Albumin Thiolation and Oxidative Stress Status in Patients with Aortic Valve Stenosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Albumin Analysis by LC–Mass Spectrometry
2.3. Quantification of Free Sulfhydryl Groups in Serum from Patients and Controls
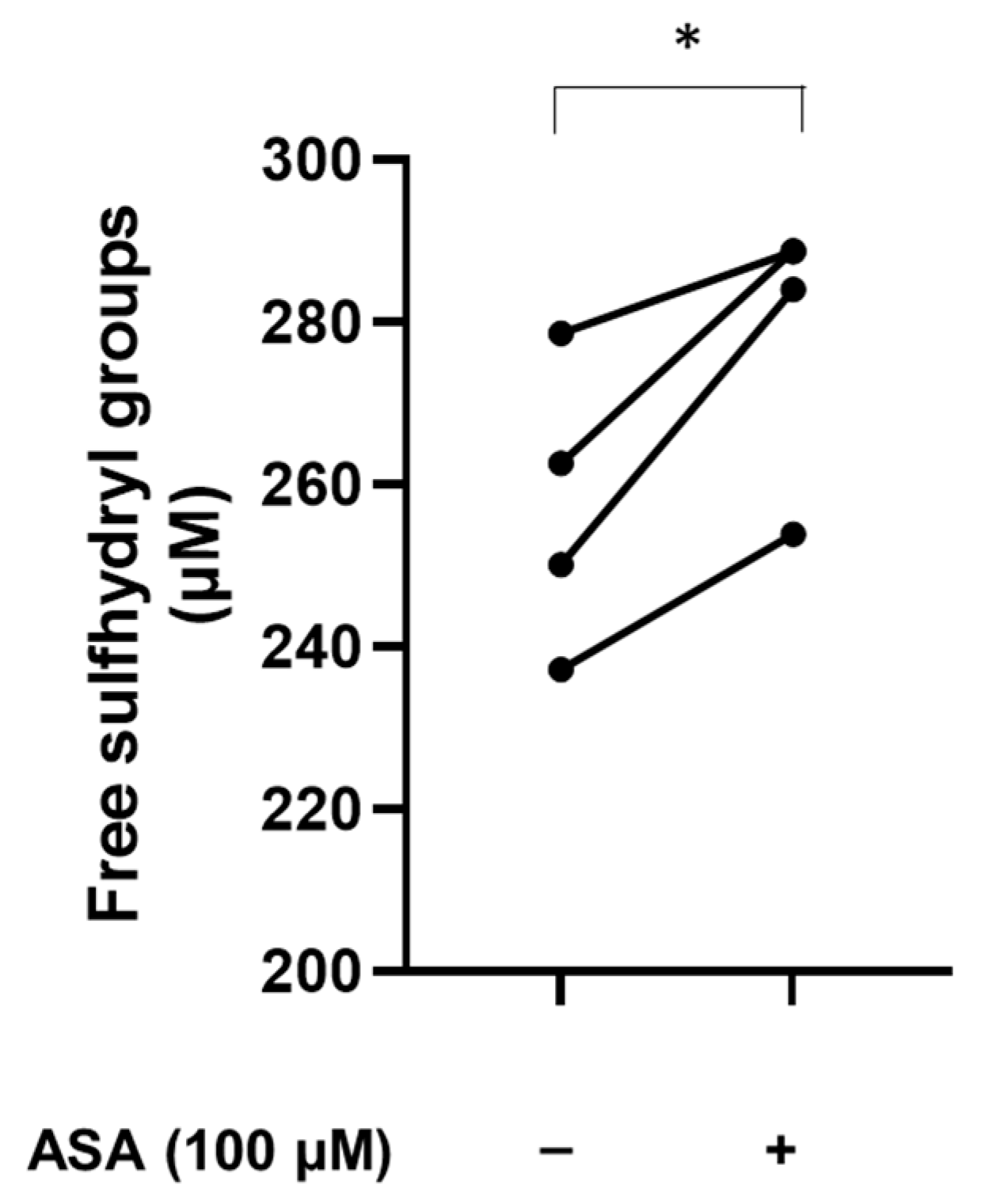
2.4. In Vitro Treatment of Plasma with Aspirin
2.5. In Vitro Treatment of Serum from Patients with N-Acetylcysteine and N-Acetylcysteine Amide AD4/NACA
2.6. Measurement of the Antioxidant Activity
2.7. Statistical Analysis
3. Results
3.1. Characteristics of the Study Participants
3.2. Albumin Proteoforms in the Study Population
3.3. Levels of Free Sulfhydryl Groups in Serum from Patients and Controls
3.4. Aspirin Increases the Levels of Free Sulfhydryl Groups
3.5. N-Acetylcysteine and N-Acetylcysteine Amide (AD4/NACA) Reduce the Serum Levels of Cysteinylated Proteoform of Albumin
3.6. N-Acetylcysteine and N-Acetylcysteine Amide (AD4/NACA) Increase the Levels of Free Sulfhydryl Groups in Serum
3.7. N-Acetylcysteine and N-Acetylcysteine Amide (AD4/NACA) Increase the Total Antioxidant Activity of Serum from Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aluru, J.S.; Barsouk, A.; Saginala, K.; Rawla, P.; Barsouk, A. Valvular Heart Disease Epidemiology. Med. Sci. 2022, 10, 32. [Google Scholar] [CrossRef] [PubMed]
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics-2020 Update: A Report from the American Heart Association. Circulation 2020, 141, e139–e596. [Google Scholar] [CrossRef] [PubMed]
- Alfieri, O.; Vahanian, A. The year in cardiology 2016: Valvular heart disease. Eur. Heart J. 2017, 38, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. J. Cardiothorac. Surg. 2021, 60, 727–800. [Google Scholar] [CrossRef]
- Sun, J.C.; Davidson, M.J.; Lamy, A.; Eikelboom, J.W. Antithrombotic management of patients with prosthetic heart valves: Current evidence and future trends. Lancet 2009, 374, 565–576. [Google Scholar] [CrossRef]
- Greenberg, H.Z.E.; Zhao, G.; Shah, A.M.; Zhang, M. Role of oxidative stress in calcific aortic valve disease and its therapeutic implications. Cardiovasc. Res. 2022, 118, 1433–1451. [Google Scholar] [CrossRef]
- Phua, K.; Chew, N.W.; Kong, W.K.; Tan, R.S.; Ye, L.; Poh, K.K. The mechanistic pathways of oxidative stress in aortic stenosis and clinical implications. Theranostics 2022, 12, 5189–5203. [Google Scholar] [CrossRef]
- Kayama, Y.; Raaz, U.; Jagger, A.; Adam, M.; Schellinger, I.N.; Sakamoto, M.; Suzuki, H.; Toyama, K.; Spin, J.M.; Tsao, P.S. Diabetic Cardiovascular Disease Induced by Oxidative Stress. Int. J. Mol. Sci. 2015, 16, 25234–25263. [Google Scholar] [CrossRef]
- Davies, M.J. Protein oxidation and peroxidation. Biochem. J. 2016, 473, 805–825. [Google Scholar] [CrossRef]
- Oliveira, P.V.S.; Laurindo, F.R.M. Implications of plasma thiol redox in disease. Clin. Sci. 2018, 132, 1257–1280. [Google Scholar] [CrossRef]
- Tabata, F.; Wada, Y.; Kawakami, S.; Miyaji, K. Serum Albumin Redox States: More Than Oxidative Stress Biomarker. Antioxidants 2021, 10, 503. [Google Scholar] [CrossRef] [PubMed]
- Turell, L.; Radi, R.; Alvarez, B. The thiol pool in human plasma: The central contribution of albumin to redox processes. Free Radic. Biol. Med. 2013, 65, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Steglich, M.; Lombide, R.; Lopez, I.; Portela, M.; Flo, M.; Marin, M.; Alvarez, B.; Turell, L. Expression, purification and initial characterization of human serum albumin domain I and its cysteine 34. PLoS ONE 2020, 15, e0240580. [Google Scholar] [CrossRef] [PubMed]
- Zoanni, B.; Brioschi, M.; Mallia, A.; Gianazza, E.; Eligini, S.; Carini, M.; Aldini, G.; Banfi, C. Novel insights about albumin in cardiovascular diseases: Focus on heart failure. Mass Spectrom. Rev. 2023, 42, 1113–1128. [Google Scholar] [CrossRef] [PubMed]
- Oettl, K.; Stauber, R.E. Physiological and pathological changes in the redox state of human serum albumin critically influence its binding properties. Br. J. Pharmacol. 2007, 151, 580–590. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, F.; Shibata, T.; Uchida, K. A unique mechanism for thiolation of serum albumins by disulphide molecules. J. Biochem. 2020, 167, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, F.; Shibata, T.; Kamiya, K.; Yoshitake, J.; Kikuchi, R.; Matsushita, T.; Ishii, I.; Gimenez-Bastida, J.A.; Schneider, C.; Uchida, K. Structural and functional insights into S-thiolation of human serum albumins. Sci. Rep. 2018, 8, 932. [Google Scholar] [CrossRef] [PubMed]
- Brioschi, M.; Gianazza, E.; Mallia, A.; Zoanni, B.; Altomare, A.; Martinez Fernandez, A.; Agostoni, P.; Aldini, G.; Banfi, C. S-Thiolation Targets Albumin in Heart Failure. Antioxidants 2020, 9, 763. [Google Scholar] [CrossRef]
- Eligini, S.; Porro, B.; Aldini, G.; Colli, S.; Banfi, C. N-Acetylcysteine Inhibits Platelet Function through the Regeneration of the Non-Oxidative Form of Albumin. Antioxidants 2022, 11, 445. [Google Scholar] [CrossRef]
- Martinez Fernandez, A.; Regazzoni, L.; Brioschi, M.; Gianazza, E.; Agostoni, P.; Aldini, G.; Banfi, C. Pro-oxidant and pro-inflammatory effects of glycated albumin on cardiomyocytes. Free Radic. Biol. Med. 2019, 144, 245–255. [Google Scholar] [CrossRef]
- Regazzoni, L.; Del Vecchio, L.; Altomare, A.; Yeum, K.J.; Cusi, D.; Locatelli, F.; Carini, M.; Aldini, G. Human serum albumin cysteinylation is increased in end stage renal disease patients and reduced by hemodialysis: Mass spectrometry studies. Free Radic. Res. 2013, 47, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Eligini, S.; Munno, M.; Atlas, D.; Banfi, C. N-acetylcysteine Amide AD4/NACA and Thioredoxin Mimetic Peptides Inhibit Platelet Aggregation and Protect against Oxidative Stress. Antioxidants 2023, 12, 1395. [Google Scholar] [CrossRef] [PubMed]
- Erel, O.; Neselioglu, S. A novel and automated assay for thiol/disulphide homeostasis. Clin. Biochem. 2014, 47, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Belinskaia, D.A.; Voronina, P.A.; Shmurak, V.I.; Jenkins, R.O.; Goncharov, N.V. Serum Albumin in Health and Disease: Esterase, Antioxidant, Transporting and Signaling Properties. Int. J. Mol. Sci. 2021, 22, 10318. [Google Scholar] [CrossRef] [PubMed]
- Sastre-Oliva, T.; Corbacho-Alonso, N.; Albo-Escalona, D.; Lopez, J.A.; Lopez-Almodovar, L.F.; Vazquez, J.; Padial, L.R.; Mourino-Alvarez, L.; Barderas, M.G. The Influence of Coronary Artery Disease in the Development of Aortic Stenosis and the Importance of the Albumin Redox State. Antioxidants 2022, 11, 317. [Google Scholar] [CrossRef]
- Dayawansa, N.H.; Baratchi, S.; Peter, K. Uncoupling the Vicious Cycle of Mechanical Stress and Inflammation in Calcific Aortic Valve Disease. Front. Cardiovasc. Med. 2022, 9, 783543. [Google Scholar] [CrossRef]
- Lee, S.H.; Choi, J.H. Involvement of Immune Cell Network in Aortic Valve Stenosis: Communication between Valvular Interstitial Cells and Immune Cells. Immune Netw. 2016, 16, 26–32. [Google Scholar] [CrossRef]
- Shu, L.; Yuan, Z.; Li, F.; Cai, Z. Oxidative stress and valvular endothelial cells in aortic valve calcification. Biomed. Pharmacother. 2023, 163, 114775. [Google Scholar] [CrossRef]
- Tanase, D.M.; Valasciuc, E.; Gosav, E.M.; Floria, M.; Costea, C.F.; Dima, N.; Tudorancea, I.; Maranduca, M.A.; Serban, I.L. Contribution of Oxidative Stress (OS) in Calcific Aortic Valve Disease (CAVD): From Pathophysiology to Therapeutic Targets. Cells 2022, 11, 2663. [Google Scholar] [CrossRef]
- Gould, S.T.; Srigunapalan, S.; Simmons, C.A.; Anseth, K.S. Hemodynamic and cellular response feedback in calcific aortic valve disease. Circ. Res. 2013, 113, 186–197. [Google Scholar] [CrossRef]
- Rutkovskiy, A.; Malashicheva, A.; Sullivan, G.; Bogdanova, M.; Kostareva, A.; Stenslokken, K.O.; Fiane, A.; Vaage, J. Valve Interstitial Cells: The Key to Understanding the Pathophysiology of Heart Valve Calcification. J. Am. Heart Assoc. 2017, 6, e006339. [Google Scholar] [CrossRef]
- Goody, P.R.; Hosen, M.R.; Christmann, D.; Niepmann, S.T.; Zietzer, A.; Adam, M.; Bonner, F.; Zimmer, S.; Nickenig, G.; Jansen, F. Aortic Valve Stenosis: From Basic Mechanisms to Novel Therapeutic Targets. Arter. Thromb. Vasc. Biol. 2020, 40, 885–900. [Google Scholar] [CrossRef] [PubMed]
- Serrano, C.V., Jr.; de Mattos, F.R.; Pitta, F.G.; Nomura, C.H.; de Lemos, J.; Ramires, J.A.F.; Kalil-Filho, R. Association between Neutrophil-Lymphocyte and Platelet-Lymphocyte Ratios and Coronary Artery Calcification Score among Asymptomatic Patients: Data from a Cross-Sectional Study. Mediat. Inflamm. 2019, 2019, 6513847. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Ying, Q.; Su, X.; Li, T. Development and application of reverse transcription loop-mediated isothermal amplification for detecting live Shewanella putrefaciens in preserved fish sample. J. Food Sci. 2012, 77, M226–M230. [Google Scholar] [CrossRef] [PubMed]
- Siudut, J.; Natorska, J.; Wypasek, E.; Wiewiorka, L.; Ostrowska-Kaim, E.; Wisniowska-Smialek, S.; Plens, K.; Legutko, J.; Undas, A. Impaired Fibrinolysis in Patients with Isolated Aortic Stenosis is Associated with Enhanced Oxidative Stress. J. Clin. Med. 2020, 9, 2002. [Google Scholar] [CrossRef] [PubMed]
- van Broekhoven, A.; Krijnen, P.A.J.; Fuijkschot, W.W.; Morrison, M.C.; Zethof, I.P.A.; van Wieringen, W.N.; Smulders, Y.M.; Niessen, H.W.M.; Vonk, A.B.A. Short-term LPS induces aortic valve thickening in ApoE*3Leiden mice. Eur. J. Clin. Investig. 2019, 49, e13121. [Google Scholar] [CrossRef]
- Aguado, B.A.; Schuetze, K.B.; Grim, J.C.; Walker, C.J.; Cox, A.C.; Ceccato, T.L.; Tan, A.C.; Sucharov, C.C.; Leinwand, L.A.; Taylor, M.R.G.; et al. Transcatheter aortic valve replacements alter circulating serum factors to mediate myofibroblast deactivation. Sci. Transl. Med. 2019, 11, eaav3233. [Google Scholar] [CrossRef]
- Hewing, B.; Ellerbroek, R.; Au, S.C.; Stangl, V.; Dreger, H.; Laule, M.; Grubitzsch, H.; Knebel, F.; Baumann, G.; Ludwig, A.; et al. Levels of Circulating Intermediate Monocytes Decrease after Aortic Valve Replacement in Patients with Severe Aortic Stenosis. Thromb. Haemost. 2017, 117, 2346–2355. [Google Scholar] [CrossRef]
- Singh, M.; Sporn, Z.A.; Schaff, H.V.; Pellikka, P.A. ACC/AHA Versus ESC Guidelines on Prosthetic Heart Valve Management: JACC Guideline Comparison. J. Am. Coll. Cardiol. 2019, 73, 1707–1718. [Google Scholar] [CrossRef]
- Hedin, U.; Matic, L.P. Recent advances in therapeutic targeting of inflammation in atherosclerosis. J. Vasc. Surg. 2019, 69, 944–951. [Google Scholar] [CrossRef]
- Chen, C.M.; Tung, Y.T.; Wei, C.H.; Lee, P.Y.; Chen, W. Anti-Inflammatory and Reactive Oxygen Species Suppression through Aspirin Pretreatment to Treat Hyperoxia-Induced Acute Lung Injury in NF-kappaB-Luciferase Inducible Transgenic Mice. Antioxidants 2020, 9, 429. [Google Scholar] [CrossRef]
- Ayyadevara, S.; Bharill, P.; Dandapat, A.; Hu, C.; Khaidakov, M.; Mitra, S.; Shmookler Reis, R.J.; Mehta, J.L. Aspirin inhibits oxidant stress, reduces age-associated functional declines, and extends lifespan of Caenorhabditis elegans. Antioxid. Redox Signal 2013, 18, 481–490. [Google Scholar] [CrossRef]
- Paseban, M.; Niazmand, S. The Comparison of Antioxidant Effect of Aspirin, Metformin, Atorvastatin and Captopril Co-administration in the Heart and Kidney Tissues of Diabetic Rats. Iran. J. Pharm. Res. 2021, 20, 27–39. [Google Scholar] [CrossRef]
- Falco, L.; Tessitore, V.; Ciccarelli, G.; Malvezzi, M.; D’Andrea, A.; Imbalzano, E.; Golino, P.; Russo, V. Antioxidant Properties of Oral Antithrombotic Therapies in Atherosclerotic Disease and Atrial Fibrillation. Antioxidants 2023, 12, 1185. [Google Scholar] [CrossRef]
- Steer, K.A.; Wallace, T.M.; Bolton, C.H.; Hartog, M. Aspirin protects low density lipoprotein from oxidative modification. Heart 1997, 77, 333–337. [Google Scholar] [CrossRef]
- Wu, R.; Lamontagne, D.; de Champlain, J. Antioxidative properties of acetylsalicylic Acid on vascular tissues from normotensive and spontaneously hypertensive rats. Circulation 2002, 105, 387–392. [Google Scholar] [CrossRef]
- Betts, W.H.; Whitehouse, M.W.; Cleland, L.G.; Vernon-Roberts, B. In vitro antioxidant properties of potential biotransformation products of salicylate, sulphasalazine and amidopyrine. J. Free Radic. Biol. Med. 1985, 1, 273–280. [Google Scholar] [CrossRef]
- Pinckard, R.N.; Hawkins, D.; Farr, R.S. In vitro acetylation of plasma proteins, enzymes and DNA by aspirin. Nature 1968, 219, 68–69. [Google Scholar] [CrossRef]
- Chen, B.; Zhao, J.; Zhang, S.; Wu, W.; Qi, R. Aspirin inhibits the production of reactive oxygen species by downregulating Nox4 and inducible nitric oxide synthase in human endothelial cells exposed to oxidized low-density lipoprotein. J. Cardiovasc. Pharmacol. 2012, 59, 405–412. [Google Scholar] [CrossRef]
- Kurban, S.; Mehmetoglu, I. Effects of acetylsalicylic acid on serum paraoxonase activity, Ox-LDL, coenzyme Q10 and other oxidative stress markers in healthy volunteers. Clin. Biochem. 2010, 43, 287–290. [Google Scholar] [CrossRef]
- Jian, Z.; Tang, L.; Yi, X.; Liu, B.; Zhang, Q.; Zhu, G.; Wang, G.; Gao, T.; Li, C. Aspirin induces Nrf2-mediated transcriptional activation of haem oxygenase-1 in protection of human melanocytes from H2 O2 -induced oxidative stress. J. Cell. Mol. Med. 2016, 20, 1307–1318. [Google Scholar] [CrossRef]
- Jorda, A.; Aldasoro, M.; Aldasoro, C.; Guerra-Ojeda, S.; Iradi, A.; Vila, J.M.; Campos-Campos, J.; Valles, S.L. Action of low doses of Aspirin in Inflammation and Oxidative Stress induced by abeta(1-42) on Astrocytes in primary culture. Int. J. Med. Sci. 2020, 17, 834–843. [Google Scholar] [CrossRef]
- Yang, J.J.; Li, P.; Wang, F.; Liang, W.J.; Ma, H.; Chen, Y.; Ma, Z.M.; Li, Q.Z.; Peng, Q.S.; Zhang, Y.; et al. Activation of activator protein 2 alpha by aspirin alleviates atherosclerotic plaque growth and instability in vivo. Oncotarget 2016, 7, 52729–52739. [Google Scholar] [CrossRef]
- Ristimae, T.; Zilmer, M.; Zilmer, K.; Kairane, C.; Kullisaar, T.; Teesalu, R. Effect of low-dose aspirin on the markers of oxidative stress. Cardiovasc. Drugs Ther. 1999, 13, 485–490. [Google Scholar] [CrossRef]
- Berg, K.; Langaas, M.; Ericsson, M.; Pleym, H.; Basu, S.; Nordrum, I.S.; Vitale, N.; Haaverstad, R. Acetylsalicylic acid treatment until surgery reduces oxidative stress and inflammation in patients undergoing coronary artery bypass grafting. Eur. J. Cardiothorac. Surg. 2013, 43, 1154–1163. [Google Scholar] [CrossRef]
- Offen, D.; Gilgun-Sherki, Y.; Barhum, Y.; Benhar, M.; Grinberg, L.; Reich, R.; Melamed, E.; Atlas, D. A low molecular weight copper chelator crosses the blood-brain barrier and attenuates experimental autoimmune encephalomyelitis. J. Neurochem. 2004, 89, 1241–1251. [Google Scholar] [CrossRef]
- Grinberg, L.; Fibach, E.; Amer, J.; Atlas, D. N-acetylcysteine amide, a novel cell-permeating thiol, restores cellular glutathione and protects human red blood cells from oxidative stress. Free Radic. Biol. Med. 2005, 38, 136–145. [Google Scholar] [CrossRef]
- Altomare, A.; Baron, G.; Brioschi, M.; Longoni, M.; Butti, R.; Valvassori, E.; Tremoli, E.; Carini, M.; Agostoni, P.; Vistoli, G.; et al. N-Acetyl-Cysteine Regenerates Albumin Cys34 by a Thiol-Disulfide Breaking Mechanism: An Explanation of Its Extracellular Antioxidant Activity. Antioxidants 2020, 9, 367. [Google Scholar] [CrossRef]
- Altomare, A.A.; Brioschi, M.; Eligini, S.; Bonomi, A.; Zoanni, B.; Iezzi, A.; Jemos, C.; Porro, B.; D’Alessandra, Y.; Guarino, A.; et al. N-Acetylcysteine Regenerates In Vivo Mercaptoalbumin. Antioxidants 2022, 11, 1758. [Google Scholar] [CrossRef]
- Velho, T.R.; Pereira, R.M.; Fernandes, F.; Guerra, N.C.; Ferreira, R.; Nobre, A. Bioprosthetic Aortic Valve Degeneration: A Review from a Basic Science Perspective. Braz. J. Cardiovasc. Surg. 2022, 37, 239–250. [Google Scholar] [CrossRef]
- Yetkin, E.; Waltenberger, J. Molecular and cellular mechanisms of aortic stenosis. Int. J. Cardiol. 2009, 135, 4–13. [Google Scholar] [CrossRef]







| Aortic Valve Replacement | Redo Aortic Valve Replacement | p | |
|---|---|---|---|
| N | 36 | 8 | / |
| Male gender | 18 (50.0) | 5 (62.5) | 0.701 |
| Age (years) | 78.0 (8.5) | 70.5 (14) | 0.100 |
| Weight (Kg) | 75.5 (12.5) | 70.0 (43) | 0.604 |
| BMI (Kg/m2) | 27.5 (3.7) | 29.2 (12.2) | 0.784 |
| Hemoglobin (g/dL) | 12.7 (2.1) | 12.8 (3.8) | 0.346 |
| Platelets (×109/L) | 196 (80) | 157.5 (89) | 0.041 |
| Neutrophils (×109/L) | / | 6.4 (53.8) | / |
| Lymphocytes (×109/L) | / | 2.0 (17.6) | / |
| Creatinine (mg/dL) | 0.91 (0.40) | 1.04 (0.36) | 0.640 |
| LDL (mg/dL) | 71 (38) | 92.5 (40) | 0.439 |
| Hypertension (n, %) | 33 (91.7) | 6 (75.0) | 0.219 |
| Dyslipidemia (n, %) | 27 (75.0) | 5 (62.5) | 0.663 |
| Diabetes (n, %) | 7 (19.4) | 2 (25.0) | 0.659 |
| Coronary artery disease (n, %) | 6 (16.7) | 1 (12.5) | 1.000 |
| LVEF (%) | 61 (11.0) | 55 (32.0) | 0.692 |
| Peak aortic gradient (mmHg) | 72 (32) | 75 (27) | 0.737 |
| Mean aortic gradient (mmHg) | 43 (15) | 48 (25) | 0.608 |
| Previous coronary artery bypass graft (n, %) | 1 (2.8) | 1 (12.5) | 0.334 |
| Oncological history (n, %) | 3 (8.3) | 1 (12.5) | 1.000 |
| Novel oral anticoagulants (n, %) | 6 (16.7) | 0 (0.0) | 0.053 |
| Statins (n, %) | 21 (58.3) | 4 (50.0) | 0.710 |
| Antidiabetic drugs (n, %) | 7 (19.4) | 2 (25.0) | 0.659 |
| Antihypertensive drugs (n, %) | 32 (88.9) | 5 (63.0) | 0.100 |
| Years since first surgery | / | 8.7 (6.1) | / |
| Albumin Proteoforms (%) | Aortic Valve Replacement | Redo Aortic Valve Replacement | Controls | p Value Valv Repl vs. Redo Valv Repl | p Value Valv Repl vs. Controls |
|---|---|---|---|---|---|
| n | 36 | 8 | 10 | / | / |
| % Cys (PA) | 32.0 (14.4) | 15.7 (3.1) | 14.3 (4.2) | <0.001 | <0.0001 |
| % Gly (PA) | 6.1 (1.8) | 5.1 (0.8) | 5.6 (1.5) | 0.012 | 0.136 |
| % SH (PA) | 61.0 (17.0) | 79.9 (4.0) | 80.1 (3.9) | <0.001 | <0.0001 |
| HSA total (PA) | 6.58 × 109 (26.2 × 109) | 4.54 × 109 (3 × 109) | 6.0 × 109 (18.3 × 109) | 0.331 | 0.564 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Savini, C.; Tenti, E.; Mikus, E.; Eligini, S.; Munno, M.; Gaspardo, A.; Gianazza, E.; Greco, A.; Ghilardi, S.; Aldini, G.; et al. Albumin Thiolation and Oxidative Stress Status in Patients with Aortic Valve Stenosis. Biomolecules 2023, 13, 1713. https://doi.org/10.3390/biom13121713
Savini C, Tenti E, Mikus E, Eligini S, Munno M, Gaspardo A, Gianazza E, Greco A, Ghilardi S, Aldini G, et al. Albumin Thiolation and Oxidative Stress Status in Patients with Aortic Valve Stenosis. Biomolecules. 2023; 13(12):1713. https://doi.org/10.3390/biom13121713
Chicago/Turabian StyleSavini, Carlo, Elena Tenti, Elisa Mikus, Sonia Eligini, Marco Munno, Anna Gaspardo, Erica Gianazza, Arianna Greco, Stefania Ghilardi, Giancarlo Aldini, and et al. 2023. "Albumin Thiolation and Oxidative Stress Status in Patients with Aortic Valve Stenosis" Biomolecules 13, no. 12: 1713. https://doi.org/10.3390/biom13121713
APA StyleSavini, C., Tenti, E., Mikus, E., Eligini, S., Munno, M., Gaspardo, A., Gianazza, E., Greco, A., Ghilardi, S., Aldini, G., Tremoli, E., & Banfi, C. (2023). Albumin Thiolation and Oxidative Stress Status in Patients with Aortic Valve Stenosis. Biomolecules, 13(12), 1713. https://doi.org/10.3390/biom13121713











