AI-Based Homology Modelling of Fatty Acid Transport Protein 1 Using AlphaFold: Structural Elucidation and Molecular Dynamics Exploration
Abstract
1. Introduction
2. Methods
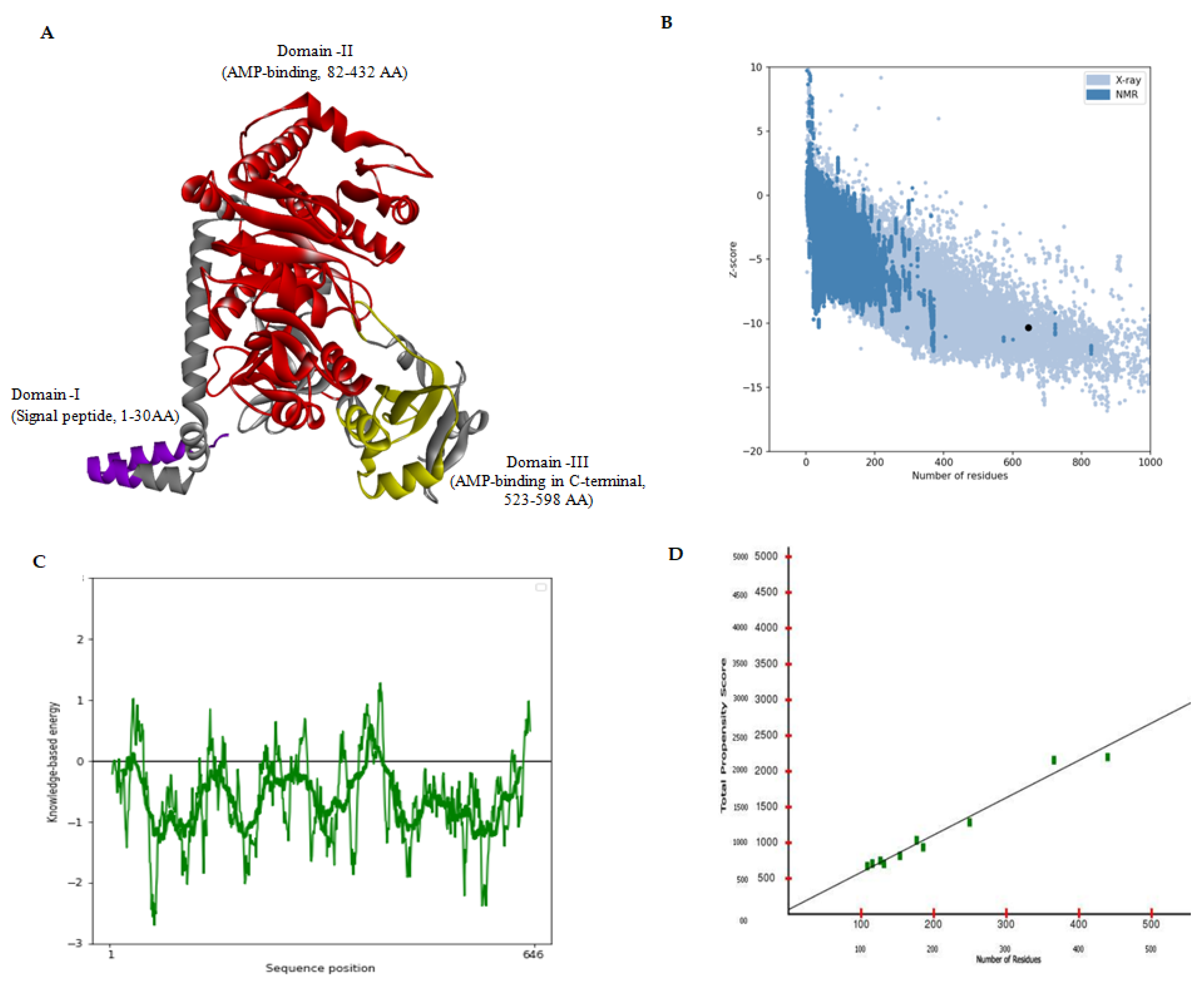
2.1. In Silico Protein Modelling
2.2. Secondary Structure Prediction
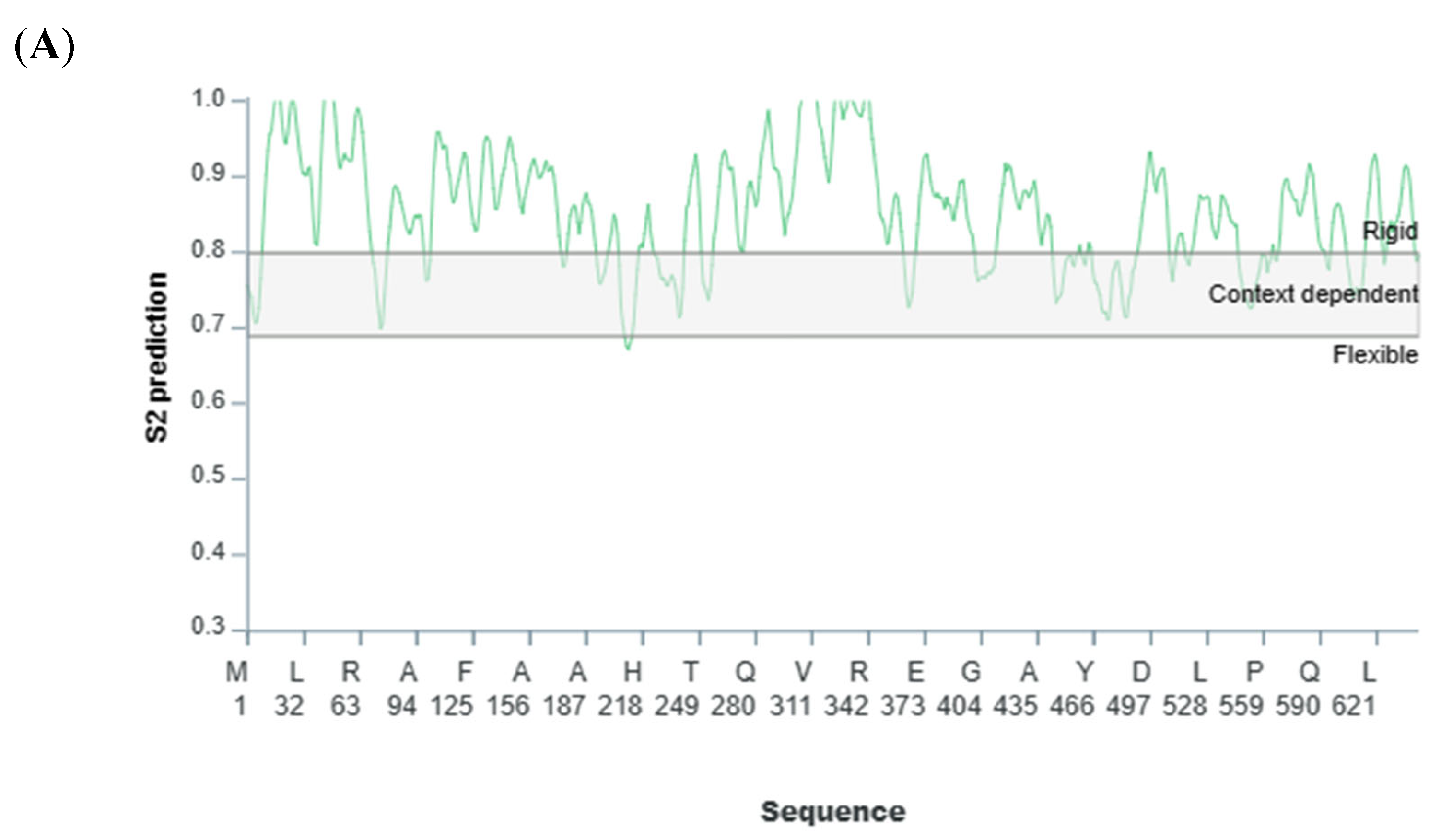
2.3. Coarse Dynamics’ Refinement and Residue Level Propensity
2.4. Model Validation
2.5. Protein Stability Analysis through Molecular Dynamic Simulation (MDS)
2.6. Protein–Protein Interaction (PPI) Network Analysis
2.7. Gene Set Enrichment and Pathway Analysis
3. Results
3.1. MDS-Based In Silico Structure Prediction and Validation
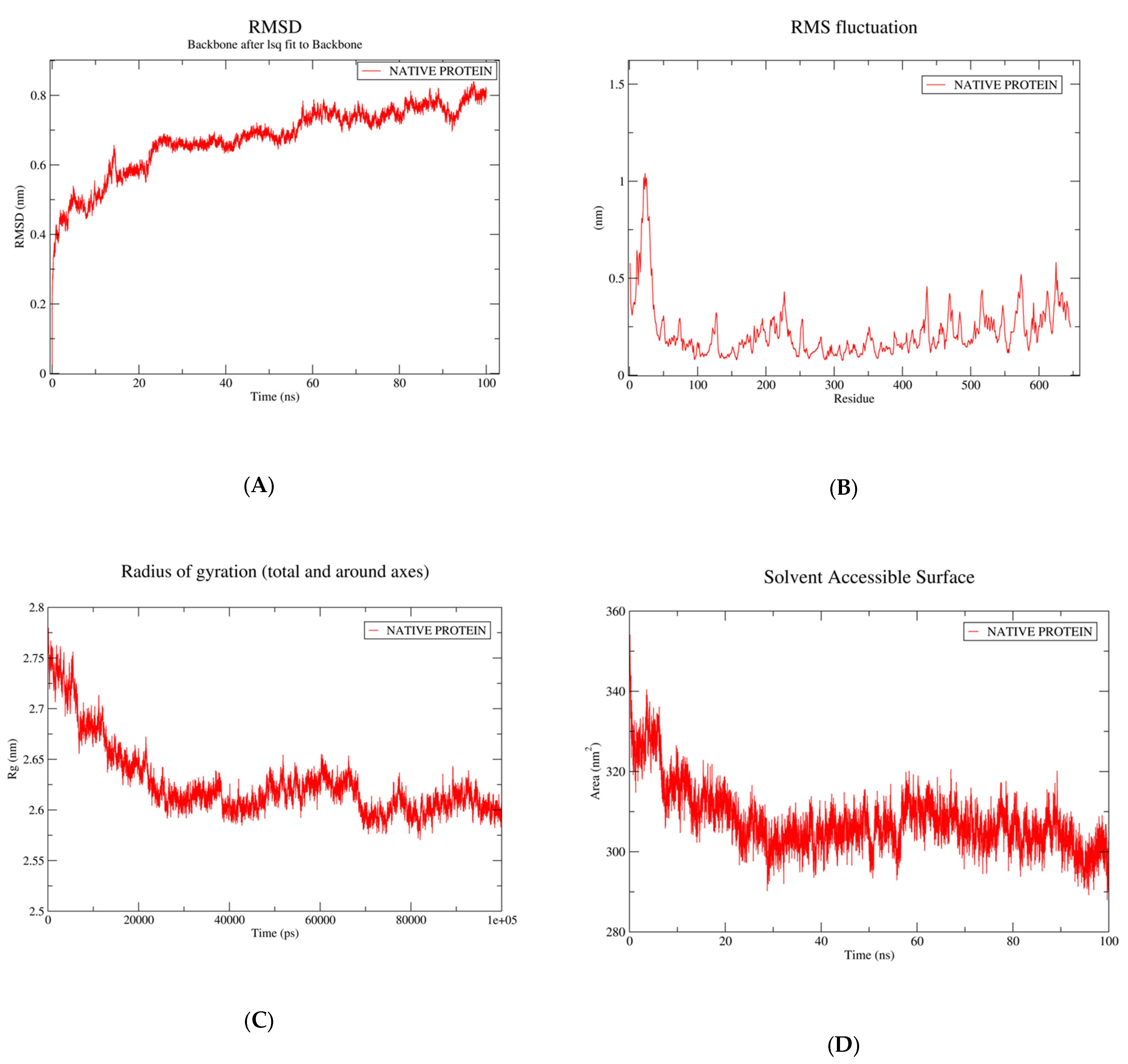
3.2. Structural Stability of Fatty Acid Transport Protein 1
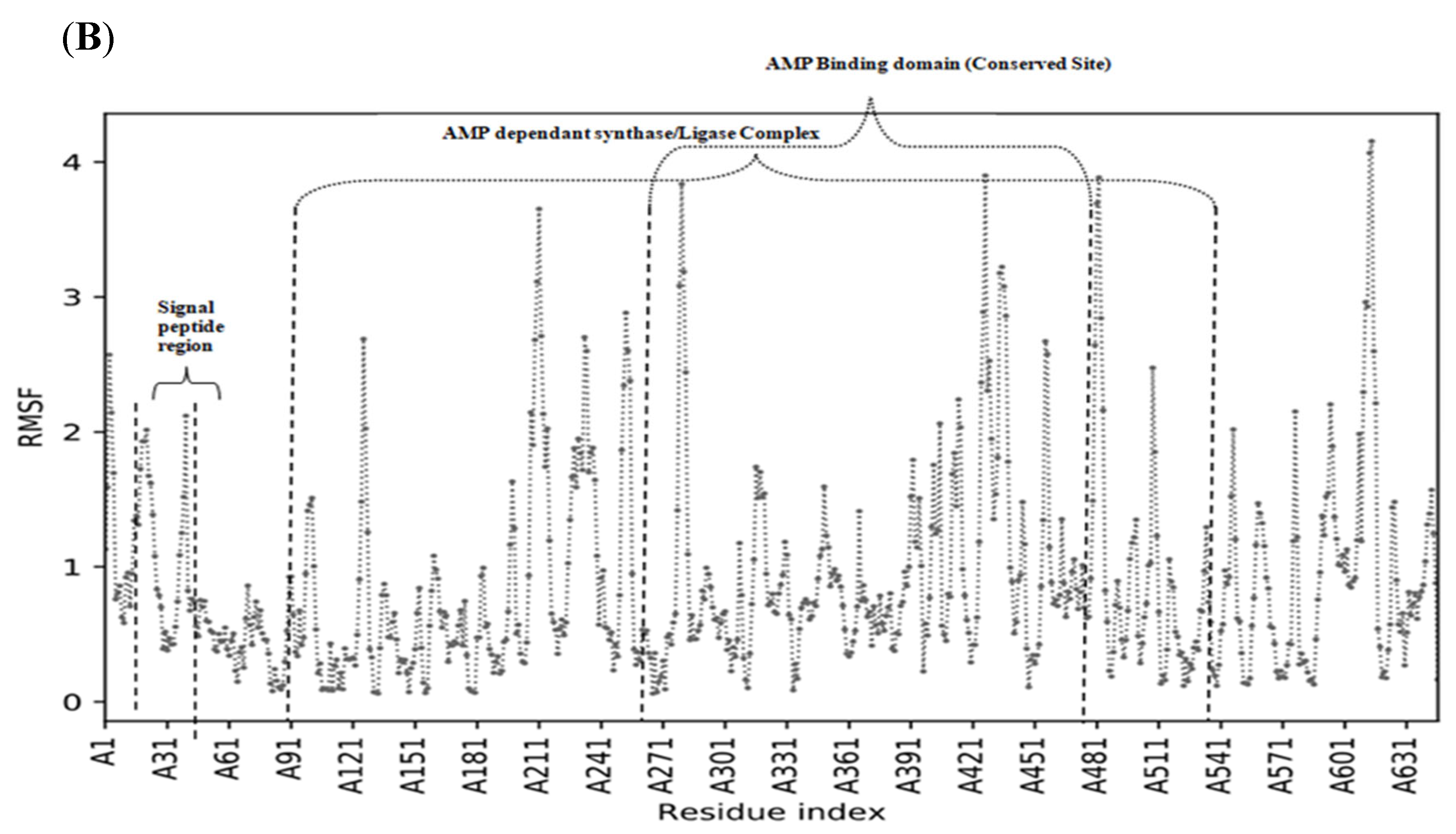
3.3. Root-Mean-Square Fluctuation and Protein Flexibility of Fatty Acid Transport Protein 1
3.4. Radius of Gyration
3.5. Calculation of Solvent Assessable Surface Area (SASA)
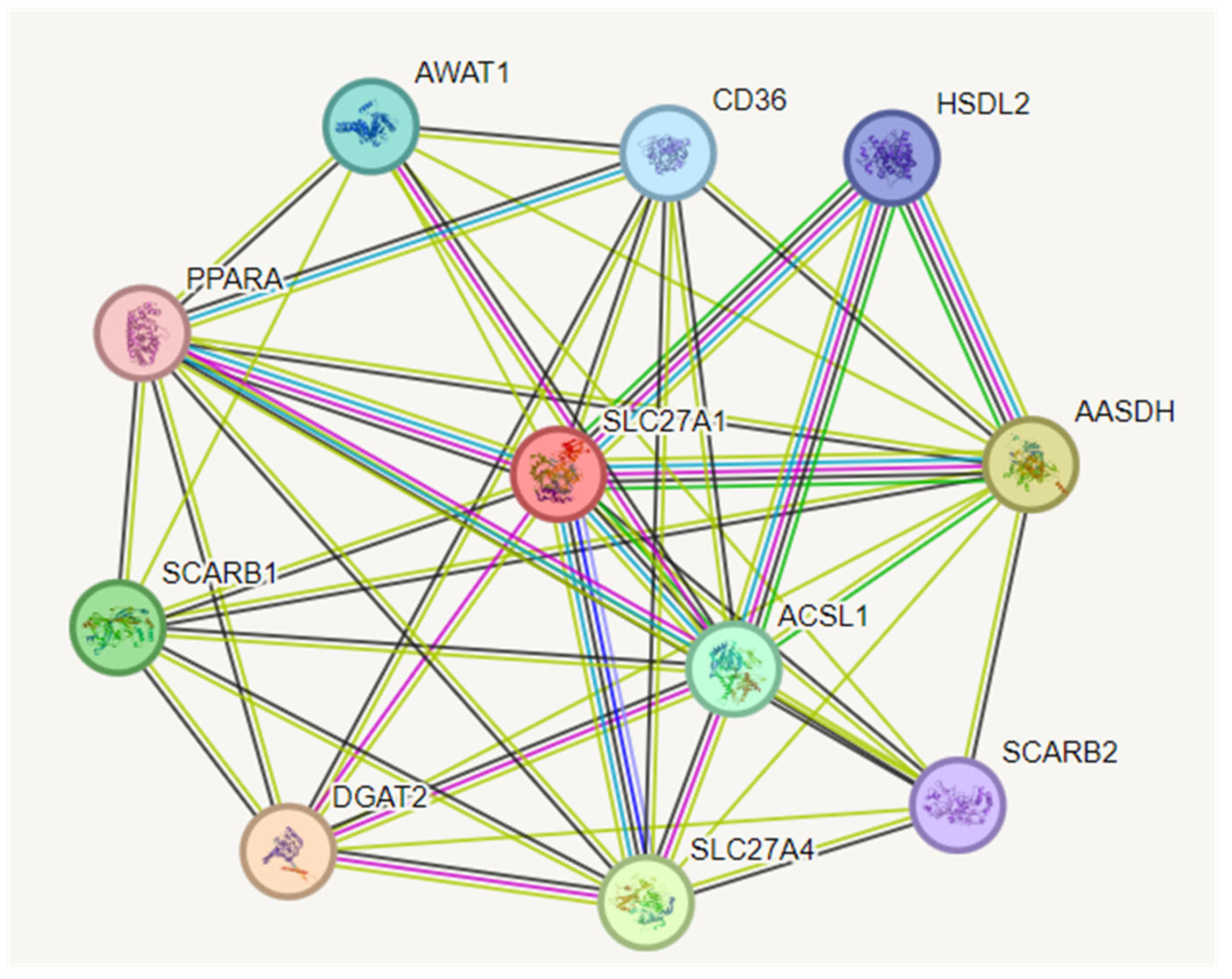
3.6. Construction of Protein–Protein Interaction (PPI) Network
3.7. Interactomics Analysis of Hub Gene
3.8. GO Enrichment Analysis
3.9. Pathway Enrichment Analysis of Target Proteins
4. Analysis of Gene–Disease Association
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| FATP1 | Fatty Acid Transport Protein 1 |
| LCFA | Long-Chain Fatty Acid |
| VLCFA | Very-Long-Chain Fatty Acid |
| SLC27A | Solute Carrier Family 27 A |
| COS1 | Fibroblast-Like Cell Lines Obtained from Green Monkey Kidney Tissue |
| ACS | Acyl CoA Synthetase |
| BCC | Breast Cancer Cells |
| PDB | Protein Data Bank |
| AI | Artificial Intelligence |
| CASP | Critical Assessment of Structure Prediction |
| SOPMA | Self-Optimised Prediction Method with Alignment |
| PSI PRED | PSI-Blast Based Secondary Structure Prediction |
| RMSF | Root Mean Square Fluctuation |
| SAVES | Structural Analysis and Verification Server |
| MDS | Molecular Dynamic Simulations |
References
- Glatz, J.F.C.; Luiken, J.J.F.P.; Bonen, A. Membrane fatty acid transporters as regulators of lipid metabolism: Implications for metabolic disease. Physiol. Rev. 2010, 90, 367–417. [Google Scholar] [CrossRef] [PubMed]
- Black, P.N.; Sandoval, A.; Arias-Barrau, E.; DiRusso, C.C. Targeting the fatty acid transport proteins (FATP) to understand the mechanisms linking fatty acid transport to metabolism. Immunol. Endocr. Metab. Agents Med. Chem. 2009, 9, 11–17. [Google Scholar] [CrossRef]
- Faergeman, N.J.; DiRusso, C.C.; Elberger, A.; Knudsen, J.; Black, P.N. Disruption of the Saccharomyces cerevisiae homologue to the murine fatty acid transport protein impairs uptake and growth on long-chain fatty acids. J. Biol. Chem. 1997, 272, 8531–8538. [Google Scholar] [CrossRef] [PubMed]
- Ferrada, E.; Superti-Furga, G. A structure and evolutionary-based classification of solute carriers. iScience 2022, 25, 105096. [Google Scholar] [CrossRef] [PubMed]
- Hui, T.Y.; Bernlohr, D.A. Fatty acid transporters in animal cells. Front. Biosci.-Landmark 1997, 2, 222–231. [Google Scholar]
- Bernlohr, D.A.; Coe, N.R.; LiCata, V.J. Fatty acid trafficking in the adipocyte. Semin. Cell Dev. Biol. 1999, 10, 43–49. [Google Scholar] [CrossRef]
- Schaffer, J.E.; Lodish, H.F. Molecular mechanism of long-chain fatty acid uptake. Trends Cardiovasc. Med. 1995, 5, 218–224. [Google Scholar] [CrossRef]
- Tokuyama, S.; Nakamoto, K. Pain as modified by polyunsaturated fatty acids. In Omega-3 Fatty Acids in Brain and Neurological Health; Elsevier: Amsterdam, The Netherlands, 2014; pp. 131–146. ISBN 9780124105270. [Google Scholar]
- Bayly, G.R. Lipids and disorders of lipoprotein metabolism. In Clinical Biochemistry: Metabolic and Clinical Aspects; Elsevier: Amsterdam, The Netherlands, 2014; pp. 702–736. ISBN 9780702051401. [Google Scholar]
- Huang, J.; Guo, D.; Zhu, R.; Feng, Y.; Li, R.; Yang, X.; Shi, D. FATP1 exerts variable effects on adipogenic differentiation and proliferation in cells derived from muscle and adipose tissue. Front. Vet. Sci. 2022, 9, 904879. [Google Scholar] [CrossRef]
- Wu, Q.; Ortegon, A.M.; Tsang, B.; Doege, H.; Feingold, K.R.; Stahl, A. FATP1 is an insulin-sensitive fatty acid transporter involved in diet-induced obesity. Mol. Cell. Biol. 2006, 26, 3455–3467. [Google Scholar] [CrossRef]
- Wang, M.; Jiao, H.; Zhao, J.; Lin, H.; Wang, X. The involvement of FATP1 regulating skeletal muscle fat deposition in stressed broilers was affected by fatty acid substrates. Front. Vet. Sci. 2022, 9, 965894. [Google Scholar] [CrossRef]
- Chabowski, A.; Coort, S.L.M.; Calles-Escandon, J.; Tandon, N.N.; Glatz, J.F.C.; Luiken, J.J.F.P.; Bonen, A. The subcellular compartmentation of fatty acid transporters is regulated differently by insulin and by AICAR. FEBS Lett. 2005, 579, 2428–2432. [Google Scholar] [CrossRef] [PubMed]
- Chiu, H.-C.; Kovacs, A.; Blanton, R.M.; Han, X.; Courtois, M.; Weinheimer, C.J.; Yamada, K.A.; Brunet, S.; Xu, H.; Nerbonne, J.M.; et al. Transgenic expression of fatty acid transport protein 1 in the heart causes lipotoxic cardiomyopathy. Circ. Res. 2005, 96, 225–233. [Google Scholar] [CrossRef]
- Binnert, C.; Koistinen, H.A.; Martin, G.; Andreelli, F.; Ebeling, P.; Koivisto, V.A.; Laville, M.; Auwerx, J.; Vidal, H. Fatty acid transport protein-1 mRNA expression in skeletal muscle and in adipose tissue in humans. Am. J. Physiol. Endocrinol. Metab. 2000, 279, E1072–E1079. [Google Scholar] [CrossRef]
- Holloway, G.P.; Chou, C.J.; Lally, J.; Stellingwerff, T.; Maher, A.C.; Gavrilova, O.; Haluzik, M.; Alkhateeb, H.; Reitman, M.L.; Bonen, A. Increasing skeletal muscle fatty acid transport protein 1 (FATP1) targets fatty acids to oxidation and does not predispose mice to diet-induced insulin resistance. Diabetologia 2011, 54, 1457–1467. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ochiai, Y.; Uchida, Y.; Ohtsuki, S.; Tachikawa, M.; Aizawa, S.; Terasaki, T. The blood-brain barrier fatty acid transport protein 1 (FATP1/SLC27A1) supplies docosahexaenoic acid to the brain, and insulin facilitates transport. J. Neurochem. 2017, 141, 400–412. [Google Scholar] [CrossRef] [PubMed]
- Schmuth, M.; Ortegon, A.M.; Mao-Qiang, M.; Elias, P.M.; Feingold, K.R.; Stahl, A. Differential expression of fatty acid transport proteins in epidermis and skin appendages. J. Investig. Dermatol. 2005, 125, 1174–1181. [Google Scholar] [CrossRef]
- Yao, H.; Gong, J.; Peterson, A.L.; Lu, X.; Zhang, P.; Dennery, P.A. Fatty Acid Oxidation Protects against Hyperoxia-induced Endothelial Cell Apoptosis and Lung Injury in Neonatal Mice. Am. J. Respir. Cell Mol. Biol. 2019, 60, 667–677. [Google Scholar] [CrossRef]
- Liu, Z.; Gao, Z.; Li, B.; Li, J.; Ou, Y.; Yu, X.; Zhang, Z.; Liu, S.; Fu, X.; Jin, H.; et al. Lipid-associated macrophages in the tumor-adipose microenvironment facilitate breast cancer progression. Oncoimmunology 2022, 11, 2085432. [Google Scholar] [CrossRef]
- Acharya, R.; Shetty, S.S.; Kumari, N.S. Fatty acid transport proteins (FATPs) in cancer. Chem. Phys. Lipids 2023, 250, 105269. [Google Scholar] [CrossRef]
- Acharya, R.; Shetty, S.S.; Monteiro, F.; Shetty, A.S.; Shetty, D.P.; Patil, P.; Kumari, N.S. Serum fatty acid transport protein 1 in women with and without breast cancer. Biomedicine 2022, 42, 1185–1190. [Google Scholar] [CrossRef]
- Watkins, P.A.; Pevsner, J.; Steinberg, S.J. Human very long-chain acyl-CoA synthetase and two human homologs: Initial characterization and relationship to fatty acid transport protein. Prostaglandins Leukot. Essent. Fat. Acids 1999, 60, 323–328. [Google Scholar] [CrossRef]
- Coe, N.R.; Smith, A.J.; Frohnert, B.I.; Watkins, P.A.; Bernlohr, D.A. The fatty acid transport protein (FATP1) is a very long chain acyl-CoA synthetase. J. Biol. Chem. 1999, 274, 36300–36304. [Google Scholar] [CrossRef]
- Schaffer, J.E.; Lodish, H.F. Expression cloning and characterization of a novel adipocyte long chain fatty acid transport protein. Cell 1994, 79, 427–436. [Google Scholar] [CrossRef]
- Mendes, C.; Lopes-Coelho, F.; Ramos, C.; Martins, F.; Santos, I.; Rodrigues, A.; Silva, F.; André, S.; Serpa, J. Unraveling FATP1, regulated by ER-β, as a targeted breast cancer innovative therapy. Sci. Rep. 2019, 9, 14107. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Di Martino, J.S.; Bowman, R.L.; Campbell, N.R.; Baksh, S.C.; Simon-Vermot, T.; Kim, I.S.; Haldeman, P.; Mondal, C.; Yong-Gonzales, V.; et al. Adipocyte-Derived Lipids Mediate Melanoma Progression via FATP Proteins. Cancer Discov. 2018, 8, 1006–1025. [Google Scholar] [CrossRef]
- Corn, K.C.; Windham, M.A.; Rafat, M. Lipids in the tumor microenvironment: From cancer progression to treatment. Prog. Lipid Res. 2020, 80, 101055. [Google Scholar] [CrossRef]
- Biochemistry of Lipids, Lipoproteins and Membranes; Elsevier: Amsterdam, The Netherlands, 2016; ISBN 9780444634382.
- Anderson, C.M.; Stahl, A. SLC27 fatty acid transport proteins. Mol. Aspects Med. 2013, 34, 516–528. [Google Scholar] [CrossRef]
- Doege, H.; Stahl, A. Protein-mediated fatty acid uptake: Novel insights from in vivo models. Physiology 2006, 21, 259–268. [Google Scholar] [CrossRef]
- Gimeno, R.E.; Ortegon, A.M.; Patel, S.; Punreddy, S.; Ge, P.; Sun, Y.; Lodish, H.F.; Stahl, A. Characterization of a heart-specific fatty acid transport protein. J. Biol. Chem. 2003, 278, 16039–16044. [Google Scholar] [CrossRef] [PubMed]
- Habets, D.D.J.; Coumans, W.A.; Voshol, P.J.; den Boer, M.A.M.; Febbraio, M.; Bonen, A.; Glatz, J.F.C.; Luiken, J.J.F.P. AMPK-mediated increase in myocardial long-chain fatty acid uptake critically depends on sarcolemmal CD36. Biochem. Biophys. Res. Commun. 2007, 355, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Heather, L.C.; Cole, M.A.; Lygate, C.A.; Evans, R.D.; Stuckey, D.J.; Murray, A.J.; Neubauer, S.; Clarke, K. Fatty acid transporter levels and palmitate oxidation rate correlate with ejection fraction in the infarcted rat heart. Cardiovasc. Res. 2006, 72, 430–437. [Google Scholar] [CrossRef]
- Wu, Q.; Li, B.; Li, Z.; Li, J.; Sun, S.; Sun, S. Cancer-associated adipocytes: Key players in breast cancer progression. J. Hematol. Oncol. 2019, 12, 95. [Google Scholar] [CrossRef]
- Chandi, J. The Role of Fatty Acid Transport in Breast Cancer Growth, Progression, and Metastasis. Bachelor’s Thesis, Department of Nutrition, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA, 2020. [Google Scholar]
- Mitchell, R.W.; On, N.H.; Del Bigio, M.R.; Miller, D.W.; Hatch, G.M. Fatty acid transport protein expression in human brain and potential role in fatty acid transport across human brain microvessel endothelial cells. J. Neurochem. 2011, 117, 735–746. [Google Scholar] [CrossRef]
- Banks, W.A.; Rhea, E.M. The Blood–Brain Barrier, Oxidative Stress, and Insulin Resistance. Antioxidants 2021, 10, 1695. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.; Simpkin, A.J.; Hartmann, M.D.; Rigden, D.J.; Keegan, R.M.; Lupas, A.N. High-accuracy protein structure prediction in CASP14. Proteins 2021, 89, 1687–1699. [Google Scholar] [CrossRef] [PubMed]
- Hekkelman, M.L.; de Vries, I.; Joosten, R.P.; Perrakis, A. AlphaFill: Enriching AlphaFold models with ligands and cofactors. Nat. Methods 2023, 20, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Tunyasuvunakool, K.; Adler, J.; Wu, Z.; Green, T.; Zielinski, M.; Žídek, A.; Bridgland, A.; Cowie, A.; Meyer, C.; Laydon, A.; et al. Highly accurate protein structure prediction for the human proteome. Nature 2021, 596, 590–596. [Google Scholar] [CrossRef]
- Varadi, M.; Anyango, S.; Deshpande, M.; Nair, S.; Natassia, C.; Yordanova, G.; Yuan, D.; Stroe, O.; Wood, G.; Laydon, A.; et al. AlphaFold Protein Structure Database: Massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Res. 2022, 50, D439–D444. [Google Scholar] [CrossRef]
- Geourjon, C.; Deléage, G. SOPMA: Significant improvements in protein secondary structure prediction by consensus prediction from multiple alignments. Comput. Appl. Biosci. 1995, 11, 681–684. [Google Scholar] [CrossRef]
- McGuffin, L.J.; Bryson, K.; Jones, D.T. The PSIPRED protein structure prediction server. Bioinformatics 2000, 16, 404–405. [Google Scholar] [CrossRef]
- Heo, L.; Park, H.; Seok, C. GalaxyRefine: Protein structure refinement driven by side-chain repacking. Nucleic Acids Res. 2013, 41, W384–W388. [Google Scholar] [CrossRef] [PubMed]
- Pugalenthi, G.; Shameer, K.; Srinivasan, N.; Sowdhamini, R. HARMONY: A server for the assessment of protein structures. Nucleic Acids Res. 2006, 34, W231–W234. [Google Scholar] [CrossRef] [PubMed]
- Cilia, E.; Pancsa, R.; Tompa, P.; Lenaerts, T.; Vranken, W.F. The DynaMine webserver: Predicting protein dynamics from sequence. Nucleic Acids Res. 2014, 42, W264–W270. [Google Scholar] [CrossRef]
- Laskowski, R.A.; MacArthur, M.W.; Moss, D.S.; Thornton, J.M. PROCHECK: A program to check the stereochemical quality of protein structures. J. Appl. Crystallogr. 1993, 26, 283–291. [Google Scholar] [CrossRef]
- Colovos, C.; Yeates, T.O. Verification of protein structures: Patterns of nonbonded atomic interactions. Protein Sci. 1993, 2, 1511–1519. [Google Scholar] [CrossRef] [PubMed]
- Lüthy, R.; Bowie, J.U.; Eisenberg, D. Assessment of protein models with three-dimensional profiles. Nature 1992, 356, 83–85. [Google Scholar] [CrossRef]
- Pontius, J.; Richelle, J.; Wodak, S.J. Deviations from standard atomic volumes as a quality measure for protein crystal structures. J. Mol. Biol. 1996, 264, 121–136. [Google Scholar] [CrossRef]
- Basu, S.; Naha, A.; Veeraraghavan, B.; Ramaiah, S.; Anbarasu, A. In silico structure evaluation of BAG3 and elucidating its association with bacterial infections through protein-protein and host-pathogen interaction analysis. J. Cell. Biochem. 2022, 123, 115–127. [Google Scholar] [CrossRef]
- Wiederstein, M.; Sippl, M.J. ProSA-web: Interactive web service for the recognition of errors in three-dimensional structures of proteins. Nucleic Acids Res. 2007, 35, W407–W410. [Google Scholar] [CrossRef]
- Lemkul, J. From proteins to perturbed Hamiltonians: A suite of tutorials for the GROMACS-2018 molecular simulation package [article v1. 0]. Liv. J. Comput. Mol. Sci. 2018, 1, 5068. [Google Scholar] [CrossRef]
- Jayaraman, M.; Rajendra, S.K.; Ramadas, K. Structural insight into conformational dynamics of non-active site mutations in KasA: A Mycobacterium tuberculosis target protein. Gene 2019, 720, 144082. [Google Scholar] [CrossRef] [PubMed]
- Muthu, K.; Panneerselvam, M.; Topno, N.S.; Jayaraman, M.; Ramadas, K. Structural perspective of ARHI mediated inhibition of STAT3 signaling: An insight into the inactive to active transition of ARHI and its interaction with STAT3 and importinβ. Cell. Signal. 2015, 27, 739–755. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Franceschini, A.; Wyder, S.; Forslund, K.; Heller, D.; Huerta-Cepas, J.; Simonovic, M.; Roth, A.; Santos, A.; Tsafou, K.P.; et al. STRING v10: Protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015, 43, D447–D452. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Franceschini, A.; Kuhn, M.; Simonovic, M.; Roth, A.; Minguez, P.; Doerks, T.; Stark, M.; Muller, J.; Bork, P.; et al. The STRING database in 2011: Functional interaction networks of proteins, globally integrated and scored. Nucleic Acids Res. 2011, 39, D561–D568. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. von STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.X.; Jung, D.; Yao, R. ShinyGO: A graphical gene-set enrichment tool for animals and plants. Bioinformatics 2020, 36, 2628–2629. [Google Scholar] [CrossRef]
- Sekaran, T.S.G.; Kedilaya, V.R.; Kumari, S.N.; Shetty, P.; Gollapalli, P. Exploring the differentially expressed genes in human lymphocytes upon response to ionizing radiation: A network biology approach. Radiat. Oncol. J. 2021, 39, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Lavanya, P.; Ramaiah, S.; Anbarasu, A. Influence of C-H...O interactions on the structural stability of β-lactamases. J. Biol. Phys. 2013, 39, 649–663. [Google Scholar] [CrossRef]
- Lavanya, P.; Ramaiah, S.; Anbarasu, A. Non-canonical H-bonds in β-lactamases: Importance of C-H···π interactions. J. Biol. Inorg. Chem. 2013, 18, 539–545. [Google Scholar] [CrossRef]
- Lavanya, P.; Ramaiah, S.; Anbarasu, A. Binding site residues in β-lactamases: Role in non-classical interactions and metal binding. J. Coord. Chem. 2014, 67, 2898–2910. [Google Scholar] [CrossRef]
- Panja, A.S.; Maiti, S.; Bandyopadhyay, B. Protein stability governed by its structural plasticity is inferred by physicochemical factors and salt bridges. Sci. Rep. 2020, 10, 1822. [Google Scholar] [CrossRef]
- Lavanya, P.; Ramaiah, S.; Singh, H.; Bahadur, R.; Anbarasu, A. Investigations on the role of CH…O interactions and its impact on stability and specificity of penicillin binding proteins. Comput. Biol. Med. 2015, 65, 85–92. [Google Scholar] [CrossRef]
- Benson, N.C.; Daggett, V. A comparison of multiscale methods for the analysis of molecular dynamics simulations. J. Phys. Chem. B 2012, 116, 8722–8731. [Google Scholar] [CrossRef]
- de Souza, A.S.; Pacheco, B.D.C.; Pinheiro, S.; Muri, E.M.F.; Dias, L.R.S.; Lima, C.H.S.; Garrett, R.; de Moraes, M.B.M.; de Souza, B.E.G.; Puzer, L. 3-Acyltetramic acids as a novel class of inhibitors for human kallikreins 5 and 7. Bioorg. Med. Chem. Lett. 2019, 29, 1094–1098. [Google Scholar] [CrossRef] [PubMed]
- Likic, V.A.; Gooley, P.R.; Speed, T.P.; Strehler, E.E. A statistical approach to the interpretation of molecular dynamics simulations of calmodulin equilibrium dynamics. Protein Sci. 2005, 14, 2955–2963. [Google Scholar] [CrossRef]
- Luck, K.; Jailkhani, N.; Cusick, M.E.; Rolland, T.; Calderwood, M.A.; Charloteaux, B.; Vidal, M. Interactomes-Scaffolds of Cellular Systems. Biology 2016, 430–443. [Google Scholar] [CrossRef]
- Oughtred, R.; Rust, J.; Chang, C.; Breitkreutz, B.-J.; Stark, C.; Willems, A.; Boucher, L.; Leung, G.; Kolas, N.; Zhang, F.; et al. The BioGRID database: A comprehensive biomedical resource of curated protein, genetic, and chemical interactions. Protein Sci. 2021, 30, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhu, R.; Shi, D. The role of FATP1 in lipid accumulation: A review. Mol. Cell. Biochem. 2021, 476, 1897–1903. [Google Scholar] [CrossRef]
- Fu, Y.; Zou, T.; Shen, X.; Nelson, P.J.; Li, J.; Wu, C.; Yang, J.; Zheng, Y.; Bruns, C.; Zhao, Y.; et al. Lipid metabolism in cancer progression and therapeutic strategies. MedComm 2021, 2, 27–59. [Google Scholar] [CrossRef]
- Azam, A.; Sounni, N.E. Lipid Metabolism Heterogeneity and Crosstalk with Mitochondria Functions Drive Breast Cancer Progression and Drug Resistance. Cancers 2022, 14, 6267. [Google Scholar] [CrossRef]
- Lopes-Coelho, F.; André, S.; Félix, A.; Serpa, J. Breast cancer metabolic cross-talk: Fibroblasts are hubs and breast cancer cells are gatherers of lipids. Mol. Cell. Endocrinol. 2018, 462, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Kuriata, A.; Gierut, A.M.; Oleniecki, T.; Ciemny, M.P.; Kolinski, A.; Kurcinski, M.; Kmiecik, S. CABS-flex 2.0: A web server for fast simulations of flexibility of protein structures. Nucleic Acids Res. 2018, 46, W338–W343. [Google Scholar] [CrossRef]
- Dolan, C.; Burke, C.S.; Byrne, A.; Keyes, T.E. Cellular uptake and sensing capability of transition metal peptide conjugates. In Inorganic and Organometallic Transition Metal Complexes with Biological Molecules and Living Cells; Elsevier: Amsterdam, The Netherlands, 2017; pp. 55–89. ISBN 9780128038147. [Google Scholar]
- Smith, D.J.; Earl, A.J.; Turner, G. The multifunctional peptide synthetase performing the first step of penicillin biosynthesis in Penicillium chrysogenum is a 421,073 dalton protein similar to Bacillus brevis peptide antibiotic synthetases. EMBO J. 1990, 9, 2743–2750. [Google Scholar] [CrossRef] [PubMed]
- Schröder, J. Protein sequence homology between plant 4-coumarate:CoA ligase and firefly luciferase. Nucleic Acids Res. 1989, 17, 460. [Google Scholar] [CrossRef] [PubMed]
- Mallonee, D.H.; White, W.B.; Hylemon, P.B. Cloning and sequencing of a bile acid-inducible operon from Eubacterium sp. strain VPI 12708. J. Bacteriol. 1990, 172, 7011–7019. [Google Scholar] [CrossRef]
- Conti, E.; Franks, N.P.; Brick, P. Crystal structure of firefly luciferase throws light on a superfamily of adenylate-forming enzymes. Structure 1996, 4, 287–298. [Google Scholar] [CrossRef]
- Jackson, D.R.; Tu, S.S.; Nguyen, M.; Barajas, J.F.; Schaub, A.J.; Krug, D.; Pistorius, D.; Luo, R.; Müller, R.; Tsai, S.-C. Structural Insights into Anthranilate Priming during Type II Polyketide Biosynthesis. ACS Chem. Biol. 2016, 11, 95–103. [Google Scholar] [CrossRef]
- DiRusso, C.C.; Li, H.; Darwis, D.; Watkins, P.A.; Berger, J.; Black, P.N. Comparative biochemical studies of the murine fatty acid transport proteins (FATP) expressed in yeast. J. Biol. Chem. 2005, 280, 16829–16837. [Google Scholar] [CrossRef]

| GO-TERM | Description | Count in Network | Strength | False Discovery Rate |
|---|---|---|---|---|
| GO:1904017 | Cellular response to Thyroglobulin triodothyronine | 2 of 2 | 3.25 | 0.0012 |
| GO:1901700 | Response to oxygen-containing compound | 8 of 1567 | 0.96 | 0.00024 |
| GO:1901576 | Organic substance biosynthetic process | 7 of 2734 | 0.66 | 0.0391 |
| GO:0071396 | Cellular response to lipid | 5 of 528 | 1.23 | 0.0027 |
| GO:0051173 | Positive regulation of nitrogen compound metabolic process | 8 of 3239 | 0.64 | 0.0166 |
| GO:0048545 | Response to steroid hormone | 5 of 328 | 1.43 | 0.0005 |
| GO:0071310 | Cellular response to organic substance | 8 of 2369 | 0.78 | 0.0026 |
| GO:0045893 | Positive regulation of transcription, DNA-templated | 6 of 1587 | 0.83 | 0.0234 |
| GO:0046320 | Regulation of fatty acid oxidation | 2 of 32 | 2.05 | 0.0341 |
| GO:0045834 | Positive regulation of lipid metabolic process | 3 of 152 | 1.55 | 0.0205 |
| GO:0045722 | Positive regulation of gluconeogenesis | 2 of 14 | 2.41 | 0.0108 |
| GO:0045017 | Glycerolipid biosynthetic process | 3 of 229 | 1.37 | 0.0433 |
| GO:0044539 | Long-chain fatty acid import into cell | 2 of 10 | 2.55 | 0.0067 |
| GO:0044249 | Cellular biosynthetic process | 7 of 2611 | 0.68 | 0.0333 |
| GO:0043401 | Steroid hormone-mediated signalling pathway | 4 of 118 | 1.78 | 0.00042 |
| GO:0043393 | Regulation of protein binding | 3 of 212 | 1.4 | 0.0391 |
| GO:0035336 | Long-chain fatty-acyl-CoA metabolic process | 2 of 25 | 2.15 | 0.0247 |
| GO:0034654 | Nucleobase-containing compound biosynthetic process | 5 of 995 | 0.95 | 0.0287 |
| GO:0034201 | Response to oleic acid | 2 of 6 | 2.77 | 0.0033 |
| GO:0033993 | Response to lipid | 7 of 858 | 1.16 | 0.00011 |
| GO:0033211 | Adiponectin-activated signalling pathway | 2 of 7 | 2.71 | 0.004 |
| GO:0033036 | Macromolecule localisation | 8 of 2473 | 0.76 | 0.0033 |
| GO:0032870 | Cellular response to hormone stimulus | 7 of 569 | 1.34 | 1.34 × 10−5 |
| GO:0032570 | Response to progesterone | 2 of 45 | 1.9 | 0.048 |
| GO:0031328 | Positive regulation of cellular biosynthetic process | 8 of 2005 | 0.85 | 0.0011 |
| GO:0031325 | Positive regulation of cellular metabolic process | 9 of 3413 | 0.67 | 0.0027 |
| GO:0030522 | Intracellular receptor signalling pathway | 4 of 166 | 1.63 | 0.0012 |
| GO:0019432 | Triglyceride biosynthetic process | 2 of 20 | 2.25 | 0.0184 |
| GO:0015908 | Fatty acid transport | 3 of 74 | 1.86 | 0.0038 |
| GO:0019216 | Regulation of lipid metabolic process | 11 of 424 | 1.66 | 7.39 × 10−15 |
| GO:0015721 | Bile acid and bile salt transport | 3 of 30 | 2.25 | 0.00057 |
| GO:0015718 | Monocarboxylic acid transport | 6 of 142 | 1.88 | 4.85 × 10−7 |
| GO:0014070 | Response to organic cyclic compound | 6 of 911 | 1.07 | 0.002 |
| GO:0010906 | Regulation of glucose metabolic process | 3 of 118 | 1.66 | 0.0111 |
| GO:0010876 | Lipid localisation | 7 of 326 | 1.58 | 5.16 × 10−7 |
| GO:0010867 | Positive regulation of triglyceride biosynthetic process | 2 of 13 | 2.44 | 0.0099 |
| GO:0010604 | Positive regulation of macromolecule metabolic process | 8 of 3600 | 0.6 | 0.0287 |
| GO:0009755 | Hormone-mediated signalling pathway | 6 of 169 | 1.8 | 6.70 × 10−7 |
| GO:0006869 | Lipid transport | 6 of 296 | 1.56 | 1.34 × 10−5 |
| GO:0006629 | Lipid metabolic process | 5 of 1190 | 0.87 | 0.0457 |
| GO:0006351 | Transcription, DNA-templated | 4 of 567 | 1.1 | 0.0391 |
| GO:0006139 | Nucleobase-containing compound metabolic process | 7 of 2659 | 0.67 | 0.034 |
| GO:1904017 | Cellular response to Thyroglobulin triiodothyronine | 2 of 2 | 3.25 | 0.0012 |
| GO:1901700 | Response to oxygen-containing compound | 8 of 1567 | 0.96 | 0.00024 |
| GO:1901576 | Organic substance biosynthetic process | 7 of 2734 | 0.66 | 0.0391 |
| GO:0071396 | Cellular response to lipid | 5 of 528 | 1.23 | 0.0027 |
| GO:0051173 | Positive regulation of nitrogen compound metabolic process | 8 of 3239 | 0.64 | 0.0166 |
| GO:0048545 | Response to steroid hormone | 5 of 328 | 1.43 | 0.0005 |
| GO:0045722 | Positive regulation of gluconeogenesis | 2 of 14 | 2.41 | 0.0108 |
| GO:0071310 | Cellular response to organic substance | 8 of 2369 | 0.78 | 0.0026 |
| GO:0045893 | Positive regulation of transcription, DNA-templated | 6 of 1587 | 0.83 | 0.0234 |
| GO:0046320 | Regulation of fatty acid oxidation | 2 of 32 | 2.05 | 0.0341 |
| GO:0045834 | Positive regulation of lipid metabolic process | 3 of 152 | 1.55 | 0.0205 |
| KEGG Pathways Involved in Functional Enrichment Analysis | ||||
|---|---|---|---|---|
| GO-TERM | Description | Count in Network | Strength | False Discovery Rate |
| hsa04975 | Fat digestion and absorption | 2 of 41 | 1.94 | 0.0215 |
| hsa04920 | Adipocytokine signalling pathway | 3 of 69 | 1.89 | 0.0013 |
| hsa03320 | PPAR signalling pathway | 4 of 75 | 1.98 | 2.66 × 10−5 |
| hsa04919 | Thyroid hormone signalling pathway | 3 of 119 | 1.65 | 0.0042 |
| hsa04975 | Fat digestion and absorption | 2 of 41 | 1.94 | 0.0215 |
| Reactome Pathways Involved in Functional Enrichment Analysis of FATP1/SLC27A1 | ||||
| HSA-9623433 | NR1H2 and NR1H3 regulate gene expression to control bile acid homeostasis | 2 of 9 | 2.6 | 0.0012 |
| HSA-9029569 | NR1H3 and NR1H2 regulate gene expression linked to cholesterol transport and efflux | 2 of 37 | 1.98 | 0.0137 |
| HSA-9018519 | Estrogen-dependent gene expression | 3 of 119 | 1.65 | 0.0027 |
| HSA-9006931 | Signalling by nuclear receptors | 4 of 265 | 1.43 | 0.00086 |
| has-556833 | Metabolism of lipids | 11 of 733 | 1.43 | 4.39 × 10−14 |
| HSA-4090294 | SUMOylation of intracellular receptors | 2 of 29 | 2.09 | 0.0093 |
| HSA-3899300 | SUMOylation of transcription co-factors | 2 of 43 | 1.92 | 0.0174 |
| HSA-383280 | Nuclear receptor transcription pathway | 2 of 53 | 1.83 | 0.024 |
| HSA-3247509 | Chromatin-modifying enzymes | 4 of 237 | 1.48 | 0.0006 |
| HSA-381340 | Transcriptional regulation of white adipocyte differentiation | 8 of 84 | 2.23 | 6.86 × 10−15 |
| HSA-3214858 | RMTs methylate histone arginines | 2 of 49 | 1.86 | 0.0212 |
| HSA-3108232 | SUMO E3 ligases SUMOylate target proteins | 4 of 166 | 1.63 | 0.00018 |
| HSA-2426168 | Activation of gene expression by SREBF(SREBP) | 8 of 42 | 2.53 | 7.16 × 10−17 |
| HSA-2151201 | Transcriptional activation of mitochondrial biogenesis | 8 of 54 | 2.42 | 3.75 × 10−16 |
| HSA-211976 | Endogenous sterols | 3 of 27 | 2.3 | 6.07 × 10−5 |
| HSA-1989781 | PPARA activates gene expression | 10 of 117 | 2.18 | 1.09 × 10−18 |
| HSA-193807 | Synthesis of bile acids and bile salts via 27- hydroxycholesterol | 3 of 15 | 2.55 | 1.46 × 10−5 |
| HSA-193368 | Synthesis of bile acids and bile salts via 7alpha- hydroxycholesterol | 3 of 24 | 2.35 | 4.62 × 10−5 |
| HSA-159418 | Recycling of bile acids and salts | 3 of 16 | 2.52 | 1.63 × 10−5 |
| HSA-1368108 | BMAL1:CLOCK, NPAS2 activates circadian gene expression | 8 of 27 | 2.72 | 3.93 × 10−18 |
| HSA-1368082 | RORA activates gene expression | 8 of 18 | 2.9 | 1.05 × 10−18 |
| S.No. | Gene | Gene id | Disease | Disease id | Gene(SLC27A1)–Disease Association Score |
|---|---|---|---|---|---|
| 1 | SLC27A1 | 376497 | Obesity | C0028754 | 0.22 |
| 2 | SLC27A1 | 376497 | Myocardial Infarction | C0027051 | 0.2 |
| 3 | SLC27A1 | 376497 | Hyperlipoproteinemias | C0020476 | 0.2 |
| 4 | SLC27A1 | 376497 | Hyperlipidemia | C0020473 | 0.2 |
| 5 | SLC27A1 | 376497 | Hyperinsulinism | C0020459 | 0.2 |
| 6 | SLC27A1 | 376497 | Impaired Glucose Tolerance | C0271650 | 0.2 |
| 7 | SLC27A1 | 376497 | Insulin Resistance | C0021655 | 0.2 |
| 8 | SLC27A1 | 376497 | Gestational Diabetes | C0085207 | 0.01 |
| 9 | SLC27A1 | 376497 | Endometrial Carcinoma | C0476089 | 0.01 |
| 10 | SLC27A1 | 376497 | Metabolic Syndrome X | C0524620 | 0.01 |
| 11 | SLC27A1 | 376497 | Breast Carcinoma | C0678222 | 0.01 |
| 12 | SLC27A1 | 376497 | Tumor Cell Invasion | C1269955 | 0.01 |
| 13 | SLC27A1 | 376497 | Photoreceptor Degeneration | C1998028 | 0.01 |
| 14 | SLC27A1 | 376497 | Experimental Organism Basal Cell Carcinoma | C3811653 | 0.01 |
| 15 | SLC27A1 | 376497 | Atherosclerotic Lesion | C4703473 | 0.01 |
| 16 | SLC27A1 | 376497 | Diffuse Large B-Cell Lymphoma | C0079744 | 0.01 |
| 17 | SLC27A1 | 376497 | Uterine Fibroids | C0042133 | 0.01 |
| 18 | SLC27A1 | 376497 | Cardiovascular Diseases | C0007222 | 0.01 |
| 19 | SLC27A1 | 376497 | Diabetes Mellitus, Non-Insulin-Dependent | C0011860 | 0.01 |
| 20 | SLC27A1 | 376497 | Fetal Growth Retardation | C0015934 | 0.01 |
| 21 | SLC27A1 | 376497 | Ichthyoses | C0020757 | 0.01 |
| 22 | SLC27A1 | 376497 | Congenital Ichthyosis | C0020758 | 0.01 |
| 23 | SLC27A1 | 376497 | Fibroid Tumour | C0023267 | 0.01 |
| 24 | SLC27A1 | 376497 | Melanoma | C0025202 | 0.01 |
| 25 | SLC27A1 | 376497 | Metabolic Diseases | C0025517 | 0.01 |
| 26 | SLC27A1 | 376497 | Malignant Neoplasm of Breast | C0006142 | 0.01 |
| 27 | SLC27A1 | 376497 | Carcinoma, Basal Cell | C4721806 | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acharya, R.; Shetty, S.S.; Pavan, G.; Monteiro, F.; Munikumar, M.; Naresh, S.; Kumari, N.S. AI-Based Homology Modelling of Fatty Acid Transport Protein 1 Using AlphaFold: Structural Elucidation and Molecular Dynamics Exploration. Biomolecules 2023, 13, 1670. https://doi.org/10.3390/biom13111670
Acharya R, Shetty SS, Pavan G, Monteiro F, Munikumar M, Naresh S, Kumari NS. AI-Based Homology Modelling of Fatty Acid Transport Protein 1 Using AlphaFold: Structural Elucidation and Molecular Dynamics Exploration. Biomolecules. 2023; 13(11):1670. https://doi.org/10.3390/biom13111670
Chicago/Turabian StyleAcharya, Ranjitha, Shilpa S. Shetty, Gollapalli Pavan, Flama Monteiro, Manne Munikumar, Sriram Naresh, and Nalilu Suchetha Kumari. 2023. "AI-Based Homology Modelling of Fatty Acid Transport Protein 1 Using AlphaFold: Structural Elucidation and Molecular Dynamics Exploration" Biomolecules 13, no. 11: 1670. https://doi.org/10.3390/biom13111670
APA StyleAcharya, R., Shetty, S. S., Pavan, G., Monteiro, F., Munikumar, M., Naresh, S., & Kumari, N. S. (2023). AI-Based Homology Modelling of Fatty Acid Transport Protein 1 Using AlphaFold: Structural Elucidation and Molecular Dynamics Exploration. Biomolecules, 13(11), 1670. https://doi.org/10.3390/biom13111670






