Progressive Exaptation of Endogenous Retroviruses in Placental Evolution in Cattle
Abstract
:1. Introduction
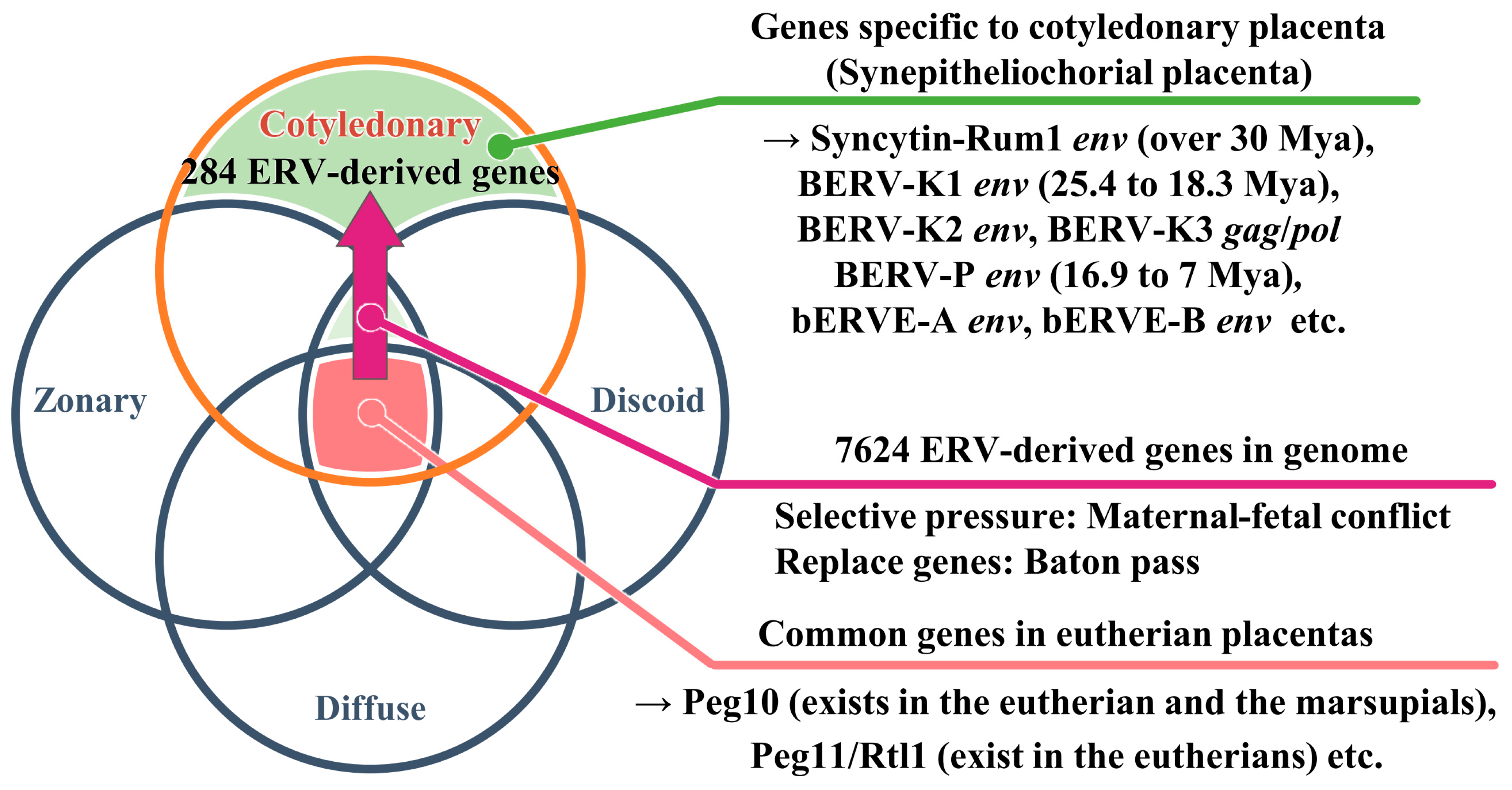
2. Diversity and Classification of Placenta in Mammals
3. The Principle of Genomic Rewiring during Waves of Retroviral Infection
4. Peg10, an LTR-Type Retrotransposon Commonly Expressed in Marsupial and Eutherian Placentas
5. The env Genes, Syncytin and Syncytin-like, Essential for Placentation in Mammals
6. Morphology of the Bovine Placenta
7. Expression of env-Derived Genes from Endogenous Retroviruses in Bovine Placenta
8. Regulatory Mechanisms Involved in BERV Gene Expression in the Bovine Placenta
9. Expression of gag-Derived Genes from Endogenous Retroviruses in Cattle Placenta
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shine, R. Life-history evolution in reptiles. Annu. Rev. Ecol. Evol. Syst. 2005, 36, 23–46. [Google Scholar] [CrossRef]
- Van Dyke, J.U.; Brandley, M.C.; Thompson, M.B. The evolution of viviparity: Molecular and genomic data from squamate reptiles advance understanding of live birth in amniotes. Reproduction 2013, 147, R15–R26. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, D.G. Evolution of vertebrate viviparity and specializations for fetal nutrition: A quantitative and qualitative analysis. J. Morphol. 2015, 276, 961–990. [Google Scholar] [CrossRef]
- Hughes, R.L.; Hall, L.S. Early development and embryology of the platypus. Philos. Trans. R. Soc. B Biol. Sci. 1998, 353, 1101–1114. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.X.; Yuan, C.X.; Meng, Q.J.; Ji, Q. A Jurassic eutherian mammal and divergence of marsupials and placentals. Nature 2011, 476, 442–445. [Google Scholar] [CrossRef] [PubMed]
- Bi, S.; Zheng, X.; Wang, X.; Cignetti, N.E.; Yang, S.; Wible, J.R. An Early Cretaceous eutherian and the placental-marsupial dichotomy. Nature 2018, 558, 390–395. [Google Scholar] [CrossRef]
- Roberts, R.M.; Green, J.A.; Schulz, L.C. The evolution of the placenta. Reproduction 2016, 152, R179–R189. [Google Scholar] [CrossRef] [PubMed]
- Foster, C.S.P.; Van Dyke, J.U.; Thompson, M.B.; Smith, N.M.A.; Simpfendorfer, C.A.; Murphy, C.R.; Whittington, C.M. Different Genes are Recruited During Convergent Evolution of Pregnancy and the Placenta. Mol. Biol. Evol. 2022, 39, msac077. [Google Scholar] [CrossRef]
- Mika, K.; Marinić, M.; Singh, M.; Muter, J.; Brosens, J.J.; Lynch, V.J. Evolutionary transcriptomics implicates new genes and pathways in human pregnancy and adverse pregnancy outcomes. Elife 2021, 10, e69584. [Google Scholar] [CrossRef]
- Carter, A.M.; Enders, A.C. Comparative aspects of trophoblast development and placentation. Reprod. Biol. Endocrinol. 2004, 2, 46. [Google Scholar] [CrossRef]
- Armstrong, D.L.; McGowen, M.R.; Weckle, A.; Pantham, P.; Caravas, J.; Agnew, D.; Benirschke, K.; Savage-Rumbaugh, S.; Nevo, E.; Kim, C.J.; et al. The core transcriptome of mammalian placentas and the divergence of expression with placental shape. Placenta 2017, 57, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Haig, D. Genetic conflicts in human pregnancy. Q. Rev. Biol. 1993, 68, 495–532. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, S.; Bai, H.; Sakurai, T.; Nakaya, Y.; Konno, T.; Miyazawa, T.; Gojobori, T.; Imakawa, K. Dynamic evolution of endogenous retrovirus-derived genes expressed in bovine conceptuses during the period of placentation. Genome Biol. Evol. 2013, 5, 296–306. [Google Scholar] [CrossRef]
- Sakurai, T.; Nakagawa, S.; Bai, H.; Bai, R.; Kusama, K.; Ideta, A.; Aoyagi, Y.; Kaneko, K.; Iga, K.; Yasuda, J.; et al. Novel endogenous retrovirus-derived transcript expressed in the bovine placenta is regulated by WNT signaling. Biochem. J. 2017, 474, 3499–3512. [Google Scholar] [CrossRef] [PubMed]
- Ohshima, K.; Hattori, M.; Yada, T.; Gojobori, T.; Sakaki, Y.; Okada, N. Whole-genome screening indicates a possible burst of formation of processed pseudogenes and Alu repeats by particular L1 subfamilies in ancestral primates. Genome Biol. 2003, 4, R74. [Google Scholar] [CrossRef] [PubMed]
- Kazazian, H.H., Jr. Mobile elements: Drivers of genome evolution. Science 2004, 303, 1626–1632. [Google Scholar] [CrossRef]
- Bovine Genome Sequencing and Analysis Consortium; Elsik, C.G.; Tellam, R.L.; Worley, K.C.; Gibbs, R.A.; Muzny, D.M.; Weinstock, G.M.; Adelson, D.L.; Eichler, E.E.; Elnitski, L.; et al. The genome sequence of taurine cattle: A window to ruminant biology and evolution. Science 2009, 324, 522–528. [Google Scholar]
- Chuong, E.B.; Elde, N.C.; Feschotte, C. Regulatory activities of transposable elements: From conflicts to benefits. Nat. Rev. Genet. 2017, 18, 71–86. [Google Scholar] [CrossRef]
- Zhao, P.; Peng, C.; Fang, L.; Wang, Z.; Liu, G.E. Taming transposable elements in livestock and poultry: A review of their roles and applications. Genet. Sel. Evol. 2023, 55, 50. [Google Scholar] [CrossRef]
- Xiong, Y.; Eickbush, T.H. Origin and evolution of retroelements based upon their reverse transcriptase sequences. EMBO J. 1990, 9, 3353–3362. [Google Scholar] [CrossRef]
- Weiss, R.A. The discovery of endogenous retroviruses. Retrovirology 2006, 3, 67. [Google Scholar] [CrossRef] [PubMed]
- Hoyt, S.J.; Storer, J.M.; Hartley, G.A.; Grady, P.G.S.; Gershman, A.; de Lima, L.G.; Limouse, C.; Halabian, R.; Wojenski, L.; Rodriguez, M.; et al. From telomere to telomere: The transcriptional and epigenetic state of human repeat elements. Science 2022, 376, eabk3112. [Google Scholar] [CrossRef] [PubMed]
- Jern, P.; Coffin, J.M. Effects of retroviruses on host genome function. Annu. Rev. Genet. 2008, 42, 709–732. [Google Scholar] [CrossRef] [PubMed]
- Adelson, D.L.; Raison, J.M.; Edgar, R.C. Characterization and distribution of retrotransposons and simple sequence repeats in the bovine genome. Proc. Natl. Acad. Sci. USA 2009, 106, 12855–12860. [Google Scholar] [CrossRef]
- Garcia-Etxebarria, K.; Jugo, B.M. Genomic environment and digital expression of bovine endogenous retroviruses. Gene 2014, 548, 14–21. [Google Scholar] [CrossRef]
- Gifford, R.; Tristem, M. The evolution, distribution and diversity of endogenous retroviruses. Virus Genes 2003, 26, 291–315. [Google Scholar] [CrossRef]
- de Parseval, N.; Heidmann, T. Human endogenous retroviruses: From infectious elements to human genes. Cytogenet. Genome Res. 2005, 110, 318–332. [Google Scholar] [CrossRef]
- Vargiu, L.; Rodriguez-Tomé, P.; Sperber, G.O.; Cadeddu, M.; Grandi, N.; Blikstad, V.; Tramontano, E.; Blomberg, J. Classification and characterization of human endogenous retroviruses; mosaic forms are common. Retrovirology 2016, 13, 7. [Google Scholar] [CrossRef]
- Dupressoir, A.; Vernochet, C.; Harper, F.; Guégan, J.; Dessen, P.; Pierron, G.; Heidmann, T. A pair of co-opted retroviral envelope syncytin genes is required for formation of the two-layered murine placental syncytiotrophoblast. Proc. Natl. Acad. Sci. USA 2011, 108, E1164–E1173. [Google Scholar] [CrossRef]
- Kaneko-Ishino, T.; Ishino, F. The role of genes domesticated from LTR retrotransposons and retroviruses in mammals. Front. Microbiol. 2012, 3, 262. [Google Scholar] [CrossRef]
- Ono, R.; Nakamura, K.; Inoue, K.; Naruse, M.; Usami, T.; Wakisaka-Saito, N.; Hino, T.; Suzuki-Migishima, R.; Ogonuki, N.; Miki, H.; et al. Deletion of Peg10, an imprinted gene acquired from a retrotransposon, causes early embryonic lethality. Nat. Genet. 2006, 38, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Edwards, C.A.; Mungall, A.J.; Matthews, L.; Ryder, E.; Gray, D.J.; Pask, A.J.; Shaw, G.; Graves, J.A.; Rogers, J.; SAVOIR consortium; et al. The evolution of the DLK1-DIO3 imprinted domain in mammals. PLoS Biol. 2008, 6, e135. [Google Scholar] [CrossRef] [PubMed]
- Sekita, Y.; Wagatsuma, H.; Nakamura, K.; Ono, R.; Kagami, M.; Wakisaka, N.; Hino, T.; Suzuki-Migishima, R.; Kohda, T.; Ogura, A.; et al. Role of retrotransposon-derived imprinted gene, Rtl1, in the feto-maternal interface of mouse placenta. Nat. Genet. 2008, 40, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Blond, J.L.; Lavillette, D.; Cheynet, V.; Bouton, O.; Oriol, G.; Chapel-Fernandes, S.; Mandrand, B.; Mallet, F.; Cosset, F.L. An envelope glycoprotein of the human endogenous retrovirus HERV-W is expressed in the human placenta and fuses cells expressing the type D mammalian retrovirus receptor. J. Virol. 2000, 74, 3321–3329. [Google Scholar] [CrossRef]
- Mi, S.; Lee, X.; Li, X.; Veldman, G.M.; Finnerty, H.; Racie, L.; LaVallie, E.; Tang, X.Y.; Edouard, P.; Howes, S.; et al. Syncytin is a captive retroviral envelope protein involved in human placental morphogenesis. Nature 2000, 403, 785–789. [Google Scholar] [CrossRef]
- Blaise, S.; de Parseval, N.; Bénit, L.; Heidmann, T. Genomewide screening for fusogenic human endogenous retrovirus envelopes identifies syncytin 2, a gene conserved on primate evolution. Proc. Natl. Acad. Sci. USA 2003, 100, 13013–13018. [Google Scholar] [CrossRef]
- Esnault, C.; Priet, S.; Ribet, D.; Vernochet, C.; Bruls, T.; Lavialle, C.; Weissenbach, J.; Heidmann, T. A placenta-specific receptor for the fusogenic, endogenous retrovirus-derived, human syncytin-2. Proc. Natl. Acad. Sci. USA 2008, 105, 17532–17537. [Google Scholar] [CrossRef]
- Lokossou, A.G.; Toudic, C.; Barbeau, B. Implication of human endogenous retrovirus envelope proteins in placental functions. Viruses 2014, 6, 4609–4627. [Google Scholar] [CrossRef]
- Dupressoir, A.; Marceau, G.; Vernochet, C.; Bénit, L.; Kanellopoulos, C.; Sapin, V.; Heidmann, T. Syncytin-A and syncytin-B, two fusogenic placenta-specific murine envelope genes of retroviral origin conserved in Muridae. Proc. Natl. Acad. Sci. USA 2005, 102, 725–730. [Google Scholar] [CrossRef]
- Dupressoir, A.; Vernochet, C.; Bawa, O.; Harper, F.; Pierron, G.; Opolon, P.; Heidmann, T. Syncytin-A knockout mice demonstrate the critical role in placentation of a fusogenic, endogenous retrovirus-derived, envelope gene. Proc. Natl. Acad. Sci. USA 2009, 106, 12127–12132. [Google Scholar] [CrossRef]
- Heidmann, O.; Vernochet, C.; Dupressoir, A.; Heidmann, T. Identification of an endogenous retroviral envelope gene with fusogenic activity and placenta-specific expression in the rabbit: A new “syncytin” in a third order of mammals. Retrovirology 2009, 6, 107. [Google Scholar] [CrossRef] [PubMed]
- Cornelis, G.; Heidmann, O.; Bernard-Stoecklin, S.; Reynaud, K.; Véron, G.; Mulot, B.; Dupressoir, A.; Heidmann, T. Ancestral capture of syncytin-Car1, a fusogenic endogenous retroviral envelope gene involved in placentation and conserved in Carnivora. Proc. Natl. Acad. Sci. USA 2012, 109, E432–E441. [Google Scholar] [CrossRef] [PubMed]
- Dunlap, K.A.; Palmarini, M.; Adelson, D.L.; Spencer, T.E. Sheep endogenous betaretroviruses (enJSRVs) and the hyaluronidase 2 (HYAL2) receptor in the ovine uterus and conceptus. Biol. Reprod. 2005, 73, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Dunlap, K.A.; Palmarini, M.; Varela, M.; Burghardt, R.C.; Hayashi, K.; Farmer, J.L.; Spencer, T.E. Endogenous retroviruses regulate periimplantation placental growth and differentiation. Proc. Natl. Acad. Sci. USA 2006, 103, 14390–14395. [Google Scholar] [CrossRef] [PubMed]
- Dunlap, K.A.; Palmarini, M.; Spencer, T.E. Ovine endogenous betaretroviruses (enJSRVs) and placental morphogenesis. Placenta 2006, 27 (Suppl. A), S135–S140. [Google Scholar] [CrossRef]
- Murcia, P.R.; Arnaud, F.; Palmarini, M. The transdominant endogenous retrovirus enJS56A1 associates with and blocks intracellular trafficking of Jaagsiekte sheep retrovirus Gag. J. Virol. 2007, 81, 1762–1772. [Google Scholar] [CrossRef]
- Armezzani, A.; Arnaud, F.; Caporale, M.; di Meo, G.; Iannuzzi, L.; Murgia, C.; Palmarini, M. The signal peptide of a recently integrated endogenous sheep betaretrovirus envelope plays a major role in eluding gag-mediated late restriction. J. Virol. 2011, 85, 7118–7128. [Google Scholar] [CrossRef]
- Morozov, V.A.; Morozov, A.V.; Lagaye, S. Endogenous JSRV-like proviruses in domestic cattle: Analysis of sequences and transcripts. Virology 2007, 367, 59–70. [Google Scholar] [CrossRef]
- Baba, K.; Nakaya, Y.; Shojima, T.; Muroi, Y.; Kizaki, K.; Hashizume, K.; Imakawa, K.; Miyazawa, T. Identification of novel endogenous betaretroviruses which are transcribed in the bovine placenta. J. Virol. 2011, 85, 1237–1245. [Google Scholar] [CrossRef]
- Koshi, K.; Suzuki, Y.; Nakaya, Y.; Imai, K.; Hosoe, M.; Takahashi, T.; Kizaki, K.; Miyazawa, T.; Hashizume, K. Bovine trophoblastic cell differentiation and binucleation involves enhanced endogenous retrovirus element expression. Reprod. Biol. Endocrinol. 2012, 10, 41. [Google Scholar] [CrossRef]
- Cornelis, G.; Heidmann, O.; Degrelle, S.A.; Vernochet, C.; Lavialle, C.; Letzelter, C.; Bernard-Stoecklin, S.; Hassanin, A.; Mulot, B.; Guillomot, M.; et al. Captured retroviral envelope syncytin gene associated with the unique placental structure of higher ruminants. Proc. Natl. Acad. Sci. USA 2013, 110, E828–E837. [Google Scholar] [CrossRef] [PubMed]
- Nakaya, Y.; Koshi, K.; Nakagawa, S.; Hashizume, K.; Miyazawa, T. Fematrin-1 is involved in fetomaternal cell-to-cell fusion in Bovinae placenta and has contributed to diversity of ruminant placentation. J. Virol. 2013, 87, 10563–10572. [Google Scholar] [CrossRef] [PubMed]
- Cornelis, G.; Vernochet, C.; Carradec, Q.; Souquere, S.; Mulot, B.; Catzeflis, F.; Nilsson, M.A.; Menzies, B.R.; Renfree, M.B.; Pierron, G.; et al. Retroviral envelope gene captures and syncytin exaptation for placentation in marsupials. Proc. Natl. Acad. Sci. USA 2015, 112, E487–E496. [Google Scholar] [CrossRef] [PubMed]
- Bazer, F.W.; Spencer, T.E.; Johnson, G.A. Interferons and uterine receptivity. Semin. Reprod. Med. 2009, 27, 90–102. [Google Scholar] [CrossRef]
- Green, J.A.; Geisert, R.D.; Johnson, G.A.; Spencer, T.E. Implantation and Placentation in Ruminants. Adv. Anat. Embryol. Cell Biol. 2021, 234, 129–154. [Google Scholar]
- Wooding, F.B. Current topic: The synepitheliochorial placenta of ruminants: Binucleate cell fusions and hormone production. Placenta 1992, 13, 101–113. [Google Scholar] [CrossRef]
- Klisch, K.; Pfarrer, C.; Schuler, G.; Hoffmann, B.; Leiser, R. Tripolar acytokinetic mitosis and formation of feto-maternal syncytia in the bovine placentome: Different modes of the generation of multinuclear cells. Anat. Embryol. 1999, 200, 229–237. [Google Scholar] [CrossRef]
- Wooding, F.B.P. The ruminant placental trophoblast binucleate cell: An evolutionary breakthrough. Biol. Reprod. 2022, 107, 705–716. [Google Scholar] [CrossRef]
- Wooding, F.B. Frequency and localization of binucleate cells in the placentomes of ruminants. Placenta 1983, 4, 527–539. [Google Scholar]
- Wooding, F.B.; Wathes, D.C. Binucleate cell migration in the bovine placentome. J. Reprod. Fertil. 1980, 59, 425–430. [Google Scholar] [CrossRef]
- Seo, H.; Bazer, F.W.; Burghardt, R.C.; Johnson, G.A. Immunohistochemical Examination of Trophoblast Syncytialization during Early Placentation in Sheep. Int. J. Mol. Sci. 2019, 20, 4530. [Google Scholar] [CrossRef] [PubMed]
- Yamada, A.; Ohtsuki, K.; Shiga, N.; Green, J.A.; Matsuno, Y.; Imakawa, K. Epithelial-mesenchymal transition and bi- and multi-nucleated trophoblast cell formation in ovine conceptuses during the peri-implantation period. J. Reprod. Dev. 2022, 68, 110–117. [Google Scholar] [CrossRef]
- Imakawa, K.; Nakagawa, S.; Miyazawa, T. Baton pass hypothesis: Successive incorporation of unconserved endogenous retroviral genes for placentation during mammalian evolution. Genes Cells 2015, 20, 771–788. [Google Scholar] [CrossRef]
- Nakaya, Y.; Miyazawa, T. Dysfunction of bovine endogenous retrovirus K2 envelope glycoprotein is related to unsuccessful intracellular trafficking. J. Virol. 2014, 88, 6896–6905. [Google Scholar] [CrossRef] [PubMed]
- Koshi, K.; Nakaya, Y.; Kizaki, K.; Ishiguro-Oonuma, T.; Miyazawa, T.; Spencer, T.E.; Hashizume, K. Induction of ovine trophoblast cell fusion by fematrin-1 in vitro. Anim. Sci. J. 2016, 87, 419–422. [Google Scholar] [CrossRef] [PubMed]
- Khazaee, E.; Farzaneh, N.; Mirshokraei, P.; Tabatabaeizadeh, S.E.; Dehghani, H. Expression of endogenous retroviruses in pre-implantation stages of bovine embryo. Reprod. Domest. Anim. 2018, 53, 1405–1414. [Google Scholar] [CrossRef]
- Garcia-Etxebarria, K.; Jugo, B.M. Genome-wide detection and characterization of endogenous retroviruses in Bos taurus. J. Virol. 2010, 84, 10852–10862. [Google Scholar] [CrossRef]
- Xiao, R.; Park, K.; Lee, H.; Kim, J.; Park, C. Identification and classification of endogenous retroviruses in cattle. J. Virol. 2008, 82, 582–587. [Google Scholar] [CrossRef]
- Garcia-Etxebarria, K.; Jugo, B.M. Evolutionary history of bovine endogenous retroviruses in the Bovidae family. BMC Evol. Biol. 2013, 13, 256. [Google Scholar] [CrossRef]
- Johnson, W.E. Endogenous Retroviruses in the Genomics Era. Annu. Rev. Virol. 2015, 2, 135–159. [Google Scholar] [CrossRef]
- Xiao, R.; Park, K.; Oh, Y.; Kim, J.; Park, C. Structural characterization of the genome of BERV gamma4, the most abundant endogenous retrovirus family in cattle. Mol. Cells 2008, 26, 404–408. [Google Scholar] [PubMed]
- Torresi, C.; Casciari, C.; Giammarioli, M.; Feliziani, F.; De Mia, G.M. Characterization of a novel full-length bovine endogenous retrovirus, BERV-β1. Arch. Virol. 2015, 160, 3105–3114. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.; Kim, J.; Choi, H.; Park, K.; Lee, H.; Park, C. Characterization of the bovine endogenous retrovirus beta3 genome. Mol. Cells 2008, 25, 142–147. [Google Scholar] [PubMed]
- Wang, X.; Liu, S. Endogenous Jaagsiekte sheep retrovirus envelope protein promotes sheep trophoblast cell fusion by activating PKA/MEK/ERK1/2 signaling. Theriogenology 2022, 193, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, J.; Sugimoto, M.; Bernstein, H.; Jinno, Y.; Schust, D. A novel human endogenous retroviral protein inhibits cell-cell fusion. Sci. Rep. 2013, 3, 1462. [Google Scholar] [CrossRef]
- Kitao, K.; Tanikaga, T.; Miyazawa, T. Identification of a post-transcriptional regulatory element in the human endogenous retroviral syncytin-1. J. Gen. Virol. 2019, 100, 662–668. [Google Scholar] [CrossRef]
- Kitao, K.; Nakagawa, S.; Miyazawa, T. An ancient retroviral RNA element hidden in mammalian genomes and its involvement in co-opted retroviral gene regulation. Retrovirology 2021, 18, 36. [Google Scholar] [CrossRef]
- Kovalskaya, E.; Buzdin, A.; Gogvadze, E.; Vinogradova, T.; Sverdlov, E. Functional human endogenous retroviral LTR transcription start sites are located between the R and U5 regions. Virology 2006, 346, 373–378. [Google Scholar] [CrossRef]
- Criscione, S.W.; Zhang, Y.; Thompson, W.; Sedivy, J.M.; Neretti, N. Transcriptional landscape of repetitive elements in normal and cancer human cells. BMC Genom. 2014, 15, 583. [Google Scholar] [CrossRef]
- Thompson, P.J.; Macfarlan, T.S.; Lorincz, M.C. Long Terminal Repeats: From Parasitic Elements to Building Blocks of the Transcriptional Regulatory Repertoire. Mol. Cell 2016, 62, 766–776. [Google Scholar] [CrossRef]
- Manghera, M.; Douville, R.N. Endogenous retrovirus-K promoter: A landing strip for inflammatory transcription factors? Retrovirology 2013, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Akopov, S.B.; Nikolaev, L.G.; Khil, P.P.; Lebedev, Y.B.; Sverdlov, E.D. Long terminal repeats of human endogenous retrovirus K family (HERV-K) specifically bind host cell nuclear proteins. FEBS Lett. 1998, 421, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.F.; Coffin, J.M. Human endogenous retrovirus K solo-LTR formation and insertional polymorphisms: Implications for human and viral evolution. Proc. Natl. Acad. Sci. USA 2004, 10S1, 1668–1672. [Google Scholar] [CrossRef] [PubMed]
- Belshaw, R.; Watson, J.; Katzourakis, A.; Howe, A.; Woolven-Allen, J.; Burt, A.; Tristem, M. Rate of recombinational deletion among human endogenous retroviruses. J. Virol. 2007, 81, 9437–9442. [Google Scholar] [CrossRef] [PubMed]
- Cohen, C.J.; Lock, W.M.; Mager, D.L. Endogenous retroviral LTRs as promoters for human genes: A critical assessment. Gene 2009, 448, 105–114. [Google Scholar] [CrossRef]
- Dunn, C.A.; Romanish, M.T.; Gutierrez, L.E.; van de Lagemaat, L.N.; Mager, D.L. Transcription of two human genes from a bidirectional endogenous retrovirus promoter. Gene 2006, 366, 335–342. [Google Scholar] [CrossRef]
- Romanish, M.T.; Lock, W.M.; van de Lagemaat, L.N.; Dunn, C.A.; Mager, D.L. Repeated recruitment of LTR retrotransposons as promoters by the anti-apoptotic locus NAIP during mammalian evolution. PLoS Genet. 2007, 3, e10. [Google Scholar] [CrossRef]
- Reiss, D.; Zhang, Y.; Mager, D.L. Widely variable endogenous retroviral methylation levels in human placenta. Nucleic Acids Res. 2007, 35, 4743–4754. [Google Scholar] [CrossRef]
- Matousková, M.; Blazková, J.; Pajer, P.; Pavlícek, A.; Hejnar, J. CpG methylation suppresses transcriptional activity of human syncytin-1 in non-placental tissues. Exp. Cell Res. 2006, 312, 1011–1020. [Google Scholar] [CrossRef]
- Davenport, K.M.; Ortega, M.S.; Liu, H.; O’Neil, E.V.; Kelleher, A.M.; Warren, W.C.; Spencer, T.E. Single-nuclei RNA sequencing (snRNA-seq) uncovers trophoblast cell types and lineages in the mature bovine placenta. Proc. Natl. Acad. Sci. USA 2023, 120, e2221526120. [Google Scholar] [CrossRef]
- Wang, Y.; Ming, H.; Yu, L.; Li, J.; Zhu, L.; Sun, H.X.; Pinzon-Arteaga, C.A.; Wu, J.; Jiang, Z. Establishment of bovine trophoblast stem cells. Cell Rep. 2023, 42, 112439. [Google Scholar] [CrossRef] [PubMed]

| Mode of Distribution of Villi | Degree of Syncytialization | Representative Species | Type of Fused Cells |
|---|---|---|---|
| Cotyledonary | Synepitheliochorial | Cow, Sheep | Fetomaternal hybrid |
| Diffuse | Epitheliochorial | Horse, Pig | None |
| Zonary | Endotheliochorial | Dog, Cat | Syncytiotrophoblast |
| Discoid | Hemochorial | Mouse, Human | Syncytiotrophoblast |
| Appearance in Host Genome | Species | Expressed Tissues (Localization) #1 | Function | References | |
|---|---|---|---|---|---|
| Syncytin-Rum1 env | Over 30 Mya | Most ruminants(except for the family Tragulidae) | Placenta (BNCs) | Fusogenic activity | [51] |
| BERV-K1 env | 25.4 to 18.3 Mya | Bovinae subfamily | Placenta (BNCs), embryonic blastomeres (two- to 16-cell stage) | Fusogenic activity | [52,63] |
| BERV-K2 env | No data | No data | Endometrium, embryonic blastomeres (2- to 16-cell stage) | No data | [49,63] |
| BERV-P env | 16.9 to 7.0 Mya | genus Bos | Conceptus during periattachment (Trophectoderm) | No data | [13] |
| bERVE-A env | No data | Bos taurus | Placenta (BNCs) | No data | [50] |
| bERVE-B env | No data | No data | Endometrium, placenta | No data | [50] |
| BERV-K3 gag/pol | No data | No data | Conceptus after attachment (Trophectoderm), placenta | No data | [64] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakurai, T.; Kusama, K.; Imakawa, K. Progressive Exaptation of Endogenous Retroviruses in Placental Evolution in Cattle. Biomolecules 2023, 13, 1680. https://doi.org/10.3390/biom13121680
Sakurai T, Kusama K, Imakawa K. Progressive Exaptation of Endogenous Retroviruses in Placental Evolution in Cattle. Biomolecules. 2023; 13(12):1680. https://doi.org/10.3390/biom13121680
Chicago/Turabian StyleSakurai, Toshihiro, Kazuya Kusama, and Kazuhiko Imakawa. 2023. "Progressive Exaptation of Endogenous Retroviruses in Placental Evolution in Cattle" Biomolecules 13, no. 12: 1680. https://doi.org/10.3390/biom13121680
APA StyleSakurai, T., Kusama, K., & Imakawa, K. (2023). Progressive Exaptation of Endogenous Retroviruses in Placental Evolution in Cattle. Biomolecules, 13(12), 1680. https://doi.org/10.3390/biom13121680






