Oxidative Stress in Pregnancy
Abstract
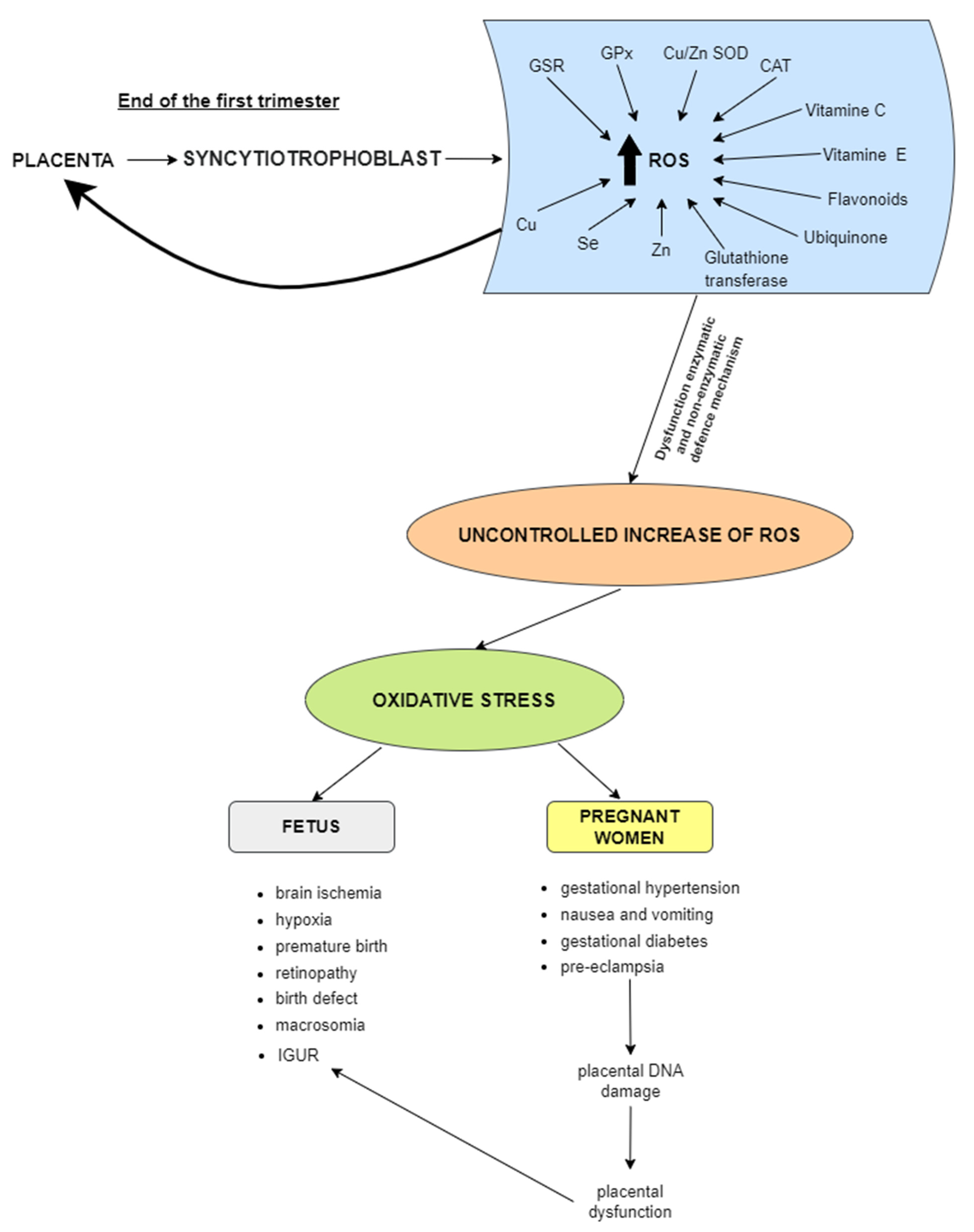
:1. Introduction
2. Reactive Oxygen and Nitrogen Species
3. Oxidative Stress
4. Antioxidant System
5. Trace Elements and Oxidative Stress
5.1. Copper
5.2. Iron
5.3. Zinc
5.4. Manganese
5.5. Selenium
5.6. Lead and Oxidative Stress
5.7. Undernutrition and Oxidative Metabolism
6. Biomarkers of Oxidative Stress
7. Research on Animal Models
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dröge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef] [PubMed]
- Kamata, H.; Hirata, H. Redox regulation of cellular signalling. Cell Signal 1999, 11, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Chandra, J. Oxidative stress by targeted agents promotes cytotoxicity in hematologic malignancies. Antioxid. Redox Signal 2009, 11, 1123–1137. [Google Scholar] [CrossRef] [PubMed]
- Rahman, I.; MacNee, W. Antioxidant pharmacological therapies for COPD. Curr. Opin. Pharmacol. 2012, 12, 256–265. [Google Scholar] [CrossRef]
- Gerdes, H.J.; Yang, M.; Heisner, J.S.; Camara, A.K.S.; Stowe, D.F. Modulation of peroxynitrite produced via mitochondrial nitric oxide synthesis during Ca2+ and succinate-induced oxidative stress in cardiac isolated mitochondria. Biochim. Biophys. Acta Bioenerg. 2020, 1861, 148290. [Google Scholar] [CrossRef] [PubMed]
- Collin, F. Chemical Basis of Reactive Oxygen Species Reactivity and Involvement in Neurodegenerative Diseases. Int. J. Mol. Sci. 2019, 20, 2407. [Google Scholar] [CrossRef]
- Gilca, M.; Stoian, I.; Atanasiu, V.; Virgolici, B. The oxidative hypothesis of senescence. J. Postgrad. Med. 2007, 53, 207–213. [Google Scholar] [CrossRef]
- Krumova, K.; Cosa, G. Chapter 1: Overview of Reactive Oxygen Species, in Singlet Oxygen: Applications in Biosciences and Nanosciences. Compr. Ser. Photochem. Photobiol. Sci. 2016, 1, 1–21. [Google Scholar] [CrossRef]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free radicals: Properties, sources, targets, and their implication in various diseases. Indian J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef]
- Trougakos, I.P.; Gonos, E.S. Regulation of clusterin/apolipoprotein J, a functional homologue to the small heat shock proteins, by oxidative stress in ageing and age-related diseases. Free Radic. Res. 2006, 40, 1324–1334. [Google Scholar] [CrossRef]
- Dhawan, S.; Myers, P.; Bailey, D.M.D.; Ostrovsky, A.D.; Evers, J.F.; Landgraf, M. Reactive oxygen species mediate activity-regulated dendritic plasticity through nadph oxidase and aquaporin regulation. Front. Cell Neurosci. 2021, 15, 641802. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Makker, K.; Sharma, R. Clinical relevance of oxidative stress in male factor infertility: An update. Am. J. Reprod. Immunol. 2008, 59, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Menezo, Y.J.; Silvestris, E.; Dale, B.; Elder, K. Oxidative stress and alterations in DNA methylation: Two sides of the same coin in reproduction. Reprod. Biomed. Online 2016, 33, 668–683. [Google Scholar] [CrossRef]
- Ahsan, H. 3-Nitrotyrosine: A biomarker of nitrogen free radical species modified proteins in systemic autoimmunogenic conditions. Hum. Immunol. 2013, 74, 1392–1399. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.C.; Martel, F. Oxidative stress in pregnancy and fertility pathologies. Cell Biol. Toxicol. 2014, 30, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Aponte-Mellado, A.; Premkumar, B.J.; Shaman, A.; Gupta, S. The effects of oxidative stress on female reproduction: A review. Reprod. Biol. Endocrinol. 2012, 10, 49. [Google Scholar] [CrossRef] [PubMed]
- Al-Gubory, K.H.; Fowler, P.A.; Garrel, C. The roles of cellular reactive oxygen species, oxidative stress and antioxidants in pregnancy outcomes. Int. J. Biochem. Cell Biol. 2010, 42, 1634–1650. [Google Scholar] [CrossRef]
- Sladek, S.M.; Magness, R.R.; Conrad, K.P. Nitric oxide and pregnancy. Am. J. Physiol. 1997, 272, R441–R463. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative stress: Harms and benefits for human health. Oxid. Med. Cell Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Burton, G.J.; Jauniaux, E. Oxidative stress. Best Pract. Res. Clin. Obstet. Gynaecol. 2011, 25, 287–299. [Google Scholar] [CrossRef]
- Cindrova-Davies, T.; Yung, H.W.; Johns, J.; Spasic-Boskovic, O.; Korolchuk, S.; Jauniaux, E.; Burton, G.J.; Charnock-Jones, D.S. Oxidative stress, gene expression, and protein changes induced in the human placenta during labor. Am. J. Pathol. 2007, 171, 1168–1179. [Google Scholar] [CrossRef]
- Ruder, E.H.; Hartman, T.J.; Goldman, M.B. Impact of oxidative stress on female fertility. Curr. Opin. Obstet. Gynecol. 2009, 21, 219–222. [Google Scholar] [CrossRef]
- Halliwell, B. Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol. 2006, 141, 312–322. [Google Scholar] [CrossRef]
- Narendhirakannan, R.T.; Hannah, M.A. Oxidative stress and skin cancer: An overview. Indian J. Clin. Biochem. 2013, 28, 110–115. [Google Scholar] [CrossRef]
- Wołonciej, M.; Milewska, E.; Roszkowska-Jakimiec, W. Trace elements as an activator of antioxidant enzymes. Postepy Hig. Med. Dosw. 2016, 70, 1483–1498. [Google Scholar] [CrossRef]
- Sharma, R.K.; Agarwal, A. Role of reactive oxygen species in male infertility. Urology 1996, 48, 835–850. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Prabakaran, S.; Allamaneni, S.S. Relationship between oxidative stress, varicocele and infertility: A meta-analysis. Reprod. Biomed. Online 2006, 12, 630–633. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Allamaneni, S.S. Role of free radicals in female reproductive diseases and assisted reproduction. Reprod. Biomed. Online 2004, 9, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Bizerea, T.O.; Dezsi, S.G.; Marginean, O.; Stroescu, R.; Rogobete, A.; Bizerea-Spiridon, O.; Ilie, C. The link between selenium, oxidative stress and pregnancy induced hypertensive disorders. Clin. Lab. 2018, 64, 1593–1610. [Google Scholar] [CrossRef]
- Lu, J.; Wang, Z.; Cao, J.; Chen, Y.; Dong, Y. A novel and compact review on the role of oxidative stress in female reproduction. Reprod. Biol. Endocrinol. 2018, 16, 80. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Choi, E.; Kim, S.; Kim, D.S.; Kim, J.H.; Chang, S.; Choi, J.S.; Park, K.J.; Roh, K.B.; Lee, J.; et al. Oxidative Stress-Protective and Anti-Melanogenic Effects of Loliolide and Ethanol Extract from Fresh Water Green Algae, Prasiola japonica. Int. J. Mol. Sci. 2018, 19, 2825. [Google Scholar] [CrossRef] [PubMed]
- Chao, H.T.; Lee, S.Y.; Lee, H.M.; Liao, T.L.; Wei, Y.H.; Kao, S.H. Repeated ovarian stimulations induce oxidative damage and mitochondrial DNA mutations in mouse ovaries. Ann. N. Y. Acad. Sci. 2005, 1042, 148–156. [Google Scholar] [CrossRef]
- Hatırnaz, Ş.; Ata, B.; Hatırnaz, E.S.; Dahan, M.H.; Tannus, S.; Tan, J.; Tan, S.L. Oocyte in vitro maturation: A sytematic review. Turk. J. Obstet. Gynecol. 2018, 15, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Lord, T.; Aitken, R.J. Oxidative stress and ageing of the post-ovulatory oocyte. Reproduction 2013, 146, R217–R227. [Google Scholar] [CrossRef] [PubMed]
- Mihalas, B.P.; Redgrove, K.A.; McLaughlin, E.A.; Nixon, B. Molecular mechanisms responsible for increased vulnerability of the ageing oocyte to oxidative damage. Oxid. Med. Cell Longev. 2017, 2017, 4015874. [Google Scholar] [CrossRef] [PubMed]
- Soma-Pillay, P.; Nelson-Piercy, C.; Tolppanen, H.; Mebazaa, A. Physiological changes in pregnancy. Cardiovasc. J. Afr. 2016, 27, 89–94. [Google Scholar] [CrossRef]
- Casanueva, E.; Viteri, F.E. Iron and oxidative stress in pregnancy. J. Nutr. 2003, 133, 1700S–1708S. [Google Scholar] [CrossRef]
- Ademuyiwa, O.; Odusoga, O.L.; Adebawo, O.O.; Ugbaja, R. Endogenous antioxidant defences in plasma and erythrocytes of pregnant women during different trimesters of pregnancy. Acta Obstet. Gynecol. Scand. 2007, 86, 1175–1182. [Google Scholar] [CrossRef]
- Hubel, C.A. Oxidative stress in the pathogenesis of preeclampsia. Proc. Soc. Exp. Biol. Med. 1999, 222, 222–235. [Google Scholar] [CrossRef]
- Myatt, L.; Cui, X. Oxidative stress in the placenta. Histochem. Cell Biol. 2004, 122, 369–382. [Google Scholar] [CrossRef]
- Fainaru, O.; Almog, B.; Gamzu, R.; Lessing, J.B.; Kupferminc, M. The management of symptomatic hydronephrosis in pregnancy. BJOG 2002, 109, 1385–1387. [Google Scholar] [CrossRef]
- Mocatta, T.J.; Winterbourn, C.C.; Inder, T.E.; Darlow, B.A. The effect of gestational age and labour on markers of lipid and protein oxidation in cord plasma. Free Radic. Res. 2004, 38, 185–191. [Google Scholar] [CrossRef]
- Pressman, E.K.; Cavanaugh, J.L.; Mingione, M.; Norkus, E.P.; Woods, J.R. Effects of maternal antioxidant supplementation on maternal and fetal antioxidant levels: A randomized, double-blind study. Am. J. Obstet. Gynecol. 2003, 189, 1720–1725. [Google Scholar] [CrossRef]
- Redman, C.W.; Sargent, I.L. Immunology of pre-eclampsia. Am. J. Reprod. Immunol. 2010, 63, 534–543. [Google Scholar] [CrossRef]
- Hung, T.H.; Skepper, J.N.; Charnock-Jones, D.S.; Burton, G.J. Hypoxia-reoxygenation: A potent inducer of apoptotic changes in the human placenta and possible etiological factor in preeclampsia. Circ. Res. 2002, 90, 1274–1281. [Google Scholar] [CrossRef]
- Aramouni, K.; Assaf, R.; Shaito, A.; Fardoun, M.; Al-Asmakh, M.; Sahebkar, A.; Eid, A.H. Biochemical and cellular basis of oxidative stress: Implications for disease onset. J. Cell Physiol. 2023, 238, 1951–1963. [Google Scholar] [CrossRef] [PubMed]
- Del Rosario, M.C.; Ossowski, V.; Knowler, W.C.; Bogardus, C.; Baier, L.J.; Hanson, R.L. Potential epigenetic dysregulation of genes associated with MODY and type 2 diabetes in humans exposed to a diabetic intrauterine environment: An analysis of genome-wide DNA methylation. Metabolism 2014, 63, 654–660. [Google Scholar] [CrossRef] [PubMed]
- Moreli, J.B.; Santos, J.H.; Rocha, C.R.; Damasceno, D.C.; Morceli, G.; Rudge, M.V.; Bevilacqua, E.; Calderon, I.M. DNA damage and its cellular response in mother and fetus exposed to hyperglycemic environment. Biomed. Res. Int. 2014, 2014, 676758. [Google Scholar] [CrossRef] [PubMed]
- Nomura, Y.; Lambertini, L.; Rialdi, A.; Lee, M.; Mystal, E.Y.; Grabie, M.; Manaster, I.; Huynh, N.; Finik, J.; Davey, M.; et al. Global methylation in the placenta and umbilical cord blood from pregnancies with maternal gestational diabetes, preeclampsia, and obesity. Reprod. Sci. 2014, 21, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Chiarello, D.I.; Abad, C.; Rojas, D.; Toledo, F.; Vázquez, C.M.; Mate, A.; Sobrevia, L.; Marín, R. Oxidative stress: Normal pregnancy versus preeclampsia. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165354. [Google Scholar] [CrossRef]
- Lima, P.H.O.; Sinzato, Y.K.; Gelaleti, R.B.; Calderon, I.M.P.; Rudge, M.V.C.; Damasceno, D.C. Genotoxicity evaluation in severe or mild diabetic pregnancy in laboratory animals. Exp. Clin. Endocrinol. Diabetes 2012, 120, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Toescu, V.; Nuttall, S.L.; Martin, U.; Nightingale, P.; Kendall, M.J.; Brydon, P.; Dunne, F. Changes in plasma lipids and markers of oxidative stress in normal pregnancy and pregnancies complicated by diabetes. Clin. Sci. 2004, 106, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Myatt, L. Review: Reactive oxygen and nitrogen species and functional adaptation of the placenta. Placenta 2010, 31, S66–S69. [Google Scholar] [CrossRef] [PubMed]
- Rani, N.; Dhingra, R.; Arya, D.S.; Kalaivani, M.; Bhatla, N.; Kumar, R. Role of oxidative stress markers and antioxidants in the placenta of preeclamptic patients. J. Obstet. Gynaecol. Res. 2010, 36, 1189–1194. [Google Scholar] [CrossRef] [PubMed]
- Jauniaux, E.; Poston, L.; Burton, G.J. Placental-related diseases of pregnancy: Involvement of oxidative stress and implications in human evolution. Hum. Reprod. Update 2006, 12, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Saugstad, L.F. Cerebral lateralisation and rate of maturation. Int. J. Psychophysiol. 1998, 28, 37–62. [Google Scholar] [CrossRef] [PubMed]
- Harrington, K.; Carpenter, R.G.; Goldfrad, C.; Campbell, S. Transvaginal Doppler ultrasound of the uteroplacental circulation in the early prediction of pre-eclampsia and intrauterine growth retardation. Br. J. Obstet. Gynaecol. 1997, 104, 674–681. [Google Scholar] [CrossRef]
- Araújo, J.R.; Correia-Branco, A.; Pereira, A.C.; Pinho, M.J.; Keating, E.; Martel, F. Oxidative stress decreases uptake of neutral amino acids in a human placental cell line (BeWo cells). Reprod. Toxicol. 2013, 40, 76–81. [Google Scholar] [CrossRef]
- Bartho, L.A.; Holland, O.J.; Moritz, K.M.; Perkins, A.V.; Cuffe, J.S.M. Maternal corticosterone in the mouse alters oxidative stress markers, antioxidant function and mitochondrial content in placentas of female fetuses. J. Physiol. 2019, 597, 3053–3067. [Google Scholar] [CrossRef]
- Umekawa, T.; Sugiyama, T.; Kihira, T.; Murabayashi, N.; Zhang, L.; Nagao, K.; Kamimoto, Y.; Ma, N.; Yodoi, J.; Sagawa, N. Overexpression of thioredoxin-1 reduces oxidative stress in the placenta of transgenic mice and promotes fetal growth via glucose metabolism. Endocrinology 2008, 149, 3980–3988. [Google Scholar] [CrossRef]
- Gupta, S.; Agarwal, A.; Banerjee, J.; Alvarez, J.G. The role of oxidative stress in spontaneous abortion and recurrent pregnancy loss: A systematic review. Obstet. Gynecol. Surv. 2007, 62, 335–347. [Google Scholar] [CrossRef]
- Webster, J.D.; Miller, M.A.; Vemulapalli, R. Encephalitozoon cuniculi-associated placentitis and perinatal death in an alpaca (Lama pacos). Vet. Pathol. 2008, 45, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Poston, L.; Raijmakers, M.; Kelly, F. Vitamin E in preeclampsia. Ann. N. Y. Acad. Sci. 2004, 1031, 242–248. [Google Scholar] [CrossRef]
- Hannah, M.E. Planned elective cesarean section: A reasonable choice for some women? CMAJ 2004, 170, 813–814. [Google Scholar] [CrossRef]
- Dennery, P.A. Effects of oxidative stress on embryonic development. Birth Defects Res. Part C Embryo Today 2007, 81, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Sengupta, P.; Das, S.; Slama, P.; Roychoudhury, S. Reactive nitrogen species and male reproduction: Physiological and pathological aspects. Int. J. Mol. Sci. 2022, 23, 10574. [Google Scholar] [CrossRef]
- Hardy, M.L.M.; Day, M.L.; Morris, M.B. redox regulation and oxidative stress in mammalian oocytes and embryos developed in vivo and in vitro. Int. J. Environ. Res. Public Health 2021, 18, 11374. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Saleh, R.A.; Bedaiwy, M.A. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil. Steril. 2003, 79, 829–843. [Google Scholar] [CrossRef]
- Takahashi, M. Oxidative stress and redox regulation on in vitro development of mammalian embryos. J. Reprod. Dev. 2012, 58, 1–9. [Google Scholar] [CrossRef]
- Pérez-Torres, I.; Manzano-Pech, L.; Rubio-Ruíz, M.E.; Soto, M.E.; Guarner-Lans, V. Nitrosative stress and its association with cardiometabolic disorders. Molecules 2020, 25, 2555. [Google Scholar] [CrossRef]
- Islam, B.U.; Habib, S.; Ahmad, P.; Allarakha, S.; Moinuddin Ali, A. Pathophysiological role of peroxynitrite induced dna damage in human diseases: A special focus on poly(ADP-ribose) polymerase (PARP). Indian J. Clin. Biochem. 2015, 30, 368–385. [Google Scholar] [CrossRef] [PubMed]
- Marangos, P.; Stevense, M.; Niaka, K.; Lagoudaki, M.; Nabti, I.; Jessberger, R.; Carroll, J. DNA damage-induced metaphase I arrest is mediated by the spindle assembly checkpoint and maternal age. Nat. Commun. 2015, 6, 8706. [Google Scholar] [CrossRef]
- Marsico, T.V.; Silva, M.V.; Valente, R.S.; Annes, K.; Rissi, V.B.; Glanzner, W.G.; Sudano, M.J. Unraveling the consequences of oxygen imbalance on early embryo development: Exploring mitigation strategies. Animals 2023, 13, 2171. [Google Scholar] [CrossRef]
- Kasterstein, E.; Strassburger, D.; Komarovsky, D.; Bern, O.; Komsky, A.; Raziel, A.; Friedler, S.; Ron-El, R. The effect of two distinct levels of oxygen concentration on embryo development in a sibling oocyte study. J. Assist. Reprod. Genet. 2013, 30, 1073–1079. [Google Scholar] [CrossRef] [PubMed]
- Yedwab, G.A.; Paz, G.; Homonnai, T.Z.; David, M.P.; Kraicer, P.F. The temperature, pH, and partial pressure of oxygen in the cervix and uterus of women and uterus of rats during the cycle. Fertil. Steril. 1976, 27, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.F.; Shi, S.L.; Jin, H.X.; Yao, G.D.; Wang, E.Y.; Yang, H.Y.; Song, W.Y.; Sun, Y.P. Impact of oxygen concentrations on fertilization, cleavage, implantation, and pregnancy rates of in vitro generated human embryos. Int. J. Clin. Exp. Med. 2015, 8, 6179–6185. [Google Scholar] [PubMed]
- Sciorio, R.; Smith, G. Embryo culture at a reduced oxygen concentration of 5%: A mini review. Zygote 2019, 27, 355–361. [Google Scholar] [CrossRef]
- Van Montfoort, A.P.A.; Arts, E.G.J.M.; Wijnandts, L.; Sluijmer, A.; Pelinck, M.J.; Land, J.A.; Van Echten-Arends, J. Reduced oxygen concentration during human IVF culture improves embryo utilization and cumulative pregnancy rates per cycle. Hum. Reprod. Open. 2020, 2020, hoz036. [Google Scholar] [CrossRef] [PubMed]
- Kelley, R.L.; Gardner, D.K. Individual culture and atmospheric oxygen during culture affect mouse preimplantation embryo metabolism and post-implantation development. Reprod. Biomed. Online 2019, 39, 3–18. [Google Scholar] [CrossRef]
- Chen, L.; Ma, S.; Xie, M.; Gong, F.; Lu, C.; Zhang, S.; Lin, G. Oxygen concentration from days 1 to 3 after insemination affects the embryo culture quality, cumulative live birth rate, and perinatal outcomes. J. Assist. Reprod. Genet. 2023, 40, 2609–2618. [Google Scholar] [CrossRef]
- Gelo, N.; Kirinec, G.; Baldani, D.P.; Vrčić, H.; Ježek, D.; Milošević, M.; Stanić, P. Influence of human embryo cultivation in a classic CO2 incubator with 20% oxygen versus benchtop incubator with 5% oxygen on live births: The randomized prospective trial. Zygote 2019, 27, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Rendón Abad, M.; Serra, V.; Gámiz, P.; de Los Santos, J.M.; Remohí, J.; Navarro, A.T.; de Los Santos, M.J. The influence of oxygen concentration during embryo culture on obstetric and neonatal outcomes: A secondary analysis of a randomized controlled trial. Hum. Reprod. 2020, 35, 2017–2025. [Google Scholar] [CrossRef] [PubMed]
- Aguilera-Castrejon, A.; Oldak, B.; Shani, T.; Ghanem, N.; Itzkovich, C.; Slomovich, S.; Tarazi, S.; Bayerl, J.; Chugaeva, V.; Ayyash, M.; et al. Ex utero mouse embryogenesis from pre-gastrulation to late organogenesis. Nature 2021, 593, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Arribas, L.; Almansa, I.; Miranda, M.; Muriach, M.; Romero, F.J.; Villar, V.M. Serum malondialdehyde concentration and glutathione peroxidase activity in a longitudinal study of gestational diabetes. PLoS ONE 2016, 11, e0155353. [Google Scholar] [CrossRef] [PubMed]
- Aycicek, A.; Iscan, A. The effects of carbamazepine, valproic acid and phenobarbital on the oxidative and antioxidative balance in epileptic children. Eur. Neurol. 2007, 57, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Fogel, I.; Pinchuk, I.; Kupferminc, M.J.; Lichtenberg, D.; Fainaru, O. Oxidative stress in the fetal circulation does not depend on mode of delivery. Am. J. Obstet. Gynecol. 2005, 193, 241–246. [Google Scholar] [CrossRef]
- Vakilian, K.; Ranjbar, A.; Zarganjfard, A.; Mortazavi, M.; Vosough-Ghanbari, S.; Mashaiee, S.; Abdollahi, M. On the relation of oxidative stress in delivery mode in pregnant women; a toxicological concern. Toxicol. Mech. Methods 2009, 19, 94–99. [Google Scholar] [CrossRef]
- Sgorbini, M.; Bonelli, F.; Percacini, G.; Pasquini, A.; Rota, A. Maternal and neonatal evaluation of derived reactive oxygen metabolites and biological antioxidant potential in donkey mares and foals. Animals 2021, 11, 2885. [Google Scholar] [CrossRef]
- Mutlu, B.; Aksoy, N.; Cakir, H.; Celik, H.; Erel, O. The effects of the mode of delivery on oxidative-antioxidative balance. J. Matern. Fetal Neonatal. Med. 2011, 24, 1367–1370. [Google Scholar] [CrossRef]
- Şimşek, Y.; Karabiyik, P.; Polat, K.; Duran, Z.; Polat, A. Mode of delivery changes oxidative and antioxidative properties of human milk: A prospective controlled clinical investigation. J. Matern. Fetal Neonatal. Med. 2015, 28, 734–738. [Google Scholar] [CrossRef]
- Saphier, O.; Schneid-Kofman, N.; Silberstein, E.; Silberstein, T. Does mode of delivery affect neonate oxidative stress in parturition? Review of literature. Arch. Gynecol. Obstet. 2013, 287, 403–406. [Google Scholar] [CrossRef] [PubMed]
- Hung, T.H.; Chen, S.F.; Hsieh, T.T.; Lo, L.M.; Li, M.J.; Yeh, Y.L. The associations between labor and delivery mode and maternal and placental oxidative stress. Reprod. Toxicol. 2011, 31, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Alcala, M.; Gutierrez-Vega, S.; Castro, E.; Guzman-Gutiérrez, E.; Ramos-Álvarez, M.P.; Viana, M. Antioxidants and oxidative stress: Focus in obese pregnancies. Front. Physiol. 2018, 9, 1569. [Google Scholar] [CrossRef] [PubMed]
- Solis-Paredes, M.; Estrada-Gutierrez, G.; Perichart-Perera, O.; Montoya-Estrada, A.; Guzmán-Huerta, M.; Borboa-Olivares, H.; Bravo-Flores, E.; Cardona-Pérez, A.; Zaga-Clavellina, V.; Garcia-Latorre, E.; et al. Key Clinical Factors Predicting Adipokine and Oxidative Stress Marker Concentrations among Normal, Overweight and Obese Pregnant Women Using Artificial Neural Networks. Int. J. Mol. Sci. 2017, 19, 86. [Google Scholar] [CrossRef] [PubMed]
- Solis Paredes, J.M.; Perichart Perera, O.; Montoya Estrada, A.; Reyes Muñoz, E.; Espino Sosa, S.; Ortega Castillo, V.; Medina Bastidas, D.; Tolentino Dolores, M.; Sanchez Martinez, M.; Nava Salazar, S.; et al. Gestational weight gain influences the adipokine-oxidative stress association during pregnancy. Obes. Facts. 2021, 14, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Peuchant, E.; Brun, J.L.; Rigalleau, V.; Dubourg, L.; Thomas, M.J.; Daniel, J.Y.; Leng, J.J.; Gin, H. Oxidative and antioxidative status in pregnant women with either gestational or type 1 diabetes. Clin. Biochem. 2004, 37, 293–298. [Google Scholar] [CrossRef]
- Longini, M.; Perrone, S.; Vezzosi, P.; Marzocchi, B.; Kenanidis, A.; Centini, G.; Rosignoli, L.; Buonocore, G. Association between oxidative stress in pregnancy and preterm premature rupture of membranes. Clin. Biochem. 2007, 40, 793–797. [Google Scholar] [CrossRef] [PubMed]
- Leguizamón, G.F.; Zeff, N.P.; Fernández, A. Hypertension and the pregnancy complicated by diabetes. Curr. Diab. Rep. 2006, 6, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Van Devanter, N.; Fenstermaker, M.; Cawkwell, P.; Sherman, S.; Weitzman, M. A study of the use, knowledge, and beliefs about cigarettes and alternative tobacco products among students at one U.S. Medical School. Acad. Med. 2015, 90, 1713–1719. [Google Scholar] [CrossRef]
- Isik, B.; Ceylan, A.; Isik, R. Oxidative stress in smokers and non-smokers. Inhal. Toxicol. 2007, 19, 767–769. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, T.; Sureka, C.; Bhuvana, S.; Begum, V.H. Oxidative stress in the brain of cigarette smoke-induced noxiousness: Neuroprotective role of Sesbania grandiflora. Metab. Brain Dis. 2015, 30, 573–582. [Google Scholar] [CrossRef]
- Kamceva, G.; Arsova-Sarafinovska, Z.; Ruskovska, T.; Zdravkovska, M.; Kamceva-Panova, L.; Stikova, E. Cigarette smoking and oxidative stress in patients with coronary artery disease. Open Access Maced. J. Med. Sci. 2016, 4, 636–640. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Tao, J.Q.; Johncola, A.; Guo, W.; Caporale, A.; Langham, M.C.; Wehrli, F.W. Acute exposure to e-cigarettes causes inflammation and pulmonary endothelial oxidative stress in nonsmoking, healthy young subjects. Am. J. Physiol. Lung Cell Mol. Physiol. 2019, 317, L155–L166. [Google Scholar] [CrossRef]
- Luo, Z.C.; Fraser, W.D.; Julien, P.; Deal, C.L.; Audibert, F.; Smith, G.N.; Xiong, X.; Walker, M. Tracing the origins of “fetal origins” of adult diseases: Programming by oxidative stress? Med. Hypotheses 2006, 66, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Akçay, A.; Tatar Aksoy, H.; Uras, N.; Dilmen, U. Reference values of oxidative stress biomarkers in healthy newborns. Pediatr. Int. 2013, 55, 604–607. [Google Scholar] [CrossRef] [PubMed]
- Friel, J.K.; Friesen, R.W.; Harding, S.V.; Roberts, L.J. Evidence of oxidative stress in full-term healthy infants. Pediatr. Res. 2004, 56, 878–882. [Google Scholar] [CrossRef] [PubMed]
- Abdel Ghany, E.A.; Alsharany, W.; Ali, A.A.; Youness, E.R.; Hussein, J.S. Anti-oxidant profiles and markers of oxidative stress in preterm neonates. Paediatr. Int. Child Health 2016, 36, 134–140. [Google Scholar] [CrossRef]
- Gitto, E.; Pellegrino, S.; Aversa, S.; Romeo, C.; Trimarchi, G.; Barberi, I.; Calabró, M.P.; Salpietro, C.D.; Reiter, R.J. Oxidative stress and persistent pulmonary hypertension of the newborn treated with inhaled nitric oxide and different oxygen concentrations. J. Matern. Fetal Neonatal. Med. 2012, 25, 1723–1726. [Google Scholar] [CrossRef]
- Gitto, E.; Pellegrino, S.; Gitto, P.; Barberi, I.; Reiter, R.J. Oxidative stress of the newborn in the pre- and postnatal period and the clinical utility of melatonin. J. Pineal Res. 2009, 46, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Negi, R.; Pande, D.; Karki, K.; Kumar, A.; Khanna, R.S.; Khanna, H.D. A novel approach to study oxidative stress in neonatal respiratory distress syndrome. BBA Clin. 2014, 3, 65–69. [Google Scholar] [CrossRef]
- Saugstad, O.D. Oxygenation of the newborn. The impact of one molecule on newborn lives. J. Perinat. Med. 2022, 51, 20–26. [Google Scholar] [CrossRef]
- Rahman, I. Pharmacological antioxidant strategies as therapeutic interventions for COPD. Biochim. Biophys. Acta 2012, 1822, 714–728. [Google Scholar] [CrossRef]
- Belviranlı, M.; Hakkı Gökbel, H. Acute exercise induced oxidative stress and antioxidant changes. Eur. J. Gen. Med. 2006, 3, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Faraci, F.M.; Didion, S.P. Vascular protection: Superoxide dismutase isoforms in the vessel wall. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1367–1373. [Google Scholar] [CrossRef] [PubMed]
- Trachootham, D.; Lu, W.; Ogasawara, M.A.; Nilsa, R.D.; Huang, P. Redox regulation of cell survival. Antioxid. Redox Signal 2008, 10, 1343–1374. [Google Scholar] [CrossRef] [PubMed]
- Jamilian, M.; Mirhosseini, N.; Eslahi, M.; Bahmani, F.; Shokrpour, M.; Chamani, M.; Asemi, Z. The effects of magnesium-zinc-calcium-vitamin D co-supplementation on biomarkers of inflammation, oxidative stress and pregnancy outcomes in gestational diabetes. BMC Pregnancy Childbirth 2019, 19, 107. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.; Garruto, R.M. Weight variation by sex and nature of risk factors in high-risk infants: An evolutionary perspective. Coll. Antropol. 2007, 31, 937–941. [Google Scholar]
- Omeljaniuk, W.J.; Socha, K.; Borawska, M.H.; Charkiewicz, A.E.; Laudański, T.; Kulikowski, M.; Kobylec, E. Antioxidant status in women who have had a miscarriage. Adv. Med. Sci. 2015, 60, 329–334. [Google Scholar] [CrossRef]
- Hernández-Trejo, M.; Montoya-Estrada, A.; Torres-Ramos, Y.; Espejel-Núñez, A.; Guzmán-Grenfell, A.; Morales-Hernández, R.; Tolentino-Dolores, M.; Laresgoiti-Servitje, E. Oxidative stress biomarkers and their relationship with cytokine concentrations in overweight/obese pregnant women and their neonates. BMC Immunol. 2017, 18, 3. [Google Scholar] [CrossRef] [PubMed]
- Michalczyk, K.; Cymbaluk-Płoska, A. The role of zinc and copper in gynecological malignancies. Nutrients 2020, 12, 3732. [Google Scholar] [CrossRef]
- Grzeszczak, K.; Kapczuk, P.; Kupnicka, P.; Simińska, D.K.; Lebdowicz-Knul, J.; Kwiatkowski, S.K.; Łanocha-Arendarczyk, N.; Chlubek, D.; Kosik-Bogacka, D.I. The trace element concentrations and oxidative stress parameters in afterbirths from women with multiple pregnancies. Biomolecules 2023, 13, 797. [Google Scholar] [CrossRef]
- Gaetke, L.M.; Chow-Johnson, H.S.; Chow, C.K. Copper: Toxicological relevance and mechanisms. Arch. Toxicol. 2014, 88, 1929–1938. [Google Scholar] [CrossRef]
- Uriu-Adams, J.Y.; Scherr, R.E.; Lanoue, L.; Keen, C.L. Influence of copper on early development: Prenatal and postnatal considerations. Biofactors 2010, 36, 136–152. [Google Scholar] [CrossRef]
- Rak, K.; Łoźna, K.; Styczyńska, M.; Bobak, Ł.; Bronkowska, M. Oxidative stress at birth is associated with the concentration of iron and copper in maternal serum. Nutrients 2021, 13, 1491. [Google Scholar] [CrossRef]
- Bresgen, N.; Eckl, P.M. Oxidative stress and the homeodynamics of iron metabolism. Biomolecules 2015, 5, 808–847. [Google Scholar] [CrossRef]
- Emerit, J.; Beaumont, C.; Trivin, F. Iron metabolism, free radicals, and oxidative injury. Biomed. Pharmacother. 2001, 55, 333–339. [Google Scholar] [CrossRef]
- Papanikolaou, G.; Pantopoulos, K. Iron metabolism and toxicity. Toxicol. Appl. Pharmacol. 2005, 202, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Devrim, E.; Tarhan, I.; Ergüder, I.B.; Durak, I. Oxidant/antioxidant status of placenta, blood, and cord blood samples from pregnant women supplemented with iron. J. Soc. Gynecol. Investig. 2006, 13, 502–505. [Google Scholar] [CrossRef] [PubMed]
- Tvrdá, E.; Kováčik, A.; Tušimová, E.; Massányi, P.; Lukáč, N. Resveratrol offers protection to oxidative stress induced by ferrous ascorbate in bovine spermatozoa. J. Environ. Sci. Health 2015, 50, 1440–1451. [Google Scholar] [CrossRef]
- Rao, R.; Georgieff, M.K. Iron in fetal and neonatal nutrition. Semin. Fetal Neonatal. Med. 2007, 12, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Friedrisch, J.R.; Friedrisch, B.K. Prophylactic Iron supplementation in pregnancy: A controversial issue. Biochem. Insights 2017, 10, 1178626417737738. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.S. Discovery of human zinc deficiency: Its impact on human health and disease. Adv. Nutr. 2013, 4, 176–190. [Google Scholar] [CrossRef]
- King, J.C.; Cousins, R.J. Zinc. In Modern Nutrition in Health and Disease; Shils, M.E., Shike, M., Ross, A.C., Caballero, B., Cousins, R.J., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2005; pp. 271–285. [Google Scholar]
- Tian, X.; Anthony, K.; Neuberger, T.; Diaz, F.J. Preconception zinc deficiency disrupts postimplantation fetal and placental development in mice. Biol. Reprod. 2014, 90, 83. [Google Scholar] [CrossRef]
- Wang, C.; Li, B.; Wang, B.; Xie, N. Degradation and antioxidant activities of peptides and zinc-peptide complexes during in vitro gastrointestinal digestion. Food Chem. 2015, 173, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Hu, C.; Zheng, Y. Maternal serum zinc level is associated with risk of preeclampsia: A systematic review and meta-analysis. Front. Public Health 2022, 10, 968045. [Google Scholar] [CrossRef] [PubMed]
- Li, H.T.; Jiao, M.; Chen, J.; Liang, Y. Roles of zinc and copper in modulating the oxidative refolding of bovine copper, zinc superoxide dismutase. Acta Biochim. Biophys. Sin. 2010, 42, 183–194. [Google Scholar] [CrossRef]
- Ahn, B.I.; Kim, M.J.; Koo, H.S.; Seo, N.; Joo, N.S.; Kim, Y.S. Serum zinc concentration is inversely associated with insulin resistance but not related with metabolic syndrome in nondiabetic Korean adults. Biol. Trace Elem. Res. 2014, 160, 169–175. [Google Scholar] [CrossRef]
- Fukunaka, A.; Fujitani, Y. Role of zinc homeostasis in the pathogenesis of diabetes and obesity. Int. J. Mol. Sci. 2018, 19, 476. [Google Scholar] [CrossRef]
- Olechnowicz, J.; Tinkov, A.; Skalny, A.; Suliburska, J. Zinc status is associated with inflammation, oxidative stress, lipid, and glucose metabolism. J. Physiol. Sci. 2018, 68, 19–31. [Google Scholar] [CrossRef]
- Jain, S.; Sharma, P.; Kulshreshtha, S.; Mohan, G.; Singh, S. The role of calcium, magnesium, and zinc in pre-eclampsia. Biol. Trace Elem. Res. 2010, 133, 162–170. [Google Scholar] [CrossRef]
- Açikgoz, S.; Harma, M.; Harma, M.; Mungan, G.; Can, M.; Demirtas, S. Comparison of angiotensin-converting enzyme, malonaldehyde, zinc, and copper levels in preeclampsia. Biol. Trace Elem. Res. 2006, 113, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Dong, H.; Zhou, J.; Luo, Y.; Yuan, M.M.; Zhan, J.F.; Liu, Y.L.; Xia, J.Y.; Zhang, L. Aged gut microbiota contribute to different changes in antioxidant defense in the heart and liver after transfer to germ-free mice. PLoS ONE 2023, 18, e0289892. [Google Scholar] [CrossRef] [PubMed]
- Bakacak, M.; Kılınç, M.; Serin, S.; Ercan, Ö.; Köstü, B.; Avcı, F.; Kıran, H.; Kıran, G. Changes in copper, zinc, and malondialdehyde levels and superoxide dismutase activities in pre-eclamptic pregnancies. Med. Sci. Monit. 2015, 21, 2414–2420. [Google Scholar] [CrossRef] [PubMed]
- Yamada, R.T.; Leone, C.R. Intrauterine growth restriction and zinc concentrations in term infants during the first month of life. J. Am. Coll. Nutr. 2008, 27, 485–491. [Google Scholar] [CrossRef]
- Mesdaghinia, E.; Naderi, F.; Bahmani, F.; Chamani, M.; Ghaderi, A.; Asemi, Z. The effects of zinc supplementation on clinical response and metabolic profiles in pregnant women at risk for intrauterine growth restriction: A randomized, double-blind, placebo-controlled trial. J. Matern. Fetal Neonatal. Med. 2021, 34, 1382–1388. [Google Scholar] [CrossRef]
- Karamali, M.; Heidarzadeh, Z.; Seifati, S.M.; Samimi, M.; Tabassi, Z.; Hajijafari, M.; Asemi, Z.; Esmaillzadeh, A. Zinc supplementation and the effects on metabolic status in gestational diabetes: A randomized, double-blind, placebo-controlled trial. J. Diabetes Complicat. 2015, 29, 1314–1319. [Google Scholar] [CrossRef]
- Dashner-Titus, E.J.; Hoover, J.; Li, L.; Lee, J.H.; Du, R.; Liu, K.J.; Traber, M.G.; Ho, E.; Lewis, J.; Hudson, L.G. Metal exposure and oxidative stress markers in pregnant Navajo Birth Cohort Study participants. Free Radic. Biol. Med. 2018, 124, 484–492. [Google Scholar] [CrossRef]
- Holley, A.K.; Bakthavatchalu, V.; Velez-Roman, J.M.; St Clair, D.K. Manganese superoxide dismutase: Guardian of the powerhouse. Int. J. Mol. Sci. 2011, 12, 7114–7162. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yang, X. The Essential element manganese, oxidative stress, and metabolic diseases: Links and interactions. Oxid. Med. Cell Longev. 2018, 2018, 7580707. [Google Scholar] [CrossRef]
- Liu, T.; Yang, Y.; Wang, C. Manganese-catalyzed hydroarylation of unactivated alkenes. Angew. Chem. Int. Ed. Engl. 2020, 59, 14256–14260. [Google Scholar] [CrossRef] [PubMed]
- Tinggi, U. Selenium: Its role as antioxidant in human health. Environ. Health Prev. Med. 2008, 13, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Yu, J.Y.; Jenkins, A.J.; Nankervis, A.J.; Hanssen, K.F.; Henriksen, T.; Lorentzen, B.; Garg, S.K.; Menard, M.K.; Hammad, S.M.; et al. Trace elements as predictors of preeclampsia in type 1 diabetic pregnancy. Nutr. Res. 2015, 35, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Razaghi, A.; Poorebrahim, M.; Sarhan, D.; Björnstedt, M. Selenium stimulates the antitumour immunity: Insights to future research. Eur. J. Cancer 2021, 155, 256–267. [Google Scholar] [CrossRef]
- Barchielli, G.; Capperucci, A.; Tanini, D. The role of selenium in pathologies: An updated review. Antioxidants 2022, 11, 251. [Google Scholar] [CrossRef] [PubMed]
- Micetić-Turk, D.; Rossipal, E.; Krachler, M.; Li, F. Maternal selenium status in Slovenia and its impact on the selenium concentration of umbilical cord serum and colostrum. Eur. J. Clin. Nutr. 2000, 54, 522–524. [Google Scholar] [CrossRef] [PubMed]
- Al-Kunani, A.S.; Knight, R.; Haswell, S.J.; Thompson, J.W.; Lindow, S.W. The selenium status of women with a history of recurrent miscarriage. Br. J. Obstet. Gynaecol. 2001, 108, 1094–1097. [Google Scholar] [CrossRef]
- Lewandowska, M.; Sajdak, S.; Lubiński, J. Serum selenium level in early healthy pregnancy as a risk marker of pregnancy induced hypertension. Nutrients 2019, 11, 1028. [Google Scholar] [CrossRef]
- Mistry, H.D.; Williams, P.J. The importance of antioxidant micronutrients in pregnancy. Oxid. Med. Cell Longev. 2011, 2011, 841749. [Google Scholar] [CrossRef]
- Rayman, M.P.; Searle, E.; Kelly, L.; Johnsen, S.; Bodman-Smith, K.; Bath, S.C.; Mao, J.; Redman, C.W. Effect of selenium on markers of risk of pre-eclampsia in UK pregnant women: A randomised, controlled pilot trial. Br. J. Nutr. 2014, 112, 99–111. [Google Scholar] [CrossRef]
- McDougall, A.R.; Dore, G.; Aboud, L.; Makama, M.; Nguyen, P.Y.; Mills, K.; Sanderson, B.; Hastie, R.; Ammerdorffer, A.; Vogel, J.P. The effect of selenium supplementation in pregnant women on maternal, fetal and newborn outcomes: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. MFM 2023, 5, 101160. [Google Scholar] [CrossRef]
- Wang, J.; Liang, C.; Hu, Y.; Xia, X.; Li, Z.; Gao, H.; Sheng, J.; Huang, K.; Wang, S.; Zhu, P.; et al. Effects of selenium levels on placental oxidative stress and inflammation during pregnancy: A prospective cohort study. J. Matern. Fetal Neonatal. Med. 2022, 35, 9956–9965. [Google Scholar] [CrossRef]
- Khera, A.; Dong, L.F.; Holland, O.; Vanderlelie, J.; Pasdar, E.A.; Neuzil, J.; Perkins, A.V. Selenium supplementation induces mitochondrial biogenesis in trophoblasts. Placenta 2015, 36, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Stoffaneller, R.; Morse, N.L. A review of dietary selenium intake and selenium status in Europe and the Middle East. Nutrients 2015, 7, 1494–1537. [Google Scholar] [CrossRef]
- Habibi, N.; Jankovic-Karasoulos, T.; Leemaqz, S.Y.; Francois, M.; Zhou, S.J.; Leifert, W.R.; Perkins, A.V.; Roberts, C.T.; Bianco-Miotto, T. Effect of iodine and selenium on proliferation, viability, and oxidative stress in HTR-8/SVneo placental cells. Biol. Trace Elem. Res. 2021, 199, 1332–1344. [Google Scholar] [CrossRef]
- Biswas, K.; McLay, J.; Campbell, F.M. Selenium supplementation in pregnancy-maternal and newborn outcomes. J. Nutr. Metab. 2022, 2022, 4715965. [Google Scholar] [CrossRef] [PubMed]
- Asemi, Z.; Jamilian, M.; Mesdaghinia, E.; Esmaillzadeh, A. Effects of selenium supplementation on glucose homeostasis, inflammation, and oxidative stress in gestational diabetes: Randomized, double-blind, placebo-controlled trial. Nutrition 2015, 31, 1235–1242. [Google Scholar] [CrossRef] [PubMed]
- Jamilian, M.; Mansury, S.; Bahmani, F.; Heidar, Z.; Amirani, E.; Asemi, Z. The effects of probiotic and selenium co-supplementation on parameters of mental health, hormonal profiles, and biomarkers of inflammation and oxidative stress in women with polycystic ovary syndrome. J. Ovarian Res. 2018, 11, 80. [Google Scholar] [CrossRef] [PubMed]
- Razavi, M.; Jamilian, M.; Kashan, Z.F.; Heidar, Z.; Mohseni, M.; Ghandi, Y.; Bagherian, T.; Asemi, Z. Selenium supplementation and the effects on reproductive outcomes, biomarkers of inflammation, and oxidative stress in women with polycystic ovary syndrome. Horm. Metab. Res. 2016, 48, 185–190. [Google Scholar] [CrossRef]
- Okunade, K.S.; John-Olabode, S.; Akinsola, O.J.; Akinajo, O.; Akanmu, S.A.; Kanki, P.J. Effects of selenium supplementation on pregnancy outcome and disease progression in HIV-infected pregnant women in Lagos, Nigeria: Study protocol for a randomised, double-blind, placebo-controlled trial. Medicine 2019, 98, e12735. [Google Scholar] [CrossRef]
- Wani, A.L.; Ara, A.; Usmani, J.A. Lead toxicity: A review. Interdiscip. Toxicol. 2015, 8, 55–64. [Google Scholar] [CrossRef]
- Ahamed, M.; Siddiqui, M.K. Low level lead exposure and oxidative stress: Current opinions. Clin. Chim. Acta 2007, 383, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Patra, R.C.; Swarup, D.; Dwivedi, S.K. Antioxidant effects of α tocopherol, ascorbic acid and L-methionine on lead induced oxidative stress to the liver, kidney and brain in rats. Toxicology 2001, 162, 81–88. [Google Scholar] [CrossRef]
- Collin, M.S.; Venkatraman, S.K.; Vijayakumar, N.; Kanimozhi, V.; Arbaaz, S.M.; Stacey, R.G.S.; Anusha, J.; Choudhary, R.; Lvov, V.; Tovar, G.I.; et al. Bioaccumulation of lead (Pb) and its effects on human: A review. J. Hazardous Mater. Adv. 2022, 7, 100094. [Google Scholar] [CrossRef]
- Tahir, I.; Alkheraije, K.A. A review of important heavy metals toxicity with special emphasis on nephrotoxicity and its management in cattle. Front. Vet. Sci. 2023, 10, 1149720. [Google Scholar] [CrossRef] [PubMed]
- Pourrut, B.; Shahid, M.; Dumat, C.; Winterton, P.; Pinelli, E. Lead uptake, toxicity, and detoxification in plants. Rev. Environ. Contam. Toxicol. 2011, 213, 113–136. [Google Scholar] [CrossRef] [PubMed]
- Patra, R.C.; Rautray, A.K.; Swarup, D. Oxidative stress in lead and cadmium toxicity and its amelioration. Vet. Med. Int. 2011, 2011, 457327. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.K.; Lee, S.B.; Won, J.; Choi, H.Y.; Kim, K.; Yang, G.M.; Dayem, A.A.; Cho, S.G. Correlation between oxidative stress, nutrition, and cancer initiation. Int. J. Mol. Sci. 2017, 18, 1544. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, P.; Ramiro-Cortijo, D.; Reyes-Hernández, C.G.; López de Pablo, A.L.; González, M.C.; Arribas, S.M. Implication of oxidative stress in fetal programming of cardiovascular disease. Front. Physiol. 2018, 9, 602. [Google Scholar] [CrossRef]
- Diamanti-Kandarakis, E.; Papalou, O.; Kandaraki, E.A.; Kassi, G. Mechanisms in Endocrinology: Nutrition as a mediator of oxidative stress in metabolic and reproductive disorders in women. Eur. J. Endocrinol. 2017, 176, R79–R99. [Google Scholar] [CrossRef]
- Sebastiani, G.; Herranz Barbero, A.; Borrás-Novell, C.; Alsina Casanova, M.; Aldecoa-Bilbao, V.; Andreu-Fernández, V.; Pascual Tutusaus, M.; Ferrero Martínez, S.; Gómez Roig, M.D.; García-Algar, O. The effects of vegetarian and vegan diet during pregnancy on the health of mothers and offspring. Nutrients 2019, 11, 557. [Google Scholar] [CrossRef]
- World Health Organization [WHO]. Global Targets 2025. To Improve Maternal, Infant and Young Child Nutrition; World Health Organization: Geneva, Switzerland, 2014.
- Black, R.E.; Victora, C.G.; Walker, S.P.; Bhutta, Z.A.; Christian, P.; de Onis, M.; Ezzati, M.; Grantham-McGregor, S.; Katz, J.; Martorell, R.; et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet 2013, 382, 427–451. [Google Scholar] [CrossRef]
- Aly, G.S.; Shaalan, A.H.; Mattar, M.K.; Hanaa Ahmed, H.H.; Zaki, M.E.; Abdallah, H.R. Oxidative stress status in nutritionally stunted children. Egypt. Pediatr. Assoc. Gaz. 2014, 62, 28–33. [Google Scholar] [CrossRef]
- Ekström, E.C.; Lindström, E.; Raqib, R.; El Arifeen, S.; Basu, S.; Brismar, K.; Selling, K.; Persson, L.Å. Effects of prenatal micronutrient and early food supplementation on metabolic status of the offspring at 4.5 years of age. The MINIMat randomized trial in rural Bangladesh. Int. J. Epidemiol. 2016, 45, 1656–1667. [Google Scholar] [CrossRef] [PubMed]
- Morales, E.; García-Serna, A.M.; Larqué, E.; Sánchez-Campillo, M.; Serrano-Munera, A.; Martinez-Graciá, C.; Santaella-Pascual, M.; Suárez-Martínez, C.; Vioque, J.; Noguera-Velasco, J.A.; et al. Dietary patterns in pregnancy and biomarkers of oxidative stress in mothers and offspring: The NELA Birth Cohort. Front. Nutr. 2022, 9, 869357. [Google Scholar] [CrossRef] [PubMed]
- Katerji, M.; Filippova, M.; Duerksen-Hughes, P. Approaches and methods to measure oxidative stress in clinical samples: Research applications in the cancer field. Oxid. Med. Cell Longev. 2019, 2019, 1279250. [Google Scholar] [CrossRef]
- Ho, M.S.; Vettese, G.F.; Morris, K.; Lloyd, J.R.; Boothman, C.; Bower, W.R.; Shaw, S.; Law, G.T.W. Retention of immobile Se(0) in flow-through aquifer column systems during bioreduction and oxic-remobilization. Sci. Total Environ. 2022, 834, 155332. [Google Scholar] [CrossRef] [PubMed]
- Drejza, M.A.; Rylewicz, K.; Majcherek, E.; Gross-Tyrkin, K.; Mizgier, M.; Plagens-Rotman, K.; Wójcik, M.; Panecka-Mysza, K.; Pisarska-Krawczyk, M.; Kędzia, W.; et al. Markers of oxidative stress in obstetrics and gynaecology-a systematic literature review. Antioxidants 2022, 11, 1477. [Google Scholar] [CrossRef]
- Squillacioti, G.; Guglieri, F.; Colombi, N.; Ghelli, F.; Berchialla, P.; Gardois, P.; Bono, R. Non-Invasive measurement of exercise-induced oxidative stress in response to physical activity: A systematic review and meta-analysis. Antioxidants 2021, 10, 2008. [Google Scholar] [CrossRef]
- Cuffe, J.S.; Xu, Z.C.; Perkins, A.V. Biomarkers of oxidative stress in pregnancy complications. Biomark. Med. 2017, 11, 295–306. [Google Scholar] [CrossRef]
- Jakovljevic, K.; Malisic, E.; Cavic, M.; Krivokuca, A.; Dobricic, J.; Jankovic, R. KRAS and BRAF mutations in Serbian patients with colorectal cancer. J. BUON 2012, 17, 575–580. [Google Scholar]
- Cui, X.; Gong, J.; Han, H.; He, L.; Teng, Y.; Tetley, T.; Sinharay, R.; Chung, K.F.; Islam, T.; Gilliland, F.; et al. Relationship between free and total malondialdehyde, a well-established marker of oxidative stress, in various types of human biospecimens. J. Thorac. Dis. 2018, 10, 3088–3097. [Google Scholar] [CrossRef] [PubMed]
- Khoubnasabjafari, M.; Ansarin, K.; Jouyban, A. Salivary malondialdehyde as an oxidative stress biomarker in oral and systemic diseases. J. Dent. Res. Dent. Clin. Dent. Prospects 2016, 10, 71–74. [Google Scholar] [CrossRef]
- Mentese, A.; Güven, S.; Demir, S.; Sümer, A.; Yaman, S.Ö.; Alver, A.; Sonmez, M.; Karahan, S.C. Circulating parameters of oxidative stress and hypoxia in normal pregnancy and HELLP syndrome. Adv. Clin. Exp. Med. 2018, 27, 1567–1572. [Google Scholar] [CrossRef] [PubMed]
- Rudra, C.B.; Qiu, C.; David, R.M.; Bralley, J.A.; Walsh, S.W.; Williams, M.A. A prospective study of early-pregnancy plasma malondialdehyde concentration and risk of preeclampsia. Clin. Biochem. 2006, 39, 722–726. [Google Scholar] [CrossRef] [PubMed]
- Van ‘t Erve, T.J.; Lih, F.B.; Kadiiska, M.B.; Deterding, L.J.; Eling, T.E.; Mason, R.P. Reinterpreting the best biomarker of oxidative stress: The 8-iso-PGF(2α)/PGF(2α) ratio distinguishes chemical from enzymatic lipid peroxidation. Free Radic. Biol. Med. 2015, 83, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Pasupathi, P.; Manivannan, U.; Manivannan, P.; Deepa, M. Cardiac troponins and oxidative stress markers in non-pregnant, pregnant and preeclampsia women. Bangladesh Med. Res. Counc. Bull. 2010, 36, 4–9. [Google Scholar] [CrossRef]
- Mistry, H.D.; Gill, C.A.; Kurlak, L.O.; Seed, P.T.; Hesketh, J.E.; Méplan, C.; Schomburg, L.; Chappell, L.C.; Morgan, L.; Poston, L.; et al. Association between maternal micronutrient status, oxidative stress, and common genetic variants in antioxidant enzymes at 15 weeks׳ gestation in nulliparous women who subsequently develop preeclampsia. Free Radic. Biol. Med. 2015, 78, 147–155. [Google Scholar] [CrossRef]
- Raijmakers, M.T.; van Tits, B.J.; Hak-Lemmers, H.L.; Roes, E.M.; Steegers, E.A.; Peters, W.H. Low plasma levels of oxidized low density lipoprotein in preeclampsia. Acta Obstet. Gynecol. Scand. 2004, 83, 1173–1177. [Google Scholar] [CrossRef]
- Draganovic, D.; Lucic, N.; Jojic, D. Oxidative stress marker and pregnancy induced hypertension. Med. Arch. 2016, 70, 437–440. [Google Scholar] [CrossRef]
- Mistry, H.D.; Wilson, V.; Ramsay, M.M.; Symonds, M.E.; Broughton Pipkin, F. Reduced selenium concentrations and glutathione peroxidase activity in preeclamptic pregnancies. Hypertension 2008, 52, 881–888. [Google Scholar] [CrossRef] [PubMed]
- Catarino, C.; Santos-Silva, A.; Belo, L.; Rocha-Pereira, P.; Rocha, S.; Patrício, B.; Quintanilha, A.; Rebelo, I. Inflammatory disturbances in preeclampsia: Relationship between maternal and umbilical cord blood. J. Pregnancy 2012, 2012, 684384. [Google Scholar] [CrossRef] [PubMed]
- Draganovic, D.; Lucic, N.; Jojic, D.; Milicevic, S. Correlation of oxidative stress markers with ultrasound and cardiotocography parameters with hypertension induced pregnancy. Acta Inform. Med. 2017, 25, 19–23. [Google Scholar] [CrossRef]
- Younus, H. Therapeutic potentials of superoxide dismutase. Int. J. Health Sci. 2018, 12, 88–93. [Google Scholar]
- Ashur-Fabian, O.; Yerushalmi, G.M.; Mazaki-Tovi, S.; Steinberg, D.M.; Goldshtein, I.; Yackobovitch-Gavan, M.; Schiff, E.; Amariglio, N.; Rechavi, G. Cell free expression of hif1α and p21 in maternal peripheral blood as a marker for preeclampsia and fetal growth restriction. PLoS ONE 2012, 7, e37273. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, L.; Jin, L.; Yi, X.; Dang, E.; Yang, Y.; Li, C.; Gao, T. Oxidative stress-induced calreticulin expression and translocation: New insights into the destruction of melanocytes. J. Invest. Dermatol. 2014, 134, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Verit, F.F.; Erel, O.; Sav, M.; Celik, N.; Cadirci, D. Oxidative stress is associated with clinical severity of nausea and vomiting of pregnancy. Am. J. Perinatol. 2007, 24, 545–548. [Google Scholar] [CrossRef]
- Xu, J.; Fang, H.; Chong, Y.; Lin, L.; Xie, T.; Ji, J.; Shen, C.; Shi, C.; Shan, J. Cyclophosphamide induces lipid and metabolite perturbation in amniotic fluid during rat embryonic development. Metabolites 2022, 12, 1105. [Google Scholar] [CrossRef]
- Erisir, M.; Kandemir, F.M.; Yüksel, M. The effects of Caesarean section on lipid peroxidation and some he effects of Caesarean section on lipid peroxidation and some antioxidants in the blood of newborn calves. Vet. Arch. 2013, 83, 153–159. [Google Scholar]
- Trocino, R.A.; Akazawa, S.; Ishibashi, M.; Matsumoto, K.; Matsuo, H.; Yamamoto, H.; Goto, S.; Urata, Y.; Kondo, T.; Nagataki, S. Significance of glutathione depletion and oxidative stress in early embryogenesis in glucose-induced rat embryo culture. Diabetes 1995, 44, 992–998. [Google Scholar] [CrossRef]
- Eriksson, U.J.; Wentzel, P. The status of diabetic embryopathy. Ups. J. Med. Sci. 2016, 121, 96–112. [Google Scholar] [CrossRef]
- Guo, Q.; Liu, X.L.; Liu, H.S.; Luo, X.Y.; Yuan, Y.; Ji, Y.M.; Liu, T.; Guo, J.L.; Zhang, J. The risk model based on the three oxidative stress-related genes evaluates the prognosis of lac patients. Oxid. Med. Cell Longev. 2022, 2022, 4022896. [Google Scholar] [CrossRef] [PubMed]
- Viana, M.; Aruoma, O.I.; Herrera, E.; Bonet, B. Oxidative damage in pregnant diabetic rats and their embryos. Free Radic. Biol. Med. 2000, 29, 1115–1121. [Google Scholar] [CrossRef] [PubMed]
- Silveira, A.S.; Aydos, R.D.; Ramalho, R.T.; Silva, I.S.; Caldas, R.A.; Santos Neto, A.T.D.; Rodrigues, C.T. Oxidative stress effects in the uterus, placenta and fetus of pregnant rats submitted to acute and chronic stress. Acta Cir. Bras. 2018, 33, 806–815. [Google Scholar] [CrossRef] [PubMed]
- Cederberg, J.; Eriksson, U.J. Decreased catalase activity in malformation-prone embryos of diabetic rats. Teratology 1997, 56, 350–357. [Google Scholar] [CrossRef]
- Sivan, E.; Chen, X.; Homko, C.J.; Reece, E.A.; Boden, G. Longitudinal study of carbohydrate metabolism in healthy obese pregnant women. Diabetes. Care 1997, 20, 1470–1475. [Google Scholar] [CrossRef]
- Lang, Q.; Wei, J.; Tian, M.; Wei, S.; Yu, X.; Zhao, C.; Zhang, J.; Huang, B. Attenuated effect of zinc gluconate on oxidative stress, inflammation, and angiogenic imbalance in pre-eclampsia rats. Life Sci. 2022, 310, 121055. [Google Scholar] [CrossRef]
| Biological Materials | Group | Value | Reference |
|---|---|---|---|
| Plasma | healthy non-pregnant women (n = 50) | 2.51 nmol/mL | Pasupathi et al. [198] |
| health pregnant women (n = 50) | 4.12 nmol/mL | ||
| pregnancy-induced hypertension (n = 50) | 6.88 nmol/mL | ||
| normotensive controls pregnant women (n = 472) | 6.7 µM | Mistry et al. [199] | |
| women with pre-eclampsia (n = 244) | 6.5 µM | ||
| women with pre-eclampsia (n = 40) | 3.8 µmol/L | Raijmakers et al. [200] | |
| normotensive pregnant controls matched for gestational age (n = 24) | 1.5 µmol/L | ||
| pregnant women without hypertension (n = 100) | 20 µmol (99% women) | Draganovic et al. [201] | |
| pregnant women with hypertension (n = 100) | 20–40 µmol (66% women), 40 µmol (34% women) | ||
| umbilical cord blood | normal pregnant (n = 27) | 0.6 μmol/L | Mistry et al. [202] |
| 25 pre-eclamptic (n = 25) | 0.8 μmol/L | ||
| healthy pregnant women (n = 42) | 1.08 μmol/L | Catarino et al. [203] | |
| PE pregnant women (n = 46) | 1.10 μmol/L | ||
| amniotic fluid | pregnant women without hypertension (n = 100) | 13.41 (96% women)–13.72 (4%) µmmol | Draganovic et al. [204] |
| pregnant women with hypertension (n = 100) | 35.17 (79%)–42.37 (21%) µmmol |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grzeszczak, K.; Łanocha-Arendarczyk, N.; Malinowski, W.; Ziętek, P.; Kosik-Bogacka, D. Oxidative Stress in Pregnancy. Biomolecules 2023, 13, 1768. https://doi.org/10.3390/biom13121768
Grzeszczak K, Łanocha-Arendarczyk N, Malinowski W, Ziętek P, Kosik-Bogacka D. Oxidative Stress in Pregnancy. Biomolecules. 2023; 13(12):1768. https://doi.org/10.3390/biom13121768
Chicago/Turabian StyleGrzeszczak, Konrad, Natalia Łanocha-Arendarczyk, Witold Malinowski, Paweł Ziętek, and Danuta Kosik-Bogacka. 2023. "Oxidative Stress in Pregnancy" Biomolecules 13, no. 12: 1768. https://doi.org/10.3390/biom13121768
APA StyleGrzeszczak, K., Łanocha-Arendarczyk, N., Malinowski, W., Ziętek, P., & Kosik-Bogacka, D. (2023). Oxidative Stress in Pregnancy. Biomolecules, 13(12), 1768. https://doi.org/10.3390/biom13121768







