mechanisms and relevance in chemical toxicology. Arch. Toxicol. 2015, 89, 155–178. [Google Scholar] [CrossRef]
- Chhabra, G.; Garvey, D.R.; Singh, C.K.; Mintie, C.A.; Ahmad, N. Effects and mechanism of nicotinamide against UVA- and/or UVB-mediated DNA damages in normal melanocytes. Photochem. Photobiol. 2019, 95, 331–337. [Google Scholar] [CrossRef]
- Lebiedzinska, M.; Karkucinska-Wieckowska, A.; Giorgi, C.; Karczmarewicz, E.; Pronicka, E.; Pinton, P.; Duszynski, J.; Pronicki, M.; Wieckowski, M.R. Oxidative stress-dependent p66Shc phosphorylation in skin fibroblasts of children with mitochondrial disorders. Biochim. Biophys. Acta 2010, 1797, 952–960. [Google Scholar] [CrossRef]
- Arany, I.; Hall, S.; Reed, D.K.; Dixit, M. The pro-oxidant gene p66Shc increases nicotine exposure-induced lipotoxic oxidative stress in renal proximal tubule cells. Mol. Med. Rep. 2016, 14, 2771–2777. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Perrini, S.; Tortosa, F.; Natalicchio, A.; Pacelli, C.; Cignarelli, A.; Palmieri, V.O.; Caccioppoli, C.; De Stefano, F.; Porro, S.; Leonardini, A. The p66Shc protein controls redox signaling and oxidation-dependent DNA damage in human liver cells. Am. J. Physiol. Gastrointest. Liver. Physiol. 2015, 309, G826–G840. [Google Scholar] [CrossRef] [PubMed]
- Roitenberg, N.; Bejerano-Sagie, M.; Boocholez, H.; Moll, L.; Marques, F.C.; Golodetzki, L.; Nevo, Y.; Elami, T.; Cohen, E. Modulation of caveolae by insulin/IGF-1 signaling regulates aging of Caenorhabditis elegans. Embo Rep. 2018, 19, e45673. [Google Scholar] [CrossRef]
- Murphy, C.T.; McCarroll, S.A.; Bargmann, C.I.; Fraser, A.; Kamath, R.S.; Ahringer, J.; Li, H.; Kenyon, C. Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature 2003, 424, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Sen, I.; Zhou, X.; Chernobrovkin, A.; Puerta-Cavanzo, N.; Kanno, T.; Salignon, J.; Stoehr, A.; Lin, X.; Baskaner, B.; Brandenburg, S.; et al. DAF-16/FOXO requires protein phosphatase 4 to initiate transcription of stress resistance and longevity promoting genes. Nat. Commun. 2020, 11, 138. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Chen, W.D.; Wang, Y.D. DAF-16/FOXO transcription factor in aging and longevity. Front. Pharmacol. 2017, 8, 548. [Google Scholar] [CrossRef]
- Zhang, Z.; Gao, T.; Yan, N.; Duan, Z.; Tang, Z.; Zhou, L.; Chen, T.; Feng, S.; Ding, C.; Yuan, S.; et al. Characterization and anti-aging activity of polysaccharides from Akebia trifoliata fruit separated by an aqueous two-phase system. Plant. Foods. Hum. Nutr. 2023, 78, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Busti, S.; Coccetti, P.; Alberghina, L.; Vanoni, M. Glucose signaling-mediated coordination of cell growth and cell cycle in Saccharomyces Cerevisiae. Sensors 2010, 10, 6195–6240. [Google Scholar] [CrossRef] [PubMed]
- Nicastro, R.; Tripodi, F.; Gaggini, M.; Castoldi, A.; Reghellin, V.; Nonnis, S.; Tedeschi, G.; Coccetti, P. Snf1 phosphorylates adenylate cyclase and negatively regulates protein kinase a-dependent transcription in Saccharomyces Cerevisiae. J. Biol. Chem. 2015, 290, 24715–24726. [Google Scholar] [CrossRef]
- Kovacs, E.J.; Palmer, J.L.; Fortin, C.F.; Fulop, T.J.; Goldstein, D.R.; Linton, P.J. Aging and innate immunity in the mouse: Impact of intrinsic and extrinsic factors. Trends. Immunol. 2009, 30, 319–324. [Google Scholar] [CrossRef]
- Budamagunta, V.; Manohar-Sindhu, S.; Yang, Y.; He, Y.; Traktuev, D.O.; Foster, T.C.; Zhou, D. Senescence-associated hyperactivation to inflammatory stimuli in vitro. Aging 2021, 13, 19088–19107. [Google Scholar] [CrossRef]
- Zhang, W.; Hwang, J.; Park, H.B.; Lim, S.M.; Go, S.; Kim, J.; Choi, I.; You, S.; Jin, J.O. Human peripheral blood dendritic cell and T cell activation by Codium fragile polysaccharide. Mar. Drugs 2020, 18, 535. [Google Scholar] [CrossRef]
- Higami, Y.; Shimokawa, I. Apoptosis in the aging process. Cell Tissue. Res. 2000, 301, 125–132. [Google Scholar] [CrossRef]
- Kim, S.; Jazwinski, S.M. The gut microbiota and healthy aging: A mini-review. Gerontology 2018, 64, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Ge, X.; Ma, X.; Zheng, M.; Cui, X.; Pan, W.; Zheng, P.; Yang, X.; Hu, M.; Hu, T.; et al. A fiber-deprived diet causes cognitive impairment and hippocampal microglia-mediated synaptic loss through the gut microbiota and metabolites. Microbiome 2021, 9, 223. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, T.S.; Rampelli, S.; Jeffery, I.; Santoro, A.; Neto, M.; Capri, M.; Giampieri, E.; Jennings, A.; Candela, M.; Turroni, S.; et al. Mediterranean diet intervention alters the gut microbiome in older people reducing frailty and improving health status: The NU-AGE 1-year dietary intervention across five European countries. Gut 2020, 69, 1218–1228. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Adil, M.Z.; Xie, X.; Zhao, S.; Zhang, J.; Huang, Z. Therapeutic targeting of mitochondria-proteostasis axis by antioxidant polysaccharides in neurodegeneration. Adv. Protein Chem. Struct. Biol. 2023, 136, 385–413. [Google Scholar]
- Deng, R.; Wang, F.; Wang, L.; Xiong, L.; Shen, X.; Song, H. Advances in plant polysaccharides as antiaging agents: Effects and signaling mechanisms. J. Agric. Food. Chem. 2023, 71, 7175–7191. [Google Scholar] [CrossRef]
- Tan, Y.; Yin, L.; Sun, Z.; Shao, S.; Chen, W.; Man, X.; Du, Y.; Chen, Y. Astragalus polysaccharide exerts anti-Parkinson via activating the PI3K/AKT/mTOR pathway to increase cellular autophagy level in vitro. Int. J. Biol. Macromol. 2020, 153, 349–356. [Google Scholar] [CrossRef]
- Yao, T.; Chen, J.M.; Shen, L.E.; Yu, Y.S.; Tang, Z.H.; Zang, G.Q.; Zhang, Y.; Chen, X.H. Astragalus polysaccharide alleviated hepatocyte senescence via autophagy pathway. Kaohsiung. J. Med. Sci. 2022, 38, 457–468. [Google Scholar] [CrossRef]
- Wang, X.; Pang, L.; Zhang, Y.; Xu, J.; Ding, D.; Yang, T.; Zhao, Q.; Wu, F.; Li, F.; Meng, H.; et al. Lycium barbarum polysaccharide promotes nigrostriatal dopamine function by modulating PTEN/AKT/mTOR pathway in a Methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) murine model of Parkinson’s disease. Neurochem. Res. 2018, 43, 938–947. [Google Scholar] [CrossRef]
- Ren, Z.L.; Wang, C.D.; Wang, T.; Ding, H.; Zhou, M.; Yang, N.; Chan, P. Ganoderma lucidum extract ameliorates MPTP-induced Parkinsonism and protects dopaminergic neurons from oxidative stress via regulating mitochondrial function, autophagy, and apoptosis. Acta Pharmacol. Sin. 2019, 40, 441–450. [Google Scholar] [CrossRef]
- Xiao, X.T.; He, S.Q.; Wu, N.N.; Lin, X.C.; Zhao, J.; Tian, C. Green tea polyphenols prevent early vascular aging induced by high-fat diet via promoting autophagy in young adult rats. Curr. Med. Sci. 2022, 42, 981–990. [Google Scholar] [CrossRef]
- He, C.L.; Tang, Y.; Wu, J.M.; Long, T.; Yu, L.; Teng, J.F.; Qiu, W.Q.; Pan, R.; Yu, C.L.; Qin, D.L.; et al. Chlorogenic acid delays the progression of Parkinson’s disease via autophagy induction in Caenorhabditis elegans. Nutr. Neurosci. 2023, 26, 11–24. [Google Scholar] [CrossRef]
- Lu, R.; He, Z.; Zhang, W.; Wang, Y.; Cheng, P.; Lv, Z.; Yuan, X.; Guo, F.; You, H.; Chen, A.M.; et al. Oroxin B alleviates osteoarthritis through anti-inflammation and inhibition of PI3K/AKT/mTOR signaling pathway and enhancement of autophagy. Front. Endocrinol. 2022, 13, 1060721. [Google Scholar] [CrossRef]
- Hosoda, R.; Nakashima, R.; Yano, M.; Iwahara, N.; Asakura, S.; Nojima, I.; Saga, Y.; Kunimoto, R.; Horio, Y.; Kuno, A. Resveratrol, a SIRT1 activator, attenuates aging-associated alterations in skeletal muscle and heart in mice. J. Pharmacol. Sci. 2023, 152, 112–122. [Google Scholar] [CrossRef]
- Lin, Y.; Kotakeyama, Y.; Li, J.; Pan, Y.; Matsuura, A.; Ohya, Y.; Yoshida, M.; Xiang, L.; Qi, J. Cucurbitacin B exerts antiaging effects in yeast by regulating autophagy and oxidative stress. Oxid. Med. Cell. Longev. 2019, 2019, 4517091. [Google Scholar] [CrossRef] [PubMed]
- Borghesan, M.; Hoogaars, W.M.H.; Varela-Eirin, M.; Talma, N.; Demaria, M.A. Senescence-centric view of aging: Implications for longevity and disease. Trends. Cell Biol. 2020, 30, 777–791. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.S.; Dreesen, O. Biomarkers of cellular senescence and skin aging. Front. Genet. 2018, 9, 247. [Google Scholar] [CrossRef] [PubMed]
- Boyajian, J.L.; Ghebretatios, M.; Schaly, S.; Islam, P.; Prakash, S. Microbiome and human aging: Probiotic and prebiotic potentials in longevity, skin health and cellular senescence. Nutrients 2021, 13, 4550. [Google Scholar] [CrossRef]
- Attaallah, A.; Lenzi, M.; Marchionni, S.; Bincoletto, G.; Cocchi, V.; Croco, E.; Hrelia, P.; Hrelia, S.; Sell, C.; Lorenzini, A. A prolongevity role for cellular senescence. GeroScience 2020, 42, 867–879. [Google Scholar] [CrossRef]
- Coppé, J.P.; Desprez, P.Y.; Krtolica, A.; Campisi, J. The senescence-associated secretory phenotype: The dark side of tumor suppression. Annu. Rev. Pathol. 2010, 5, 99–118. [Google Scholar] [CrossRef]
- Demaria, M.; Desprez, P.Y.; Campisi, J.; Velarde, M.C. Cell autonomous and non-autonomous effects of senescent cells in the skin. J. Investig. Dermatol. 2015, 135, 1722–1726. [Google Scholar] [CrossRef]
- Lee, J.H.; Yun, C.W.; Hur, J.; Lee, S.H. Fucoidan rescues p-cresol-induced cellular senescence in mesenchymal stem cells via FAK-Akt-TWIST axis. Mar. Drugs 2018, 16, 121. [Google Scholar] [CrossRef] [PubMed]
- Jing, P.; Song, X.; Xiong, L.; Wang, B.; Wang, Y.; Wang, L. Angelica sinensis polysaccharides prevents hematopoietic regression in D-Galactose-induced aging model via attenuation of oxidative stress in hematopoietic microenvironment. Mol. Biol. Rep. 2023, 50, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Novais, E.J.; Tran, V.A.; Johnston, S.N.; Darris, K.R.; Roupas, A.J.; Sessions, G.A.; Shapiro, I.M.; Diekman, B.O.; Risbud, M.V. Long-term treatment with senolytic drugs Dasatinib and Quercetin ameliorates age-dependent intervertebral disc degeneration in mice. Nat. Commun. 2021, 12, 5213. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.M.; Cheng, M.Y.; Xun, M.H.; Zhao, Z.W.; Zhang, Y.; Tang, W.; Cheng, J.; Ni, J.; Wang, W. Possible mechanisms of oxidative stress-induced skin cellular senescence, inflammation, and cancer and the therapeutic potential of plant polyphenols. Int. J. Mol. Sci. 2023, 24, 3755. [Google Scholar] [CrossRef] [PubMed]
- Rivas-Chacón, L.D.M.; Yanes-Díaz, J.; de Lucas, B.; Riestra-Ayora, J.I.; Madrid-García, R.; Sanz-Fernández, R.; Sánchez-Rodríguez, C. Cocoa polyphenol extract inhibits cellular senescence via modulation of SIRT1 and SIRT3 in auditory cells. Nutrients 2023, 15, 544. [Google Scholar] [CrossRef]
- Zhang, Y.; Mao, H.; Li, Y.; Xiong, Y.; Liu, X.; Wang, L.; Chen, Z. β-Cryptoxanthin maintains mitochondrial function by promoting NRF2 nuclear translocation to inhibit oxidative stress-induced senescence in HK-2 cells. Int. J. Mol. Sci. 2023, 24, 3851. [Google Scholar] [CrossRef]
- Chen, L.; Holder, R.; Porter, C.; Shah, Z. Vitamin D3 attenuates doxorubicin-induced senescence of human aortic endothelial cells by upregulation of IL-10 via the pAMPKα/Sirt1/Foxo3a signaling pathway. PLoS ONE 2021, 16, e0252816. [Google Scholar] [CrossRef]
- Odama, M.; Maegawa, E.; Suzuki, K.; Fujii, Y.; Maeda, R.; Murakami, S.; Ito, T. Effects of betulinic acid on the proliferation, cellular senescence, and type 1 interferon-related signaling pathways in human dermal fibroblasts. J. Agric. Food. Chem. 2023, 71, 6935–6943. [Google Scholar] [CrossRef]
- De Lange, T. Shelterin: The protein complex that shapes and safeguards human telomeres. Genes. Dev. 2005, 19, 2100–2110. [Google Scholar] [CrossRef]
- Blackburn, E.H. Switching and signaling at the telomere. Cell 2001, 106, 661–673. [Google Scholar] [CrossRef]
- Allsopp, R.C.; Vaziri, H.; Patterson, C.; Goldstein, S.; Younglai, E.V.; Futcher, A.B.; Greider, C.W.; Harley, C.B. Telomere length predicts replicative capacity of human fibroblasts. Proc. Natl. Acad. Sci. USA 1992, 89, 10114–10118. [Google Scholar] [CrossRef] [PubMed]
- de Jesus, B.B.; Schneeberger, K.; Vera, E.; Tejera, A.; Harley, C.B.; Blasco, M.A. The telomerase activator TA-65 elongates short telomeres and increases health span of adult/old mice without increasing cancer incidence. Aging Cell 2011, 10, 604–621. [Google Scholar] [CrossRef] [PubMed]
- Dow, C.T.; Harley, C.B. Evaluation of an oral telomerase activator for early age-related macular degeneration-A pilot study. Clin. Ophthalmol. 2016, 10, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Jäger, K.; Walter, M. Therapeutic targeting of telomerase. Genes 2016, 7, 39. [Google Scholar] [CrossRef]
- Harley, C.B.; Liu, W.; Blasco, M.; Vera, E.; Andrews, W.H.; Briggs, L.A.; Raffaele, J.M. A natural product telomerase activator as part of a health maintenance program. Rejuvenation Res. 2011, 14, 45–56. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y.; Xia, Q.; Xia, Q.; Wang, B.; Yang, C.; Liang, J.; Liu, X. Potential roles of telomeres and telomerase in neurodegenerative diseases. Int. J. Biol. Macromol. 2020, 163, 1060–1078. [Google Scholar] [CrossRef]
- Duman, S.; Ekiz, G.; Yılmaz, S.; Yusufoglu, H.; Ballar Kırmızıbayrak, P.; Bedir, E. Telomerase activators from 20(27)-octanor-cycloastragenol via biotransformation by the fungal endophytes. Bioorg. Chem. 2021, 109, 104708. [Google Scholar] [CrossRef]
- Küçüksolak, M.; Yılmaz, S.; Ballar-Kırmızıbayrak, P.; Bedir, E. Potent telomerase activators from a novel sapogenin via biotransformation utilizing Camarosporium laburnicola, an endophytic fungus. Microb. Cell Fact. 2023, 22, 66. [Google Scholar] [CrossRef]
- Cai, Y.; Zhong, Y.D.; Zhang, H.; Lu, P.L.; Liang, Y.Y.; Hu, B.; Wu, H. Association between dietary vitamin C and telomere length: A cross-sectional study. Front. Nutr. 2023, 10, 1025936. [Google Scholar] [CrossRef]
- Pagano, G.; Pahardo, F.V.; Lyakhovich, A.; Tiano, L.; Fittipaldi, M.R.; Toscanesi, M.; Trifuoggi, M. Aging-related disorders and mitochondrial dysfunction: A critical review for prospect mitoprotective strategies based on mitochondrial nutrient mixtures. Int. J. Mol. Sci. 2020, 21, 16. [Google Scholar] [CrossRef]
- Natarajan, V.; Chawla, R.; Mah, T.; Vivekanandan, R.; Tan, S.Y.; Sato, P.Y.; Mallilankaraman, K. Mitochondrial dysfunction in age-related metabolic disorders. Proteomics 2020, 20, 11. [Google Scholar] [CrossRef] [PubMed]
- Lagouge, M.; Argmann, C.; Gerhart-Hines, Z.; Meziane, H.; Lerin, C.; Daussin, F.; Messadeq, N.; Milne, J.; Lambert, P.; Elliott, P.; et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell 2006, 127, 1109–1122. [Google Scholar] [CrossRef] [PubMed]
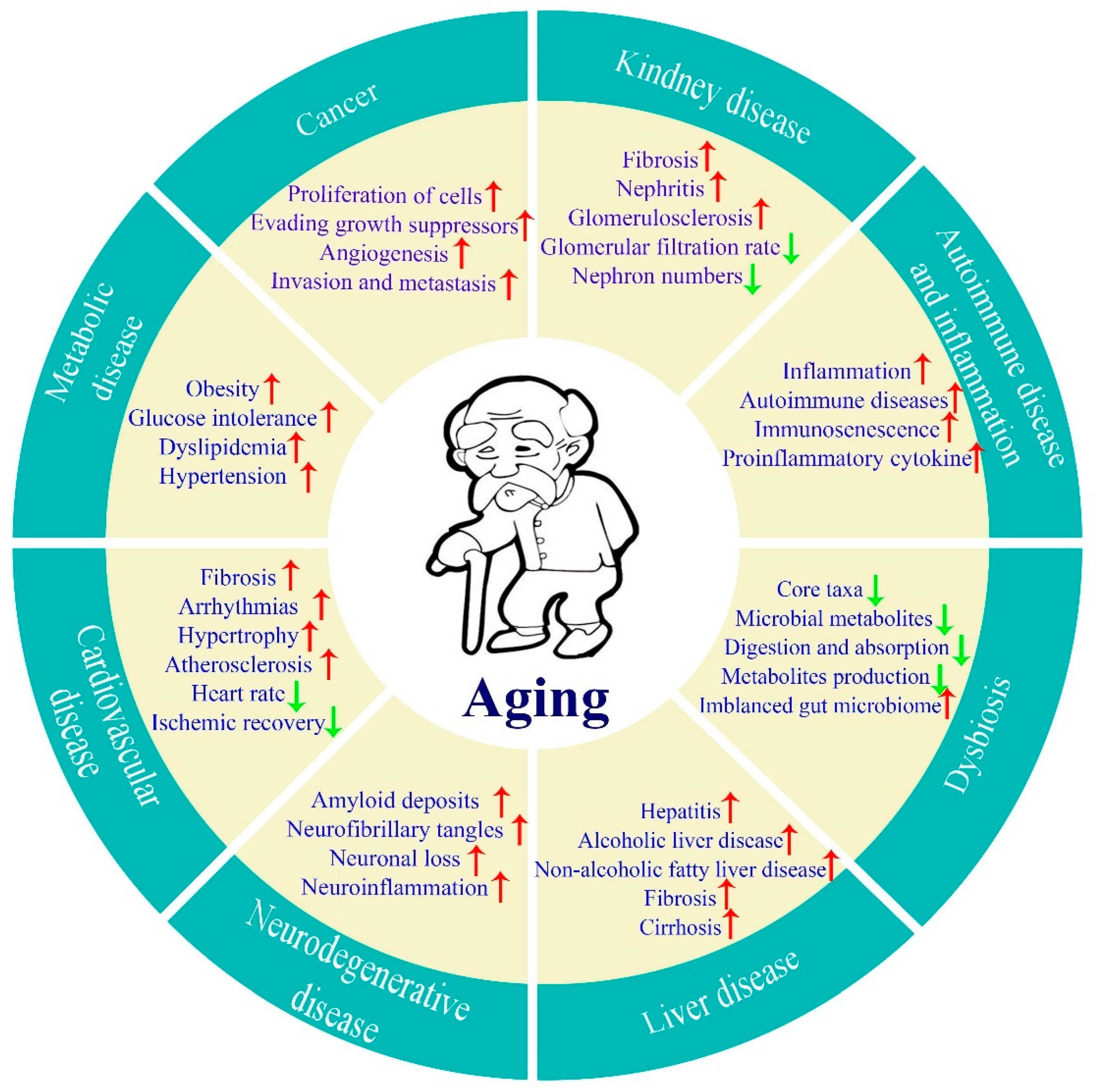
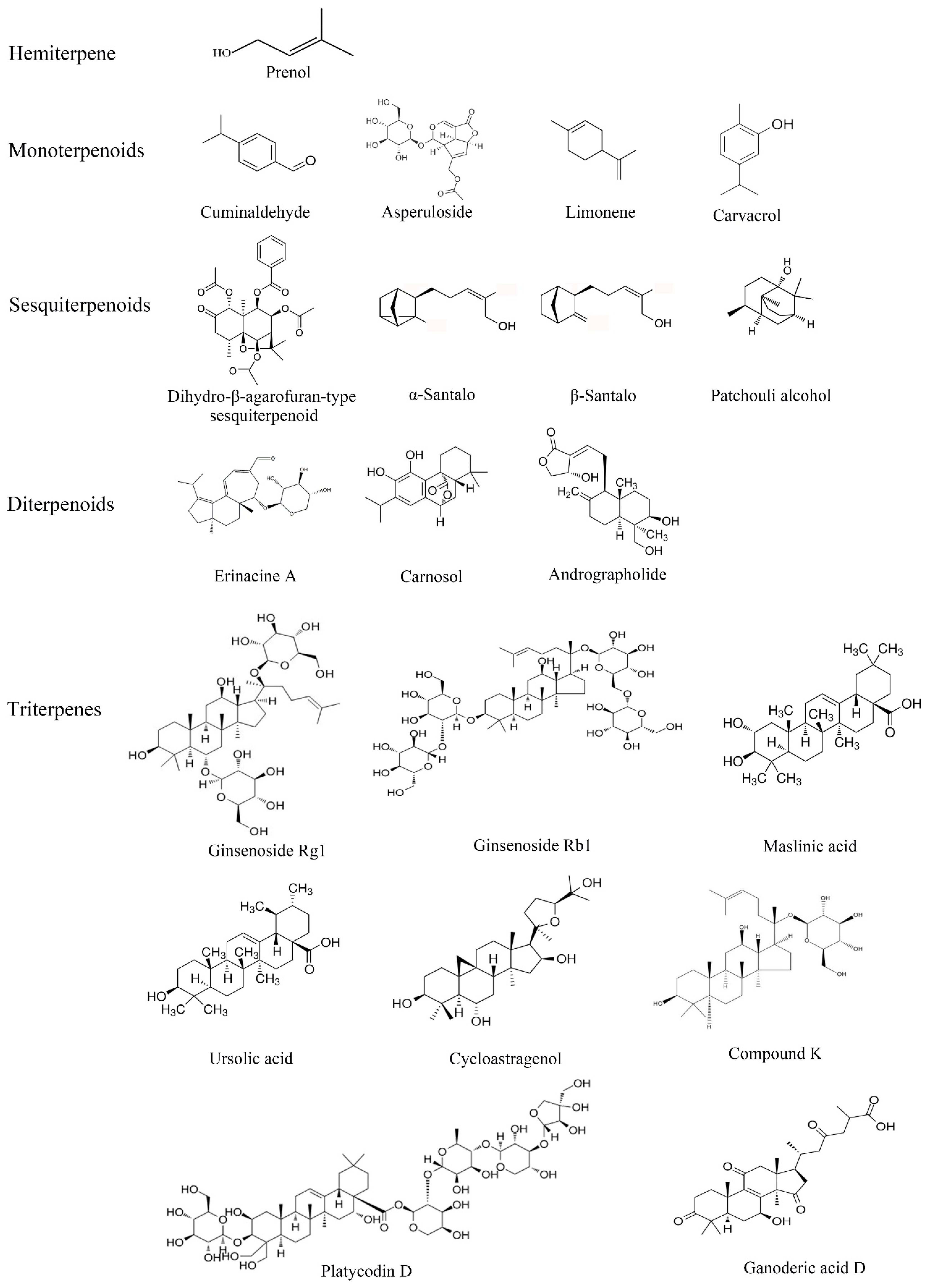
| Subclasses of Polysaccharides | Representative Components | Models | Main Indicators of Aging | Modes of Action | Reference |
|---|---|---|---|---|---|
| Plant polysaccharides | Chinese wolfberry Lycium barbarum polysaccharide (LBP) | D. melanogaster | Lifespan; MDA content; CAT and SOD activity; The relative mRNA expression of aging-related genes (mapk, tor and s6k) and longevity genes (hep, mth and rpn11). | LBP exerts anti-aging activity by regulation of age-related genes and signaling pathway. | [31] |
| LBP | C. elegans | In-vitro study: 2,2-diphenyl-1-(2,4,6-trinitrophenyl) hydrazyl (DPPH), hydroxyl, superoxide anion radical scavenging activity assay. In-vivo study: Lifespan; Oxidative-stress resistance; Heat-stress resistance; The relative mRNA levels of hsp-16.2, sod-3, daf-16 and daf-2. | LBP prolongs the lifespan of C. elegans by upregulating the expression of daf-16, sod-3 and hsp-16.2. | [32] | |
| LBP | Aging mice induced by estrogen deficiency | Learning and memory impairment assay; Levels of myeloid differentiation factor 88, NF-κB, TNF-α, IL-6 and IL-1β associated with TLR4/NF-κB signaling pathway. | LBP alleviates estrogen deficiency-induced cognitive impairments by downregulating the factors associated with TLR4/NF-κB signaling pathway. | [33] | |
| LBP | UVB-irradiation-treated HaCaT cells | Cell viability; ROS level; DNA damage; Levels of p-p38, p38, mmp-9, caspase-3 and Nrf2; The relative mRNA expression of Nrf2 target genes (AKR1C2, APOE, GCLC, GCLM, HBEGF, HO-1 and NQO1); SOD activity. | LBP protects against UVB irradiation-induced photodamage partially through activation of Nrf2/ARE pathway and suppression of UVB-induced p38 MAPK pathway. | [34] | |
| LBP | PM2.5-treated HaCaT cells | Cell viability and apoptosis assay; ROS level; MDA content; SOD activity; Mitochondrial damage; Autophagosome level. | LBP protects skin cells from PM2.5-induced cytotoxicity by regulation of oxidative stress-ER stress-autophagy-apoptosis signaling axis. | [35] | |
| LBP | Zebrafish embryos | SA-β-gal activity assay; Survival rate assay; The relative mRNA expression of p53, p21, Bax, Mdm2 and TERT. | The effects of LBP on cell apoptosis and aging might be mediated by the p53-mediated pathway. | [36] | |
| Ginseng Chuanminshen violaceum polysaccharides (CVPs) | D-Gal-induced-aging mice | In-vitro study: DPPH, hydroxyl, and superoxide anion radicals scavenging activity assay. In-vivo study: Body weight and organ indexes assay; T-SOD, Mn-SOD, Cu/Zn-SOD and CAT activity assay; MDA content; The relative mRNA expression of Cu/Zn-SOD, Mn-SOD, CAT, GPx, thioredoxin 1 (Trx1), and thioredoxin 2 (Trx2). | CVPs show anti-aging effect by increasing antioxidant activity. | [37] | |
| Ginsenoside residue polysaccharides (GRPs) | C. elegans | Lifespan assay; LF and ROS level; SOD activity; Microbiomic and transcriptomic analysis. | GRPs prolong lifespan and alleviate aging of C. elegans without affecting locomotive behaviors by increasing antioxidant activity, altering composition of gut flora and inducing preferential synthesis of beneficial fatty acids. | [38] | |
| Sweet tea Rubus suavissmus polysaccharides (STP-60a) | C. elegans and its mutant strains | Lifespan assay; LF and MDA content; The survival rate under heat stress and oxidative stress assay; Pharyngeal pump rate; The activities of SOD, CAT and GPX; ROS level; The relative mRNA expression of daf-16, skn-1, hsf-1, and their target genes. | STP-60a activates the autophagy system of nematodes through insulin and mitochondrial signaling pathways, enhances the antioxidant capacity, and ultimately promotes the longevity of C. elegans. | [39] | |
| Dangshen Codonopsis pilosula polysaccharides (CPs); Sulfated Codonopsis pilosula polysaccharides (SCPs) | H2O2-treated RAW 264.7 cells; Mice with acute oxidative injury | Cell viability and apoptosis assay; Biochemical index assay; SOD, CAT and GPX activity; MDA and ROS level; The relative mRNA expression of Keap1 and Nrf2. | Anti-aging activities of CP and SCP are mediated by activating Keap1/Nrf2 signaling pathway to enhance activity of antioxidant system. | [40] | |
| Yulangsan Millettia pulchra Kurz polysaccharide (YLSP) | D-Gal-induced-aging mice | General appearance, body weight, and organ index assay; The activities of SOD, CAT, T-AOC and GPX; The level of MDA, IL-2, IL-6 and AGEs; The content of p53 and p21. | YLSP may have a protective effect suppressing the aging process by enhancing antioxidant activity and immunity, as well as modulating aging-related gene expression. | [41] | |
| Akebia trifoliata fruit polysaccharides (ATFPs) | C. elegans | In-vitro study: 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonate) (ABTS) and hydroxyl radical-scavenging assay. In-vivo study: Lifespan; SOD, CAT, GPX activity; MDA and ROS level; LF content; mRNA levels of sir-2.1, daf-16, and daf-12. | ATFPs prolong lifespan of C. elegans through improving activity of antioxidant enzymes, that are regulated by sir-2.1, daf-16, and daf-12 in insulin/IGF signaling pathway. | [42] | |
| Red ginseng by-product polysaccharides (RGBPs) | HaCaT cells; SD rats; Male NC/Nga mice with atopic dermatitis-like symptoms and skin lesions | ABTS radical scavenging activity; Toxicity test; Cell viability; MMP-1, MMP-2 and AP-1 content; Typical Th2 chemokine (MDC/CCL22 and TARC/CCL17) level. | RGBPs alleviate skin aging caused by sUV and atopic dermatitis through increasing antioxidant activity, inhibiting AP-1 transactivation, and reducing MDC/CCL22 and TARC/CCL17 level inducted by TNF-α/IFN-γ. | [43] | |
| Angelica sinensis polysaccharide (ASP); Astragalus membranaceus polysaccharide (AMP) | H2O2-treated human fetal lung fibroblast WI-38 cells | Cell viability assay; SA-β-gal activity assay; Telomerase activity assay; Cell-cycle analysis; The content of p53 and p16INK4. | ASP and AMP exert anti-aging activity through improving telomerase activity and regulating p53-p21 and p16-pRb pathways. | [44] | |
| Algal polysaccharides | Kelp Laminaria japonica polysaccharide (LJP) | C. elegans | In-vitro study: DPPH, ABTS and hydroxyl radical-scavenging assay. In-vivo study: Lifespan assay; MDA, ROS, and LF level; T-SOD activity; The relative mRNA expression of Sod-3, Glp-1, Skn-1, Daf-2, Daf-16 and Age-1; Metabolic profiling. | LJP prolongs lifespan of C. elegans and shows anti-aging activity by resisting oxidative stress and promoting alanine, aspartate, and glutamate metabolism, TCA cycle, butanoate metabolism and FoxO signaling pathway. | [45] |
| Red alga Pyropia haitanensis polysaccharide, porphyrin | AD mice | In-vitro study: DPPH-radical scavenging assay. In-vivo study: Learning and memory assay; Activity of AChE and ChAT. | Porphyrin ameliorates learning and memory impairment of AD mice by increasing cerebral acetylcholine content in cortical and hippocampal tissue. | [46] | |
| Asparagus Gracilaria lemaneiformis polysaccharides (GPs) | C. elegans | Lifespan assay; Reproduction; Pharyngeal pumping; Thermotolerance; Oxidative stress; Polyglutamic acid content; DAF-16 level. | Anti-aging activity of GPs is not dependent upon calorie restriction but on insulin pathway DAF-16. | [47] | |
| Hijiki Sargassum fusiforme polysaccharides (SFPs) | Old male ICR mice | Proteomics study. | SFPs promote longevity of mice, which is intensively related to amino acid metabolism, antioxidation and energy metabolism. | [48] | |
| SFPs | HL-7702 cell line; D-Gal-induced-aging mice; Male chronic aging mice | In-vitro study: DPPH and hydroxyl radical-scavenging assay; Cells viability assay. In-vivo study: Ub-Nrf-2, Nrf-2 and p21 level; The relative mRNA expression of Nrf2, Keap1, Nqo1, Mn-SOD, CuZn-SOD, GCLM, GCLC and GPx1. | SFPs decelerate aging process by enhancing Nrf2-dependent cytoprotection, especially antioxidant defense. | [49] | |
| SFPs | Young, middle-aged and aged male ICR mice | The relative mRNA expression of Nrf2, SOD-2, CAT, NQO1 and HO-1; Small intestine microbiota assay. | SFPs ameliorate declined cytoprotective capacity of small intestine in aging process of mice by upregulating the Nrf2/ARE signaling pathway and partially rejuvenating the overall status of small intestine microbiota. | [50] | |
| Fungal polysaccharides | Bolete Lanmaoa asiatica polysaccharides (FLAPs); Yellow mushroom Hohenbuehelia serotina polysaccharides (FHSPs) | C. elegans | Lifespan assay; ROS, LF and MDA level; SOD and CAT activity; Specific gut bacteria and metabolites assay. | FLAPs and FHSPs prolong lifespan of C. elegans by improving the antioxidant system and enriching specific gut bacteria and metabolites without reproductive toxicity. | [51] |
| Maitake Grifola frondosa β-glucans (GFGs); Monkey head mushroom Hericium erinaceus β-glucans (HEGs) | Yeast cells; D. melanogaster | Lifespan assay; ROS level; The relative mRNA levels of RAS2, HXT7, CTT1, and SOD2; α-Synuclein toxicity and its aggregation. | β-Glucans prolong lifespan through inhibiting the Ras/PKA pathway, reducing the toxicity of α-synuclein, and suppressing its aggregation. | [52] | |
| Grifola frondosa intracellular zinc polysaccharides (IZPs) | D-Gal-induced-aging mice | In-vitro study: Hydroxyl radical, DPPH radical, superoxide radical, hydrogen peroxide-scavenging assay; Antibacterial activity. In-vivo study: T-AOC and SOD activity; MDA content; Histopathology analysis. | IZPs show anti-aging effect by increasing antioxidant and antibacterial activities of mice. | [53] | |
| Golden mushroom Flammulina velutipes sulfated polysaccharides (SFPs) | D-Gal-induced-aging mice | In-vitro study: Hydroxyl radical, DPPH radical, superoxide radical-scavenging effect assay. In-vivo study: Hematological parameters and serum biochemical indexes assay; SOD, GPX, CAT and T-AOC activity; MDA and LPO content; AchE and nitric oxide synthase (NOS) activity; Histopathology analysis. | SFPs show protective abilities against aging by increasing antioxidant activities, improving inflammatory response and ameliorating the anile condition of mice without disturbing general condition and physiology. | [54] | |
| White button mushroom Agaricus bisporus polysaccharides (WMPs) | D-Gal-induced-aging mice | Animal behavioral tests; SOD and GPX activity; MDA content; TNF-α, IL-1β and IL-6 level; Gut microbiota assay; Short-chain fatty acid (SCFA) level. | WMPs improve locomotor activity and the spatial and recognition memory of aging mice by alleviating oxidative stress, decreasing pro-inflammatory cytokine levels, and increasing the SCFA level and abundance of beneficial bacteria. | [55] | |
| Agaricus bisporus acidic-extractable polysaccharides (AcAPs) | D-Gal-induced-aging mice | Serum biochemical indexes assay; SOD, GPX, CAT activity; MDA content; Histopathology analysis. | AcAPs show anti-aging effects by increasing antioxidant activity and alleviating oxidative stress. | [56] | |
| Agaricus bisporus enzyme-assisted polysaccharides (EnAPs) | D-Gal-induced-aging mice | In-vitro study: Reducing power assay; hydroxyl radical and DPPH radical-scavenging effect assay; Fe2+-chelating rates. In-vivo study: SOD, GPX, CAT activity; MDA content; Histopathology analysis. | EnAPs show anti-aging effects by increasing antioxidant activity, improving organ function and remitting lipid metabolism. | [57] | |
| β-1,3-Glucans | Aged N. guentheri | Lifespan assay; LF content; SA-β-Gal activity; ROS, protein oxidation and lipid peroxidation level; CAT, SOD and GPX activity; The level of p66Shc. | β-1,3-Glucans show anti-aging effects by increasing antioxidant activity. | [58] | |
| β-1,3-Glucans | Young, middle-aged and aged mice | DTH response assay; Hematologic index and biochemical parameter assay; The levels of KLH-specific IgG, IgG1 and IgG2a; Cellular composition of splenocytes and proliferation of splenocytes assay. | β-1,3-Glucans exert anti-aging effects by enhancing the adaptive immune responses of aged mice without disturbing their general condition and physiology. | [59] | |
| Tremella fuciformis polysaccharides (TFPs) | H2O2-treated human dermal fibroblasts-neonatal (HDF-n) | Cell viability and apoptosis assay; ROS level; Levels of p16, p21, p53 and SIRT-1. | TFPs attenuate H2O2-induced cell oxidative stress and apoptosis through the upregulation of the SIRT1 pathway. | [60] |
| Subclasses of Polyphenols | Representative Components | Models | Main Indicators of Aging | Modes of Action | Reference |
|---|---|---|---|---|---|
| Phenolic acids | Chlorogenic acid (CGA) extracted from coffee and tea | C. elegans | Lifespan assay; Thermotolerance and stress resistance assay; The relative mRNA expression of FoxO transcription factors daf-16, hsf-1, skn-1 and hif-1. | CGA extends lifespan of C. elegans via DAF-16 in insulin/IGF-1 signaling pathway. | [74] |
| p-Coumaric acid | C. elegans | Lifespan assay; Stress resistance assay; ROS level; The relative mRNA expression of daf-16, skn-1, sod-3, osr-1, osm-7, osm-11 and ama-1. | p-Coumaric acid increases oxidative resistance of C. elegans by regulating skn-1, an ortholog of Nrf2. | [75] | |
| Caffeic acid (CA); Dihydrocaffeic acid (DHCA) | C. elegans | Lifespan and stress assay; The relative mRNA expression of the genes daf-16, daf-18, skn-1, ctl-1, hsp-16.2, hsf-1, sod-3, and sir-2.1. | CA and DHCA promote longevity and increase stress resistance in C. elegans by activating the DAF-16/FoxO transcription factor and modulating the expression of stress-related genes. | [76] | |
| Flavonoids | Quercetin | C. elegans | Lifespan assay; Evaluation of motility; Stress resistance assay; ROS level; The relative mRNA expression of daf-2, age-1, daf-16, nsy-1, sek-1, pmk-1, skn-1 and hsf-1. | Quercetin induces heat-stress tolerance in C. elegans by modulating HSF-1 expression and/or activity. | [77] |
| Quercetin | H2O2-induced senescence vascular smooth muscle cell (VSMC) | Cell viability assay; SA-β-gal activity; Apoptosis assay; The content of Bax and Bcl-2. | Quercetin alleviates H2O2-induced VSMC senescence by regulating apoptosis through the P53-P21 and P16 pathways. | [78] | |
| Quercetin; Rutin | AD model mice | GSH/GSSG ratio; MDA content; CAT, SOD and GPX activity; BACE1 activity; The relative mRNA expression of cat, sod, gpx, APP, BACE1, ADAM10, caspase-3, caspase-6, IL-1β, TNF-α and IFN-γ. | Quercetin and rutin both alleviate the aging phenotype of AD mice by augmenting intracellular redox homeostasis in the brain. | [79] | |
| Anthocyanins from purple wheat | C. elegans and its mutant strains | In-vitro study: DPPH free radical scavenging assay. In-vivo study: Lifespan assay; The relative mRNA expression of hsp-16.2; ROS level; DAF-16 location. | Anthocyanins extend the lifespan of C. elegans mainly by regulating the DAF-16/FoxO signaling pathway. | [80] | |
| Anthocyanins from honeysuckle Lonicera pallasii | D. melanogaster | Lifespan assay; Stress resistance, locomotor activity and intestinal integrity assay; The relative mRNA expression of eEF1α2, RpL32, Hsp27, Hsp68, Hsp83, Keap1, NRF, Sod1, HIF1, Clk and Per; Radical scavenging and antioxidant activity assay. | Honeysuckle anthocyanins prolong lifespan and improve the health span of D. melanogaster through enhancing antioxidant activities. | [81] | |
| Tart cherry extract (TCE) | C. elegans and its mutant strains | Lifespan assay; The relative mRNA expression of daf-16, daf-2, daf-18, aak-2, akt-1, lin-14, skn-1, ucp-4, sod-2/3; Mitochondrial respiration analysis. | TCE confers health span benefits to C. elegans through enhanced mitochondrial function and reduced oxidative stress, mainly via the DAF-16 pathway. | [82] | |
| Stilbenes | Resveratrol | D. melanogaster | Longevity and fecundity assay; Locomotor activity; SOD and CAT activity assay. | Resveratrol prolongs the lifespan of both male and female adult fruit flies by scavenging ROS and neuroprotection through increasing antioxidant enzyme activity. | [83] |
| Resveratrol | Aged N. guentheri | Lifespan assay; Cognitive ability and locomotor activity assay; Neurofibrillary degeneration assay; SA-β-gal activity; LF content. | Resveratrol prolongs the lifespan of aged fish by retarding neurodegeneration and aging-related histological markers. | [84] | |
| Resveratrol | Aged N. guentheri | SA-β-gal activity; LF content; Histological evaluation of ovarian development; PCNA, SIRT1 and GRP78 content; The relative mRNA expression of NRF2, NF-κB, IL-1β, TNF-α, IL-8, GRP78 and CHOP. | Resveratrol activates SIRT1/NRF2 to reduce inflammation and ER stress so as to delay ovarian aging in N. guentheri. | [85] | |
| Resveratrol | HtrA2 knockout mice | Motor phenotype assay; Survival assay; The relative mRNA expression of chop, atf4, p53, noxa, bcl2, bax, dr5; NeuN staining assay. | Resveratrol alleviates neural aging by attenuating apoptosis at the level of Bax. | [86] | |
| Resveratrol | 18-month-old SD rats anaesthetized by sevoflurane and nitrous oxide | Learning and memory assay; The content of SIRT1, Poly ADP-ribose polymerases-1 (PARP-1), cleaved caspase-3 and Bax. | Resveratrol improves learning and memory ability and inhibits neuronal apoptosis by increasing the expression of SIRT1 in aged rats after general anesthesia. | [87] | |
| Resveratrol | 6-, 9-, 12-month-old N. guentheri | SA-β-gal activity assay; The relative mRNA level of IL-8, IL-10, SIRT1, NF-κB; The content of TNFα, SIRT1, RelA/p65, p-IκBα, IκBα and LGR5. | Resveratrol delays the aging of the annual fish N. guentheri by inhibiting SASP through the SIRT1/NF-κB signaling pathway. | [88] | |
| Resveratrol | Young, middle-aged and aged rats | DTH response assay; Hematologic index and biochemical parameter assay; The levels of KLH-specific IgG, IgG1 and IgG2a; Cellular composition of splenocytes and proliferation of splenocytes assay. | Resveratrol exerts an anti-aging effect by enhancing the adaptive immune responses of aged rats without disturbing their general condition and physiology. | [89] | |
| Resveratrol | Senescence-accelerated mice (SAM) | The relative mRNA expression of TNF-α, IL-1β, IL-10, Bcl-2-associated death promoter (BAD), Bcl-2-associated X protein (BAX), B-cell lymphoma 2 (Bcl-2), X-linked inhibitor of apoptosis protein (XIAP), SIRT1, FoxO1 and FoxO3A; The content of TNF-α, IL-1β, IL-10, NF-κB p65, NF-κB p50–105, NF-κB p52–100, inhibitor of NF-κB (IκB)-β and -α, BAD, BAX, and Bcl-2; The activity of GPX, glutathione reductase (GR), and glutathione transferase (GST); Plasma glucose and insulin assay. | Resveratrol improves the pancreas aging of SAM by modulating the inflammatory, oxidative and apoptotic status related to aging. | [90] | |
| Lignans | Sesamin from sesame seed | β-Amyloid-induced-aging C. elegans model | Aβ-induced paralysis assay; The relative mRNA expression of Aβ transgene; β-Amyloid content; Chemotaxis behavior assay; Lifespan assay. | Sesamin prolongs the lifespan of an β-amyloid-induced-aging C. elegans model by reducing Aβ toxicity. | [91] |
| Sesamin | C. elegans Bristol strain N2 and its mutant strains | Lifespan assay; Bacterial infection assay; Locomotory scoring assay; Stress resistance assay; LF content; Protein oxidant assay; Brood size assay. | Sesamin enhances the host defense of C. elegans and increases average lifespan via the activation of both skn-1 (encoding a component of the p38 MAPK pathway) and daf-16 (encoding a component of the IGF-1 pathway). | [92] | |
| Tannins | Tannic acid (TA) | C. elegans strain N2 and its mutant strains | Lifespan assay; Stress resistance; Nematode length and reproduction assay; Pharynx pumping rate. | TA influences the aging process by regulating sek-1. | [93] |
| ellagic acid (EA) | C. elegans strain N2 and its mutant strains | Lifespan assay; Stress resistance; Antimicrobial capacity assay; Length and reproduction assay; Pharynx pumping rate; Triglyceride assay. | EA influences the aging process by regulating antimicrobial effects and hormetic action. | [94] | |
| Oenothein B (OEB) | Wild-type C. elegans strain and its mutant strains | Lifespan assay; Reproduction assay; Pharynx pumping rate; Locomotion and thermotolerance assay; ROS level; SOD content; Age pigment content. | OEB might modulate multiple genetic pathways involved in insulin/IGF-1 signaling (IIS) via age-1 and daf-16, the dietary restriction (DR) pathway via eat-2 and sir-2.1, and the mitochondrial electron transport chain via isp-1 to promote healthy lifespan. | [95] | |
| Pentagalloyl glucose (PGG) | Wild-type C. elegans strain and its mutant strains | Lifespan assay; Reproduction assay; Pharynx pumping rate; Locomotion and thermotolerance assay; ROS level; SOD content; Age pigment content. | PGG and its metabolites promote healthy lifespan by regulating the IIS and DR pathway and the mitochondrial electron transport chain. | [96] |
| Subclasses of Carotenoids | Representative Components | Models | Main Indicators of Aging | Modes of Action | Reference |
|---|---|---|---|---|---|
| Carotenes | β-Carotene | Aged MSCs induced by H2O2; Aged mice | In-vitro study: Content of p16 and p21; DNA damage and cell proliferation assay; Levels of IL-1 β, IL-6 and TNF-α; Levels of ROS and MDA; SOD activity; Level of KAT7 and P15. In-vivo study: Psychology and physiology behaviors of aged mice; Inflammation and tissue fibrosis levels. | β-Carotene inhibits aging by regulating the KAT7-P15 signaling axis. | [112] |
| Lycopene | 10-month-old adult mice | Hematology and clinical biochemistry indexes assay; MDA content; CAT, SOD and GPX activity. | Lycopene alleviates aging by increasing antioxidant enzyme activity. | [113] | |
| Lycopene | D-Gal-induced and naturally aging hens (Gallus domesticus) | Morphological and ultrastructure assay; Somatic cell proliferation assay; Levels of Bax, Bcl-xL, PCNA, CDK2 and CCND1; GSH, ROS and MDA contents; T-SOD, CAT, GPX and T-AOC activity; Levels of Nrf2, p-Nrf2, HO-1 and NQO1. | Lycopene ameliorates the aging of hen ovaries by inhibiting oxidant stress and apoptosis via the activation of the Nrf2/HO-1 pathway. | [114] | |
| trans-Lycopene from tomato juice | 28 patients (mean age 69.7 ± 3.1 years; mean BMI 31.5 ± 3.6 kg/m2) at high cardiovascular risk | Plasmatic carotenoids, ICAM-1, VCAM-1, and C-reactive protein (CRP) levels assay. | Trans-lycopene may attenuate the risk of cardiovascular disease by reducing the concentration of important inflammatory molecules related to atherosclerosis. | [115] | |
| Lycopene | Aβ1–42-induced AD rats | Behavioral parameters assay; Serum levels of TNF-α, IL-1β and IL-6β; The mRNA expressions of TLR4, p65; The content of TLR4, NF-κB-p65, β-APP, PS-1 | Lycopene significantly improves cognitive deficits by blocking the activation of NF-κB p65 and TLR4 expressions and the production of cytokines, thereby endorsing its usefulness for diminishing β-amyloid deposition in the hippocampus tissues. | [116] | |
| Crocin | Middle-aged (15 months old) rats | Serum parameters assay; SOD, CAT, GPX, GR, GST, Na+/K+ ATPase, Ca2+ ATPase, AChE, CS and CCOX activity; GSH, LPO, PCC, ROS, NO, LF and acetylcholine levels; Histopathology assay. | Oral supplementation of crocin reverses the aging of rats through the suppression of oxidative stress and neuroinflammatory responses. | [117] | |
| Xanthophylls | Astaxanthin (ATX) | D-Gal-induced-aging rats | Activities of SOD, CAT and GPX; MDA content; Serum levels of IL-2, IL-1β, IL-6, IgM and IgG; Nrf2, Keap1, NF-κB (p65) and IκBα level. | The anti-aging effect of astaxanthin is in part due to Nrf2/Keap1 and NF-κB pathways, which regulate oxidative stress and immune impairment, respectively. | [118] |
| ATX | Young and aged male mice | Level of MDA, NO, APOPs and GSH; CAT and SOD activity; | ATX alleviates brain aging by suppressing oxidative stress. | [119] | |
| ATX | Wild-type yeast Saccharomyces cerevisiae and antioxidant-deficient strains | Survivability assay; Antioxidant biomarkers assay; Apoptotic markers assay. | ATX enhances the longevity of yeast S. cerevisiae by reducing oxidative stress and apoptosis. | [120] | |
| ATX | Glutamate-treated mouse hippocampal HT22 cells | Cell viability assay; LDH activity assay; ROS content; Caspase activity and PARP cleavage assay; The relative mRNA expression of HO-1; The content of HO-1, Nrf2, p-Akt, Akt, GSK3b, p-GSK-3b (Ser9), Bcl-2, Bax, AIF, cytochrome-c (Cyto-c), PARP. | ATX alleviates glutamate excitotoxicity-related neuronal loss associated with Alzheimer’s disease by regulating oxidative stress and apoptosis through the Akt/glycogen synthase kinase-3b (GSK-3b) signaling pathway. | [121] | |
| Krill oil (rich in ATX) | Aged N. guentheri | Lifespan assay; Histological assay; LF content; SA-β-Gal activity; ROS, protein oxidation, lipid peroxidation and p66Shc level; CAT, SOD and GPX activity; The relative mRNA expression of interferon-γ, tnf-α and il10; The content of IκBα, p-IκBα, p65 and p-p65. | KO exerts its anti-aging and rejuvenation effects via the enhancement of the antioxidant system and suppression of the NF-κB signaling pathway. | [122] | |
| Canthaxanthin | Aging model liver cell (AML12) exposed to H2O2; Liver fibrosis mice model induced by CCL4 | In-vitro study: SA-β-Gal activity; P16, P21, γ-H2A, 53BP1, HP1α and H3K9me3 levels; The relative mRNA expression of IL-6, IL-1β, TNF-α, CXCL1 and MMP-1; Level of SIRT6, NF-κB, p-NF-κB, JAK2, p-JAK2, STAT1 and p-STAT1; Levels of ROS, MDA, SOD and GSH. In-vivo study: Histological analysis; Serum levels of ALT and AST; Levels of TNF-α, IL6, IL-1β, COL1a1 and α-SMA; Phosphorylation level of Smad2/3 protein. | Canthaxanthin alleviates liver aging and fibrosis by suppressing inflammation and oxidative stress, which it achieves by regulating SIRT6. | [123] | |
| Lutein | Fibroblasts exposed to UVA or UVB | Cell viability assay; Membrane integrity; MMP-1, MMP-2, TIMP-1, TIMP-2 and elastin protein levels; MMP-1 promoter activity. | Lutein alleviates fibroblast aging induced by UVA or UVB radiation through the inhibition of the MMP to TIMP ratio, cell loss, membrane damage and elastin expression. | [124] | |
| Lutein | Human RPE cell line ARPE-19 treated with H2O2 | Cell viability assay; Lysosome and ROS content; SA-β-Gal activity; Cell cycle; The content of p-SIRT1, SIRT1, SIRT3, p-p53, p53, p21, HO-1, NQO1 and Nrf2. | Lutein protects cells from cellular senescence induced by oxidative stress by upregulating antioxidant effectors. | [125] |
| Subclasses of Sterols | Representative Components | Models | Main Indicators of Aging | Modes of Action | Reference |
|---|---|---|---|---|---|
| Phytosterols | Diosgenin (DG) | Aged N. guentheri | Lifespan; LF content; SA-β-Gal activity; ROS, protein oxidation and lipid peroxidation level; CAT, SOD and GPX activity; 20S proteasome activity; PI3K/AKT/mTOR activity; The relative mRNA expression of pten, tsc1 and tsc2. | DG exerts its rejuvenation and anti-aging activity by inhibiting the PI3K/AKT/mTOR signal pathway and promoting the ubiquitin–proteasome pathway and antioxidant enzyme activity; they all play prominent roles in ROS production. | [138] |
| β-Sitosterol | H2O2-treated HDF and HaCaT cells | Cytotoxicity of β-sitosterol; Hyaluronic acid (HA) content; Levels of hyaluronic acid synthases (HAS1, -2, -3) and hyaluronidases (HYAL1, -2, -3); Levels of aquaporin3 (AQP3), loricrin (LOR), filaggrin (FLG) and involucrin (IVL). | β-Sitosterol prevents skin aging by promoting the biosynthesis of HA and enhancing skin barrier function. | [139] | |
| Daucosterol palmitate (DSP) extracted from Alpinia oxyphylla Miq | Male AD rat model | Spatial learning and memory test; ROS level; Histological examination; Synaptophysin level. | DSP ameliorates Aβ-induced learning and memory impairment in rats by inhibiting ROS production, preventing hippocampal CA1 neuronal damage and restoring hippocampal synaptophysin. | [140] | |
| β-Sitosterol | Dexamethasone-induced muscle atrophy mice model and C2C12 myoblasts | Mice grip strength and treadmill analysis; Weight of muscles; Muscle fibers; Muscle tissue histological analysis; MAFbx, MuRF1, FoxO1 and FoxO3 level. | β-Sitosterol alleviates aging sarcopenia by downregulating transcriptional factor FoxO1, making FoxO1 unable to affect the expression of muscle atrophy F-box (MAFbx), ultimately inhibiting muscle atrophy. | [141] | |
| β-Sitosterol | TNF-α-treated GT1-7 cells (a cell line of GnRH neurons) | Membrane sterols test; Gonadotropin-releasing hormone (GnRH) release assay; Levels of NF-κB and p-IκBa. | β-Sitosterol prevents TNF-α-induced GnRH decline through the inhibition of NF-κB activation via the ER-mediated inhibition of IκB processing. | [142] | |
| Animal sterols | Vitamin D3 | D-Gal-induced-aging male rats | MDA content; SOD, GSH and CAT activity; GCNA and PCNA content; Anti-apoptotic (BCL2) and apoptotic (BAX and active caspase-3) assay; TUNEL immunohistochemical assay; Heat shock protein1 a1 (HSP1A1), AGE-receptor (AGER), vitamin D and advanced glycation end products (AGE) level. | Vitamin D3 improves age-associated spermatogenesis impairment by regulating apoptosis and the antioxidant system, which are involved in the AGER and HSP1A1 pathway. | [143] |
| Vitamin D | Human lung carcinoma A549 cells, human B-cell lymphoblastoid TK6 cells, and human peripheral blood lymphocytes treated with H2O2 | Detection of histone H2AX phosphorylation and ATM activation; ROS level; Analysis of cellular fluorescence. | Vitamin D exerts an anti-aging effect by attenuating DNA damage. | [144] | |
| Mussel (Mytilidae) sterols | Wild-type and mutant-type Saccharomyces cerevisiae | Lifespan assay; ROS level; MDA content; The relative mRNA expression of uth1, skn7, tub1. | The anti-aging effect of mussel sterols depends on their antioxidative ability and regulation of uth1, skn7, tub1 expression. | [145] | |
| Fungal sterols | Ergosterols from Ganoderma lucidum spores | Wild-type and mutant-type Saccharomyces cerevisiae | Replicative lifespan; The relative mRNA expression of sod1 and sod2. | Ganoderma lucidum ergosterols prolong the replicative lifespan of yeast by regulating uth1 expression. | [146] |
| Chinese cordyceps cerevisterol | H9C2 cells; ICR mice | In-vitro study: Cell survival rate assay; Level of MAPK1, MAPK3, VEGFA, AKT1, PIK3CA and RAC1. In-vivo study: Angiogenic test. | Chinese cordyceps cerevisterol exhibits anti-aging and anti-fatigue effects by improving the body’s hypoxia tolerance through the VEGF signal pathway. | [147] |
| Subclasses of Terpenoids | Representative Components | Models | Main indicators of Aging | Modes of Action | Reference |
|---|---|---|---|---|---|
| Hemiterpene | Prenol | Wild-type and mutant-type C. elegans | Toxicity assessment; Lifespan assay; Amyloid-β-mediated paralysis, stress resistance and chemotaxis assays; Level of α-synuclein and ROS; The relative mRNA expression of sod-1, sod-2, sod-3, gst-3, gst-4, hsp 16.2, hsp-70, daf-16, skn-1 and hsf-1. | Prenol improves the lifespan and health span of worms by regulating transcription factors DAF-16, HSF-1 and SKN-1. | [170] |
| Monoterpenoids | Cuminaldehyde extracted from cumin | SH-SY5Y cells; C57BL/6J male mice | In-vitro study: Cell survival assay. In-vivo study: Spatial learning and memory assay; The relative mRNA expression of Bdnf, Icam, ApoE, TNF-α and IL-6. | Cuminaldehyde exerts neuroprotective effects through the modulation of genes coding for neurotrophic factors and inflammatory factors. | [171] |
| Asperuloside extracted from Du Zhong Eucommia ulmoides male flower | C. elegans | Motor competency assay; Mitochondrial respiratory capacity; ATP content; ROS level; DAF-16 level; Metabolomics detection; RNAi interference assay for daf-16. | Asperuloside delays the muscle aging of C. elegans through a daf-16-mediated improvement in mitochondrial dysfunction. | [172] | |
| Limonene | HaCaT cells treated with UVB | Cell viability assay; ROS level; HO-1, NQO-1 and γ-GCLC content; Nrf2 knockdown assay; Levels of α-MSH, p53, claudin, occuludin, ZO-1 and MMP-2. | Limonene displays a dermato-protective effect in skin cells by activating the Nrf2-dependent cellular antioxidant defense system. | [173] | |
| Carvacrol (CAR) | D-Gal-induced-aging rats | TAC and GPX activity; GST level; MDA content; The relative mRNA expression of p53, p21 and Bax. | CAR attenuates aging by suppressing oxidative stress. | [174] | |
| Sesquiterpenoids | Dihydro-β-agarofuran-type sesquiterpenoid | C. elegans | Lifespan assay; The relative mRNA expression of skn-1, hsf-1 and daf-16. | Dihydro-β-agarofuran-type sesquiterpenoids prolong the lifespan of worms by regulating transcription factors skn-1 and hsf-1. | [175] |
| α- and β-Santalol | Wild-type and mutant-type C. elegans | Lifespan assay; ROS level; Stress resistance assay; Toxic amyloid-β and polyglutamine repeat (Q35, Q40, and HtnQ150) aggregation assay; Neuronal survival assay; LF accumulation; RNAi interference assay for skn-1 and let-23; The relative mRNA expression of gst-4, gcs-1, gst-10, gsr-1, hsp-4 and skr-5. | Both α- and β-Santalol alleviate aging by selectively regulating SKN-1/Nrf2 and EOR-1/PLZF transcription factors through the RTK/Ras/MAPK-dependent signaling axis. | [176] | |
| Patchouli alcohol (PA) extracted from patchouli Pogostemon cablin | D-Gal-induced-aging mice; Chondrocytes isolated from D-Gal induced-aging mice | In-vitro study: Cell survival assay; The relative mRNA expression of col2a1, mmp13, Tp53, CDKN1A, cat, gss, sod, Nrf2, HO-1, Keap1 and NQO1; Content of Acan, Col2a1, Adamts5, Mmp13, TP53 and P21. In-vivo study: Mental state, body weight and organ index assay; Histopathological assay; Cartilage quality assay; The relative mRNA expression of Tp53, Cdkn1a/p21Cip1/Waf1, Cdkn2a/p16INK4a, Nrf2, HO-1, cat and sod; CAT and SOD activity; GSH and MDA level. | PA inhibits D-Gal-induced chondrocyte senescence via the activation of the antioxidative system, which is attributable to the activation of the Nrf2/HO-1 pathway. | [177] | |
| Diterpenoids | Mushroom Hericium erinaceus mycelium (HEM) and its diterpenoid derivative, erinacine A (EA) | 15-month-old mice | Spatial learning ability assay; The mRNA expression of TNFα, IL-1β, NGF and NeuN; Body weight and fat pad weight assay; Serum chemistry analysis. | HEM and EA minimize the progression of aging and obesity-induced neurodegeneration by reducing metabolic abnormalities and neuroinflammatory cytokines and increasing neurogenesis factors. | [178] |
| Carnosol extracted from Rosmarinus | Wild-type and mutant-type C. elegans | Lifespan assay; ROS level; CAT, SOD and GPX activity; MDA content; Stress resistance, mobility, fertility and paralysis assay; Age pigment and body fat accumulation; The relative mRNA expression of sod-3, sod-5, hsf-1, hsp-16.1, hsp-16.2, daf-16 and hsf-1; Subcellular localization of DAF-16. | Carnosol-mediated longevity requires the upregulated expression of sod-3, sod-5, hsf-1, hsp-16.1 and hsp-16.2 and is dependent on hsf-1 gene. | [179] | |
| Andrographolide (ANDRO) extracted from Chuan Xin Lian Andrographis paniculata | Adult and aged Octodon degus | Recognition memory, preference for novel experiences, social recognition and long-term memory assay; Level of glutamate ionotropic receptor AMPA type subunit 1 (GLUR-1), glycogen synthase kinase-3β (GSK-3β), phosphorylated glycogen synthase kinase-3β (p-GSK-3β), Homer, N-methyl D-aspartate receptor subtype 2B (NR2B), post-synaptic density 95 (PSD95), synaptotagmin I/II (SYT), synaptophysin (SYP) and gamma-aminobutyric acid receptor (GABAA). | ANDRO administration shows improved complex behaviors related to age-detrimental effects by modulating mechanisms of synaptic transmission and proteins. | [180] | |
| Triterpenes | Compound K (CK) extracted from herb Panax ginseng | Hydrobromide-induced memory impairment mice | Production and clearance of Aβ; SOD, MDA, and GSH levels; Bcl-2, Bax and Caspase-3 levels; The relative mRNA expression of Nrf2 signaling pathway-related factors Nrf2, Keap1 and HO-1. | CK improves impaired memory function by inhibiting apoptosis and enhancing stress resistance through the Nrf2/Keap1 signaling pathway. | [181] |
| CK | Vascular-dementia (VD) rats | Cognitive ability assay; Histopathological alterations assay; The deposition of Aβ1–42; The contents of Ser473-Akt/Akt, pSer9-GSK3β/GSK3β and insulin degrading enzyme (IDE). | CK might attenuate cognitive deficits and Aβ1–42 deposition in the hippocampus by enhancing the expression of pSer9-GSK-3β and IDE. | [182] | |
| CK | Mouse embryonic fibroblast NIH3T3 cells treated with UVB | Cell viability assay; The mRNA expression levels of MMP1, COX-2, filaggrin (FLG), transglutaminase (TGM), hyaluronic acid synthases (HAS)-1, 2, and 3, type I collagen; Type I collagen promoter activity assay; Melanin formation and secretion assay; Tyrosinase activity assay; The content of κBα/p-κBα, and JNK/p-JNK, ERK/p-ERK, and p38/p-p38. | CK has ability to increase skin moisture levels and melanin synthesis as well as protect against UVB-induced photo-aging by regulating the p38/AP-1/CREB pathway. | [183] | |
| Ginsenoside Rg1 (Rg1) | Sca-1+ HSC/HPC cells harvested from D-Gal-induced-aging rats | SA-β-Gal activity; Cell-cycle assay; CFU-mix assay; The mRNA expression of cleaved caspase 3, Bcl-2, Bax, SIRT3 and SOD2; The content of SIRT3 and SOD2. | Rg1 conducts functions of anti-aging in Sca-1+ HSC/HPC cells in D-gal-induced-aging rats by inhibiting mitochondrial pathway-mediated apoptosis and activating the SIRT3/SOD2 signaling pathway. | [184] | |
| Ginsenoside Rb1 (GRb1) | Young and natural aging C57BL/6J mice | Morphological and histological analysis; The relative mRNA expression of PAI-1, Mmp12, Tert, p53, p21, Cdk2, Bax, Caspase-3, IL-1β, IL-6, IL-8 and TNF-α; The content of p53, p21, Cdk2, Bax, Cleaved caspase-3, NF-κB, p-NF-κB; Metabolomics analysis. | GRb1 retards the aging process in natural-aging C57BL/6J mice by regulating the cell cycle and apoptotic pathway, which are associated with the alleviation of metabolic disorders. | [185] | |
| 20-O-β-D-glucopyranosyl20(S)-protopanaxadiol (20GPPD), the primary bioactive metabolite of ginsenoside Rb1 | Human keratinocytes HaCaT | Cell viability assay; The content of HA and hyaluronan synthase 2 (HAS2); The level of ERK, Akt, p-ERK, p-Akt, Src and p-Src. | 20GPPD enhances the production of HA by acting as an upstream modulator of ERK and Akt activity mediated by Src kinase in human keratinocytes. | [186] | |
| Ganoderic acid D (GA-D) extracted from Ganoderma lucidum | Human amniotic mesenchymal stem cell (hAMSC) treated with H2O2 | SA-β-Gal activity; Telomerase activity assay; Cell viability assay; ROS level; Osteogenic and chondrogenic differentiation of hAMSCs assay; Cell-cycle assay; The relative mRNA expression of 14-3-3ζ, PRDX3, SIRT1-7, β-Catenin, ERK1, ERK2; The content of p21, p16, Nrf2, PERK, p-PERK, and peroxidase III (PRDX3). | GA-D retards hAMSC senescence through the activation of the PERK/NRF2 signaling pathway. | [187] | |
| GA-D | hAMSCs treated with or without GA-D; Mice treated with or without GA-D | In-vitro study: 14-3-3ε-Encoding gene (YWHAE) knockdown assay; SA-β-Gal activity assay; Cell viability assay; ROS level; Differentiation of hAMSCs assay; Cell-cycle assay; The content of p21, p16, 14-3-3ζ, t-Nrf2, n-Nrf2, HO-1, NQO1, CaM, p-CaMKII, CaMKII, p16INK4a; The relative mRNA expression levels of 14-3-3ε, n-Nrf2, t-Nrf2, CaM, CaMKII, p-CaMKII, HO-1, NQO1, p16INK4a, and p21; In-vivo study: Histopathological assay; Activity of T-AOC, SOD, GPX, and content of MDA, AGEs, RAGE assay in the sera. | GA-D retards hAMSC senescence by targeting 14-3-3ε to activate the CaM/CaMKII/Nrf2 signaling pathway. | [188] | |
| Maslinic acid (MA) obtained from Olea europae | Rats pretreated with CCl4 | Plasma lipoperoxide levels and liver lipid peroxidation assay. | MA may offer some advantages in the anti-aging process by decreasing plasma lipoperoxide levels and inhibiting liver lipid peroxidation. | [189] | |
| Ursolic acid (UA) | Skeletal muscle satellite cells, isolated from 10-days old mice, treated with or without UA; 10 months aged mice treated with UA | In-vitro study: Cell viability assay; The relative mRNA levels of paired-box 7 (PAX7), Myogenin, peroxisome proliferator-activated receptor gamma (PGC-1α) and SIRT1; Immunostaining assay. In-vivo study: The content of myoglobin; The concentration of ATP and ADP; Histopathological assay; Satellite cell proliferation assay. | UA promotes skeletal muscle rejuvenation by enhancing SIRT1 expression and satellite cell proliferation. | [190] | |
| Ursolic Acid (UA), a compound which is extensively present in apple peels | Aged-mice C5BL/6 (20 months old) | The content of SIRT1, SIRT6, PGC-1β and α-Klotho. | UA treatment reverses the aging of the liver by enhancing SIRT1 and SIRT6 levels and promoting the production of PGC-1β and Klotho. | [191] | |
| Cycloastragenol (CA) | C. elegans | Lifespan assay. | CA and its new derivatives could significantly extend the lifespan of C. elegans by regulating SKN-1. | [192] | |
| CA | Human T cells | Cellular proliferative capacity; Telomerase activity; Surface markers and cytokine secretion of human CD4+ and CD8+ T cells. | CA extends T cell proliferation by increasing telomerase activity. | [193] | |
| Platycodin D extracted from Platycodon grandiflorum | C2C12 cells treated with H2O2 | Cell cytotoxicity assay; ROS level; The relative mRNA levels of Bcl-2, Bax and caspase-3; Level of Keap1, Nrf2 and HO-1. | Platycodin D protects C2C12 cells against H2O2-induced oxidative stress and apoptosis through the Keap1/Nrf2/HO-1 signal pathway. | [194] | |
| Ginsenoside Re (GRe) | 4-month-old young male mice and 14-month-old aged male mice, including Klotho wild-type, Klotho-deficient (±) mice, wild-type, GPx-1 KO, non-transgenic (non-TG), and GPx-1 TG mice | Cognitive and memory ability assay; DNA-binding activity assay; The content of JAK2, p-JAK2, STAT3, p-STAT3, angiotensin II AT1 receptor, ET1, GPx-1, ERK, p-ERK, cAMP response element-binding protein (CREB), and p-CREB; NOX activity assay; ROS, lipid peroxidation and protein carbonyl level assay; The relative mRNA level of glutamate-cysteine ligase catalytic subunit (GCLc) and glutamate-cysteine ligase modifier subunit (GCLm); The level of GSH and GSSG; SOD, GPX and GR activity assay. | GRe attenuates all alterations, such as AT1 receptor expression, NOX, ROS, and GPX levels, and cognitive dysfunction in aged Klotho-deficient (±) mice via the upregulation of Nrf2/GPx1/ERK/CREB signaling. | [195] | |
| Ginsenoside Rg1 (Rg1) | Sca-1+ HSC/HPC cells isolated from D-Gal-induced-aging rats | SA-β-gal activity; Cell-cycle analysis; CFU-mix assay; The relative mRNA expression of cleaved caspase 3, B-cell lymphoma-2 (Bcl-2), Bcl-2 associated X protein (Bax), SIRT3, SOD2; The content of SIRT3 and SOD2. | Rg1 conducts functions of anti-aging in Sca-1+ HSC/HPC cells in the D-gal-induced-aging model by inhibiting mitochondrial pathway-mediated apoptosis and activating the SIRT3/SOD2 signaling pathway. | [184] | |
| Rg1 | SAMP8 mice | ROS level; The relative mRNA expression of NOX4, p22phox and p47phox; Pathological examination; Level of collagen IV, TGF-β and NLRP3. | Rg1 reduces age-related liver fibrosis by reducing NOX4-mediated ROS-induced oxidative stress and inhibiting the activation of the NLRP3 inflammasome. | [196] | |
| Rg1 | AlCl3-induced-aging mice | Spatial learning and memory assay; Level of BDNF, TrkB, FGF2, Akt, Bcl-2 and Caspase3. | Rg1 ameliorates cognitive deficits in aging mice by regulating FGF2-Akt and BDNF-TrkB signaling pathways. | [197] |
| Subclasses of Vitamins | Representative Components | Models | Main Indicators of Aging | Modes of Action | Reference |
|---|---|---|---|---|---|
| Water-soluble vitamins | Riboflavin (RF) | D. melanogaster treated with H2O2 | Lifespan assay; Reproductive capacity assay; LF content; Activities of SOD and CAT. | RF prolongs the lifespan and increases the reproduction of fruit flies through the antioxidative stress pathway. | [206] |
| Topical liquid formula of polydeoxyribonucleotide (PDRN), vitamin C and niacinamide (a kind of vitamin B3 derivant) | Human primary epidermal keratinocyte, human primary epidermal melanocyte, human fibroblast treated with UV-B; HRM-2 mice treated with UV-B | Melanin content; Contents of NF-κB, Nrf, p-Nrf, HO-1, P53, MITF, Col1a1, Fibrillin1, Fibrillin2, Fibrillin5; mRNA expression of p53, mitf, Mmp2, Mmp3, Mmp9; Activity of NADPH oxidase and SOD. | A topical liquid formula delays skin-aging induced by UV-B by regulating Nrf2 signaling. | [207] | |
| Combination of vitamin C, rice and lupin bio-peptides, hyaluronic acid, and Vichy volcanic mineralizing water | Human keratinocytes and fibroblasts treated with UV-A and pollution | Anti-general oxidant, anti-lipid peroxidation and anti-protein glycosylation ability assays; Activity of collagenase, elastase and hyaluronidase. | A combination of vitamin C, rice and lupin bio-peptides, hyaluronic acid, and Vichy volcanic mineralizing water shows high global antioxidant capacity as well as a protective effect against oxidative stress induced by UV-A, pollution, or both combined factors by stimulating collagen synthesis. | [208] | |
| Vitamin C | Zebrafish treated with LiCl | Expressions of GSK-3β, TSC2, mTOR, FAS, ACC, and ACL; Content of TC, TG and NEFA. | Vitamin C delays liver aging by reducing lipid deposition by regulating GSK-3β/mTOR signaling. | [209] | |
| Fat-soluble vitamins | Vitamin E | DNA repair-deficient mutant mouse (Xpg−/−) | Body weight and tremors assay; SS/SH redox ratio assay; Protein oxidation assay; Histological assay; p53-positive cells assay. | Vitamin E supplementation exerts obvious neuroprotective effect by reducing the number of p53-positive cells. | [210] |
| Vitamin E; Velvet antler polypeptide | D-gal-induced-aging mice | Learning and cognitive abilities assay; Activities of SOD, GPX, and CAT; MDA, PPARα, ACOX1, CPT1A and APOE4 contents; Intestinal microecological analysis. | Vitamin E, or velvet antler polypeptide, exerts anti-aging activity by modulating the gut microbiota and regulating the PPARα/APOE4 pathway. | [211] | |
| Vitamin E and quercetin | Aged breeder hens | Intestinal morphology assay; Serum D-lactate and diamine oxidase content assay; Secretory immunoglobulin A level assay; Expression of Mucin-2, occludin, ZO1, claudin-1, TNF-α, IL-6, IL-1β, IL-10, IL-4, Bax, Bcl-2, SOD1 and GPx-2. The levels of SOD, GPX, CAT and MDA. Total antioxidant capacity assay. | Quercetin and vitamin E improve intestinal function in aged breeder hens by protecting intestinal structure and integrity. | [212] | |
| Vitamin K and vitamin D | Human osteoblasts cultured in presence of hydroxyapatite-based biomaterials | Cell viability assay; ROS and GSH level assay; GPX and ALP activity; DNA proliferation assay. | Vitamins D and K protect redox balance and support growth of osteoblasts affected by hydroxyapatite-based biomaterials due to antioxidant properties. | [213] | |
| Vitamin K2 | Wild-type and mutant-type C. elegans | Longevity and survival assay; RNA-sequencing analysis; Progeny and reproductive activity assay; Expression of daf-12, fard-1, fat-5/6/7, and lipl-4. | Vitamin K2 extends the lifespan of C. elegans and improves the resistance to pathogen infection, heat stress and H2O2-induced inner oxidative stress by enhancing fat metabolism. | [214] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, L.; Zhang, S. Anti-Aging Activity and Modes of Action of Compounds from Natural Food Sources. Biomolecules 2023, 13, 1600. https://doi.org/10.3390/biom13111600
Song L, Zhang S. Anti-Aging Activity and Modes of Action of Compounds from Natural Food Sources. Biomolecules. 2023; 13(11):1600. https://doi.org/10.3390/biom13111600
Chicago/Turabian StyleSong, Lili, and Shicui Zhang. 2023. "Anti-Aging Activity and Modes of Action of Compounds from Natural Food Sources" Biomolecules 13, no. 11: 1600. https://doi.org/10.3390/biom13111600
APA StyleSong, L., & Zhang, S. (2023). Anti-Aging Activity and Modes of Action of Compounds from Natural Food Sources. Biomolecules, 13(11), 1600. https://doi.org/10.3390/biom13111600







