A Novel Form of Neuregulin 1 Type III Caused by N-Terminal Processing
Abstract
:1. Introduction
2. Methods
2.1. Cell Line and Culture Condition
2.2. Plasmids
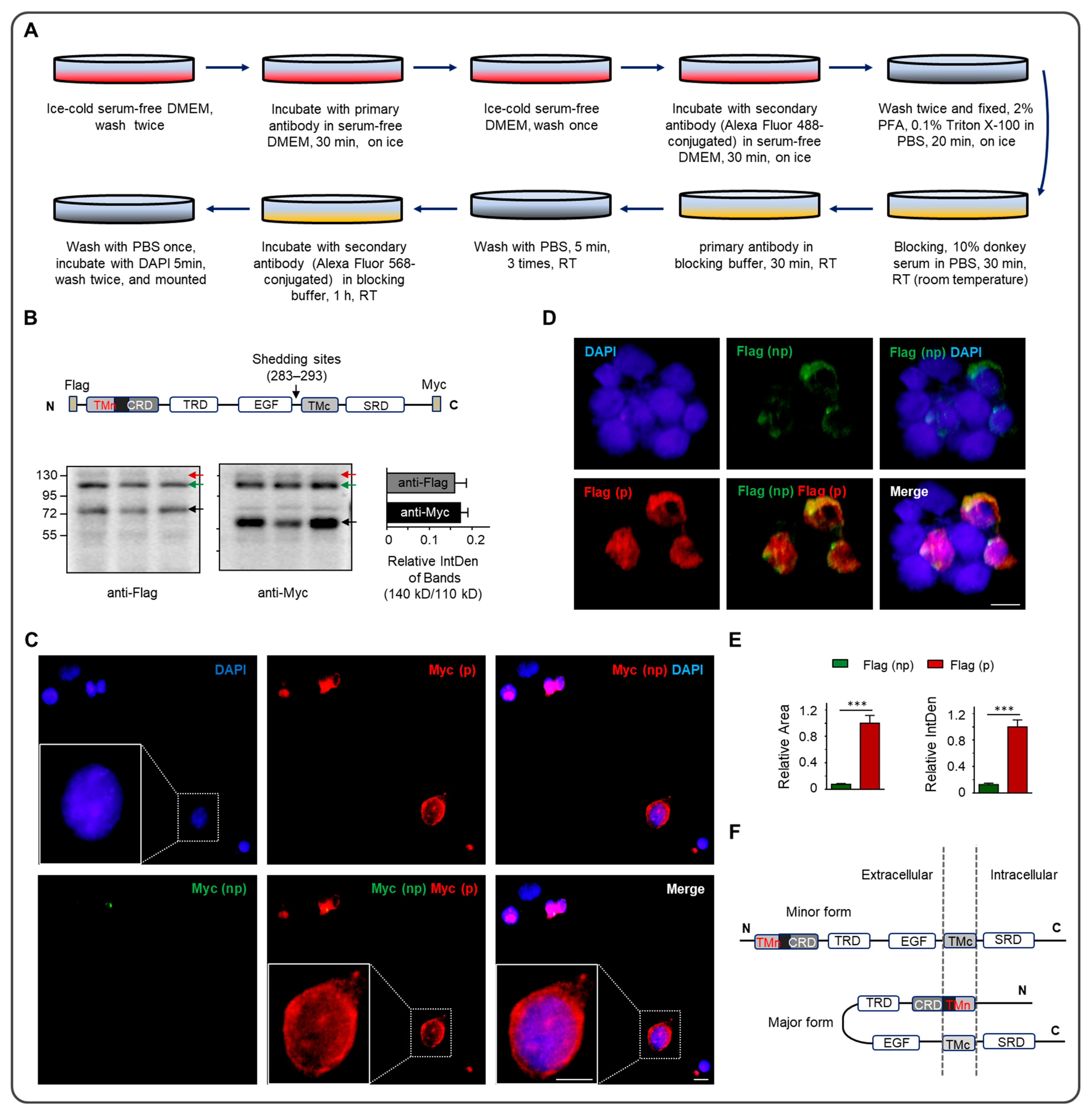
2.3. Western Blotting (WB)
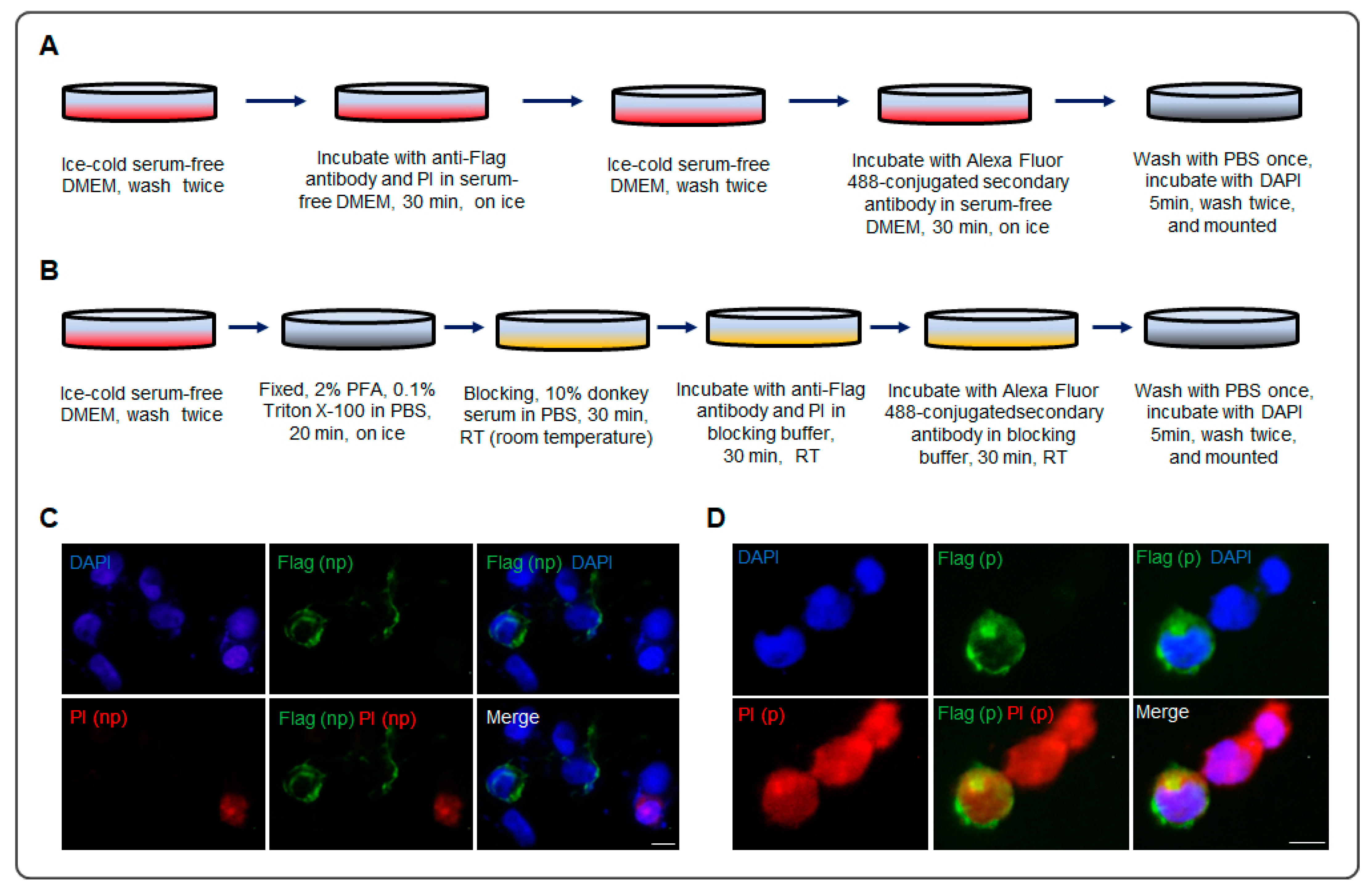
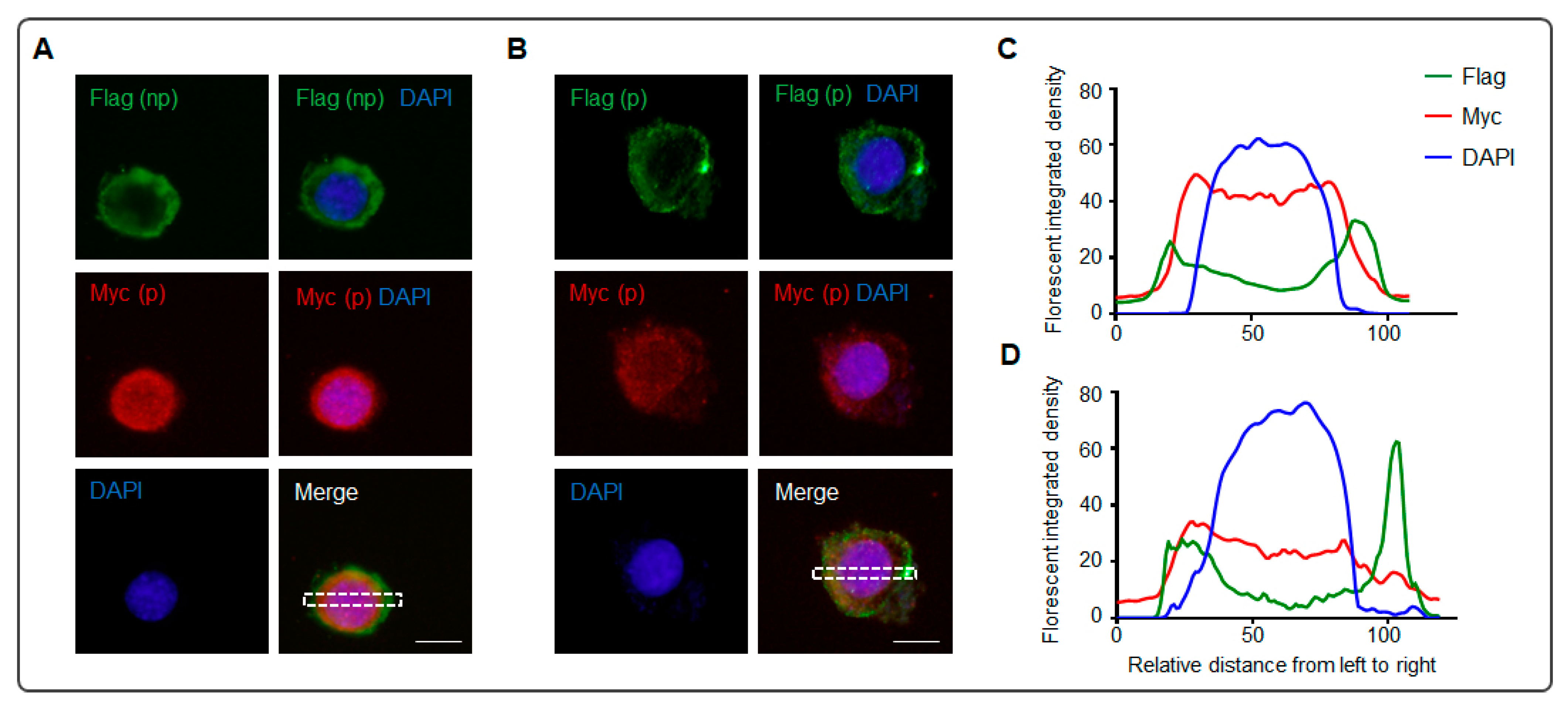
2.4. Immunocytochemistry
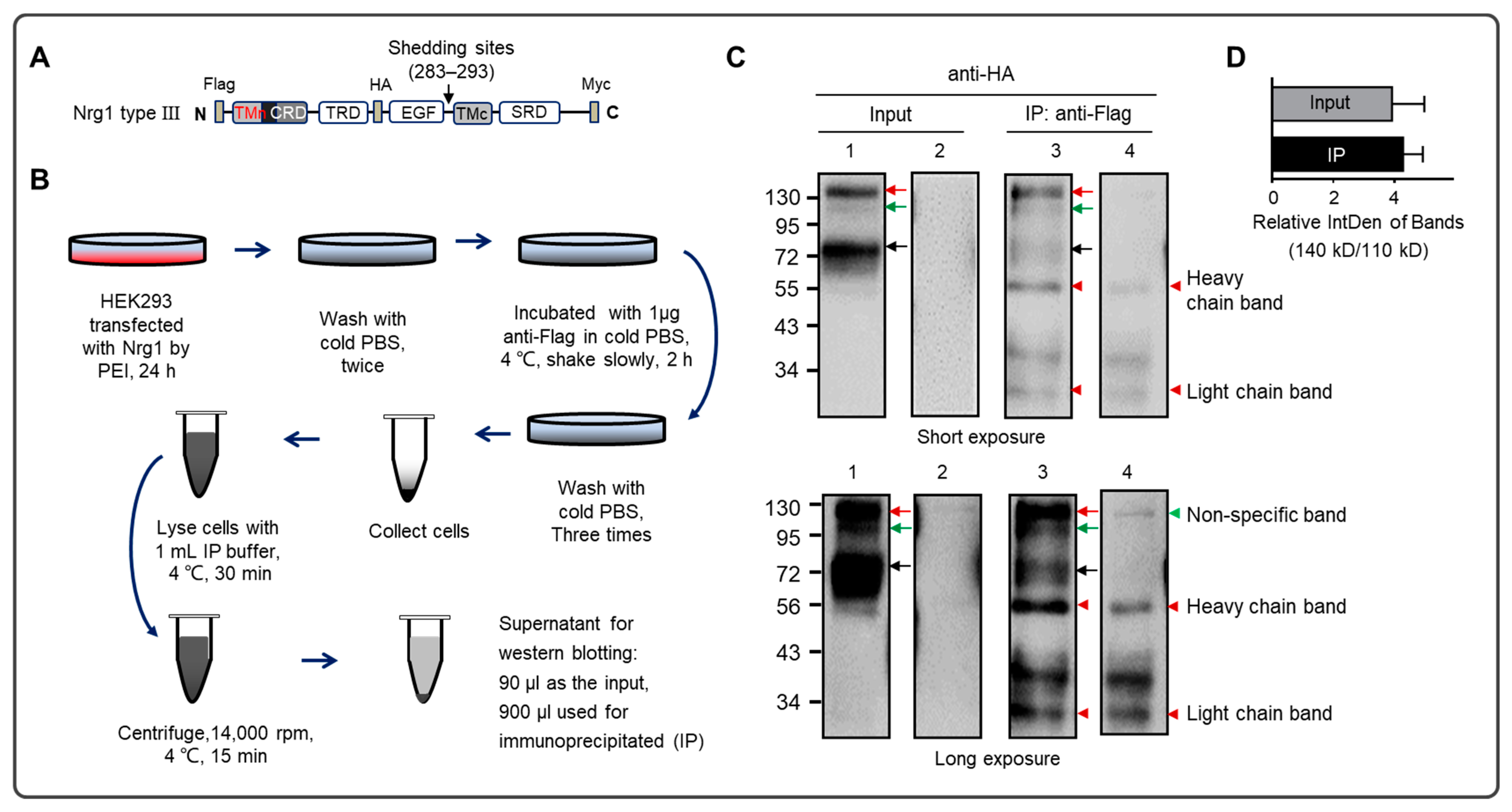
2.5. Immunoprecipitation (IP)
2.6. Analysis of the Images
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Mei, L.; Xiong, W.C. Neuregulin 1 in neural development, synaptic plasticity and schizophrenia. Nat. Rev. Neurosci. 2008, 9, 437–452. [Google Scholar] [CrossRef] [PubMed]
- Weickert, C.S.; Tiwari, Y.; Schofield, P.R.; Mowry, B.J.; Fullerton, J.M. Schizophrenia-associated HapICE haplotype is associated with increased NRG1 type III expression and high nucleotide diversity. Transl. Psychiatry 2012, 2, e104. [Google Scholar] [CrossRef] [PubMed]
- Mostaid, M.S.; Lloyd, D.; Liberg, B.; Sundram, S.; Pereira, A.; Pantelis, C.; Karl, T.; Weickert, C.S.; Everall, I.P.; Bousman, C.A. Neuregulin-1 and schizophrenia in the genome-wide association study era. Neurosci. Biobehav. Rev. 2016, 68, 387–409. [Google Scholar] [CrossRef]
- Su, N.; Zhang, W.; Eter, N.; Heiduschka, P.; Zhang, M. Overexpression of Neuregulin-1 Type III Has Impact on Visual Function in Mice. Int. J. Mol. Sci. 2022, 23, 4489. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Bates, R.; Yin, D.M.; Shen, C.; Wang, F.; Su, N.; Kirov, S.A.; Luo, Y.; Wang, J.Z.; Xiong, W.C.; et al. Specific regulation of NRG1 isoform expression by neuronal activity. J. Neurosci. 2011, 31, 8491–8501. [Google Scholar] [CrossRef]
- Taveggia, C.; Zanazzi, G.; Petrylak, A.; Yano, H.; Rosenbluth, J.; Einheber, S.; Xu, X.; Esper, R.M.; Loeb, J.A.; Shrager, P.; et al. Neuregulin-1 type III determines the ensheathment fate of axons. Neuron 2005, 47, 681–694. [Google Scholar] [CrossRef] [PubMed]
- Perlin, J.R.; Lush, M.E.; Stephens, W.Z.; Piotrowski, T.; Talbot, W.S. Neuronal Neuregulin 1 type III directs Schwann cell migration. Development 2011, 138, 4639–4648. [Google Scholar] [CrossRef]
- Heermann, S.; Schmucker, J.; Hinz, U.; Rickmann, M.; Unterbarnscheidt, T.; Schwab, M.H.; Krieglstein, K. Neuregulin 1 type III/ErbB signaling is crucial for Schwann cell colonization of sympathetic axons. PLoS ONE 2011, 6, e28692. [Google Scholar] [CrossRef]
- Limpert, A.S.; Carter, B.D. Axonal neuregulin 1 type III activates NF-kappaB in Schwann cells during myelin formation. J. Biol. Chem. 2010, 285, 16614–16622. [Google Scholar] [CrossRef]
- Miyamoto, Y.; Torii, T.; Tanoue, A.; Kawahara, K.; Arai, M.; Tsumura, H.; Ogata, T.; Nagao, M.; Terada, N.; Yamamoto, M.; et al. Neuregulin-1 type III knockout mice exhibit delayed migration of Schwann cell precursors. Biochem. Biophys. Res. Commun. 2017, 486, 506–513. [Google Scholar] [CrossRef]
- Belin, S.; Ornaghi, F.; Shackleford, G.; Wang, J.; Scapin, C.; Lopez-Anido, C.; Silvestri, N.; Robertson, N.; Williamson, C.; Ishii, A.; et al. Neuregulin 1 type III improves peripheral nerve myelination in a mouse model of congenital hypomyelinating neuropathy. Hum. Mol. Genet. 2019, 28, 1260–1273. [Google Scholar] [CrossRef]
- Scapin, C.; Ferri, C.; Pettinato, E.; Zambroni, D.; Bianchi, F.; Del Carro, U.; Belin, S.; Caruso, D.; Mitro, N.; Pellegatta, M.; et al. Enhanced axonal neuregulin-1 type-III signaling ameliorates neurophysiology and hypomyelination in a Charcot-Marie-Tooth type 1B mouse model. Hum. Mol. Genet. 2019, 28, 992–1006. [Google Scholar] [CrossRef] [PubMed]
- Ghidinelli, M.; Poitelon, Y.; Shin, Y.K.; Ameroso, D.; Williamson, C.; Ferri, C.; Pellegatta, M.; Espino, K.; Mogha, A.; Monk, K.; et al. Laminin 211 inhibits protein kinase A in Schwann cells to modulate neuregulin 1 type III-driven myelination. PLoS Biol. 2017, 15, e2001408. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Y.; Zou, S. A Glance at the Molecules That Regulate Oligodendrocyte Myelination. Curr. Issues Mol. Biol. 2022, 44, 2194–2216. [Google Scholar] [CrossRef] [PubMed]
- Chukhlieb, M.; Raasakka, A.; Ruskamo, S.; Kursula, P. The N-terminal cytoplasmic domain of neuregulin 1 type III is intrinsically disordered. Amino Acids 2015, 47, 1567–1577. [Google Scholar] [CrossRef]
- Law, A.J.; Lipska, B.K.; Weickert, C.S.; Hyde, T.M.; Straub, R.E.; Hashimoto, R.; Harrison, P.J.; Kleinman, J.E.; Weinberger, D.R. Neuregulin 1 transcripts are differentially expressed in schizophrenia and regulated by 5′ SNPs associated with the disease. Proc. Natl. Acad. Sci. USA 2006, 103, 6747–6752. [Google Scholar] [CrossRef]
- Bartus, K.; Galino, J.; James, N.D.; Hernandez-Miranda, L.R.; Dawes, J.M.; Fricker, F.R.; Garratt, A.N.; McMahon, S.B.; Ramer, M.S.; Birchmeier, C.; et al. Neuregulin-1 controls an endogenous repair mechanism after spinal cord injury. Brain 2016, 139 Pt 5, 1394–1416. [Google Scholar] [CrossRef] [PubMed]
- Bare, D.J.; Becker-Catania, S.G.; DeVries, G.H. Differential localization of neuregulin-1 type III in the central and peripheral nervous system. Brain Res. 2011, 1369, 10–20. [Google Scholar] [CrossRef]
- Huang, L.L.; Liu, Z.Y.; Huang, J.H.; Luo, Z.J. Expression pattern of neuregulin-1 type III during the development of the peripheral nervous system. Neural. Regen. Res. 2015, 10, 65–70. [Google Scholar] [CrossRef]
- Olaya, J.C.; Heusner, C.L.; Matsumoto, M.; Shannon Weickert, C.; Karl, T. Schizophrenia-relevant behaviours of female mice overexpressing neuregulin 1 type III. Behav. Brain Res. 2018, 353, 227–235. [Google Scholar] [CrossRef]
- Zieba, J.; Morris, M.J.; Weickert, C.S.; Karl, T. Behavioural effects of high fat diet in adult Nrg1 type III transgenic mice. Behav. Brain Res. 2020, 377, 112217. [Google Scholar] [CrossRef]
- Mòdol-Caballero, G.; Herrando-Grabulosa, M.; Verdés, S.; García-Lareu, B.; Hernández, N.; Francos-Quijorna, I.; López-Vales, R.; Bosch, A.; Navarro, X. Gene Therapy Overexpressing Neuregulin 1 Type I in Combination With Neuregulin 1 Type III Promotes Functional Improvement in the SOD1(G93A) ALS Mice. Front. Neurol. 2021, 12, 693309. [Google Scholar] [CrossRef] [PubMed]
- Mòdol-Caballero, G.; García-Lareu, B.; Verdés, S.; Ariza, L.; Sánchez-Brualla, I.; Brocard, F.; Bosch, A.; Navarro, X.; Herrando-Grabulosa, M. Therapeutic Role of Neuregulin 1 Type III in SOD1-Linked Amyotrophic Lateral Sclerosis. Neurotherapeutics 2020, 17, 1048–1060. [Google Scholar] [CrossRef] [PubMed]
- Chong, V.Z.; Thompson, M.; Beltaifa, S.; Webster, M.J.; Law, A.J.; Weickert, C.S. Elevated neuregulin-1 and ErbB4 protein in the prefrontal cortex of schizophrenic patients. Schizophr. Res. 2008, 100, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Chesworth, R.; Rosa-Porto, R.; Yao, S.; Karl, T. Sex-specific sensitivity to methamphetamine-induced schizophrenia-relevant behaviours in neuregulin 1 type III overexpressing mice. J. Psychopharmacol. 2021, 35, 50–64. [Google Scholar] [CrossRef]
- Chen, C.; Hsu, F.C.; Li, C.W.; Huang, M.C. Structural, functional, and neurochemical neuroimaging of methamphetamine-associated psychosis: A systematic review. Psychiatry Res. Neuroimaging 2019, 292, 23–31. [Google Scholar] [CrossRef]
- Wearne, T.A.; Cornish, J.L. A Comparison of Methamphetamine-Induced Psychosis and Schizophrenia: A Review of Positive, Negative, and Cognitive Symptomatology. Front. Psychiatry 2018, 9, 491. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Y.; Miller, S.J.; Falls, D.L. The N-terminal Region of Neuregulin Isoforms Determines the Accumulation of Cell Surface and Released Neuregulin Ectodomain. J. Biol. Chem. 2001, 276, 2841–2851. [Google Scholar] [CrossRef]
- Fleck, D.; van Bebber, F.; Colombo, A.; Galante, C.; Schwenk, B.M.; Rabe, L.; Hampel, H.; Novak, B.; Kremmer, E.; Tahirovic, S.; et al. Dual cleavage of neuregulin 1 type III by BACE1 and ADAM17 liberates its EGF-like domain and allows paracrine signaling. J. Neurosci. 2013, 33, 7856–7869. [Google Scholar] [CrossRef]
- Fleck, D.; Voss, M.; Brankatschk, B.; Giudici, C.; Hampel, H.; Schwenk, B.; Edbauer, D.; Fukumori, A.; Steiner, H.; Kremmer, E.; et al. Proteolytic Processing of Neuregulin 1 Type III by Three Intramembrane-cleaving Proteases. J. Biol. Chem. 2016, 291, 318–333. [Google Scholar] [CrossRef]
- Willem, M. Proteolytic processing of Neuregulin-1. Brain Res. Bull. 2016, 126 Pt 2, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Prior, M.; He, W.; Hu, X.; Tang, X.; Shen, W.; Yadav, S.; Kiryu-Seo, S.; Miller, R.; Trapp, B.D.; et al. Cleavage of neuregulin-1 by BACE1 or ADAM10 protein produces differential effects on myelination. J. Biol. Chem. 2011, 286, 23967–23974. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Lin, D.; Zhong, Y.; Luo, B.; Liu, S.; Fei, E.; Lai, X.; Zou, S.; Wang, S. Transmembrane protein 108 involves in adult neurogenesis in the hippocampal dentate gyrus. Cell Biosci. 2019, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Huang, S.; Zhao, X.; Zhang, Q.; Wu, M.; Sun, F.; Han, G.; Wu, D. Enrichment of prostate cancer stem cells from primary prostate cancer cultures of biopsy samples. Int. J. Clin. Exp. Pathol. 2014, 7, 184–193. [Google Scholar]
- Yan, M.; Xiong, M.; Wu, Y.; Lin, D.; Chen, P.; Chen, J.; Liu, Z.; Zhang, H.; Ren, D.; Fei, E.; et al. LRP4 is required for the olfactory association task in the piriform cortex. Cell Biosci. 2022, 12, 54. [Google Scholar] [CrossRef]
- Wu, Y.; Zhong, Y.; Liao, X.; Miao, X.; Yu, J.; Lai, X.; Zhang, Y.; Ma, C.; Pan, H.; Wang, S. Transmembrane protein 108 inhibits the proliferation and myelination of oligodendrocyte lineage cells in the corpus callosum. Mol. Brain 2022, 15, 33. [Google Scholar] [CrossRef] [PubMed]
- Velanac, V.; Unterbarnscheidt, T.; Hinrichs, W.; Gummert, M.N.; Fischer, T.M.; Rossner, M.J.; Trimarco, A.; Brivio, V.; Taveggia, C.; Willem, M.; et al. Bace1 processing of NRG1 type III produces a myelin-inducing signal but is not essential for the stimulation of myelination. Glia 2012, 60, 203–217. [Google Scholar] [CrossRef]
- Mei, L.; Nave, K.A. Neuregulin-ERBB signaling in the nervous system and neuropsychiatric diseases. Neuron 2014, 83, 27–49. [Google Scholar] [CrossRef]
- Michailov, G.V.; Sereda, M.W.; Brinkmann, B.G.; Fischer, T.M.; Haug, B.; Birchmeier, C.; Role, L.; Lai, C.; Schwab, M.H.; Nave, K.A. Axonal neuregulin-1 regulates myelin sheath thickness. Science 2004, 304, 700–703. [Google Scholar] [CrossRef] [PubMed]
- Ho, W.H.; Armanini, M.P.; Nuijens, A.; Phillips, H.S.; Osherof, P.L. Sensory and Motor Neuron-derived Factor. J. Biol. Chem. 1995, 270, 14523–14532. [Google Scholar] [CrossRef]
- Syed, N.; Reddy, K.; Yang, D.P.; Taveggia, C.; Salzer, J.L.; Maurel, P.; Kim, H.A. Soluble neuregulin-1 has bifunctional, concentration-dependent effects on Schwann cell myelination. J. Neurosci. 2010, 30, 6122–6131. [Google Scholar] [CrossRef] [PubMed]
- Syed, N.; Kim, H.A. Soluble Neuregulin and Schwann Cell Myelination: A Therapeutic Potential for Improving Remyelination of Adult Axons. Mol. Cell. Pharmacol. 2010, 2, 161–167. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhang, Y.; Wang, Y.; Chen, H.; Pan, L.; Liao, X.; Wang, S. A Novel Form of Neuregulin 1 Type III Caused by N-Terminal Processing. Biomolecules 2023, 13, 1756. https://doi.org/10.3390/biom13121756
Wang Y, Zhang Y, Wang Y, Chen H, Pan L, Liao X, Wang S. A Novel Form of Neuregulin 1 Type III Caused by N-Terminal Processing. Biomolecules. 2023; 13(12):1756. https://doi.org/10.3390/biom13121756
Chicago/Turabian StyleWang, Yukai, Yu Zhang, Yingxing Wang, Hong Chen, Liangjing Pan, Xufeng Liao, and Shunqi Wang. 2023. "A Novel Form of Neuregulin 1 Type III Caused by N-Terminal Processing" Biomolecules 13, no. 12: 1756. https://doi.org/10.3390/biom13121756
APA StyleWang, Y., Zhang, Y., Wang, Y., Chen, H., Pan, L., Liao, X., & Wang, S. (2023). A Novel Form of Neuregulin 1 Type III Caused by N-Terminal Processing. Biomolecules, 13(12), 1756. https://doi.org/10.3390/biom13121756







