Plasma Androstenedione Concentration Can Discriminate Frail versus Non-Frail Men with Prostate Cancer under Androgen Deprivation Therapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design and Study Population
2.2. Assessment of Frailty Syndrome
2.3. Geriatric Assessment
2.4. Measurement of Hormones Present in Blood Plasma
2.5. Statistical Analysis
3. Results
3.1. Sociodemographic and Clinical Data
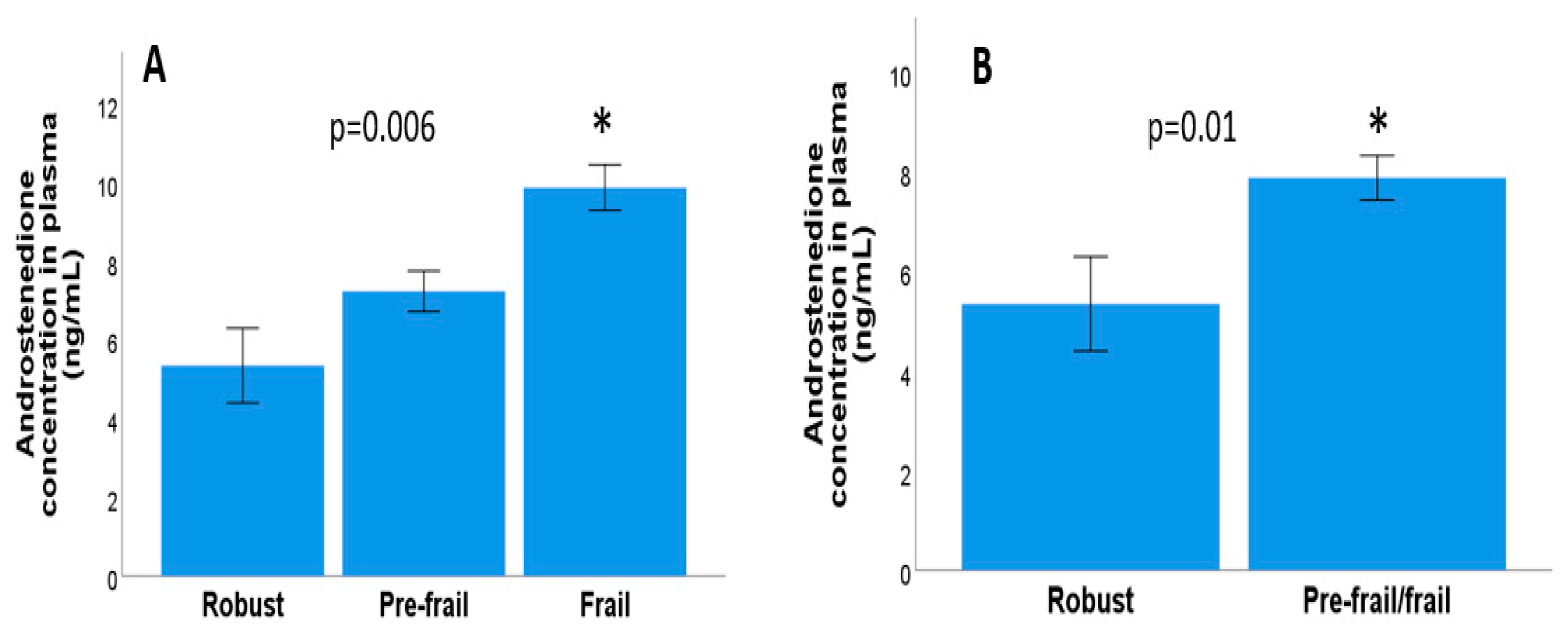
3.2. Relationship between Frailty Syndrome and Androgen Concentration in Metastatic and Localised Prostate Cancer
3.3. Relationship between Frailty Syndrome and Androgen Concentration in Metastatic Prostate Cancer
3.4. Relationship between Frailty Syndrome and Androgen Concentration in Localised Prostrate Cancer
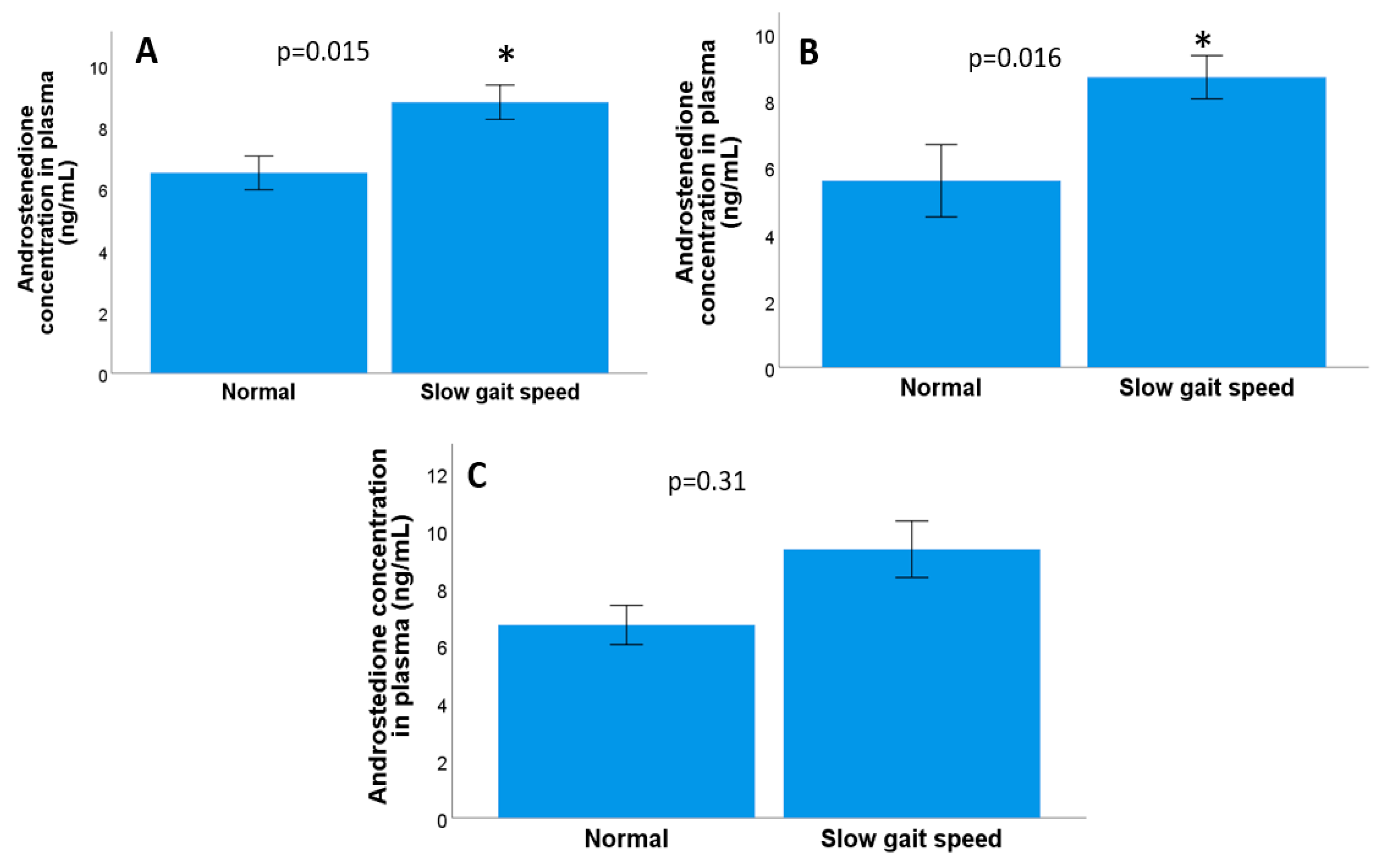
3.5. Relationship between Androgen Concentration and the Geriatric Assessment Criteria in Metastatic and Localised Prostate Cancer
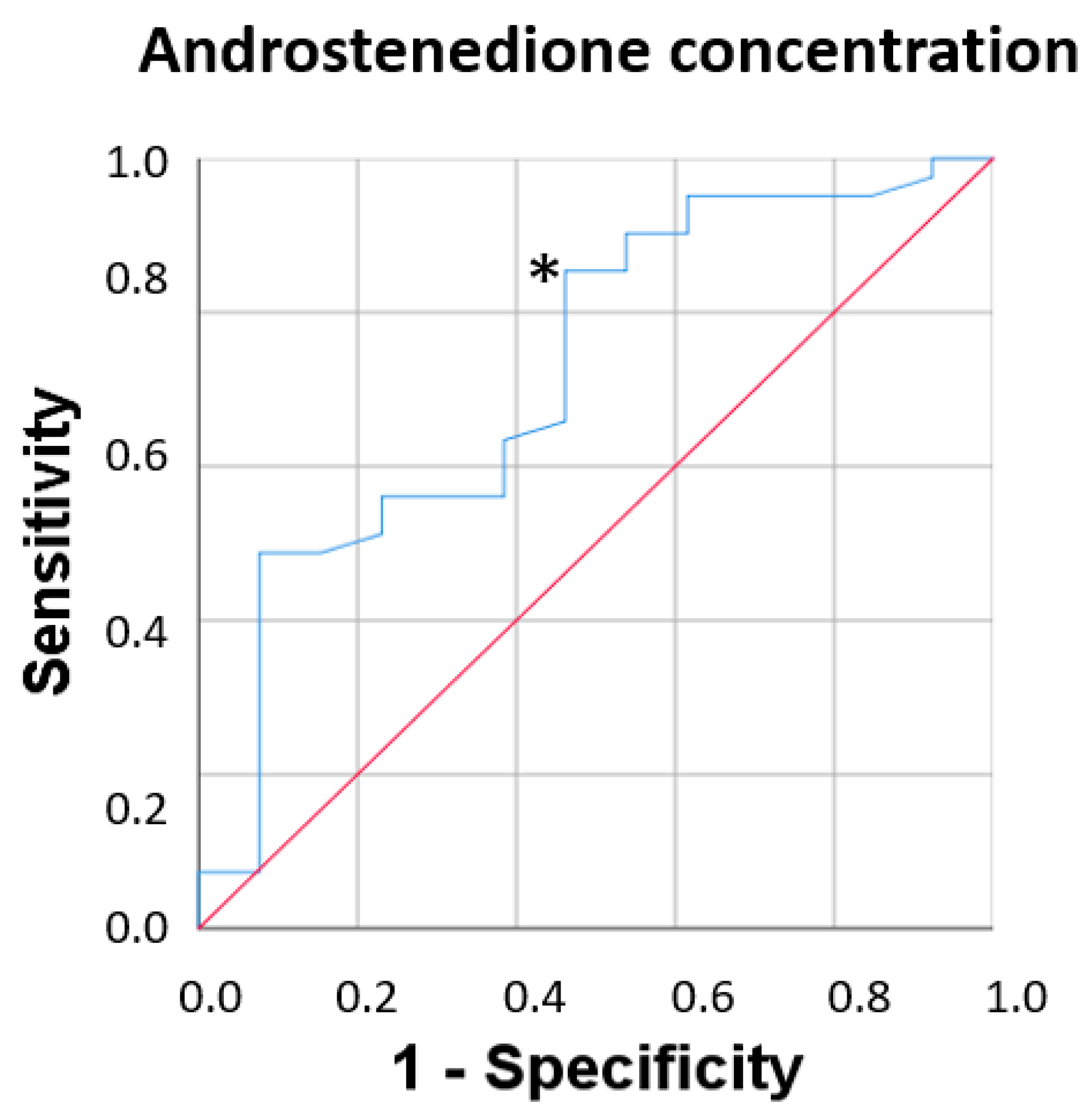
3.6. Fragility Syndrome and Plasma Androstenedione Concentration
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aurilio:, G.; Cimadamore, A.; Mazzucchelli, R.; Lopez-Beltran, A.; Verri, E.; Scarpelli, M.; Massari, F.; Cheng, L.; Santoni, M.; Montironi, R. Androgen Receptor Signaling Pathway in Prostate Cancer: From Genetics to Clinical Applications. Cells 2020, 9, 2653. [Google Scholar] [CrossRef] [PubMed]
- Sorić, T.; Vidić, I. Is testosterone prognostic in prostate cancer treatment? The urological standpoint. Acta Clin. Croat. 2019, 58, 64. [Google Scholar] [PubMed]
- Sharifi, N.; Gulley, J.L.; Dahut, W.L. Androgen Deprivation Therapy for Prostate Cancer. JAMA 2005, 294, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Balk, S.P. Intratumoral Androgen Biosynthesis in Prostate Cancer Pathogenesis and Response to Therapy. Endocr. Relat. Cancer 2011, 18, R175–R182. [Google Scholar] [CrossRef]
- Scher, H.I.; Halabi, S.; Tannock, I.; Morris, M.; Sternberg, C.N.; Carducci, M.A.; Eisenberger, M.A.; Higano, C.; Bubley, G.J.; Dreicer, R.; et al. Design and End Points of Clinical Trials for Patients with Progressive Prostate Cancer and Castrate Levels of Testosterone: Recommendations of the Prostate Cancer Clinical Trials Working Group. J. Clin. Oncol. 2008, 26, 1148–1159. [Google Scholar] [CrossRef]
- Desai, K.; McManus, J.M.; Sharifi, N. Hormonal Therapy for Prostate Cancer. Endocr. Rev. 2021, 42, 354–373. [Google Scholar] [CrossRef]
- Ryan, C.J.; Molina, A.; Li, J.; Kheoh, T.; Small, E.J.; Haqq, C.M.; Grant, R.P.; De Bono, J.S.; Scher, H.I. Serum Androgens As Prognostic Biomarkers in Castration-Resistant Prostate Cancer: Results from an Analysis of a Randomized Phase III Trial. J. Clin. Oncol. 2013, 31, 2791–2798. [Google Scholar] [CrossRef]
- Locke, J.A.; Guns, E.S.; Lubik, A.A.; Adomat, H.H.; Hendy, S.C.; Wood, C.A.; Ettinger, S.L.; Gleave, M.E.; Nelson, C.C. Androgen Levels Increase by Intratumoral De Novo Steroidogenesis during Progression of Castration-Resistant Prostate Cancer. Cancer Res. 2008, 68, 6407–6415. [Google Scholar] [CrossRef]
- Dent, E.; Martin, F.C.; Bergman, H.; Woo, J.; Romero-Ortuno, R.; Walston, J.D. Management of Frailty: Opportunities, Challenges, and Future Directions. Lancet 2019, 394, 1376–1386. [Google Scholar] [CrossRef]
- Yu, R.; Wong, M.; Chong, K.C.; Chang, B.; Lum, C.M.; Auyeung, T.W.; Lee, J.; Lee, R.; Woo, J. Trajectories of Frailty among Chinese Older People in Hong Kong between 2001 and 2012: An Age-Period-Cohort Analysis. Age Ageing 2018, 47, 254–261. [Google Scholar] [CrossRef]
- Hughes, V.A.; Frontera, W.R.; Roubenoff, R.; Evans, W.J.; Fiatarone Singh, M.A. Longitudinal Changes in Body Composition in Older Men and Women: Role of Body Weight Change and Physical Activity. Am. J. Clin. Nutr. 2002, 76, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Walston, J.; Hadley, E.C.; Ferrucci, L.; Guralnik, J.M.; Newman, A.B.; Studenski, S.A.; Ershler, W.B.; Harris, T.; Fried, L.P. Research Agenda for Frailty in Older Adults: Toward a Better Understanding of Physiology and Etiology: Summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J. Am. Geriatr. Soc. 2006, 54, 991–1001. [Google Scholar] [CrossRef] [PubMed]
- Hughes, V.A.; Frontera, W.R.; Wood, M.; Evans, W.J.; Dallal, G.E.; Roubenoff, R.; Fiatarone Singh, M.A. Longitudinal Muscle Strength Changes in Older Adults: Influence of Muscle Mass, Physical Activity, and Health. J. Gerontol.–Ser. A Biol. Sci. Med. Sci. 2001, 56, B209–B217. [Google Scholar] [CrossRef] [PubMed]
- Cheung, A.S.; Hoermann, R.; Dupuis, P.; Joon, D.L.; Zajac, J.D.; Grossmann, M. Relationships between Insulin Resistance and Frailty with Body Composition and Testosterone in Men Undergoing Androgen Deprivation Therapy for Prostate Cancer. Eur. J. Endocrinol. 2016, 175, 229–237. [Google Scholar] [CrossRef]
- Smith, M.R.; Finkelstein, J.S.; McGovern, F.J.; Zietman, A.L.; Fallon, M.A.; Schoenfeld, D.A.; Kantoff, P.W. Changes in Body Composition during Androgen Deprivation Therapy for Prostate Cancer. J. Clin. Endocrinol. Metab. 2002, 87, 599–603. [Google Scholar] [CrossRef]
- Galvão, D.A.; Spry, N.A.; Taaffe, D.R.; Newton, R.U.; Stanley, J.; Shannon, T.; Rowling, C.; Prince, R. Changes in Muscle, Fat and Bone Mass after 36 Weeks of Maximal Androgen Blockade for Prostate Cancer. BJU Int. 2008, 102, 44–47. [Google Scholar] [CrossRef]
- Winters-Stone, K.M.; Moe, E.; Graff, J.N.; Dieckmann, N.F.; Stoyles, S.; Borsch, C.; Alumkal, J.J.; Amling, C.L.; Beer, T.M. Falls and Frailty in Prostate Cancer Survivors: Current, Past and Never Users of Androgen Deprivation Therapy. J. Am. Geriatr. Soc. 2017, 65, 1414–1419. [Google Scholar] [CrossRef]
- Buigues, C.; Navarro-Martínez, R.; Sánchez-Martínez, V.; Serrano-Carrascosa, M.; Rubio-Briones, J.; Cauli, O. Interleukin-6 and Lymphocyte Count Associated and Predicted the Progression of Frailty Syndrome in Prostate Cancer Patients Undergoing Antiandrogen Therapy. Cancers 2020, 12, 1716. [Google Scholar] [CrossRef]
- Bylow, K.; Hemmerich, J.; Mohile, S.G.; Stadler, W.M.; Sajid, S.; Dale, W. Obese Frailty, Physical Performance Deficits, and Falls in Older Men with Biochemical Recurrence of Prostate Cancer on Androgen Deprivation Therapy: A Case-Control Study. Urology 2011, 77, 934–940. [Google Scholar] [CrossRef]
- Hamaya, T.; Hatakeyama, S.; Momota, M.; Narita, T.; Iwamura, H.; Kojima, Y.; Hamano, I.; Fujita, N.; Okamoto, T.; Togashi, K.; et al. Association between the Baseline Frailty and Quality of Life in Patients with Prostate Cancer (FRAQ-PC Study). Int. J. Clin. Oncol. 2021, 26, 199–206. [Google Scholar] [CrossRef]
- Wu, I.C.; Lin, X.Z.; Liu, P.F.; Tsai, W.L.; Shiesh, S.C. Low Serum Testosterone and Frailty in Older Men and Women. Maturitas 2010, 67, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Cawthon, P.M.; Ensrud, K.E.; Laughlin, G.A.; Cauley, J.A.; Dam, T.T.L.; Barrett-Connor, E.; Fink, H.A.; Hoffman, A.R.; Lau, E.; Lane, N.E.; et al. Sex Hormones and Frailty in Older Men: The Osteoporotic Fractures in Men (MrOS) Study. J. Clin. Endocrinol. Metab. 2009, 94, 3806–3815. [Google Scholar] [CrossRef] [PubMed]
- García-Sánchez, J.; Mafla-España, M.A.; Torregrosa, M.D.; Cauli, O. Androstenedione and Follicle-Stimulating Hormone Concentration Predict the Progression of Frailty Syndrome at One Year Follow-Up in Patients with Localized Breast Cancer Treated with Aromatase Inhibitors. Biomedicines 2022, 10, 1634. [Google Scholar] [CrossRef] [PubMed]
- Mafla-España, M.A.; Torregrosa, M.D.; Beamud-Cortés, M.; Bermell-Marco, L.; Rubio-Briones, J.; Cauli, O. Comparison of Frailty Criteria, Cognitive Function, Depressive and Insomnia Symptoms in Men with Localized and Advanced Prostate Cancer under Androgen Deprivation Therapy. Healthcare 2023, 11, 1266. [Google Scholar] [CrossRef] [PubMed]
- Lobo, A.; Saz, P.; Marcos, G.; Día, J.L.; De La Cámara, C.; Ventura, T.; Asín, F.M.; Pascual, L.F.; Montañés, J.Á.; Aznar, S. Revalidation and Standardization of the Cognition Mini-Exam (First Spanish Version of the Mini-Mental Status Examination) in the General Geriatric Population. Med. Clin. 1999, 112, 767–774. [Google Scholar]
- Soldatos, C.R.; Dikeos, D.G.; Paparrigopoulos, T.J. Athens Insomnia Scale: Validation of an Instrument Based on ICD-10 Criteria. J. Psychosom. Res. 2000, 48, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Yesavage, J.A.; Brink, T.L.; Rose, T.L.; Lum, O.; Huang, V.; Adey, M.; Leirer, V.O. Development and Validation of a Geriatric Depression Screening Scale: A Preliminary Report. J. Psychiatr. Res. 1982, 17, 37–49. [Google Scholar] [CrossRef]
- Badawy, M.T.; Sobeh, M.; Xiao, J.; Farag, M.A. Androstenedione (a Natural Steroid and a Drug Supplement): A Comprehensive Review of Its Consumption, Metabolism, Health Effects, and Toxicity with Sex Differences. Molecules 2021, 26, 6210. [Google Scholar] [CrossRef]
- Nunes, V.O.; Vanzellotti, N.d.C.; Fraga, J.L.; Pessoa, F.L.P.; Ferreira, T.F.; Amaral, P.F.F. Biotransformation of Phytosterols into Androstenedione—A Technological Prospecting Study. Molecules 2022, 27, 3164. [Google Scholar] [CrossRef]
- Crawford, E.D. Understanding the Epidemiology, Natural History, and Key Pathways Involved in Prostate Cancer. Urology 2009, 73, S4–S10. [Google Scholar] [CrossRef]
- Van, L.T. Assessment of Steroidogenesis and Steroidogenic Enzyme Functions. J. Steroid Biochem. Mol. Biol. 2013, 137, 176–182. [Google Scholar]
- Siiteri, P.K. Adipose Tissue as a Source of Hormones. Am. J. Clin. Nutr. 1987, 45, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Kuryłowicz, A. Estrogens in Adipose Tissue Physiology and Obesity-Related Dysfunction. Biomedicines 2023, 11, 690. [Google Scholar] [CrossRef]
- Li, J.; Papadopoulos, V.; Vihma, V. Steroid Biosynthesis in Adipose Tissue. Steroids 2015, 103, 89–104. [Google Scholar] [CrossRef]
- Deb, S.; Chin, M.Y.; Pham, S.; Adomat, H.; Hurtado-Coll, A.; Gleave, M.E.; Guns, E.S.T. Steroidogenesis in Peripheral and Transition Zones of Human Prostate Cancer Tissue. Int. J. Mol. Sci. 2021, 22, 487. [Google Scholar] [CrossRef] [PubMed]
- Mostaghel, E.A. Steroid Hormone Synthetic Pathways in Prostate Cancer. Transl. Androl. Urol. 2013, 2, 212–227. [Google Scholar]
- Breuer, B.; Trungold, S.; Martucci, C.; Wallenstein, S.; Likourezos, A.; Libow, L.S.; Zumoff, B. Relationships of Sex Hormone Levels to Dependence in Activities of Daily Living in the Frail Elderly. Maturitas 2001, 39, 147–159. [Google Scholar] [CrossRef]
- Eisenhofer, G.; Peitzsch, M.; Kaden, D.; Langton, K.; Pamporaki, C.; Masjkur, J.; Tsatsaronis, G.; Mangelis, A.; Williams, T.A.; Reincke, M.; et al. Reference Intervals for Plasma Concentrations of Adrenal Steroids Measured by LC-MS/MS: Impact of Gender, Age, Oral Contraceptives, Body Mass Index and Blood Pressure Status. Clin. Chim. Acta 2017, 470, 115–124. [Google Scholar] [CrossRef]
- Higano, C.S. Side Effects of Androgen Deprivation Therapy: Monitoring and Minimizing Toxicity. Urology 2003, 61, 32–38. [Google Scholar] [CrossRef]
- Rosenberg, I.H. Sarcopenia: Origins and Clinical Relevance. J. Nutr. 1997, 127, 990S–991S. [Google Scholar] [CrossRef]
- Shin, M.J.; Jeon, Y.K.; Kim, I.J. Testosterone and Sarcopenia. World J. Mens. Health 2018, 36, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Barone, B.; Napolitano, L.; Abate, M.; Cirillo, L.; Reccia, P.; Passaro, F.; Turco, C.; Morra, S.; Mastrangelo, F.; Scarpato, A.; et al. The Role of Testosterone in the Elderly: What Do We Know? Int. J. Mol. Sci. 2022, 23, 3535. [Google Scholar] [CrossRef] [PubMed]
- Araujo, A.B.; Travison, T.G.; Bhasin, S.; Esche, G.R.; Williams, R.E.; Clark, R.V.; McKinlay, J.B. Association between Testosterone and Estradiol and Age-Related Decline in Physical Function in a Diverse Sample of Men. J. Am. Geriatr. Soc. 2008, 56, 2000–2008. [Google Scholar] [CrossRef] [PubMed]
- Ruan, G.T.; Ge, Y.Z.; Xie, H.L.; Hu, C.L.; Zhang, Q.; Zhang, X.; Tang, M.; Song, M.M.; Zhang, X.W.; Liu, T.; et al. Association Between Systemic Inflammation and Malnutrition with Survival in Patients with Cancer Sarcopenia—A Prospective Multicenter Study. Front. Nutr. 2022, 8, 811288. [Google Scholar] [CrossRef]
- Dalle, S.; Rossmeislova, L.; Koppo, K. The Role of Inflammation in Age-Related Sarcopenia. Front. Physiol. 2017, 8, 1045. [Google Scholar] [CrossRef]
- Yuenyongchaiwat, K.; Akekawatchai, C. Systemic Inflammation in Sarcopenia Alter Functional Capacity in Thai Community-Dwelling Older People: A Preliminary Observational Study. Curr. Aging Sci. 2022, 15, 274–281. [Google Scholar] [CrossRef]
- Bylow, K.; Mohile, S.G.; Stadler, W.M.; Dale, W. Does Androgen-Deprivation Therapy Accelerate the Development of Frailty in Older Men with Prostate Cancer? A Conceptual Review. Cancer 2007, 110, 2604–2613. [Google Scholar] [CrossRef]
- Watson, E.; Shinkins, B.; Frith, E.; Neal, D.; Hamdy, F.; Walter, F.; Weller, D.; Wilkinson, C.; Faithfull, S.; Wolstenholme, J.; et al. Symptoms, Unmet Needs, Psychological Well-Being and Health Status in Survivors of Prostate Cancer: Implications for Redesigning Follow-Up. BJU Int. 2016, 117, E10–E19. [Google Scholar] [CrossRef]
- Dale, W.; Klepin, H.D.; Williams, G.R.; Alibhai, S.M.H.; Bergerot, C.; Brintzenhofeszoc, K.; Hopkins, J.O.; Jhawer, M.P.; Katheria, V.; Loh, K.P.; et al. Practical Assessment and Management of Vulnerabilities in Older Patients Receiving Systemic Cancer Therapy: ASCO Guideline Update. J. Clin. Oncol. 2023, 41, 4293–4312. [Google Scholar] [CrossRef]
- Li, D.; Sun, C.L.; Kim, H.; Soto-Perez-De-Celis, E.; Chung, V.; Koczywas, M.; Fakih, M.; Chao, J.; Cabrera Chien, L.; Charles, K.; et al. Geriatric Assessment-Driven Intervention (GAIN) on Chemotherapy-Related Toxic Effects in Older Adults with Cancer: A Randomized Clinical Trial. JAMA Oncol. 2021, 7, e214158. [Google Scholar] [CrossRef]
- Mohile, S.G.; Mohamed, M.R.; Xu, H.; Culakova, E.; Loh, K.P.; Magnuson, A.; Flannery, M.A.; Obrecht, S.; Gilmore, N.; Ramsdale, E.; et al. Evaluation of Geriatric Assessment and Management on the Toxic Effects of Cancer Treatment (GAP70+): A Cluster-Randomised Study. Lancet 2021, 398, 1894–1904. [Google Scholar] [CrossRef] [PubMed]
- Walsh, P.C.; Siiteri, P.K. Suppression of Plasma Androgens by Spironolactone in Castrated Men with Carcinoma of the Prostate. J. Urol. 1975, 114, 254–256. [Google Scholar] [CrossRef] [PubMed]
| Variables | Percentage Frequency for Categorical Variables or Mean ± Standard Error of the Mean (Range Min–Max) for Discrete Variables | Patients with Metastatic Prostate Cancer (n = 29) | Patients with Localised Prostate Cancer (n = 36) |
|---|---|---|---|
| Age | 73.7 ± 1.11 (55–92) | 74.6 ± 1.5 | 72.9 ± 1.6 |
| Previous prostatectomy | |||
| Yes | 38 (61.9%) | Yes (58.6%) | Yes (66.7%) |
| No | 25 (38.1%) | No (41.4%) | No (33.3%) |
| BMI (kg/m2) | 0 | ||
| Underweight (<18.5) | |||
| Normal (18.5–24.9) | 15 (23.1%) | 9 (31%) | 6 (16.7%) |
| Overweight (25–29.9) | 36 (55.4%) | 16 (55.2%) | 20 (55.6%) |
| Obese (>30) | 14 (21.5%) | 4 (13.7%) | 10 (27.8%) |
| Gleason Index | 7.30 ± 0.14 (5–10) | 7.5 ± 0.22 | 7.1 ± 0.17 |
| Charlson Comorbidity Index | 2.87 ± 0.20 (0–7) | 2.6 ± 0.39 | 3.3 ± 0.23 |
| Number of Fried criteria | 1.46 ± 0.13 (0–4) | 1.55 ± 0.20 (0–4) | 1.38 ± 0.18 (0–4) |
| Frailty syndrome criteria: | |||
| Robust (0 criteria) | 16 (24.6%) | 6 (20.7%) | 10 (27.8%) |
| Pre-frail (1–2 criteria) | 38 (58.5%) | 17 (58.6%) | 21 (58.3%) |
| Frail (>3 criteria) | 11 (16.9%) | 6 (20.7%) | 5 (13.9) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mafla-España, M.A.; Torregrosa, M.D.; Beamud-Cortés, M.; Bermell-Marco, L.; Rubio-Briones, J.; Cauli, O. Plasma Androstenedione Concentration Can Discriminate Frail versus Non-Frail Men with Prostate Cancer under Androgen Deprivation Therapy. Biomolecules 2023, 13, 1642. https://doi.org/10.3390/biom13111642
Mafla-España MA, Torregrosa MD, Beamud-Cortés M, Bermell-Marco L, Rubio-Briones J, Cauli O. Plasma Androstenedione Concentration Can Discriminate Frail versus Non-Frail Men with Prostate Cancer under Androgen Deprivation Therapy. Biomolecules. 2023; 13(11):1642. https://doi.org/10.3390/biom13111642
Chicago/Turabian StyleMafla-España, Mayra Alejandra, María Dolores Torregrosa, Manel Beamud-Cortés, Lorena Bermell-Marco, José Rubio-Briones, and Omar Cauli. 2023. "Plasma Androstenedione Concentration Can Discriminate Frail versus Non-Frail Men with Prostate Cancer under Androgen Deprivation Therapy" Biomolecules 13, no. 11: 1642. https://doi.org/10.3390/biom13111642







