Apiaceae Family an Important Source of Petroselinic Fatty Acid: Abundance, Biosynthesis, Chemistry, and Biological Proprieties
Abstract
:1. Introduction
2. Natural Sources of Petroselinic Acid
3. Biosynthesis of Petroselinic Acid
4. Identification and Quantification of Petroselinic Acid
5. Isolation of Petroselinic Acid
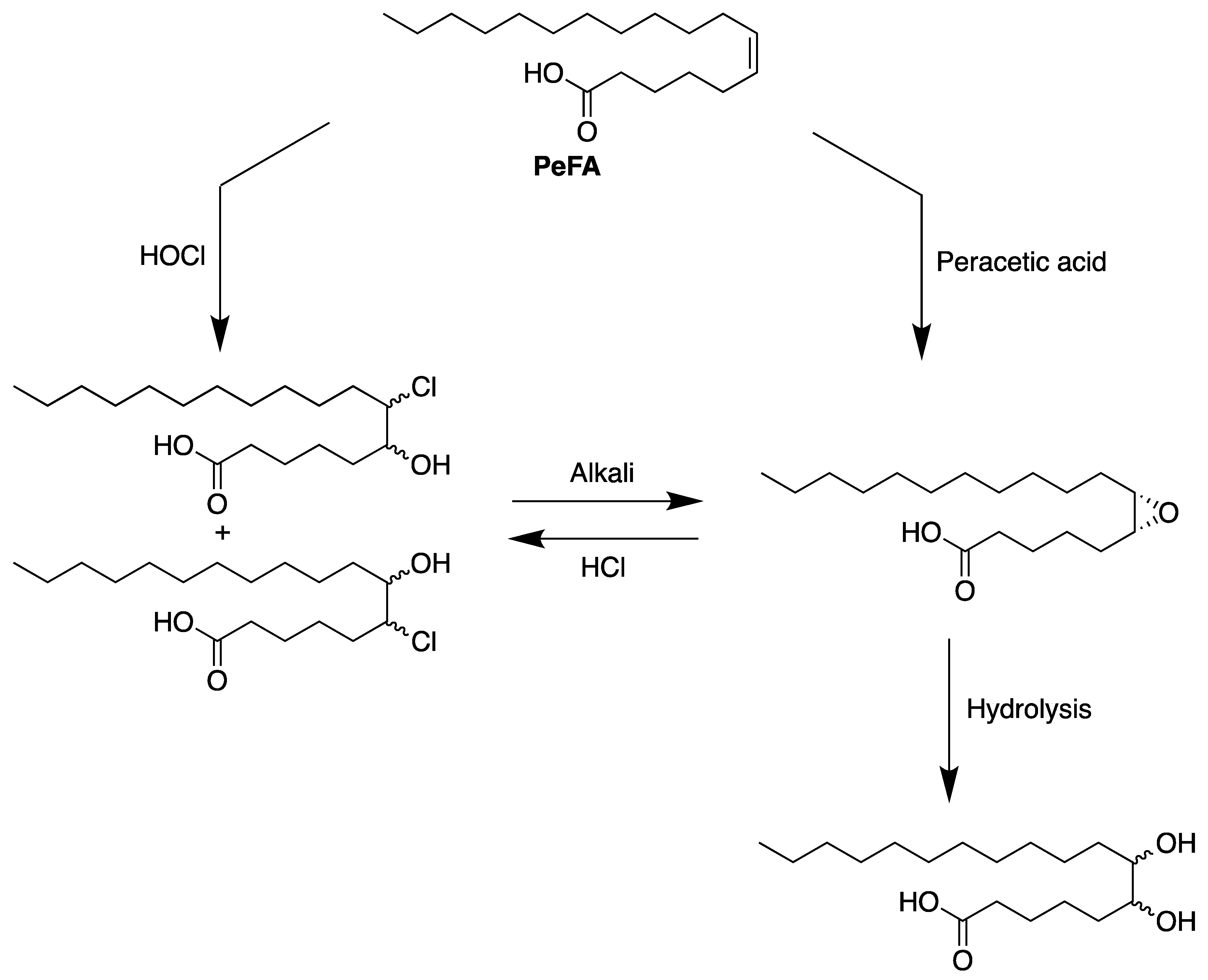
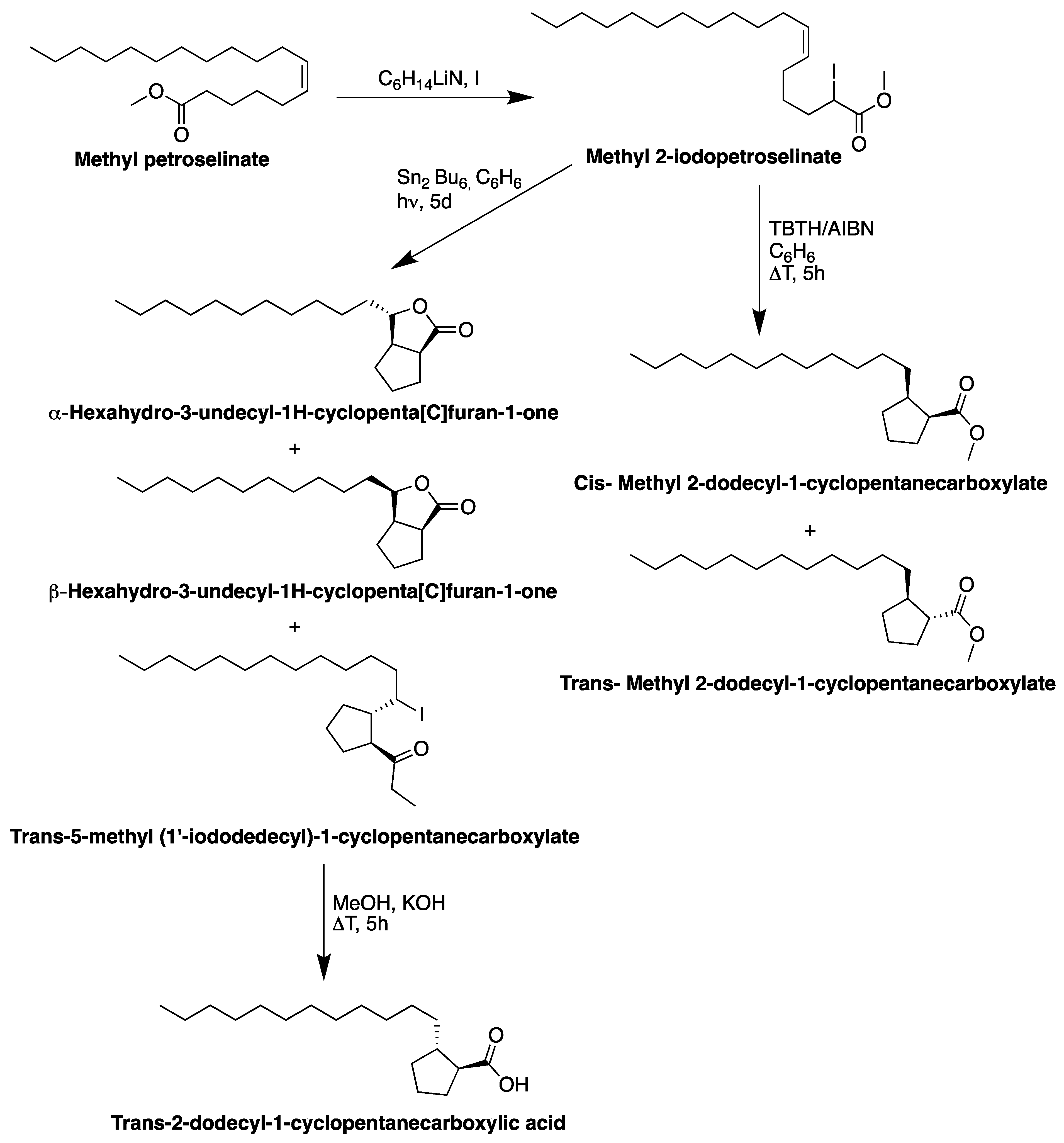
6. Reactivity of Petroselinic Acid
7. Biological Properties of Petroselinic Acid
8. Potential Application of PeFA
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Avato, P.; Tava, A. Rare fatty acids and lipids in plant oilseeds: Occurrence and bioactivity. Phytochem. Rev. 2022, 21, 401–428. [Google Scholar]
- Jie, M.S.L.K. The synthesis of rare and unusual fatty acids. Prog. Lipid Res. 1993, 32, 151–194. [Google Scholar]
- Green, A. The occurrence of ricinoleic acid in Linum seed oils. J. Am. Oil Chem. Soc. 1984, 61, 939–940. [Google Scholar] [CrossRef]
- Cahoon, E.B.; Li-Beisson, Y. Plant unusual fatty acids: Learning from the less common. Curr. Opin. Plant Biol. 2020, 55, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.-Y.; Guo, X.; Smith, M.A. Identification of Crepenynic Acid in the Seed Oil of Atractylodes lancea and A. macrocephala. J. Am. Oil Chem. Soc. 2017, 94, 655–660. [Google Scholar] [CrossRef]
- Fatope, M.O.; Adoum, O.A.; Takeda, Y. C18 acetylenic fatty acids of Ximenia americana with potential pesticidal activity. J. Agric. Food Chem. 2000, 48, 1872–1874. [Google Scholar] [CrossRef]
- Okada, S.; Zhou, X.-R.; Damcevski, K.; Gibb, N.; Wood, C.; Hamberg, M.; Haritos, V.S. Diversity of Δ12 fatty acid desaturases in santalaceae and their role in production of seed oil acetylenic fatty acids. J. Biol. Chem. 2013, 288, 32405–32413. [Google Scholar] [CrossRef]
- Spitzer, V.; Aitzetmüller, K.; Vosmann, K. The seed oil of Bernardia pulchella (Euphorbiaceae)—A rich source of vernolic acid. J. Am. Oil Chem. Soc. 1996, 73, 1733–1735. [Google Scholar] [CrossRef]
- Morris, L.; Wharry, D. Naturally occurring epoxy acids. IV. The absolute optical configuration of vernolic acid. Lipids 1966, 1, 41–46. [Google Scholar] [CrossRef]
- Tsevegsuren, N.; Aitzetmuller, K.; Vosmann, K. Geranium sanguineum (Geraniaceae) seed oil: A new source of petroselinic and vernolic acid. Lipids 2004, 39, 571–576. [Google Scholar] [CrossRef]
- Bao, X.; Katz, S.; Pollard, M.; Ohlrogge, J. Carbocyclic fatty acids in plants: Biochemical and molecular genetic characterization of cyclopropane fatty acid synthesis of Sterculia foetida. Proc. Natl. Acad. Sci. USA 2002, 99, 7172–7177. [Google Scholar] [CrossRef] [PubMed]
- Chaves, M.; Araújo, F.; Moura, C.; Tozetto, L.; Aued-Pimentel, S.; Caruso, M. Chemical characterization and stability of the Bombacopsis glabra nut oil. Food Public Health 2012, 2, 104–109. [Google Scholar] [CrossRef]
- Hajib, A.; Nounah, I.; Harhar, H.; Gharby, S.; Kartah, B.; Matthäus, B.; Bougrin, K.; Charrouf, Z. Oil content, lipid profiling and oxidative stability of “Sefri” Moroccan pomegranate (Punica granatum L.) seed oil. OCL 2021, 28, 5. [Google Scholar] [CrossRef]
- Nagao, K.; Yanagita, T. Conjugated fatty acids in food and their health benefits. J. Biosci. Bioeng. 2005, 100, 152–157. [Google Scholar] [PubMed]
- Knothe, G.; Steidley, K.R. Composition of Some Apiaceae Seed Oils Includes Phytochemicals, and Mass Spectrometry of Fatty Acid 2-Methoxyethyl Esters. Eur. J. Lipid. Sci. Technol. 2019, 121, 1800386. [Google Scholar] [CrossRef]
- Avato, P.; Fanizzi, F.P.; Rosito, I. The genus Thapsia as a source of petroselinic acid. Lipids 2001, 36, 845–850. [Google Scholar] [CrossRef]
- Thiviya, P.; Gamage, A.; Piumali, D.; Merah, O.; Madhujith, T. Apiaceae as an important source of antioxidants and their applications. Cosmetics 2021, 8, 111. [Google Scholar] [CrossRef]
- Hajib, A.; Danton, O.; Keller, M.; Potterat, O.; Bougrin, K.; Charrouf, Z.; Hamburger, M. Polyacetylenic caffeoyl amides from Ammodaucus leucotrichus. Phytochemistry 2023, 206, 113555. [Google Scholar] [CrossRef]
- Thiviya, P.; Gunawardena, N.; Gamage, A.; Madhujith, T.; Merah, O. Apiaceae family as a valuable source of biocidal components and their potential uses in agriculture. Horticulturae 2022, 8, 614. [Google Scholar] [CrossRef]
- Zidorn, C.; Jöhrer, K.; Ganzera, M.; Schubert, B.; Sigmund, E.M.; Mader, J.; Greil, R.; Ellmerer, E.P.; Stuppner, H. Polyacetylenes from the Apiaceae vegetables carrot, celery, fennel, parsley, and parsnip and their cytotoxic activities. J. Agric. Food Chem. 2005, 53, 2518–2523. [Google Scholar] [CrossRef]
- Balbino, S.; Repajić, M.; Obranović, M.; Medved, A.M.; Tonković, P.; Dragović-Uzelac, V.; Plants, A. Characterization of lipid fraction of Apiaceae family seed spices: Impact of species and extraction method. J. Appl. Res. Med. Aromat. Plants 2021, 25, 100326. [Google Scholar]
- Sayed-Ahmad, B.; Talou, T.; Saad, Z.; Hijazi, A.; Merah, O. The Apiaceae: Ethnomedicinal family as source for industrial uses. Ind. Crops Prod. 2017, 109, 661–671. [Google Scholar] [CrossRef]
- Delbeke, E.I.; Everaert, J.; Uitterhaegen, E.; Verweire, S.; Verlee, A.; Talou, T.; Soetaert, W.; Van Bogaert, I.N.; Stevens, C.V. Petroselinic acid purification and its use for the fermentation of new sophorolipids. AMB Express 2016, 6, 28. [Google Scholar] [CrossRef] [PubMed]
- Placek, L.L. A review on petroselinic acid and its derivatives. J. Am. Oil Chem. Soc. 1963, 40, 319–329. [Google Scholar] [CrossRef]
- Hajib, A.; Harhar, H.; Gharby, S.; Nounah, I.; Matthäus, B.; Guillaume, D.; Charrouf, Z. Is geographic origin a good marker for cumin seed oil (Cuminum cyminum L.). Riv. Ital. Sostanze Grasse 2018, 95, 155–159. [Google Scholar]
- Bettaieb Rebey, I.; Bourgou, S.; Aidi Wannes, W.; Hamrouni Selami, I.; Saidani Tounsi, M.; Marzouk, B.; Fauconnier, M.-L.; Ksouri, R. Comparative assessment of phytochemical profiles and antioxidant properties of Tunisian and Egyptian anise (Pimpinella anisum L.) seeds. Plant Biosyst. 2018, 152, 971–978. [Google Scholar] [CrossRef]
- Laribi, B.; Kouki, K.; Bettaieb, T.; Mougou, A.; Marzouk, B. Essential oils and fatty acids composition of Tunisian, German and Egyptian caraway (Carum carvi L.) seed ecotypes: A comparative study. Ind. Crops Prod. 2013, 41, 312–318. [Google Scholar] [CrossRef]
- Ksouda, G.; Hajji, M.; Sellimi, S.; Merlier, F.; Falcimaigne-Cordin, A.; Nasri, M.; Thomasset, B. A systematic comparison of 25 Tunisian plant species based on oil and phenolic contents, fatty acid composition and antioxidant activity. Ind. Crops Prod. 2018, 123, 768–778. [Google Scholar] [CrossRef]
- Al-Snafi, A.E. Pharmacological and therapeutic activities of Hedera helix-A review. Iosr J. Pharm. 2018, 8, 41–53. [Google Scholar]
- Shibahara, A.; Yamamoto, K.; Nakayama, T.; Kajimoto, G. cis-Vaccenic acid in pulp lipids of commonly available fruits. J. Am. Oil Chem. Soc. 1987, 64, 397–401. [Google Scholar] [CrossRef]
- Spitzer, V. Fatty acid composition of some seed oils of the Sapindaceae. Phytochemistry 1996, 42, 1357–1360. [Google Scholar] [CrossRef]
- Wolff, R.L.; Christie, W.W.; Pédrono, F.; Marpeau, A.M.; Tsevegsüren, N.; Aitzetmüller, K.; Gunstone, F.D. Δ5-Olefinic acids in the seed lipids from four Ephedra species and their distribution between the α and β positions of triacylglycerols. Characteristics common to coniferophytes and cycadophytes. Lipids 1999, 34, 855–864. [Google Scholar] [CrossRef]
- Rebey, I.B.; Jabri-Karoui, I.; Hamrouni-Sellami, I.; Bourgou, S.; Limam, F.; Marzouk, B. Effect of drought on the biochemical composition and antioxidant activities of cumin (Cuminum cyminum L.) seeds. Ind. Crops Prod. 2012, 36, 238–245. [Google Scholar] [CrossRef]
- Laribi, B.; Bettaieb, I.; Kouki, K.; Sahli, A.; Mougou, A.; Marzouk, B. Water deficit effects on caraway (Carum carvi L.) growth, essential oil and fatty acid composition. Ind. Crops Prod. 2009, 30, 372–379. [Google Scholar] [CrossRef]
- Rebey, I.B.; Wannes, W.A.; Kaab, S.B.; Bourgou, S.; Tounsi, M.S.; Ksouri, R.; Fauconnier, M.L. Bioactive compounds and antioxidant activity of Pimpinella anisum L. accessions at different ripening stages. Sci. Hortic. 2019, 246, 453–461. [Google Scholar] [CrossRef]
- Msaada, K.; Hosni, K.; Taarit, M.B.; Hammami, M.; Marzouk, B. Effects of growing region and maturity stages on oil yield and fatty acid composition of coriander (Coriandrum sativum L.) fruit. Sci. Hortic. 2009, 120, 525–531. [Google Scholar] [CrossRef]
- Kenar, J.A.; Moser, B.R.; List, G.R. Naturally occurring fatty acids: Source, chemistry, and uses. In Fatty Acids; Elsevier: Amsterdam, The Netherlands, 2017; pp. 23–82. [Google Scholar]
- Krist, S. Vegetable Fats and Oils; Springer Nature: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Ashawat, M.; Banchhor, M.; Saraf, S.; Saraf, S. Herbal Cosmetics: “Trends in Skin Care Formulation”. Pharmacogn. Rev. 2009, 3, 82. [Google Scholar]
- Tong, Y.F.; Zhang, P.; Chen, F.; Hao, L.H.; Ye, F.; Tian, J.Y.; Wu, S. Synthesis and biological evaluation of novel N-(alkoxyphenyl)-aminocarbonylbenzoic acid derivatives as PTP1B inhibitors. Chin. Chem. Lett. 2010, 21, 1415–1418. [Google Scholar] [CrossRef]
- Novak, A.F.; Clark, G.C.; Dupuy, H.P. Antimicrobial activity of some ricinoleic acid oleic acid derivatives. J. Am. Oil Chem. Soc. 1961, 38, 321–324. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kim, Y.-G.; Lee, J. Inhibition of Staphylococcus aureus Biofilm Formation and Virulence Factor Production by Petroselinic Acid and Other Unsaturated C18 Fatty Acids. Microbiol. Spectr. 2022, 10, e01330-22. [Google Scholar] [CrossRef]
- Chbani, M.; El Harkaoui, S.; Willenberg, I.; Matthäus, B. Analytical Extraction Methods, Physicochemical Properties and Chemical Composition of Cactus (Opuntia ficus-indica) Seed Oil and Its Biological Activity. Food Rev. Int. 2022, 1–17. [Google Scholar] [CrossRef]
- Bettaieb Rebey, I.; Bourgou, S.; Detry, P.; Wannes, W.A.; Kenny, T.; Ksouri, R.; Sellami, I.H.; Fauconnier, M.-L. Green extraction of fennel and anise edible oils using bio-based solvent and supercritical fluid: Assessment of chemical composition, antioxidant property, and oxidative stability. Food Bioproc. Technol. 2019, 12, 1798–1807. [Google Scholar] [CrossRef]
- Nguyen, T.; Aparicio, M.; Saleh, M.A. Accurate mass GC/LC-quadrupole time of flight mass spectrometry analysis of fatty acids and triacylglycerols of spicy fruits from the Apiaceae family. Molecules 2015, 20, 21421–21432. [Google Scholar] [CrossRef]
- Sriti, J.; Talou, T.; Msaada, K.; Marzouk, B. Comparative analysis of fatty acid, sterol and tocol composition of Tunisian and Canadian Coriander (Coriandrum sativum L.) fruit. Anal. Chem. Lett. 2011, 1, 375–383. [Google Scholar] [CrossRef]
- Denev, R.V.; Kuzmanova, I.S.; Momchilova, S.M.; Nikolova-Damyanova, B.M. Resolution and quantification of isomeric fatty acids by silver ion HPLC: Fatty acid composition of aniseed oil (Pimpinella anisum, Apiaceae). J. AOAC Int. 2011, 94, 4–8. [Google Scholar] [CrossRef]
- Beyzi, E.; Karaman, K.; Gunes, A.; Beyzi, S.B. Change in some biochemical and bioactive properties and essential oil composition of coriander seed (Coriandrum sativum L.) varieties from Turkey. Ind. Crops Prod. 2017, 109, 74–78. [Google Scholar] [CrossRef]
- Uitterhaegen, E.; Sampaio, K.A.; Delbeke, E.I.; De Greyt, W.; Cerny, M.; Evon, P.; Merah, O.; Talou, T.; Stevens, C.V. Characterization of French coriander oil as source of petroselinic acid. Molecules 2016, 21, 1202. [Google Scholar] [CrossRef]
- Nguyen, Q.-H.; Talou, T.; Evon, P.; Cerny, M.; Merah, O. Fatty acid composition and oil content during coriander fruit development. Food Chem. 2020, 326, 127034. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Q.-H.; Talou, T.; Cerny, M.; Evon, P.; Merah, O. Oil and fatty acid accumulation during coriander (Coriandrum sativum L.) fruit ripening under organic cultivation. Crop J. 2015, 3, 366–369. [Google Scholar] [CrossRef]
- Sriti, J.; Talou, T.; Faye, M.; Vilarem, G.; Marzouk, B.; Products. Oil extraction from coriander fruits by extrusion and comparison with solvent extraction processes. Ind. Crops Prod. 2011, 33, 659–664. [Google Scholar] [CrossRef]
- Senrayan, J.; Venkatachalam, S. Optimization of ultrasound-assisted solvent extraction (UASE) based on oil yield, antioxidant activity and evaluation of fatty acid composition and thermal stability of Coriandrum sativum L. seed oil. Food Sci. Biotechnol. 2019, 28, 377–386. [Google Scholar] [CrossRef]
- Kozłowska, M.; Gruczyńska, E.; Ścibisz, I.; Rudzińska, M. Fatty acids and sterols composition, and antioxidant activity of oils extracted from plant seeds. Food chem. 2016, 213, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Assefa, A.D.; Keum, Y.-S. Spices in the Apiaceae Family Represent the Healthiest Fatty Acid Profile: A Systematic Comparison of 34 Widely Used Spices and Herbs. Foods 2021, 10, 854. [Google Scholar] [CrossRef] [PubMed]
- Ngo-Duy, C.C.; Destaillats, F.; Keskitalo, M.; Arul, J.; Angers, P. Triacylglycerols of Apiaceae seed oils: Composition and regiodistribution of fatty acids. Eur. J. Lipid. Sci. Technol. 2009, 111, 164–169. [Google Scholar] [CrossRef]
- Laribi, B.; Kouki, K.; Mougou, A.; Marzouk, B. Fatty acid and essential oil composition of three Tunisian caraway (Carum carvi L.) seed ecotypes. J. Sci. Food Agric. 2010, 90, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Bourgou, S.; Bettaieb Rebey, I.; Dakhlaoui, S.; Msaada, K.; Saidani Tounsi, M.; Ksouri, R.; Fauconnier, M.L.; Hamrouni-Sellami, I. Green extraction of oil from Carum carvi seeds using bio-based solvent and supercritical fluid: Evaluation of its antioxidant and anti-inflammatory activities. Phytochem. Anal. 2020, 31, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Bettaieb, I.; Bourgou, S.; Sriti, J.; Msaada, K.; Limam, F.; Marzouk, B. Essential oils and fatty acids composition of Tunisian and Indian cumin (Cuminum cyminum L.) seeds: A comparative study. J. Sci. Food Agric. 2011, 91, 2100–2107. [Google Scholar] [CrossRef] [PubMed]
- Merah, O.; Sayed-Ahmad, B.; Talou, T.; Saad, Z.; Cerny, M.; Grivot, S.; Evon, P.; Hijazi, A. Biochemical composition of cumin seeds, and biorefining study. Biomolecules 2020, 10, 1054. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, M.F.; Asker, M.M.S.; Tadros, M. Antiradical and antimicrobial properties of cold-pressed black cumin and cumin oils. Eur. Food Res. Technol. 2012, 234, 833–844. [Google Scholar] [CrossRef]
- Ahmad, B.S.; Talou, T.; Saad, Z.; Hijazi, A.; Cerny, M.; Kanaan, H.; Chokr, A.; Merah, O. Fennel oil and by-products seed characterization and their potential applications. Ind. Crops Prod. 2018, 111, 92–98. [Google Scholar] [CrossRef]
- Hayat, K.; Abbas, S.; Hussain, S.; Shahzad, S.A.; Tahir, M.U. Effect of microwave and conventional oven heating on phenolic constituents, fatty acids, minerals and antioxidant potential of fennel seed. Ind. Crops Prod. 2019, 140, 111610. [Google Scholar] [CrossRef]
- Piras, A.; Porcedda, S.; Falconieri, D.; Fais, A.; Era, B.; Carta, G.; Rosa, A. Supercritical extraction of volatile and fixed oils from Petroselinum crispum L. seeds: Chemical composition and biological activity. Nat. Prod. Res. 2022, 36, 1883–1888. [Google Scholar] [CrossRef]
- Shams, K.A.; Abdel-Azim, N.S.; Tawfik, W.A.; Hassanein, H.D.; Saleh, M.A.; Hammouda, F.M. Green extraction techniques: Effect of extraction method on lipid contents of three medicinal plants of Apiaceae. J. Chem. Pharm. Res. 2015, 7, 1080–1088. [Google Scholar]
- Cahoon, E.B.; Shanklin, J.; Ohlrogge, J.B. Expression of a coriander desaturase results in petroselinic acid production in transgenic tobacco. Proc. Natl. Acad. Sci. USA 1992, 89, 11184–11188. [Google Scholar] [CrossRef] [PubMed]
- Coşge, B.; Kiralan, M.; Gürbüz, B. Characteristics of fatty acids and essential oil from sweet fennel (Foeniculum vulgare Mill. var. dulce) and bitter fennel fruits (F. vulgare Mill. var. vulgare) growing in Turkey. Nat. Prod. Res. 2008, 22, 1011–1016. [Google Scholar] [PubMed]
- Yaldiz, G.; Camlica, M. Variation in the fruit phytochemical and mineral composition, and phenolic content and antioxidant activity of the fruit extracts of different fennel (Foeniculum vulgare L.) genotypes. Ind. Crops Prod. 2019, 142, 111852. [Google Scholar] [CrossRef]
- Mhemdi, H.; Rodier, E.; Kechaou, N.; Fages, J. A supercritical tuneable process for the selective extraction of fats and essential oil from coriander seeds. J. Food Eng. 2011, 105, 609–616. [Google Scholar] [CrossRef]
- Thelen, J.J.; Ohlrogge, J.B. Metabolic engineering of fatty acid biosynthesis in plants. Metab. Eng. 2002, 4, 12–21. [Google Scholar] [CrossRef]
- Mekhedov, S.; Cahoon, E.B.; Ohlrogge, J. An unusual seed-specific 3-ketoacyl-ACP synthase associated with the biosynthesis of petroselinic acid in coriander. Plant Mol. Biol. 2001, 47, 507–518. [Google Scholar] [CrossRef]
- Cahoon, E.B.; Dörmann, P.; Ohlrogge, J.B. Petroselinic acid biosynthesis and production in transgenic plants. Prog. Lipid Res. 1994, 33, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Li, C.; Jia, Q.; Zhao, C.; Taylor, D.C.; Li, D.; Zhang, M. Transcriptome analysis reveals candidate genes for petroselinic acid biosynthesis in fruits of Coriandrum sativum L. J. Agric. Food Chem. 2020, 68, 5507–5520. [Google Scholar] [CrossRef]
- Cahoon, E.B.; Ohlrogge, J.B. Apparent role of phosphatidylcholine in the metabolism of petroselinic acid in developing Umbelliferae endosperm. Plant Physiol. 1994, 104, 845–855. [Google Scholar] [CrossRef] [PubMed]
- Dormann, P.; Frentzen, M.; Ohlrogge, J.B. Specificities of the acyl-acyl carrier protein (ACP) thioesterase and glycerol-3-phosphate acyltransferase for octadecenoyl-ACP isomers (identification of a petroselinoyl-ACP thioesterase in umbelliferae). Plant Physiol. 1994, 104, 839–844. [Google Scholar] [CrossRef] [PubMed]
- Voelker, T. Plant acyl-ACP thioesterases: Chain-length determining enzymes in plant fatty acid biosynthesis. In Genetic Engineering; Springer: Boston, MA, USA, 1996; Volume 18, pp. 111–133. [Google Scholar]
- Biłas, R.; Szafran, K.; Hnatuszko-Konka, K.; Kononowicz, A.K. Cis-regulatory elements used to control gene expression in plants. Plant Cell, Tissue Organ Cult. 2016, 127, 269–287. [Google Scholar] [CrossRef]
- Guiltinan, M.J.; Marcotte Jr, W.R.; Quatrano, R.S. A plant leucine zipper protein that recognizes an abscisic acid response element. Science 1990, 250, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Washida, H.; Wu, C.-Y.; Suzuki, A.; Yamanouchi, U.; Akihama, T.; Harada, K.; Takaiwa, F. Identification of cis-regulatory elements required for endosperm expression of the rice storage protein glutelin gene GluB-1. Plant Mol. Biol. 1999, 40, 1–12. [Google Scholar] [CrossRef]
- Wu, C.Y.; Washida, H.; Onodera, Y.; Harada, K.; Takaiwa, F. Quantitative nature of the Prolamin-box, ACGT and AACA motifs in a rice glutelin gene promoter: Minimal cis-element requirements for endosperm-specific gene expression. Plant J. 2000, 23, 415–421. [Google Scholar] [CrossRef]
- Kim, M.J.; Shin, J.S.; Kim, J.-K.; Suh, M.C. Genomic structures and characterization of the 5′-flanking regions of acyl carrier protein and Δ4-palmitoyl-ACP desaturase genes from Coriandrum sativum. Biochim. Biophys. Acta-Gene Struct. Expr. 2005, 1730, 235–244. [Google Scholar] [CrossRef]
- Kang, J.; Yim, S.; Choi, H.; Kim, A.; Lee, K.P.; Lopez-Molina, L.; Martinoia, E.; Lee, Y. Abscisic acid transporters cooperate to control seed germination. Nat. Commun. 2015, 6, 8113. [Google Scholar] [CrossRef]
- Vega, A.; O’Brien, J.A.; Gutiérrez, R.A. Nitrate and hormonal signaling crosstalk for plant growth and development. Curr. Opin. Plant Biol. 2019, 52, 155–163. [Google Scholar] [CrossRef]
- Von Rudloff, E. Periodate-permanganate oxidations. IV. Determination of the position of double bonds in unsaturated fatty acids and esters. J. Am. Oil Chem. Soc. 1956, 33, 126–128. [Google Scholar] [CrossRef]
- Wolff, R.L.; Vandamme, F.F. Separation of petroselinic (cis-6 18: 1) and oleic (cis-9 18: 1) acids by gas-liquid chromatography of their isopropyl esters. J. Am. Oil Chem. Soc. 1992, 69, 1228–1231. [Google Scholar] [CrossRef]
- Santinelli, F.; Damiani, P. A simple and rapid method for concurrent determination of petroselinic and oleic acids in oils. J. Am. Oil Chem. Soc. 1997, 74, 935–938. [Google Scholar] [CrossRef]
- Liu, L.; Hammond, E. Phenylethyl esters of fatty acids for the analytical resolution of petroselinate and oleate. J. Am. Oil Chem. Soc. 1995, 72, 749–751. [Google Scholar] [CrossRef]
- Thies, W. Determination of the petroselinic acid in seeds of Coriandrum sativum by gas liquid chromatography as n-butyl esters. Lipid/Fett 1995, 97, 411–413. [Google Scholar] [CrossRef]
- Sansa-ard, C.; Aryusuk, K.; Lilitchan, S.; Krisnangkura, K. Free energy contribution to gas chromatographic separation of petroselinate and oleate esters. Chromatogr. Res. Int. 2011, 2011, 252543. [Google Scholar] [CrossRef]
- Radin, N.S. The hydroxy fatty acids: Isolation, structure determination, quantitation. J. Am. Oil Chem. Soc. 1965, 42, 569–580. [Google Scholar] [CrossRef] [PubMed]
- Charvet, A.; Comeau, L.; Gaydou, E. New preparation of pure petroselinic acid from fennel oil (Foeniculum vulgare). J. Am. Oil Chem. Soc. 1991, 68, 604–607. [Google Scholar] [CrossRef]
- Fore, S.P.; Holmes, R.L.; Bickford, W. Preparation of petroselinic acid. J. Am. Oil Chem. Soc. 1960, 37, 490–491. [Google Scholar] [CrossRef]
- Mbayhoudel, K.; Comeau, L.-C. Obtention sélective de l’acide pétrosélinique à partir de l’huile de fenouil par hydrolyse enzymatique. Rev. Française Corps Gras 1989, 36, 427–431. [Google Scholar]
- Ackman, R.G.; Retson, M.; Gallay, L.; Vandenheuvel, F. Ozonolysis of unsaturated fatty acids: I. Ozonolysis of oleic acid. Can. J. Chem. 1961, 39, 1956–1963. [Google Scholar] [CrossRef]
- Holmes, R.; Moreau, J. Preparation of 2-decyl-2-hydroxymethyl-1,3-propanediol from dodecanal and from petroselinic acid. J. Am. Oil Chem. Soc. 1965, 42, 833–835. [Google Scholar] [CrossRef]
- Holmes, R.; Moreau, J.; Sumrell, G. Preparation of 2-hydroxytridecanenitrile from petroselinic acid. J. Am. Oil Chem. Soc. 1965, 42, 841–842. [Google Scholar] [CrossRef]
- Holmes, R.; Moreau, J.; Sumrell, G. Application of the ritter reaction to petroselinic acid. J. Am. Oil Chem. Soc. 1965, 42, 922–923. [Google Scholar] [CrossRef]
- Farooq, M.; Osman, S.; Siddiqui, M.S. Studies on the hypohalogenation of isomeric 6,7-octadecenoic acids: Petroselinic and petroselaidic acids. Recl. Trav. Chim. 1961, 80, 415–421. [Google Scholar] [CrossRef]
- Metzger, J.O.; Mahler, R. Free-radical Cyclization of Petroselinic Acid. Justus Liebigs Ann. Chem. 1993, 1993, 203–205. [Google Scholar] [CrossRef]
- Placek, L.L.; Dollear, F. The preparation and properties of some nitrogen-containing derivatives of petroselinic acid. J. Am. Oil Chem. Soc. 1962, 39, 347–350. [Google Scholar] [CrossRef]
- Cermak, S.C.; Isbell, T.A.; Evangelista, R.L.; Johnson, B.L. Synthesis and physical properties of petroselinic based estolide esters. Ind. Crops Prod. 2011, 33, 132–139. [Google Scholar] [CrossRef]
- Dierker, M.; Schäfer, H.J. Surfactants from oleic, erucic and petroselinic acid: Synthesis and properties. Eur. J. Lipid. Sci. Technol. 2010, 112, 122–136. [Google Scholar] [CrossRef]
- Rao, P.; Kamalakar, K.; Jyothirmayi, T.; Karuna, M.; Prasad, R. Esters of petroselinic acid containing Trachyspermum copticum seed oil: Potential industrial lubricant base stocks. CSIR-NIScPR 2020, 59B, 126–134. [Google Scholar]
- Suzuki, K.; Shono, F.; Kai, H.; Uno, T.; Uyeda, M. Inhibition of topoisomerases by fatty acids. J. Enzyme Inhib. Med. Chem. 2000, 15, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, K.; Yamaoka, Y.; Kamiyabu, S.; Kishimoto, S.; Fukushima, S.; Suzuki, M.; Kobayashi, M.; Takeuchi, Y. Petroselinic Acid and Oleic Acid: Comparison of Enhanced Skin Permeability Relative to Affinity for Saturated Fatty Acids of Stratum Corneum. J. Pharm. Sci. Technol. 2000, 60, 53–61. [Google Scholar]
- Novak, A.; Fisher, M.J.; Fore, S.P.; Dupuy, H.P. Antimycotic activity of some fatty acid derivatives. J. Am. Oil Chem. Soc. 1964, 41, 503–505. [Google Scholar] [CrossRef]
- Mahe, Y.; Bru, C.; Gueniche, A. Use of Petroselinic Acid to Fight against Aesthetic Disorders of the Body Figure. U.S. Patent 14/890,064, 5 July 2014. [Google Scholar]
- Mahe, Y.; BRU, C.; Gueniche, A. Petroselinic Acid or a Combination of Active Ingredients Comprising at Least Petroselinic Acid for Promoting Weight Loss and/or Weight Maintenance. U.S. Patent 14/889,895, 5 June 2014. [Google Scholar]
- Mahe, Y.; Bru, C. Combination of Petroselinic Acid and Zinc for Oral Administration for Hair Aging Control. U.S. Patent 214/889,984, 5 June 2014. [Google Scholar]
- Gueniche, A.; Castiel, I. Use of Petroselinic Acid for the Treatment of Fragile Scalps. U.S. Patent ES06291929.5T, 14 December 2006. [Google Scholar]
- Weinkauf, R.; Santhanam, U.; Rose, L.; Palanker; Januario, E.T.; Brinker, A. Petroselinic Acid as an Anti-Irritant in Compositions Containing Alpha-Hydroxy Acids. U.S. Patent 6,022,896, 10 September 1998. [Google Scholar]
- Gueniche, A.; Castiel, I.; Cruz-Hernandez, C.; Guitard, M.M.-C.; Destaillats, F. Monounsaturated Fatty Acid for Nailcare. U.S. Patent 10/555,882, 8 November 2012. [Google Scholar]
- Mahe, Y.; Bru, C.; Piccardi, N.; Gueniche, A. Combination of Active agents for Oral Administration for Improving the Quality of Nails. U.S. Patent 9,682,025, 6 May 2014. [Google Scholar]
- Li, S.; Li, J.; Huang, W. New Application of Petroselinic Acid. C.N. Patent CN11510549.2A, 17 March 2021. [Google Scholar]
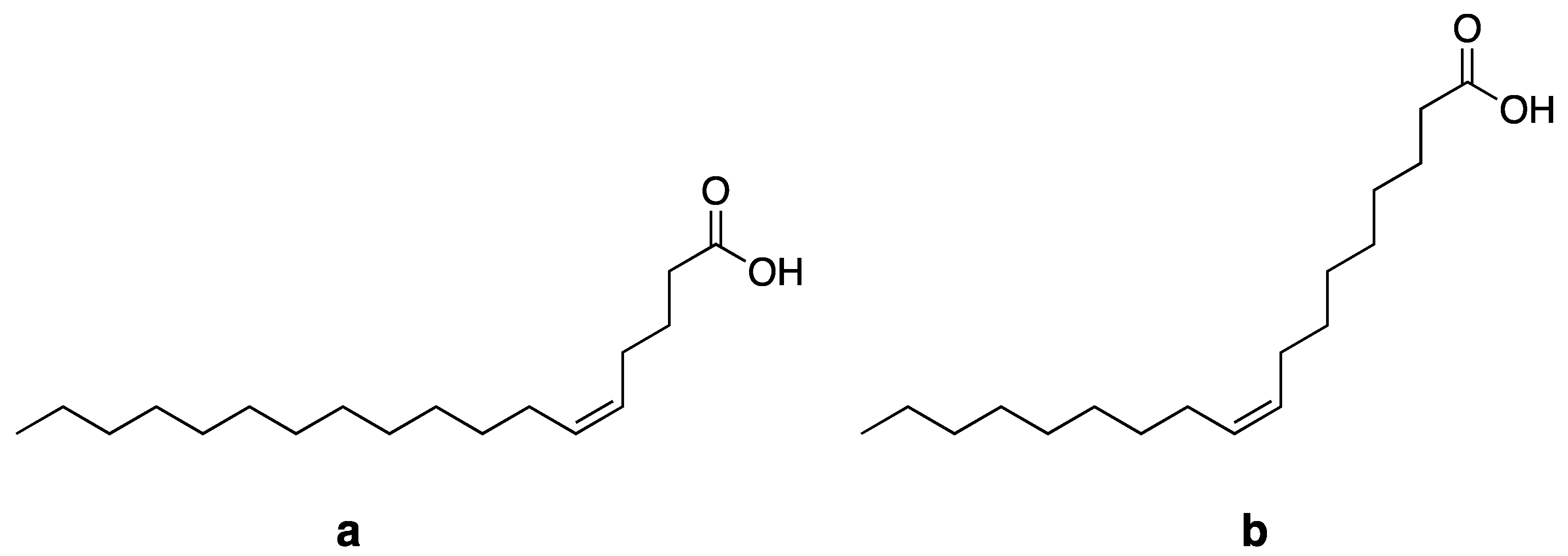


| Plant Name | Origin | Extraction Method/Solvent | Oil Yield (% Based on Dry Matter Weight) | Petroselinic Acid (as % of Total Fatty Acids) | References |
|---|---|---|---|---|---|
| Anise (Pimpinella anisum) | Tunisia | Soxhlet/n-Hex | 16.8 | 46.7 | [44] |
| Floch method/CHCl3:MeOH (2:1, v/v) | 24.0 | 42.3 | |||
| Soxhlet/MeTHF | 23.6 | 48.9 | |||
| Sc-CO2 | ≈16.5 | 47.0 | |||
| Tunisia | Precellys Homogenizer/diethyl ether | 25.0 | 31.3 | [28] | |
| Egypt * | Percolation/CHCl3:MeOH (2:1, v/v) | 15.3 | 66.2 | [45] | |
| Tunisia * | Soxhlet/n-Hex | 21.8 | 75.6 | [46] | |
| Canada * | Soxhlet/n-Hex | 15.8 | 73.2 | ||
| Tunisia | Bligh and Dyer’s method/ CHCl3:MeOH:Hex (1:2:1, v/v/v) | 6.7–13.8 | 13.5–46.6 (maturity) | [35] | |
| Serbia | Bligh and Dyer’s method/ CHCl3:MeOH:Hex (1:2:1, v/v/v) | 7.1–11.9 | 11.1–41.3 (maturity) | ||
| Egypt | Bligh and Dyer’s method/ CHCl3:MeOH:Hex (1:2:1, v/v/v) | 7.2–12.2 | 14.4–40.2 (maturity) | ||
| Turkey | Bligh and Dyer’s method/ CHCl3:MeOH:Hex (1:2:1, v/v/v) | 7.8–13.4 | 10.4–45.1 (maturity) | ||
| Tunisia | Soxhlet/Hex (for yield calculation) Bligh and Dyer’s method/ CHCl3:MeOH:Hex (1:2:1, v/v/v) (for further analysis) | 11.6 | 46.6 | [26] | |
| Egypt * | Soxhlet/Hex (for yield calculation) Bligh and Dyer’s method/ CHCl3:MeOH:Hex (1:2:1, v/v/v) (for further analysis) | 9.8 | 38.4 | ||
| Bulgaria * | Soxhlet/n-Hex | n.i. | 53.7 | [47] | |
| Coriander (Coriandrum sativum) | Turkey | Automatic analyzer/n.i | 4.7–6.2 | 79.7–81.9 (plant variety) | [48] |
| France | Soxhlet/n-Hex | 22.9 | 72.6 | [49] | |
| France | Soxhlet/n-cyclohexane | 5.8–24.9 | 1.0–74.3 (maturity) | [50] | |
| France | Soxhlet/n-cyclohexane | 4.6–25.1 | 2.8–76.4 (maturity) | [51] | |
| Tunisia | Soxhlet/Hex (for yield calculation) Bligh and Dyer’s method/ CHCl3:MeOH:Hex (1:2:1, v/v/v) (for further analysis) | 2.7–25.9 | 40.2–81.2 (maturity) | [36] | |
| Tunisia | OMEGA 20 single-screw extruder | 7.1–15.7 | 74.9–77.4 | [52] | |
| Soxhlet/hexane | 21.3 | 75.9 | |||
| Tunisia | Precellys Homogenizer/diethyl ether | 16.6 | 56.1 | [28] | |
| India * | UASE /hexane | 30.7 (at the optimum conditions) | 76.2 | [53] | |
| Poland * | Soxhlet/n-Hex | 20.0 | 73.4 | [54] | |
| Floch method/CHCl3:MeOH (2:1, v/v) | 22.1 | 73.8 | |||
| Korea * | Unspecific method (refer to article) | n.i. | 62.0 | [55] | |
| Egypt * | Percolation/ CHCl3:MeOH (2:1 v/v) | 16.8 | 79.7 | [45] | |
| Caraway (Carum carvi) | Egypt * | Percolation/ CHCl3:MeOH (2:1 v/v) | 14.3 | 57.6 | [45] |
| Canada * | Soxhlet/n-Hex | 19.2 | 40.6 | [56] | |
| Korea * | Unspecific method (refer to the article) | n.i. | 34.09 | [55] | |
| Poland * | Soxhlet/n-Hex | 20.1 | 33.3 | [54] | |
| Floch method/CHCl3:MeOH (2:1, v/v) | 18.9 | 33.5 | |||
| Tunisian | Bligh and Dyer’s method/ CHCl3:MeOH:Hex (4:3:2 v/v/v) | 7.3 | 31.1 | [27] | |
| Germany | Bligh and Dyer’s method/ CHCl3:MeOH:Hex (4:3:2 v/v/v) | 5.8 | 30.8 | ||
| Egypt | Bligh and Dyer’s method/ CHCl3:MeOH:Hex (4:3:2 v/v/v) | 2.9 | 29.4 | ||
| Tunisian | Bligh and Dyer’s method/ CHCl3:MeOH:Hex (4:3:2 v/v/v) | 2.9–5.6 | 31.5–38.3 (ecotypes) | [57] | |
| Tunisia * | Soxhlet/n-Hex | 13.0 | 43.4 | [58] | |
| Soxhlet/MeTHF | 16.0 | 40.3 | |||
| Floch method/CHCl3:MeOH (2:1, v/v) | 18.0 | 39.2 | |||
| Sc-CO2 | ≈11.5 | 43.5 | |||
| Tunisian | Bligh and Dyer’s method/ CHCl3:MeOH:Hex (4:3:2, v/v/v) | ≈3.0, 4.0 and 7.0 | 28.5–35.0 (water deficit effect) | [34] | |
| Cumin (Cuminum cyminum) | Tunisia | Soxhlet/Hex (for yield calculation) Bligh and Dyer’s method/ CHCl3:MeOH:Hex (1:2:1, v/v/v) for further analysis) | ≈9.0, 13.0 and 18.0 | 43.4–55.9 (effect of drought) | [33] |
| Tunisian * | Soxhlet/Hex (for yield calculation) Bligh and Dyer’s method/ CHCl3:MeOH:Hex (1:2:1, v/v/v) (for further analysis) | 17.7 | 55.9 | [59] | |
| Indian * | Soxhlet/Hex (for yield calculation) Bligh and Dyer’s method/ CHCl3:MeOH:Hex (1:2:1, v/v/v) (for further analysis) | 15.0 | 41.4 | [59] | |
| Morocco | Soxhlet/Hex | 16.3–25.7 | 54.9–60.9 (locations) | [25] | |
| Lebanon * | Soxhlet/cyclohexane | 23.1 | 49.2 | [60] | |
| France * | Soxhlet/cyclohexane | 29.1 | 51.5 | ||
| Algeria * | Soxhlet/cyclohexane | 13.4 | 51.6 | ||
| Syria * | Soxhlet/cyclohexane | 14.6 | 47.4 | ||
| Korea * | Unspecific method (refer to the article) | n.i. | 49.8 | [55] | |
| Egypt * | Percolation/CHCl3:MeOH (2:1, v/v) | 23.4 | 61.8 | [45] | |
| Egypt * | Cold pressing | n.i. | 41.3 | [61] | |
| Dill (Anethum graveolens) | Tunisia | Precellys Homogenizer/diethyl ether | 15.9 | 87.2 | [28] |
| Korea * | Unspecific method (refer to the article) | n.i. | 50.3 | [55] | |
| Egypt * | Percolation/CHCl3:MeOH (2:1, v/v) | 20.5 | 79.9 | [45] | |
| Fennel (Foeniculum vulgare) | Tunisia | Precellys Homogenizer/diethyl ether | 5.8 | 77.8 | [28] |
| n.i | Soxhlet/cyclohexane | 19.8 | 74.8 | [62] | |
| Tunisia | Soxhlet/n-Hex | ≈16.5 | 54.2 | [44] | |
| Floch method/CHCl3:MeOH (2:1, v/v) | 20.0 | 58.1 | |||
| Soxhlet/MeTHF | 18.7 | 61.2 | |||
| Sc-CO2 | ≈14.0 | 60.8 | |||
| Pakistan * | Petroleum ether/n-Hex | n.i. | 69.2–71.3 (roasting) | [63] | |
| Korea * | Unspecific method (refer to the article) | n.i. | 63.3 | [55] | |
| Egypt * | Percolation/CHCl3:MeOH (2:1, v/v) | 14.6 | 81.9 | [45] | |
| Egypt * | Percolation/CHCl3:MeOH (2:1, v/v) | 13.6 | 61.4 | [56] | |
| UASE/CHCl3:MeOH (2:1, v/v) | 17.9 | 76.4 | |||
| Sc-CO2 | 13.7 | 43.1 | |||
| Parsley (Petroselinum crispum) | Italy | Supercritical carbon dioxide | 0.4 | 50% (GC-FID) 181 mg/g of oil (HPLC-DAD) | [64] |
| Soxhlet/n-Hex | n.i. | ≈35% (GC-FID) 75.7 mg/g of oil (HPLC-DAD) | |||
| Egypt * | Percolation/CHCl3:MeOH (2:1, v/v) | 9.7 | 62.8 | [65] | |
| UASE/CHCl3:MeOH (2:1, v/v) | 11.3 | 71.6 | |||
| Sc-CO2 | 9.4 | 69.8 | |||
| Canada * | Soxhlet/n-Hex | 27.6 | 75.1 | [56] | |
| Celery (Apium graveolens) | Egypt * | Percolation/CHCl3:MeOH (2:1, v/v) | 9.8 | 65.7 | [45] |
| Korea * | Unspecific method (refer to the article) | n.i. | 49.4 | [55] | |
| Tunisia | Precellys Homogenizer/diethyl ether | 29.0 | 56.1 | [28] | |
| Canada * | Soxhlet/n-Hex | 25.4 | 64.3 | [56] | |
| Egypt * | Percolation/CHCl3:MeOH (2:1, v/v) | 9.8 | 58.7 | [65] | |
| UASE/ CHCl3:MeOH (2:1, v/v) | 14.4 | 75.6 | |||
| Sc-CO2 | 8.7 | 61.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hajib, A.; El Harkaoui, S.; Choukri, H.; Khouchlaa, A.; Aourabi, S.; El Menyiy, N.; Bouyahya, A.; Matthaeus, B. Apiaceae Family an Important Source of Petroselinic Fatty Acid: Abundance, Biosynthesis, Chemistry, and Biological Proprieties. Biomolecules 2023, 13, 1675. https://doi.org/10.3390/biom13111675
Hajib A, El Harkaoui S, Choukri H, Khouchlaa A, Aourabi S, El Menyiy N, Bouyahya A, Matthaeus B. Apiaceae Family an Important Source of Petroselinic Fatty Acid: Abundance, Biosynthesis, Chemistry, and Biological Proprieties. Biomolecules. 2023; 13(11):1675. https://doi.org/10.3390/biom13111675
Chicago/Turabian StyleHajib, Ahmed, Said El Harkaoui, Hasnae Choukri, Aya Khouchlaa, Sarra Aourabi, Naoual El Menyiy, Abdelhakim Bouyahya, and Bertrand Matthaeus. 2023. "Apiaceae Family an Important Source of Petroselinic Fatty Acid: Abundance, Biosynthesis, Chemistry, and Biological Proprieties" Biomolecules 13, no. 11: 1675. https://doi.org/10.3390/biom13111675
APA StyleHajib, A., El Harkaoui, S., Choukri, H., Khouchlaa, A., Aourabi, S., El Menyiy, N., Bouyahya, A., & Matthaeus, B. (2023). Apiaceae Family an Important Source of Petroselinic Fatty Acid: Abundance, Biosynthesis, Chemistry, and Biological Proprieties. Biomolecules, 13(11), 1675. https://doi.org/10.3390/biom13111675






