Functional and Transcriptomic Characterization of Postnatal Maturation of ENS and SIP Syncytium in Mice Colon
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Total RNA Isolation and Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.3. RNA Sequencing and Data Analyses
2.4. Wes Simple Western Automated Capillary Electrophoresis and Immunodetection
2.5. Western Blot
2.6. Isometric Force Recording (IFR)
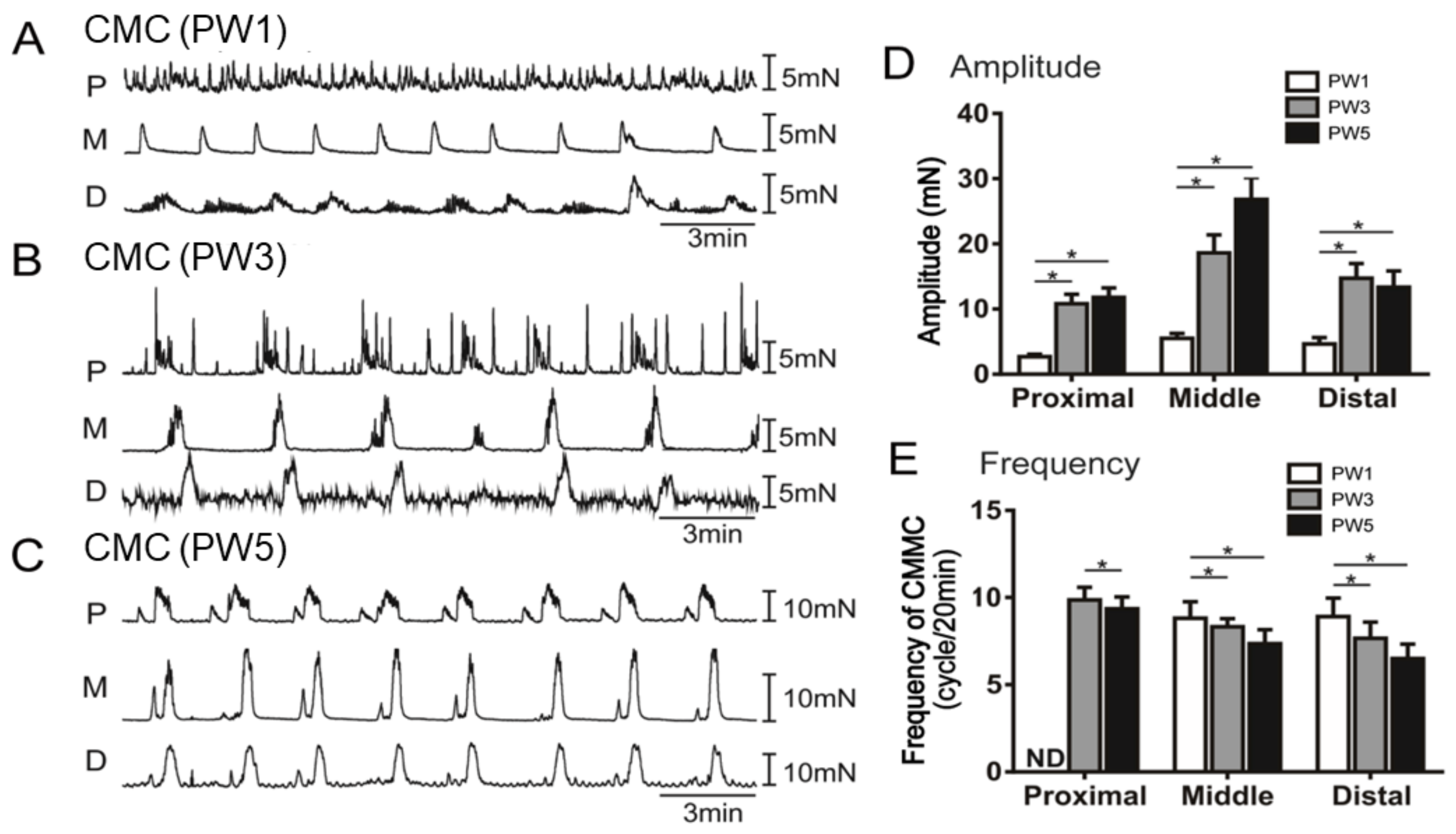
2.7. Colonic Motor Complexes
2.8. Statistical Analysis (Clarify Transcriptional, Protein, IFC, and CMC Experiments)
3. Results
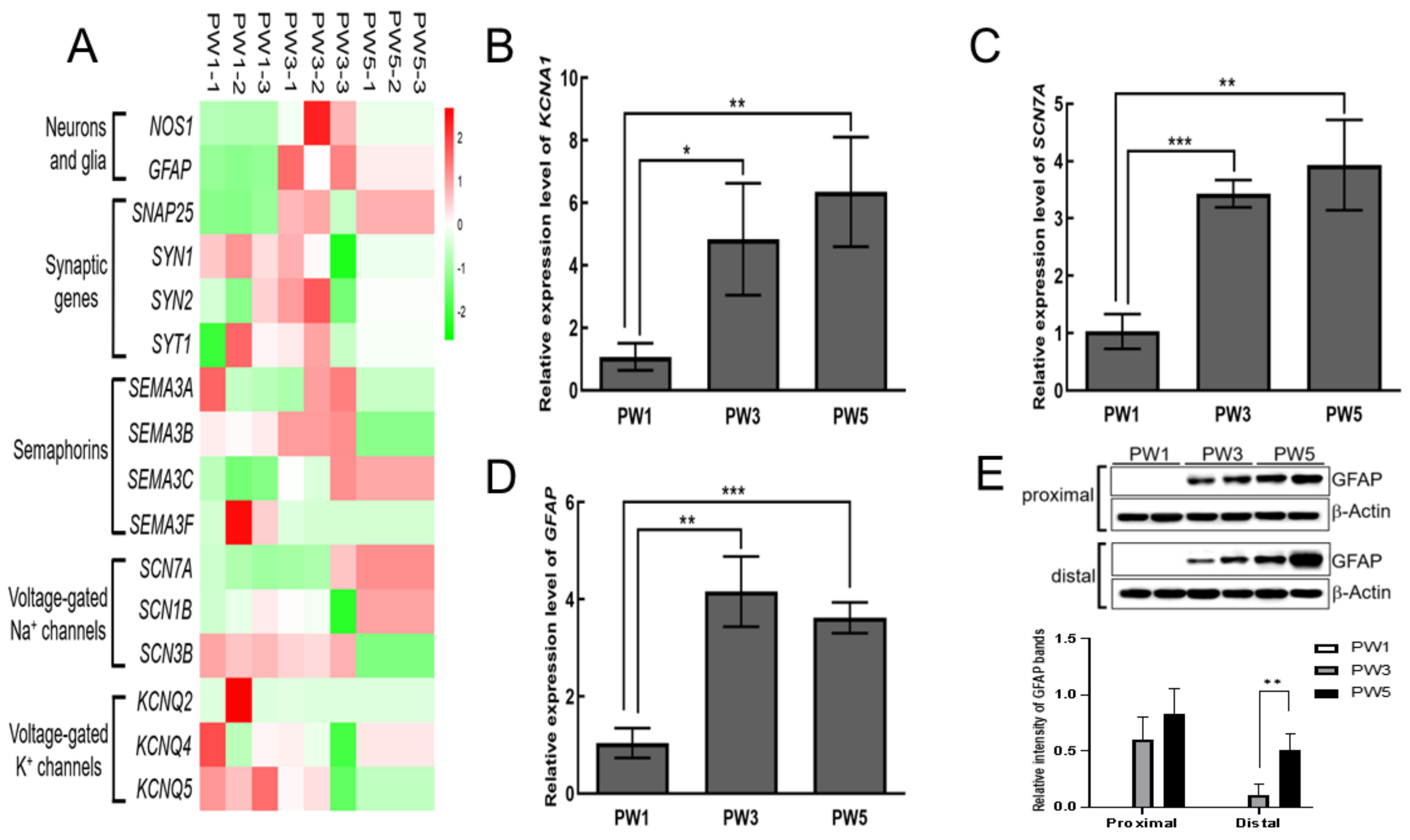
3.1. Characterization of the Transcriptome in Young Murine Colon
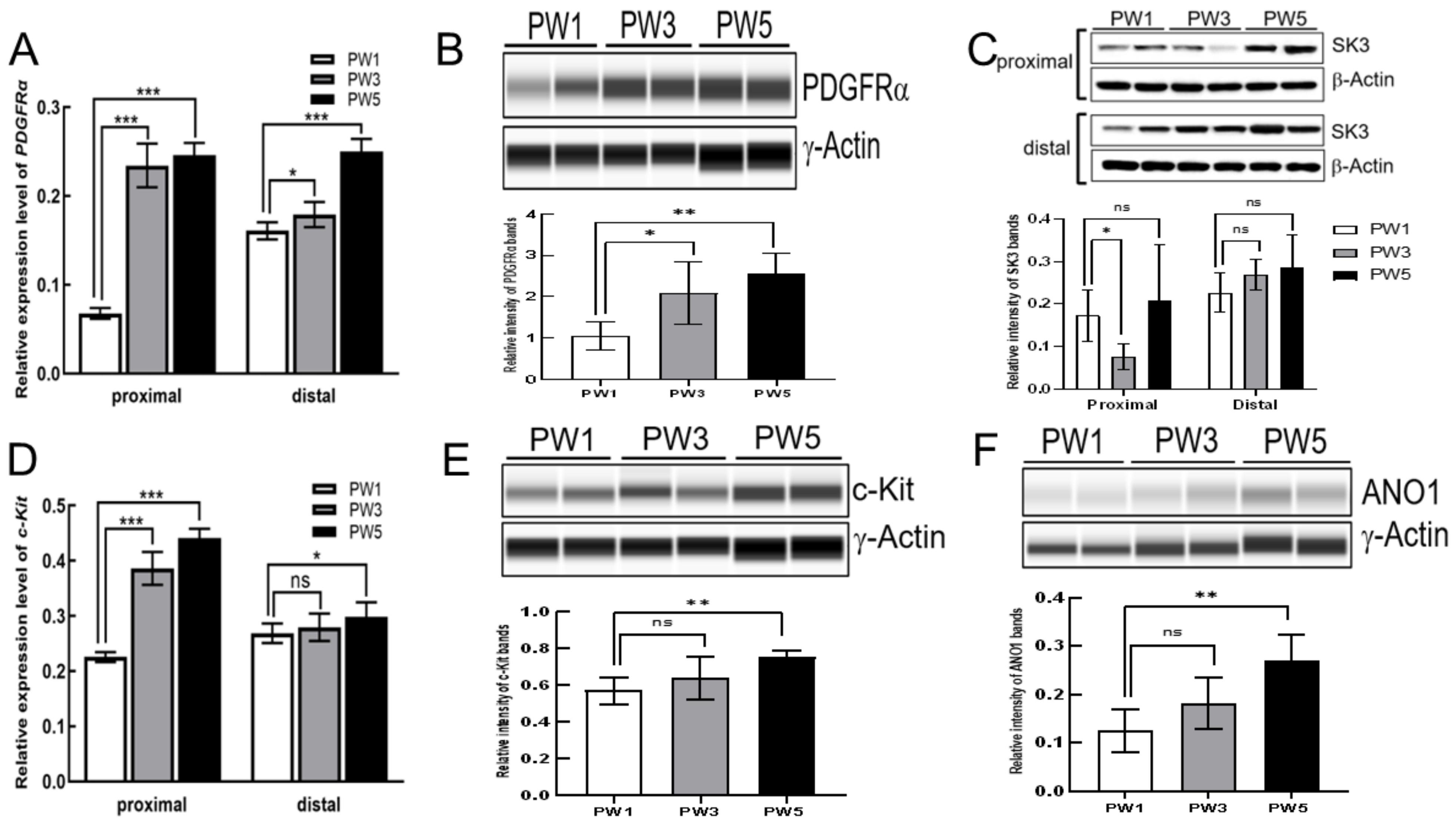
3.2. Expression of Key Genes and Proteins in SIP Syncytium following Postnatal Development
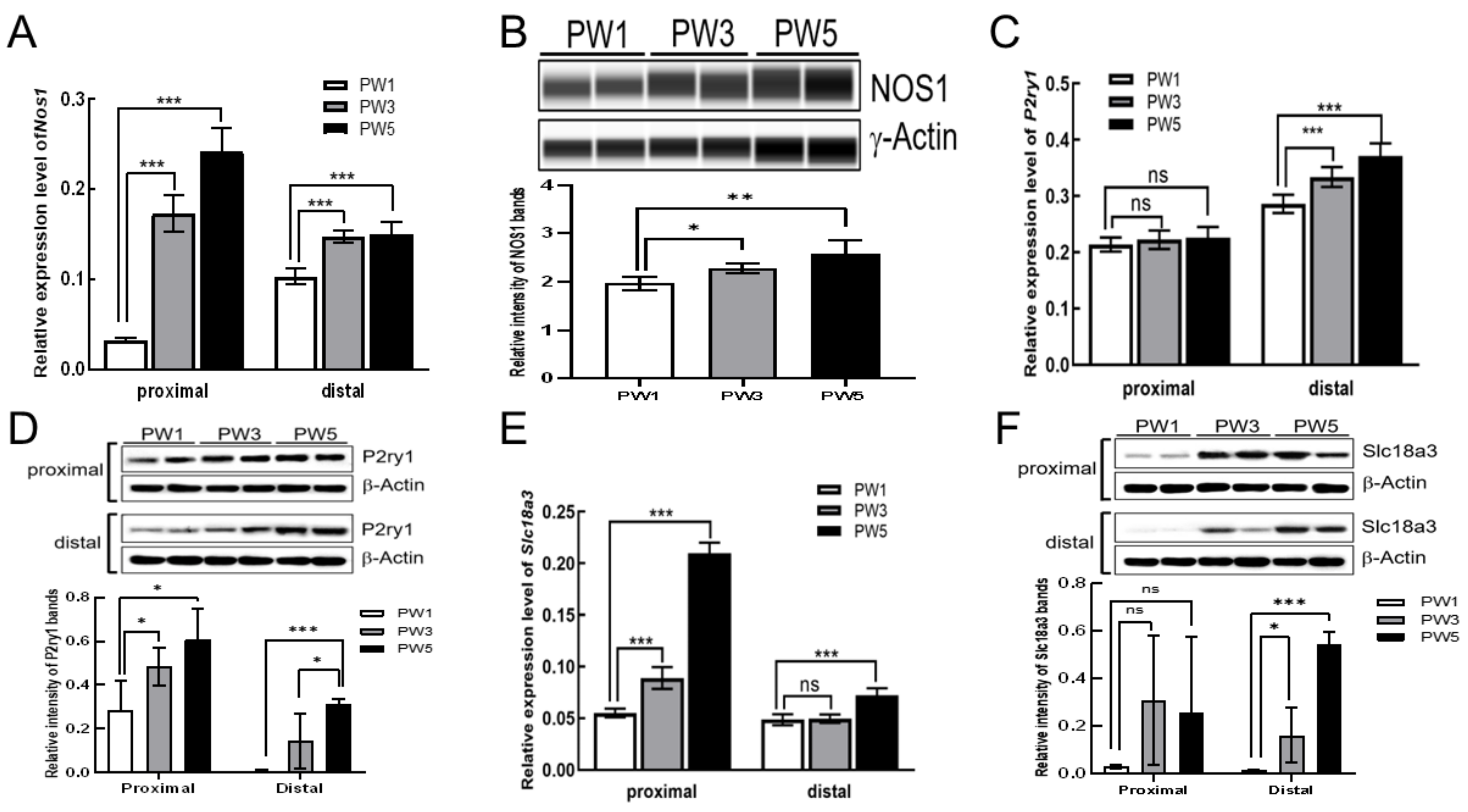
3.3. Expression of Excitatory and Inhibitory Motor Neurons following Postnatal Development
3.4. Excitatory and Inhibitory Motor Responses of Colonic Contractions
3.4.1. Responses of Colonic Contractions in the Proximal Colon
3.4.2. Responses of Colonic Contractions in the Distal Colon
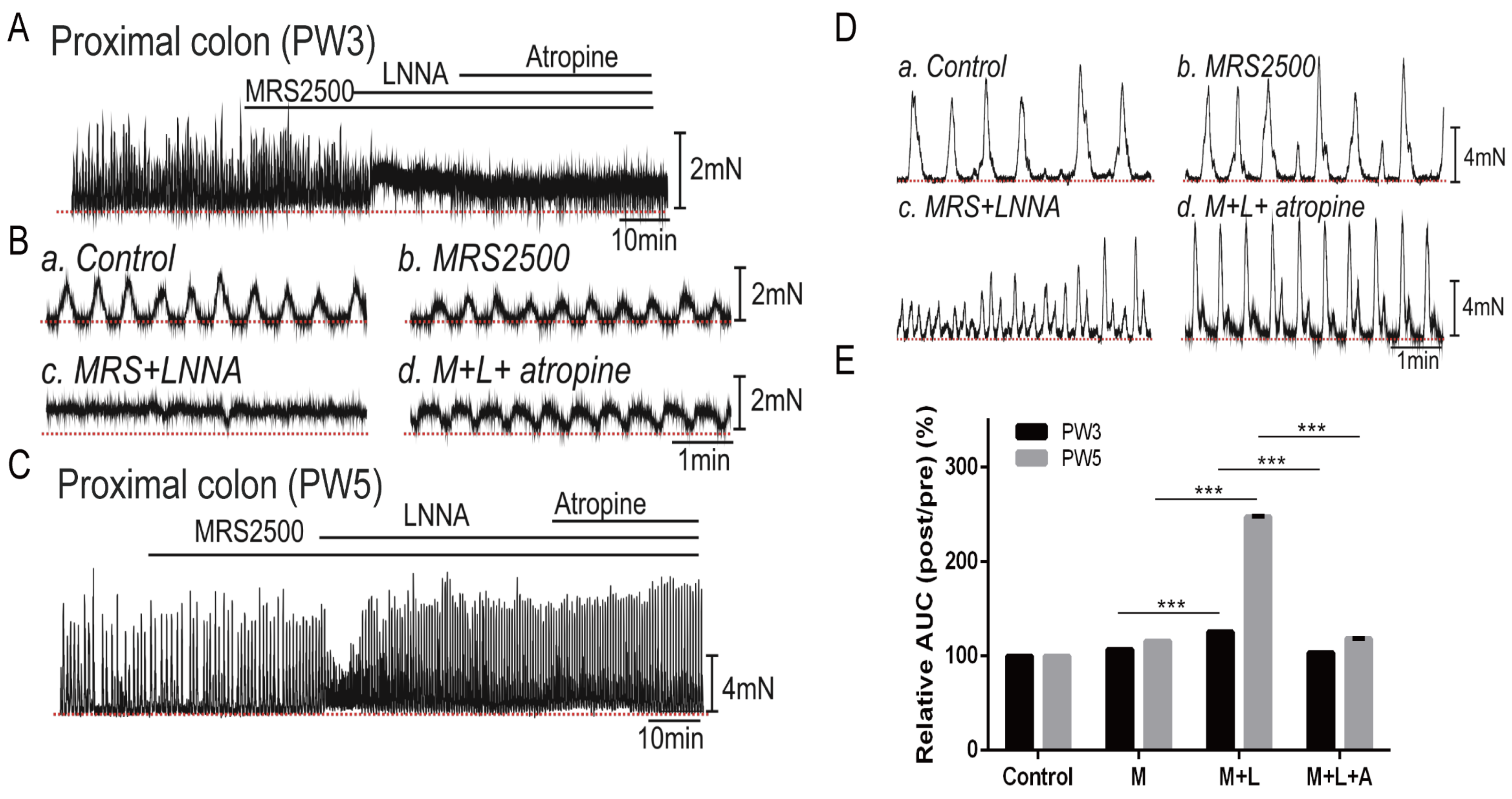
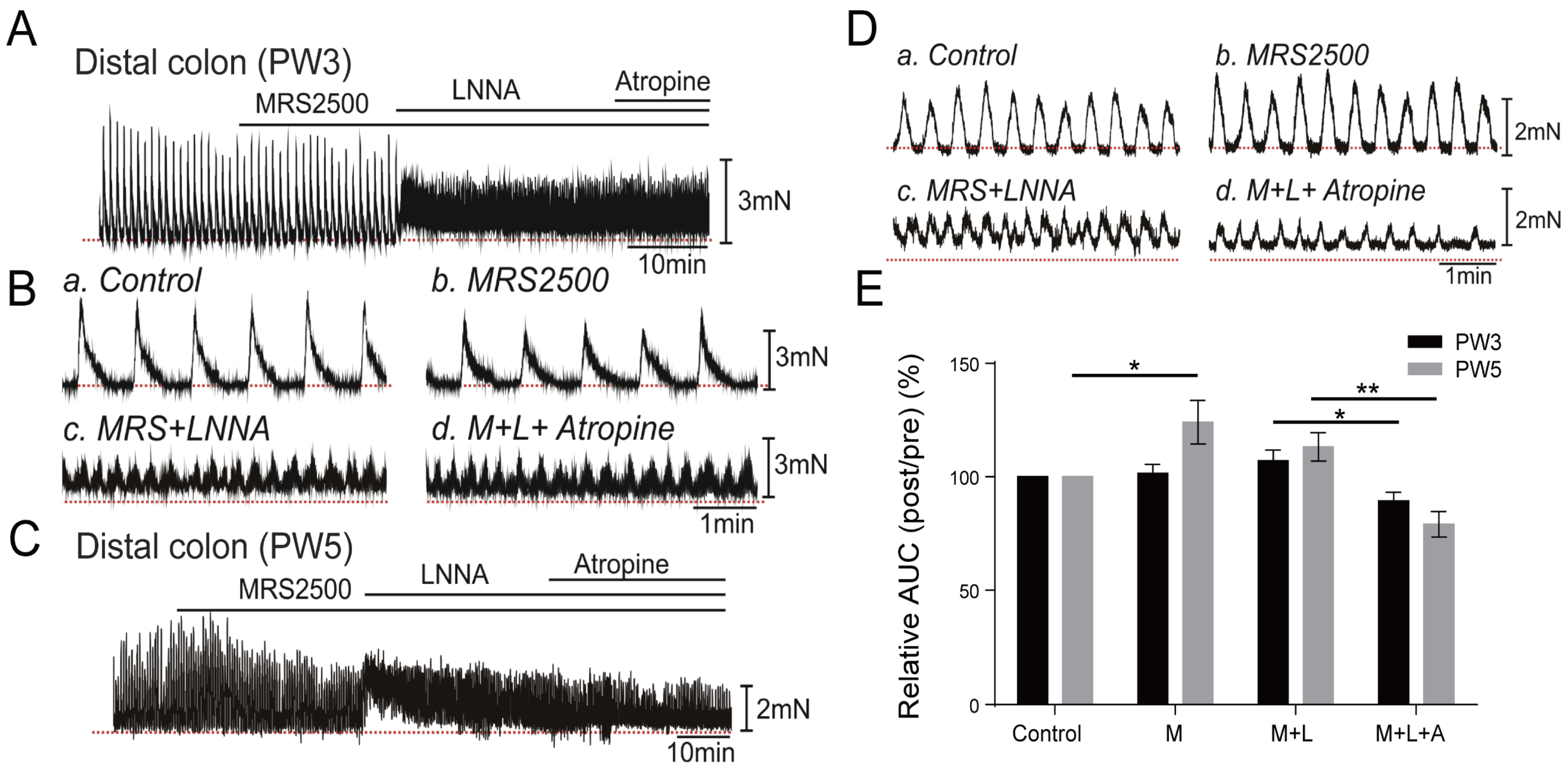
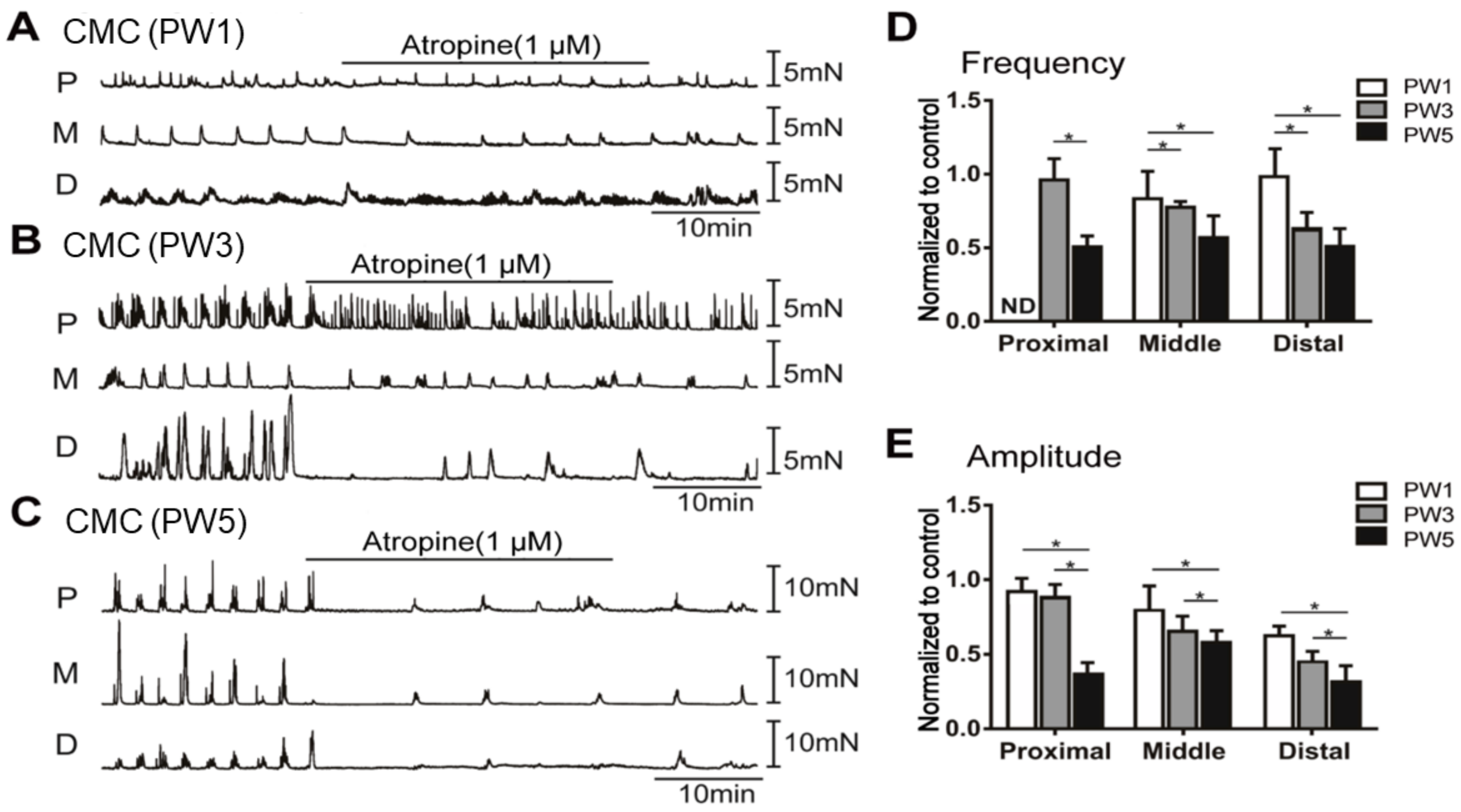
3.5. Impact of Neuron Regulators on the CMCs
3.5.1. Responses of CMCs with the Application of LNNA
3.5.2. Responses of CMCs with the Application of Atropine
3.5.3. Responses of CMCs with the Application of MRS2500
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Albertí, E.; Mikkelsen, H.B.; Larsen, J.O.; Jiménez, M. Motility patterns and distribution of interstitial cells of Cajal and nitrergic neurons in the proximal, mid- and distal-colon of the rat. Neurogastroenterol. Motil. 2005, 17, 133–147. [Google Scholar] [CrossRef] [PubMed]
- Vetri, T.; Bonvissuto, F.; Marino, A.; Postorino, A. Nitrergic and purinergic interplay in inhibitory transmission in rat gastric fundus. Auton Autacoid Pharmacol. 2007, 27, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Foong, J.P.; Tough, I.R.; Cox, H.M.; Bornstein, J.C. Properties of cholinergic and non-cholinergic submucosal neurons along the mouse colon. J. Physiol. 2014, 592, 777–793. [Google Scholar] [CrossRef] [PubMed]
- Yntema, C.L.; Hammond, W.S. The origin of intrinsic ganglia of trunk viscera from vagal neural crest in the chick embryo. J. Comp. Neurol. 1954, 101, 515–541. [Google Scholar] [CrossRef]
- Burns, A.J.; Douarin, N.M. The sacral neural crest contributes neurons and glia to the post-umbilical gut: Spatiotemporal analysis of the development of the enteric nervous system. Development 1998, 125, 4335–4347. [Google Scholar] [CrossRef]
- Burns, A.J.; Le Douarin, N.M. Enteric nervous system development: Analysis of the selective developmental potentialities of vagal and sacral neural crest cells using quail-chick chimeras. Anat. Rec. 2001, 262, 16–28. [Google Scholar] [CrossRef]
- Iino, S.; Horiguchi, K.; Horiguchi, S.; Nojyo, Y. c-Kit-negative fibroblast-like cells express platelet-derived growth factor receptor alpha in the murine gastrointestinal musculature. Histochem. Cell Biol. 2009, 131, 691–702. [Google Scholar] [CrossRef]
- Kurahashi, M.; Zheng, H.; Dwyer, L.; Ward, S.M.; Koh, S.D.; Sanders, K.M. A functional role for the ‘fibroblast-like cells’ in gastrointestinal smooth muscles. J. Physiol. 2011, 589, 697–710. [Google Scholar] [CrossRef]
- Koh, S.D.; Ward, S.M.; Sanders, K.M. Ionic conductances regulating the excitability of colonic smooth muscles. Neurogastroenterol. Motil. 2012, 24, 705–718. [Google Scholar] [CrossRef]
- Blair, P.J.; Rhee, P.L.; Sanders, K.M.; Ward, S.M. The significance of interstitial cells in neurogastroenterology. J. Neurogastroenterol. Motil. 2014, 20, 294–317. [Google Scholar] [CrossRef]
- Lies, B.; Groneberg, D.; Friebe, A. Toward a better understanding of gastrointestinal nitrergic neuromuscular transmission. Neurogastroenterol. Motil. 2014, 26, 901–912. [Google Scholar] [CrossRef] [PubMed]
- Beck, K.; Voussen, B.; Reigl, A.; Vincent, A.D.; Parsons, S.P.; Huizinga, J.D.; Friebe, A. Cell-specific effects of nitric oxide on the efficiency and frequency of long distance contractions in murine colon. Neurogastroenterol. Motil. 2019, 31, e13589. [Google Scholar] [CrossRef]
- Kim, T.W.; Koh, S.D.; Ordög, T.; Ward, S.M.; Sanders, K.M. Muscarinic regulation of pacemaker frequency in murine gastric interstitial cells of Cajal. J. Physiol. 2003, 546, 415–425. [Google Scholar] [CrossRef]
- Lee, H.; Koh, B.H.; Peri, L.E.; Sanders, K.M.; Koh, S.D. Purinergic inhibitory regulation of murine detrusor muscles mediated by PDGFRα+ interstitial cells. J. Physiol. 2014, 592, 1283–1293. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, C.; Uesaka, T.; Manabe, T.; Yonekura, Y.; Nagasawa, T.; Newgreen, D.F.; Young, H.M.; Enomoto, H. Trans-mesenteric neural crest cells are the principal source of the colonic enteric nervous system. Nat. Neurosci. 2012, 15, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Lake, J.I.; Heuckeroth, R.O. Enteric nervous system development: Migration, differentiation, and disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 305, G1–G24. [Google Scholar] [CrossRef] [PubMed]
- Hao, M.M.; Foong, J.P.; Bornstein, J.C.; Li, Z.L.; Vanden Berghe, P.; Boesmans, W. Enteric nervous system assembly: Functional integration within the developing gut. Dev. Biol. 2016, 417, 168–181. [Google Scholar] [CrossRef] [PubMed]
- McCann, C.J.; Alves, M.M.; Brosens, E.; Natarajan, D.; Perin, S.; Chapman, C.; Hofstra, R.M.; Burns, A.J.; Thapar, N. Neuronal Development and Onset of Electrical Activity in the Human Enteric Nervous System. Gastroenterology 2019, 156, 1483–1495.e6. [Google Scholar] [CrossRef] [PubMed]
- Hao, M.M.; Boesmans, W.; Van den Abbeel, V.; Jennings, E.A.; Bornstein, J.C.; Young, H.M.; Vanden Berghe, P. Early emergence of neural activity in the developing mouse enteric nervous system. J. Neurosci. 2011, 31, 15352–15361. [Google Scholar] [CrossRef]
- Hao, M.M.; Bornstein, J.C.; Young, H.M. Development of myenteric cholinergic neurons in ChAT-Cre;R26R-YFP mice. J. Comp. Neurol. 2013, 521, 3358–3370. [Google Scholar] [CrossRef]
- Hao, M.M.; Bergner, A.J.; Hirst, C.S.; Stamp, L.A.; Casagranda, F.; Bornstein, J.C.; Boesmans, W.; Vanden Berghe, P.; Young, H.M. Spontaneous calcium waves in the developing enteric nervous system. Dev. Biol. 2017, 428, 74–87. [Google Scholar] [CrossRef] [PubMed]
- Parathan, P.; Wang, Y.; Leembruggen, A.J.; Bornstein, J.C.; Foong, J.P. The enteric nervous system undergoes significant chemical and synaptic maturation during adolescence in mice. Dev. Biol. 2020, 458, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Foong, J.P.; Nguyen, T.V.; Furness, J.B.; Bornstein, J.C.; Young, H.M. Myenteric neurons of the mouse small intestine undergo significant electrophysiological and morphological changes during postnatal development. J. Physiol. 2012, 590, 2375–2390. [Google Scholar] [CrossRef] [PubMed]
- Foong, J.P. Postnatal Development of the Mouse Enteric Nervous System. Adv. Exp. Med. Biol. 2016, 891, 135–143. [Google Scholar] [PubMed]
- Collins, J.; Borojevic, R.; Verdu, E.F.; Huizinga, J.D.; Ratcliffe, E.M. Intestinal microbiota influence the early postnatal development of the enteric nervous system. Neurogastroenterol. Motil. 2014, 26, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Kabouridis, P.S.; Lasrado, R.; McCallum, S.; Chng, S.H.; Snippert, H.J.; Clevers, H.; Pettersson, S.; Pachnis, V. Microbiota controls the homeostasis of glial cells in the gut lamina propria. Neuron 2015, 85, 289–295. [Google Scholar] [CrossRef] [PubMed]
- De Vadder, F.; Grasset, E.; Mannerås Holm, L.; Karsenty, G.; Macpherson, A.J.; Olofsson, L.E.; Bäckhed, F. Gut microbiota regulates maturation of the adult enteric nervous system via enteric serotonin networks. Proc. Natl. Acad. Sci. USA 2018, 115, 6458–6463. [Google Scholar] [CrossRef]
- Obata, Y.; Castaño, Á.; Boeing, S.; Bon-Frauches, A.C.; Fung, C.; Fallesen, T.; de Agüero, M.G.; Yilmaz, B.; Lopes, R.; Huseynova, A.; et al. Neuronal programming by microbiota regulates intestinal physiology. Nature 2020, 578, 284–289. [Google Scholar] [CrossRef]
- Curley, J.P.; Jordan, E.R.; Swaney, W.T.; Izraelit, A.; Kammel, S.; Champagne, F.A. The meaning of weaning: Influence of the weaning period on behavioral development in mice. Dev. Neurosci. 2009, 31, 318–331. [Google Scholar] [CrossRef]
- Roberts, R.R.; Murphy, J.F.; Young, H.M.; Bornstein, J.C. Development of colonic motility in the neonatal mouse-studies using spatiotemporal maps. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 292, G930–G938. [Google Scholar] [CrossRef]
- Hao, M.M.; Moore, R.E.; Roberts, R.R.; Nguyen, T.; Furness, J.B.; Anderson, R.B.; Young, H.M. The role of neural activity in the migration and differentiation of enteric neuron precursors. Neurogastroenterol. Motil. 2010, 22, e127–e137. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Sengupta, P. Men and mice: Relating their ages. Life Sci. 2016, 152, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Hamnett, R.; Dershowitz, L.B.; Sampathkumar, V.; Wang, Z.; Gomez-Frittelli, J.; De Andrade, V.; Kasthuri, N.; Druckmann, S.; Kaltschmidt, J.A. Regional cytoarchitecture of the adult and developing mouse enteric nervous system. Curr. Biol. 2022, 32, 4483–4492.e5. [Google Scholar] [CrossRef]
- Spencer, N.J.; Costa, M.; Hibberd, T.J.; Wood, J.D. Advances in colonic motor complexes in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2021, 320, G12–G29. [Google Scholar] [CrossRef]
- Corsetti, M.; Costa, M.; Bassotti, G.; Bharucha, A.E.; Borrelli, O.; Dinning, P.; Di Lorenzo, C.; Huizinga, J.D.; Jimenez, M.; Rao, S.; et al. First translational consensus on terminology and definitions of colonic motility in animals and humans studied by manometric and other techniques. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 559–579. [Google Scholar] [CrossRef] [PubMed]
- Barnes, K.J.; Spencer, N.J. Can colonic migrating motor complexes occur in mice lacking the endothelin-3 gene? Clin. Exp. Pharmacol. Physiol. 2015, 42, 485–495. [Google Scholar] [CrossRef]
- Li, W.; Sasse, K.C.; Bayguinov, Y.; Ward, S.M.; Perrino, B.A. Contractile Protein Expression and Phosphorylation and Contractility of Gastric Smooth Muscles from Obese Patients and Patients with Obesity and Diabetes. J. Diabetes Res. 2018, 2018, 8743874. [Google Scholar] [CrossRef]
- Dudem, S.; Large, R.J.; Kulkarni, S.; McClafferty, H.; Tikhonova, I.G.; Sergeant, G.P.; Thornbury, K.D.; Shipston, M.J.; Perrino, B.A.; Hollywood, M.A. LINGO1 is a regulatory subunit of large conductance, Ca(2+)-activated potassium channels. Proc. Natl. Acad. Sci. USA 2020, 117, 2194–2200. [Google Scholar] [CrossRef]
- Sanders, K.M.; Ward, S.M.; Koh, S.D. Interstitial cells: Regulators of smooth muscle function. Physiol. Rev. 2014, 94, 859–907. [Google Scholar] [CrossRef]
- Zhu, M.H.; Kim, T.W.; Ro, S.; Yan, W.; Ward, S.M.; Koh, S.D.; Sanders, K.M. A Ca(2+)-activated Cl(-) conductance in interstitial cells of Cajal linked to slow wave currents and pacemaker activity. J. Physiol. 2009, 587, 4905–4918. [Google Scholar] [CrossRef]
- Furness, J.B. The enteric nervous system and neurogastroenterology. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 286–294. [Google Scholar] [CrossRef]
- Schneider, S.; Wright, C.M.; Heuckeroth, R.O. Unexpected Roles for the Second Brain: Enteric Nervous System as Master Regulator of Bowel Function. Annu. Rev. Physiol. 2019, 81, 235–259. [Google Scholar] [CrossRef] [PubMed]
- Spencer, N.J.; Hu, H. Enteric nervous system: Sensory transduction, neural circuits and gastrointestinal motility. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 338–351. [Google Scholar] [CrossRef] [PubMed]
- Kurahashi, M.; Mutafova-Yambolieva, V.; Koh, S.D.; Sanders, K.M. Platelet-derived growth factor receptor-α-positive cells and not smooth muscle cells mediate purinergic hyperpolarization in murine colonic muscles. Am. J. Physiol. Cell Physiol. 2014, 307, C561–C570. [Google Scholar] [CrossRef] [PubMed]
- Patti, L.; Raiteri, L.; Grilli, M.; Parodi, M.; Raiteri, M.; Marchi, M. P2X(7) receptors exert a permissive role on the activation of release-enhancing presynaptic alpha7 nicotinic receptors co-existing on rat neocortex glutamatergic terminals. Neuropharmacology 2006, 50, 705–713. [Google Scholar] [CrossRef]
- Gallego, D.; Gil, V.; Aleu, J.; Aulí, M.; Clavé, P.; Jiménez, M. Purinergic and nitrergic junction potential in the human colon. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 295, G522–G533. [Google Scholar] [CrossRef]
- Mañé, N.; Gil, V.; Martínez-Cutillas, M.; Clavé, P.; Gallego, D.; Jiménez, M. Differential functional role of purinergic and nitrergic inhibitory cotransmitters in human colonic relaxation. Acta Physiol. 2014, 212, 293–305. [Google Scholar] [CrossRef]
- Lies, B.; Gil, V.; Groneberg, D.; Seidler, B.; Saur, D.; Wischmeyer, E.; Jiménez, M.; Friebe, A. Interstitial cells of Cajal mediate nitrergic inhibitory neurotransmission in the murine gastrointestinal tract. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 307, G98–G106. [Google Scholar] [CrossRef] [PubMed]
- Sanders, K.M.; Ward, S.M. Nitric oxide and its role as a non-adrenergic, non-cholinergic inhibitory neurotransmitter in the gastrointestinal tract. Br. J. Pharmacol. 2019, 176, 212–227. [Google Scholar] [CrossRef] [PubMed]
- Fida, R.; Lyster, D.J.; Bywater, R.A.; Taylor, G.S. Colonic migrating motor complexes (CMMCs) in the isolated mouse colon. Neurogastroenterol. Motil. 1997, 9, 99–107. [Google Scholar] [CrossRef]
- Leembruggen, A.J.L.; Balasuriya, G.K.; Zhang, J.; Schokman, S.; Swiderski, K.; Bornstein, J.C.; Nithianantharajah, J.; Hill-Yardin, E.L. Colonic dilation and altered ex vivo gastrointestinal motility in the neuroligin-3 knockout mouse. Autism Res. 2020, 13, 691–701. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Huang, X.; Lu, H.L.; Liu, S.H.; Zang, J.Y.; Li, Y.J.; Chen, J.; Xu, W.X. Different distributions of interstitial cells of Cajal and platelet-derived growth factor receptor-α positive cells in colonic smooth muscle cell/interstitial cell of Cajal/platelet-derived growth factor receptor-α positive cell syncytium in mice. World J. Gastroenterol. 2018, 24, 4989–5004. [Google Scholar] [CrossRef] [PubMed]
- Ward, S.M.; Beckett, E.A.; Wang, X.; Baker, F.; Khoyi, M.; Sanders, K.M. Interstitial cells of Cajal mediate cholinergic neurotransmission from enteric motor neurons. J. Neurosci. 2000, 20, 1393–1403. [Google Scholar] [CrossRef] [PubMed]
- Cole, W.C. ANO1-ther brick in the wall–role of Ca2+-activated Cl− channels of interstitial cells of Cajal in cholinergic motor control of gastrointestinal smooth muscle. J. Physiol. 2011, 589, 4641–4642. [Google Scholar] [CrossRef]
- Drumm, B.T.; Rembetski, B.E.; Huynh, K.; Nizar, A.; Baker, S.A.; Sanders, K.M. Excitatory cholinergic responses in mouse colon intramuscular interstitial cells of Cajal are due to enhanced Ca(2+) release via M(3) receptor activation. FASEB J. 2020, 34, 10073–10095. [Google Scholar] [CrossRef]
- Spencer, N.J.; Hibberd, T.J.; Travis, L.; Wiklendt, L.; Costa, M.; Hu, H.; Brookes, S.J.; Wattchow, D.A.; Dinning, P.G.; Keating, D.J.; et al. Identification of a Rhythmic Firing Pattern in the Enteric Nervous System That Generates Rhythmic Electrical Activity in Smooth Muscle. J. Neurosci. 2018, 38, 5507–5522. [Google Scholar] [CrossRef]
- Hata, F.; Kataoka, T.; Takeuchi, T.; Yagasaki, O.; Yamano, N. Differences in control of descending inhibition in the proximal and distal regions of rat colon. Br. J. Pharmacol. 1990, 101, 1011–1015. [Google Scholar] [CrossRef]
- Gonda, T.; Oki, M. Distribution of cholinergic and catecholaminergic nerves in the colon of the rat with aganglionosis. Exp. Anim. 1991, 40, 471–484. [Google Scholar] [CrossRef][Green Version]
- Wang, Q.; Zang, J.; Huang, X.; Lu, H.; Xu, W.; Chen, J. Colonic Dysmotility in Murine Partial Colonic Obstruction Due to Functional Changes in Interstitial Cells. J. Neurogastroenterol. Motil. 2019, 25, 589–601. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Z.; Wang, Q.; Yang, F.; Wang, J.; Zhao, Y.; Perrino, B.A.; Chen, J. Functional and Transcriptomic Characterization of Postnatal Maturation of ENS and SIP Syncytium in Mice Colon. Biomolecules 2023, 13, 1688. https://doi.org/10.3390/biom13121688
Wu Z, Wang Q, Yang F, Wang J, Zhao Y, Perrino BA, Chen J. Functional and Transcriptomic Characterization of Postnatal Maturation of ENS and SIP Syncytium in Mice Colon. Biomolecules. 2023; 13(12):1688. https://doi.org/10.3390/biom13121688
Chicago/Turabian StyleWu, Zhihao, Qianqian Wang, Fan Yang, Jiaxuan Wang, Yuying Zhao, Brian A. Perrino, and Jie Chen. 2023. "Functional and Transcriptomic Characterization of Postnatal Maturation of ENS and SIP Syncytium in Mice Colon" Biomolecules 13, no. 12: 1688. https://doi.org/10.3390/biom13121688
APA StyleWu, Z., Wang, Q., Yang, F., Wang, J., Zhao, Y., Perrino, B. A., & Chen, J. (2023). Functional and Transcriptomic Characterization of Postnatal Maturation of ENS and SIP Syncytium in Mice Colon. Biomolecules, 13(12), 1688. https://doi.org/10.3390/biom13121688





